Dual-Functional Organosilicon Additives Containing Methacrylate and Trimethoxysilyl Groups Enhancing Impact Toughness of Polylactide (PLA): Structure–Property Relationship
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Synthesis Procedure of the Difunctional Organosilicon Derivatives
2.4. Technology Procedure for PLA Modification
3. Results and Discussion
3.1. Mechanical Properties Evaluation
3.1.1. Impact Resistance
3.1.2. Static Tensile and Flexural Test Results
3.2. Microstructure Evaluation
3.2.1. Distribution of Additives in PLA Composites—SEM-EDS Analysis
3.2.2. A Fractography Study of the Breakthrough Surface After Dynamic Tension—Optical Microscopy (OM) and SEM Observations
3.3. X-Ray Diffraction (XRD) Analysis Results
3.4. Thermal Analysis Results
3.4.1. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
3.4.2. Heat Deflection Temperature (HDT)
3.5. Rheology
3.5.1. Melt Flow Rate (MFR)
3.5.2. Oscillatory Rheometry
3.6. Water Contact Angle (WCA)
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morão, A.; De Bie, F. Life Cycle Impact Assessment of Polylactic Acid (PLA) Produced from Sugarcane in Thailand. J. Polym. Environ. 2019, 27, 2523–2539. [Google Scholar] [CrossRef]
- Vink, E.T.H.; Rábago, K.R.; Glassner, D.A.; Gruber, P.R. Applications of Life Cycle Assessment to NatureWorksTM Polylactide (PLA) Production. Polym. Degrad. Stab. 2003, 80, 403–419. [Google Scholar] [CrossRef]
- De Albuquerque, T.L.; Marques Júnior, J.E.; De Queiroz, L.P.; Ricardo, A.D.S.; Rocha, M.V.P. Polylactic Acid Production from Biotechnological Routes: A Review. Int. J. Biol. Macromol. 2021, 186, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides—Methods of Synthesis and Characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the Barrier Properties of PLA: A State-of-the-Art Review for Food Packaging Applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Ingrao, C.; Tricase, C.; Cholewa-Wójcik, A.; Kawecka, A.; Rana, R.; Siracusa, V. Polylactic Acid Trays for Fresh-Food Packaging: A Carbon Footprint Assessment. Sci. Total Environ. 2015, 537, 385–398. [Google Scholar] [CrossRef]
- Torki, M.M.; Hassanajili, S.; Jalisi, M.M. Design Optimizations of PLA Stent Structure by FEM and Investigating Its Function in a Simulated Plaque Artery. Math. Comput. Simul. 2020, 169, 103–116. [Google Scholar] [CrossRef]
- Capuana, E.; Lopresti, F.; Ceraulo, M.; La Carrubba, V. Poly-l-Lactic Acid (PLLA)-Based Biomaterials for Regenerative Medicine: A Review on Processing and Applications. Polymers 2022, 14, 1153. [Google Scholar] [CrossRef]
- Da Silva, D.; Kaduri, M.; Poley, M.; Adir, O.; Krinsky, N.; Shainsky-Roitman, J.; Schroeder, A. Biocompatibility, Biodegradation and Excretion of Polylactic Acid (PLA) in Medical Implants and Theranostic Systems. Chem. Eng. J. 2018, 340, 9–14. [Google Scholar] [CrossRef]
- Tümer, E.H.; Erbil, H.Y. Extrusion-Based 3D Printing Applications of PLA Composites: A Review. Coatings 2021, 11, 390. [Google Scholar] [CrossRef]
- Bhagia, S.; Bornani, K.; Agrawal, R.; Satlewal, A.; Ďurkovič, J.; Lagaňa, R.; Bhagia, M.; Yoo, C.G.; Zhao, X.; Kunc, V.; et al. Critical Review of FDM 3D Printing of PLA Biocomposites Filled with Biomass Resources, Characterization, Biodegradability, Upcycling and Opportunities for Biorefineries. Appl. Mater. Today 2021, 24, 101078. [Google Scholar] [CrossRef]
- Sztorch, B.; Brząkalski, D.; Pakuła, D.; Frydrych, M.; Špitalský, Z.; Przekop, R.E. Natural and Synthetic Polymer Fillers for Applications in 3D Printing—FDM Technology Area. Solids 2022, 3, 508–548. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed]
- Baniasadi, H.; Äkräs, L.; Madani, Z.; Silvenius, F.; Fazeli, M.; Lipponen, S.; Vapaavuori, J.; Seppälä, J. Development and Characterization of Polylactic Acid/Starch Biocomposites—From Melt Blending to Preliminary Life Cycle Assessment. Int. J. Biol. Macromol. 2024, 279, 135173. [Google Scholar] [CrossRef]
- Sirin, H.; Kodal, M.; Ozkoc, G. The Influence of POSS Type on the Properties of PLA. Polym. Compos. 2016, 37, 1497–1506. [Google Scholar] [CrossRef]
- Meyva-Zeybek, Y.; Kaynak, C. A Comparative Study for the Behavior of 3D-PRINTED and Compression Molded PLA/POSS Nanocomposites. J. Appl. Polym. Sci. 2021, 138, 50246. [Google Scholar] [CrossRef]
- Doganci, M.D.; Caner, D.; Doganci, E.; Ozkoc, G. Effects of Hetero-armed Star-shaped PCL-PLA Polymers with POSS Core on Thermal, Mechanical, and Morphological Properties of PLA. J. Appl. Polym. Sci. 2021, 138, 50712. [Google Scholar] [CrossRef]
- Zhao, Y.; Jiang, X.; Zhang, X.; Hou, L. Toughened Elastomer/Polyhedral Oligomeric Silsesquioxane (POSS)-intercalated Rectorite Nanocomposites: Preparation, Microstructure, and Mechanical Properties. Polym. Compos. 2017, 38, E443–E450. [Google Scholar] [CrossRef]
- Rezaei, H.; Seifi, S.; Moeinifar, E.; Hejazi, I.; Seyfi, J.; Khonakdar, H.A. Effect of Nanoparticle Type and Content on Morphology, Rheology, and Crystallinity of Poly(Lactic Acid) Using Titanium Dioxide and Polyhedral Oligomeric Silsesquioxane Nanoparticles. Polym. Compos. 2020, 41, 1551–1560. [Google Scholar] [CrossRef]
- Sztorch, B.; Brząkalski, D.; Głowacka, J.; Pakuła, D.; Frydrych, M.; Przekop, R.E. Trimming Flow, Plasticity, and Mechanical Properties by Cubic Silsesquioxane Chemistry. Sci. Rep. 2023, 13, 14156. [Google Scholar] [CrossRef]
- Brząkalski, D.; Sztorch, B.; Frydrych, M.; Pakuła, D.; Dydek, K.; Kozera, R.; Boczkowska, A.; Marciniec, B.; Przekop, R.E. Limonene Derivative of Spherosilicate as a Polylactide Modifier for Applications in 3D Printing Technology. Molecules 2020, 25, 5882. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.; Zhou, Y.; Zeng, G.; Liu, W. Biodegradable PLA-Based Composites Modified by POSS Particles. Polym. Plast. Technol. Mater. 2020, 59, 998–1009. [Google Scholar] [CrossRef]
- Niemczyk, A.; Dziubek, K.; Sacher-Majewska, B.; Czaja, K.; Dutkiewicz, M.; Marciniec, B. Study of Thermal Properties of Polyethylene and Polypropylene Nanocomposites with Long Alkyl Chain-Substituted POSS Fillers. J. Therm. Anal. Calorim. 2016, 125, 1287–1299. [Google Scholar] [CrossRef]
- Wei, L.; Shicheng, H.; Hongfu, Z. Effect of Octa(Epoxycyclohexyl) POSS on Thermal, Rheology Property, and Foaming Behavior of PLA Composites. J. Appl. Polym. Sci. 2018, 135, 46399. [Google Scholar] [CrossRef]
- Chua, M.H.; Zhou, H.; Xu, J. POSS as Fire Retardant. In Polymer/POSS Nanocomposites and Hybrid Materials; Kalia, S., Pielichowski, K., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2018; pp. 337–372. ISBN 978-3-030-02326-3. [Google Scholar]
- Turgut, G.; Dogan, M.; Tayfun, U.; Ozkoc, G. The Effects of POSS Particles on the Flame Retardancy of Intumescent Polypropylene Composites and the Structure-Property Relationship. Polym. Degrad. Stab. 2018, 149, 96–111. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, K.; Zhou, Y.; Shang, P.; Yang, S.; Zhang, B.; Liu, A.; Liu, J.; Xie, J.; Xu, J. POSS(Epoxy)8 Reinforced Poly (Butylene Adipate-Co-Terephthalate)/Lignin Biodegradable Films: Fabrication, Enhanced Mechanical Properties and UV Aging Resistance. Int. J. Biol. Macromol. 2024, 255, 127921. [Google Scholar] [CrossRef]
- Sztorch, B.; Pakuła, D.; Kustosz, M.; Romanczuk-Ruszuk, E.; Gabriel, E.; Przekop, R.E. The Influence of Organofunctional Substituents of Spherosilicates on the Functional Properties of PLA/TiO2 Composites Used in 3D Printing (FDM/FFF). Polymers 2022, 14, 5493. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Ni, H.; Pittman, C.U., Jr. Polyhedral Oligomeric Silsesquioxane (POSS) Polymers and Copolymers: A Review. J. Inorg. Organomet. Polym. 2001, 11, 123–154. [Google Scholar] [CrossRef]
- Turan, D.; Sirin, H.; Ozkoc, G. Effects of POSS Particles on the Mechanical, Thermal, and Morphological Properties of PLA and Plasticised PLA. J. Appl. Polym. Sci. 2011, 121, 1067–1075. [Google Scholar] [CrossRef]
