Next-Generation Bioplastics for Food Packaging: Sustainable Materials and Applications
Abstract
:1. Introduction
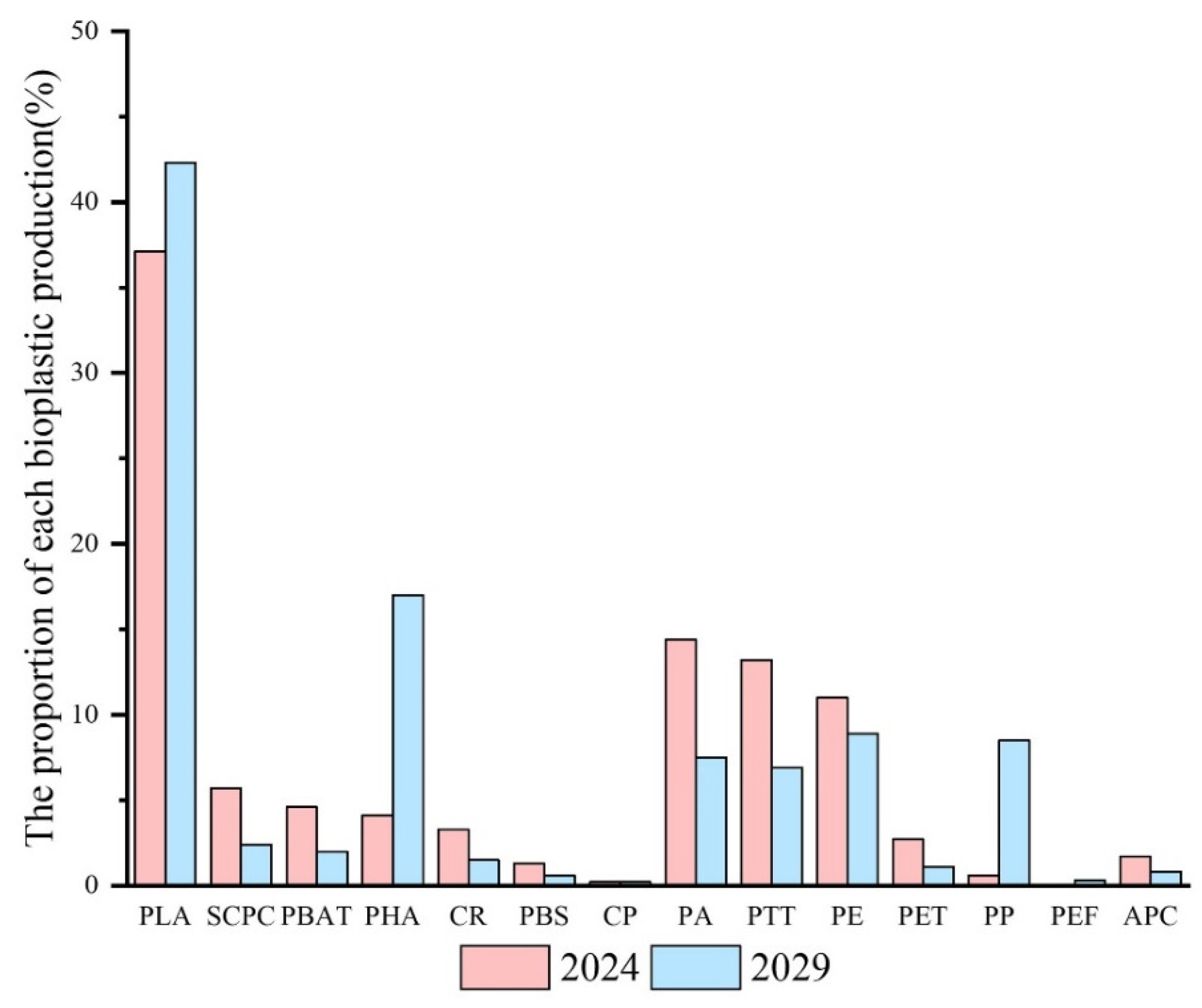
2. Classification and Characterization of Bioplastics
2.1. Biobased Biodegradable Bioplastics
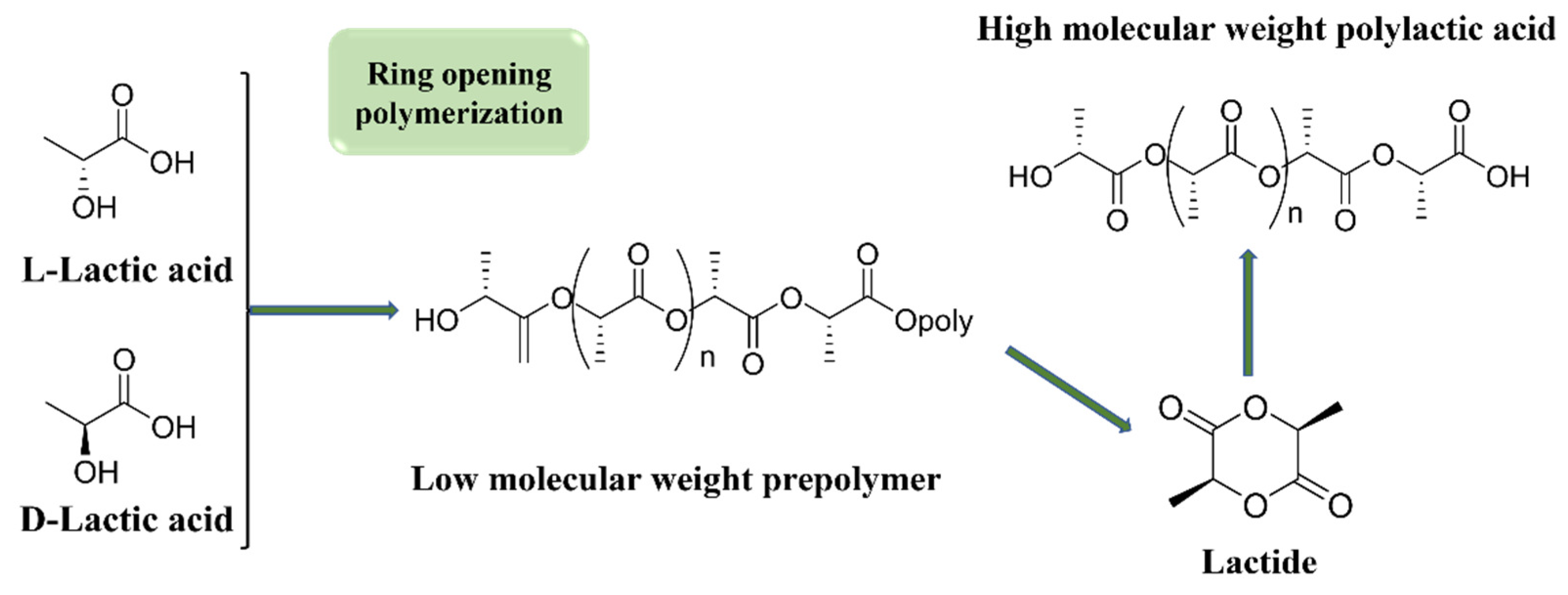
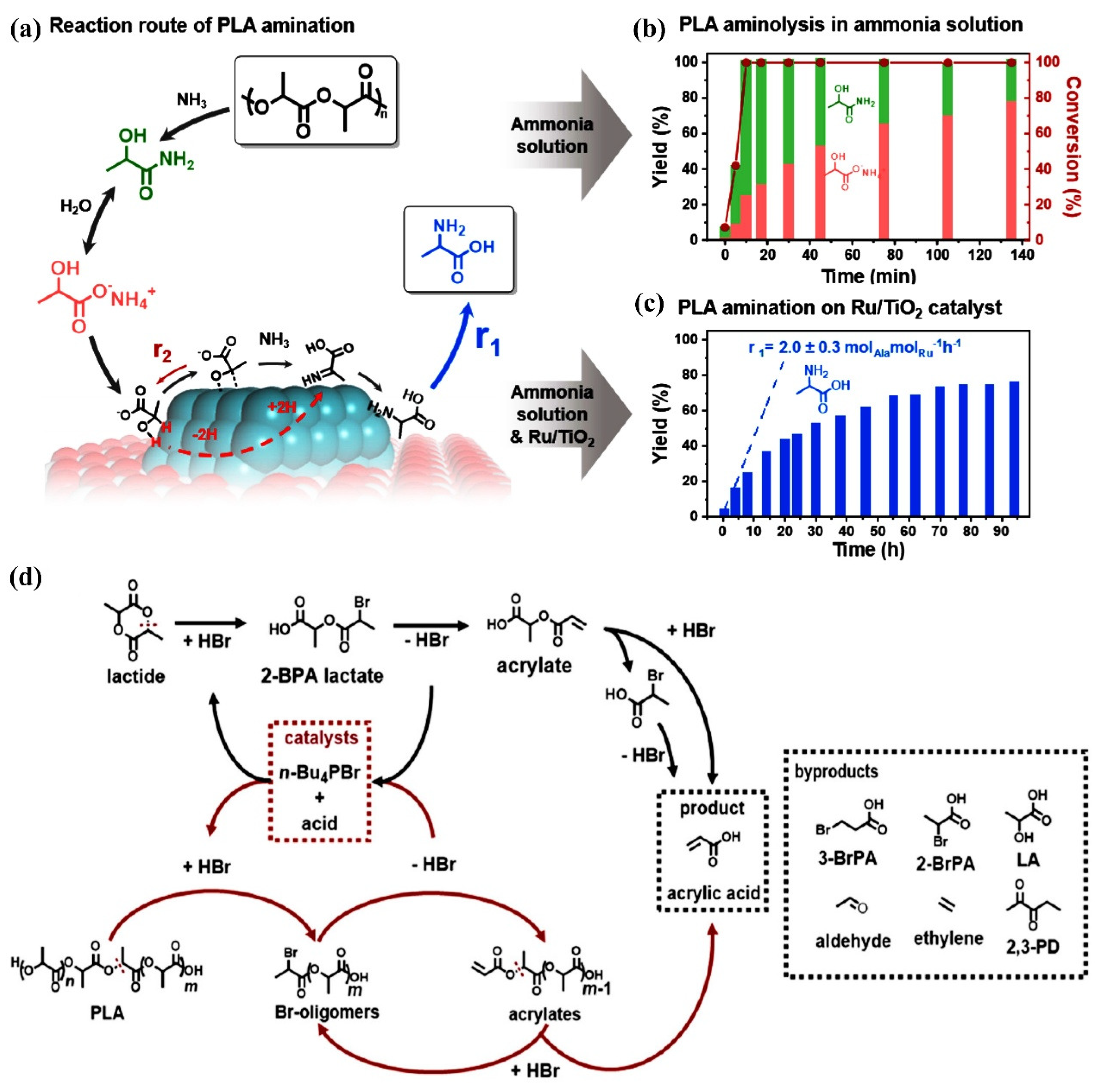
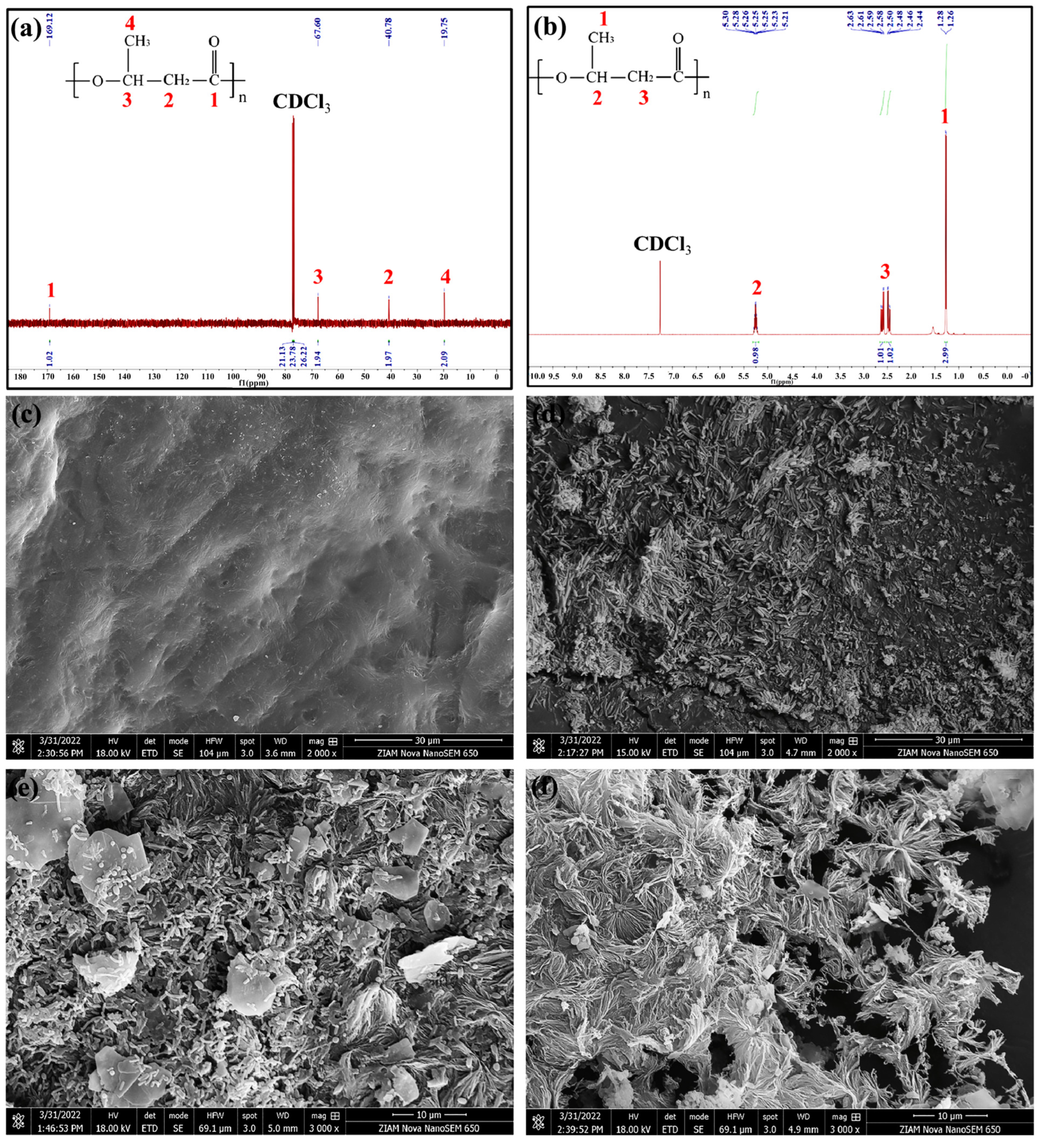
2.1.1. PLA
2.1.2. PHA
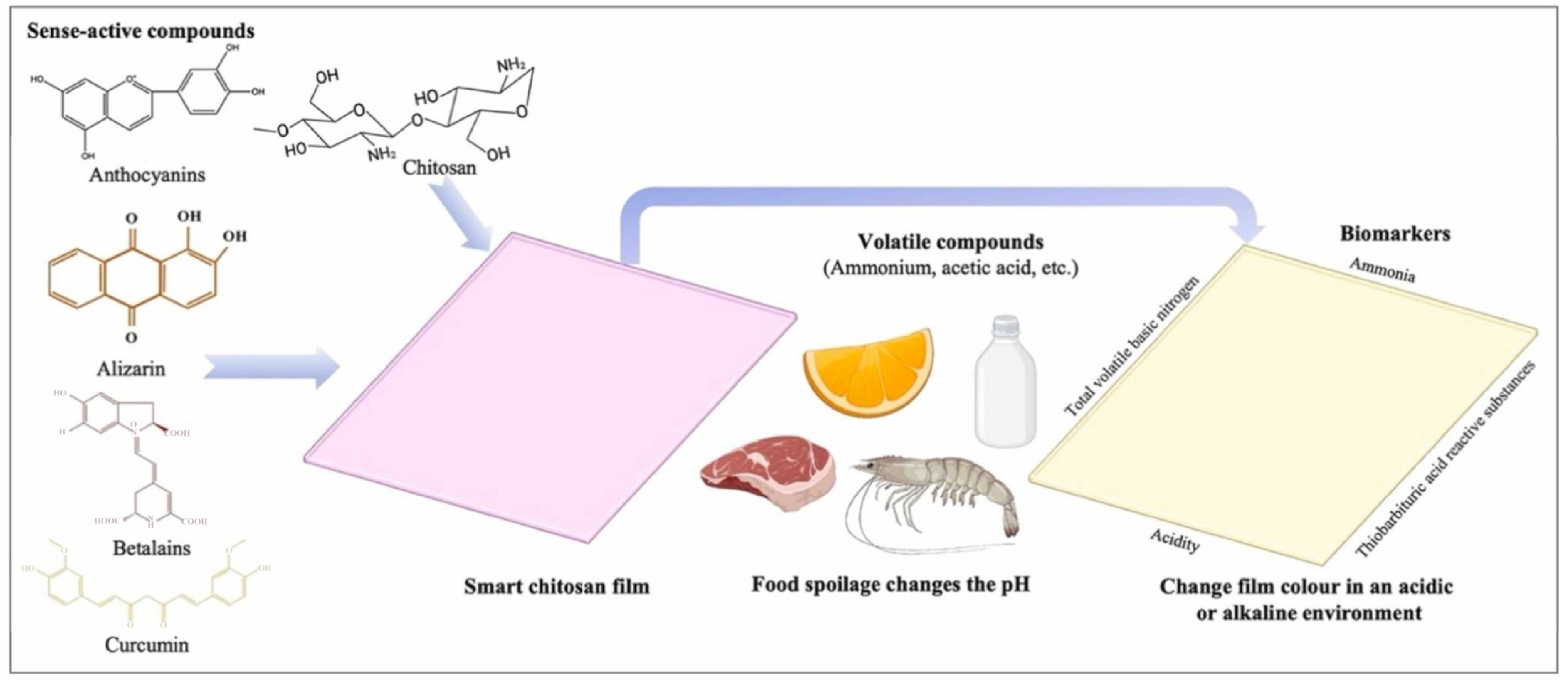
2.1.3. Chitosan
2.1.4. Cellulose
2.2. Biobased Non-Biodegradable Bioplastics
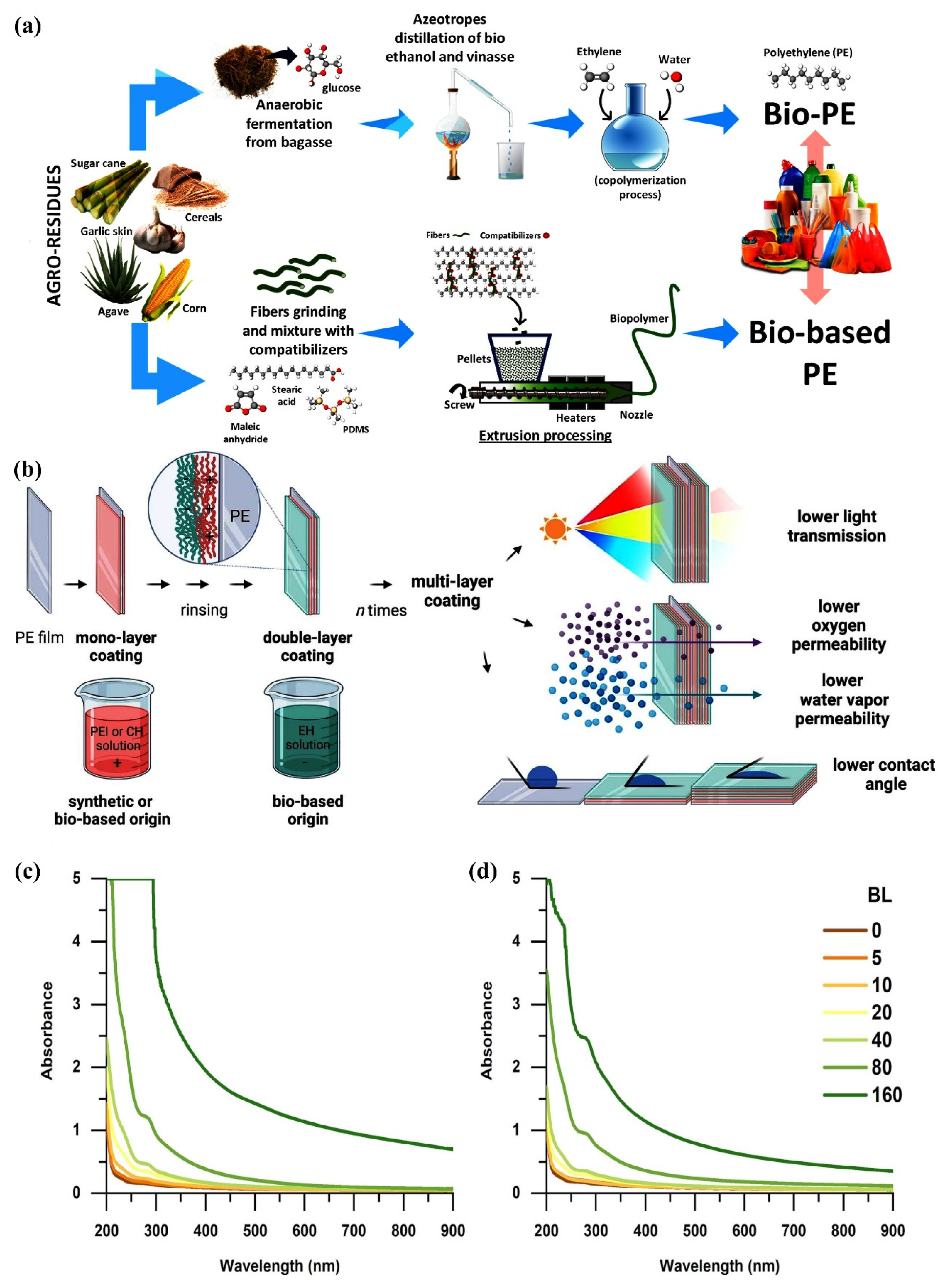
2.2.1. Bio-PE
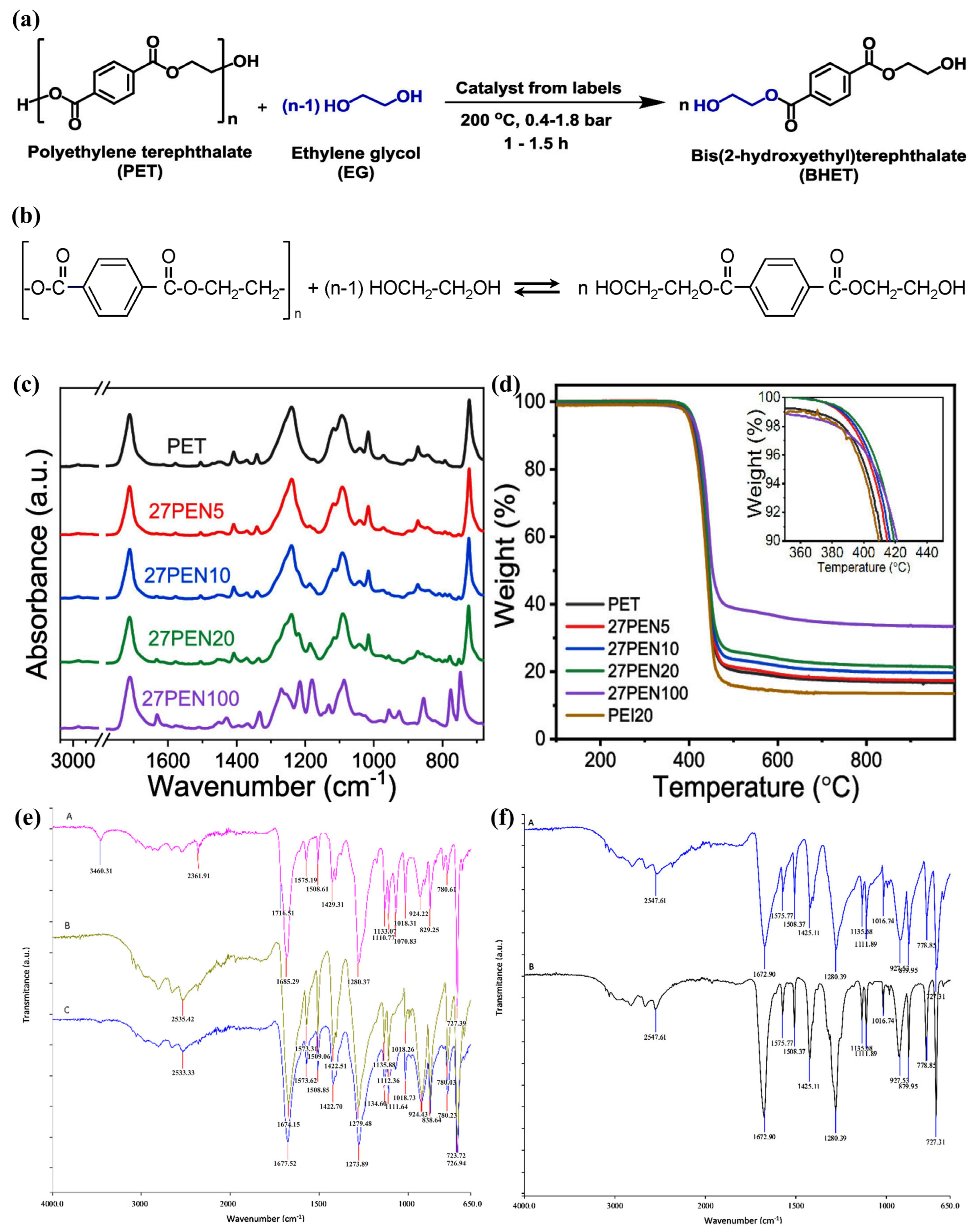
2.2.2. Bio-PET
| Add Ingredients | Function | Processing Technology | Degradation Rate | Reference |
|---|---|---|---|---|
| Co-CeO2 | Catalyst | Photocatalytic reaction | 91.61 ± 1.50% | [170] |
| TFA | Activator | Depolymerization reaction | 96% | [171] |
| maleic acid/DSS | Test agent | Subcritical water conditions | 100% | [172] |
| Glutathione S-Transferase | Catalyst | Catalytic reaction | 98.9% | [173] |
| FAST-PETase hydrolase | Hydrolytic | Hydrolytic reaction | 100% | [174] |
2.3. Non-Biobased Biodegradable Bioplastics
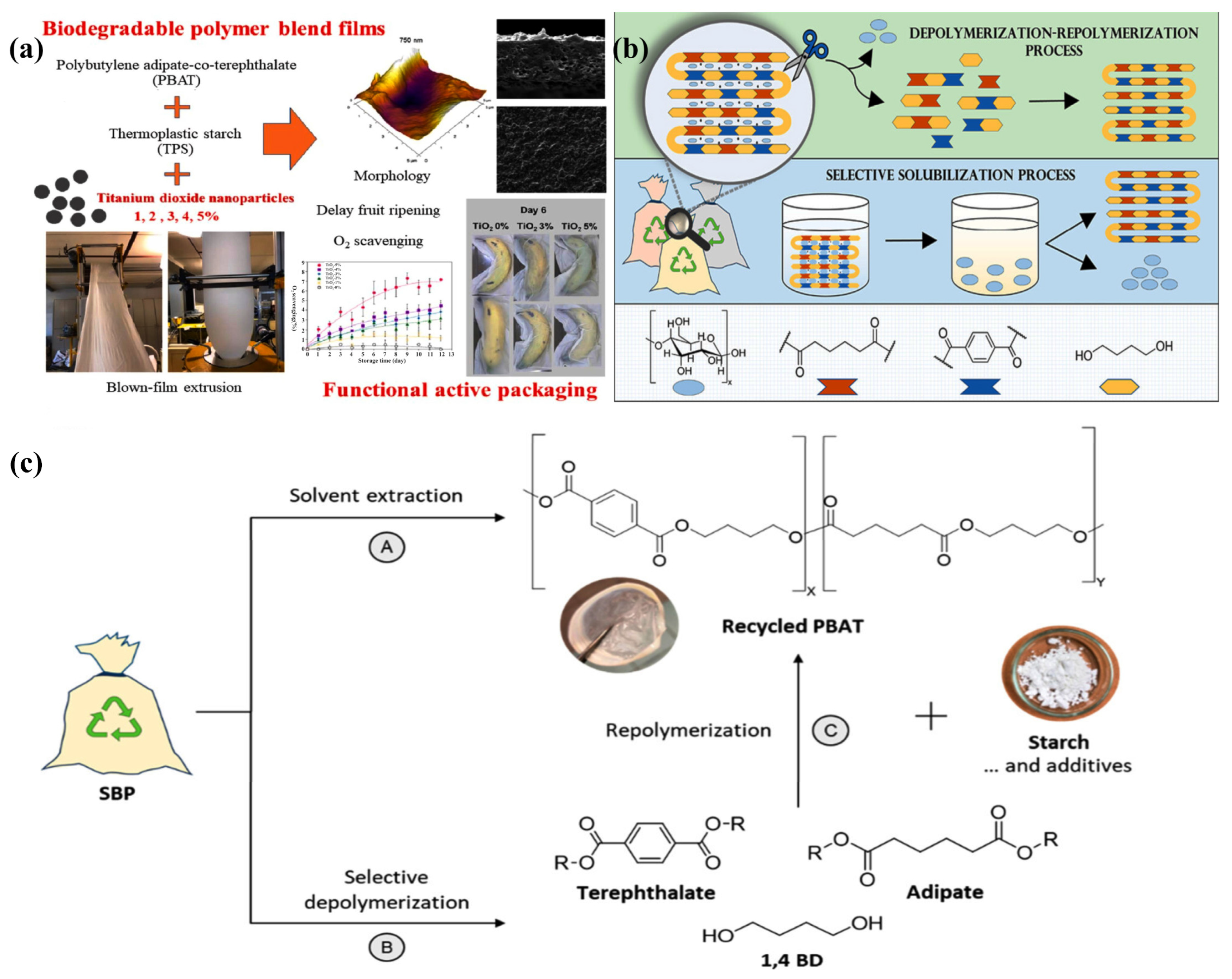
2.3.1. PBAT
2.3.2. PCL
2.3.3. PBS
3. Processing Technologies and Applications of Bioplastics
3.1. Thermoforming
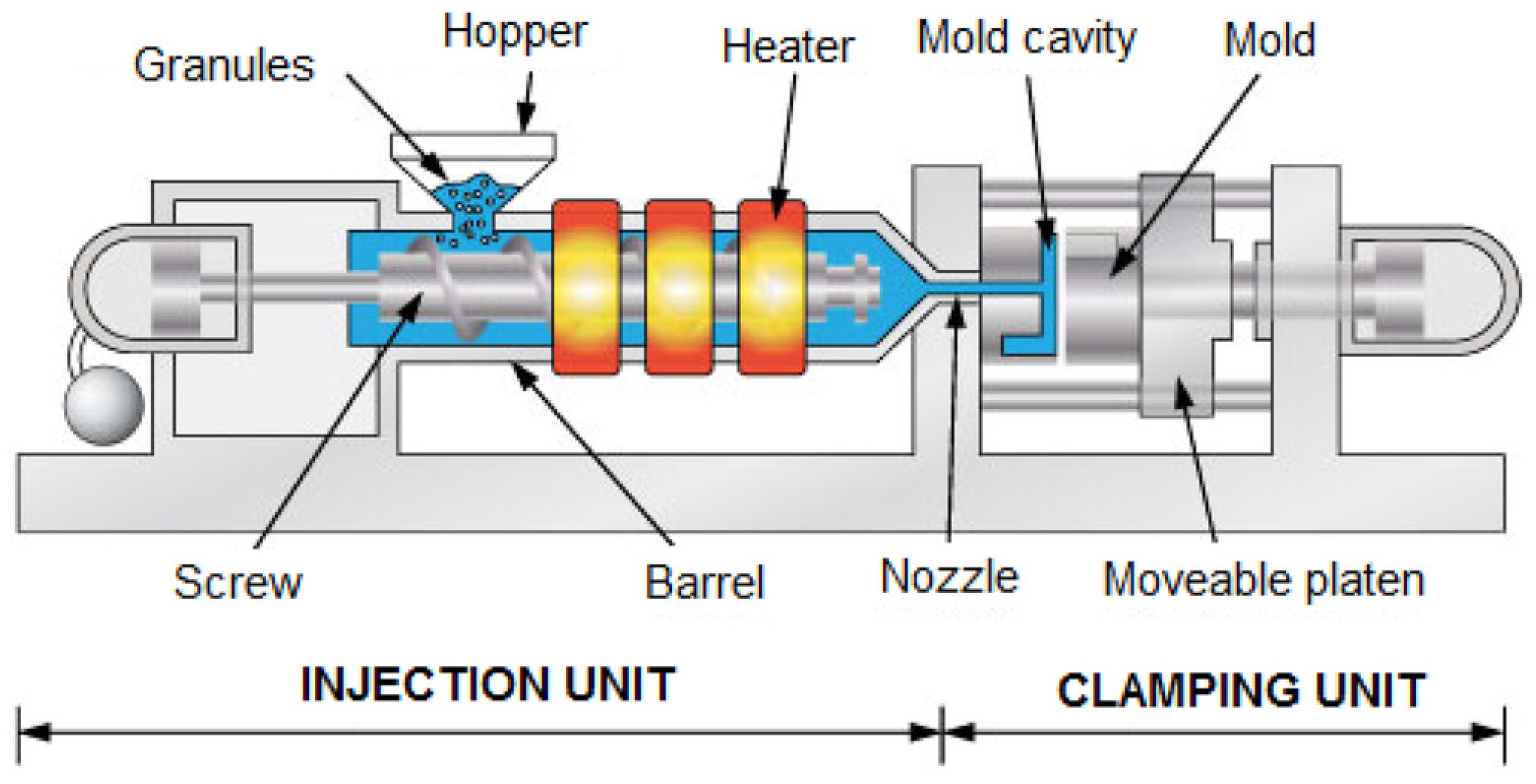
3.2. Injection Molding
3.3. Extrusion Molding
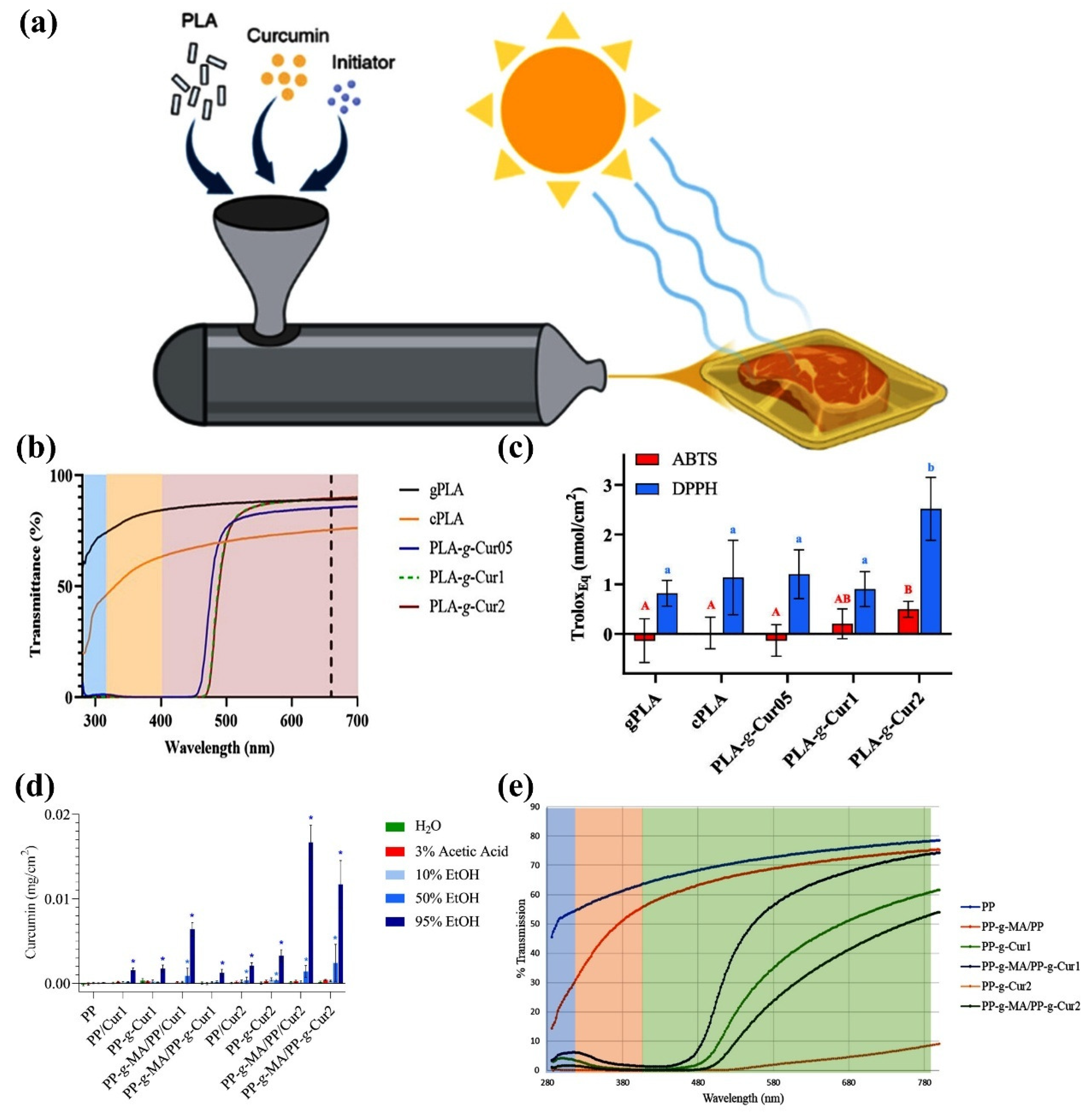
3.4. Coating Technology
4. Current Challenges and Future Developments
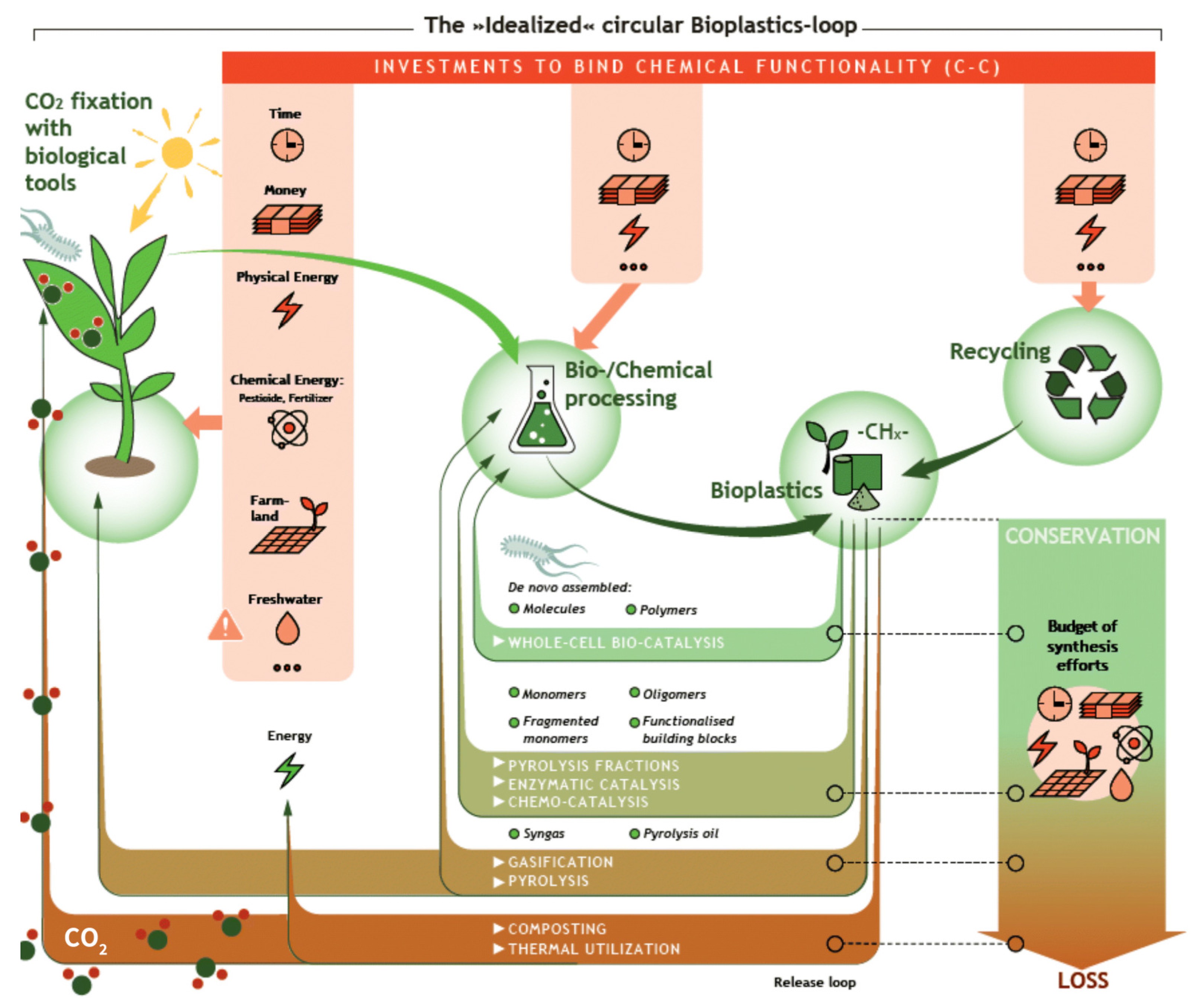
5. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Landrigan Philip, J. Plastics, Fossil Carbon, and the Heart. N. Engl. J. Med. 2024, 390, 948–950. [Google Scholar] [CrossRef] [PubMed]
- On the plastics crisis. Nat. Sustain. 2023, 6, 1137. [CrossRef]
- Lima, T.C.H.; Machado, E.L.; Schneider, R.d.C.d.S. Scientometric analysis of the development of plastic packaging considering the circular economy and clean technologies: A review. Waste Manag. Res. 2023, 41, 1188–1202. [Google Scholar] [CrossRef] [PubMed]
- Navarre, N.; Mogollon, J.M.; Tukker, A.; Barbarossa, V. Recycled plastic packaging from the Dutch food sector pollutes Asian oceans. Resour. Conserv. Recycl. 2022, 185, 106508. [Google Scholar] [CrossRef]
- Novakovic, K.; Thumbarathy, D.; Peeters, M.; Geoghegan, M.; Jefferies, J.G.; Hicks, C.; Manika, D.; Dai, S. Zero-waste circular economy of plastic packaging: The bottlenecks and a way forward. Sustain. Mater. Technol. 2023, 38, e00735. [Google Scholar] [CrossRef]
- Plastics give and plastics take. Nat. Rev. Mater. 2022, 7, 67. [CrossRef]
- Cowger, W.; Willis, K.A.; Bullock, S.; Conlon, K.; Emmanuel, J.; Erdle, L.M.; Eriksen, M.; Farrelly, T.A.; Hardesty, B.D.; Kerge, K.; et al. Global producer responsibility for plastic pollution. Sci. Adv. 2024, 10, eadj8275. [Google Scholar] [CrossRef]
