Effect of Heat Treatment Conditions on Mechanical Properties of Die-Casting Al–Si–Cu–xLa Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
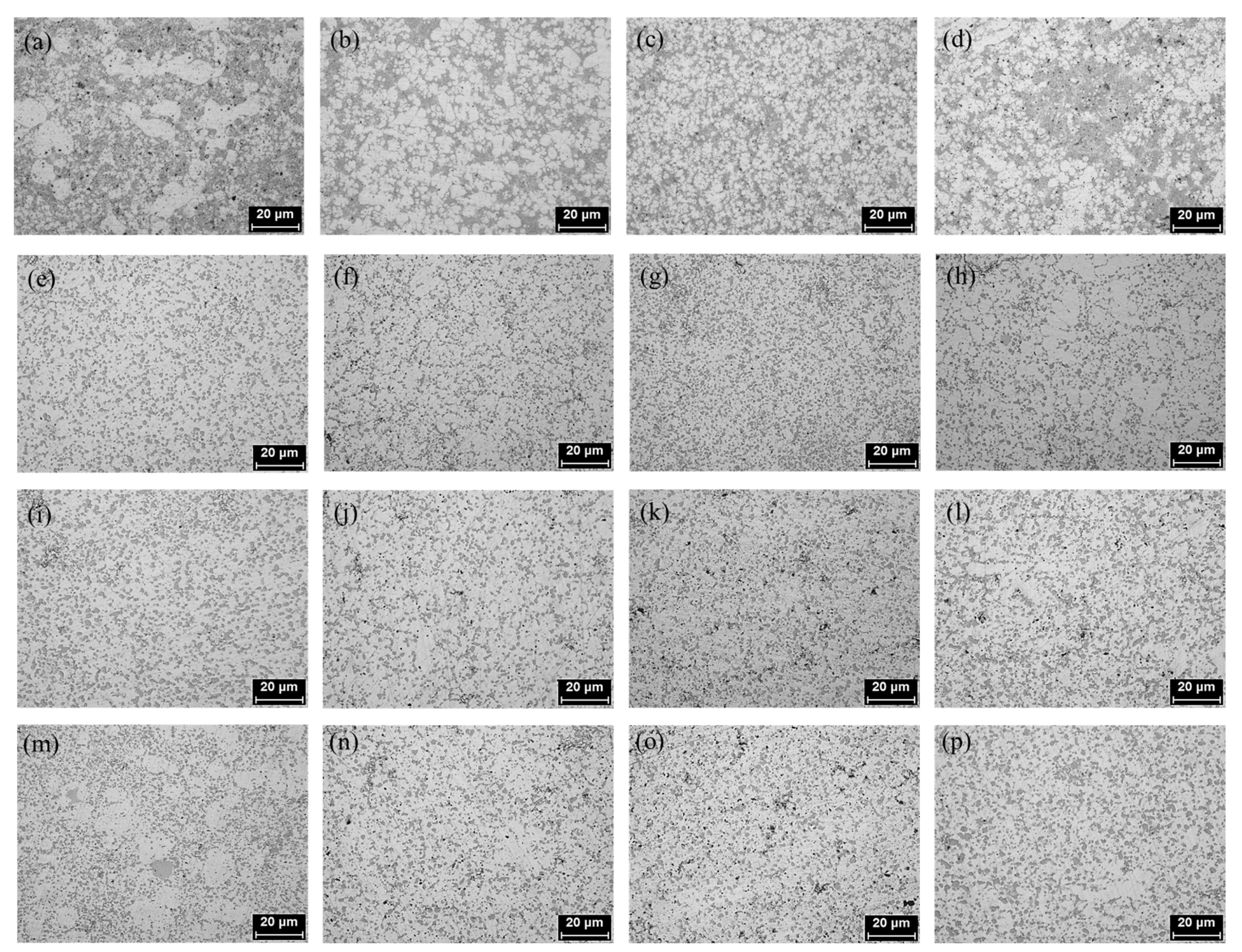
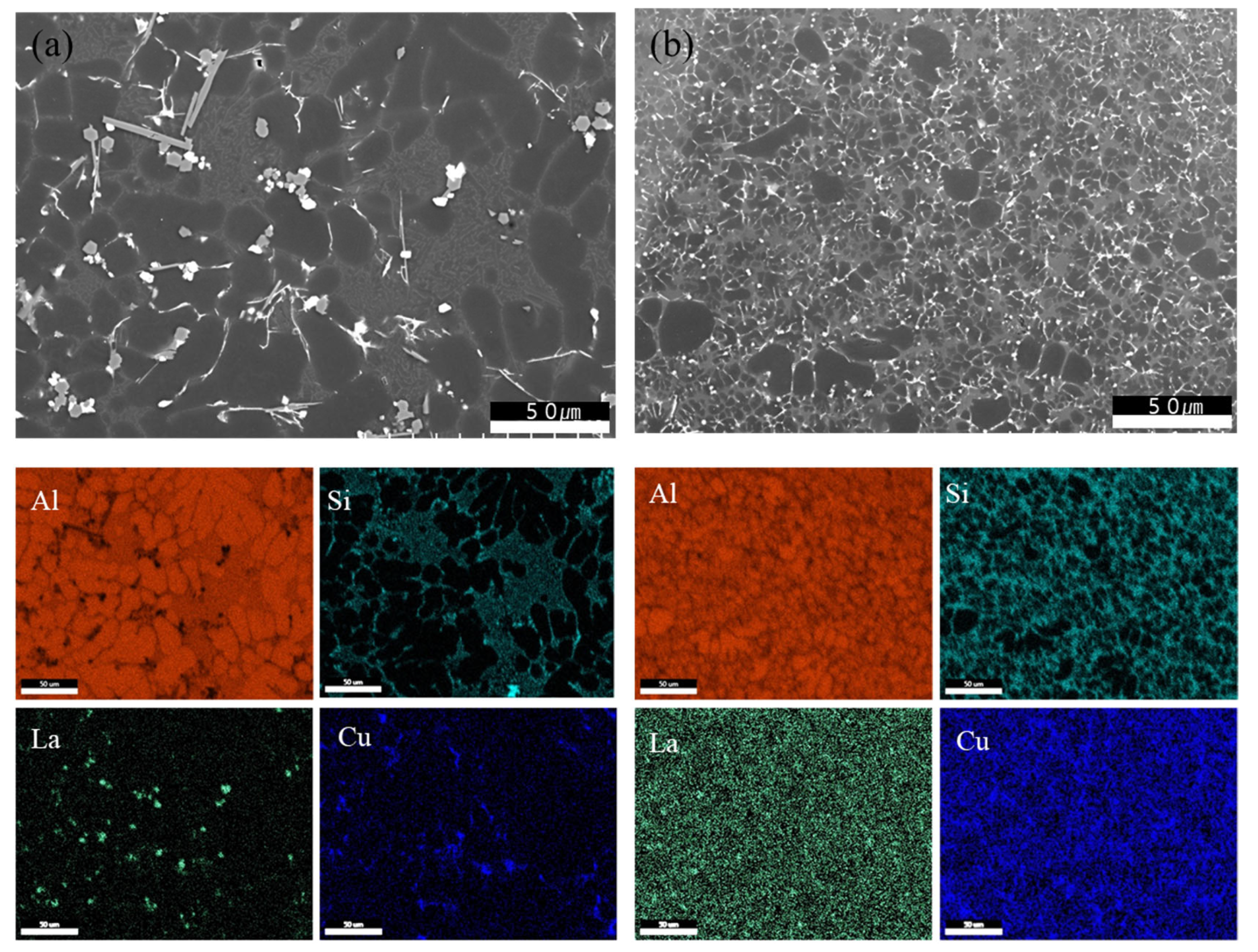
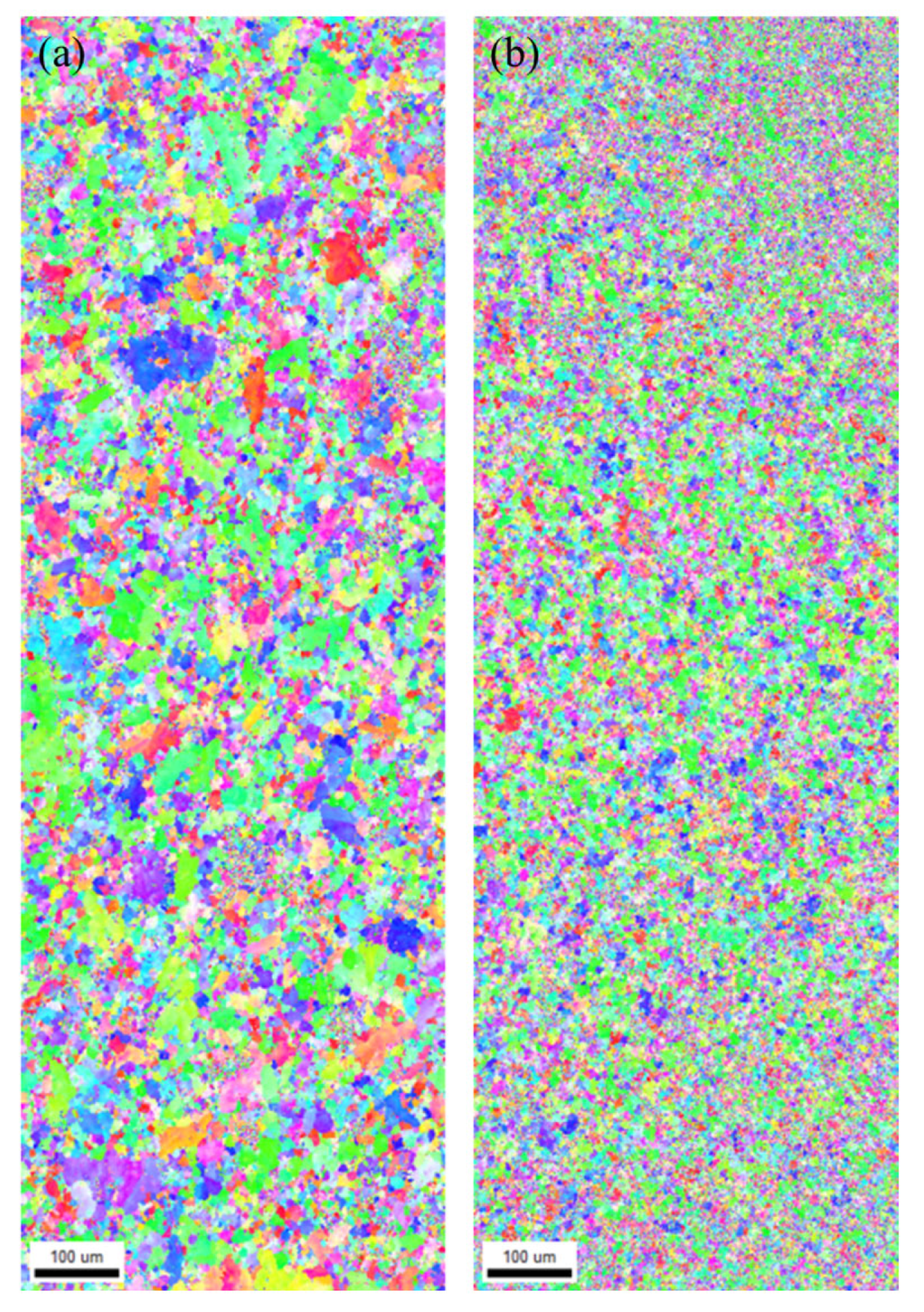
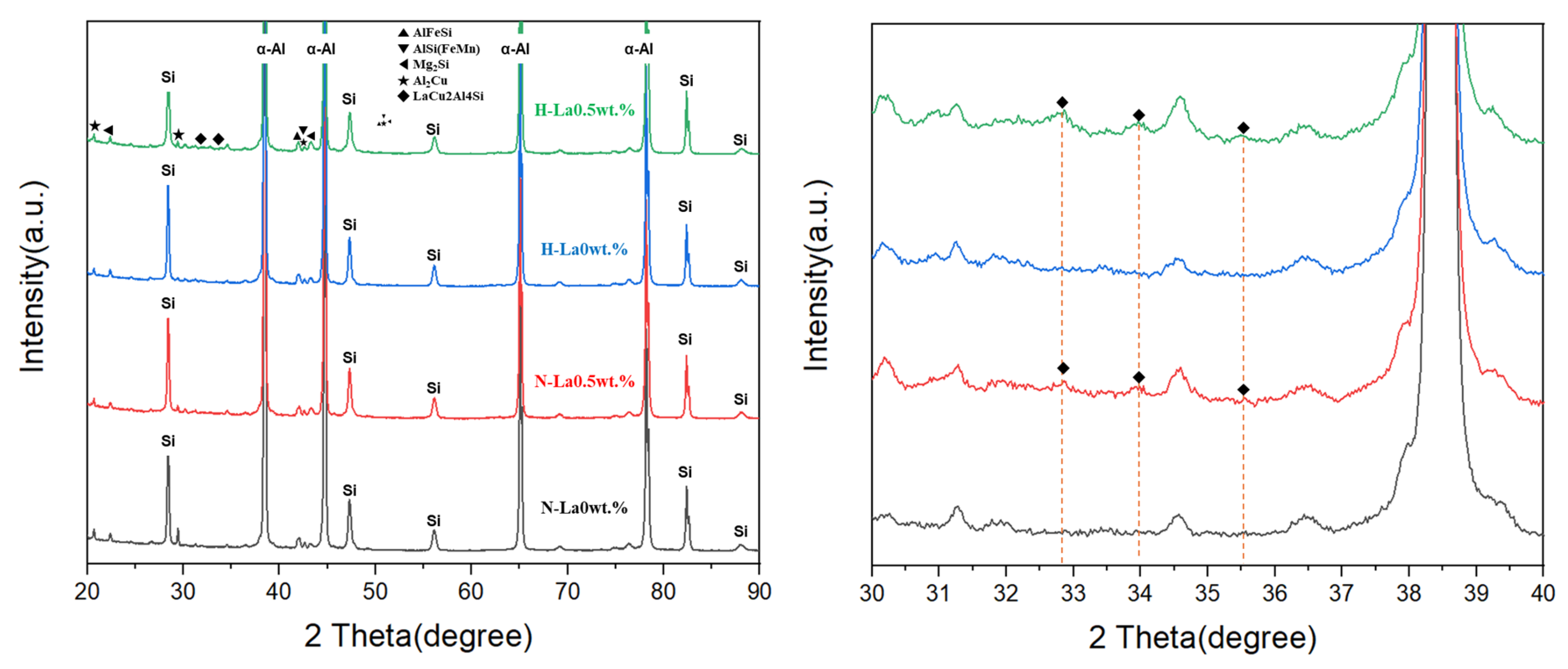
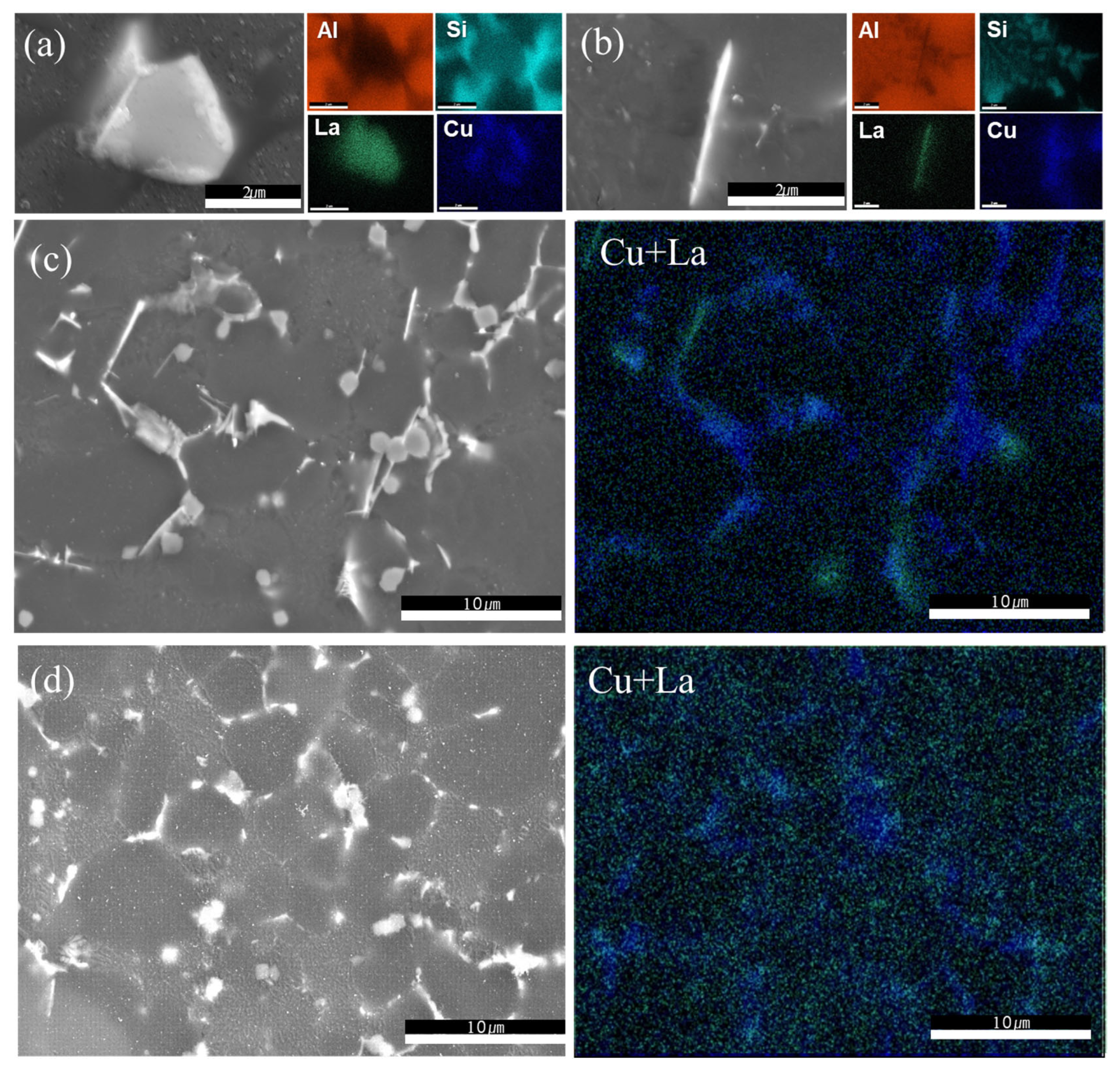
3.1. Results of Microstructure Analysis According to Heat Treatment and La Addition
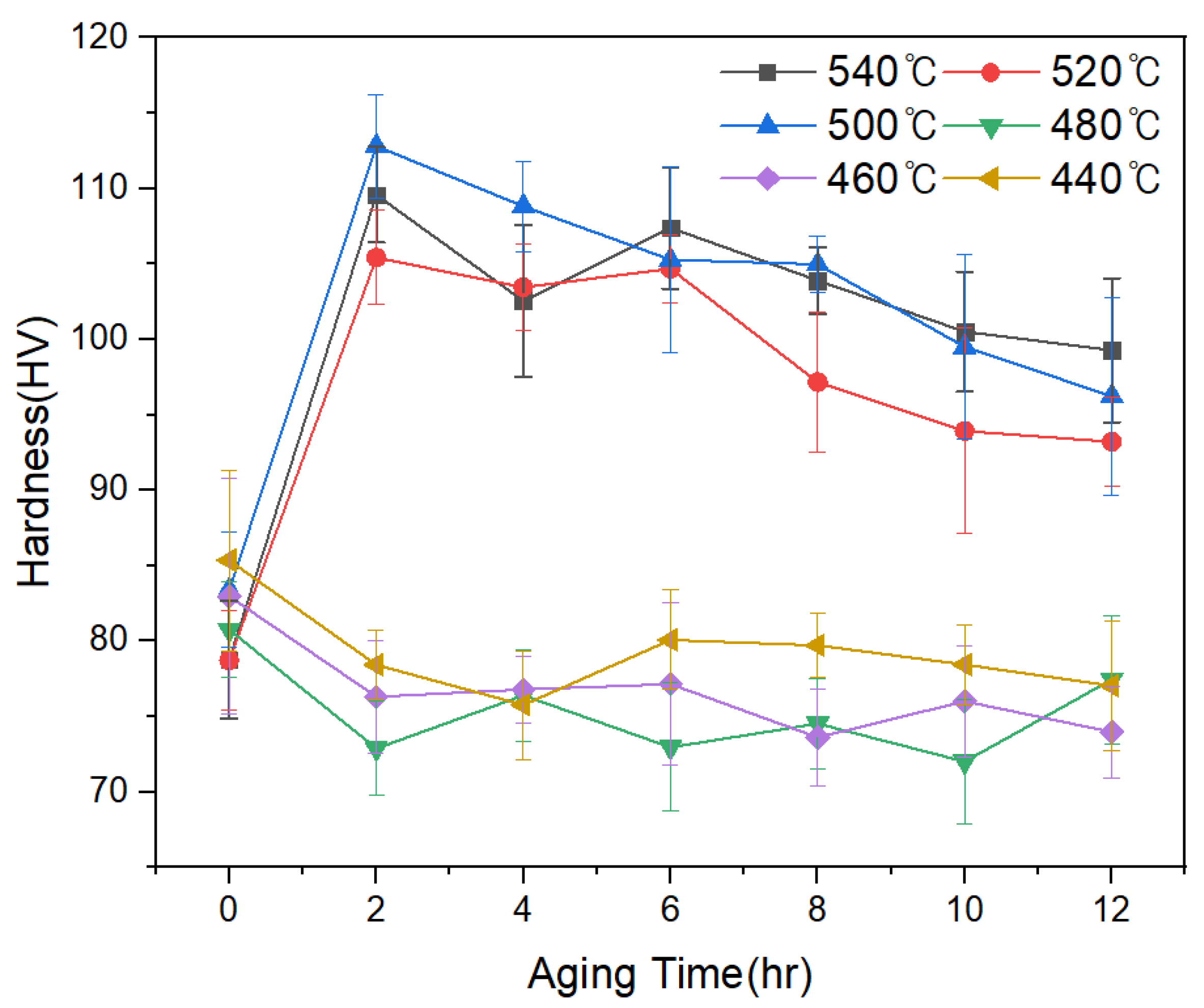
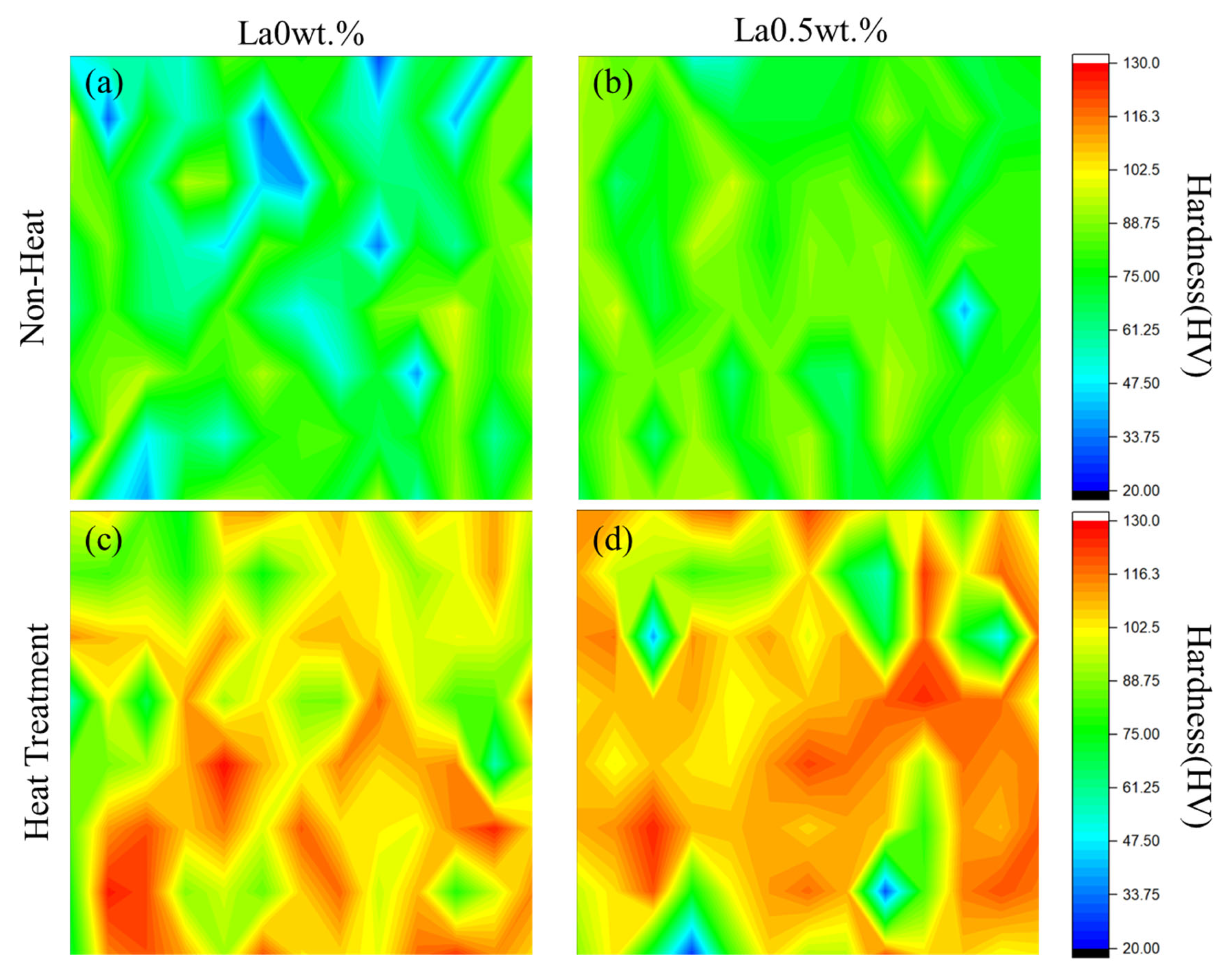
3.2. Analysis of Mechanical Properties by Heat Treatment and La Addition
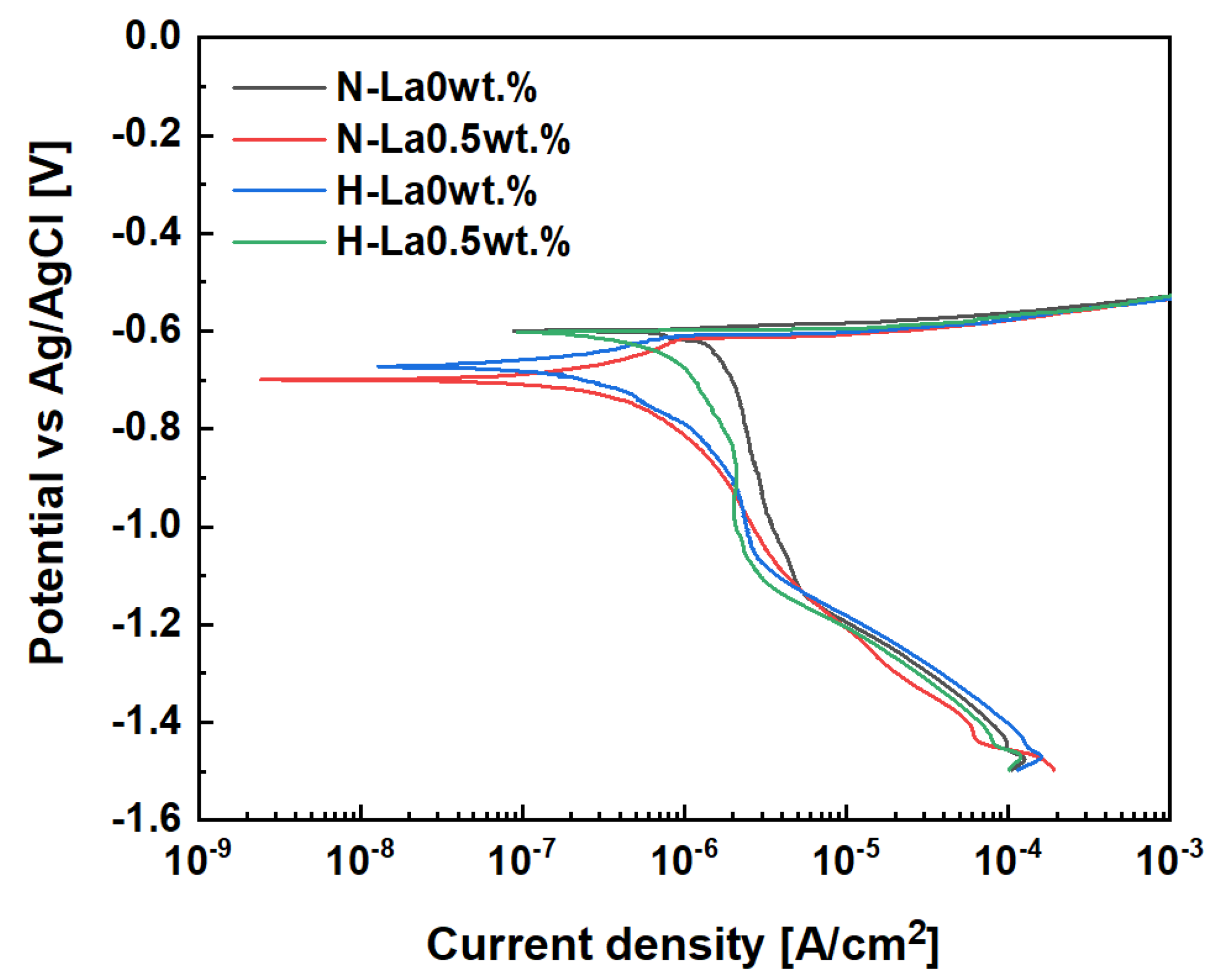
3.3. Analysis of Corrosion Resistance Characteristics According to Heat Treatment and La Addition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakai, M.; Eto, T. New aspect of development of high strength aluminum alloys for aerospace applications. Mater. Sci. Eng. A 2000, 285, 62–68. [Google Scholar] [CrossRef]
- Benedyk, J. Aluminum alloys for lightweight automotive structures. In Materials, Design and Manufacturing for Lightweight Vehicles; Woodhead Publishing: Sawston, UK, 2010; pp. 79–113. [Google Scholar]
- Gruzleski, J.E.; Closset, B.M. The treatment of liquid aluminum-silicon alloys. In Des Plaines; American Foundarymen’s Society Inc.: Schaumburg, IL, USA, 1990; p. 25. [Google Scholar]
