Influence of Post-Printing Polymerization Time on the Elution of Residual Monomers and Water Sorption of 3D-Printed Resin Composite
Abstract
1. Introduction
2. Materials and Methods
2.1. Elution of Residual Monomers
2.1.1. Specimen Preparation
2.1.2. Identification and Quantification of Eluted Monomers
2.2. Water Sorption and Solubility
Wso (µg/mm3) = (m2 − m3)/V
Sol (µg/mm3) = (m1 − m3)/V
2.3. Statistical Analyses
3. Results
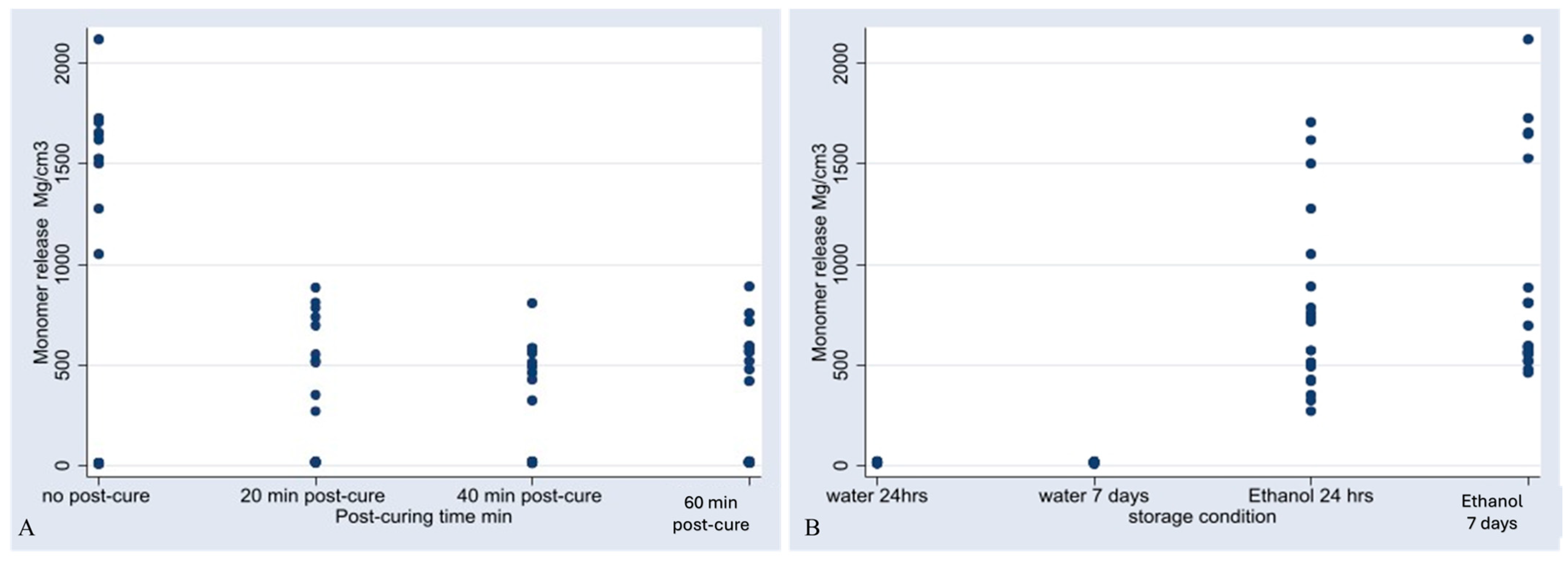
3.1. Monomer Release
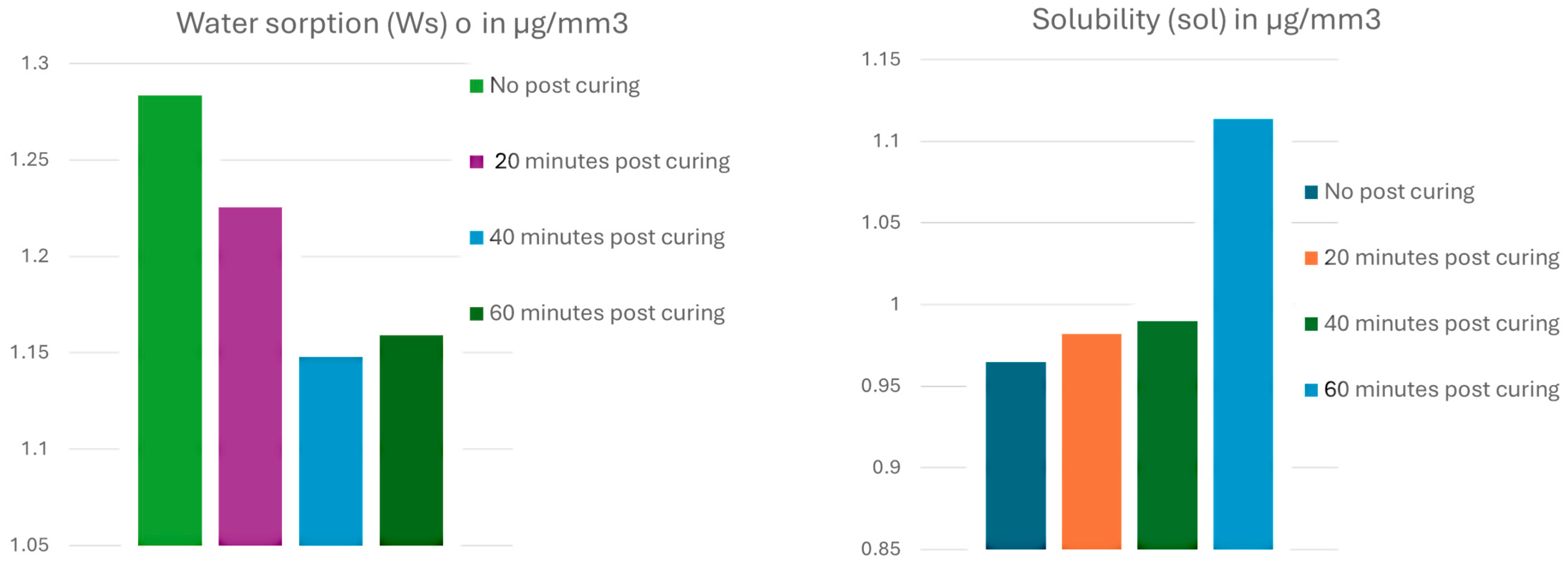
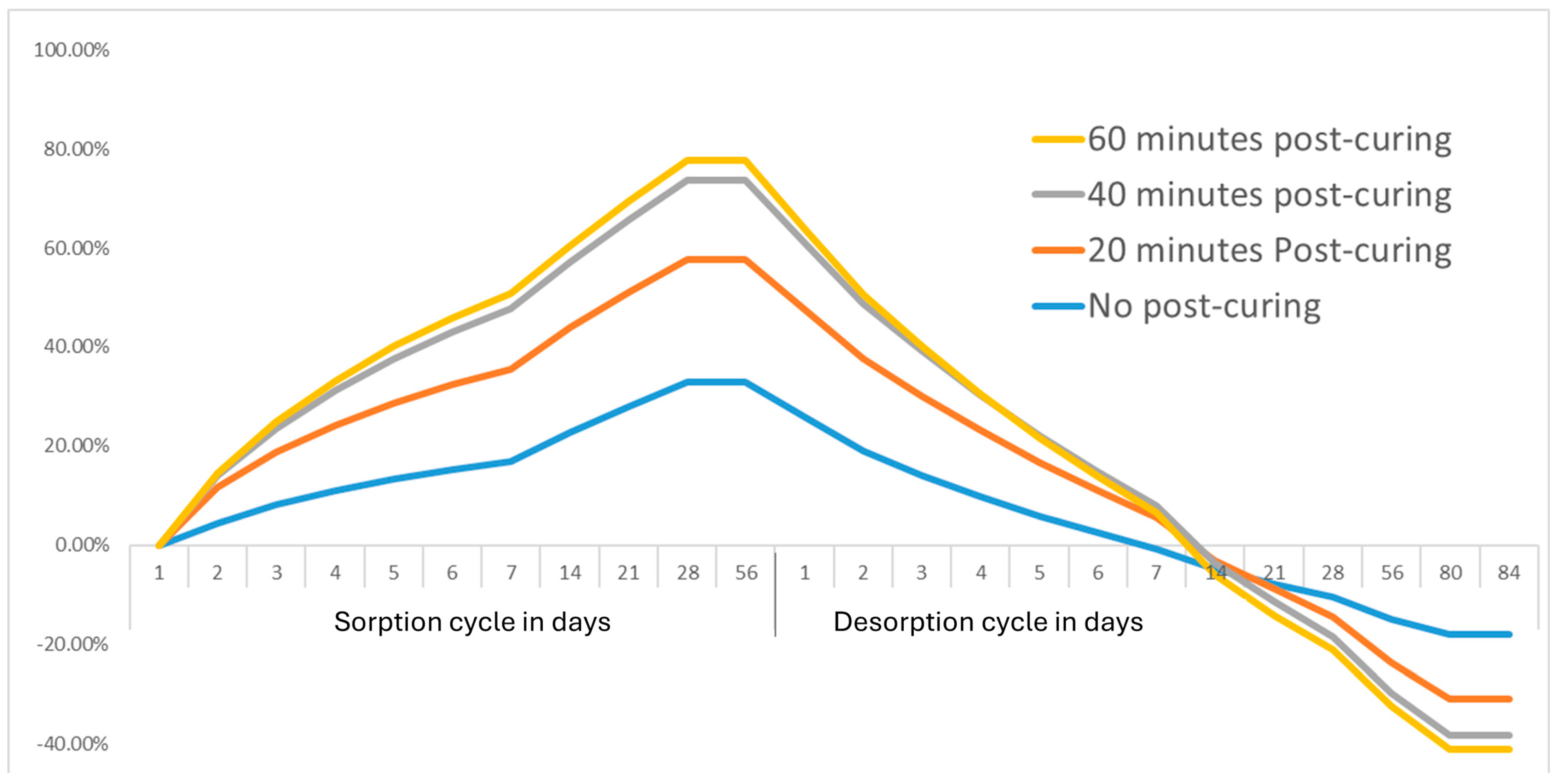
3.2. Water Sorption and Solubility
4. Discussion
5. Conclusions
- Forty minutes of post-curing was the most effective duration for minimizing the release of residual monomers, particularly BisEMA, and reducing water sorption.
- Prolonged post-curing beyond 40 min did not result in further improvements.
- The storage medium significantly impacted monomer release, with ethanol demonstrating higher aggressiveness than water.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Wso | Water sorption |
| Sol | Solubility |
| HPLC | High-performance liquid chromatography |
Appendix A





References
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Wiertelak-Makała, K.; Szymczak-Pajor, I.; Bociong, K.; Kaczmarek, D.; Czemplik, M.; Tanasiewicz, M.; Lewandowski, B.; Maciejewska, B.; Cieślik, M.; Wojtkowiak, M.; et al. Monomers used in resin composites: Degree of conversion, mechanical properties and water sorption/solubility. Braz. Dent. J. 2012, 23, 508–514. [Google Scholar]