- Issifu, I.; Dahmouni, I.; Sumaila, U.R. Assessing the ecological and economic transformation pathways of plastic production system. J. Environ. Manag. 2025, 374, 124104. [Google Scholar] [CrossRef]
- Drewniok, M.P.; Gao, Y.; Cullen, J.M.; Cabrera Serrenho, A. What to Do about Plastics? Lessons from a Study of United Kingdom Plastics Flows. Environ. Sci. Technol. 2023, 57, 4513–4521. [Google Scholar] [CrossRef]
- Lakhiar, I.A.; Yan, H.F.; Zhang, J.Y.; Wang, G.Q.; Deng, S.S.; Bao, R.X.; Zhang, C.; Syed, T.N.; Wang, B.Y.; Zhou, R.; et al. Plastic Pollution in Agriculture as a Threat to Food Security, the Ecosystem, and the Environment: An Overview. Agronomy 2024, 14, 548. [Google Scholar] [CrossRef]
- Law, K.L.; Rochman, C.M. Collaborations uncover extent of plastic pollution. Nature 2023, 619, 254–255. [Google Scholar] [CrossRef] [PubMed]
- MacLeo, M.; Arp, H.P.H.; Tekman, M.B.; Jahnke, A. The global threat from plastic pollution. Science 2021, 373, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Mihai, F.-C.; Gundogdu, S.; Markley, L.A.; Olivelli, A.; Khan, F.R.; Gwinnett, C.; Gutberlet, J.; Reyna-Bensusan, N.; Llanquileo-Melgarejo, P.; Meidiana, C.; et al. Plastic Pollution, Waste Management Issues, and Circular Economy Opportunities in Rural Communities. Sustainability 2022, 14, 20. [Google Scholar] [CrossRef]
- Asgher, M.; Qamar, S.A.; Bilal, M.; Iqbal, H.M.N. Bio-based active food packaging materials: Sustainable alternative to conventional petrochemical-based packaging materials. Food Res. Int. 2020, 137, 109625. [Google Scholar] [CrossRef]
- Kumar, S.; Dubey, N.; Kumar, V.; Choi, I.; Jeon, J.; Kim, M. Combating micro/nano plastic pollution with bioplastic: Sustainable food packaging, challenges, and future perspectives. Environ. Pollut. 2024, 363, 125077. [Google Scholar] [CrossRef]
- Iqbal, B.; Zhao, T.; Yin, W.; Zhao, X.; Xie, Q.; Khan, K.Y.; Zhao, X.; Nazar, M.; Li, G.; Du, D. Impacts of soil microplastics on crops: A review. Appl. Soil Ecol. 2023, 181, 104680. [Google Scholar] [CrossRef]
- Zhai, X.D.; Li, Z.H.; Zhang, J.J.; Shi, J.Y.; Zou, X.Y.; Huang, X.W.; Zhang, D.; Sun, Y.; Yang, Z.K.; Holmes, M.; et al. Natural Biomaterial-Based Edible and pH-Sensitive Films Combined with Electrochemical Writing for Intelligent Food Packaging. J. Agric. Food Chem. 2018, 66, 12836–12846. [Google Scholar] [CrossRef]
- Zhou, W.J.; Luo, J.Y.; Zhao, L.H.; Zhao, M.; Ouyang, Z.; Yang, M.H. Influences of different storage environments and packaging materials on the quality of the traditional Chinese health food Herba Menthae Haplocalycis. Food Packag. Shelf Life 2018, 15, 52–61. [Google Scholar] [CrossRef]
- Atta, O.M.; Manan, S.; Shahzad, A.; Ul-Islam, M.; Ullah, M.W.; Yang, G. Biobased materials for active food packaging: A review. Food Hydrocoll. 2022, 125, 107419. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.; Cui, H. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Edible films from chitosan-gelatin: Physical properties and food packaging application. Food Biosci. 2021, 40, 100871. [Google Scholar] [CrossRef]