- Monticelli, O.; Putti, M.; Gardella, L.; Cavallo, D.; Basso, A.; Prato, M.; Nitti, S. New Stereocomplex PLA-Based Fibers: Effect of POSS on Polymer Functionalization and Properties. Macromolecules 2014, 47, 4718–4727. [Google Scholar] [CrossRef]
- Sirin, H.; Turan, D.; Ozkoc, G.; Gurdag, S. POSS Reinforced PET Based Composite Fibers: “Effect of POSS Type and Loading Level. ” Compos. Part B Eng. 2013, 53, 395–403. [Google Scholar] [CrossRef]
- Karamishamloo, M.; Mirmohammadi, S.A.; Davachi, S.M. Polyethylene Glycol/Polyhedral Oligomeric Silsesquioxane as an in Situ Photocrosslinkable Polymeric Nanohybrid. Polym. Int. 2020, 69, 492–501. [Google Scholar] [CrossRef]
- Xu, Y.; Long, J.; Zhang, R.; Du, Y.; Guan, S.; Wang, Y.; Huang, L.; Wei, H.; Liu, L.; Huang, Y. Greatly Improving Thermal Stability of Silicone Resins by Modification with POSS. Polym. Degrad. Stab. 2020, 174, 109082. [Google Scholar] [CrossRef]
- Hu, Z.; Wang, Y.; Liu, X.; Wang, Q.; Cui, X.; Jin, S.; Yang, B.; Xia, Y.; Huang, S.; Qiang, Z.; et al. Rational Design of POSS Containing Low Dielectric Resin for SLA Printing Electronic Circuit Plate Composites. Compos. Sci. Technol. 2022, 223, 109403. [Google Scholar] [CrossRef]
- Soldatov, M.; Liu, H. Hybrid Porous Polymers Based on Cage-like Organosiloxanes: Synthesis, Properties and Applications. Prog. Polym. Sci. 2021, 119, 101419. [Google Scholar] [CrossRef]
- Zhang, W.; Camino, G.; Yang, R. Polymer/Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposites: An Overview of Fire Retardance. Prog. Polym. Sci. 2017, 67, 77–125. [Google Scholar] [CrossRef]
- Seidi, F.; Jouyandeh, M.; Taghizadeh, A.; Taghizadeh, M.; Habibzadeh, S.; Jin, Y.; Xiao, H.; Zarrintaj, P.; Saeb, M.R. Polyhedral Oligomeric Silsesquioxane/Epoxy Coatings: A Review. Surf. Innov. 2021, 9, 3–16. [Google Scholar] [CrossRef]
- Ayandele, E.; Sarkar, B.; Alexandridis, P. Polyhedral Oligomeric Silsesquioxane (POSS)-Containing Polymer Nanocomposites. Nanomaterials 2012, 2, 445–475. [Google Scholar] [CrossRef]
- Dou, Q.; Abdul Karim, A.; Loh, X. Modification of Thermal and Mechanical Properties of PEG-PPG-PEG Copolymer (F127) with MA-POSS. Polymers 2016, 8, 341. [Google Scholar] [CrossRef]
- Hajiali, F.; Marić, M. Incorporation of POSS to Improve Thermal Stability of BIO-BASED Polymethacrylates by NITROXIDE-MEDIATED Polymerization: Polymerization Kinetics and Characterization. J. Polym. Sci. 2020, 58, 1503–1520. [Google Scholar] [CrossRef]
- Tunstall-Garcia, H.; Charles, B.L.; Evans, R.C. The Role of Polyhedral Oligomeric Silsesquioxanes in Optical Applications. Adv. Photonics Res. 2021, 2, 2000196. [Google Scholar] [CrossRef]
- Ma, Y.; He, L. POSS-Pendanted in Epoxy Chain Inorganic-Organic Hybrid for Highly Thermo-Mechanical, Permeable and Hydrothermal-Resistant Coatings. Mater. Chem. Phys. 2017, 201, 120–129. [Google Scholar] [CrossRef]
- Zhang, Z.; Tian, D.; Niu, Z.; Zhou, Y.; Hou, X.; Ma, X. Enhanced Toughness and Lowered Dielectric Loss of Reactive POSS Modified Bismaleimide Resin as Well as the Silica Fiber Reinforced Composites. Polym. Compos. 2021, 42, 6900–6911. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, X.; Xu, K.; Liu, H.; Su, J.; Liu, X.; Fu, Z.; Guo, Y.; Chen, M. Preparation and Properties of Polycarbonate/Polyhedral Oligomeric Silsesquioxanes (POSS) Hybrid Composites. Polym. Adv. Technol. 2012, 23, 765–775. [Google Scholar] [CrossRef]
- Vahabi, H.; Ferry, L.; Longuet, C.; Otazaghine, B.; Negrell-Guirao, C.; David, G.; Lopez-Cuesta, J.-M. Combination Effect of Polyhedral Oligomeric Silsesquioxane (POSS) and a Phosphorus Modified PMMA, Flammability and Thermal Stability Properties. Mater. Chem. Phys. 2012, 136, 762–770. [Google Scholar] [CrossRef]
- Wu, J.; Mather, P.T. POSS Polymers: Physical Properties and Biomaterials Applications. Polym. Rev. 2009, 49, 25–63. [Google Scholar] [CrossRef]
- Yahyaei, H.; Mohseni, M.; Ghanbari, H. POSS Hybrid Materials for Medical Applications. In Polymer/POSS Nanocomposites and Hybrid Materials; Kalia, S., Pielichowski, K., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2018; pp. 373–394. ISBN 978-3-030-02326-3. [Google Scholar]
- Dankert, F.; Von Hänisch, C. Siloxane Coordination Revisited: Si−O Bond Character, Reactivity and Magnificent Molecular Shapes. Eur. J. Inorg. Chem. 2021, 2021, 2907–2927. [Google Scholar] [CrossRef]
- Sokolnicki, T.; Franczyk, A.; Kozak, R.; Walkowiak, J. Coupling Agents with 2,4,6,8-Tetramethylcyclotetrasiloxane Core—Synthesis and Application in Styrene–Butadiene Rubber Production. Inorg. Chem. Front. 2023, 10, 5897–5907. [Google Scholar] [CrossRef]
- Tao, Y.; Mei, S.; Yi, H.; Pan, X.; Zhang, R.; Li, Z. Enhancing Fracture Toughness of Polydimethylsiloxane with Cyclosiloxane Hybrid Polymer Microspheres. Compos. Sci. Technol. 2023, 244, 110314. [Google Scholar] [CrossRef]
- Yuan, W.; Wei, X.; Peng, Q.; Fan, L.; Li, X.; Hu, H.; Huang, Y.; Ma, J.; Yang, J. Silacyclobutane-functionalized Cyclosiloxanes as Photoactive Precursors for High Thermal Stability, Low Dielectric Constant and Low Dielectric Loss Polymers. J. Appl. Polym. Sci. 2021, 138, 51376. [Google Scholar] [CrossRef]
- Bailey, F.E.; Vandenberg, E.J.; Blumstein, A.; Bowden, M.J.; Arthur, J.C.; Lal, J.; Ottenbrite, R.M. (Eds.) Initiation of Polymerization; ACS Symposium Series; American Chemical Society: Washingtong, DC, USA, 1983; Volume 212, ISBN 978-0-8412-0765-3. [Google Scholar]
- Zhu, H.; Watanabe, Y.; Yoshida, N.; Ishizaki, Y.; Ohwada, M.; Tang, R.; Mitsuishi, M. Facile Synthesis of Amine-Substituted Cyclosiloxanes via a Photocatalytic Thiol-Ene Reaction to Generate Ketoenamine-Linked Hybrid Networks. Polym. J. 2022, 54, 1257–1265. [Google Scholar] [CrossRef]
- Li, G.; Liu, Y. Cyclosiloxane-Containing Polymers and the Formation of Highly Stable Elastomer. Chem. Lett. 2020, 49, 299–302. [Google Scholar] [CrossRef]
- Sun, R.; Feng, S.; Zhou, B.; Chen, Z.; Wang, D.; Liu, H. Flexible Cyclosiloxane-Linked Fluorescent Porous Polymers for Multifunctional Chemical Sensors. ACS Macro Lett. 2020, 9, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhou, X.; Xu, H.; Song, Y.; Li, B.; Liu, M. Recently Process in the Preparation, Properties, and Applications of Cyclosiloxane-Containing Polymers. Polymer 2024, 299, 126956. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, D.; Liu, M.; Song, Y.; Wang, J. Cyclosiloxane Hybrid Polymer as a Robust Transparent Eco-Friendly Anti-Fouling Coating. Eng. Sci. 2022, 20, 110–116. [Google Scholar] [CrossRef]
- Li, Q.Y.; Xu, J.; Zhang, W.Z.; Li, P. Preparation and Characterization of Chiral Cyclosiloxane-Based Liquid-Crystalline Elastomers Bearing Menthyl Groups. Adv. Mater. Res. 2012, 466–467, 445–448. [Google Scholar] [CrossRef]
- Zhu, H.; Buchtal, T.J.; Mitsuishi, M. Self-Assembling Superstructures of Cyclosiloxane Amphiphiles with Complex Flower Shapes and Superhydrophobic Properties. Appl. Surf. Sci. 2021, 563, 150245. [Google Scholar] [CrossRef]
- Gan, H.; Seraji, S.M.; Khan, M.J.; Zhang, J.; Swan, S.R.; Senanayake, R.B.; Varley, R.J. Reactivity, Processability, and Thermal Stability of Tetrafunctional Glycidyl Ether Cyclic Siloxane Epoxy Hybrid Networks. J. Appl. Polym. Sci. 2024, 141, e55849. [Google Scholar] [CrossRef]
- Kong, D.; Liu, J.; Zhang, Z.; Wang, S.; Lu, Z. Preparation of Synergistic Silicon, Phosphorus and Nitrogen Flame Retardant Based on Cyclosiloxane and Its Application to Cotton Fabric. Cellulose 2021, 28, 8115–8128. [Google Scholar] [CrossRef]
- Herc, A.S.; Wlodarska, M.; Nowacka, M.; Bojda, J.; Szymanski, W.; Kowalewska, A. Supramolecular Interactions between Polylactide and Model Cyclosiloxanes with Hydrogen Bonding-Capable Functional Groups. Express Polym. Lett. 2020, 14, 134–153. [Google Scholar] [CrossRef]
- PN-EN ISO 179-1:2023; Plastics—Charpy Impact Determination—Part 1: Non-Instrumental Impact Testing. ISO: Geneva, Switzerland, 2023.
- PN-EN ISO 178:2019; Plastics—Determination of Flexural Properties. ISO: Geneva, Switzerland, 2019.
- PN-EN ISO 527:2019; Plastics—Determination of Tensile Properties. ISO: Geneva, Switzerland, 2019.