- Liao, H.; Sun, G. Mutual poisoning effect between Sr and B in Al-Si casting alloys. Scripta Mater. 2003, 48, 1035–1039. [Google Scholar] [CrossRef]
- Chen, S.; Chen, K.; Peng, G.; Chen, X.; Ceng, Q. Effect of heat treatment on hot deformation behavior and microstructure evolution of 7085 aluminum alloy. J. Alloys Compd. 2012, 537, 338–345. [Google Scholar] [CrossRef]
- Toda, H.; Qu, P.C.; Ito, S.; Shimizu, K.; Uesugi, K.; Takeuchi, A.; Suzuki, Y.; Kobayashi, M. Formation behaviour of blister in cast aluminium alloy. Int. J. Cast Metal Res. 2014, 27, 369–377. [Google Scholar] [CrossRef]
- Timelli, G.; Lohne, O.; Arnberg, L.; Laukli, H.I. Effect of solution heat treatments on the microstructure and mechanical properties of a die-cast AlSi7MgMn alloy. Metall. Mater. Trans. A 2008, 39, 1747–1758. [Google Scholar] [CrossRef]
- Han, L.; Wu, X.; Zhu, J.; Bai, M.; Zheng, X.; Zhang, Z. Study on microstructures and properties of the Al. alloy vacuum die-cast parts of TL117 and C611. J. Phys. Conf. Ser. 2023, 2468, 012111. [Google Scholar] [CrossRef]
- Yang, H.; Ji, S.; Fan, Z. Effect of heat treatment and Fe content on the microstructure and mechanical properties of die-cast Al–Si–Cu alloys. Mater. Design 2015, 85, 823–832. [Google Scholar] [CrossRef]
- Lumley, R.N.; Gunasegaram, D.R.; Gershenzon, M.; O’Donnell, R.G. Effect of alloying elements on heat treatment response of aluminium high pressure die castings. Int. Heat Treat. Surf. Eng. 2010, 4, 25–32. [Google Scholar] [CrossRef]