- Cucina, M.; Carlet, L.; De Nisi, P.; Somensi, C.A.; Giordano, A.; Adani, F. Degradation of biodegradable bioplastics under thermophilic anaerobic digestion: A full-scale approach. J. Clean. Prod. 2022, 368, 133232. [Google Scholar] [CrossRef]
- Delavar, M.A.; Wang, J. Illumination and fluid flow effects on bioplastic production and biohydrogen generation in microbioreactors with different geometries. Energy 2023, 282, 128610. [Google Scholar] [CrossRef]
- Kong, U.; Rawi, N.F.M.; Tay, G.S. The Potential Applications of Reinforced Bioplastics in Various Industries: A Review. Polymers 2023, 15, 2399. [Google Scholar] [CrossRef]
- Cruz, R.M.S.; Krauter, V.; Krauter, S.; Agriopoulou, S.; Weinrich, R.; Herbes, C.; Scholten, P.B.V.; Uysal-Unalan, I.; Sogut, E.; Kopacic, S.; et al. Bioplastics for Food Packaging: Environmental Impact, Trends and Regulatory Aspects. Foods 2022, 11, 3087. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Barrow, C.J.; Adhikari, B. The future of bioplastics in food packaging: An industrial perspective. Food Packag. Shelf Life 2024, 43, 101279. [Google Scholar] [CrossRef]
- Eissenberger, K.; Ballesteros, A.; De Bisschop, R.; Bugnicourt, E.; Cinelli, P.; Defoin, M.; Demeyer, E.; Fuertauer, S.; Gioia, C.; Gomez, L.; et al. Approaches in Sustainable, Biobased Multilayer Packaging Solutions. Polymers 2023, 15, 1184. [Google Scholar] [CrossRef]
- Jha, S.; Akula, B.; Enyioma, H.; Novak, M.; Amin, V.; Liang, H. Biodegradable Biobased Polymers: A Review of the State of the Art, Challenges, and Future Directions. Polymers 2024, 16, 2262. [Google Scholar] [CrossRef]
- Sedita, S.R.; Di Maria, E.; Mazzoni, L.; Bekele, N.A. Decoding the Biobased Blueprint: Key Players and Evolutionary Trends in Materials Innovation. Polymers 2025, 17, 177. [Google Scholar] [CrossRef]
- Iqbal, M.W.; Riaz, T.; Yasmin, I.; Leghari, A.A.; Amin, S.; Bilal, M.; Qi, X.H. Chitosan-Based Materials as Edible Coating of Cheese: A Review. Starch-Starke 2021, 73, 2100088. [Google Scholar] [CrossRef]
- Hamadou, A.H.; Zhang, J.Y.; Li, H.T.; Chen, C.; Xu, B. Modulating the glycemic response of starch-based foods using organic nanomaterials: Strategies and opportunities. Crit. Rev. Food Sci. Nutr. 2023, 63, 11942–11966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wang, Y.; Chen, X.; Yu, X.; Li, W.; Zhang, S.; Meng, X.; Zhao, Z.-M.; Dong, T.; Anderson, A.; et al. Sustainable bioplastics derived from renewable natural resources for food packaging. Matter 2023, 6, 97–127. [Google Scholar] [CrossRef]
- Market—European Bioplastics e.V. Available online: https://www.european-bioplastics.org/market/ (accessed on 16 June 2025).