- PN-EN ISO 75-1:2020; Plastics—Determination of Temperature of Deflection Under Load Part 1: General Test Method. ISO: Geneva, Switzerland, 2020.
- PN-EN ISO 1133-1:2022; Plastics—Determination of the Melt Mass-Flow Rate (MFR) and Melt Volume-Flow Rate (MVR) of Thermoplastics Part 1: Standard Method. ISO: Geneva, Switzerland, 2022.
- Carreau, P.J. Rheology of Filled Polymeric Systems. In Transport Processes in Bubbles, Drops and Particles; Springer: Berlin, Germany, 1992; pp. 90–165. [Google Scholar]
- Sztorch, B.; Głowacka, J.; Brząkalski, D.; Romanczuk-Ruszuk, E.; Marciniec, B.; Przekop, R.E. High Flexural Modulus of Polilactide Composites for 3D Printing Technology Using Multifunctional Octaspherosilicates. J. Mater. Res. 2024, 39, 2507–2521. [Google Scholar] [CrossRef]
- PN-EN ISO 20753:2019; Plastics—Test Specimens. ISO: Geneva, Switzerland, 2019.
- Xu, S.; Tahon, J.-F.; De-Waele, I.; Stoclet, G.; Gaucher, V. Brittle-to-Ductile Transition of PLA Induced by Macromolecular Orientation. Express Polym. Lett. 2020, 14, 1034–1047. [Google Scholar] [CrossRef]
- Kaiser, M.; Anuar, H.; Razak, S. Ductile–Brittle Transition Temperature of Polylactic Acid-Based Biocomposite. J. Thermoplast. Compos. Mater. 2013, 26, 216–226. [Google Scholar] [CrossRef]
- Liu, Y.; Tseng, M.; Fangchiang, M. Polymerization and Nanocomposites Properties of Multifunctional Methylmethacrylate POSS. J. Polym. Sci. Part Polym. Chem. 2008, 46, 5157–5166. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Matinlinna, J.P. Aspects of Silane Coupling Agents and Surface Conditioning in Dentistry: An Overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef]
- Xie, Y.; Hill, C.A.S.; Xiao, Z.; Militz, H.; Mai, C. Silane Coupling Agents Used for Natural Fiber/Polymer Composites: A Review. Compos. Part Appl. Sci. Manuf. 2010, 41, 806–819. [Google Scholar] [CrossRef]
- Swapna, V.P.; Nambissan, P.M.G.; Thomas, S.P.; Vayyaprontavida Kaliyathan, A.; Jose, T.; George, S.C.; Thomas, S.; Stephen, R. Free Volume Defects and Transport Properties of Mechanically Stable Polyhedral Oligomeric Silsesquioxane Embedded Poly(Vinyl Alcohol)-poly(Ethylene Oxide) Blend Membranes. Polym. Int. 2019, 68, 1280–1291. [Google Scholar] [CrossRef]
- Dintcheva, N.T.; Morici, E.; Arrigo, R.; La Mantia, F.P.; Malatesta, V.; Schwab, J.J. Structure-Properties Relationships of Polyhedral Oligomeric Silsesquioxane (POSS) Filled PS Nanocomposites. Express Polym. Lett. 2012, 6, 561–571. [Google Scholar] [CrossRef]
- Chaos, A.; Sangroniz, A.; Gonzalez, A.; Iriarte, M.; Sarasua, J.; Del Río, J.; Etxeberria, A. Tributyl Citrate as an Effective Plasticizer for Biodegradable Polymers: Effect of Plasticizer on Free Volume and Transport and Mechanical Properties. Polym. Int. 2019, 68, 125–133. [Google Scholar] [CrossRef]
- Van Krevelen, D.W.; Te Nijenhuis, K. Product Properties (I). In Properties of Polymers; Elsevier: Amsterdam, The Netherlands, 2009; pp. 819–845. ISBN 978-0-08-054819-7. [Google Scholar]
- Greenhalgh, E.S. Delamination-Dominated Failures in Polymer Composites. In Failure Analysis and Fractography of Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2009; pp. 164–237. ISBN 978-1-84569-217-9. [Google Scholar]