- Cingi, C.; Rauta, V.; Suikkanen, E.; Orkas, J. Effect of heat treatment on thermal conductivity of aluminum die casting alloys. Adv. Mat. Res. 2012, 538–541, 2047–2052. [Google Scholar] [CrossRef]
- Cai, Q.; Mendis, C.L.; Wang, S.; Chang, I.T.H.; Fan, Z. Effect of heat treatment on microstructure and tensile properties of die-cast Al-Cu-Si-Mg alloys. J. Alloys Compd. 2021, 881, 160559. [Google Scholar] [CrossRef]
- Sjölander, E.; Seifeddine, S. The heat treatment of Al–Si–Cu–Mg casting alloys. J. Mater. Process. Technol. 2010, 210, 1249–1259. [Google Scholar] [CrossRef]
- Torfeh, M.; Niu, Z.; Assadi, H. Phase-field modelling of bimodal dendritic solidification during Al alloy die casting. Metals 2025, 15, 66. [Google Scholar] [CrossRef]
- Liu, W.Y.; Xu, C.; Xiao, W.L.; Liu, M.W.; Zhang, J.B.; Chen, J.X.; Ma, C.L. Effects of cooling rate on morphology of eutectic Si in RE modified Al-10wt.% Si alloy. Mater. Sci. Forum 2016, 850, 587–593. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Feng, J.; Zhang, Y.; Kang, Y. Effect of cooling rates on the microstructure and mechanical property of La modified Al7SiMg alloys processed by gravity die casting and semi-solid die casting. Metals 2020, 10, 549. [Google Scholar] [CrossRef]