- Ali, W.; Ali, H.; Gillani, S.; Zinck, P.; Souissi, S. Polylactic acid synthesis, biodegradability, conversion to microplastics and toxicity: A review. Environ. Chem. Lett. 2023, 21, 1761–1786. [Google Scholar] [CrossRef]
- Chen, X.; Kroell, N.; Li, K.; Feil, A.; Pretz, T. Influences of bioplastic polylactic acid on near-infrared-based sorting of conventional plastic. Waste Manag. Res. 2021, 39, 1210–1213. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Suri, S.; Barua, S.; Phimolsiripol, Y. Native and modified biodegradable starch-based packaging for shelf-life extension and safety of fruits/vegetables. Int. J. Food Sci. Technol. 2023, 58, 862–870. [Google Scholar] [CrossRef]
- Donati, N.; Spada, J.C.; Tessaro, I.C. Recycling rice husk ash as a filler on biodegradable cassava starch-based foams. Polym. Bull. 2023, 80, 10231–10248. [Google Scholar] [CrossRef]
- Xu, H.; Cheng, H.; McClements, D.J.; Chen, L.; Long, J.; Jin, Z. Enhancing the physicochemical properties and functional performance of starch-based films using inorganic carbon materials: A review. Carbohydr. Polym. 2022, 295, 119743. [Google Scholar] [CrossRef]
- Nanda, S.; Patra, B.R.; Patel, R.; Bakos, J.; Dalai, A.K. Innovations in applications and prospects of bioplastics and biopolymers: A review. Environ. Chem. Lett. 2022, 20, 379–395. [Google Scholar] [CrossRef]
- Li, C.Z.; Chen, W.Q.; Siva, S.; Cui, H.Y.; Lin, L. Electrospun phospholipid nanofibers encapsulated with cinnamaldehyde/HP-β-CD inclusion complex as a novel food packaging material. Food Packag. Shelf Life 2021, 28, 100647. [Google Scholar] [CrossRef]
- Wang, H.; Ding, F.; Ma, L.; Zhang, Y. Recent advances in gelatine and chitosan complex material for practical food preservation application. Int. J. Food Sci. Technol. 2021, 56, 6279–6300. [Google Scholar] [CrossRef]
- Kongkaoroptham, P.; Piroonpan, T.; Pasanphan, W. Chitosan nanoparticles based on their derivatives as antioxidant and antibacterial additives for active bioplastic packaging. Carbohydr. Polym. 2021, 257, 117610. [Google Scholar] [CrossRef] [PubMed]
- Acquavia, M.A.; Benítez, J.J.; Guzmán-Puyol, S.; Porras-Vázquez, J.M.; Hierrezuelo, J.; Grifé-Ruiz, M.; Romero, D.; Di Capua, A.; Bochicchio, R.; Laurenza, S.; et al. Enhanced extraction of bioactive compounds from tea waste for sustainable polylactide-based bioplastic applications in active food packaging. Food Packag. Shelf Life 2024, 46, 101410. [Google Scholar] [CrossRef]
- Abe, M.M.; Martins, J.R.; Sanvezzo, P.B.; Macedo, J.V.; Branciforti, M.C.; Halley, P.; Botaro, V.R.; Brienzo, M. Advantages and Disadvantages of Bioplastics Production from Starch and Lignocellulosic Components. Polymers 2021, 13, 2484. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Kambanis, J.; Sorenson, T.L.; Sunde, M.; Shen, Y. From Fundamental Amyloid Protein Self-Assembly to Development of Bioplastics. Biomacromolecules 2023, 25, 5–23. [Google Scholar] [CrossRef]
- Rosenboom, J.-G.; Langer, R.; Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 2022, 7, 117–137. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Yang, Z.; Astruc, T.; Amdursky, N. Aqueous-Based Assembly of Plant-Derived Proteins Yields a Crosslinker-Free Biodegradable Bioplastic Consistent with Green Chemistry Principles. Chemsuschem 2025, 18, e202401567. [Google Scholar] [CrossRef]
- Siddiqui, S.A.; Yang, X.; Deshmukh, R.K.; Gaikwad, K.K.; Bahmid, N.A.; Castro-Muñoz, R. Recent advances in reinforced bioplastics for food packaging—A critical review. Int. J. Biol. Macromol. 2024, 263, 130399. [Google Scholar] [CrossRef]
- Abookleesh, F.; Upadhyay, P.; Ullah, A. Rapeseed Protein-Based Bioplastic Nanocomposite Films Containing Cellulose Nanocrystals, Montmorillonite, and Hydroxyapatite for Food Packaging. ACS Appl. Nano Mater. 2024, 7, 28393–28407. [Google Scholar] [CrossRef]
- Karaca, A.E.; Ozel, C.; Ozarslan, A.C.; Yucel, S. The simultaneous extraction of cellulose fiber and crystal biogenic silica from the same rice husk and evaluation in cellulose-based composite bioplastic films. Polym. Compos. 2022, 43, 6838–6853. [Google Scholar] [CrossRef]
- Lisuzzo, L.; Cavallaro, G.; Milioto, S.; Lazzara, G. Effects of halloysite content on the thermo-mechanical performances of composite bioplastics. Appl. Clay Sci. 2020, 185, 105416. [Google Scholar] [CrossRef]
- Ding, F.Y.; Long, S.M.; Huang, X.W.; Shi, J.Y.; Povey, M.; Zou, X.B. Emerging Pickering emulsion films for bio-based food packaging applications. Food Packag. Shelf Life 2024, 42, 101242. [Google Scholar] [CrossRef]
- Mu, R.J.; Hong, X.; Ni, Y.S.; Li, Y.Z.; Pang, J.; Wang, Q.; Xiao, J.B.; Zheng, Y.F. Recent trends and applications of cellulose nanocrystals in food industry. Trends Food Sci. Technol. 2019, 93, 136–144. [Google Scholar] [CrossRef]
- Jariyasakoolroj, P.; Leelaphiwat, P.; Harnkarnsujarit, N. Advances in research and development of bioplastic for food packaging. J. Sci. Food Agric. 2020, 100, 5032–5045. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.H.; Bhoi, P.R. An overview of non-biodegradable bioplastics. J. Clean. Prod. 2021, 294, 126218. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Aisyah, H.A.; Asyraf, M.R.M.; Hassan, S.A.; Zainudin, E.S.; Sapuan, S.M.; Sharma, S.; Bangar, S.P.; Jumaidin, R.; et al. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef]
- Chellali, J.E.; Alverson, A.K.; Robinson, J.R. Zinc Aryl/Alkyl β-diketiminates: Balancing Accessibility and Stability for High-Activity Ring-Opening Polymerization of rac-Lactide. ACS Catal. 2022, 12, 5585–5594. [Google Scholar] [CrossRef]
- Ghosh, S.; Huse, K.; Woelper, C.; Tjaberings, A.; Groeschel, A.H.; Schulz, S. Fluorinated β-Ketoiminate Zinc Complexes: Synthesis, Structure and Catalytic Activity in Ring Opening Polymerization of Lactide. Z. fur Anorg. und Allgemeine Chemie 2021, 647, 1744–1750. [Google Scholar] [CrossRef]
- Grillo, A.; Rusconi, Y.; D’Alterio, M.C.; De Rosa, C.; Talarico, G.; Poater, A. Ring Opening Polymerization of Six- and Eight-Membered Racemic Cyclic Esters for Biodegradable Materials. Int. J. Mol. Sci. 2024, 25, 1647. [Google Scholar] [CrossRef]
- Li, X.; Lin, Y.; Liu, M.; Meng, L.; Li, C. A review of research and application of polylactic acid composites. J. Appl. Polym. Sci. 2023, 140, e53477. [Google Scholar] [CrossRef]
- Ferreira, P.S.; Ribeiro, S.M.; Pontes, R.; Nunes, J. Production methods and applications of bioactive polylactic acid: A review. Environ. Chem. Lett. 2024, 22, 1831–1859. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.R.; Nair, S.S.; Zhang, X.; Tanguy, N.; Yan, N. Starch Maleate/Epoxidized Soybean Oil/Polylactic Acid Films with Improved Ductility and Biodegradation Potential for Packaging Fatty Foods. ACS Sustain. Chem. Eng. 2022, 10, 14185–14194. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, M.; Wang, L.; Tao, N.; Li, L.; Zhu, W.; Xu, C.; Deng, S.; Wang, Y. Mechanism of antimicrobials immobilized on packaging film inhabiting foodborne pathogens. LWT Food Sci. Technol. 2022, 169, 114037. [Google Scholar] [CrossRef]
- Zhang, H.; Sablani, S. Biodegradable packaging reinforced with plant-based food waste and by-products. Curr. Opin. Food Sci. 2021, 42, 61–68. [Google Scholar] [CrossRef]
- Holler, M.; Alberdi-Cedeño, J.; Auñon-Lopez, A.; Pointner, T.; Martínez-Yusta, A.; König, J.; Pignitter, M. Polylactic acid as a promising sustainable plastic packaging for edible oils. Food Packag. Shelf Life 2023, 36, 101051. [Google Scholar] [CrossRef]
- Dong, X.; Liang, X.; Zhou, Y.; Bao, K.; Sameen, D.E.; Ahmed, S.; Dai, J.; Qin, W.; Liu, Y. Preparation of polylactic acid/TiO2/GO nano-fibrous films and their preservation effect on green peppers. Int. J. Biol. Macromol. 2021, 177, 135–148. [Google Scholar] [CrossRef]
- Fiorentini, C.; Leni, G.; de Apodaca, E.D.; Fernández-de-Castro, L.; Rocchetti, G.; Cortimiglia, C.; Spigno, G.; Bassani, A. Development of Coated PLA Films Containing a Commercial Olive Leaf Extract for the Food Packaging Sector. Antioxidants 2024, 13, 519. [Google Scholar] [CrossRef]
- Godoy Zúniga, M.M.; Ding, R.; Oh, E.; Nguyen, T.B.; Tran, T.T.; Nam, J.-D.; Suhr, J. Avocado seed starch utilized in eco-friendly, UV-blocking, and high-barrier polylactic acid (PLA) biocomposites for active food packaging applications. Int. J. Biol. Macromol. 2024, 265, 130837. [Google Scholar] [CrossRef]
- Tian, S.; Jiao, Y.; Gao, Z.; Xu, Y.; Fu, L.; Fu, H.; Zhou, W.; Hu, C.; Liu, G.; Wang, M.; et al. Catalytic Amination of Polylactic Acid to Alanine. J. Am. Chem. Soc. 2021, 143, 16358–16363. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, M.; Ma, D. Catalytic Cracking of Polylactic Acid to Acrylic Acid. Chin. J. Chem. 2023, 41, 2071–2076. [Google Scholar] [CrossRef]
- Mürtz, S.D.; Lehnertz, M.S.; Kümper, J.; Häger, E.; Markus, A.; Becker, T.; Herres-Pawlis, S.; Palkovits, R. Electrochemical depolymerisation of polylactic acid. Green Chem. 2024, 26, 6423–6428. [Google Scholar] [CrossRef]
- Myburgh, M.W.; Favaro, L.; van Zyl, W.H.; Viljoen-Bloom, M. Engineered yeast for the efficient hydrolysis of polylactic acid. Bioresour. Technol. 2023, 378, 129008. [Google Scholar] [CrossRef] [PubMed]
- Atarés, L.; Chiralt, A.; González-Martínez, C.; Vargas, M. Production of Polyhydroxyalkanoates for Biodegradable Food Packaging Applications Using Haloferax mediterranei and Agrifood Wastes. Foods 2024, 13, 950. [Google Scholar] [CrossRef] [PubMed]
- Corrado, I.; Di Girolamo, R.; Regalado-Gonzalez, C.; Pezzella, C. Polyhydroxyalkanoates-Based Nanoparticles as Essential Oil Carriers. Polymers 2022, 14, 166. [Google Scholar] [CrossRef]
- Ferri, M.; Papchenko, K.; Degli Esposti, M.; Tondi, G.; De Angelis, M.G.; Morselli, D.; Fabbri, P. Fully Biobased Polyhydroxyalkanoate/Tannin Films as Multifunctional Materials for Smart Food Packaging Applications. ACS Appl. Mater. Interfaces 2023, 15, 28594–28605. [Google Scholar] [CrossRef]
- Mineo, A.; Isern-Cazorla, L.; Rizzo, C.; Piccionello, A.P.; Suarez-Ojeda, M.E.; Mannina, G. Polyhydroxyalkanoates production by an advanced food-on-demand strategy: The effect of operational conditions. Chem. Eng. J. 2023, 472, 145007. [Google Scholar] [CrossRef]
- Paul, V.; Pandhi, S.; Mahato, D.K.; Agarwal, A.; Tripathi, A.D. Polyhydroxyalkanoates (PHAs) and its copolymer nanocarrier application in cancer treatment: An overview and challenges. Int. J. Biol. Macromol. 2024, 277, 134201. [Google Scholar] [CrossRef]
- Amaro, T.M.M.M.; Rosa, D.; Comi, G.; Iacumin, L. Prospects for the Use of Whey for Polyhydroxyalkanoate (PHA) Production. Front. Microbiol. 2019, 10, 992. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
- Chandra, R.; Thakor, A.; Mekonnen, T.H.; Charles, T.C.; Lee, H.-S. Production of polyhydroxyalkanoate (PHA) copolymer from food waste using mixed culture for carboxylate production and Pseudomonas putida for PHA synthesis. J. Environ. Manag. 2023, 336, 117650. [Google Scholar] [CrossRef]
- Dietrich, K.; Dumont, M.-J.; Del Rio, L.F.; Orsat, V. Sustainable PHA production in integrated lignocellulose biorefineries. New Biotechnol. 2019, 49, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; He, H.; Yan, X.; Liu, X.; Scrutton, N.S.; Chen, G.-Q. PHA is not just a bioplastic! Biotechnol. Adv. 2024, 71, 108320. [Google Scholar] [CrossRef] [PubMed]
- Chavan, S.; Yadav, B.; Tyagi, R.D.; Wong, J.W.C.; Drogui, P. Trends and challenges in the valorization of kitchen waste to polyhydroxyalkanoates. Bioresour. Technol. 2023, 369, 128323. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, M.; De Anda, A.R.; Michely, L.; Colin, C.S.; Renard, E.; Langlois, V. Synthesis of Fluorinated Polyhydroxyalkanoates from Marine Bioresources as a Promising Biomaterial Coating. Biomacromolecules 2021, 22, 4510–4520. [Google Scholar] [CrossRef]
- Qie, Z.; Kosuge, K.; Sakurai, T.; Ramamoorthi, S.M.; Miyahara, Y.; Tsuge, T. Biodegradability of oxidized films of polyhydroxyalkanoate copolymers containing 2-hydroxy-4-methylthiobutyrate unit in seawater. Polym. Degrad. Stab. 2024, 229, 110975. [Google Scholar] [CrossRef]
- Tan, H.T.; Chek, M.F.; Neoh, S.Z.; Ang, S.L.; Yoshida, S.; Hakoshima, T.; Sudesh, K. Characterization of the polyhydroxyalkanoate (PHA) synthase from Ideonella sakaiensis, a bacterium that is capable of degrading and assimilating poly(ethylene terephthalate). Polym. Degrad. Stab. 2022, 206, 110160. [Google Scholar] [CrossRef]
- Tateiwa, J.; Hsu, Y.-I.; Uyama, H.; Kasuya, K.-i.; Iwata, T. Surface oxidation of polyhydroxyalkanoate films with different molecular structure via photo-activated chlorine dioxide radical and comparison of the influence on the properties. Polym. Degrad. Stab. 2023, 214, 110391. [Google Scholar] [CrossRef]
- Medeiros Garcia Alcântara, J.; Distante, F.; Storti, G.; Moscatelli, D.; Morbidelli, M.; Sponchioni, M. Current trends in the production of biodegradable bioplastics: The case of polyhydroxyalkanoates. Biotechnol. Adv. 2020, 42, 107582. [Google Scholar] [CrossRef]
- Raunhan, R.; Jantharadej, K.; Mhuantong, W.; Chanprateep Napathorn, S.; Boonchayaanant Suwannasilp, B. Valorization of food waste derived anaerobic digestate into polyhydroxyalkanoate (PHA) using Thauera mechernichensis TL1. Waste Manag. 2023, 171, 248–258. [Google Scholar] [CrossRef]
- Zhou, W.; Colpa, D.I.; Permentier, H.; Offringa, R.A.; Rohrbach, L.; Euverink, G.-J.W.; Krooneman, J. Insight into polyhydroxyalkanoate (PHA) production from xylose and extracellular PHA degradation by a thermophilic Schlegelella thermodepolymerans. Resour. Conserv. Recycl. 2023, 194, 107006. [Google Scholar] [CrossRef]
