Preparation of Low-Salt-Rejection Membrane by Sodium Hypochlorite Chlorination for Concentration of Low-Concentration Magnesium Chloride Solution
Abstract
1. Introduction
2. Experimental Materials and Methods
2.1. Materials
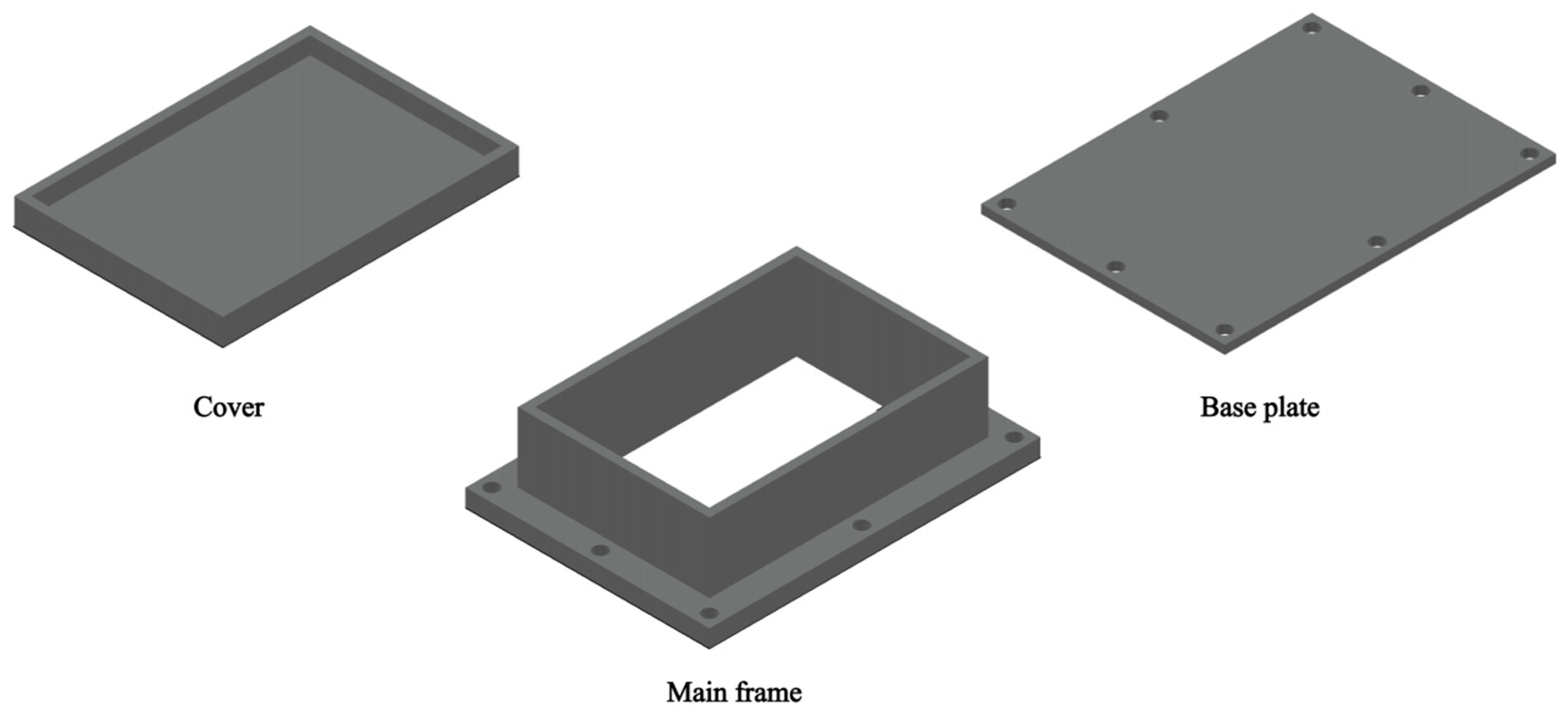
2.2. Preparation and Performance Testing of Low-Salt-Rejection Membrane
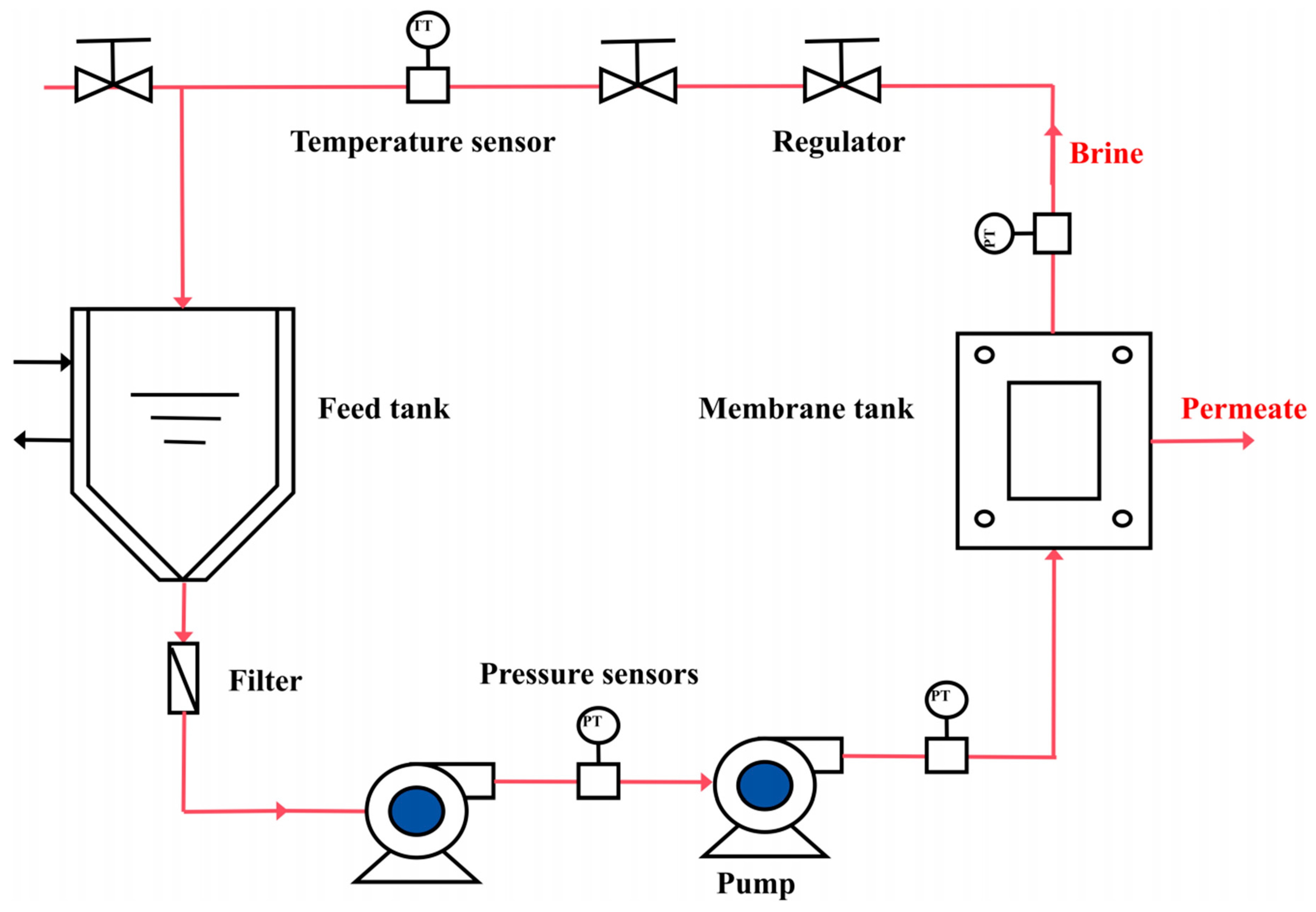
2.3. Gradient Concentration Experiment
2.4. Characterization of Membranes
3. Results and Discussion
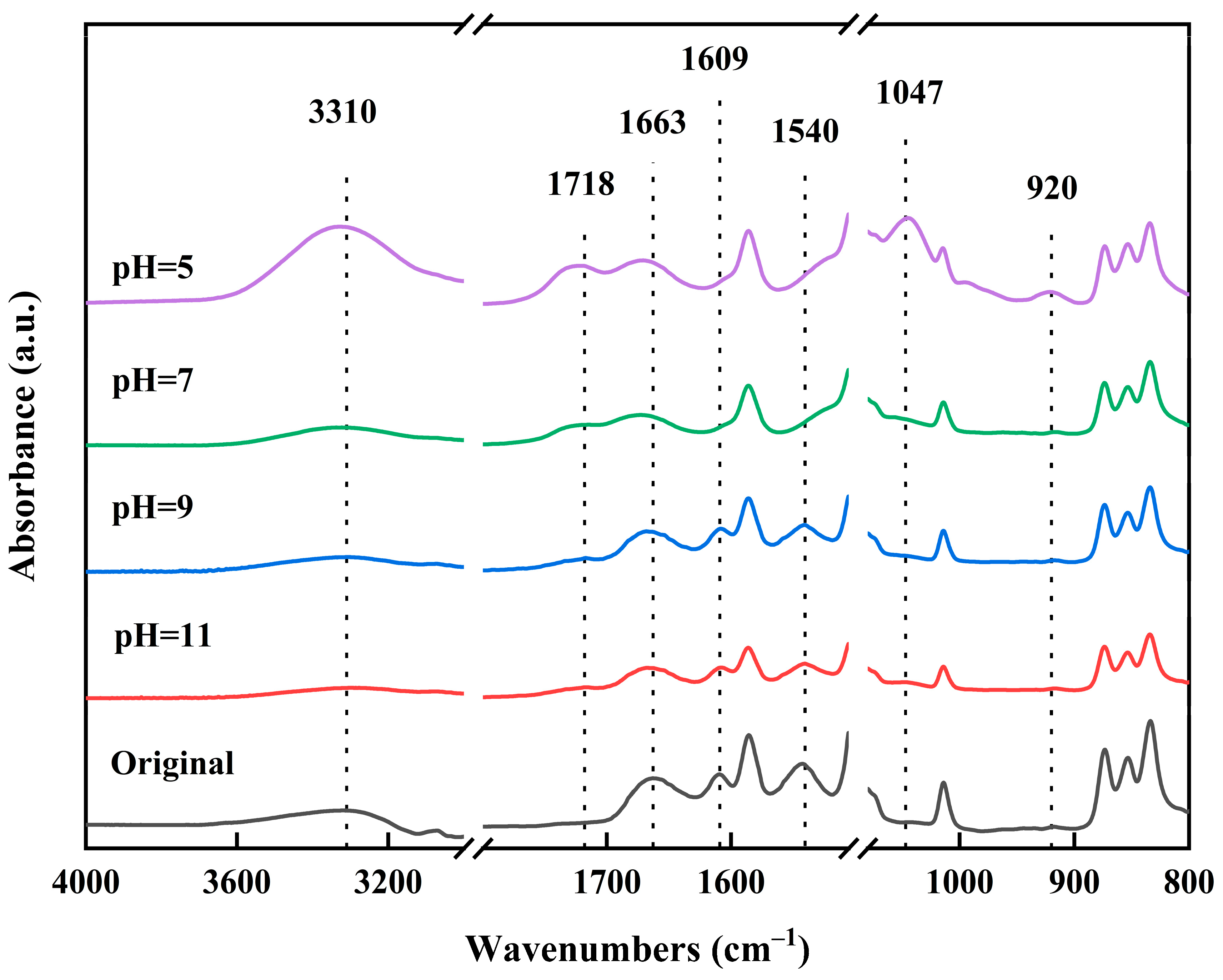
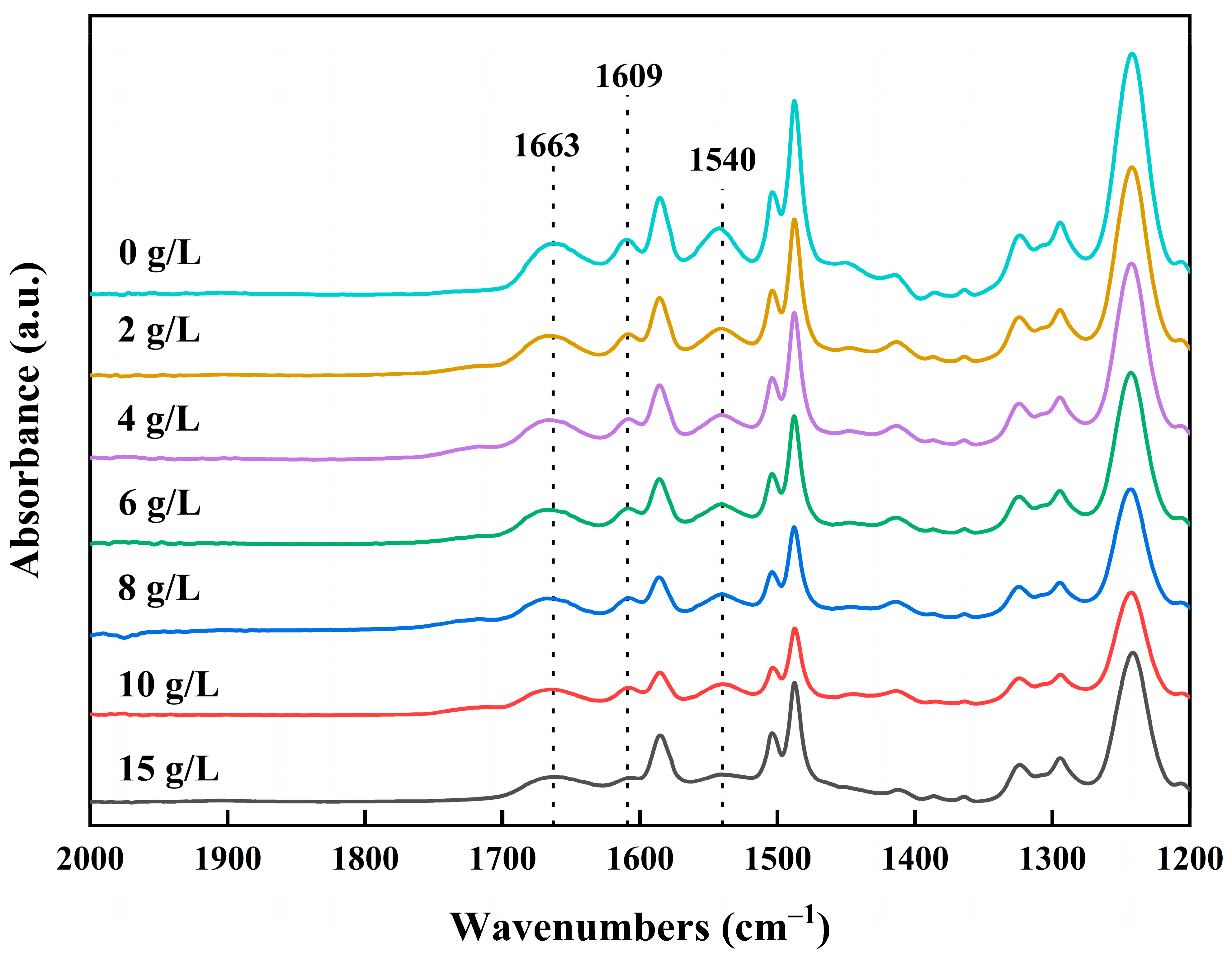
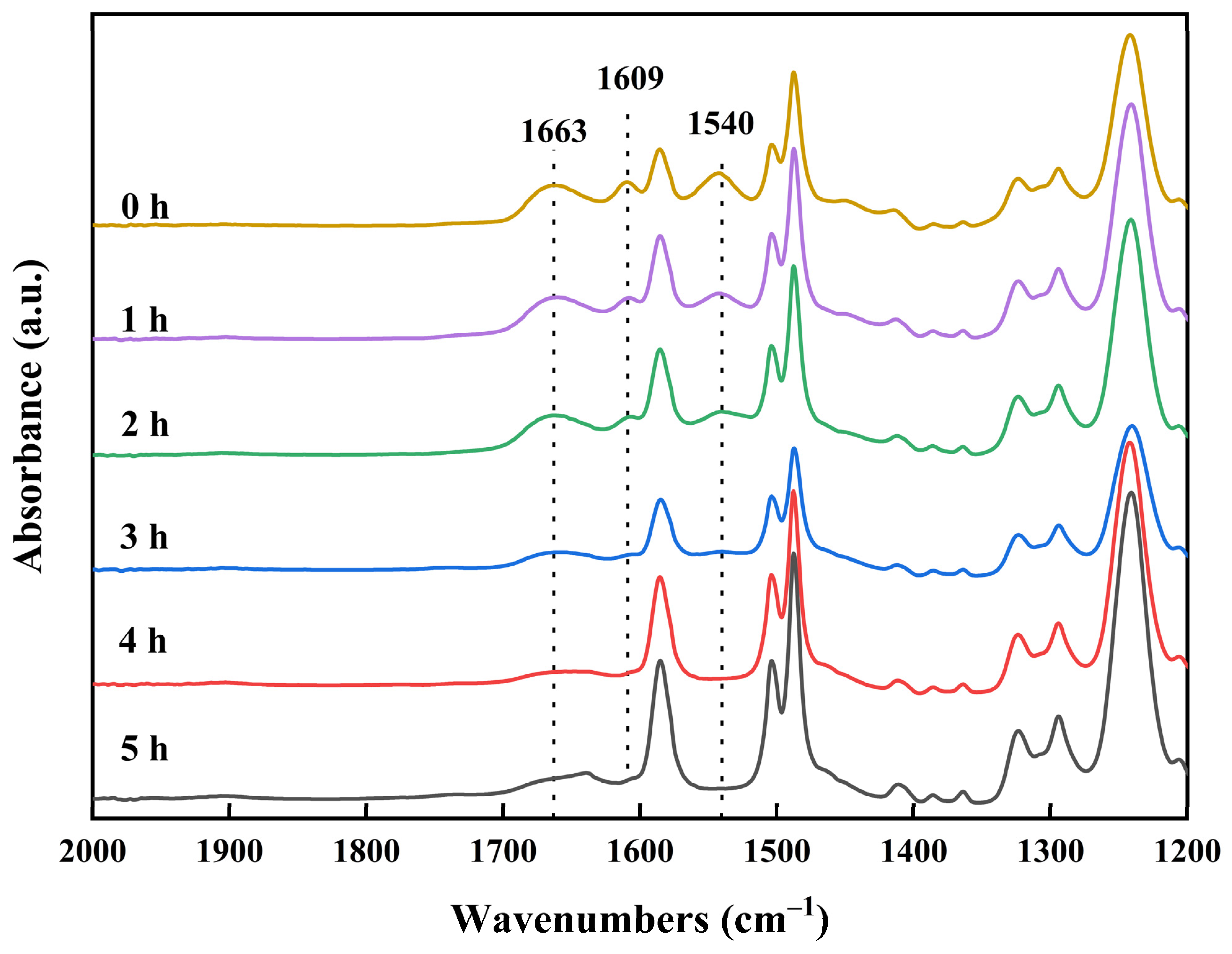
3.1. Infrared Spectral Analysis of Membranes
3.1.1. Effect of pH of NaClO Solution
3.1.2. Effect of Concentration of NaClO Solution
3.1.3. Effect of Chlorination Time with NaClO Solution
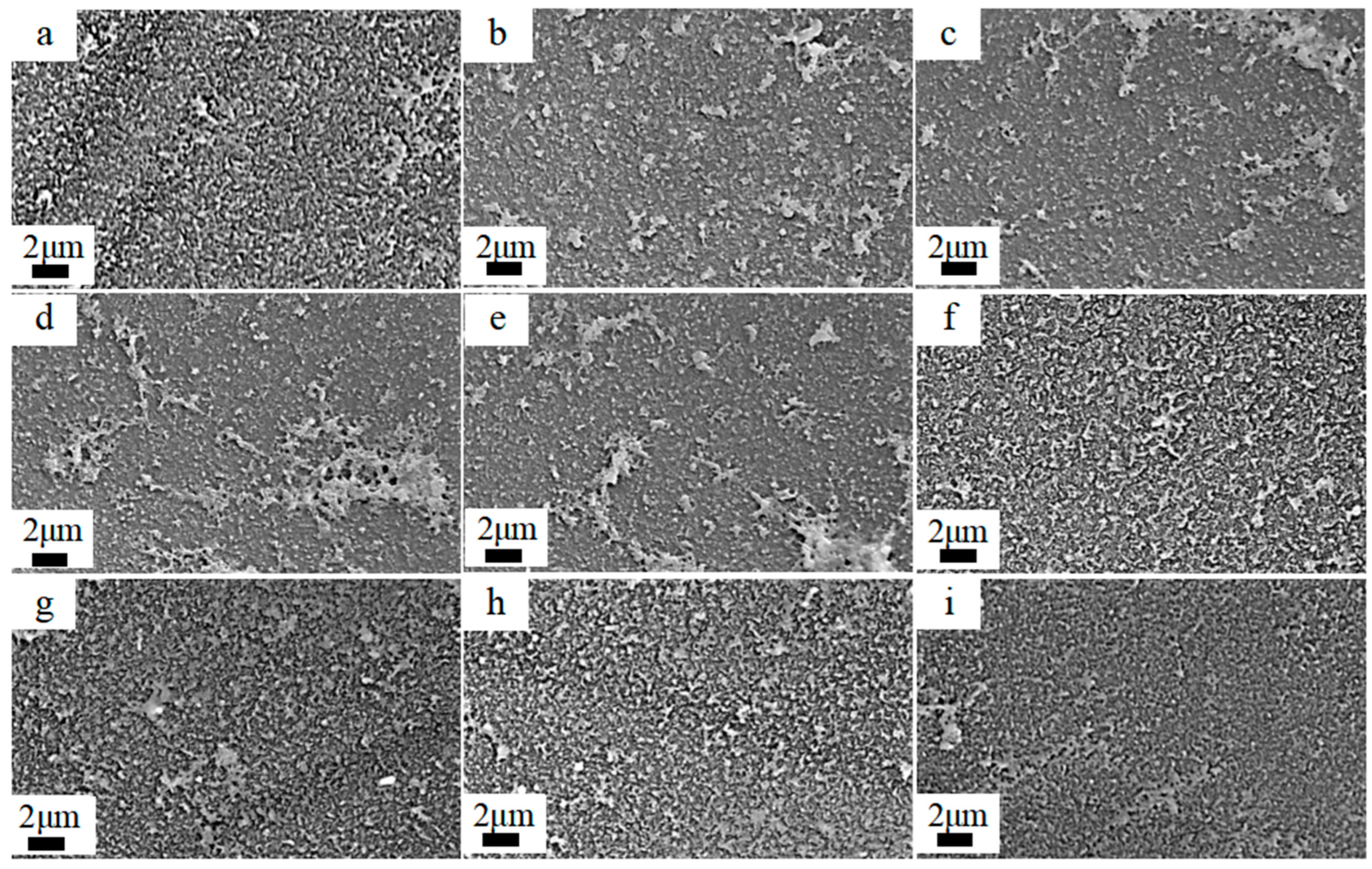
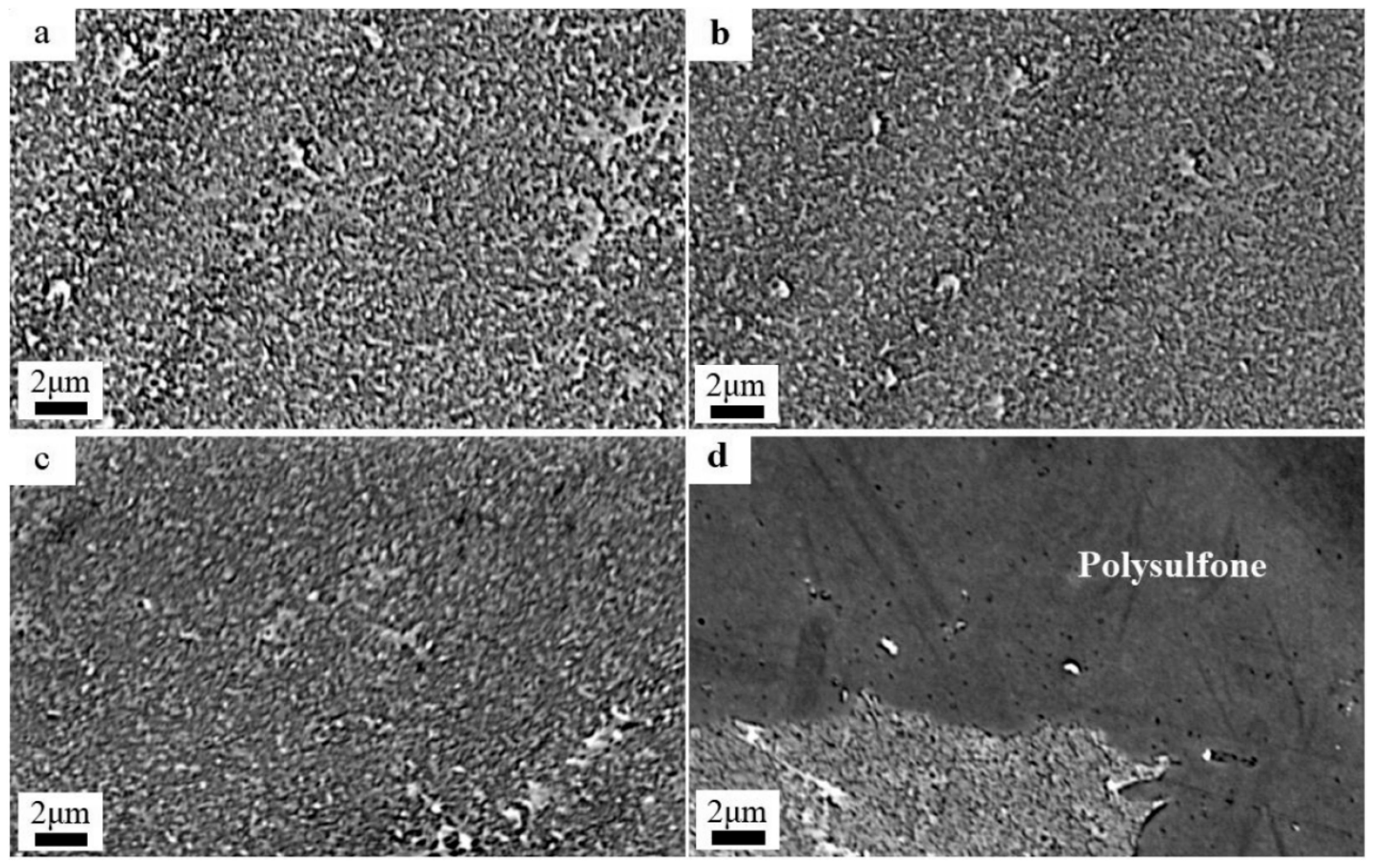
3.2. Surface Morphology Analysis of Membranes
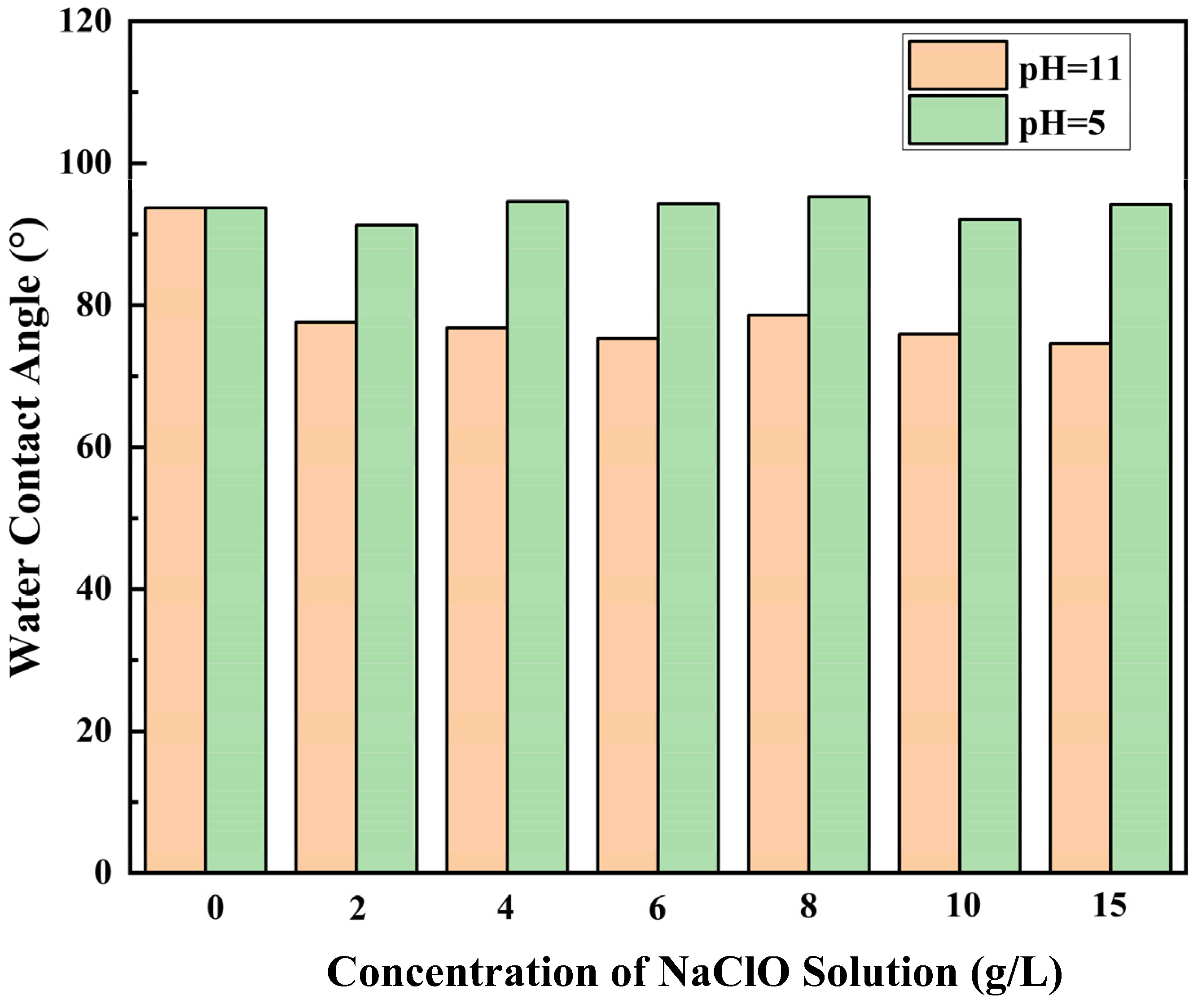
3.2.1. Effect of pH and Concentration of NaClO Solution
3.2.2. Effect of Chlorination Time with NaClO Solution
3.3. Changes in Water Flux and Salt Rejection of Membranes
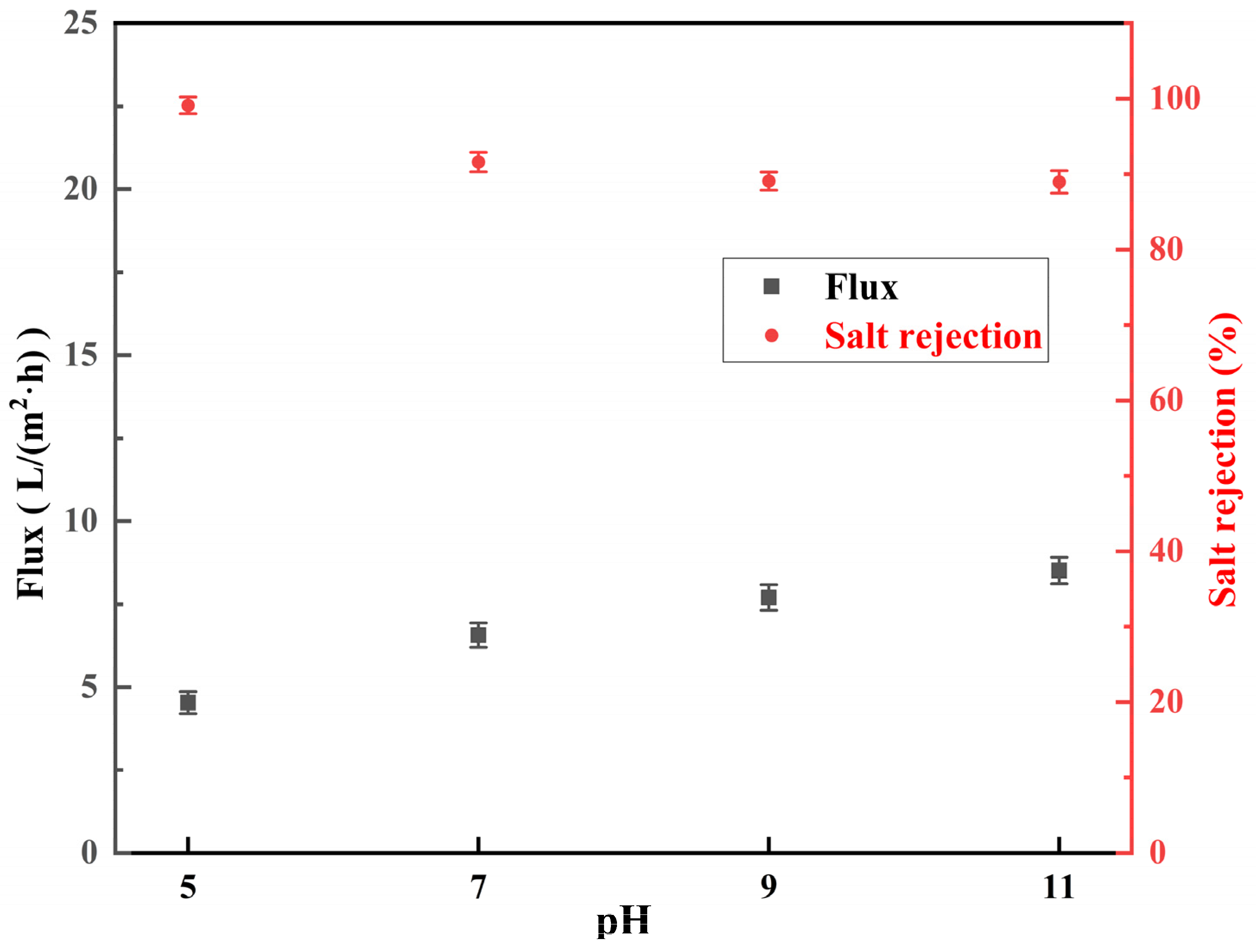
3.3.1. Effect of pH of NaClO Solution
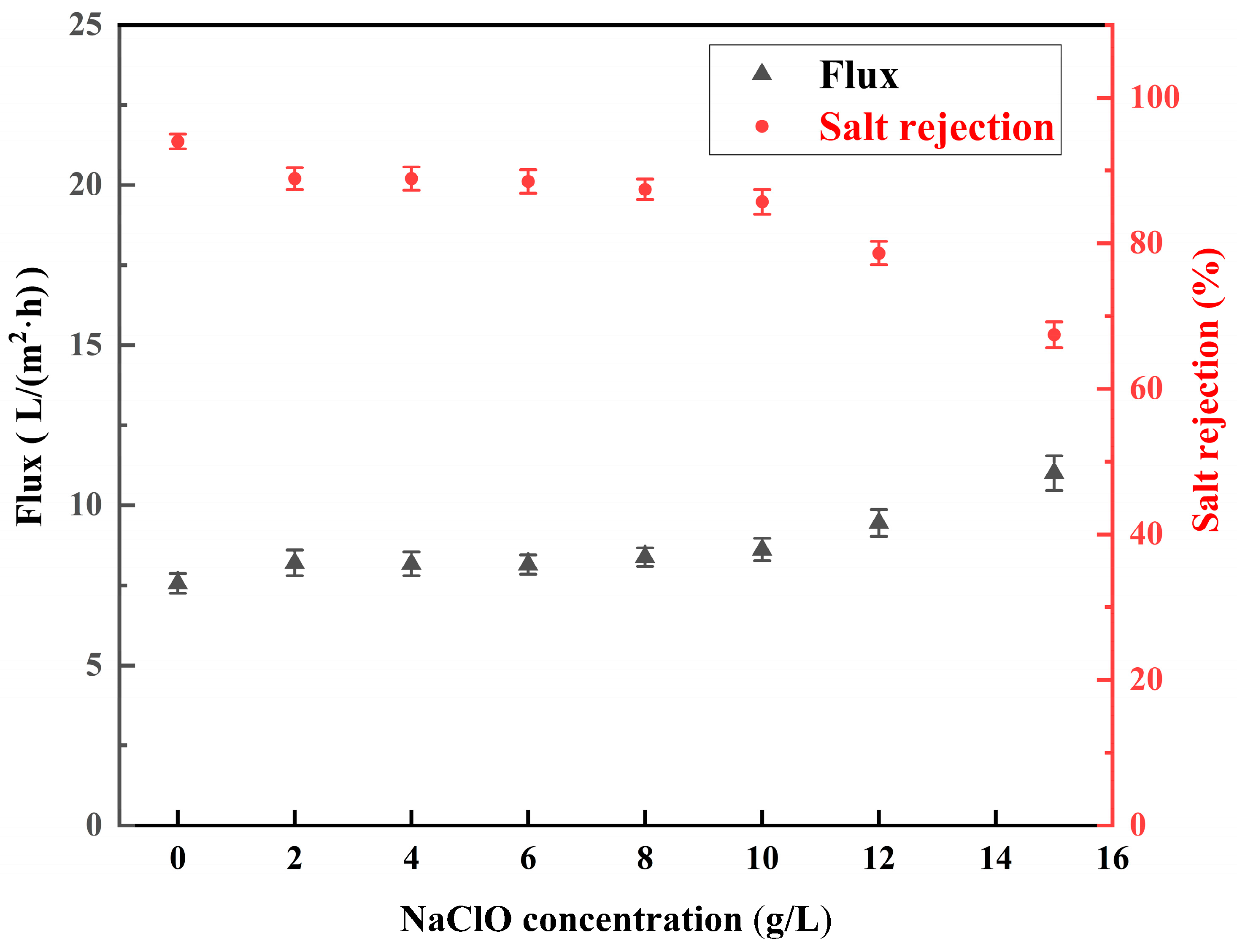
3.3.2. Effect of Concentration of NaClO Solution
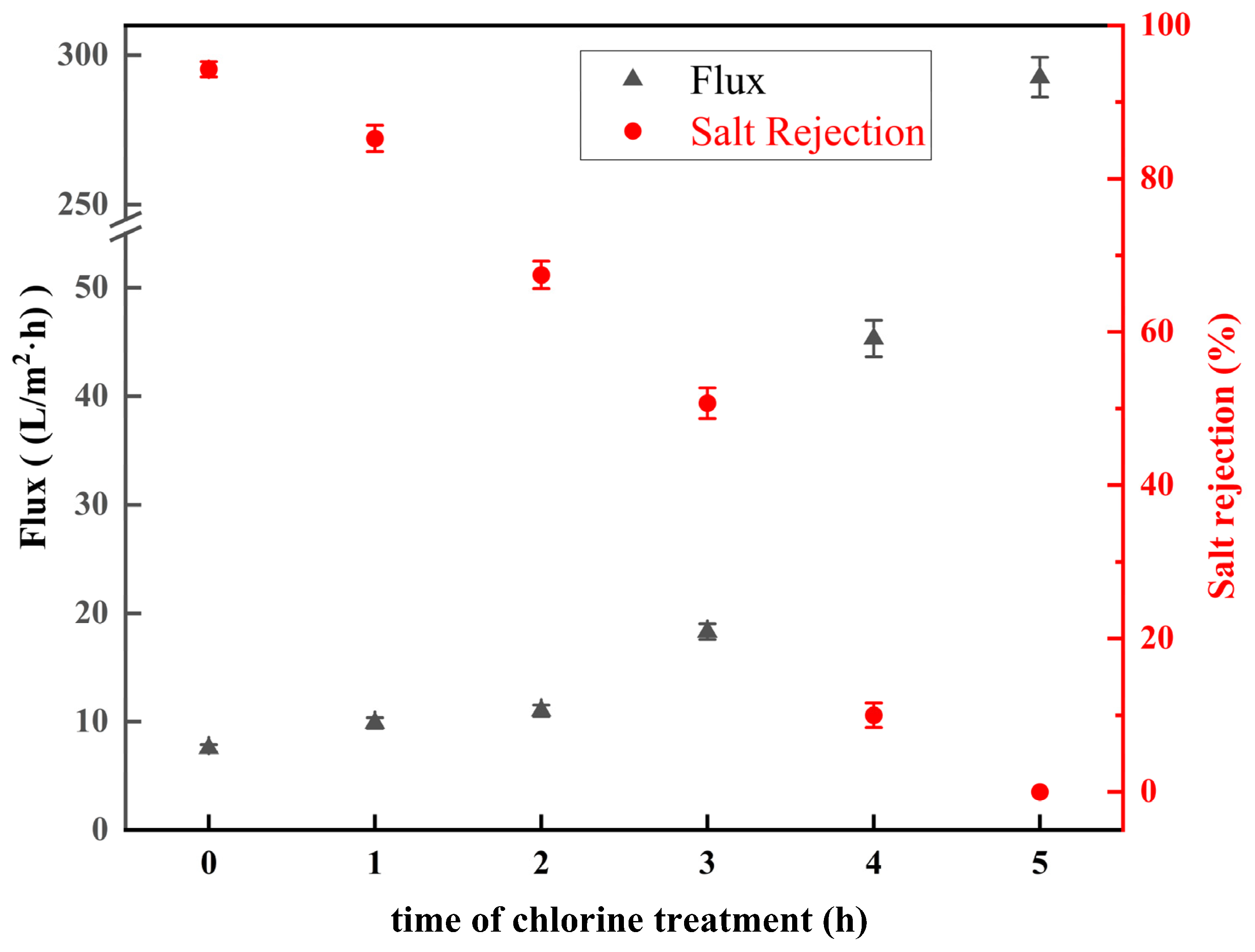
3.3.3. Effect of Chlorination Time with NaClO Solution
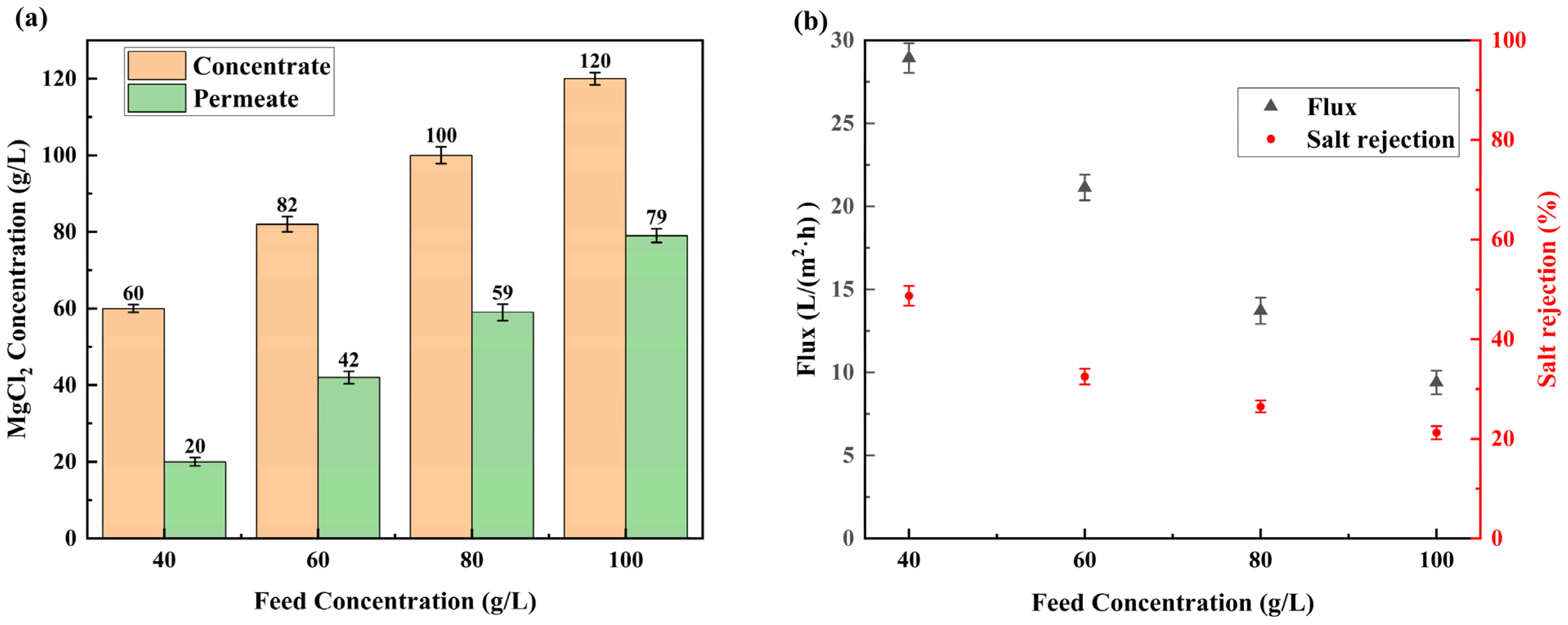
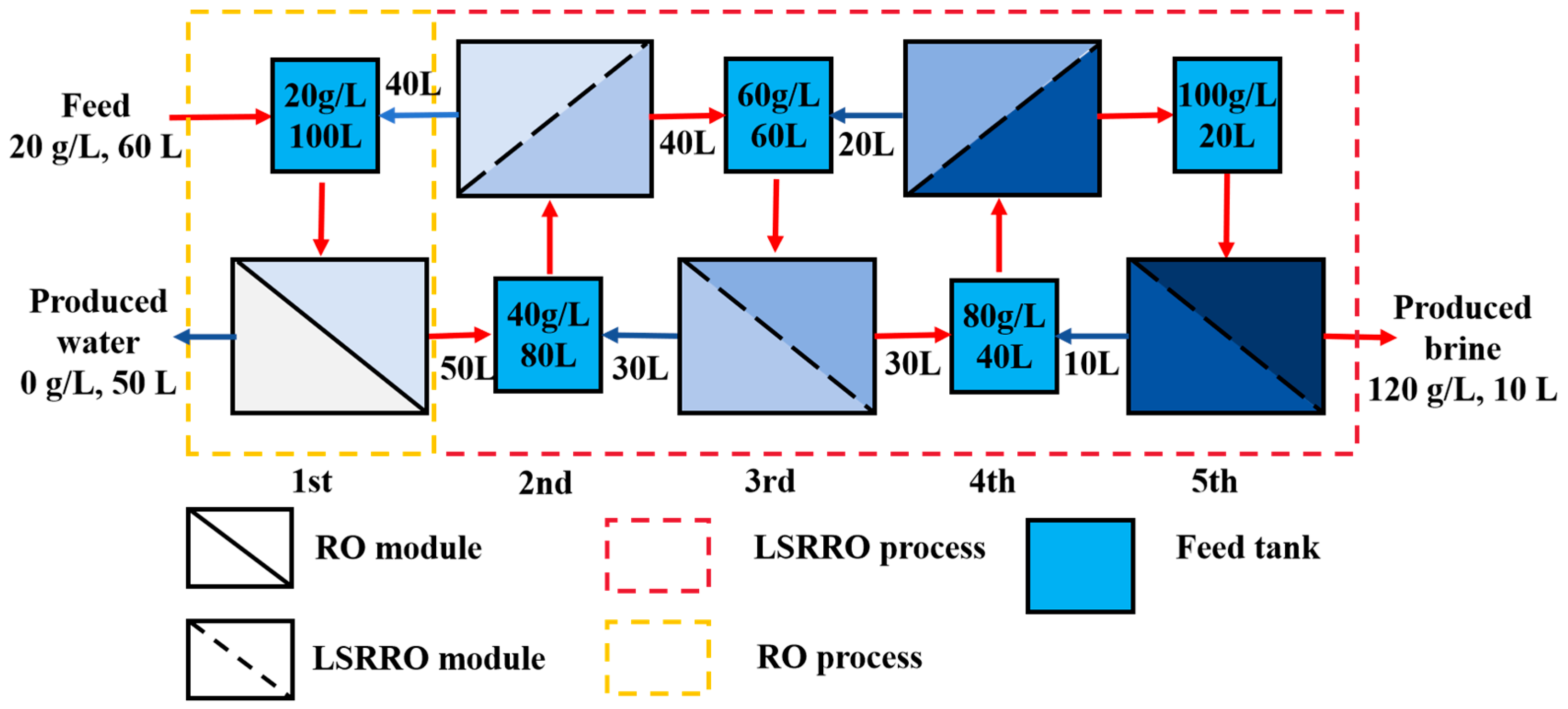
3.4. Multi-Stage LSRRO of Low Concentration MgCl2 Solution
3.5. Challenges and Future Outlook
4. Conclusions
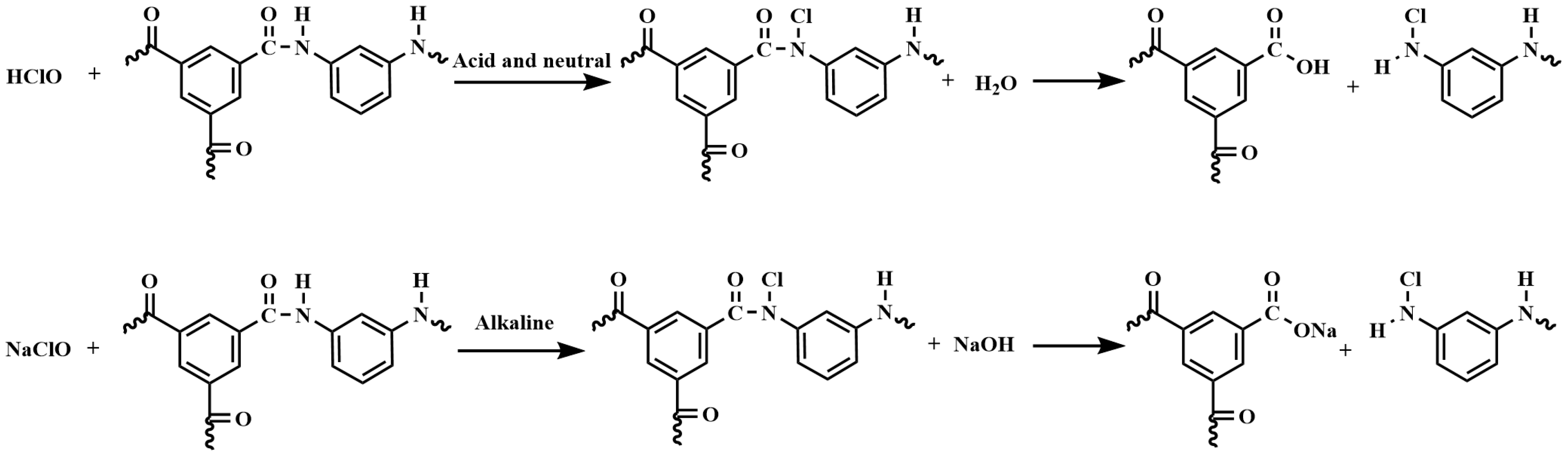