- Mitrašinović, A.M.; Robles Hernández, F.C. Determination of the growth restriction factor and grain size for aluminum alloys by a quasi-binary equivalent method. Mater. Sci. Eng. A 2012, 540, 63–69. [Google Scholar] [CrossRef]
- Zhao, B.; Xing, S.; Sun, H.; Yan, G.; Gao, W.; Ou, L. Effect of rare-earth La on microstructure and mechanical properties of Al7Si4CuMg alloys prepared by squeeze casting. J. Mater. Sci. 2022, 57, 12064–12083. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhang, L.; Jiang, H.; Zhao, J.; He, J. Effect mechanisms of micro-alloying element La on microstructure and mechanical properties of hypoeutectic Al-Si alloys. J. Mater. Sci. Technol. 2020, 47, 142–151. [Google Scholar] [CrossRef]
- Liang, G.; Ali, Y.; You, G.; Zhang, M.-X. Effect of cooling rate on grain refinement of cast aluminum alloys. Materialia 2018, 3, 113–121. [Google Scholar] [CrossRef]
- Liu, W.; Yan, H.; Zhu, J.-B. Effect of the addition of rare earth element La on the tribological behavior of AlSi5Cu1Mg alloy. Appl. Sci. 2018, 8, 163. [Google Scholar] [CrossRef]
- Mi, B.; Wang, Q.; Xu, Y.; Qin, Z.; Chen, Z.; Wang, H. Improvement in corrosion resistance and interfacial contact resistance properties of 316L stainless steel by coating with Cr, Ti Co-doped amorphous carbon films in the environment of the PEMFCs. Molecules 2023, 28, 2821. [Google Scholar] [CrossRef]