- Wiertelak-Makała, K.; Szymczak-Pajor, I.; Bociong, K.; Śliwińska, A. Considerations about Cytotoxicity of Resin-Based Composite Dental Materials: A Systematic Review. Int. J. Mol. Sci. 2023, 25, 152. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H. Craig’s restorative dental materials. Br. Dent. J. 2019, 226, 9. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef]
- Leprince, J.G.; Palin, W.M.; Hadis, M.A.; Devaux, J.; Leloup, G. Progress in dimethacrylate-based dental composite technology and curing efficiency. Dent. Mater. 2013, 29, 139–156. [Google Scholar] [CrossRef]
- Ferracane, J.L. Hygroscopic and hydrolytic effects in dental polymer networks. Dent. Mater. 2006, 22, 211–222. [Google Scholar] [CrossRef]
- Sideridou, I. Study of water sorption, solubility and modulus of elasticity of light-cured dimethacrylate-based dental resins. Biomaterials 2003, 24, 655–665. [Google Scholar] [CrossRef]
- Wulff, J.; Merle, C.L.; Hahnel, S.; Rosentritt, M. Wear Behavior and Water Sorption of Additively Manufactured Resin-Based Splint Materials. Materials 2024, 17, 5880. [Google Scholar] [CrossRef]
- Gad, M.M.; Alshehri, S.Z.; Alhamid, S.A.; Albarrak, A.; Khan, S.Q.; Alshahrani, F.A.; Alqarawi, F.K. Water Sorption, Solubility, and Translucency of 3D-Printed Denture Base Resins. Dent. J. 2022, 10, 42. [Google Scholar] [CrossRef]
- Dimitrova, M.; Vlahova, A.; Hristov, I.; Kazakova, R.; Chuchulska, B.; Kazakov, S.; Forte, M.; Granberg, V.; Barile, G.; Capodiferro, S.; et al. Evaluation of Water Sorption and Solubility of 3D-Printed, CAD/CAM Milled, and PMMA Denture Base Materials Subjected to Artificial Aging. J. Compos. Sci. 2023, 7, 339. [Google Scholar] [CrossRef]