- The reaction mechanism of the NaClO treatment on polyamide RO membranes involved the chlorination and hydrolysis of polyamide into carboxylic acid and amine groups. Since the carboxylic acid groups were insoluble in water in acidic and neutral environments, and dissolved in water in an alkaline environment, the crosslinking degree and salt rejection of the membrane were lower under the alkaline conditions. Low-salt-rejection membranes can be prepared under alkaline conditions. A higher concentration of the NaClO solution can strengthen the reaction process, and the increase in reaction time had a significant effect.
- Under acidic conditions, the ridge-and-valley topography on the membrane surface became more pronounced, and surface roughness increased. In contrast, under alkaline conditions, the ridge-and-valley topography and surface roughness reduced, which resulted in a higher water flux. The morphology of the membranes did not change significantly with the increasing NaClO concentration. Furthermore, with a prolonged reaction time, the ridge-and-valley topography and surface roughness were further reduced. Upon reaching 4 h exposure, almost all of the polyamide active layer had undergone degradation.
- The salt rejection of the membrane decreased with the increase in the pH, concentration, and reaction time of the NaClO solution. The water flux of the membrane increased with the increase in the pH, concentration, and reaction time of the NaClO solution. The salt rejection of the RO membrane prepared by treating for 3 h at a NaClO concentration of 15 g/L and a pH of 11 was 50.7%. Further, the salt rejection and water flux of the low desalination membrane could be regulated by changing the treatment concentration and chlorination time to realize the controllable preparation of the low desalination membrane.