- Sabapathy, P.C.; Devaraj, S.; Meixner, K.; Anburajan, P.; Kathirvel, P.; Ravikumar, Y.; Zabed, H.M.; Qi, X. Recent developments in Polyhydroxyalkanoates (PHAs) production—A review. Bioresour. Technol. 2020, 306, 123132. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.; Wang, Y.; Tong, Y.; Chen, G.-Q. Grand Challenges for Industrializing Polyhydroxyalkanoates (PHAs). Trends Biotechnol. 2021, 39, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Chen, Z.; Wen, Q.; Liu, S.; Wang, Y.; Wang, Z. Sequential recovery of extracellular alginate and intracellular polyhydroxyalkanoate (PHA) from mixed microbial culture PHA production system. J. Clean. Prod. 2024, 448, 141668. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Dong, Q.; Xu, C.; Deng, S.; Kang, Y.; Fan, M.; Li, L. Application of functionalized chitosan in food: A review. Int. J. Biol. Macromol. 2023, 235, 123716. [Google Scholar] [CrossRef]
- Wu, S.; Xu, C.; Zhao, Y.; Shi, W.; Li, H.; Cai, J.; Ding, F.; Qu, P. Recent Advances in Chitosan-Based Hydrogels for Flexible Wearable Sensors. Chemosensors 2023, 11, 39. [Google Scholar] [CrossRef]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.H.; Li, J.H.; Li, M.; Xing, D.K.; An, H.M.; Wu, X.M.; Wu, Y.Y. A Chitosan Composite Film Sprayed before Pathogen Infection Effectively Controls Postharvest Soft Rot in Kiwifruit. Agronomy 2020, 10, 265. [Google Scholar] [CrossRef]
- Tamzid, F.; Sakhawat, S.B.; Rashid, T.U. Chitosan based electrospun nanofibrous materials: A sustainable alternative for food packaging. Trends Food Sci. Technol. 2024, 151, 104617. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Al-Gheethi, A.A.S.; Ghaleb, A.D.S.; Mahdi, A.A.; Al-Ansi, W.; Noman, A.E.; Al-Adeeb, A.; Odjo, A.K.O.; Du, Y.H.; Wei, M.P.; et al. Fabrication and characterization of chitosan/gelatin films loaded with microcapsules of Pulicaria jaubertii extract. Food Hydrocoll. 2022, 129, 107624. [Google Scholar] [CrossRef]
- Fu, X.; Chang, X.; Xu, S.; Xu, H.; Ge, S.; Xie, Y.; Wang, R.; Xu, Y.; Luo, Z.; Shan, Y.; et al. Development of a chitosan/pectin-based multi-active food packaging with both UV and microbial defense functions for effectively preserving of strawberry. Int. J. Biol. Macromol. 2024, 254, 127968. [Google Scholar] [CrossRef]
- Lin, L.; Mao, X.; Sun, Y.; Rajivgandhi, G.; Cui, H. Antibacterial properties of nanofibers containing chrysanthemum essential oil and their application as beef packaging. Int. J. Food Microbiol. 2019, 292, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Fatima, M.; Mir, S.; Ali, M.; Hassan, S.; Ul Haq Khan, Z.; Waqar, K. Synthesis and applications of chitosan derivatives in food preservation-A review. Eur. Polym. J. 2024, 216, 113242. [Google Scholar] [CrossRef]
- Zhu, Y.; Gu, Z.Q.; Liao, Y.W.; Li, S.; Xue, Y.Y.; Firempong, M.A.; Xu, Y.; Yu, J.N.; Smyth, H.D.C.; Xu, X.M. Improved intestinal absorption and oral bioavailability of astaxanthin using poly (ethylene glycol)-graft-chitosan nanoparticles: Preparation, in vitro evaluation, and pharmacokinetics in rats. J. Sci. Food Agric. 2022, 102, 1002–1011. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.X.; Zou, J.; Pu, Q.Q.; Shi, K.; Xu, B.G.; Ma, Y.K. One-step preparation of hydrogel based on different molecular weights of chitosan with citric acid. J. Sci. Food Agric. 2022, 102, 3826–3834. [Google Scholar] [CrossRef]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Anti-listeria effects of chitosan-coated nisin-silica liposome on Cheddar cheese. J. Dairy Sci. 2016, 99, 8598–8606. [Google Scholar] [CrossRef]
- Cui, H.; Surendhiran, D.; Li, C.; Lin, L. Biodegradable zein active film containing chitosan nanoparticle encapsulated with pomegranate peel extract for food packaging. Food Packag. Shelf Life 2020, 24, 100511. [Google Scholar] [CrossRef]
- Shi, Q.; Nian, Y.; You, Y.; Li, Z.; Li, Y.; Zhao, R.; Chen, Z.; Huang, T.; Hu, B. Heterogeneity in Diameters of Protein Fibrils and Chitosan for a High CO2/O2 Selectivity and Desired Mechanical Properties of Edible Bioplastic Films. Small 2025, 21, 2405346. [Google Scholar] [CrossRef]
- Cui, H.Y.; Bai, M.; Rashed, M.M.A.; Lin, L. The antibacterial activity of clove oil/chitosan nanoparticles embedded gelatin nanofibers against Escherichia coli O157:H7 biofilms on cucumber. Int. J. Food Microbiol. 2018, 266, 69–78. [Google Scholar] [CrossRef]
- Zhang, C.; Long, Y.H.; Wang, Q.P.; Li, J.H.; An, H.M.; Wu, X.M.; Li, M. The effect of preharvest 28.6% chitosan composite film sprays for controlling the soft rot on kiwifruit and its defence responses. Hortic. Sci. 2019, 46, 180–194. [Google Scholar] [CrossRef]
- Bhowmik, S.; Agyei, D.; Ali, A. Smart chitosan films as intelligent food packaging: An approach to monitoring food freshness and biomarkers. Food Packag. Shelf Life 2024, 46, 101370. [Google Scholar] [CrossRef]
- Li, N.; Liu, C.; Niu, L.; Li, X.; Feng, J.; Liu, Z. Freshness monitoring of some food products using pH-responsive smart films containing cyanidin cation. Food Control 2025, 171, 111047. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, D.; Gu, Y.; Xu, Y.; Jiang, Q.; Yang, F.; Regenstein, J.M.; Yu, D.; Xia, W. Green halochromic smart and active packaging materials based on chitosan film loading nanoparticles: Functionality, physicochemical properties and application. Food Hydrocoll. 2024, 150, 109667. [Google Scholar] [CrossRef]
- Flórez, M.; Guerra-Rodríguez, E.; Cazón, P.; Vázquez, M. Chitosan for food packaging: Recent advances in active and intelligent films. Food Hydrocoll. 2022, 124, 107328. [Google Scholar] [CrossRef]
- Subramani, G.; Manian, R. Bioactive chitosan films: Integrating antibacterial, antioxidant, and antifungal properties in food packaging. Int. J. Biol. Macromol. 2024, 278, 134596. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Pirouzi, S.; Yaghoubi, M.; Karimi-Dehkordi, M.; Jafarzadeh, S.; Mousavi Khaneghah, A. Packaging of beef fillet with active chitosan film incorporated with ɛ-polylysine: An assessment of quality indices and shelf life. Meat Sci. 2021, 176, 108475. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Z.; Sun, Y.; Wang, X.; Li, L. Combined antioxidant and sensory effects of active chitosan/zein film containing α-tocopherol on Agaricus bisporus. Food Packag. Shelf Life 2020, 24, 100470. [Google Scholar] [CrossRef]
- Yan, J.; Cui, R.; Qin, Y.; Li, L.; Yuan, M. A pH indicator film based on chitosan and butterfly pudding extract for monitoring fish freshness. Int. J. Biol. Macromol. 2021, 177, 328–336. [Google Scholar] [CrossRef]
- Li, Y.; Wu, K.; Wang, B.; Li, X. Colorimetric indicator based on purple tomato anthocyanins and chitosan for application in intelligent packaging. Int. J. Biol. Macromol. 2021, 174, 370–376. [Google Scholar] [CrossRef]
- Ma, M.; Gu, M.; Zhang, S.; Yuan, Y. Effect of tea polyphenols on chitosan packaging for food preservation: Physicochemical properties, bioactivity, and nutrition. Int. J. Biol. Macromol. 2024, 259, 129267. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Shi, J.; Zou, X.; Zhai, X.; Huang, X.; Li, Z.; Holmes, M.; Daglia, M.; Xiao, J. Physical properties and bioactivities of chitosan/gelatin-based films loaded with tannic acid and its application on the preservation of fresh-cut apples. LWT 2021, 144, 111223. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, H.; Luo, W.; Chen, G.; Xiao, N.; Xiao, G.; Liu, C. Development of functional hydroxyethyl cellulose-based composite films for food packaging applications. Front. Bioeng. Biotechnol. 2022, 10, 989893. [Google Scholar] [CrossRef] [PubMed]
- Jiao, H.; Shi, Y.; Sun, J.; Lu, X.; Zhang, H.; Li, Y.; Fu, Y.; Guo, J.; Wang, Q.; Liu, H.; et al. Sawdust-derived cellulose nanofibrils with high biosafety for potential bioprinting. Ind. Crops Prod. 2024, 209, 118025. [Google Scholar] [CrossRef]
- Lu, A.P.; Yu, X.J.; Ji, Q.H.; Chen, L.; Yagoub, A.E.G.; Olugbenga, F.; Zhou, C.S. Preparation and characterization of lignin-containing cellulose nanocrystals from peanut shells using a deep eutectic solvent containing lignin-derived phenol. Ind. Crops Prod. 2023, 195, 116415. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Tian, Y.H.; Dar, M.A.; Yun, J.H.; Zhao, M.; Ragauskas, A.J.; Li, J.; Qi, X.H. Assessing microbial systems and process configurations for improved ethanol production from sugary stovers by integrating soluble sugars and holocellulose. Ind. Crops Prod. 2024, 212, 118269. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Sorrentino, A.; Sorrentino, L.; Notarnicola, M.; Kuchta, K.; Picuno, C.; Oliviero, M. Effect of Cellulose-Based Bioplastics on Current LDPE Recycling. Materials 2023, 16, 4869. [Google Scholar] [CrossRef]
- Huang, C.; Yu, H.; Gao, Y.; Chen, Y.; Abdalkarim, S.Y.H.; Tam, K.C. Recent Advances in Green and Efficient Cellulose Utilization Through Structure Deconstruction and Regeneration. Adv. Funct. Mater. 2025. [Google Scholar] [CrossRef]
- Gadaleta, G.; De Gisi, S.; Picuno, C.; Heerenklage, J.; Kuchta, K.; Sorrentino, A.; Notarnicola, M.; Oliviero, M. Assessment of methane production, disintegration, and biodegradation potential of bioplastic waste in anaerobic digestion systems. J. Environ. Chem. Eng. 2024, 12, 111658. [Google Scholar] [CrossRef]
- Gadaleta, G.; Ferrara, C.; De Gisi, S.; Notarnicola, M.; De Feo, G. Life cycle assessment of end-of-life options for cellulose-based bioplastics when introduced into a municipal solid waste management system. Sci. Total Environ. 2023, 871, 161958. [Google Scholar] [CrossRef]
- Zeng, J.; Ma, Y.; Li, P.; Zhang, X.; Gao, W.; Wang, B.; Xu, J.; Chen, K. Development of high-barrier composite films for sustainable reduction of non-biodegradable materials in food packaging application. Carbohydr. Polym. 2024, 330, 121824. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Tedeschi, G.; Goldoni, L.; Benítez, J.J.; Ceseracciu, L.; Koschella, A.; Heinze, T.; Athanassiou, A.; Heredia-Guerrero, J.A. Greaseproof, hydrophobic, and biodegradable food packaging bioplastics from C6-fluorinated cellulose esters. Food Hydrocoll. 2022, 128, 107562. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhai, X.D.; Zou, X.B.; Li, Z.H.; Shi, J.Y.; Yang, Z.K.; Sun, Y.; Arslan, M.; Chen, Z.Y.; Xiao, J.B. Novel hydrophobic colorimetric films based on ethylcellulose/castor oil/anthocyanins for pork freshness monitoring. LWT Food Sci. Technol. 2022, 164, 113631. [Google Scholar] [CrossRef]
- Liu, H.; Li, P.P.; Qiu, F.X.; Zhang, T.; Xu, J.C. Controllable preparation of FeOOH/CuO@WBC composite based on water bamboo cellulose applied for enhanced arsenic removal. Food Bioprod. Process. 2020, 123, 177–187. [Google Scholar] [CrossRef]
- Ormanli, E.; Amca Uluturk, B.; Bozdogan, N.; Bayraktar, O.; Tavman, S.; Kumcuoglu, S. Development of a novel, sustainable, cellulose-based food packaging material and its application for pears. Food Chem. 2023, 429, 136719. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Seidi, F.; Ahmad, M.; Liu, Y.; Saeb, M.R.; Akbari, A.; Xiao, H. Recent Advances in Functional Cellulose-based Films with Antimicrobial and Antioxidant Properties for Food Packaging. J. Agric. Food Chem. 2023, 71, 16469–16487. [Google Scholar] [CrossRef]
- Benitez, J.J.; Florido-Moreno, P.; Porras-Vázquez, J.M.; Tedeschi, G.; Athanassiou, A.; Heredia-Guerrero, J.A.; Guzman-Puyol, S. Transparent, plasticized cellulose-glycerol bioplastics for food packaging applications. Int. J. Biol. Macromol. 2024, 273, 132956. [Google Scholar] [CrossRef]
- Guzman-Puyol, S.; Hierrezuelo, J.; Benítez, J.J.; Tedeschi, G.; Porras-Vázquez, J.M.; Heredia, A.; Athanassiou, A.; Romero, D.; Heredia-Guerrero, J.A. Transparent, UV-blocking, and high barrier cellulose-based bioplastics with naringin as active food packaging materials. Int. J. Biol. Macromol. 2022, 209, 1985–1994. [Google Scholar] [CrossRef]
- Baniasadi, H.; Fathi, Z.; Lizundia, E.; Cruz, C.D.; Abidnejad, R.; Fazeli, M.; Tammela, P.; Kontturi, E.; Lipponen, J.; Niskanen, J. Development and characterization of pomegranate peel extract-infused carboxymethyl cellulose composite films for functional, sustainable food packaging. Food Hydrocoll. 2025, 158, 110525. [Google Scholar] [CrossRef]
- Ahmad, K.; Din, Z.-u.; Ullah, H.; Ouyang, Q.; Rani, S.; Jan, I.; Alam, M.; Rahman, Z.; Kamal, T.; Ali, S.; et al. Preparation and Characterization of Bio-based Nanocomposites Packaging Films Reinforced with Cellulose Nanofibers from Unripe Banana Peels. Starch-Starke 2022, 74, 2100283. [Google Scholar] [CrossRef]
- Li, Y.; Liang, W.; Huang, M.G.; Huang, W.Y.; Feng, J. Green preparation of holocellulose nanocrystals from burdock and their inhibitory effects against α-amylase and α-glucosidase. Food Funct. 2022, 13, 170–185. [Google Scholar] [CrossRef]
- Lu, Q.M.; Yu, X.J.; Yagoub, A.A.; Wahia, H.; Zhou, C.S. Application and challenge of nanocellulose in the food industry. Food Biosci. 2021, 43, 101285. [Google Scholar] [CrossRef]
- Li, Y.; Liang, W.; Huang, W.Y.; Huang, M.G.; Feng, J. Complexation between burdock holocellulose nanocrystals and corn starch: Gelatinization properties, microstructure, and digestibility in vitro. Food Funct. 2022, 13, 548–560. [Google Scholar] [CrossRef] [PubMed]
- Burelo, M.; Hernández-Varela, J.D.; Medina, D.I.; Treviño-Quintanilla, C.D. Recent developments in bio-based polyethylene: Degradation studies, waste management and recycling. Heliyon 2023, 9, e21374. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Chen, K.; Morgan, H.M.; Liang, J.; Zhang, X.; Yan, L.; Mao, H. Thermal behavior and kinetic study of the effects of zinc-modified biochar catalyst on lignin and low-density polyethylene (LDPE) co-pyrolysis. Trans. ASABE 2018, 61, 1783–1793. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Cui, H.Y.; Li, C.Z.; Lin, L. A novel polyethylene oxide/Dendrobium officinale nanofiber: Preparation, characterization and application in pork packaging. Food Packag. Shelf Life 2019, 21, 100329. [Google Scholar] [CrossRef]
- Anisko, J.; Salasinska, K.; Barczewski, M. Study on thermal stability and degradation kinetics of bio-based low-density polyethylene. Polimery 2023, 68, 451–460. [Google Scholar] [CrossRef]
