Road Map for the Use of Electron Spin Resonance Spectroscopy in the Study of Functionalized Magnetic Nanoparticles
Abstract
1. Introduction: Basics of the EPR Technique in Nanoparticle Research
2. EPR for Characterization of Nanoparticle Cores
3. Advanced Methods for Analyzing X-Band EPR Spectra of Nanoparticles
4. Applications of EPR in Nanomaterial Characterizations
4.1. EPR of Functionalized Nanoparticles
4.2. EPR Study of Nanoparticles in Various Media
4.3. EPR for Cargo Characterization
4.4. EPR in the Study of Redox Activity of Nanoparticles
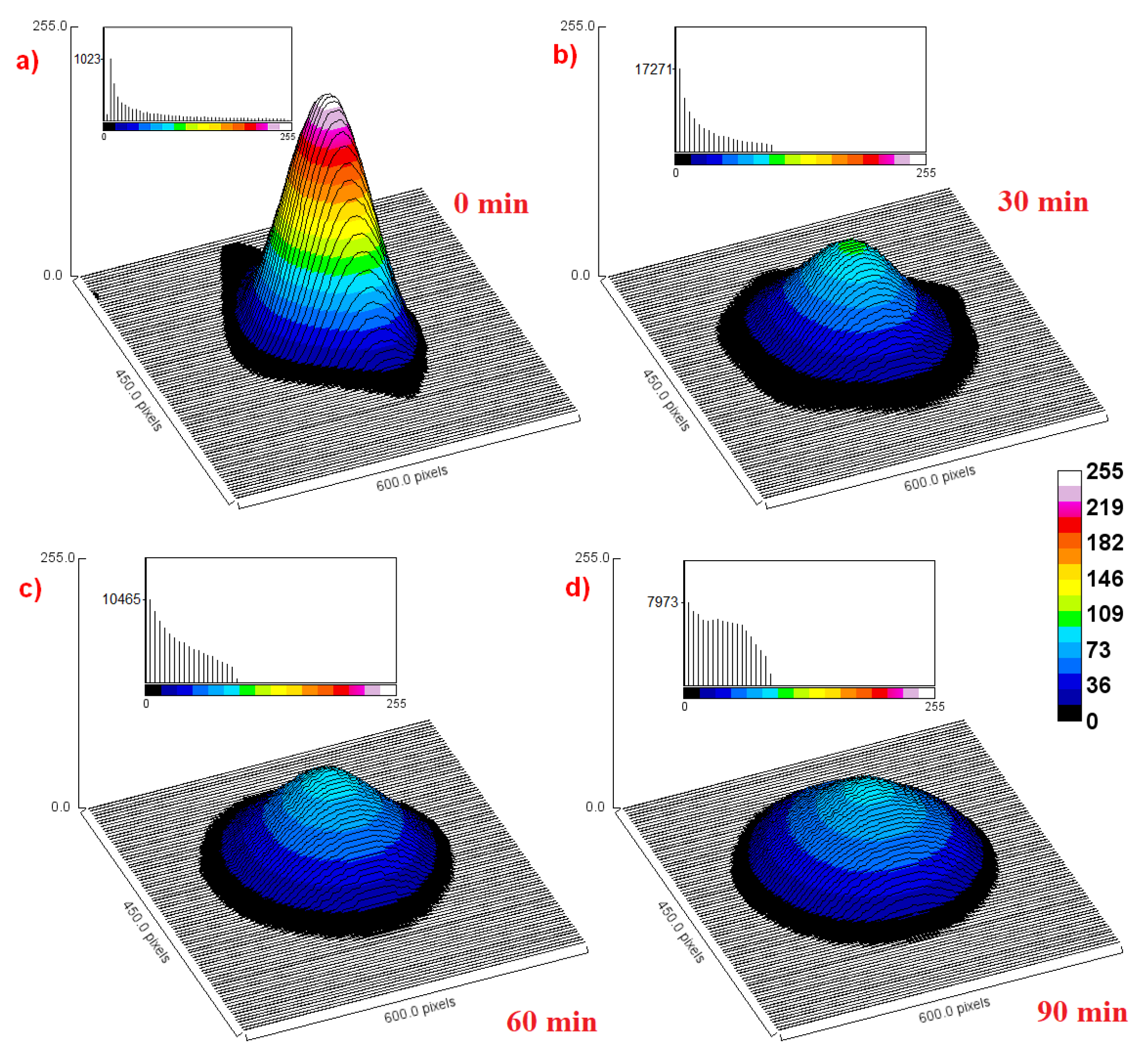
4.5. EPR Imaging of Nanoparticles
4.6. Quantitative EPR of Nanoparticles
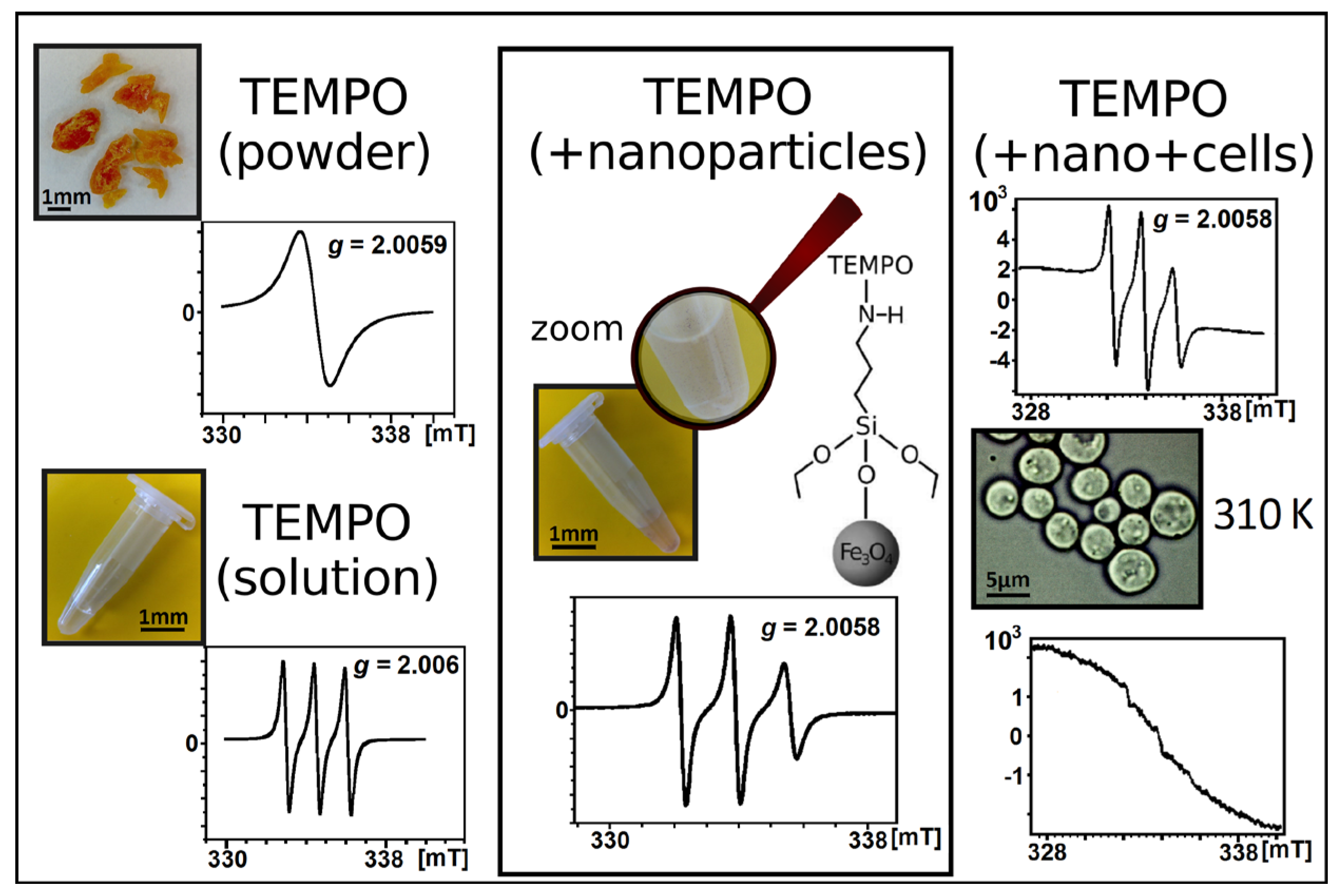
4.7. Cell-Based EPR Research
5. Perspectives for Future Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CREM | computer resolution enhancement method |
| DMPO | 5,5-dimethyl-1-pyrroline-N-oxide |
| EPR | electron paramagnetic resonance |
| EPRI | electron paramagnetic resonance imaging |
| ROS | reactive oxygen species |
| TEMPO | 2,2,6,6-tetramethylpiperidine-1-oxyl |
References
- Junk, M.J.N. Electron Paramagnetic Resonance Theory. In Assessing the Functional Structure of Molecular Transporters by EPR Spectroscopy; Junk, M.J.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 7–52. [Google Scholar]
- Odom, B.; Hanneke, D.; D’Urso, B.; Gabrielse, G. New Measurement of the Electron Magnetic Moment Using a One-Electron Quantum Cyclotron. Phys. Rev. Lett. 2006, 97, 030801. [Google Scholar] [CrossRef] [PubMed]
- Bielas, R.; Kubiak, T.; Molcan, M.; Dobosz, B.; Rajnak, M.; Józefczak, A. Biocompatible Hydrogel-Based Liquid Marbles with Magnetosomes. Materials 2024, 17, 99. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Pardo, R.; Valenzuela, R. Characterization of Magnetic Phases in Nanostructured Ferrites by Electron Spin Resonance. In Advanced Electromagnetic Waves; Osman, B.S., Ed.; IntechOpen: Rijeka, Croatia, 2015; Chapter 6. [Google Scholar]
- Shukla, A.K. EMR/ESR/EPR Spectroscopy for Characterization of Nanomaterials; Springer: New Delhi, India, 2018. [Google Scholar]
- Mørup, S.; Hansen, M.F.; Frandsen, C. Magnetic interactions between nanoparticles. Beilstein J. Nanotechnol. 2010, 1, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Can, M.M.; Coşkun, M.; Fırat, T. A comparative study of nanosized iron oxide particles; magnetite (Fe3O4), maghemite (γ-Fe2O3) and hematite (α-Fe2O3), using ferromagnetic resonance. J. Alloys Compd. 2012, 542, 241–247. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Tran, H.-V.; Xu, S.; Lee, T.R. Fe3O4 Nanoparticles: Structures, Synthesis, Magnetic Properties, Surface Functionalization, and Emerging Applications. Appl. Sci. 2021, 11, 11301. [Google Scholar] [CrossRef]
- Liang, J.; Ma, H.; Luo, W.; Wang, S. Synthesis of magnetite submicrospheres with tunable size and superparamagnetism by a facile polyol process. Mater. Chem. Phys. 2013, 139, 383–388. [Google Scholar] [CrossRef]
- Busquets, M.A.; Fernández-Pradas, J.M.; Serra, P.; Estelrich, J. Superparamagnetic Nanoparticles with Efficient Near-Infrared Photothermal Effect at the Second Biological Window. Molecules 2020, 25, 5315. [Google Scholar] [CrossRef]
- Ferreira, M.; Sousa, J.; Pais, A.; Vitorino, C. The Role of Magnetic Nanoparticles in Cancer Nanotheranostics. Materials 2020, 13, 266. [Google Scholar] [CrossRef]
- Kolhatkar, A.G.; Jamison, A.C.; Litvinov, D.; Willson, R.C.; Lee, T.R. Tuning the Magnetic Properties of Nanoparticles. Int. J. Mol. Sci. 2013, 14, 15977–16009. [Google Scholar] [CrossRef]
- Vargas, J.M.; Lima, E.; Zysler, R.D.; Duque, J.G.S.; De Biasi, E.; Knobel, M. Effective anisotropy field variation of magnetite nanoparticles with size reduction. Eur. Phys. J. B 2008, 64, 211–218. [Google Scholar] [CrossRef]
- Flores-Arias, Y.; Vázquez-Victorio, G.; Ortega-Zempoalteca, R.; Acevedo-Salas, U.; Ammar, S.; Valenzuela, R. Magnetic phase transitions in ferrite nanoparticles characterized by electron spin resonance. J. Appl. Phys. 2015, 117, 17A503. [Google Scholar] [CrossRef]