- Kopp, J.-B.; Girardot, J. Dynamic Fracture in a Semicrystalline Biobased Polymer: An Analysis of the Fracture Surface. Int. J. Fract. 2020, 226, 121–132. [Google Scholar] [CrossRef]
- Robertson, R.E.; Mindroiu, V.E. Fracture Surface Characteristics of Unfilled Thermosets. Polym. Eng. Sci. 1987, 27, 55–62. [Google Scholar] [CrossRef]
- Barbosa, P.; Campos, J.; Turygin, A.; Shur, V.Y.; Kholkin, A.; Barros-Timmons, A.; Figueiredo, F.M. Piezoelectric Poly(Lactide) Stereocomplexes with a Cholinium Organic Ionic Plastic Crystal. J. Mater. Chem. C 2017, 5, 12134–12142. [Google Scholar] [CrossRef]
- Hsieh, Y.-T.; Nozaki, S.; Kido, M.; Kamitani, K.; Kojio, K.; Takahara, A. Crystal Polymorphism of Polylactide and Its Composites by X-Ray Diffraction Study. Polym. J. 2020, 52, 755–763. [Google Scholar] [CrossRef]
- Di Lorenzo, M.L.; Androsch, R. Influence of A′-/A-crystal Polymorphism on Properties of Poly(L -lactic Acid). Polym. Int. 2019, 68, 320–334. [Google Scholar] [CrossRef]
- Song, P.; Chen, G.; Wei, Z.; Zhang, W.; Liang, J. Calorimetric Analysis of the Multiple Melting Behavior of Melt-Crystallized Poly(l-Lactic Acid) with a Low Optical Purity. J. Therm. Anal. Calorim. 2013, 111, 1507–1514. [Google Scholar] [CrossRef]
- Silva, A.L.N.; Cipriano, T.F.; ASilva, N.H.M.D.F.T.D.; Rocha, M.C.C.G.; Sousa, A.F.; Silva, G.M.D. Thermal, Rheological and Morphological Properties of Poly (Lactic Acid) (PLA) and Talc Composites. Polímeros Ciênc. E Tecnol. 2014, 24, 276–282. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Y.; Xu, T.; Zhang, J.; Liu, C.; Shen, C. Crystallization Kinetics and Morphology of Partially Melted Poly(Lactic Acid). Polym. Test. 2014, 37, 179–185. [Google Scholar] [CrossRef]
- Tabi, T.; Sajo, I.E.; Szabo, F.; Luyt, A.S.; Kovacs, J.G. Crystalline Structure of Annealed Polylactic Acid and Its Relation to Processing. Express Polym. Lett. 2010, 4, 659–668. [Google Scholar] [CrossRef]
- Gracia-Fernández, C.A.; Gómez-Barreiro, S.; López-Beceiro, J.; Naya, S.; Artiaga, R. New Approach to the Double Melting Peak of Poly(L -Lactic Acid) Observed by DSC. J. Mater. Res. 2012, 27, 1379–1382. [Google Scholar] [CrossRef]
- Kodal, M.; Sirin, H.; Ozkoc, G. Effects of Screw Speed on the Properties of Plasticized PLA/POSS Composites. In Proceedings of the PPS-29: The 29th International Conference of the Polymer Processing Society, Nuremberg, Germany, 15–19 July 2013; pp. 420–423. [Google Scholar]
- Yazdaninia, A.; Khonakdar, H.A.; Jafari, S.H.; Asadi, V. Influence of Trifluoropropyl-POSS Nanoparticles on the Microstructure, Rheological, Thermal and Thermomechanical Properties of PLA. RSC Adv. 2016, 6, 37149–37159. [Google Scholar] [CrossRef]
- Pielichowski, K.; Majka, T.M.; Raftopoulos, K.N. Rheology and Processing of Polymer/POSS Nanocomposites. In Rheology and Processing of Polymer Nanocomposites; Thomas, S., Muller, R., Abraham, J., Eds.; Wiley: Hoboken, NJ, USA, 2016; pp. 293–327. ISBN 978-1-118-96979-3. [Google Scholar]
- Hato, M.J.; Ray, S.S.; Luyt, A.S. Melt-State Viscoelastic Properties of POSS-Containing Polyethylene Nanocomposites. Adv. Sci. Lett. 2011, 4, 3585–3589. [Google Scholar] [CrossRef]