- A five-stage LSRRO process was designed based on experimental data at the level of actual process practice. The results demonstrated significant effectiveness in concentrating the MgCl2 solution by increasing its concentration from 20 g/L to 120 g/L under 5 MPa. This concentration was substantially higher than the theoretical maximum of 64 g/L achievable with traditional RO membranes at 5 MPa, representing an 87% improvement. The SEC for concentrating a 20 g/L solution of magnesium chloride to 120 g/L at a pressure of 5 MPa was calculated as 4.17 kWh/m3, which was decreased by about 80% compared to that of MVR. These findings provide evidence of the superior performance of LSRRO technology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, X.W.; Long, Z.Q.; Wang, L.S.; Feng, Z.Y. Technology development for rare earth cleaner hydrometallurgy in China. Rare Met. 2015, 34, 215–222. [Google Scholar] [CrossRef]
- Wu, S.X.; Zhao, L.S.; Wang, L.S.; Huang, X.W.; Zhang, Y.H.; Feng, Z.Y.; Cui, D.L. Simultaneous recovery of rare earth elements and phosphorus from phosphate rock by phosphoric acid leaching and selective precipitation: Towards green process. J. Rare Earths 2019, 37, 652–658. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Zhang, D.; Gao, K.; Xu, W.; Wang, H.; Geng, J.; Ma, X.; Huang, L. Clean production technology of selective decomposition of Bayan Obo rare earth concentrate by NaOH. J. Clean. Prod. 2019, 236, 117616. [Google Scholar] [CrossRef]
- Wang, M.; Huang, X.W.; Xia, C.; Feng, Z.Y.; Xu, Y.; Meng, D.L.; Peng, X.L. Efficient preparation of magnesium bicarbonate from magnesium sulfate solution and saponification-extraction for rare earth separation. Trans. Nonferrous Met. Soc. China 2023, 33, 584–595. [Google Scholar] [CrossRef]