- Fischer, H.; Luster, J.; Gehring, A.U. EPR evidence for maghemitization of magnetite in a tropical soil. Geophys. J. Int. 2007, 169, 909–916. [Google Scholar] [CrossRef][Green Version]
- Berger, R.; Bissey, J.-C.; Kliava, J.; Daubric, H.; Estournès, C. Temperature dependence of superparamagnetic resonance of iron oxide nanoparticles. J. Magn. Magn. Mater. 2001, 234, 535–544. [Google Scholar] [CrossRef]
- Ozkaya, T.; Toprak, M.S.; Baykal, A.; Kavas, H.; Köseoğlu, Y.; Aktaş, B. Synthesis of Fe3O4 nanoparticles at 100 °C and its magnetic characterization. J. Alloys Compd. 2009, 472, 18–23. [Google Scholar] [CrossRef]
- Ennas, G.; Musinu, A.; Piccaluga, G.; Zedda, D.; Gatteschi, D.; Sangregorio, C.; Stanger, J.L.; Concas, G.; Spano, G. Characterization of Iron Oxide Nanoparticles in an Fe2O3−SiO2 Composite Prepared by a Sol−Gel Method. Chem. Mater. 1998, 10, 495–502. [Google Scholar] [CrossRef]
- Subin, J.P.; Jacob, M.M. Determination of ferrimagnetic and superparamagnetic components of magnetization and the effect of particle size on structural, magnetic and hyperfine properties of Mg0.5Zn0.5Fe2O4 nanoparticles. J. Alloys Compd. 2021, 869, 159242. [Google Scholar] [CrossRef]
- Berkowitz, A.E.; Takano, K. Exchange anisotropy—A review. J. Magn. Magn. Mater. 1999, 200, 552–570. [Google Scholar] [CrossRef]
- Kliava, J.; Berger, R. Size and shape distribution of magnetic nanoparticles in disordered systems: Computer simulations of superparamagnetic resonance spectra. J. Magn. Magn. Mater. 1999, 205, 328–342. [Google Scholar] [CrossRef]
- Sharma, V.K.; Waldner, F. Superparamagnetic and ferrimagnetic resonance of ultrafine Fe3O4 particles in ferrofluids. J. Appl. Phys. 1977, 48, 4298–4302. [Google Scholar] [CrossRef]
- Hsu, K.H.; Wu, J.H.; Huang, Y.Y.; Wang, L.Y.; Lee, H.Y.; Lin, J.G. Critical size effects on the magnetic resonance in Fe3O4 nanoparticles. J. Appl. Phys. 2005, 97, 114322. [Google Scholar] [CrossRef]
- Atsarkin, V.A.; Noginova, N. Electron Spin Resonance on the Border Between Para- and Ferromagnetism: Quantum versus Classical. Appl. Magn. Reson. 2020, 51, 1467–1480. [Google Scholar] [CrossRef]
- Figueiredo, L.C.; Lacava, B.M.; Skeff Neto, K.; Pelegrini, F.; Morais, P.C. Magnetic resonance study of maghemite-based magnetic fluid. J. Magn. Magn. Mater. 2008, 320, e347–e350. [Google Scholar] [CrossRef]
- Atrei, A.; Mahdizadeh, F.F.; Baratto, M.C.; Scala, A. Effect of Citrate on the Size and the Magnetic Properties of Primary Fe3O4 Nanoparticles and Their Aggregates. Appl. Sci. 2021, 11, 6974. [Google Scholar] [CrossRef]
- Gazeau, F.; Bacri, J.C.; Gendron, F.; Perzynski, R.; Raikher, Y.L.; Stepanov, V.I.; Dubois, E. Magnetic resonance of ferrite nanoparticles:: Evidence of surface effects. J. Magn. Magn. Mater. 1998, 186, 175–187. [Google Scholar] [CrossRef]
- Vázquez-Victorio, G.; Acevedo-Salas, U.; Valenzuela, R. Microwave Absorption in Nanostructured Spinel Ferrites. In Ferromagnetic Resonance; Orhan, Y., Ed.; IntechOpen: Rijeka, Croatia, 2013; Chapter 7. [Google Scholar]
- Kodama, R.H.; Berkowitz, A.E.; McNiff, J.E.J.; Foner, S. Surface Spin Disorder in NiFe2O4 Nanoparticles. Phys. Rev. Lett. 1996, 77, 394–397. [Google Scholar] [CrossRef]
- Perzynski, R.; Raikher, Y.L. Effect of Surface Anisotropy on the Magnetic Resonance Properties of Nanosize Ferroparticles. In Surface Effects in Magnetic Nanoparticles; Fiorani, D., Ed.; Springer: Boston, MA, USA, 2005; pp. 141–187. [Google Scholar]
- Bødker, F.; Mørup, S.; Linderoth, S. Surface effects in metallic iron nanoparticles. Phys. Rev. Lett. 1994, 72, 282–285. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Schroeder, G.; Kurczewska, J. Electron paramagnetic resonance as an effective method for a characterization of functionalized iron oxide. J. Phys. Chem. Solids 2014, 75, 594–598. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Kubiak, T.; Dobosz, B.; Schroeder, G.; Kurczewska, J. EPR spectroscopy and imaging of TEMPO-labeled magnetite nanoparticles. Curr. Appl. Phys. 2014, 14, 798–804. [Google Scholar] [CrossRef]