- Zhang, J.; Koubaa, A.; Xing, D.; Wang, H.; Wang, F.; Wang, X.-M.; Wang, Q. Flammability, thermal stability, and mechanical properties of wood flour/polycarbonate/polyethylene bio-based composites. Ind. Crops Prod. 2021, 169, 113638. [Google Scholar] [CrossRef]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(ethylene terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Wang, J.; Yang, T.; Wei, T.; Chen, R.; Yuan, S.Q. Experimental determination of local head loss of non-coaxial emitters in thin-wall lay-flat polyethylene pipes. Biosyst. Eng. 2020, 190, 71–86. [Google Scholar] [CrossRef]
- Lin, L.; Mahdi, A.A.; Li, C.Z.; Al-Ansi, W.; Al-Maqtari, Q.A.; Hashim, S.B.H.; Cui, H.Y. Enhancing the properties of Litsea cubeba essential oil/peach gum/polyethylene oxide nanofibers packaging by ultrasonication br. Food Packag. Shelf Life 2022, 34, 100951. [Google Scholar] [CrossRef]
- Abbadessa, A.; Dogaris, I.; Kishani Farahani, S.; Reid, M.S.; Rautkoski, H.; Holopainen-Mantila, U.; Oinonen, P.; Henriksson, G. Layer-by-layer assembly of sustainable lignin-based coatings for food packaging applications. Prog. Org. Coat. 2023, 182, 107676. [Google Scholar] [CrossRef]
- Lu, N.; Chen, Z.; Zhang, W.; Yang, G.; Liu, Q.; Böttger, R.; Zhou, S.; Liu, Y. Effect of silver ion implantation on antibacterial ability of polyethylene food packing films. Food Packag. Shelf Life 2021, 28, 100650. [Google Scholar] [CrossRef]
- Carullo, D.; Casson, A.; Rovera, C.; Ghaani, M.; Bellesia, T.; Guidetti, R.; Farris, S. Testing a coated PE-based mono-material for food packaging applications: An in-depth performance comparison with conventional multi-layer configurations. Food Packag. Shelf Life 2023, 39, 101143. [Google Scholar] [CrossRef]
- Cui, Y.; Deng, C.; Fan, L.; Qiu, Y.; Zhao, L. Progress in the biosynthesis of bio-based PET and PEF polyester monomers. Green Chem. 2023, 25, 5836–5857. [Google Scholar] [CrossRef]
- Lee, T.-H.; Liu, H.; Forrester, M.J.; Shen, L.; Wang, T.-p.; Yu, H.; He, J.-H.; Li, W.; Kraus, G.A.; Cochran, E.W. Next-Generation High-Performance Biobased Naphthalate-Modified PET for Sustainable Food Packaging Applications. Macromolecules 2022, 55, 7785–7797. [Google Scholar] [CrossRef]
- Salmi-Mani, H.; Aymes-Chodur, C.; Atkins, C.J.; Terreros, G.; Barroca-Aubry, N.; Regeard, C.; Roger, P. An eco-friendly process for the elaboration of poly(ethylene terephthalate) surfaces grafted with biobased network embedding silver nanoparticles with multiple antibacterial modes. Eur. Polym. J. 2022, 181, 111638. [Google Scholar] [CrossRef]
- Weiland, F.; Kohlstedt, M.; Wittmann, C. Biobased de novo synthesis, upcycling, and recycling—The heartbeat toward a green and sustainable polyethylene terephthalate industry. Curr. Opin. Biotechnol. 2024, 86, 103079. [Google Scholar] [CrossRef]
- Cai, J.; Li, K.; Wu, S. Recent advances in catalytic conversion of biomass derived 5-hydroxymethylfurfural into 2,5-furandicarboxylic acid. Biomass Bioenergy 2022, 158, 106358. [Google Scholar] [CrossRef]
- Kim, D.Y.; Park, T.H.; Choo, J.E.; Hwang, S.W. Evaluation of PET recyclability and characterization of modified reprocessed-PET for industrial application. J. Appl. Polym. Sci. 2024, 141, e55506. [Google Scholar] [CrossRef]
- Andreasi Bassi, S.; Tonini, D.; Saveyn, H.; Astrup, T.F. Environmental and Socioeconomic Impacts of Poly(ethylene terephthalate) (PET) Packaging Management Strategies in the EU. Environ. Sci. Technol. 2022, 56, 501–511. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
- Jones, H.; Saffar, F.; Koutsos, V.; Ray, D. Polyolefins and Polyethylene Terephthalate Package Wastes: Recycling and Use in Composites. Energies 2021, 14, 7306. [Google Scholar] [CrossRef]
- Killinger, L.; Hanich-Spahn, R.; Flick, K.; Hashmi, A.S.K. Lewis-pair-catalytic depolymerization of post-consumer polyethylene terephthalate (PET) waste. Polym. Degrad. Stab. 2025, 233, 111163. [Google Scholar] [CrossRef]
- Sanchez-Caballero, S.; Selles, M.A.; Peydro, M.A.; Cherukuri, H.P. Development of a constitutive model for the compaction of recovered polyethylene terephthalate packages. Waste Manag. 2021, 133, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, K.A.; Spyrowski, M.; Romańska, P.; Majka, T.; Piech, R.; Zawadzka, Z.; Furtak, K.; Bednarowski, D. PET composites and their applications in the face of green chemistry challenges—An overview. Polimery 2025, 70, 227–239. [Google Scholar] [CrossRef]
- Rorrer, N.A.; Nicholson, S.; Carpenter, A.; Biddy, M.J.; Grundl, N.J.; Beckham, G.T. Combining Reclaimed PET with Bio-based Monomers Enables Plastics Upcycling. Joule 2019, 3, 1006–1027. [Google Scholar] [CrossRef]
- Enayati, M.; Mohammadi, S.; Bouldo, M.G. Sustainable PET Waste Recycling: Labels from PET Water Bottles Used as a Catalyst for the Chemical Recycling of the Same Bottles. ACS Sustain. Chem. Eng. 2023, 11, 16618–16626. [Google Scholar] [CrossRef]
- Volmajer Valh, J.; Stopar, D.; Selaya Berodia, I.; Erjavec, A.; Šauperl, O.; Fras Zemljič, L. Economical Chemical Recycling of Complex PET Waste in the Form of Active Packaging Material. Polymers 2022, 14, 3244. [Google Scholar] [CrossRef]
- Asueta, A.; Arnaiz, S.; Miguel-Fernández, R.; Leivar, J.; Amundarain, I.; Aramburu, B.; Gutiérrez-Ortiz, J.I.; López-Fonseca, R. Viability of Glycolysis for the Chemical Recycling of Highly Coloured and Multi-Layered Actual PET Wastes. Polymers 2023, 15, 4196. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, H.; Liu, J.; Li, J.; Zhou, W.; Zhang, J.; Liu, X.; Song, X.; Wang, H.; Huo, P. Removal of polyethylene terephthalate plastics waste via Co–CeO2 photocatalyst–activated peroxymonosulfate strategy. Chem. Eng. J. 2024, 479, 147781. [Google Scholar] [CrossRef]
- Ji, L.; Meng, J.; Li, C.; Wang, M.; Jiang, X. From Polyester Plastics to Diverse Monomers via Low-Energy Upcycling. Adv. Sci. 2024, 11, 2403002. [Google Scholar] [CrossRef]
- Jaime-Azuara, A.; Lemming, M.; Wimmer, R.; Kohansal, K.; Hinge, M.; Helmer Pedersen, T. Continuous hydrothermal processing of poly(ethylene terephthalate) (PET) under subcritical water conditions: A proof-of-principle closed-loop study. Chem. Eng. J. 2024, 495, 153223. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Shu, Z.; Huang, L.; Liu, Q.; Jiang, G. High-Efficiency Degradation of PET Plastics by Glutathione S-Transferase under Mild Conditions. Environ. Sci. Technol. 2024, 58, 13358–13369. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Li, Q.; Zhang, Y.; Zhou, H. New possibility for PET plastic recycling by a tailored hydrolytic enzyme. Green Energy Environ. 2024, 9, 163–165. [Google Scholar] [CrossRef]
- Coralli, I.; Rombolà, A.G.; Fabbri, D. Analytical pyrolysis of the bioplastic PBAT poly(butylene adipate-co-terephthalate). J. Anal. Appl. Pyrolysis 2024, 181, 106577. [Google Scholar] [CrossRef]
- Wongphan, P.; Nerín, C.; Harnkarnsujarit, N. Enhanced compatibility and functionality of thermoplastic cassava starch blended PBAT blown films with erythorbate and nitrite. Food Chem. 2023, 420, 136107. [Google Scholar] [CrossRef]
- Roy, S.; Ghosh, T.; Zhang, W.; Rhim, J.-W. Recent progress in PBAT-based films and food packaging applications: A mini-review. Food Chem. 2024, 437, 137822. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Waterhouse, G.I.N.; Gong, R.; Xie, J.; Zhang, K.; Xu, J. Optimizing interfacial adhesion in PBAT/PLA nanocomposite for biodegradable packaging films. Food Chem. 2021, 334, 127487. [Google Scholar] [CrossRef]
- Fernandes, M.; Salvador, A.F.; Vicente, A.A. Biodegradation of PHB/PBAT films and isolation of novel PBAT biodegraders from soil microbiomes. Chemosphere 2024, 362, 142696. [Google Scholar] [CrossRef]
- Wang, S.; Tang, K.; Zhang, Z.; Liu, H.; Yao, Y.; Liao, X. PBAT/lignin-ZnO composite film for food packaging: Photo-stability, better barrier and antibacterial properties. Int. J. Biol. Macromol. 2024, 275, 133651. [Google Scholar] [CrossRef]
- Xiao, L.; Yao, Z.; He, Y.; Han, Z.; Zhang, X.; Li, C.; Xu, P.; Yang, W.; Ma, P. Antioxidant and antibacterial PBAT/lignin-ZnO nanocomposite films for active food packaging. Ind. Crops Prod. 2022, 187, 115515. [Google Scholar] [CrossRef]
- Venkatesan, R.; Sana, S.S.; Ramkumar, V.; Alagumalai, K.; Kim, S.-C. Development and characterization of poly(butylene adipate-co-terephthalate) (PBAT) composites with N, P-doped carbons for food packaging. Carbon Lett. 2023, 33, 1679–1687. [Google Scholar] [CrossRef]
- de Castro, L.L.R.L.; Silva, L.G.L.; Abreu, I.R.; Braz, C.J.F.; Rodrigues, S.C.S.; Moreira-Araújo, R.S.d.R.; Folkersma, R.; de Carvalho, L.H.; Barbosa, R.; Alves, T.S. Biodegradable PBAT/PLA blend films incorporated with turmeric and cinnamomum powder: A potential alternative for active food packaging. Food Chem. 2024, 439, 138146. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Arpaia, V.; Samorì, C.; Mazzocchetti, L.; Galletti, P. Novel Strategies for Recycling Poly(butylene adipate-co-terephthalate)-Starch-Based Plastics: Selective Solubilization and Depolymerization–Repolymerization Processes. ACS Sustain. Chem. Eng. 2023, 11, 14518–14527. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, M.-S.; Park, S.B.; Kim, S.; Lee, M.; Park, S.-A.; Hwang, S.Y.; Koo, J.M.; Oh, D.X.; Park, J. Improved mechanical properties of biodegradable polycaprolactone nanocomposites prepared using cellulose nanocrystals. Cellulose 2023, 30, 11561–11574. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Norrrahim, M.N.; Misenan, M.S.; Jenol, M.A.; Samsudin, S.A.; Nurazzi, N.M.; Asyraf, M.R.M.; Supian, A.B.M.; Bangar, S.P.; et al. Natural Fiber-Reinforced Polycaprolactone Green and Hybrid Biocomposites for Various Advanced Applications. Polymers 2022, 14, 182. [Google Scholar] [CrossRef]
- Pang, J.; Jiang, T.; Ke, Z.; Xiao, Y.; Li, W.; Zhang, S.; Guo, P. Wood Cellulose Nanofibers Grafted with Poly(ε-caprolactone) Catalyzed by ZnEu-MOF for Functionalization and Surface Modification of PCL Films. Nanomaterials 2023, 13, 1904. [Google Scholar] [CrossRef]
- Gutiérrez, T.J.; Mendieta, J.R.; Ortega-Toro, R. In-depth study from gluten/PCL-based food packaging films obtained under reactive extrusion conditions using chrome octanoate as a potential food grade catalyst. Food Hydrocoll. 2021, 111, 106255. [Google Scholar] [CrossRef]
- Hu, G.; Huang, Q.; Li, J.; Wang, Z.; Yu, Y.; Yang, W.; Hu, Y. PCL/Fucoidan nanofiber membrane loaded HP-β-CD/EGC inclusion complexes for food packaging based on self-assembly strategy. Food Hydrocoll. 2024, 151, 109836. [Google Scholar] [CrossRef]
- Gürler, N.; Pekdemir, M.E.; Torğut, G.; Kök, M. Binary PCL–waste photopolymer blends for biodegradable food packaging applications. J. Mol. Struct. 2023, 1279, 134990. [Google Scholar] [CrossRef]
- Alkassfarity, A.N.; Yassin, M.A.; Abdel Rehim, M.H.; Liu, L.; Jiao, Z.; Wang, B.; Wei, Z. Modified cellulose nanocrystals enhanced polycaprolactone multifunctional films with barrier, UV-blocking and antimicrobial properties for food packaging. Int. J. Biol. Macromol. 2024, 261, 129871. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Zhou, A.; Fang, D.; Lu, T.; Wang, J.; Song, Y.; Lyu, L.; Wu, W.; Huang, C.; Li, W. Oregano essential oil/β-cyclodextrin inclusion compound polylactic acid/polycaprolactone electrospun nanofibers for active food packaging. Chem. Eng. J. 2022, 445, 136746. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Poly(butylene succinate) (PBS): Materials, processing, and industrial applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Rajgond, V.; Mohite, A.; More, N.; More, A. Biodegradable polyester-polybutylene succinate (PBS): A review. Polym. Bull. 2024, 81, 5703–5752. [Google Scholar] [CrossRef]
- Bourg, V.; Valette, R.; Le Moigne, N.; Ienny, P.; Guillard, V.; Bergeret, A. Shear and Extensional Rheology of Linear and Branched Polybutylene Succinate Blends. Polymers 2021, 13, 652. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.L.; Fine, R.L.; Stankowski, D.S.; Koch, C.A.; Limoges, K.A.; Robertson, N.J. Highly selective pressure-dependent (transfer) hydrogenative depolymerization of polybutylene succinate. Chem. Commun. 2024, 60, 702–705. [Google Scholar] [CrossRef]
- Rova, L.; Kurita, H.; Kudo, S.; Hatayama, S.; Kanno, T.; Gallet-Pandelle, A.; Narita, F. Variation of the Tensile Properties of Basalt-Fiber-Reinforced Polybutylene Succinate Matrix Composites during Microbial Degradation. Polymers 2023, 15, 1796. [Google Scholar] [CrossRef]
- Savetlana, S.; Gough, T.; Kelly, A. Properties of polybutylene succinate and polybutylene succinate -polycaprolactone based composite reinforced with coconut shell particles. Compos. Interfaces 2023, 30, 1119–1144. [Google Scholar] [CrossRef]
- Zabidi, N.A.; Zainal, N.N.; Tawakkal, I.S.M.A.; Mohd Basri, M.S.; Ariffin, S.H.; Naim, M.N. Effect of thymol on properties of bionanocomposites from poly (lactic acid)/poly (butylene succinate)/nanofibrillated cellulose for food packaging application. Int. J. Biol. Macromol. 2023, 251, 126212. [Google Scholar] [CrossRef]
- Nuamduang, P.; Auras, R.; Winotapun, C.; Hararak, B.; Wanmolee, W.; Leelaphiwat, P. Enhanced antifungal properties of poly(butylene succinate) film with lignin nanoparticles and trans-cinnamaldehyde for mango packaging. Int. J. Biol. Macromol. 2024, 267, 131185. [Google Scholar] [CrossRef]
- Moe, N.C.; Basbasan, A., Jr.; Winotapun, C.; Hararak, B.; Wanmolee, W.; Suwanamornlert, P.; Leelaphiwat, P.; Boonruang, K.; Chinsirikul, W.; Chonhenchob, V. Application of lignin nanoparticles in polybutylene succinate based antifungal packaging for extending the shelf life of bread. Food Packag. Shelf Life 2023, 39, 101127. [Google Scholar] [CrossRef]
- Ucpinar Durmaz, B.; Ugur Nigiz, F.; Aytac, A. Active packaging films based on poly(butylene succinate) films reinforced with alkaline halloysite nanotubes: Production, properties, and fruit packaging applications. Appl. Clay Sci. 2024, 259, 107517. [Google Scholar] [CrossRef]