- Feng, Z.Y.; Huang, X.W.; Wang, M.; Zhang, G.C. Progress and Trend of Green Chemistry in Extraction and Separation of Typical Rare Earth Resources. Chin. J. Rare Metals 2017, 41, 604–612. [Google Scholar]
- Feng, Z.Y.; Wang, M.; Zhao, L.; Xu, Y.; Zhang, Y.; Huang, X. Development Status and Prospect of Rare Earth Extraction and Separation Technology. J. Chin. Soc. Rare Earths 2021, 39, 469–478. [Google Scholar]
- Si, Z.; Han, D.; Xiang, J. Experimental investigation on the mechanical vapor recompression evaporation system coupled with multiple vacuum membrane distillation modules to treat industrial wastewater. Sep. Purif. Technol. 2021, 275, 119178. [Google Scholar] [CrossRef]
- Al-Othman, A.; Tawalbeh, M.; Assad, M.E.H.; Alkayyali, T.; Eisa, A. Novel multi-stage flash (MSF) desalination plant driven by parabolic trough collectors and a solar pond: A simulation study in UAE. Desalination 2018, 443, 237–244. [Google Scholar] [CrossRef]
- Razmi, A.; Soltani, M.; Tayefeh, M.; Torabi, M.; Dusseault, M.B. Thermodynamic analysis of compressed air energy storage (CAES) hybridized with a multi-effect desalination (MED) system. Energy Convers. Manag. 2019, 199, 112047. [Google Scholar] [CrossRef]
- Qasim, M.; Badrelzaman, M.; Darwish, N.N.; Darwish, N.A.; Hilal, N. Reverse osmosis desalination: A state-of-the-art review. Desalination 2019, 459, 59–104. [Google Scholar] [CrossRef]
- Ali, A.; Tufa, R.A.; Macedonio, F.; Curcio, E.; Drioli, E. Membrane technology in renewable-energy-driven desalination. Renew. Sustain. Energy Rev. 2018, 81, 1–21. [Google Scholar] [CrossRef]
- Tang, C.Y.; Yang, Z.; Guo, H.; Wen, J.J.; Nghiem, L.D.; Cornelissen, E. Potable Water Reuse through Advanced Membrane Technology. Environ. Sci. Technol. 2018, 52, 10215–10223. [Google Scholar] [CrossRef] [PubMed]