- ASTM G102; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. National Standard of the People’s Republic of China: Beijing, China, 2023.
- Stern, M.; Geary, A.L. Electrochemical Polarization: I. A Theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56. [Google Scholar] [CrossRef]
- Fusco, M.A.; Ay, Y.; Casey, A.H.M.; Bourham, M.A.; Winfrey, A.L. Corrosion of single layer thin film protective coatings on steel substrates for high level waste containers. Prog. Nucl. Energy 2016, 89, 159–169. [Google Scholar] [CrossRef]
- Yanagimoto, H.; Saito, K.; Takahashi, H.; Chiba, M. Changes in the structure and corrosion protection ability of porous anodic oxide films on pure Al and Al alloys by pore sealing treatment. Materials 2022, 15, 8544. [Google Scholar] [CrossRef] [PubMed]
- Serizawa, A.; Oda, T.; Watanabe, K.; Mori, K.; Yokomizo, T.; Ishizaki, T. Formation of anticorrosive film for suppressing pitting corrosion on Al-Mg-Si alloy by steam coating. Coatings 2018, 8, 23. [Google Scholar] [CrossRef]
- Kim, K.; Heo, U.; Yang, H.; Kang, N. Enhancement of the corrosion properties of Al–10% Si–2% Cu alloys with La addition. Materials 2024, 17, 2496. [Google Scholar] [CrossRef]
- Wan, H.; Shuai, L.; Ling, L.; Hu, Z.; Yan, H. Mechanical properties and corrosion resistance of La2O3/A356 composites fabricated by ultrasonic-assisted casting. Metals 2025, 15, 184. [Google Scholar] [CrossRef]