- Kubiak, T. EPR Study of Functionalized Magnetite Nanoparticles in Serum and Whole Human Blood (Badanie Metodą EPR Funkcjonalizowanych Nanocząstek Magnetytu w Surowicy i Pełnej Krwi Ludzkiej). Ph.D. Thesis, Adam Mickiewicz University, Poznan, Poland, 2016. [Google Scholar]
- Bakuzis, A.F.; Morais, P.C.; Pelegrini, F. Surface and exchange anisotropy fields in MnFe2O4 nanoparticles: Size and temperature effects. J. Appl. Phys. 1999, 85, 7480–7482. [Google Scholar] [CrossRef]
- Upadhyay, R.V.; Parekh, K.; Mehta, R.V. Spin-glass transition in a model magnetic fluid: Electron spin resonance investigation of Mn0.5Zn0.5Fe2O4 nanoparticles dispersed in kerosene. Phys. Rev. B 2003, 68, 224434. [Google Scholar] [CrossRef]
- Nikiforov, V.N.; Koksharov, Y.A.; Polyakov, S.N.; Malakho, A.P.; Volkov, A.V.; Moskvina, M.A.; Khomutov, G.B.; Irkhin, V.Y. Magnetism and Verwey transition in magnetite nanoparticles in thin polymer film. J. Alloys Compd. 2013, 569, 58–61. [Google Scholar] [CrossRef]
- Cini, A.; Ceci, P.; Falvo, E.; Gatteschi, D.; Fittipaldi, M. An EPR Study of Small Magnetic Nanoparticles. Z. Phys. Chem. 2017, 231, 745–757. [Google Scholar] [CrossRef][Green Version]
- Domracheva, N.E.; Vorobeva, V.E.; Gruzdev, M.S.; Shvachko, Y.N.; Starichenko, D.V. EPR detection of presumable quantum behavior of iron oxide nanoparticles in dendrimeric nanocomposite. Inorg. Chim. Acta 2017, 465, 38–43. [Google Scholar] [CrossRef]
- Ganguly, S.; Neelam; Grinberg, I.; Margel, S. Layer by layer controlled synthesis at room temperature of tri-modal (MRI, fluorescence and CT) core/shell superparamagnetic IO/human serum albumin nanoparticles for diagnostic applications. Polym. Adv. Technol. 2021, 32, 3909–3921. [Google Scholar] [CrossRef]
- Singh, S.; Goswami, N. Structural, magnetic and dielectric study of Fe2O3 nanoparticles obtained through exploding wire technique. Curr. Appl. Phys. 2021, 22, 20–29. [Google Scholar] [CrossRef]
- Raita, O.; Popa, A.; Toloman, D.; Badilita, V.; Piticesu, R.; Giurgiu, L.i. Superparamagnetic behavior of ZnFe2O4 nanoparticles as evidenced by EPR. J. Optoelectron. Adv. Mater. 2015, 17, 1314–1318. [Google Scholar]
- Lin, P.-Y.; Chalise, D.; Cahill, D.G. Improving nuclear magnetic resonance and electron spin resonance thermometry with size reduction of superparamagnetic iron oxide nanoparticles. Phys. Rev. Appl. 2024, 22, 044082. [Google Scholar] [CrossRef]
- Chandekar, K.V.; Kant, K.M. Estimation of the spin-spin relaxation time of surfactant coated CoFe2O4 nanoparticles by electron paramagnetic resonance spectroscopy. Phys. E Low-Dimens. Syst. Nanostruct. 2018, 104, 192–205. [Google Scholar] [CrossRef]
- Ashok, K.; Usha, P.; Nagaraju, R.; Ramesh, T.; Kumar, N.P.; Sulaiman, G.M. Multifunctional Characterization and Anticancer Properties of Magnetic Zinc Ferrite Nanoparticles by Modified Ultrasonic Assisted Co-precipitation Method. ECS J. Solid State Sci. Technol. 2024, 13, 083011. [Google Scholar] [CrossRef]
- Lund, A.; Sagstuen, E.; Sanderud, A.; Maruani, J. Relaxation-Time Determination from Continuous-Microwave Saturation of EPR Spectra. Radiat. Res. 2009, 172, 753–760. [Google Scholar] [CrossRef]
- Li, M.; Wang, X. Beating the Size-Dependent Limit with Spin–Lattice Coupling in Nanomagnetism. J. Am. Chem. Soc. 2025, 147, 1732–1739. [Google Scholar] [CrossRef] [PubMed]
- Fittipaldi, M.; Innocenti, C.; Ceci, P.; Sangregorio, C.; Castelli, L.; Sorace, L.; Gatteschi, D. Looking for quantum effects in magnetic nanoparticles using the molecular nanomagnet approach. Phys. Rev. B 2011, 83, 104409. [Google Scholar] [CrossRef]
- Misra, S.K.; Andronenko, S.I.; Thurber, A.; Punnoose, A.; Nalepa, A. An X- and Q-band Fe3+ EPR study of nanoparticles of magnetic semiconductor Zn1−xFexO. J. Magn. Magn. Mater. 2014, 363, 82–87. [Google Scholar] [CrossRef]
- Radu, L.; Caruntu, D.; White, M.; Wiley, J.; Connor, C.J.O.; Hanson, P. Ligand-dependent changes in the SPR of magnetic nanoparticles. In 2006 NSTI Nanotechnology Conference and Trade Show—NSTI Nanotech 2006 Technical Proceedings; CRC Press: Boca Raton, FL, USA, 2006; Volume 2. [Google Scholar]
- Piekarz, P.; Parlinski, K.; Oleś, A.M. Origin of the Verwey transition in magnetite: Group theory, electronic structure, and lattice dynamics study. Phys. Rev. B 2007, 76, 165124. [Google Scholar] [CrossRef]