- Barczewski, M.; Mysiukiewicz, O.; Szulc, J.; Kloziński, A. Poly(Lactic Acid) Green Composites Filled with Linseed Cake as an Agricultural Waste Filler. Influence of Oil Content within the Filler on the Rheological Behavior. J. Appl. Polym. Sci. 2019, 136, 47651. [Google Scholar] [CrossRef]
- Gupta, A.; Simmons, W.; Schueneman, G.T.; Hylton, D.; Mintz, E.A. Rheological and Thermo-Mechanical Properties of Poly(Lactic Acid)/Lignin-Coated Cellulose Nanocrystal Composites. ACS Sustain. Chem. Eng. 2017, 5, 1711–1720. [Google Scholar] [CrossRef]
- Romo-Uribe, A. Co-(POSS#-styrene) Nanocomposites Reduced the Glass-transition Temperature, Rubbery Modulus, and Melt Viscosity of Entangled Polystyrene. Polym. Eng. Sci. 2019, 59, 2377–2386. [Google Scholar] [CrossRef]
- Muralisrinivasan, N.S. Polymer Blends and Composites: Chemistry and Technology; Wiley: Hoboken, NJ, USA; Scrivener Publishing: Beverly, MA, USA, 2017; ISBN 978-1-118-11889-4. [Google Scholar]
- Dealy, J.M.; Wissbrun, K.F. Melt Rheology and Its Role in Plastics Processing: Theory and Applications; Springer: Dordrecht, The Netherlands, 1999; ISBN 978-0-7923-5886-2. [Google Scholar]
- Barczewski, M.; Mysiukiewicz, O.; Lewandowski, K.; Nowak, D.; Matykiewicz, D.; Andrzejewski, J.; Skórczewska, K.; Piasecki, A. Effect of Basalt Powder Surface Treatments on Mechanical and Processing Properties of Polylactide-Based Composites. Materials 2020, 13, 5436. [Google Scholar] [CrossRef]
- Guo, M.; David, É.; Fréchette, M.; Demarquette, N.R. Polyethylene/Polyhedral Oligomeric Silsesquioxanes Composites: Dielectric, Thermal and Rheological Properties. Polymer 2017, 115, 60–69. [Google Scholar] [CrossRef]
- Lipińska, M. The Effect of Various Polyhedral Oligomeric Silsesquioxanes on Viscoelastic, Thermal Properties and Crystallization of Poly(ε-Caprolactone) Nanocomposites. Polymers 2022, 14, 5078. [Google Scholar] [CrossRef]
- Latko-Durałek, P.; Macutkevic, J.; Kay, C.; Boczkowska, A.; McNally, T. Hot-melt Adhesives Based on Co-polyamide and Multiwalled Carbon Nanotubes. J. Appl. Polym. Sci. 2018, 135, 45999. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zhang, Y.; Yin, N. Rheological Behavior of Polypropylene/Octavinyl Polyhedral Oligomeric Silsesquioxane Composites. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 526–533. [Google Scholar] [CrossRef]
- Rahman, M.; Brazel, C. The Plasticizer Market: An Assessment of Traditional Plasticizers and Research Trends to Meet New Challenges. Prog. Polym. Sci. 2004, 29, 1223–1248. [Google Scholar] [CrossRef]
- Bodaghi, A. An Overview on the Recent Developments in Reactive Plasticizers in Polymers. Polym. Adv. Technol. 2020, 31, 355–367. [Google Scholar] [CrossRef]
- Trinkle, S.; Friedrich, C. Van Gurp-Palmen-Plot: A Way to Characterize Polydispersity of Linear Polymers. Rheol. Acta 2001, 40, 322–328. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A. Reactive Extrusion of PLA, PBAT with a Multi-Functional Epoxide: Physico-Chemical and Rheological Properties. Eur. Polym. J. 2014, 58, 90–102. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Fu, Z.; Zhao, X.; Li, Y.; Li, J. Rheology of Nanosilica-Compatibilized Immiscible Polymer Blends: Formation of a “Heterogeneous Network” Facilitated by Interfacially Anchored Hybrid Nanosilica. Macromolecules 2017, 50, 9494–9506. [Google Scholar] [CrossRef]