- Goh, P.S.; Lau, W.J.; Othman, M.H.D.; Ismail, A.F. Membrane fouling in desalination and its mitigation strategies. Desalination 2018, 425, 130–155. [Google Scholar] [CrossRef]
- Lim, Y.J.; Goh, K.; Kurihara, M.; Wang, R. Seawater desalination by reverse osmosis: Current development and future challenges in membrane fabrication? A review. J. Membr. Sci. 2021, 629, 119292. [Google Scholar] [CrossRef]
- Kim, J.; Park, K.; Yang, D.R.; Hong, S. A comprehensive review of energy consumption of seawater reverse osmosis desalination plants. Appl. Energy 2019, 254, 113652. [Google Scholar] [CrossRef]
- Panagopoulos, A. Techno-economic assessment of minimal liquid discharge (MLD) treatment systems for saline wastewater (brine) management and treatment. Process Saf. Environ. Prot. 2021, 146, 656–669. [Google Scholar] [CrossRef]
- Davenport, D.M.; Deshmukh, A.; Werber, J.R.; Elimelech, M. High-pressure reverse osmosis for energy-efficient hypersaline brine desalination: Current status, design considerations, and Research needs. Environ. Sci. Technol. Lett. 2018, 5, 467–475. [Google Scholar] [CrossRef]
- Bartholomew, T.V.; Mey, L.; Arena, J.T.; Siefert, N.S.; Mauter, M.S. Osmotically assisted reverse osmosis for high salinity brine treatment. Desalination 2017, 421, 3–11. [Google Scholar] [CrossRef]
- Ibrar, I.; Altaee, A.; Zhou, J.L.; Naji, O.; Khanafer, D. Challenges and potentials of forward osmosis process in the treatment of wastewater. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1339–1383. [Google Scholar] [CrossRef]
- Al-Amshawee, S.; Yunus, M.Y.; Azoddein, A.A.; Hassell, D.G.; Dakhil, I.H.; Hasan, H.A. Electrodialysis desalination for water and wastewater: A review. Chem. Eng. J. 2020, 380, 122231. [Google Scholar] [CrossRef]