- Stankowski, J.; Kempiński, W.; Łoś, S.; Bednarski, W.; Waplak, S.; Micnas, R. Two paramagnetic iron states at the Verwey phase transition in magnetite. J. Magn. Magn. Mater. 2006, 301, 88–93. [Google Scholar] [CrossRef]
- Adler, P.; Jeglič, P.; Reehuis, M.; Geiß, M.; Merz, P.; Knaflič, T.; Komelj, M.; Hoser, A.; Sans, A.; Janek, J.; et al. Verwey-type charge ordering transition in an open-shell p-electron compound. Sci. Adv. 2018, 4, eaap7581. [Google Scholar] [CrossRef]
- Ahmad, Y.; Raina, B.; Thakur, S.; Bamzai, K.K. Magnesium and yttrium doped superparamagnetic manganese ferrite nanoparticles for magnetic and microwave applications. J. Magn. Magn. Mater. 2022, 552, 169178. [Google Scholar] [CrossRef]
- Monisha, P.; Priyadharshini, P.; Gomathi, S.S.; Pushpanathan, K. Influence of Mn dopant on the crystallite size, optical and magnetic behaviour of CoFe2O4 magnetic nanoparticles. J. Phys. Chem. Solids 2021, 148, 109654. [Google Scholar] [CrossRef]
- Victory, M.; Pant, R.P.; Phanjoubam, S. Synthesis and characterization of oleic acid coated Fe–Mn ferrite based ferrofluid. Mater. Chem. Phys. 2020, 240, 122210. [Google Scholar] [CrossRef]
- Masur, S.; Zingsem, B.; Marzi, T.; Meckenstock, R.; Farle, M. Characterization of the oleic acid/iron oxide nanoparticle interface by magnetic resonance. J. Magn. Magn. Mater. 2016, 415, 8–12. [Google Scholar] [CrossRef]
- Koper, A.; Krzyminiewski, R. Analysis of resonance excitations by linear transformation technique theory. Acta Magn. 1985, 2, 3–23. [Google Scholar]
- Krzyminiewski, R.; Kowalczyk, R.M.; Bielewicz-Mordalska, A.; Pająk, Z.; Czarnecki, P. Computer enhancement of CW-EPR experimental spectra resolution as a new method in investigation of molecular dynamics in pyridinium tetrafluoroborate. J. Mol. Struct. 1998, 471, 243–249. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Koralewski, M.; Hałupka-Bryl, M. Computer enhancement of ESR spectra of magnetite nanoparticles. J. Magn. Magn. Mater. 2016, 407, 114–121. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Kurczewska, J.; Schroeder, G. The influence of surface modification, coating agents and pH value of aqueous solutions on physical properties of magnetite nanoparticles investigated by ESR method. J. Magn. Magn. Mater. 2017, 429, 203–210. [Google Scholar] [CrossRef]
- Noginova, N.; Chen, F.; Weaver, T.; Giannelis, E.P.; Bourlinos, A.B.; Atsarkin, V.A. Magnetic resonance in nanoparticles: Between ferro- and paramagnetism. J. Phys. Condens. Matter 2007, 19, 246208. [Google Scholar] [CrossRef]
- Upadhyay, R.V.; Srinivas, D.; Mehta, R.V. Magnetic resonance in nanoscopic particles of a ferrofluid. J. Magn. Magn. Mater. 2000, 214, 105–111. [Google Scholar] [CrossRef]
- Thamarai Selvi, E.; Meenakshi Sundar, S. Effect of size on structural, optical and magnetic properties of SnO2 nanoparticles. Mater. Res. Express 2017, 4, 075903. [Google Scholar] [CrossRef]
- Erdem, E. Microwave power, temperature, atmospheric and light dependence of intrinsic defects in ZnO nanoparticles: A study of electron paramagnetic resonance (EPR) spectroscopy. J. Alloys Compd. 2014, 605, 34–44. [Google Scholar] [CrossRef]
- Galyametdinov, Y.G.; Sagdeev, D.O.; Voronkova, V.K.; Sukhanov, A.A.; Shamilov, R.R. The dependence of paramagnetic and optical characteristics of Mn:CdS nanoparticles on high-temperature synthesis conditions. Mater. Res. Express 2018, 5, 075009. [Google Scholar] [CrossRef]
- Naik, R.; Prashantha, S.C.; Nagabhushana, H.; Girish, K.M. Electrochemical, photoluminescence and EPR studies of Fe3+ doped nano Forsterite: Effect of doping on tetra and octahedral sites. J. Lumin. 2018, 197, 233–241. [Google Scholar] [CrossRef]
- Al Boukhari, J.; Zeidan, L.; Khalaf, A.; Awad, R. Synthesis, characterization, optical and magnetic properties of pure and Mn, Fe and Zn doped NiO nanoparticles. Chem. Phys. 2019, 516, 116–124. [Google Scholar] [CrossRef]
- Atkins, T.M.; Walton, J.H.; Singh, M.P.; Ganguly, S.; Janka, O.; Louie, A.Y.; Kauzlarich, S.M. EPR and Structural Characterization of Water-Soluble Mn2+-Doped Si Nanoparticles. J. Phys. Chem. C 2017, 121, 1948–1956. [Google Scholar] [CrossRef] [PubMed]