- Jain, S.; Sayed, M.E.; Shetty, M.; Alqahtani, S.M.; Al Wadei, M.H.D.; Gupta, S.G.; Othman, A.A.A.; Alshehri, A.H.; Alqarni, H.; Mobarki, A.H.; et al. Physical and Mechanical Properties of 3D-Printed Provisional Crowns and Fixed Dental Prosthesis Resins Compared to CAD/CAM Milled and Conventional Provisional Resins: A Systematic Review and Meta-Analysis. Polymers 2022, 14, 2691. [Google Scholar] [CrossRef]
- Hassanpour, M.; Narongdej, P.; Alterman, N.; Moghtadernejad, S.; Barjasteh, E. Effects of Post-Processing Parameters on 3D-Printed Dental Appliances: A Review. Polymers 2024, 16, 2795. [Google Scholar] [CrossRef]
- Aati, S.; Akram, Z.; Shrestha, B.; Patel, J.; Shih, B.; Shearston, K.; Ngo, H.; Fawzy, A. Effect of post-curing light exposure time on the physico–mechanical properties and cytotoxicity of 3D-printed denture base material. Dent. Mater. 2022, 38, 57–67. [Google Scholar] [CrossRef]
- Berghaus, E.; Klocke, T.; Maletz, R.; Petersen, S. Degree of conversion and residual monomer elution of 3D-printed, milled and self-cured resin-based composite materials for temporary dental crowns and bridges. J. Mater. Sci. Mater. Med. 2023, 34, 23. [Google Scholar] [CrossRef]
- Wedekind, L.; Güth, J.-F.; Schweiger, J.; Kollmuss, M.; Reichl, F.-X.; Edelhoff, D.; Högg, C. Elution behavior of a 3D-printed, milled and conventional resin-based occlusal splint material. Dent. Mater. 2021, 37, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Reichl, F.-X.; Folwaczny, M.; Högg, C. Monomer release from surgical guide resins manufactured with different 3D printing devices. Dent. Mater. 2020, 36, 1486–1492. [Google Scholar] [CrossRef]