- Ivanova, R.; Kotsilkova, R. Rheological Study of Poly(Lactic) Acid Nanocomposites with Carbon Nanotubes and Graphene Additives as a Tool for Materials Characterization for 3D Printing Application. Appl. Rheol. 2018, 28, 54014. [Google Scholar] [CrossRef]
- Münstedt, H. Rheological Measurements and Structural Analysis of Polymeric Materials. Polymers 2021, 13, 1123. [Google Scholar] [CrossRef]
- Yu, T.; Wilkes, G.L. Influence of Molecular Weight Distribution on the Melt Extrusion of High Density Polyethylene (HDPE): Effects of Melt Relaxation Behavior on Morphology and Orientation in HDPE Extruded Tubular Films. J. Rheol. 1996, 40, 1079–1093. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Matykiewicz, D. Correlation between Processing Parameters and Degradation of Different Polylactide Grades during Twin-Screw Extrusion. Polymers 2020, 12, 1333. [Google Scholar] [CrossRef]
- Romo-Uribe, A. Viscoelasticity and Microstructure of POSS-Methyl Methacrylate Nanocomposites. Dynamics and Entanglement Dilution. Polymer 2018, 148, 27–38. [Google Scholar] [CrossRef]
- Romo-Uribe, A.; Reyes-Mayer, A.; Paredes-Pérez, M.; Lichtenhan, J.; Yañez-Lino, M.; Sarmiento-Bustos, E. POSS Driven Chain Disentanglements, Decreased the Melt Viscosity and Reduced O2 Transmission in Polyethylene. Polymer 2019, 165, 61–71. [Google Scholar] [CrossRef]
- Dong, F.; Padua, G.W.; Wang, Y. Controlled Formation of Hydrophobic Surfaces by Self-Assembly of an Amphiphilic Natural Protein from Aqueous Solutions. Soft Matter 2013, 9, 5933. [Google Scholar] [CrossRef]
- Brochier Salon, M.-C.; Belgacem, M.N. Hydrolysis-Condensation Kinetics of Different Silane Coupling Agents. Phosphorus Sulfur Silicon Relat. Elem. 2011, 186, 240–254. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Kinetics of the Thermal Decomposition of Processed Poly(Lactic Acid). Polym. Degrad. Stab. 2010, 95, 2508–2514. [Google Scholar] [CrossRef]
- Riegel, B.; Blittersdorf, S.; Kiefer, W.; Hofacker, S.; Müller, M.; Schottner, G. Kinetic Investigations of Hydrolysis and Condensation of the Glycidoxypropyltrimethoxysilane/Aminopropyltriethoxy-Silane System by Means of FT-Raman Spectroscopy I. J. Non-Cryst. Solids 1998, 226, 76–84. [Google Scholar] [CrossRef]

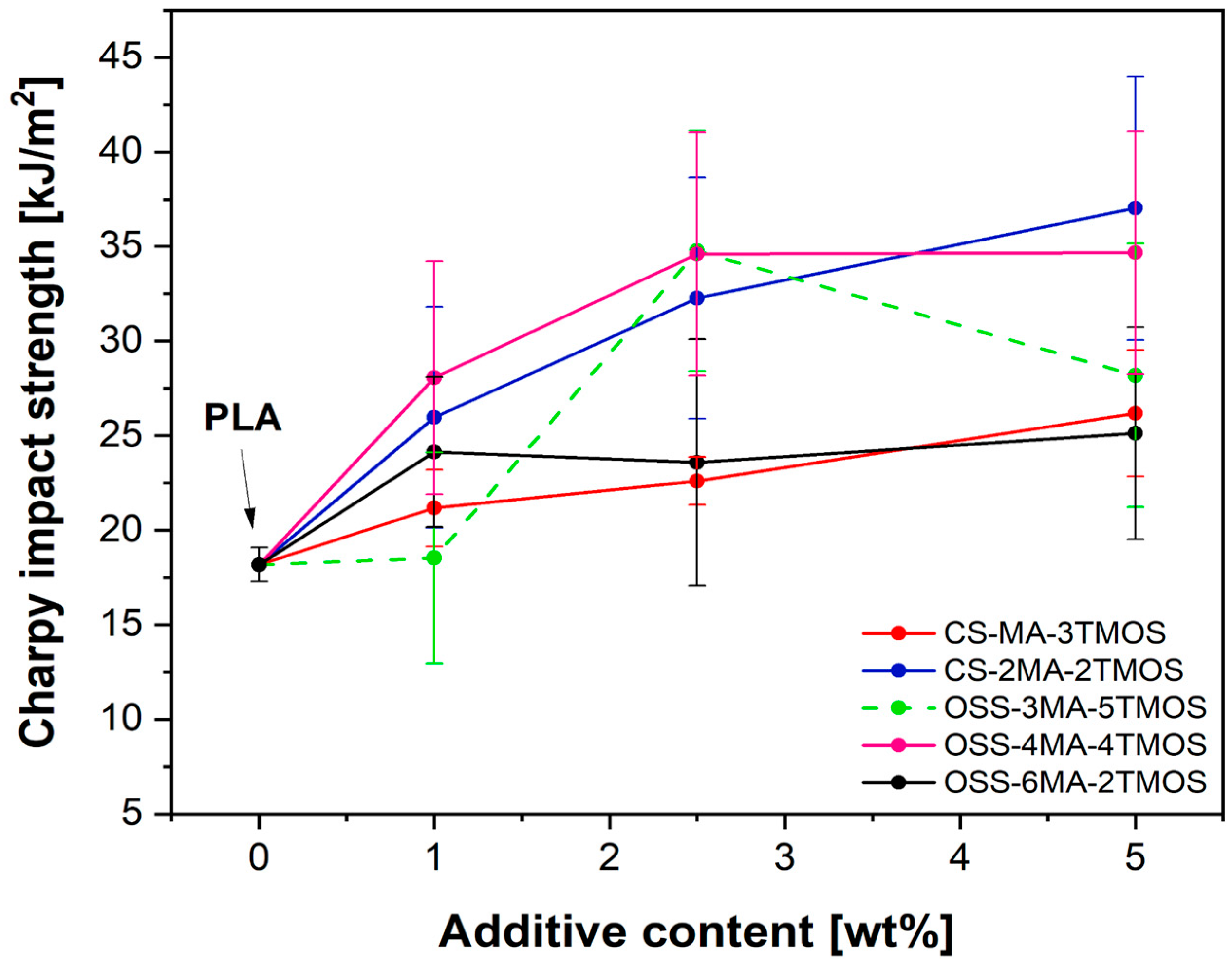

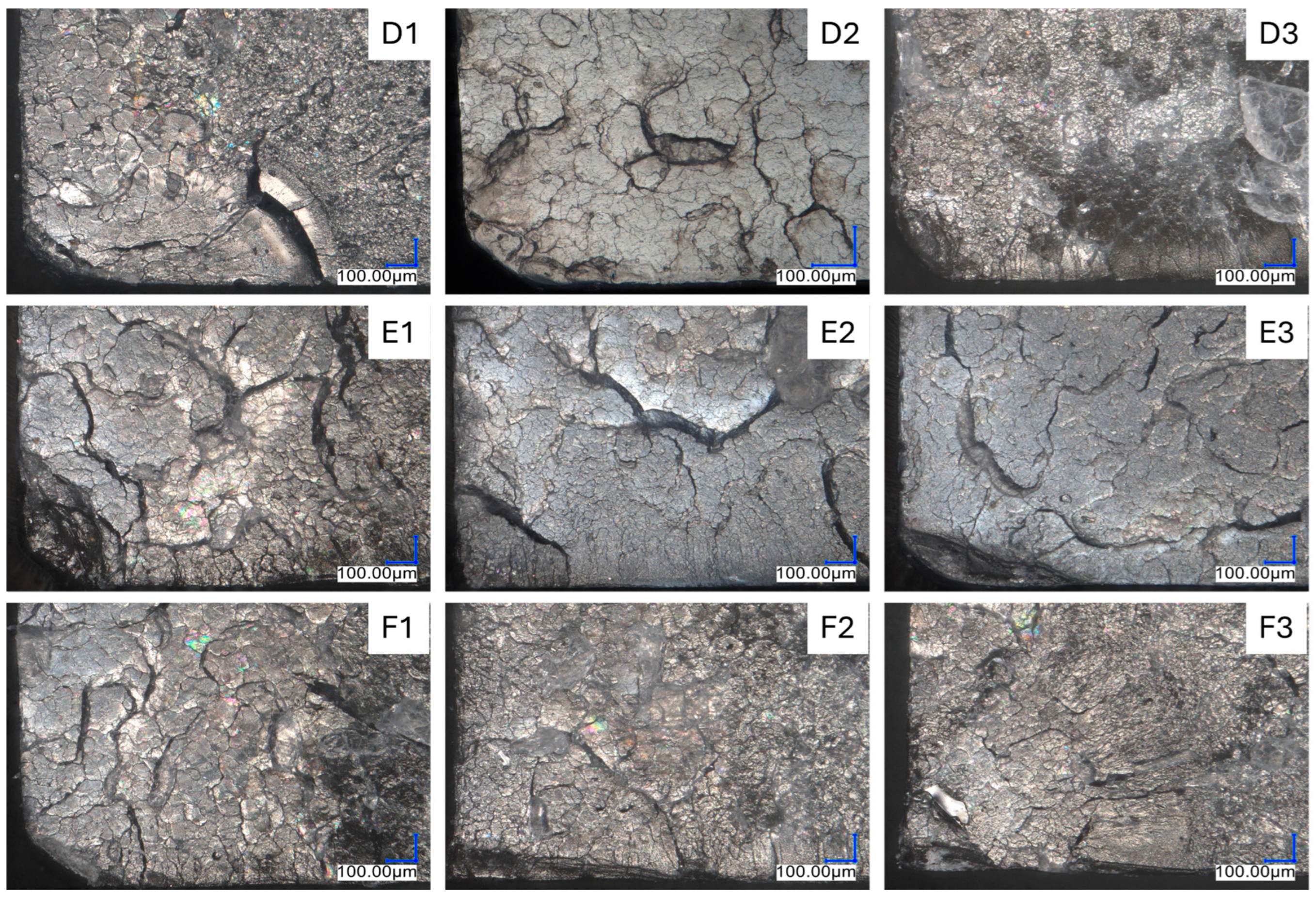
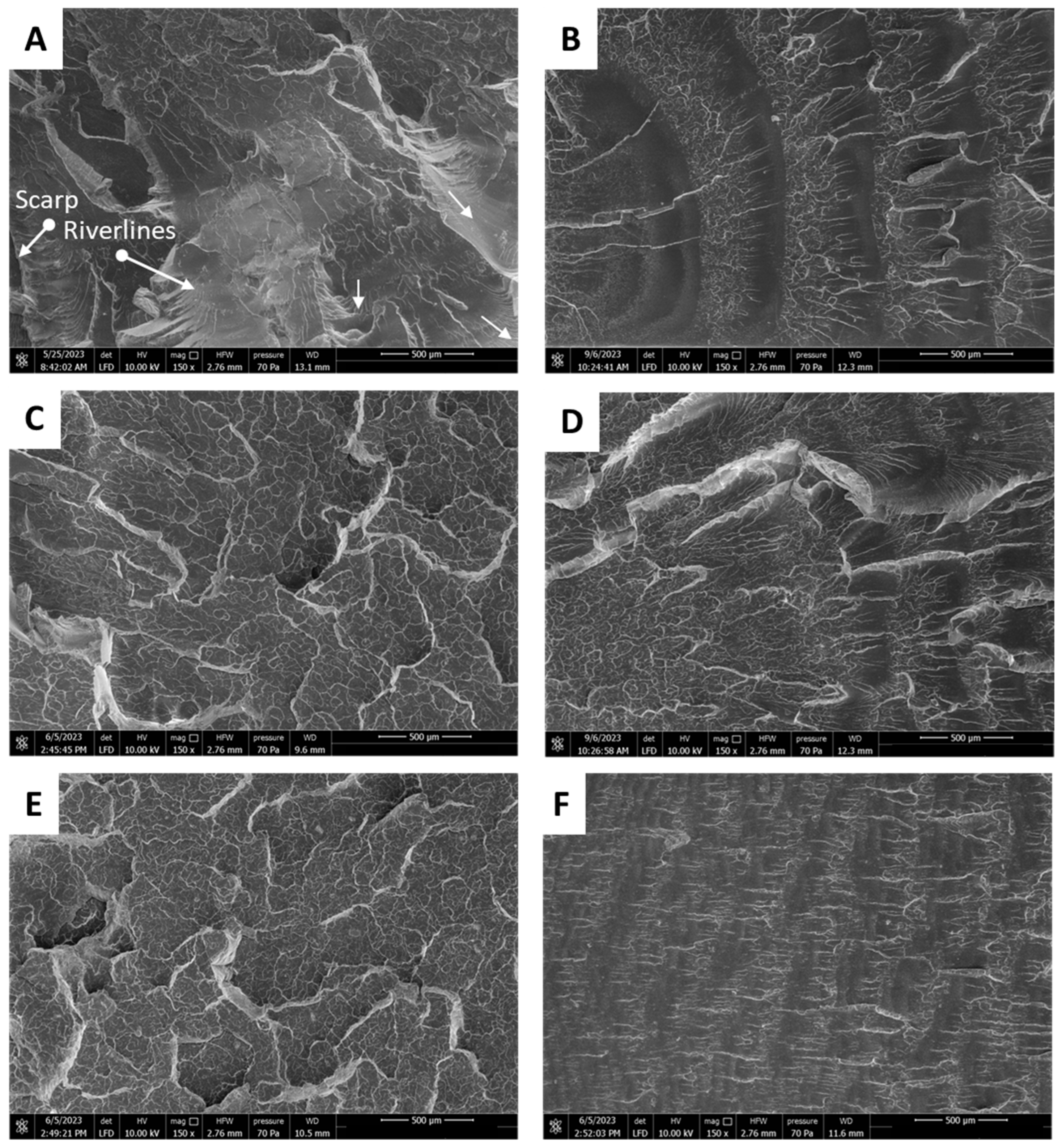
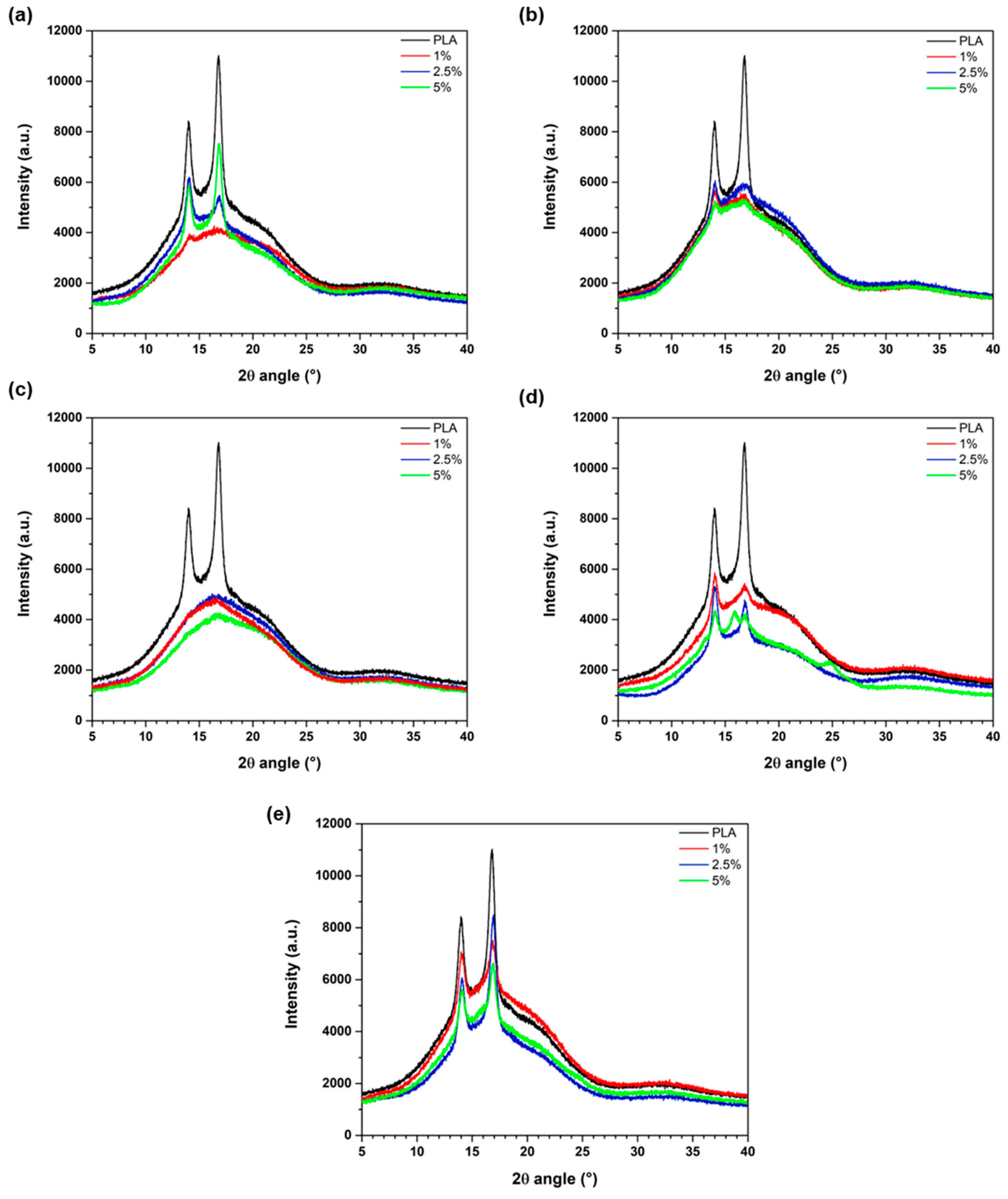

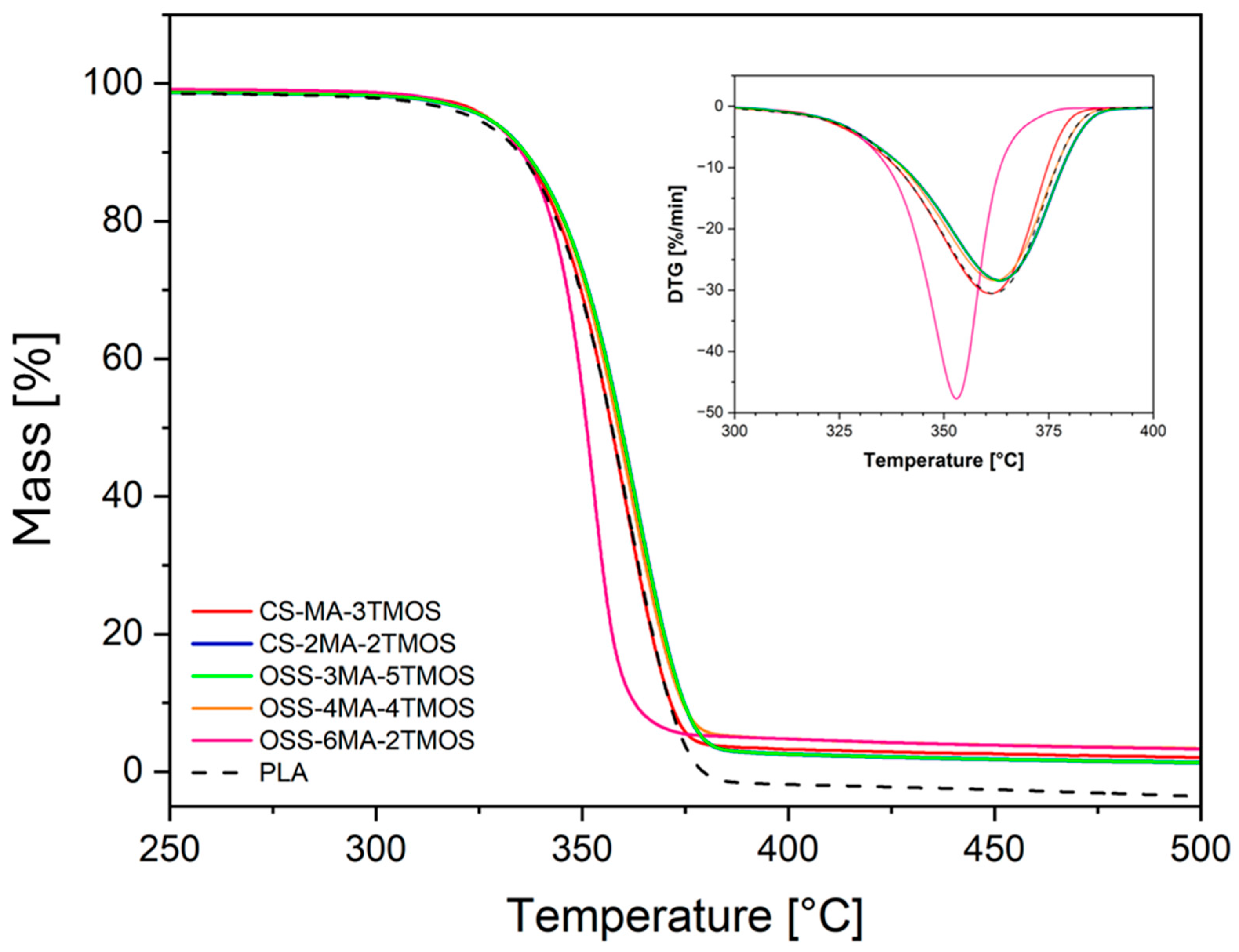
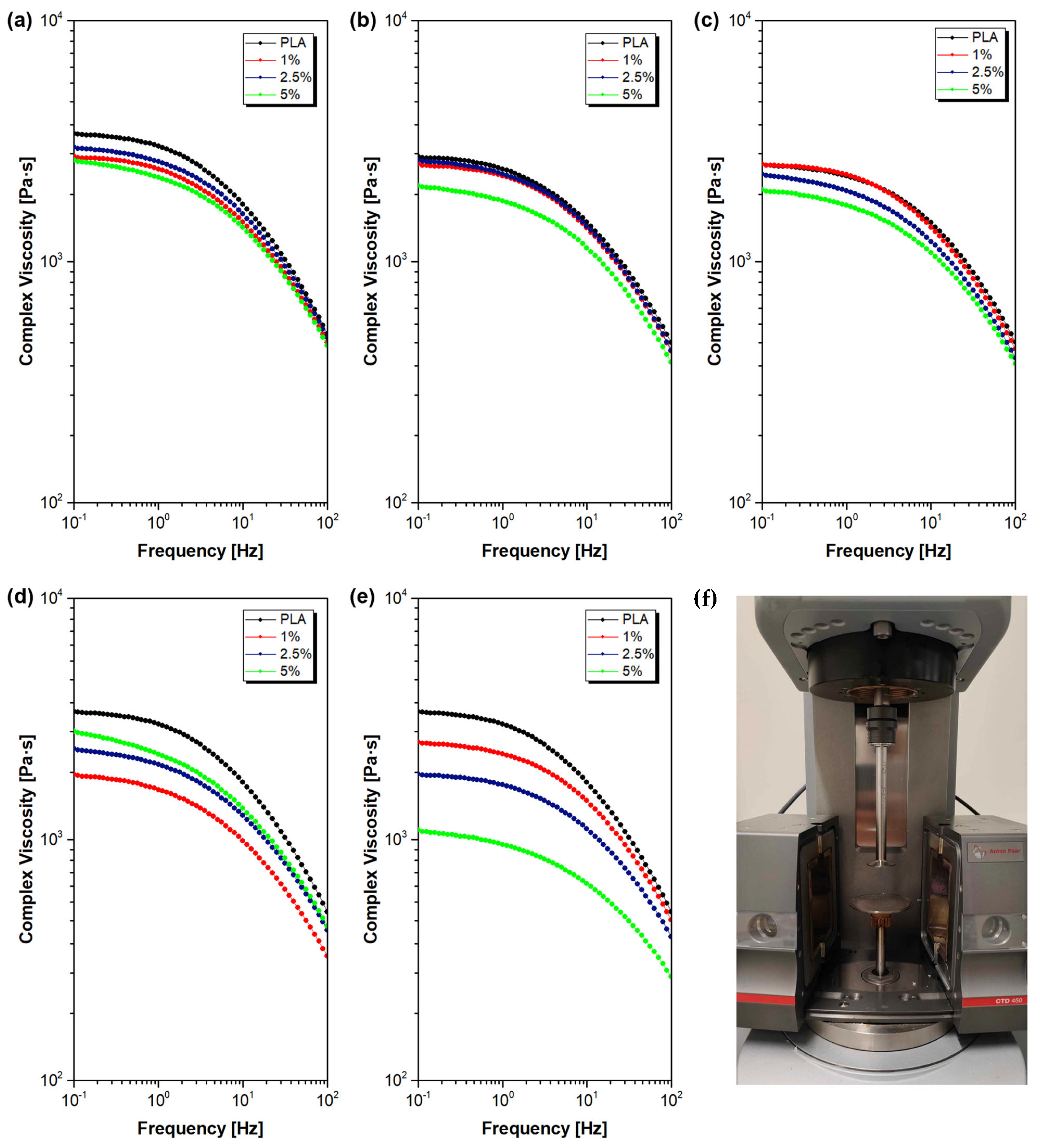
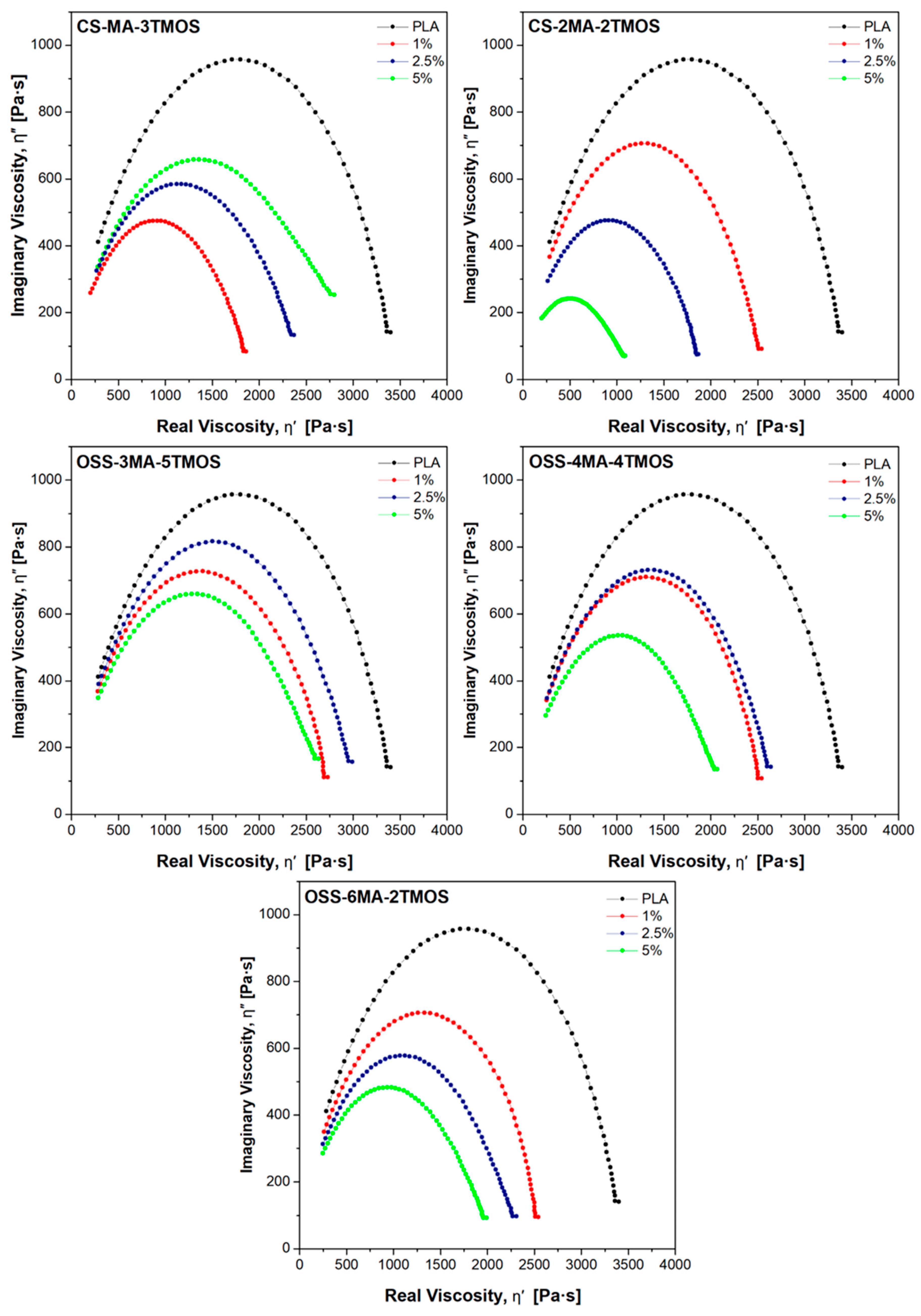
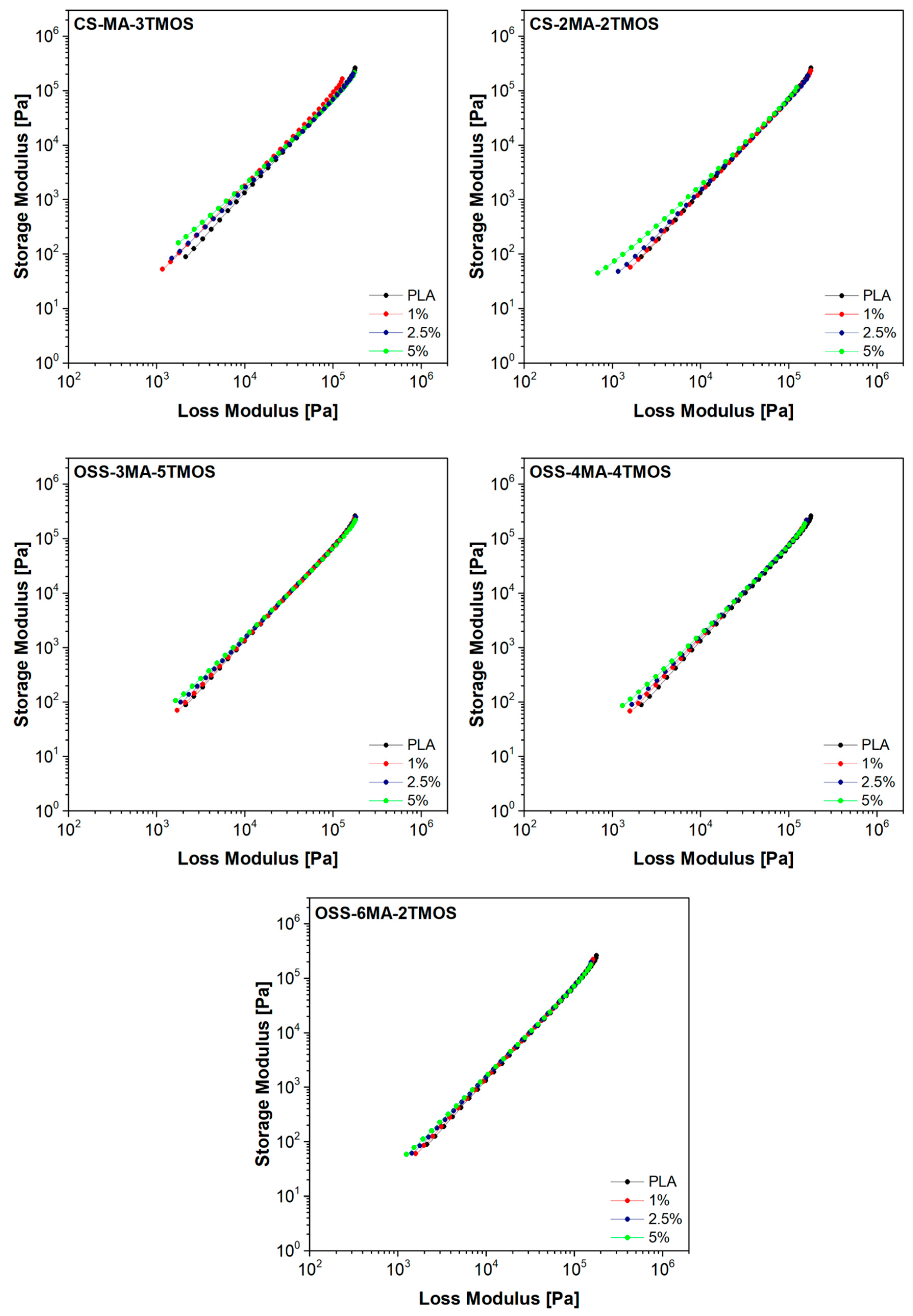
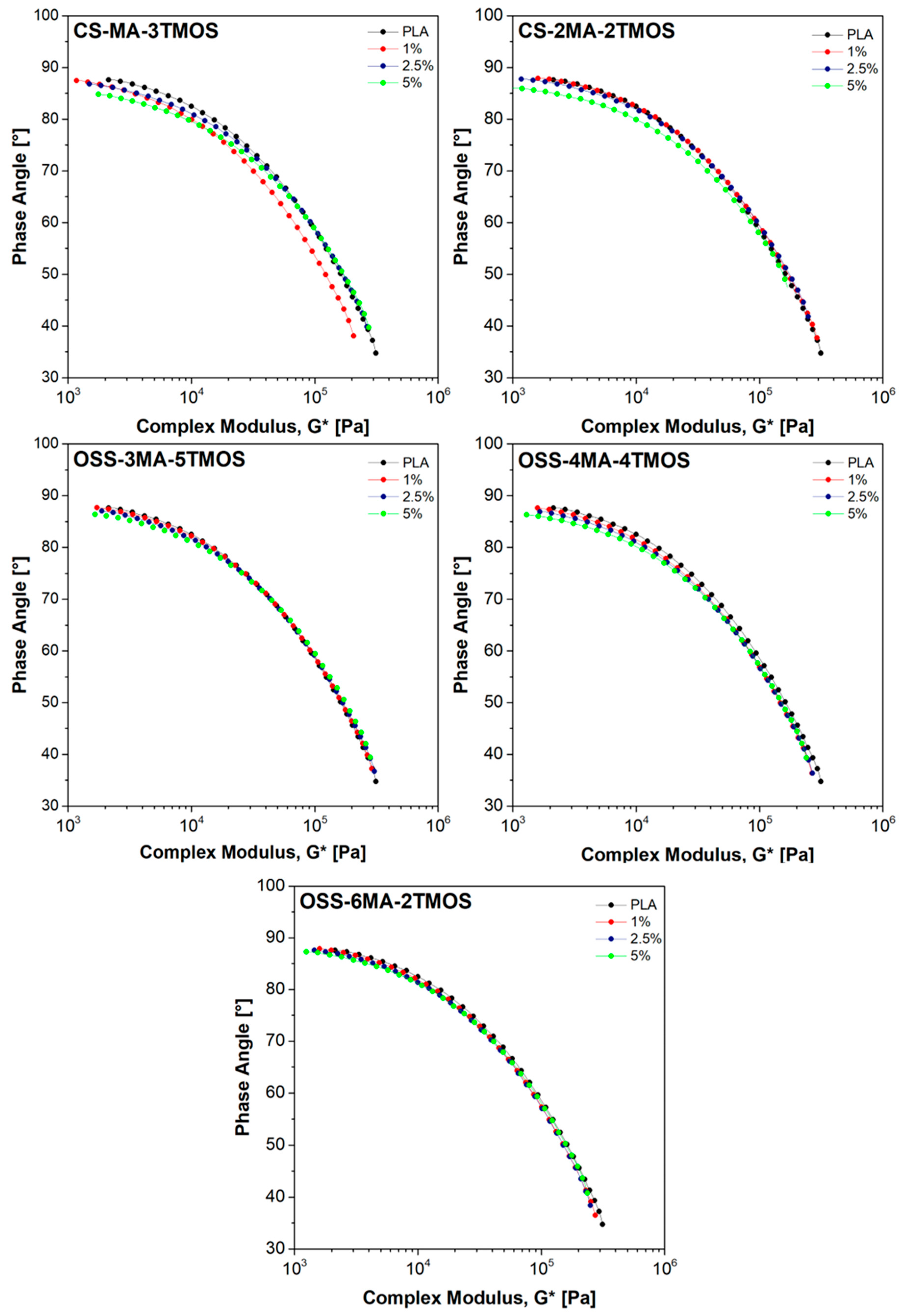
| Sample Name | Modifier (wt%) | Rf (MPa) | Ef (MPa) | Rm (MPa) | ε (%) | Ey (MPa) |
|---|---|---|---|---|---|---|
| PLA | - | 101.03 ± 0.28 | 3497.18 ± 13.00 | 66.11 ± 1.70 | 2.61 ± 0.07 | 3284.19 ± 52.65 |
| PLA/CS-MA-3TMOS | 1 | 100.27 ± 1.54 | 3690.38 ± 46.40 | 64.13 ± 1.25 | 2.59 ± 0.07 | 3201.63 ± 47.09 |