- Daniel, J.E.; Weaver, S.I.; Matthias, B.R.; Golden, R.; George, G.M.; Kerpal, C.; Donley, C.L.; Jarocha, L.E.; Anderson, M.E. Investigating Cu-Site Doped Cu–Sb–S Nanoparticles Using Photoelectron and Electron Paramagnetic Resonance Spectroscopy. J. Phys. Chem. C 2024, 128, 13888–13899. [Google Scholar] [CrossRef] [PubMed]
- Misra, S.K.; Andronenko, S.I.; Srinivasa Rao, S.; Chess, J.; Punnoose, A. An X-band Co2+ EPR study of Zn1−xCoxO (x = 0.005–0.1) nanoparticles prepared by chemical hydrolysis methods using diethylene glycol and denaturated alcohol at 5K. J. Magn. Magn. Mater. 2015, 394, 138–142. [Google Scholar] [CrossRef]
- Choudhury, B.; Dey, M.; Choudhury, A. Defect generation, d-d transition, and band gap reduction in Cu-doped TiO2 nanoparticles. Int. Nano Lett. 2013, 3, 25. [Google Scholar] [CrossRef]
- Smirnov, A.I. Post-processing of EPR spectra by convolution filtering: Calculation of a harmonics’ series and automatic separation of fast-motion components from spin-label EPR spectra. J. Magn. Reson. 2008, 190, 154–159. [Google Scholar] [CrossRef]
- Travin, S.O.; Kokorin, A.I. Kinetic Analysis and Resolution of Overlapping EPR Spectra. Appl. Magn. Reson. 2022, 53, 1069–1088. [Google Scholar] [CrossRef]
- Kara, G.; Malekghasemi, S.; Ozpolat, B.; Denkbas, E.B. Development of novel poly-l-lysine-modified sericin-coated superparamagnetic iron oxide nanoparticles as siRNA carrier. Colloids Surf. A Physicochem. Eng. Asp. 2021, 630, 127622. [Google Scholar] [CrossRef]
- Jamir, M.; Islam, R.; Pandey, L.M.; Borah, J.P. Effect of surface functionalization on the heating efficiency of magnetite nanoclusters for hyperthermia application. J. Alloys Compd. 2021, 854, 157248. [Google Scholar] [CrossRef]
- Kavas, H.; Kasapoğlu, N.; Baykal, A.; Köseoğlu, Y. Characterization of NiFe2O4 nanoparticles synthesized by various methods. Chem. Pap. 2009, 63, 450–455. [Google Scholar] [CrossRef]
- Nedelcu, G.G.; Nastro, A.; Filippelli, L.; Cazacu, M.; Iacob, M.; Rossi, C.O.; Popa, A.; Toloman, D.; Dobromir, M.; Iacomi, F. Structural characterization of copolymer embedded magnetic nanoparticles. Appl. Surf. Sci. 2015, 352, 109–116. [Google Scholar] [CrossRef]
- Adams, S.; Bonabi, S.; Allen, A.L.; Roseman, G.; Ramirez, A.P.; Millhauser, G.; Zhang, J.Z. The effect of polymer and gold functionalization on the magnetic properties of magnetite nanoparticles. Biomed. Spectrosc. Imaging 2018, 7, 115–124. [Google Scholar] [CrossRef]
- Deliormanlı, A.M.; Almisned, G.; Tekin, H.O. Nanoarchitectonics and properties of sol-gel-derived bioactive glasses containing maghemite@ZnO core-shell nanoparticles. Appl. Phys. A 2024, 130, 538. [Google Scholar] [CrossRef]
- Çimen, D.; Bereli, N.; Denizli, A. Metal-chelated magnetic nanoparticles for protein C purification. Sep. Sci. Technol. 2020, 55, 2259–2268. [Google Scholar] [CrossRef]
- Orhan, H.; Aktaş Uygun, D. Immobilization of L-Asparaginase on Magnetic Nanoparticles for Cancer Treatment. Appl. Biochem. Biotechnol. 2020, 191, 1432–1443. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Konstantinova, E.A.; Kokorin, A.I.; Gyergyek, S.; Leitgeb, M. Structural and magnetic characteristics of carboxymethyl dextran coated magnetic nanoparticles: From characterization to immobilization application. React. Funct. Polym. 2020, 148, 104481. [Google Scholar] [CrossRef]
- Lloveras, V.; Badetti, E.; Chechik, V.; Vidal-Gancedo, J. Magnetic Interactions in Spin-Labeled Au Nanoparticles. J. Phys. Chem. C 2014, 118, 21622–21629. [Google Scholar] [CrossRef]
- Zhang, M.; Li, X.H.; Gong, Y.D.; Zhao, N.M.; Zhang, X.F. Properties and biocompatibility of chitosan films modified by blending with PEG. Biomaterials 2002, 23, 2641–2648. [Google Scholar] [CrossRef]
- Hirota, Y.; Haida, M.; Mohtarami, F.; Takeda, K.; Iwamoto, T.; Shioya, S.; Tsuji, C.; Hasumi, K.; Nakazawa, H. Implication of ESR signals from ceruloplasmin (Cu2+) and transferrin (Fe3+) in pleural effusion of lung diseases. Pathophysiology 2000, 7, 41–45. [Google Scholar] [CrossRef]