- Bartawi, E.H.; Marioara, C.D.; Shaban, G.; Rahimi, E.; Mishin, O.V.; Sunde, J.K.; Gonzalez-Garcia, Y.; Holmestad, R.; Ambat, R. Effects of grain boundary chemistry and precipitate structure on intergranular corrosion in Al-Mg-Si alloys doped with Cu and Zn. Corros. Sci. 2024, 236, 112227. [Google Scholar] [CrossRef]
- Mao, H.; Kong, Y.; Cai, D.; Yang, M.; Peng, Y.; Zeng, Y.; Zhang, G.; Shuai, X.; Huang, Q.; Li, K.; et al. β″needle-shape precipitate formation in Al-Mg-Si alloy: Phase field simulation and experimental verification. Comput. Mater. Sci. 2020, 184, 109878. [Google Scholar] [CrossRef]
- Kayani, S.H.; Ha, H.-Y.; Kim, B.-J.; Cho, Y.-H.; Son, H.-W.; Lee, J.-M. Impact of intermetallic phases on the localized pitting corrosion and high-temperature tensile strength of Al–SiMgCuNi alloys. Corros. Sci. 2024, 233, 112064. [Google Scholar] [CrossRef]
- Vončina, M.; Medved, J.; Bombač, D.; Ozimič, K. The influence of minor additions of La and Ce on the microstructural components and forming properties of Al-1.4 Fe Alloys. Appl. Sci. 2024, 14, 8194. [Google Scholar] [CrossRef]
- Taheri, M.L.; Molodov, D.; Gottstein, G.; Rollett, A.D. Grain boundary mobility under a stored-energy driving force: A comparison to curvature-driven boundary migration. Int. J. Mater. Res. 2022, 96, 1166–1170. [Google Scholar] [CrossRef]