- Wang, Z.; Deshmukh, A.; Du, Y.; Elimelech, M. Minimal and zero liquid discharge with reverse osmosis using low-salt-rejection membranes. Water Res. 2020, 170, 115317. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Togo, N.; Takagi, R.; Shintani, T.; Yoshioka, T.; Kamio, E.; Matsuyama, H. Multistage osmotically assisted reverse osmosis process for concentrating solutions using hollow fiber membrane modules. Chem. Eng. Res. Des. 2020, 162, 117–124. [Google Scholar] [CrossRef]
- Atia, A.A.; Yip, N.Y.; Fthenakis, V. Pathways for minimal and zero liquid discharge with enhanced reverse osmosis technologies: Module-scale modeling and techno-economic assessment. Desalination 2021, 509, 115069. [Google Scholar] [CrossRef]
- Bargeman, G. Maximum allowable retention for low-salt-rejection reverse osmosis membranes and its effect on concentrating undersaturated NaCl solutions to saturation. Sep. Purif. Technol. 2023, 317, 123854. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.; Chen, Y. A theoretical analysis on upgrading desalination plants with low-salt-rejection reverse osmosis. Desalination 2023, 565, 116827. [Google Scholar] [CrossRef]
- Du, Y.; Wang, Z.; Cooper, N.J.; Gilron, J.; Elimelech, M. Module-scale analysis of low-salt-rejection reverse osmosis: Design guidelines and system performance. Water Res. 2022, 209, 117936. [Google Scholar] [CrossRef]
- Du, Y.; Wang, L.; Belgada, A.; Younssi, S.A.; Gilron, J.; Elimelech, M. A mechanistic model for salt and water transport in leaky membranes: Implications for low-salt-rejection reverse osmosis membranes. J. Membr. Sci. 2023, 678, 121642. [Google Scholar] [CrossRef]