- Preoteasa, E.A.; Schianchi, G.; Camillo Giori, D.; Duliu, O.G.; Butturini, A.; Izzi, G. Unexpected Detection of Low- and High-Spin Ferrihemoglobin Derivatives in Blood Serum of Polytransfused Patients with Homozygous β-Thalassemia under Chelation Therapy. An EPR Study. Dig. J. Nanomater. Biostruct. 2013, 8, 469–499. [Google Scholar]
- Shukla, A.K. Electron Spin Resonance Spectroscopy in Medicine; Springer: Singapore, 2019; pp. 1–22. [Google Scholar]
- Kubiak, T.; Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J.; Hałupka-Bryl, M. A study of magnetite nanoparticles in whole human blood by means of electron paramagnetic resonance. Acta Bio-Opt. Inform. Medica Inżynieria Biomed. 2015, 21, 9–15. [Google Scholar]
- Gamarra, L.F.; Pontuschka, W.M.; Amaro, E.; Costa-Filho, A.J.; Brito, G.E.S.; Vieira, E.D.; Carneiro, S.M.; Escriba, D.M.; Falleiros, A.M.F.; Salvador, V.L. Kinetics of elimination and distribution in blood and liver of biocompatible ferrofluids based on Fe3O4 nanoparticles: An EPR and XRF study. Mater. Sci. Eng. C 2008, 28, 519–525. [Google Scholar] [CrossRef]
- Kaczmarek, K.; Hornowski, T.; Dobosz, B.; Józefczak, A. Influence of Magnetic Nanoparticles on the Focused Ultrasound Hyperthermia. Materials 2018, 11, 1607. [Google Scholar] [CrossRef] [PubMed]
- Kubiak, T. The Influence of Blood and Serum Microenvironment on Spin-Labeled Magnetic Nanoparticles. Magnetism 2024, 4, 114–124. [Google Scholar] [CrossRef]
- Chechik, V.; Wellsted, H.J.; Korte, A.; Gilbert, B.C.; Caldararu, H.; Ionita, P.; Caragheorgheopol, A. Spin-labelled Au nanoparticles. Faraday Discuss. 2004, 125, 279–291. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 2006, 178, 42–55. [Google Scholar] [CrossRef]
- Stoll, S.; Schweiger, A. EasySpin: Simulating cw ESR spectra. Biol. Magn. Reson. 2007, 27, 299–321. [Google Scholar]
- Dobosz, B.; Krzyminiewski, R.; Kurczewska, J.; Schroeder, G. The dynamics of functionalized magnetite nanoparticles in various solutions studied by ESR method. Mater. Chem. Phys. 2017, 198, 297–302. [Google Scholar] [CrossRef]
- Krzyminiewski, R.; Dobosz, B.; Krist, B.; Schroeder, G.; Kurczewska, J.; Bluyssen, H.A.R. ESR Method in Monitoring of Nanoparticle Endocytosis in Cancer Cells. Int. J. Mol. Sci. 2020, 21, 4388. [Google Scholar] [CrossRef]
- Saeidpour, S.; Lohan, S.B.; Anske, M.; Unbehauen, M.; Fleige, E.; Haag, R.; Meinke, M.C.; Bittl, R.; Teutloff, C. Localization of dexamethasone within dendritic core-multishell (CMS) nanoparticles and skin penetration properties studied by multi-frequency electron paramagnetic resonance (EPR) spectroscopy. Eur. J. Pharm. Biopharm. 2017, 116, 94–101. [Google Scholar] [CrossRef]
- Haag, S.F.; Chen, M.; Peters, D.; Keck, C.M.; Taskoparan, B.; Fahr, A.; Teutloff, C.; Bittl, R.; Lademann, J.; Schäfer-Korting, M.; et al. Nanostructured lipid carriers as nitroxide depot system measured by electron paramagnetic resonance spectroscopy. Int. J. Pharm. 2011, 421, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-M.; Li, R.; Kao, Y.-C.J.; Hsu, C.-H.; Chu, H.-L.; Lu, K.-Y.; Changou, C.A.; Chang, C.-C.; Chang, L.-H.; Tsai, M.-L.; et al. Synthesis and characterization of Gd-DTPA/fucoidan/peptide complex nanoparticle and in vitro magnetic resonance imaging of inflamed endothelial cells. Mater. Sci. Eng. C 2020, 114, 111064. [Google Scholar] [CrossRef] [PubMed]
- Hałupka-Bryl, M.; Bednarowicz, M.; Dobosz, B.; Krzyminiewski, R.; Zalewski, T.; Wereszczyńska, B.; Nowaczyk, G.; Jarek, M.; Nagasaki, Y. Doxorubicin loaded PEG-b-poly(4-vinylbenzylphosphonate) coated magnetic iron oxide nanoparticles for targeted drug delivery. J. Magn. Magn. Mater. 2015, 384, 320–327. [Google Scholar] [CrossRef]
- Davies, M.J. Detection and characterisation of radicals using electron paramagnetic resonance (EPR) spin trapping and related methods. Methods 2016, 109, 21–30. [Google Scholar] [CrossRef]
- Jeong, M.S.; Yu, K.-N.; Chung, H.H.; Park, S.J.; Lee, A.Y.; Song, M.R.; Cho, M.-H.; Kim, J.S. Methodological considerations of electron spin resonance spin trapping techniques for measuring reactive oxygen species generated from metal oxide nanomaterials. Sci. Rep. 2016, 6, 26347. [Google Scholar] [CrossRef]