- Pedroni, M.; Vassallo, E.; Aloisio, M.; Brasca, M.; Chen, H.; Donnini, R.; Firpo, G.; Morandi, S.; Pietralunga, S.M.; Silvetti, T.; et al. Nature-inspired antibacterial poly (butylene succinate) (PBS) by plasma etching nanotexturing for food packaging applications. Surf. Coat. Technol. 2023, 471, 129828. [Google Scholar] [CrossRef]
- Pulikkalparambil, H.; Phothisarattana, D.; Promhuad, K.; Harnkarnsujarit, N. Effect of silicon dioxide nanoparticle on microstructure, mechanical and barrier properties of biodegradable PBAT/PBS food packaging. Food Biosci. 2023, 55, 103023. [Google Scholar] [CrossRef]
- Łopusiewicz, Ł.; Zdanowicz, M.; Macieja, S.; Kowalczyk, K.; Bartkowiak, A. Development and Characterization of Bioactive Poly(butylene-succinate) Films Modified with Quercetin for Food Packaging Applications. Polymers 2021, 13, 1798. [Google Scholar] [CrossRef]
- Wei, H. Optimisation on Thermoforming of Biodegradable Poly (Lactic Acid) (PLA) by Numerical Modelling. Polymers 2021, 13, 654. [Google Scholar] [CrossRef]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Genovesi, A.; Koca, N.; Barletta, M.; Aversa, C. Extrusion and thermoforming of poly(butylene succinate-co-butylene adipate)/poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) bio-based blends for the fabrication of disposable packaging. J. Appl. Polym. Sci. 2024, 141, e55464. [Google Scholar] [CrossRef]
- Oksuz, M.; Alsac, C.; Ates, M. The effects of thermoform molding conditions on polyvinylchloride and polyethylene double layer package materials. Polym. Eng. Sci. 2009, 49, 2234–2241. [Google Scholar] [CrossRef]
- Semple, K.E.; Zhou, C.; Rojas, O.J.; Nkeuwa, W.N.; Dai, C. Moulded pulp fibers for disposable food packaging: A state-of-the-art review. Food Packag. Shelf Life 2022, 33, 100908. [Google Scholar] [CrossRef]
- Lachance, C.; Barnabe, S.; Deshaies, D.; Bideau, B. The impact of cellulosic pulps on thermoforming process: Effects on formation time and drainage efficiency. Nord. Pulp Pap. Res. J. 2025. [Google Scholar] [CrossRef]
- Morcillo-Martín, R.; Rabasco-Vílchez, L.; Jiménez-Jiménez, F.; Espinosa, E.; Tarrés, Q.; Rodríguez, A. Thermoformed Fiber-Polyethylene Biocomposites: A Circular Food Packaging on Cherry Tomatoes. Food Bioprocess Technol. 2025, 18, 2447–2461. [Google Scholar] [CrossRef]
- Bernardez-Morales, G.M.M.; Nichols, B.W.W.; Douglas, S.L.L.; Belk, A.D.D.; Brandebourg, T.D.D.; Reyes, T.M.M.; Sawyer, J.T.T. Extended Storage of Beef Steaks Using Thermoforming Vacuum Packaging. Foods 2023, 12, 2922. [Google Scholar] [CrossRef] [PubMed]
- Nichols, B.W.; Bernardez-Morales, G.M.; Douglas, S.L.; Johnson, G.F.; Barrazueta-Cordero, R.J.; Belk, A.D.; Ball, J.J.; Sawyer, J.T. Thermoforming Vacuum Packaging Influences Fresh Pork Loin Chop Characteristics. Foods 2024, 13, 2701. [Google Scholar] [CrossRef]
- Barletta, M.; Puopolo, M. Thermoforming of compostable PLA/PBS blends reinforced with highly hygroscopic calcium carbonate. J. Manuf. Process. 2020, 56, 1185–1192. [Google Scholar] [CrossRef]
- Jia, S.; Feng, Z.; Lv, Z.; Yan, X.; Pan, J.; Zhang, Z.; Rao, J.; Peng, P.; Peng, F. Xylan thermoplastics with closed-loop recyclability. Carbohydr. Polym. 2025, 352, 123161. [Google Scholar] [CrossRef]
- Friedrich, D. Thermoplastic moulding of Wood-Polymer Composites (WPC): A review on physical and mechanical behaviour under hot-pressing technique. Compos. Struct. 2021, 262, 113649. [Google Scholar] [CrossRef]
- Yue, J.; Chao, C.; Hong, L.; Xiang, Q.J. Influences of nozzle parameters and low-pressure on jet breakup and droplet characteristics. Int. J. Agric. Biol. Eng. 2016, 9, 22–32. [Google Scholar]
- Tang, P.; Li, H.; Issaka, Z.; Chen, C. Methodology to investigate the hydraulic characteristics of a water-powered piston-type proportional injector used for agricultural chemigation. Appl. Eng. Agric. 2018, 34, 545–553. [Google Scholar] [CrossRef]
- Formas, K.; Kurowska, A.; Janusz, J.; Szczygie, P.; Rajzer, I. Injection Molding Process Simulation of Polycaprolactone Sticks for Further 3D Printing of Medical Implants. Materials 2022, 15, 7295. [Google Scholar] [CrossRef]
- Murata, Y.; Machiya, R.; Komori, T. Influence of Processing Conditions on the Generation of Surface Defects in a Heat-and-Cool Hybrid Injection Molding Technique for Carbon Fiber-Reinforced Thermoplastic Sheets and Development of a Suitable Mold Heated by Far-Infrared Radiation. Polymers 2023, 15, 4437. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.T.; Seefried, A.; Gehde, M. Investigation of the Influence of Fiber Content, Processing Conditions and Surface Roughness on the Polymer Filling Behavior in Thermoset Injection Molding. Polymers 2023, 15, 1244. [Google Scholar] [CrossRef] [PubMed]
- Czepiel, M.; Bańkosz, M.; Sobczak-Kupiec, A. Advanced Injection Molding Methods: Review. Materials 2023, 16, 5802. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Krantz, J.; Ferki, O.; Nieduzak, Z.; Perry, S.; Sobkowicz, M.J.; Masato, D. Thermo-mechanical recycling via ultrahigh-speed extrusion of film-grade recycled LDPE and injection molding. Sustain. Mater. Technol. 2023, 38, e00719. [Google Scholar] [CrossRef]
- Kathuria, A.; Abiad, M.G.; Auras, R. PLLA-ZIF-8 metal organic framework composites for potential use in food applications: Production, characterization and migration studies. Packag. Technol. Sci. 2021, 34, 393–400. [Google Scholar] [CrossRef]
- Ott, C.; Drummer, D. Low-stress over-molding of media-tight electronics using thermoplastic foam injection molding. Polym. Eng. Sci. 2021, 61, 1518–1528. [Google Scholar] [CrossRef]
- Zhou, S.; Hrymak, A.N. Injection Molding of Polymers and Polymer Composites. Polymers 2024, 16, 1796. [Google Scholar] [CrossRef]
- Svečko, R.; Kusić, D.; Kek, T.; Sarjaš, A.; Hančič, A.; Grum, J. Acoustic Emission Detection of Macro-Cracks on Engraving Tool Steel Inserts during the Injection Molding Cycle Using PZT Sensors. Sensors 2013, 13, 6365–6379. [Google Scholar] [CrossRef]
- Alonso-González, M.; Castro-Criado, D.; Felix, M.; Romero, A. Evaluation of rice bran varieties and heat treatment for the development of protein/starch-based bioplastics via injection molding. Int. J. Biol. Macromol. 2023, 253, 127503. [Google Scholar] [CrossRef]
- Babu, A.; Kumar, A.R.; Amrutha, N.R.; Madhurya, S.; Punil Kumar, H.N.; Reddy, J.P.; Murthy, P.S.K.; Varaprasad, K. Utilizing foxtail millet husk waste for sustainable new bioplastic composites with enhanced thermal stability and biodegradability. Int. J. Biol. Macromol. 2024, 282, 137283. [Google Scholar] [CrossRef]
- Tábi, T.; Ageyeva, T.; Kovács, J.G. Improving the ductility and heat deflection temperature of injection molded Poly(lactic acid) products: A comprehensive review. Polym. Test. 2021, 101, 107282. [Google Scholar] [CrossRef]
- Otálora González, C.M.; Alvarez Castillo, E.; Flores, S.; Gerschenson, L.N.; Bengoechea, C. Effect of plasticizer composition on the properties of injection molded cassava starch-based bioplastics. Food Packag. Shelf Life 2023, 40, 101218. [Google Scholar] [CrossRef]
- Gamero, S.; Jiménez-Rosado, M.; Romero, A.; Bengoechea, C.; Guerrero, A. Reinforcement of Soy Protein-Based Bioplastics Through Addition of Lignocellulose and Injection Molding Processing Conditions. J. Polym. Environ. 2019, 27, 1285–1293. [Google Scholar] [CrossRef]
- Ahmad, N.; Hussain, A.; Khan, S.; Korma, S.A.; Hussain, G.; Aadil, R.M.; Siddique, R.; Ali, A.; Shabbir, U.; Haq, A.U.; et al. Impact of thermal extrusion and microwave vacuum drying on fatty acids profile during fish powder preparation. Food Sci. Nutr. 2021, 9, 2743–2753. [Google Scholar] [CrossRef]
- Guo, Z.; Arslan, M.; Li, Z.H.; Cen, S.Y.; Shi, J.Y.; Huang, X.W.; Xiao, J.B.; Zou, X.B. Application of Protein in Extrusion-Based 3D Food Printing: Current Status and Prospectus. Foods 2022, 11, 1902. [Google Scholar] [CrossRef]
- Alkarri, S.; Naveed, M.; Alali, F.; Vachon, J.; Walworth, A.; Vanderberg, A. Anti-Microbial, Thermal, Mechanical, and Gas Barrier Properties of Linear Low-Density Polyethylene Extrusion Blow-Molded Bottles. Polymers 2024, 16, 1914. [Google Scholar] [CrossRef]
- Aversa, C.; Barletta, M.; Gisario, A.; Pizzi, E.; Prati, R.; Vesco, S. Corotating twin-screw extrusion of poly(lactic acid) PLA/poly(butylene succinate) PBS/micro-lamellar talc blends for extrusion blow molding of biobased bottles for alcoholic beverages. J. Appl. Polym. Sci. 2021, 138, 51294. [Google Scholar] [CrossRef]
- Li, T.; Li, R.; Luo, H.; Peng, L.; Wang, J.; Li, S.; Zhou, M.; Yuan, X.; Zhang, Z.; Wu, H. Eggshell powder as a bio-filler for starch and gelatin: Ternary biodegradable composite films manufactured by extrusion compression molding. Food Hydrocoll. 2024, 150, 109632. [Google Scholar] [CrossRef]
- Yu, X.; Chen, L.; Jin, Z.; Jiao, A. Research progress of starch-based biodegradable materials: A review. J. Mater. Sci. 2021, 56, 11187–11208. [Google Scholar] [CrossRef]
- Fehlberg, J.; McKay, S.; Matuana, L.M.; Almenar, E. Use of orange juice processing waste to produce films using blown film extrusion for food packaging. J. Food Eng. 2023, 341, 111337. [Google Scholar] [CrossRef]
- Ignacio, M.C.C.D.; Tumu, K.N.; Munshi, M.; Vorst, K.L.; Curtzwiler, G.W. Suitability of MRF Recovered Post-Consumer Polypropylene Applications in Extrusion Blow Molded Bottle Food Packaging. Polymers 2023, 15, 3471. [Google Scholar] [CrossRef] [PubMed]
- Redfearn, H.N.; Warren, M.K.; Goddard, J.M. Reactive Extrusion of Nonmigratory Active and Intelligent Packaging. ACS Appl. Mater. Interfaces 2023, 15, 29511–29524. [Google Scholar] [CrossRef] [PubMed]
- Redfearn, H.N.; Goddard, J.M. Reactive Extrusion of Poly(lactic acid)-graft-Curcumin Antioxidant and Intelligent Packaging. ACS Appl. Polym. Mater. 2024, 6, 192–206. [Google Scholar] [CrossRef]
- Zhai, X.; Wang, X.; Zhang, J.; Yang, Z.; Sun, Y.; Li, Z.; Huang, X.; Holmes, M.; Gong, Y.; Povey, M.; et al. Extruded low density polyethylene-curcumin film: A hydrophobic ammonia sensor for intelligent food packaging. Food Packag. Shelf Life 2020, 26, 100595. [Google Scholar] [CrossRef]
- Shlush, E.; Davidovich-Pinhas, M. Fabrication of bioplastic material based on ethyl-cellulose using hot-melt extrusion. Food Packag. Shelf Life 2023, 40, 101206. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Maigret, J.-E.; Perez-Puyana, V.; Romero, A.; Lourdin, D. Revaluation of a Soy Protein By-product in Eco-friendly Bioplastics by Extrusion. J. Polym. Environ. 2022, 30, 1587–1599. [Google Scholar] [CrossRef]
- Jiménez-Rosado, M.; Zarate-Ramírez, L.S.; Romero, A.; Bengoechea, C.; Partal, P.; Guerrero, A. Bioplastics based on wheat gluten processed by extrusion. J. Clean. Prod. 2019, 239, 117994. [Google Scholar] [CrossRef]
- Panariello, L.; Coltelli, M.-B.; Hadrich, A.; Braca, F.; Fiori, S.; Haviv, A.; Miketa, F.; Lazzeri, A.; Staebler, A.; Gigante, V.; et al. Antimicrobial and Gas Barrier Crustaceans and Fungal Chitin-Based Coatings on Biodegradable Bioplastic Films. Polymers 2022, 14. [Google Scholar] [CrossRef]
- Luo, B.; Xuan, S.; Wang, X.; Ding, K.; Jin, P.; Zheng, Y.; Wu, Z. Liposome/chitosan coating film bioplastic packaging for Litchi fruit preservation. Food Chem. 2025, 464, 141850. [Google Scholar] [CrossRef]
- Wang, B.; Wei, W.L.; Aputexiakere, J.; Li, Y.L.; Ma, H.L. Surface decontamination of whole eggs using pulsed light technology and shelf life study of combined pulsed light and vaseline coating during room temperature storage. Food Control 2022, 137, 108411. [Google Scholar] [CrossRef]
- Tahir, H.E.; Li, Z.; Mahunu, G.K.; Zou, X.; Arslan, M.; Huang, X.; Yang, Z.K.; Mariod, A.A. Effect of gum arabic edible coating incorporated with African baobab pulp extract on postharvest quality of cold stored blueberries. Food Sci. Biotechnol. 2020, 29, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, C.; Salsabila, S.; Muna, A.; Rusliman, D.; Wasisto, H.S. Advanced biopolymer-based edible coating technologies for food preservation and packaging. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13275. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Quilez-Molina, A.I.; Perotto, G.; Bassani, A.; Spigno, G.; Athanassiou, A. A second life for fruit and vegetable waste: A review on bioplastic films and coatings for potential food protection applications. Green Chem. 2022, 24, 4703–4727. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A review of multilayer and composite films and coatings for active biodegradable packaging. npj Sci. Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Tahir, H.E.; Zou, X.B.; Shi, J.Y.; Mahunu, G.K.; Zhai, X.D.; Mariod, A.A. Quality and postharvest-shelf life of cold-stored strawberry fruit as affected by gum arabic (Acacia senegal) edible coating. J. Food Biochem. 2018, 42, e12527. [Google Scholar] [CrossRef]
- Hassane Hamadou, A.; Zhang, J.; Chao, C.; Xu, B. Stability of rutin using pectin-chitosan dual coating nanoliposomes. LWT 2022, 170, 114084. [Google Scholar] [CrossRef]
- Shorey, R.; Mekonnen, T.H. Sustainable paper coating with enhanced barrier properties based on esterified lignin and PBAT blend. Int. J. Biol. Macromol. 2022, 209, 472–484. [Google Scholar] [CrossRef]
- Piroonpan, T.; Booncharoen, K.; Pasanphan, W. Sugar-Core Synthesized Multibranched Polylactic Acid and Its Diacrylate Blends as a UV LED-Curable Coating with Enhanced Toughness and Performance. ACS Sustain. Chem. Eng. 2022, 10, 17027–17042. [Google Scholar] [CrossRef]
- Shen, C.; Chen, W.; Li, C.; Aziz, T.; Cui, H.; Lin, L. Topical advances of edible coating based on the nanoemulsions encapsulated with plant essential oils for foodborne pathogen control. Food Control 2023, 145, 109419. [Google Scholar] [CrossRef]