- Madduri, S.; Sodaye, H.S.; Debnath, A.K.; Adak, A.K.; Prasad, T.L. Transformation of brackish water Reverse Osmosis membranes to nanofiltration & ultrafiltration membranes by NaOCl treatment: Kinetic and characterization studies. J. Water Process Eng. 2023, 56, 104549. [Google Scholar]
- Garcia-Pacheco, R.; Landaburu-Aguirre, J.; Molina, S.; Rodriguez-Saez, L.; Teli, S.B.; Garcia-Calvo, E. Transformation of end-of-life RO membranes into NF and UF membranes: Evaluation of membrane performance. J. Membr. Sci. 2015, 495, 305–315. [Google Scholar] [CrossRef]
- Atia, A.A.; Allen, J.; Young, E.; Knueven, B.; Bartholomew, T.V. Cost optimization of low-salt-rejection reverse osmosis. Desalination 2023, 551, 116407. [Google Scholar] [CrossRef]
- Wang, Z.; Feng, D.; Chen, Y.; He, D.; Elimelech, M. Comparison of Energy Consumption of Osmotically Assisted Reverse Osmosis and Low-Salt-Rejection Reverse Osmosis for Brine Management. Environ. Sci. Technol. 2021, 55, 10714–10723. [Google Scholar] [CrossRef] [PubMed]
- Verbeke, R.; Gomez, V.; Koschine, T.; Eyley, S.; Szymczyk, A.; Dickmann, M.; Stimpel-Lindner, T.; Egger, W.; Thielemans, W.; Vankelecom, I.F. Real-scale chlorination at pH4 of BW30 TFC membranes and their physicochemical characterization. J. Membr. Sci. 2018, 551, 123–135. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Lejarazu-Larrañaga, A.; Rodríguez-Sáez, L.; Molina, S.; Ransome, T.; García-Calvo, E. Free chlorine exposure dose (ppm.h) and its impact on RO membranes ageing and recycling potential. Desalination 2019, 457, 133–143. [Google Scholar] [CrossRef]
- Yang, L.; Yu, H.; Zhao, H.; Xia, C.; Yu, Q.; Chen, X.; Cao, G.; Cai, L.; Meng, S.; Tang, C.Y. Degradation of polyamide nanofiltration membranes by free chlorine and halide ions: Kinetics, mechanisms, and implications. Water Res. 2025, 272, 122963. [Google Scholar] [CrossRef]