- Dai, Z.; Tang, J.; Gu, Z.; Wang, Y.; Yang, Y.; Yang, Y.; Yu, C. Eliciting Immunogenic Cell Death via a Unitized Nanoinducer. Nano Lett. 2020, 20, 6246–6254. [Google Scholar] [CrossRef]
- Zhang, H.; Li, S.; Liu, Y.; Yu, Y.; Lin, S.; Wang, Q.; Miao, L.; Wei, H.; Sun, W. Fe3O4@GO magnetic nanocomposites protect mesenchymal stem cells and promote osteogenic differentiation of rat bone marrow mesenchymal stem cells. Biomater. Sci. 2020, 8, 5984–5993. [Google Scholar] [CrossRef]
- Liu, Y.; Quan, X.; Li, J.; Huo, J.; Li, X.; Zhao, Z.; Li, S.; Wan, J.; Li, J.; Liu, S.; et al. Liposomes embedded with PEGylated iron oxide nanoparticles enable ferroptosis and combination therapy in cancer. Natl. Sci. Rev. 2023, 10, nwac167. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, J.; He, J.; Ding, Y.; Huo, D.; Hu, Y. Long-term monitoring of tumor-related autophagy in vivo by Fe3O4NO· nanoparticles. Biomaterials 2018, 179, 186–198. [Google Scholar] [CrossRef]
- Silva, M.A.S.; Romo, A.I.B.; Abreu, D.S.; Carepo, M.S.P.; Lemus, L.; Jafelicci, M.; Paulo, T.F.; Nascimento, O.R.; Vargas, E.; Denardin, J.C.; et al. Magnetic nanoparticles as a support for a copper (II) complex with nuclease activity. J. Inorg. Biochem. 2018, 186, 294–300. [Google Scholar] [CrossRef]
- Moreno Maldonado, A.C.; Winkler, E.L.; Raineri, M.; Toro Córdova, A.; Rodríguez, L.M.; Troiani, H.E.; Mojica Pisciotti, M.L.; Mansilla, M.V.; Tobia, D.; Nadal, M.S.; et al. Free-Radical Formation by the Peroxidase-Like Catalytic Activity of MFe2O4 (M = Fe, Ni, and Mn) Nanoparticles. J. Phys. Chem. C 2019, 123, 20617–20627. [Google Scholar] [CrossRef]
- Chen, Z.; Yin, J.-J.; Zhou, Y.-T.; Zhang, Y.; Song, L.; Song, M.; Hu, S.; Gu, N. Dual Enzyme-like Activities of Iron Oxide Nanoparticles and Their Implication for Diminishing Cytotoxicity. ACS Nano 2012, 6, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- Valgimigli, L.; Baschieri, A.; Amorati, R. Antioxidant activity of nanomaterials. J. Mater. Chem. B 2018, 6, 2036–2051. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; He, X.; Zhou, C.; Reaume, M.; Wu, M.; Liu, B.; Lee, B.P. Iron Magnetic Nanoparticle-Induced ROS Generation from Catechol-Containing Microgel for Environmental and Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 21210–21220. [Google Scholar] [CrossRef]
- Lazarova, D.; Semkova, S.; Zlateva, G.; Tatsuya, H.; Aoki, I.; Bakalova, R. Quantum Sensors To Track Total Redox-Status and Oxidative Stress in Cells and Tissues Using Electron-Paramagnetic Resonance, Magnetic Resonance Imaging, and Optical Imaging. Anal. Chem. 2021, 93, 2828–2837. [Google Scholar] [CrossRef]
- Elas, M.; Krzykawska-Serda, M.; Gonet, M.; Kozińska, A.; Płonka, P.M. Electron Paramagnetic Resonance Imaging-Solo and Orchestra. In Medical Imaging Methods: Recent Trends; Shukla, A.K., Ed.; Springer: Singapore, 2019; pp. 1–42. [Google Scholar]
- Danhier, P.; De Preter, G.; Magat, J.; Godechal, Q.; Porporato, P.E.; Jordan, B.F.; Feron, O.; Sonveaux, P.; Gallez, B. Multimodal cell tracking of a spontaneous metastasis model: Comparison between MRI, electron paramagnetic resonance and bioluminescence. Contrast Media Mol. Imaging 2014, 9, 143–153. [Google Scholar] [CrossRef]
- Rana, S.; Chawla, R.; Kumar, R.; Singh, S.; Zheleva, A.; Dimitrova, Y.; Gadjeva, V.; Arora, R.; Sultana, S.; Sharma, R.K. Electron paramagnetic resonance spectroscopy in radiation research: Current status and perspectives. J. Pharm. Bioallied Sci. 2010, 2, 80–87. [Google Scholar] [CrossRef]
- Dobosz, B.; Krzyminiewski, R.; Schroeder, G.; Kurczewska, J. Diffusion of functionalized magnetite nanoparticles forced by a magnetic field studied by EPR method. Curr. Appl. Phys. 2016, 16, 562–567. [Google Scholar] [CrossRef]
- Bakker, M.G.; Fowler, B.; Bowman, M.K.; Patience, G.S. Experimental methods in chemical engineering: Electron paramagnetic resonance spectroscopy-EPR/ESR. Can. J. Chem. Eng. 2020, 98, 1668–1681. [Google Scholar] [CrossRef]