- Chu, Y.; Gao, C.; Liu, X.; Zhang, N.; Xu, T.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Improvement of storage quality of strawberries by pullulan coatings incorporated with cinnamon essential oil nanoemulsion. LWT 2020, 122, 109054. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Saravanakumar, K.; Mariadoss, A.V.A.; Ramachandran, C.; Hu, X.; Oh, D.-H.; Wang, M.-H. Chitosan-tea tree oil nanoemulsion and calcium chloride tailored edible coating increase the shelf life of fresh cut red bell pepper. Prog. Org. Coat. 2021, 151, 106010. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Raza, H.; Chi, Z.; Chi, R.; Ren, X.; Ma, H. Preparation and characterization of ultrasound-assisted essential oil-loaded nanoemulsions stimulated pullulan-based bioactive film for strawberry fruit preservation. Food Chem. 2023, 422, 136254. [Google Scholar] [CrossRef] [PubMed]
- Ben-Fadhel, Y.; Cingolani, M.C.; Li, L.; Chazot, G.; Salmieri, S.; Horak, C.; Lacroix, M. Effect of γ-irradiation and the use of combined treatments with edible bioactive coating on carrot preservation. Food Packag. Shelf Life 2021, 28, 100635. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, M.; Bhandari, B.; Xu, J.; Yang, C. Effects of nanoemulsion-based active coatings with composite mixture of star anise essential oil, polylysine, and nisin on the quality and shelf life of ready-to-eat Yao meat products. Food Control 2020, 107, 106771. [Google Scholar] [CrossRef]
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for food packaging. Trends Food Sci. Technol. 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Huang, S.S.; Deng, C.; Zhang, H.L.; Yue, X.J.; Qiu, F.X.; Zhang, T. Antibacterial cellulose-based membrane with heat dissipation and liquid transportation for food packaging applications. Food Bioprod. Process. 2025, 149, 284–293. [Google Scholar] [CrossRef]
- Ding, F.Y.; Wu, R.K.; Huang, X.W.; Shi, J.Y.; Zou, X.B. Anthocyanin loaded composite gelatin films crosslinked with oxidized alginate for monitoring spoilage of flesh foods. Food Packag. Shelf Life 2024, 42, 101255. [Google Scholar] [CrossRef]
- Boisseaux, P.; Hopkinson, P.; Santillo, D.; Smith, C.; Garmulewicz, A.; Powell, Z.; Galloway, T. Environmental safety of second and third generation bioplastics in the context of the circular economy. Ecotoxicol. Environ. Saf. 2023, 256, 114835. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Zhang, J.J.; Hu, X.T.; Huang, X.W.; Zhang, X.A.; Zou, X.B.; Shi, J.Y. Preparation of edible antibacterial films based on corn starch/carbon nanodots for bioactive food packaging. Food Chem. 2024, 444, 138467. [Google Scholar] [CrossRef]
- Cui, H.Y.; Cheng, Q.; Li, C.Z.; Khin, M.N.; Lin, L. Schiff base cross-linked dialdehyde β-cyclodextrin/gelatin-carrageenan active packaging film for the application of carvacrol on ready-to-eat foods. Food Hydrocoll. 2023, 141, 108744. [Google Scholar] [CrossRef]
- Reshmy, R.; Thomas, D.; Philip, E.; Paul, S.A.; Madhavan, A.; Sindhu, R.; Sirohi, R.; Varjani, S.; Pugazhendhi, A.; Pandey, A.; et al. Bioplastic production from renewable lignocellulosic feedstocks: A review. Rev. Environ. Sci. Bio/Technol. 2021, 20, 167–187. [Google Scholar] [CrossRef]
- Cui, H.Y.; Yang, X.J.; Li, C.Z.; Ye, Y.; Chen, X.C.; Lin, L. Enhancing anti-E. coli O157:H7 activity of composite phage nanofiber film by D-phenylalanine for food packaging. Int. J. Food Microbiol. 2022, 376, 109762. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Luo, C.C.; Li, C.Z.; Abdel-Samie, M.A.; Cui, H.Y. Eugenol/silk fibroin nanoparticles embedded Lycium barbarum polysaccharide nanofibers for active food packaging. Food Packag. Shelf Life 2022, 32, 100841. [Google Scholar] [CrossRef]
- Pascoli, D.U.; Dichiara, A.; Roumeli, E.; Gustafson, R.; Bura, R. Lignocellulosic nanomaterials production from wheat straw via peracetic acid pretreatment and their application in plastic composites. Carbohydr. Polym. 2022, 295, 119857. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, X.; Zhai, X.; Huang, X.; Jiang, C.; Holmes, M. Preparation of an intelligent pH film based on biodegradable polymers and roselle anthocyanins for monitoring pork freshness. Food Chem. 2019, 272, 306–312. [Google Scholar] [CrossRef]
- Adamu Ugya, Y.; Chen, H.; Sheng, Y.; Ajibade, F.O.; Wang, Q. A review of microalgae biofilm as an eco-friendly approach to bioplastics, promoting environmental sustainability. Environ. Res. 2023, 236, 116833. [Google Scholar] [CrossRef]
- Sid, S.; Mor, R.S.; Kishore, A.; Sharanagat, V.S. Bio-sourced polymers as alternatives to conventional food packaging materials: A review. Trends Food Sci. Technol. 2021, 115, 87–104. [Google Scholar] [CrossRef]
- Ren, M.N.; Kong, F.G.; Zhou, C.S.; Fakayode, O.A.; Liang, J.K.; Li, H.X.; Zhou, M.; Fan, X.Y. Green, one-pot biomass hierarchical utilization strategy for lignin-containing cellulose nanofibrils and fractionated lignin preparation. Ind. Crops Prod. 2023, 203, 87–104. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, Z.F.; Zhang, T.; Chen, Q.Y.; Qiu, F.X.; Peng, Y.X.; Tang, S. Fabrication of functional biomass carbon aerogels derived from sisal fibers for application in selenium extraction. Food Bioprod. Process. 2018, 111, 93–103. [Google Scholar] [CrossRef]
- Xu, W. A study on the synthesis, modification and current market status of PBAT. E3S Web Conf. 2023, 385, 04007. [Google Scholar] [CrossRef]
- Filho, W.L.; Salvia, A.L.; Bonoli, A.; Saari, U.A.; Voronova, V.; Klõga, M.; Kumbhar, S.S.; Olszewski, K.; De Quevedo, D.M.; Barbir, J. An assessment of attitudes towards plastics and bioplastics in Europe. Sci. Total Environ. 2021, 755, 142732. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Improving anti-listeria activity of cheese packaging via nanofiber containing nisin-loaded nanoparticles. LWT Food Sci. Technol. 2017, 81, 233–242. [Google Scholar] [CrossRef]
- Idris, S.N.; Amelia, T.S.M.; Bhubalan, K.; Lazim, A.M.M.; Zakwan, N.A.M.A.; Jamaluddin, M.I.; Santhanam, R.; Amirul, A.-A.A.; Vigneswari, S.; Ramakrishna, S. The degradation of single-use plastics and commercially viable bioplastics in the environment: A review. Environ. Res. 2023, 231, 115988. [Google Scholar] [CrossRef] [PubMed]
- Ali, W.; Ali, H.; Souissi, S.; Zinck, P. Are bioplastics an ecofriendly alternative to fossil fuel plastics? Environ. Chem. Lett. 2023, 21, 1991–2002. [Google Scholar] [CrossRef]
- Varghese, S.; Dhanraj, N.D.; Rebello, S.; Sindhu, R.; Binod, P.; Pandey, A.; Jisha, M.S.; Awasthi, M.K. Leads and hurdles to sustainable microbial bioplastic production. Chemosphere 2022, 305, 135390. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Wemyss, A.M.; Iacovidou, E.; Wan, C. Design and Control of Compostability in Synthetic Biopolyesters. ACS Sustain. Chem. Eng. 2021, 9, 9151–9164. [Google Scholar] [CrossRef]
- Mastropetros, S.G.; Pispas, K.; Zagklis, D.; Ali, S.S.; Kornaros, M. Biopolymers production from microalgae and cyanobacteria cultivated in wastewater: Recent advances. Biotechnol. Adv. 2022, 60, 107999. [Google Scholar] [CrossRef]
- Cui, H.Y.; Abdel-Samie, M.A.S.; Lin, L. Novel packaging systems in grape storage—A review. J. Food Process Eng. 2019, 42, e13162. [Google Scholar] [CrossRef]
- Bangar, S.P.; Kajla, P.; Ghosh, T. Valorization of wheat straw in food packaging: A source of cellulose. Int. J. Biol. Macromol. 2023, 227, 762–776. [Google Scholar] [CrossRef]
- Chelliah, R.; Wei, S.; Vijayalakshmi, S.; Barathikannan, K.; Sultan, G.; Liu, S.; Oh, D.-H. A Comprehensive Mini-Review on Lignin-Based Nanomaterials for Food Applications: Systemic Advancement and Future Trends. Molecules 2023, 28, 6470. [Google Scholar] [CrossRef]
- Cheng, H.; Lambert, D.M.; DeLong, K.L.; Jensen, K.L. Inattention, availability bias, and attribute premium estimation for a biobased product. Agric. Econ. 2022, 53, 274–288. [Google Scholar] [CrossRef]
- Tournier, V.; Topham, C.M.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.L.; Texier, H.; Gavalda, S.; et al. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Merchan, A.L.; Fischöder, T.; Hee, J.; Lehnertz, M.S.; Osterthun, O.; Pielsticker, S.; Schleier, J.; Tiso, T.; Blank, L.M.; Klankermayer, J.; et al. Chemical recycling of bioplastics: Technical opportunities to preserve chemical functionality as path towards a circular economy. Green Chem. 2022, 24, 9428–9449. [Google Scholar] [CrossRef]
- Myburgh, M.W.; van Zyl, W.H.; Modesti, M.; Viljoen-Bloom, M.; Favaro, L. Enzymatic hydrolysis of single-use bioplastic items by improved recombinant yeast strains. Bioresour. Technol. 2023, 390, 129908. [Google Scholar] [CrossRef]
- El-Mesery, H.S.; Sarpong, F.; Atress, A.S.H. Statistical interpretation of shelf-life indicators of tomato (Lycopersicon esculentum) in correlation to storage packaging materials and temperature. J. Food Meas. Charact. 2022, 16, 366–376. [Google Scholar] [CrossRef]
- Vidal, F.; van der Marel, E.R.; Kerr, R.W.F.; McElroy, C.; Schroeder, N.; Mitchell, C.; Rosetto, G.; Chen, T.T.D.; Bailey, R.M.; Hepburn, C.; et al. Designing a circular carbon and plastics economy for a sustainable future. Nature 2024, 626, 45–57. [Google Scholar] [CrossRef]
- Ding, F.Y.; Hu, B.; Lan, S.; Wang, H.X. Flexographic and screen printing of carboxymethyl chitosan based edible inks for food packaging applications. Food Packag. Shelf Life 2020, 26, 100559. [Google Scholar] [CrossRef]
- Zhou, C.Q.; Abdel-Samie, M.A.; Li, C.Z.; Cui, H.Y.; Lin, L. Active packaging based on swim bladder gelatin/galangal root oil nanofibers: Preparation, properties and antibacterial application. Food Packag. Shelf Life 2020, 26, 100586. [Google Scholar] [CrossRef]



| Company | PHA Type | Technology | Scale (ton/year) | Websites |
|---|---|---|---|---|
| PhaBuilder, Beijing, China | All types | Halomonas spp. (NGIBa) | 1000–10,000 | www.phabuilder.com |
| Medpha, Zhuhai, China | P3HB4HB | Halomonas spp. (NGIB) | 100 | www.medpha.cn |
| COFCO, Beijing, China | PHB | Halomonas spp. (NGIB) | 1000 | www.cofco.com |
| Bluepha, Beijing, China | PHBHHx | Ralstonia eutropha and NGIB | 1000 | www.bluepha.com |
| TianAnBiopolymer, Ningbo, China | PHBV | R. eutropha | 2000 | www.tianan-enmat.com |
| DanimerScientific, Bainbridge, GA, USA | PHBHHx | R. eutropha | 10,000 | danimerscientific.com |
| Kaneka, Osaka, Japan | PHBHHx | R. eutropha | 5000 | www.kaneka.be |
| RWDC, Singapore and Athens, GA, USA | PHBHHx | R. eutropha | Unknown | www.rwdc-industries.com |
| GreenBio, Taizhou, China | P3HB4HB | Escherichia coli | 10,000 | www.greenbio.cn |
| Active Components | Function | Food | Reference |
|---|---|---|---|
| gelatine | Antioxidant | fruits | [41] |
| ϵ-polylysine | AntioxidantAntimicrobial | Beef fillet | [116] |
| α-tocopherol | Antioxidant | Mushroom | [117] |
| MO | Antioxidant | Cheese | [20] |
| Butterfly pudding extract | pH indicator | Fish | [118] |
| Purple tomato | Freshness | Milk and fish | [119] |
| Tea polyphenols | Freshness | Meat, fruits and vegetables | [120] |
| TA | UV/Antioxidant | Apple | [121] |
| Essential Oils | Preparation Method of Nanoemulsions | Formula of Coating Solution | Foods | Main Effects | References |
|---|---|---|---|---|---|
| Cinnamon | Ultrasonication | Pullulan solution (2 g/100 mL), glycerol (15 g/100 g pullulan polysaccharide) + Tween 80 and 8% of CEO | Strawberries | Pullulan-CEO NE coating remarkably lowered the loss in fruit mass, firmness, and showed the strongest antimicrobial activity against bacteria and molds, respectively. | [261] |
| Tea tree | Ultrasonication | TTO (1% dissolved in 10 mL of ethanol) + Tween 80 (0.3%) incorporated with LMWCS (low molecular weight chitosan) solution | Fresh cut red bell pepper (FCRBP) | The texture, sensory behavior, and overall quality of FCRBP were maintained for 18 days through controlling the contamination of foodborne pathogenic fungi and bacteria including Salmonella enterica, and Listeria monocytes. | [262] |
| Curcumin and Orange essential oils | Ultrasonication | The nanoemulsions comprise of 85% aqueous phase, 5% (w/v) soy protein, and 10% (v/v), curcumin or orange essential oil | Strawberries | Exhibits the highest EAB and lowest WVP, highly effective bacterial inhibition, excellent freshness retention | [263] |
| Cinnamon, lemongrass, oregano and citronella | Ultrasonication | Ca-Cas (5% w/v) and glycerol (2.5% w/v) + emulsifiers and EOs (cinnamon, lemongrass, oregano, and citronella) with citrus extract and cranberry juice | Carrot | The coating showed a synergistic potential and a higher efficiency to extend the shelf-life of carrots and maintain their quality throughout storage, compared to single treatments. | [264] |
| Star anise | Ultrasonication | SPI (0.5%, 1%, 1.5% (w/v)) and lecithin 0.05% (w/w) + water-soluble polyline (0.067 wt.%) and nisin (0.133% wt.) + 0.4% wt. essential oil and 99.6% wt. water phase + glycerol | Ready-to-eat Yao meat | Samples with NEAC showed the best color, odor and overall acceptance, and the effect of coating with essential oil on sensory acceptability was improved | [265] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Cui, L.; Xu, C.; Wu, S. Next-Generation Bioplastics for Food Packaging: Sustainable Materials and Applications. Materials 2025, 18, 2919. https://doi.org/10.3390/ma18122919
Shi X, Cui L, Xu C, Wu S. Next-Generation Bioplastics for Food Packaging: Sustainable Materials and Applications. Materials. 2025; 18(12):2919. https://doi.org/10.3390/ma18122919
Chicago/Turabian StyleShi, Xiaokun, Lijuan Cui, Chao Xu, and Shuping Wu. 2025. "Next-Generation Bioplastics for Food Packaging: Sustainable Materials and Applications" Materials 18, no. 12: 2919. https://doi.org/10.3390/ma18122919
APA StyleShi, X., Cui, L., Xu, C., & Wu, S. (2025). Next-Generation Bioplastics for Food Packaging: Sustainable Materials and Applications. Materials, 18(12), 2919. https://doi.org/10.3390/ma18122919