- Duarte, S.; Phark, J. Advances in Dental Restorations: A Comprehensive Review of Machinable and 3D-Printed Ceramic-Reinforced Composites. J. Esthet. Restor. Dent. 2025, 37, 257–276. [Google Scholar] [CrossRef]
- ISO 10993-13:2010; Biological Evaluation of Medical Devices—Part 13: Identification and Quantification of Degradation Products from Polymeric Medical Devices. International Organization for Standardization: Geneva, Switzerland, 2010.
- Formlabs GmbH. Safety Data Sheet: Permanent Crown A2, A3, B1, C2; Formlabs GmbH: Berlin, Germany, 2020. [Google Scholar]
- Penzenstadler, M.; Intarak, N.; Kamnoedboon, P.; Nantanapiboon, D.; Suwanchaikasem, P.; Porntaveetus, T.; Srinivasan, M. In vitro analysis of composition profiles of resins for 3D printing of dentures. J. Dent. 2025, 154, 105565. [Google Scholar] [CrossRef]
- Blum, F. High performance liquid chromatography. Br. J. Hosp. Med. 2014, 75, C18–C21. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. A Guide through the Dental Dimethacrylate Polymer Network Structural Characterization and Interpretation of Physico-Mechanical Properties. Materials 2019, 12, 4057. [Google Scholar] [CrossRef] [PubMed]
- Australian Industrial Chemicals Introduction Scheme. EVA00130—Evaluation Statement. 26 June 2024. Available online: https://www.industrialchemicals.gov.au/sites/default/files/2024-06/EVA00130%20-%20Evaluation%20Statement%20-%2026%20June%202024.pdf (accessed on 18 June 2025).