- Kus, E.; Lee, Z.; Nutt, S.; Mansfeld, F. A comparison of the corrosion behavior of nanocrystalline and conventional Al 5083 samples. Corrosion 2006, 62, 152–161. [Google Scholar] [CrossRef]
- Ralston, K.D.; Fabijanic, D.; Birbilis, N. Effect of grain size on corrosion of high purity aluminum. Electrochim. Acta 2011, 56, 1729–1736. [Google Scholar] [CrossRef]


| Alloys | Si | Mg | Ti | Mn | Fe | Cu | Zn | Sr | La | Al |
|---|---|---|---|---|---|---|---|---|---|---|
| ASCD-0 | 9.18 | 0.25 | 0.03 | 0.22 | 0.69 | 1.50 | 0.94 | 0.02 | - | Bal. |
| ASCD-0.25 | 9.44 | 0.28 | 0.08 | 0.21 | 1.06 | 1.50 | 1.07 | 0.02 | 0.28 | |
| ASCD-0.5 | 8.96 | 0.24 | 0.02 | 0.21 | 0.66 | 1.42 | 0.90 | 0.02 | 0.45 | |
| ASCD-0.75 | 8.48 | 0.26 | 0.02 | 0.22 | 0.65 | 1.41 | 0.89 | 0.02 | 0.66 |
| Alloys Aging Time (h) | La0wt.% | La0.25wt.% | La0.5wt.% | La0.75wt.% | ||||
|---|---|---|---|---|---|---|---|---|
| Area Fraction (%) | SD. | Area Fraction (%) | SD. | Area Fraction (%) | SD. | Area Fraction (%) | SD. | |
| 0 | 74.73 | 2.67 | 70.86 | 0.30 | 65.97 | 0.31 | 71.18 | 1.06 |
| 2 | 60.63 | 0.62 | 57.27 | 1.29 | 54.92 | 0.23 | 57.22 | 0.32 |
| 6 | 70.33 | 1.20 | 67.41 | 1.24 | 61.66 | 1.63 | 68.18 | 0.37 |
| 12 | 76.47 | 2.40 | 71.81 | 0.38 | 67.41 | 1.66 | 73.51 | 2.30 |
| Alloys | Icorr (A/cm2) | Ecorr (V) | Corrosion Rate (mmy) | Βanodic (V/Decade) | βcathodic (V/Decade) | Rp (Ω·cm2) | Icorr (A/cm2) |