| 2.5 | 98.85 ± 0.32 | 3661.71 ± 17.11 | 63.25 ± 0.77 | 2.55 ± 0.04 | 3173.26 ± 41.52 | |
| 5 | 95.01 ± 0.67 | 3609.20 ± 6.06 | 63.18 ± 1.32 | 2.73 ± 0.08 | 2971.66 ± 98.88 | |
| PLA/CS-2MA-2TMOS | 1 | 99.20 ± 0.42 | 3697.11 ± 13.67 | 63.87 ± 0.55 | 2.53 ± 0.04 | 3241.24 ± 38.84 |
| 2.5 | 95.35 ± 1.09 | 3648.86 ± 28.18 | 60.46 ± 1.25 | 2.38 ± 0.03 | 3230.36 ± 23.96 | |
| 5 | 93.34 ± 1.57 | 3615.38 ± 42.36 | 58.39 ± 1.19 | 2.44 ± 0.03 | 3109.54 ± 58.21 | |
| PLA/OSS-3MA-5TMOS | 1 | 98.03 ± 0.77 | 3701.57 ± 25.84 | 61.09 ± 1.48 | 2.40 ± 0.05 | 3241.47 ± 20.23 |
| 2.5 | 96.22 ± 0.32 | 3610.46 ± 23.86 | 62.61 ± 1.48 | 2.57 ± 0.13 | 3111.34 ± 80.10 | |
| 5 | 93.08 ± 1.26 | 3297.12 ± 30.5 | 60.55 ± 1.08 | 2.61 ± 0.12 | 3043.58 ± 112.90 | |
| PLA/OSS-4MA-4TMOS | 1 | 97.01 ± 0.23 | 3442.67 ± 16.84 | 61.55 ± 0.7 | 2.36 ± 0.02 | 3322.9 ± 30.20 |
| 2.5 | 96.36 ± 0.45 | 3638.49 ± 29.00 | 58.60 ± 1.43 | 2.39 ± 0.08 | 3236.02 ± 17.84 | |
| 5 | 95.21 ± 0.53 | 3575.26 ± 43.47 | 58.83 ± 1.17 | 2.57 ± 0.06 | 3119.93 ± 20.72 | |
| PLA/OSS-6MA-2TMOS | 1 | 97.21 ± 0.54 | 3616.06 ± 96.63 | 62.11 ± 0.93 | 2.50 ± 0.05 | 3244.85 ± 39.27 |
| 2.5 | 95.90 ± 0.76 | 3636.19 ± 12.97 | 60.72 ± 0.28 | 2.49 ± 0.05 | 3158.08 ± 54.62 | |
| 5 | 92.88 ± 0.88 | 3576.5 ± 24.50 | 59.14 ± 0.84 | 2.62 ± 0.11 | 3066.76 ± 37.99 |
| Modifier (wt%) | PLA | PLA/CS-MA-3TMOS | PLA/CS-2MA-2TMOS | PLA/OSS-3MA-5TMOS | PLA/OSS-4MA-4TMOS | PLA/OSS-6MA-2TMOS | |
|---|---|---|---|---|---|---|---|
| Crystallinity degree Xc (%) | - | 30.49 | - | - | - | - | - |
| 1 | - | 25.2 | 28.4 | 21.7 | 31.7 | 36.3 | |
| 2.5 | - | 29.4 | 29.5 | 25.9 | 33.4 | 35.8 | |
| 5 | - | 31.7 | 29.9 | 27.3 | 38.2 | 42.3 |
| Sample Name | Modifier (wt%) | Tg (°C) | Tcc (°C) | Tm1/Tm2 (°C) | T5% (°C) | Tonset (°C) | Tmax (°C) | HDT (°C) |
|---|---|---|---|---|---|---|---|---|
| PLA | - | 63.2 | 127.2 | 155.4 | 324.2 | 342.2 | 362.1 | 52.80 ± 0.15 |
| PLA/CS-MA-3TMOS | 1 | 62.8 | 129.2 | 153.8 | - | - | - | 57.50 ± 0.26 |
| 2.5 | 62.7 | 119.4 | 151.9 | - | - | - | 56.80 ± 0.20 | |
| 5 | 61.8 | 116.9 | 150.1 | 327.4 | 341.8 | 361.2 | 54.70 ± 0.20 | |
| PLA/CS-2MA-2TMOS | 1 | 62.6 | 121.2 | 152.3 | - | - | - | 57.50 ± 0.10 |
| 2.5 | 61.8 | 113.2 | 150.7/155.5 | - | - | - | 56.83 ± 0.15 | |
| 5 | 62.4 | 114.5 | 151.3 | 327.0 | 343.1 | 363.3 | 54.93 ± 0.32 | |
| PLA/OSS-3MA-5TMOS | 1 | 62.0 | 111.8 | 149.6/156.1 | - | - | - | 57.75 ± 0.15 |
| 2.5 | 62.8 | 120.9 | 152.8 | - | - | - | 56.60 ± 0.10 | |
| 5 | 62.4 | 120.5 | 152.0 | 329.7 | 345.3 | 360.5 | 55.87 ± 0.21 | |
| PLA/OSS-4MA-4TMOS | 1 | 63.2 | 122.6 | 153.2 | - | - | - | 56.90 ± 0.00 |
| 2.5 | 62.7 | 121.2 | 152.7 | - | - | - | 57.23 ± 0.23 | |
| 5 | 62.7 | 119.9 | 152.6 | 327.2 | 342.5 | 362.1 | 56.57 ± 0.23 | |
| PLA/OSS-6MA-2TMOS | 1 | 63.2 | 120.5 | 152.7 | - | - | - | 57.71 ± 0.28 |
| 2.5 | 62.8 | 128.7 | 153.5 | - | - | - | 57.36 ± 0.05 | |
| 5 | 62.7 | 129.0 | 154.1 | 327.1 | 342.1 | 353.2 | 56.27 ± 0.06 |
| Sample Name | Modifier [wt%] | ωcross [rad/s] | G′ = G″ [kPa] | λM [ms] | λC-Y [ms] | η0 [Pa·s] | Han Plot Slope | MFR(210 °C, 2.16 kg) [g/10 min] |
|---|---|---|---|---|---|---|---|---|