- Barszczewska-Rybarek, I.; Jurczyk, S. Comparative Study of Structure-Property Relationships in Polymer Networks Based on Bis-GMA, TEGDMA and Various Urethane-Dimethacrylates. Materials 2015, 8, 1230–1248. [Google Scholar] [CrossRef]
- Sideridou, I.; Achilias, D.S.; Kyrikou, E. Thermal expansion characteristics of light-cured dental resins and resin composites. Biomaterials 2004, 25, 3087–3097. [Google Scholar] [CrossRef]
- Mjøsund, H.; Wikant, A. The Influence of the Bis-EMA Content on the Sorption and Solubility of Dental Composite Resins; Universitetet i Tromsø: Tromsø, Norway, 2012. [Google Scholar]
- Luo, S.; Zhu, W.; Liu, F.; He, J. Preparation of a Bis-GMA-Free Dental Resin System with Synthesized Fluorinated Dimethacrylate Monomers. Int. J. Mol. Sci. 2016, 17, 2014. [Google Scholar] [CrossRef]
- Dressano, D.; Salvador, M.V.; Oliveira, M.T.; Marchi, G.M.; Fronza, B.M.; Hadis, M.; Palin, W.M.; Lima, A.F. Chemistry of novel and contemporary resin-based dental adhesives. J. Mech. Behav. Biomed. Mater. 2020, 110, 103875. [Google Scholar] [CrossRef] [PubMed]
- Tichy, A.; Simkova, M.; Vrbova, R.; Roubickova, A.; Duskova, M.; Bradna, P. Bisphenol A Release from Dental Composites and Resin-Modified Glass Ionomers under Two Polymerization Conditions. Polymers 2021, 14, 46. [Google Scholar] [CrossRef]
- De Nys, S.; Duca, R.C.; Vervliet, P.; Covaci, A.; Boonen, I.; Elskens, M.; Vanoirbeek, J.; Godderis, L.; Van Meerbeek, B.; Van Landuyt, K.L. Bisphenol A as degradation product of monomers used in resin-based dental materials. Dent. Mater. 2021, 37, 1020–1029. [Google Scholar] [CrossRef]
- American Dental Association. Bisphenol A. ADA Library & Archives. Available online: https://www.ada.org/resources/research/science-and-research-institute/oral-health-topics/bisphenol-a (accessed on 18 June 2025).
- Jung, Y.S.; Ro, S.T.; Kang, S.W.; Lee, H.; Lee, J.S.; Chae, Y.K.; Lee, K.E.; Lee, H.-S.; Kwack, K.H.; Kim, S.K.; et al. Bisphenol A release from commercially available 3-dimensionally printed resins and human cell apoptosis to bisphenol A: An in-vitro study. J. Clin. Pediatr. Dent. 2023, 47, 89–95. [Google Scholar]