|---|---|---|---|---|---|---|---|
| N-La0wt.% | 1.27 × 10−6 | 0.603 | 1.38 × 10−2 | 0.0292 | 0.398 | 9.29 × 103 | 1.27 × 10−6 |
| N-La0.5wt.% | 6.65 × 10−7 | 0.603 | 7.25 × 10−3 | 0.0409 | 0.366 | 2.40 × 104 | 6.65 × 10−7 |
| H-La0wt.% | 4.34 × 10−7 | 0.673 | 4.74 × 10−3 | 0.0354 | 0.312 | 3.91 × 104 | 4.34 × 10−7 |
| H-La0.5wt.% | 2.20 × 10−7 | 0.692 | 2.41 × 10−3 | 0.0353 | 0.408 | 6.42 × 104 | 2.20 × 10−7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Heo, U.; Bae, Y.; Kim, S.; Kang, N.; Yang, H. Effect of Heat Treatment Conditions on Mechanical Properties of Die-Casting Al–Si–Cu–xLa Alloys. Materials 2025, 18, 3046. https://doi.org/10.3390/ma18133046
Kim K, Heo U, Bae Y, Kim S, Kang N, Yang H. Effect of Heat Treatment Conditions on Mechanical Properties of Die-Casting Al–Si–Cu–xLa Alloys. Materials. 2025; 18(13):3046. https://doi.org/10.3390/ma18133046
Chicago/Turabian StyleKim, Kyeonghun, Uro Heo, Younghun Bae, Seongtak Kim, NamHyun Kang, and Haewoong Yang. 2025. "Effect of Heat Treatment Conditions on Mechanical Properties of Die-Casting Al–Si–Cu–xLa Alloys" Materials 18, no. 13: 3046. https://doi.org/10.3390/ma18133046
APA StyleKim, K., Heo, U., Bae, Y., Kim, S., Kang, N., & Yang, H. (2025). Effect of Heat Treatment Conditions on Mechanical Properties of Die-Casting Al–Si–Cu–xLa Alloys. Materials, 18(13), 3046. https://doi.org/10.3390/ma18133046