| PLA | - | 212.42 | 148.24 | 4.71 | 19,12 | 3486.10 | 1.76 | 7.11 ± 0.03 |
| PLA/CS-MA-3TMOS | 1 | 324.19 | 111.22 | 3.08 | 10.07 | 3031.80 | 1.68 | 9.55 ± 0.01 |
| 2.5 | 385.28 | 154.75 | 2.60 | 8.57 | 2483.20 | 1.63 | 9.50 ± 0.03 | |
| 5 | 369.71 | 158.48 | 2.70 | 4.46 | 1937.30 | 1.55 | 10.98 ± 0.05 | |
| PLA/CS-2MA-2TMOS | 1 | 300.25 | 155.03 | 3.33 | 9.78 | 2590.10 | 1.73 | 9.58 ± 0.02 |
| 2.5 | 480.21 | 158.16 | 2.08 | 8.85 | 1921.50 | 1.66 | 10.72 ± 0.08 | |
| 5 | - | - | - | 1.99 | 1147.90 | 1.52 | 13.45 ± 0.03 | |
| PLA/OSS-3MA-5TMOS | 1 | 284.90 | 150.55 | 3.51 | 13.81 | 3074.00 | 1.72 | 10.59 ± 0.29 |
| 2.5 | 260.91 | 153.52 | 3.83 | 11.41 | 2823.70 | 1.68 | 15.50 ± 0.91 | |
| 5 | 361.11 | 161.84 | 2.77 | 5.81 | 2748.50 | 1.61 | 16.30 ± 0.27 | |
| PLA/OSS-4MA-4TMOS | 1 | 258.54 | 133.74 | 3.87 | 16.43 | 2582.10 | 1.72 | 10.84 ± 0.05 |
| 2.5 | 255.36 | 134.86 | 3.92 | 12.17 | 2704.50 | 1.68 | 9.29 ± 0.03 | |
| 5 | 371.55 | 138.39 | 2.69 | 5.20 | 2137.60 | 1.60 | 13.23 ± 0.05 | |
| PLA/OSS-6MA-2TMOS | 1 | 264.99 | 137.46 | 3.77 | 17.06 | 2593.70 | 1.74 | 10.27 ± 0.09 |
| 2.5 | 332.49 | 138.07 | 3.01 | 6.06 | 2417.00 | 1.70 | 9.02 ± 0.07 | |
| 5 | 434.56 | 144.68 | 2.30 | 4.49 | 2090.50 | 1.65 | 13.16 ± 0.03 |
| Modifier (wt%) | PLA | PLA/CS-MA-3TMOS | PLA/CS-2MA-2TMOS | PLA/OSS-3MA-5TMOS | PLA/OSS-4MA-4TMOS | PLA/OSS-6MA-2TMOS | |
|---|---|---|---|---|---|---|---|
| WCA (°) | - | 78.83 ± 0.96 | - | - | - | - | - |
| 1 | - | 83.93 ± 1.55 | 88.76 ± 1.58 | 37.40 ± 1.30 | 77.57 ± 1.31 | 83.13 ± 1.93 | |
| 2.5 | - | 83.68 ± 0.78 | 89.07 ± 0.21 | 44.93 ± 1.32 | 76.65 ± 1.85 | 83.67 ± 2.40 | |
| 5 | - | 82.78 ± 1.05 | 92.33 ± 1.73 | 50.40 ± 1.56 | 84.13 ± 1.80 | 87.03 ± 2.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Głowacka, J.; Frydrych, M.; Romańczuk-Ruszuk, E.; Gao, Y.; Zhou, H.; Przekop, R.E.; Sztorch, B. Dual-Functional Organosilicon Additives Containing Methacrylate and Trimethoxysilyl Groups Enhancing Impact Toughness of Polylactide (PLA): Structure–Property Relationship. Materials 2025, 18, 2903. https://doi.org/10.3390/ma18122903
Głowacka J, Frydrych M, Romańczuk-Ruszuk E, Gao Y, Zhou H, Przekop RE, Sztorch B. Dual-Functional Organosilicon Additives Containing Methacrylate and Trimethoxysilyl Groups Enhancing Impact Toughness of Polylactide (PLA): Structure–Property Relationship. Materials. 2025; 18(12):2903. https://doi.org/10.3390/ma18122903
Chicago/Turabian StyleGłowacka, Julia, Miłosz Frydrych, Eliza Romańczuk-Ruszuk, Yi Gao, Hui Zhou, Robert E. Przekop, and Bogna Sztorch. 2025. "Dual-Functional Organosilicon Additives Containing Methacrylate and Trimethoxysilyl Groups Enhancing Impact Toughness of Polylactide (PLA): Structure–Property Relationship" Materials 18, no. 12: 2903. https://doi.org/10.3390/ma18122903
APA StyleGłowacka, J., Frydrych, M., Romańczuk-Ruszuk, E., Gao, Y., Zhou, H., Przekop, R. E., & Sztorch, B. (2025). Dual-Functional Organosilicon Additives Containing Methacrylate and Trimethoxysilyl Groups Enhancing Impact Toughness of Polylactide (PLA): Structure–Property Relationship. Materials, 18(12), 2903. https://doi.org/10.3390/ma18122903