- Šimková, M.; Tichý, A.; Dušková, M.; Bradna, P. Dental Composites—A Low-Dose Source of Bisphenol A? Physiol. Res. 2020, 69, S295–S304. [Google Scholar] [CrossRef]
- Lopes-Rocha, L.; Ribeiro-Gonçalves, L.; Henriques, B.; Özcan, M.; Tiritan, M.E.; Souza, J.C.M. An integrative review on the toxicity of Bisphenol A (BPA) released from resin composites used in dentistry. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1942–1952. [Google Scholar] [CrossRef]
- Lakkala, P.; Munnangi, S.R.; Bandari, S.; Repka, M. Additive manufacturing technologies with emphasis on stereolithography 3D printing in pharmaceutical and medical applications: A review. Int. J. Pharm. X 2023, 5, 100159. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Fabbri, P.; Leoni, E.; Mazzanti, F.; Akbari, R.; Antonini, C. Additive manufacturing by digital light processing: A review. Prog. Addit. Manuf. 2023, 8, 331–351. [Google Scholar] [CrossRef]
- Zhu, G.; Hou, Y.; Xu, J.; Zhao, N. Reprintable Polymers for Digital Light Processing 3D Printing. Adv. Funct. Mater. 2021, 31, 2007173. [Google Scholar] [CrossRef]
- Mahmood, A.; Perveen, F.; Chen, S.; Akram, T.; Irfan, A. Polymer Composites in 3D/4D Printing: Materials, Advances, and Prospects. Molecules 2024, 29, 319. [Google Scholar] [CrossRef]
- Stansbury, J.W.; Idacavage, M.J. 3D printing with polymers: Challenges among expanding options and opportunities. Dent. Mater. 2016, 32, 54–64. [Google Scholar] [CrossRef]
- Shaukat, U.; Rossegger, E.; Schlögl, S. A Review of Multi-Material 3D Printing of Functional Materials via Vat Photopolymerization. Polymers 2022, 14, 2449. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Francos, X.; Konuray, O.; Ramis, X.; Serra, À.; De la Flor, S. Enhancement of 3D-Printable Materials by Dual-Curing Procedures. Materials 2020, 14, 107. [Google Scholar] [CrossRef]