- Irfan, M.; Dogan, N.; Bingolbali, A.; Aliew, F. Synthesis and characterization of NiFe2O4 magnetic nanoparticles with different coating materials for magnetic particle imaging (MPI). J. Magn. Magn. Mater. 2021, 537, 168150. [Google Scholar] [CrossRef]
- Eaton, G.R.; Eaton, S.; Barr, D.; Weber, R.T. Quantitative EPR; Springer: Vienna, Austria, 2010. [Google Scholar]
- Marnautov, N.A.; Serezhenkov, V.A.; Komissarova, L.K.; Tkachev, N.A.; Tatikolov, A.S.; Goloshchapov, A.N.; Vanin, A.F. Evaluation of the Biodistribution of Magnetoliposomes in a Tumor and Organs of Mice by Electron Paramagnetic Resonance Spectroscopy. Biophysics 2020, 65, 656–659. [Google Scholar] [CrossRef]
- Chertok, B.; Cole, A.J.; David, A.E.; Yang, V.C. Comparison of Electron Spin Resonance Spectroscopy and Inductively-Coupled Plasma Optical Emission Spectroscopy for Biodistribution Analysis of Iron-Oxide Nanoparticles. Mol. Pharm. 2010, 7, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Paulini, F.; Marangon, A.R.M.; Azevedo, C.L.; Brito, J.L.M.; Lemos, M.S.; Sousa, M.H.; Veiga-Souza, F.H.; Souza, P.E.N.; Lucci, C.M.; Azevedo, R.B. In Vivo Evaluation of DMSA-Coated Magnetic Nanoparticle Toxicity and Biodistribution in Rats: A Long-Term Follow-Up. Nanomaterials 2022, 12, 3513. [Google Scholar] [CrossRef] [PubMed]
- Partain, B.D.; Unni, M.; Rinaldi, C.; Allen, K.D. The clearance and biodistribution of magnetic composite nanoparticles in healthy and osteoarthritic rat knees. J. Control. Release 2020, 321, 259–271. [Google Scholar] [CrossRef]
- Shimizu, M.; Yoshitomi, T.; Nagasaki, Y. The behavior of ROS-scavenging nanoparticles in blood. J. Clin. Biochem. Nutr. 2014, 54, 166–173. [Google Scholar] [CrossRef][Green Version]
- Fuchs, J.; Groth, N.; Herrling, T. Cutaneous Tolerance to Nitroxide Free Radicals in Human Skin. Free Radical Biol. Med. 1998, 24, 643–648. [Google Scholar] [CrossRef]
- Lohan, S.B.; Saeidpour, S.; Colombo, M.; Staufenbiel, S.; Unbehauen, M.; Wolde-Kidan, A.; Netz, R.R.; Bodmeier, R.; Haag, R.; Teutloff, C.; et al. Nanocrystals for Improved Drug Delivery of Dexamethasone in Skin Investigated by EPR Spectroscopy. Pharmaceutics 2020, 12, 400. [Google Scholar] [CrossRef]
- Huang, W.-C.; Tang, I.C. Bacterial and Yeast Cultures—Process Characteristics, Products, and Applications. In Bioprocessing for Value-Added Products from Renewable Resources; Yang, S.-T., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 185–223. [Google Scholar]
- Krzyminiewski, R.; Dobosz, B.; Schroeder, G.; Kurczewska, J. ESR as a monitoring method of the interactions between TEMPO-functionalized magnetic nanoparticles and yeast cells. Sci. Rep. 2019, 9, 18733. [Google Scholar] [CrossRef]
- Dobosz, B.; Gunia, E.; Kotarska, K.; Schroeder, G.; Kurczewska, J. The Effect of a Magnetic Field on the Transport of Functionalized Magnetite Nanoparticles into Yeast Cells. Appl. Sci. 2024, 14, 1343. [Google Scholar] [CrossRef]
- Khmelinskii, I.; Makarov, V.I. EPR hyperthermia of S. cerevisiae using superparamagnetic Fe3O4 nanoparticles. J. Therm. Biol. 2018, 77, 55–61. [Google Scholar] [CrossRef]
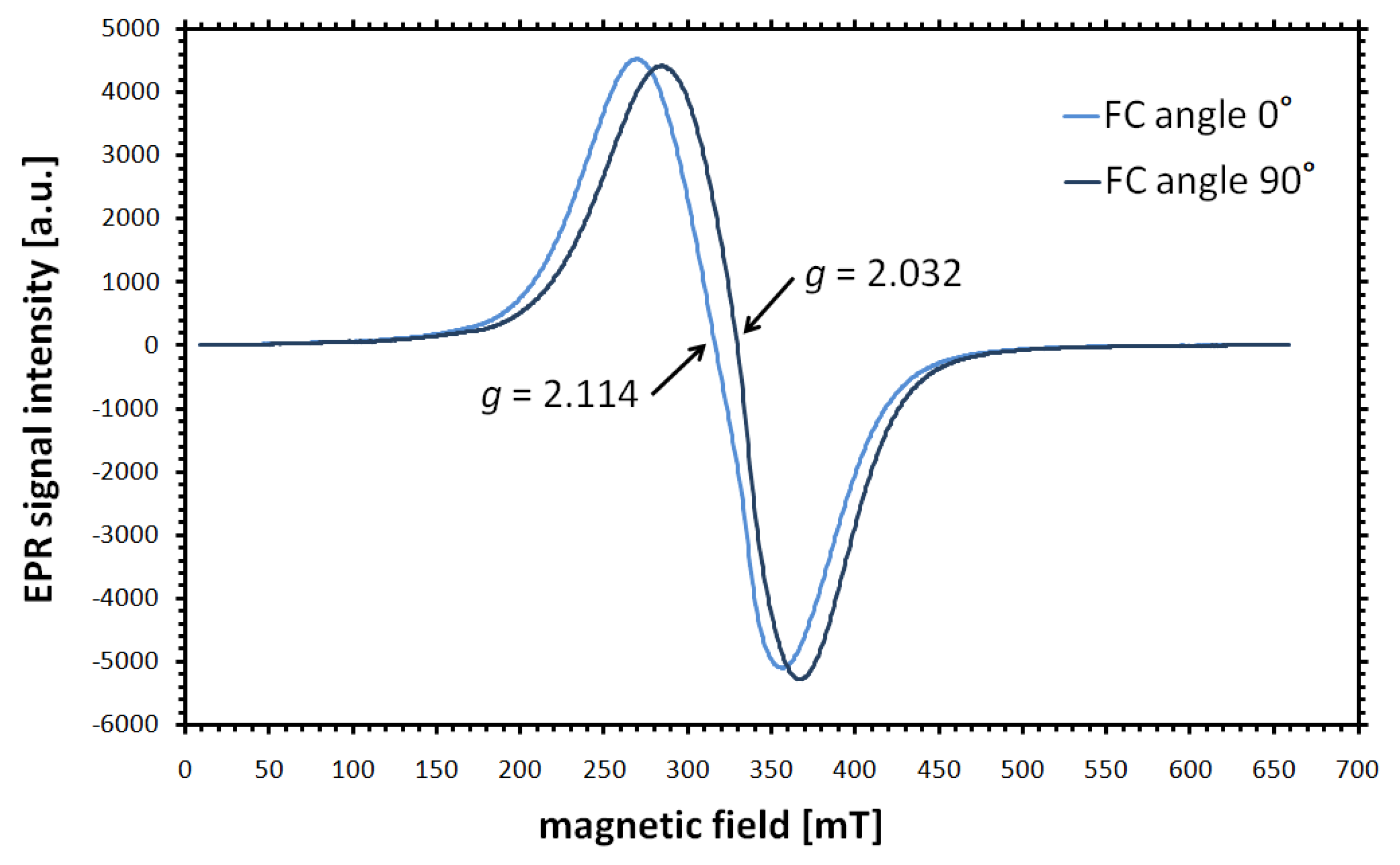
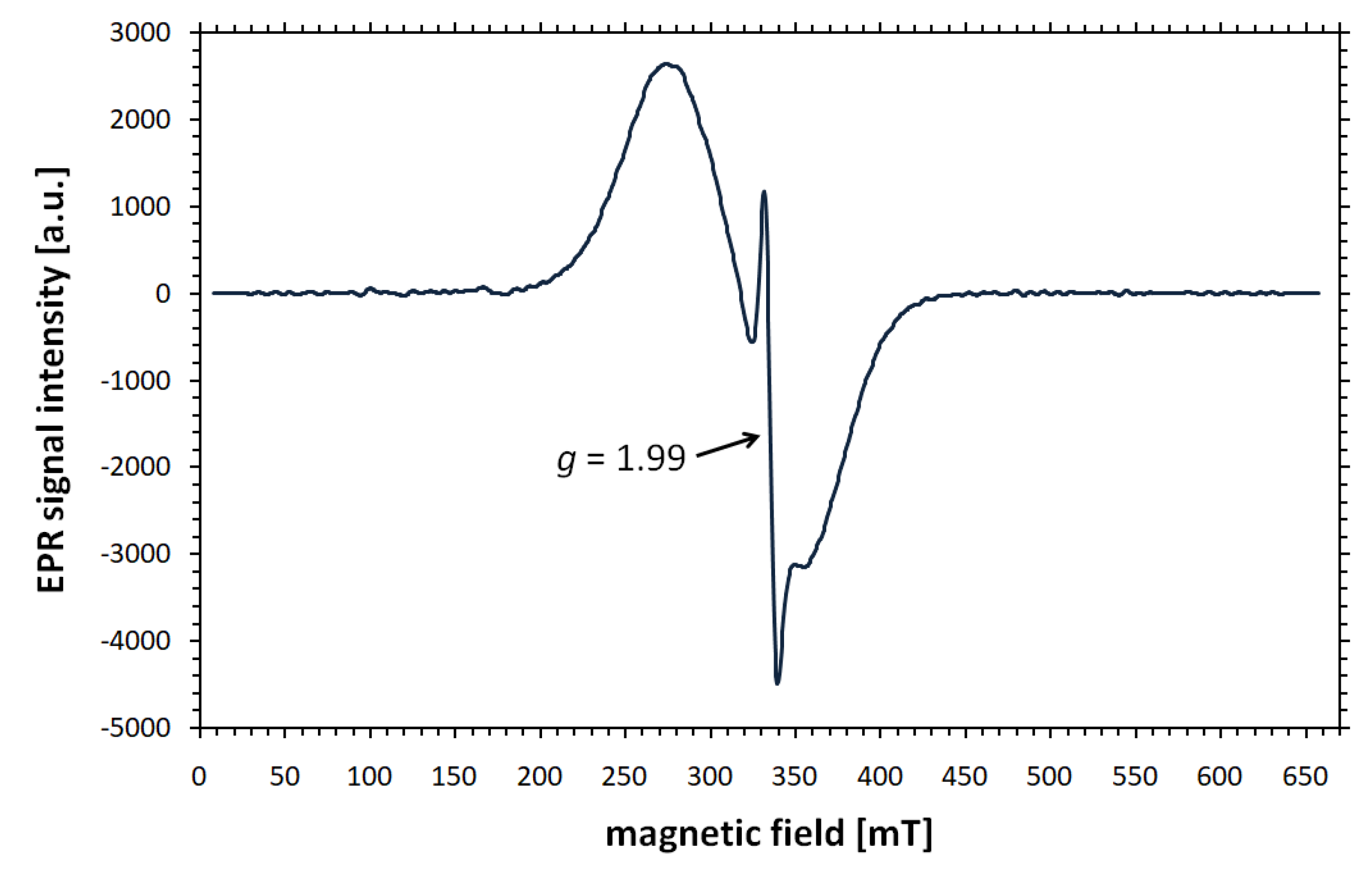
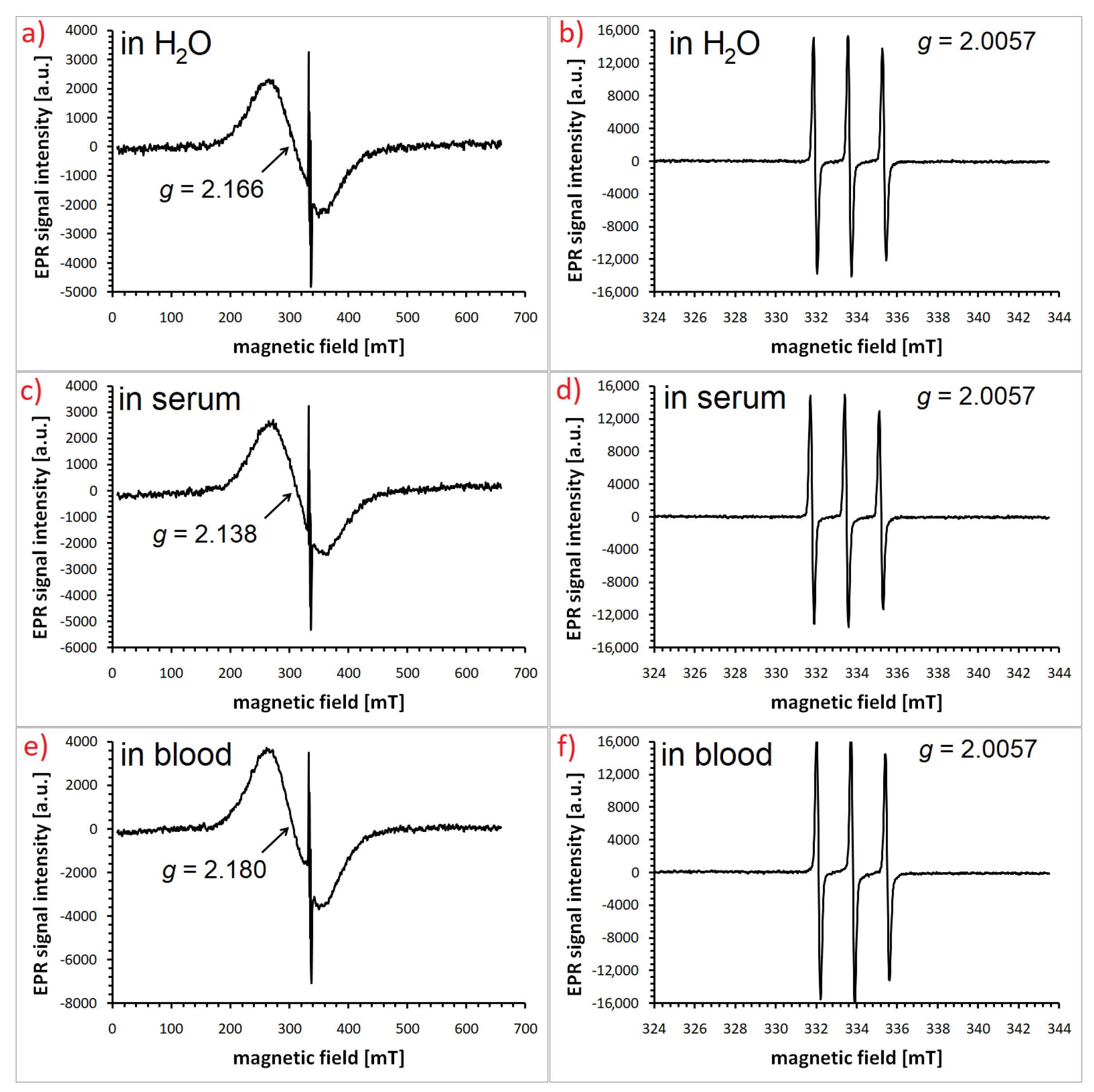
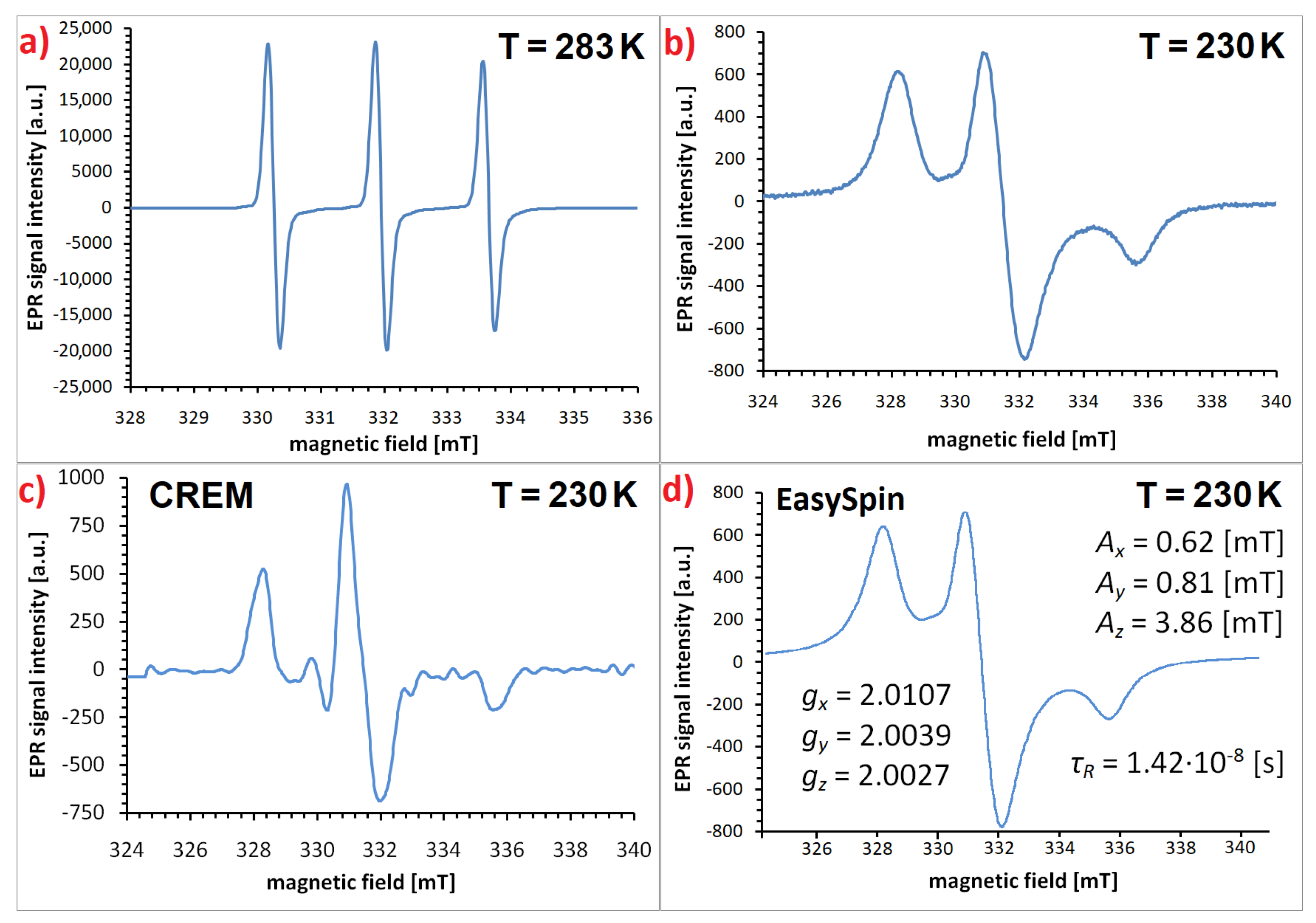
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubiak, T.; Dobosz, B. Road Map for the Use of Electron Spin Resonance Spectroscopy in the Study of Functionalized Magnetic Nanoparticles. Materials 2025, 18, 2841. https://doi.org/10.3390/ma18122841
Kubiak T, Dobosz B. Road Map for the Use of Electron Spin Resonance Spectroscopy in the Study of Functionalized Magnetic Nanoparticles. Materials. 2025; 18(12):2841. https://doi.org/10.3390/ma18122841
Chicago/Turabian StyleKubiak, Tomasz, and Bernadeta Dobosz. 2025. "Road Map for the Use of Electron Spin Resonance Spectroscopy in the Study of Functionalized Magnetic Nanoparticles" Materials 18, no. 12: 2841. https://doi.org/10.3390/ma18122841
APA StyleKubiak, T., & Dobosz, B. (2025). Road Map for the Use of Electron Spin Resonance Spectroscopy in the Study of Functionalized Magnetic Nanoparticles. Materials, 18(12), 2841. https://doi.org/10.3390/ma18122841