- Donose, B.C.; Sukumar, S.; Pidou, M.; Poussade, Y.; Keller, J.; Gernjak, W. Effect of pH on the ageing of reverse osmosis membranes upon exposure to hypochlorite. Desalination 2013, 309, 97–105. [Google Scholar] [CrossRef]
- You, M.; Feng, G.; Fei, P.; Zhang, Y.; Cao, Z.; Xia, J.; Lau, W.J.; Meng, J. Probing and relating the morphology, structure and performance evolution of low pressure RO membranes under chlorine exposure. J. Environ. Chem. Eng. 2021, 9, 106223. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.; Feng, Z.; Zhao, L.; Li, Z.; Wang, M.; Xia, C. Preparation of Low-Salt-Rejection Membrane by Sodium Hypochlorite Chlorination for Concentration of Low-Concentration Magnesium Chloride Solution. Materials 2025, 18, 2824. https://doi.org/10.3390/ma18122824
Wu Z, Feng Z, Zhao L, Li Z, Wang M, Xia C. Preparation of Low-Salt-Rejection Membrane by Sodium Hypochlorite Chlorination for Concentration of Low-Concentration Magnesium Chloride Solution. Materials. 2025; 18(12):2824. https://doi.org/10.3390/ma18122824
Chicago/Turabian StyleWu, Zhengyang, Zongyu Feng, Longsheng Zhao, Zheng Li, Meng Wang, and Chao Xia. 2025. "Preparation of Low-Salt-Rejection Membrane by Sodium Hypochlorite Chlorination for Concentration of Low-Concentration Magnesium Chloride Solution" Materials 18, no. 12: 2824. https://doi.org/10.3390/ma18122824
APA StyleWu, Z., Feng, Z., Zhao, L., Li, Z., Wang, M., & Xia, C. (2025). Preparation of Low-Salt-Rejection Membrane by Sodium Hypochlorite Chlorination for Concentration of Low-Concentration Magnesium Chloride Solution. Materials, 18(12), 2824. https://doi.org/10.3390/ma18122824