- Schulz, S.D.; Laquai, T.; Kümmerer, K.; Bolek, R.; Mersch-Sundermann, V.; Polydorou, O. Elution of Monomers from Provisional Composite Materials. Int. J. Polym. Sci. 2015, 2015, 617407. [Google Scholar] [CrossRef]
- Rothmund, L.; Shehata, M.; Van Landuyt, K.L.; Schweikl, H.; Carell, T.; Geurtsen, W.; Hellwig, E.; Hickel, R.; Reichl, F.-X.; Högg, C. Release and protein binding of components from resin based composites in native saliva and other extraction media. Dent. Mater. 2015, 31, 496–504. [Google Scholar] [CrossRef]
- Decker, C.; Masson, F.; Bianchi, C. Kinetic Study of Photoinitiated Polymerization Reactions by Real-Time Infrared Spectroscopy. In In Situ Spectroscopy of Monomer and Polymer Synthesis; Springer: Boston, MA, USA, 2003; pp. 109–124. [Google Scholar]
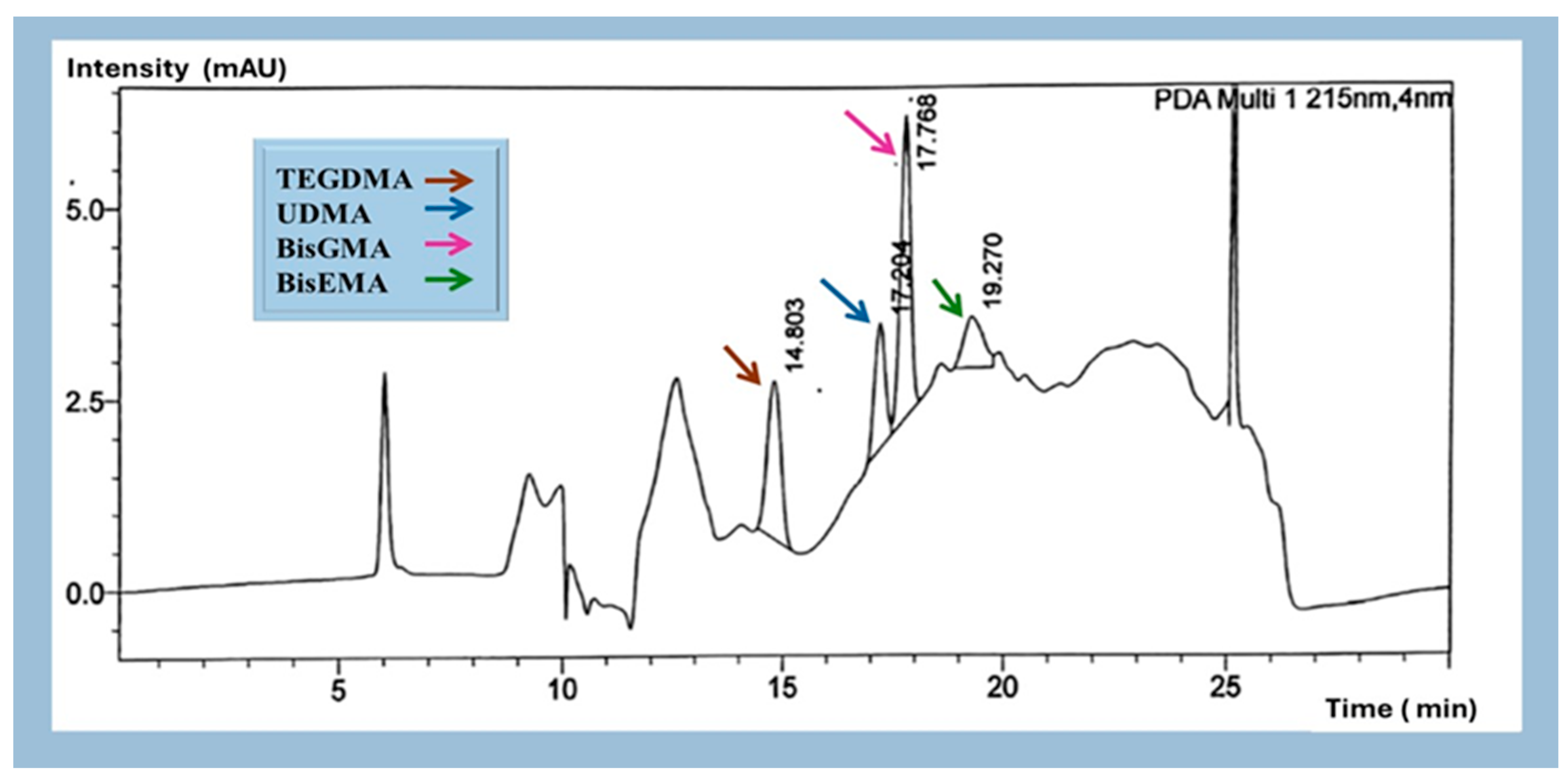
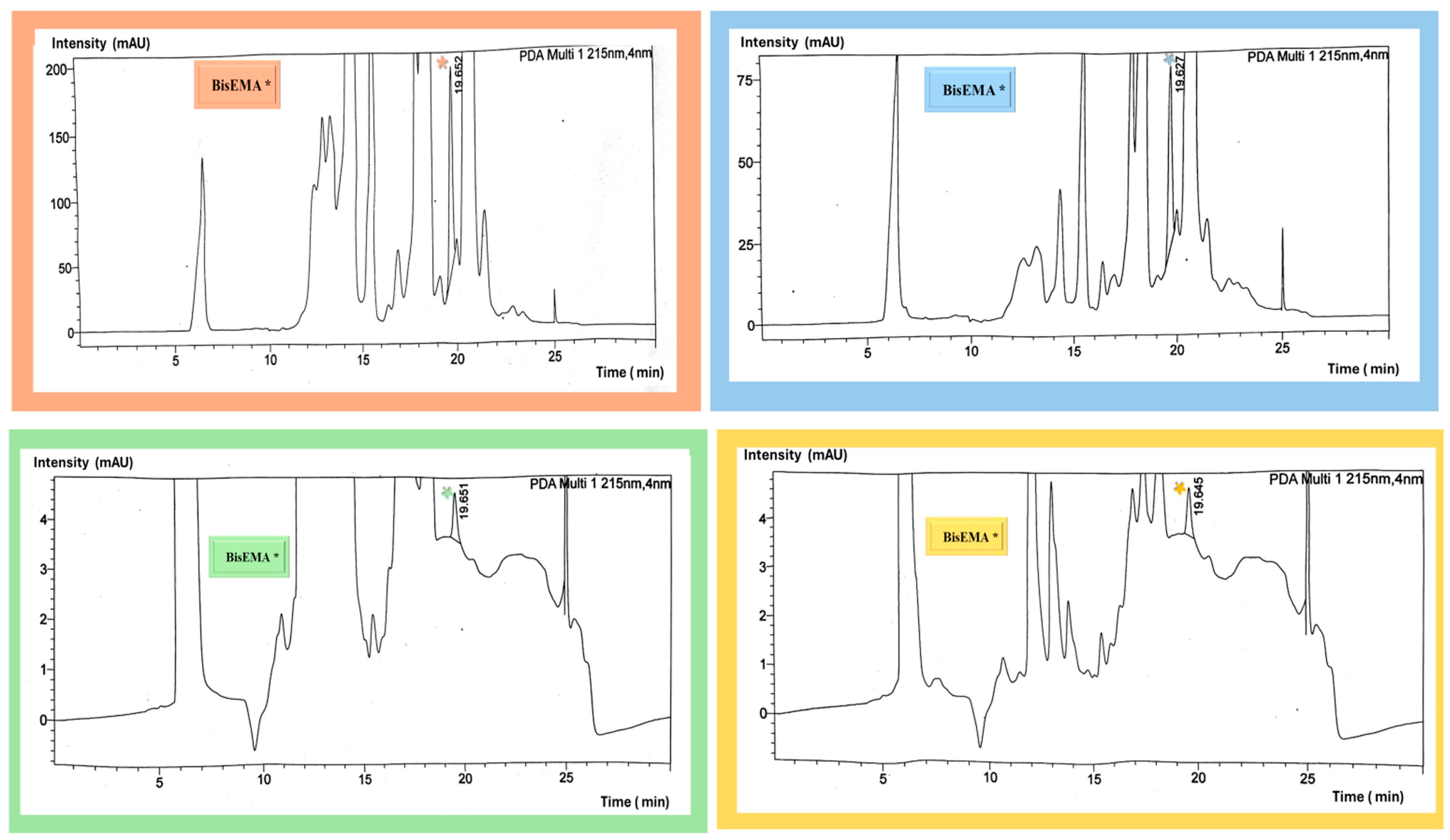

| Material | Manufacturer (Company) | Resin Composition | 3D Printer |
|---|---|---|---|
| Permanent Crown Shade A3 (Lot Number 600164) | Formlabs Inc., Somerville, MA, USA. | Inorganic fillers: Ceramic micro-filler Organic Polymers: Bis-EMA Photoinitiator system: TPO, photoinitiator | Pionext D128, Piocreat 3d, Shenzhen, China. |
| Reference Substance (Abbreviation) | Chemical Nomenclature | Function | CAS# * | Manufacturer | Product Number |
|---|---|---|---|---|---|
| BisGMA | Bisphenol A glycerolate dimethacrylate | Monomer | 1565-94-2 | Sigma-Aldrich, St. Louis, MO, USA | 494356 |
| BisEMA | Bisphenol A ethoxylate dimethacrylate | Monomer | 41637-38-1 | Sigma-Aldrich, St. Louis, MO, USA | 455059 |
| UDMA | Urethane-di-methacrylate | Co-Monomer | 72869-86-4 | Sigma-Aldrich, St. Louis, MO, USA | 436909 |
| TEGDMA | Triethylenglycol-dimethacrylate | Co-Monomer | 109-16-0 | Sigma-Aldrich, St. Louis, MO, USA | 261548 |
| Effect | Comparison | Crude Analysis | SE | z-Value | p-Value | 95% Confidence Interval |
|---|---|---|---|---|---|---|
| Mean Concentration | 425.3676 | 59.75953 | 7.12 | <0.001 | [304.0363, 548.1826] | |
| Effect of post-curing time | 20 min post-curing vs. no post-curing | −478.95 | 80.15424 | −5.98 | <0.001 | [−630.0968, −336.0107] |
| 40 min post-curing vs. no post-curing | −519.963 | 80.8881 | −6.43 | <0.001 | [−661.6359, −365.1328] | |
| 60 min post-curing vs. no post-curing | −480.1584 | 78.91344 | −6.08 | <0.001 | [−630.2413, −333.1834] | |
| Effect of storage condition | H2O 7 d vs. H2O 24 h | 0.27259 | 19.15515 | 0.01 | 0.989 | [−8.618262, 15.89887] |
| ethanol 24 h vs. water 24 h | 754.668 | 68.29158 | 11.05 | <0.001 | [608.6126, 886.7524] | |
| ethanol 7 d vs. water 24 h | 872.4947 | 66.50705 | 13.12 | <0.001 | [729.3719, 988.1243] | |
| Post-Curing Duration | Average Wso (μg/mm3) | Average Sol (μg/mm3) |
|---|---|---|
| No post-curing | 1.28 ± 0.12 a | 0.96 ± 0.09 a |
| 20 min post-curing | 1.22 ± 0.21 b | 0.98 ± 0.14 b |
| 40 min post-curing | 1.14 ± 0.15 ab | 0.99 ± 0.12 ab |
| 60 min post-curing | 1.15 ± 0.05 a | 1.11 ± 0.07 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharbi, S.; Alshabib, A.; Algamaiah, H.; Aldosari, M.; Alayad, A. Influence of Post-Printing Polymerization Time on the Elution of Residual Monomers and Water Sorption of 3D-Printed Resin Composite. Materials 2025, 18, 2905. https://doi.org/10.3390/ma18122905
Alharbi S, Alshabib A, Algamaiah H, Aldosari M, Alayad A. Influence of Post-Printing Polymerization Time on the Elution of Residual Monomers and Water Sorption of 3D-Printed Resin Composite. Materials. 2025; 18(12):2905. https://doi.org/10.3390/ma18122905
Chicago/Turabian StyleAlharbi, Shaima, Abdulrahman Alshabib, Hamad Algamaiah, Muath Aldosari, and Abdullah Alayad. 2025. "Influence of Post-Printing Polymerization Time on the Elution of Residual Monomers and Water Sorption of 3D-Printed Resin Composite" Materials 18, no. 12: 2905. https://doi.org/10.3390/ma18122905
APA StyleAlharbi, S., Alshabib, A., Algamaiah, H., Aldosari, M., & Alayad, A. (2025). Influence of Post-Printing Polymerization Time on the Elution of Residual Monomers and Water Sorption of 3D-Printed Resin Composite. Materials, 18(12), 2905. https://doi.org/10.3390/ma18122905







