Spark Plasma Sintering and Electrospark Deposition of High Entropy Alloys with Elemental Variation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Spark Plasma Sintering—HEA Consolidation
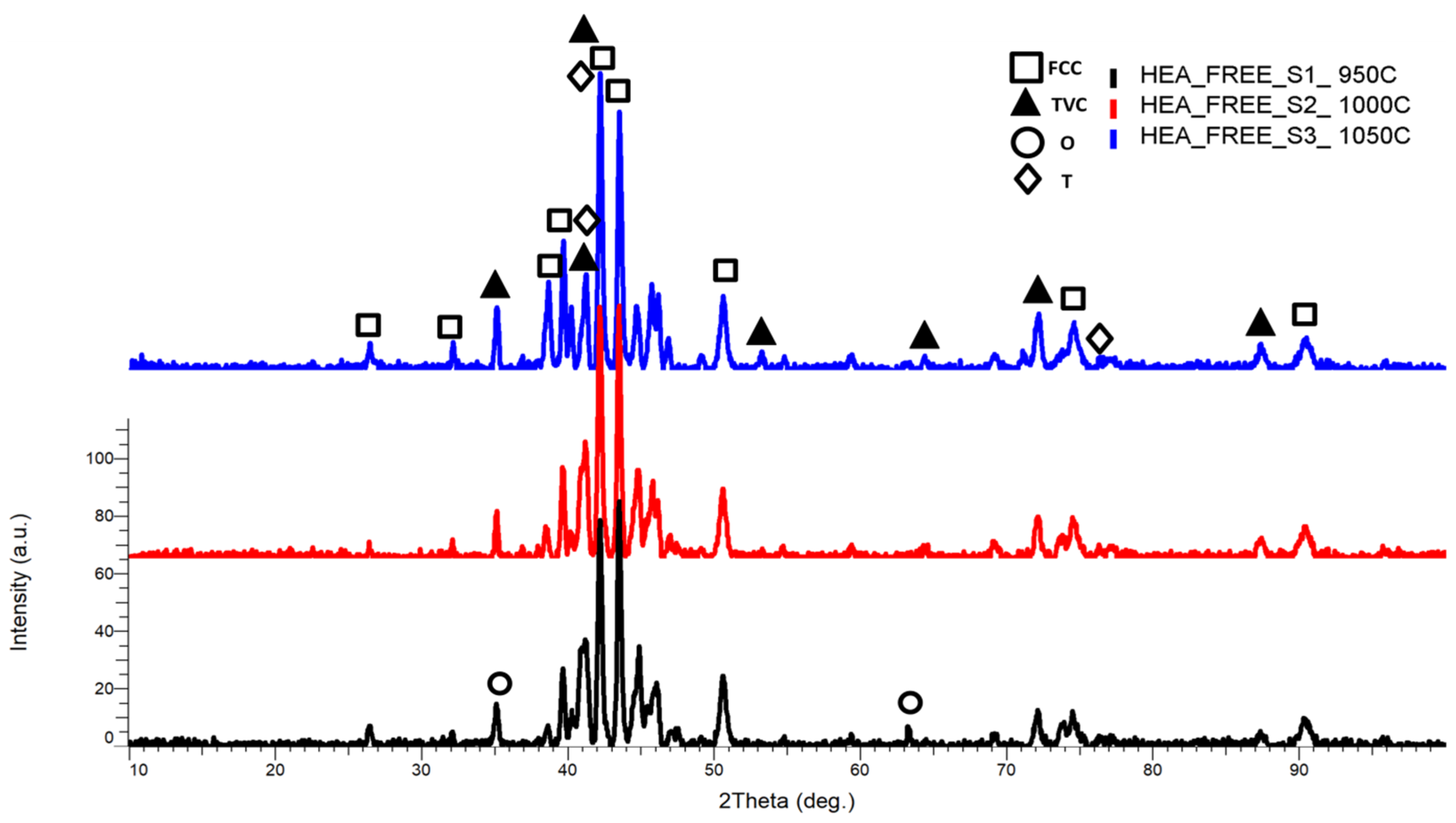
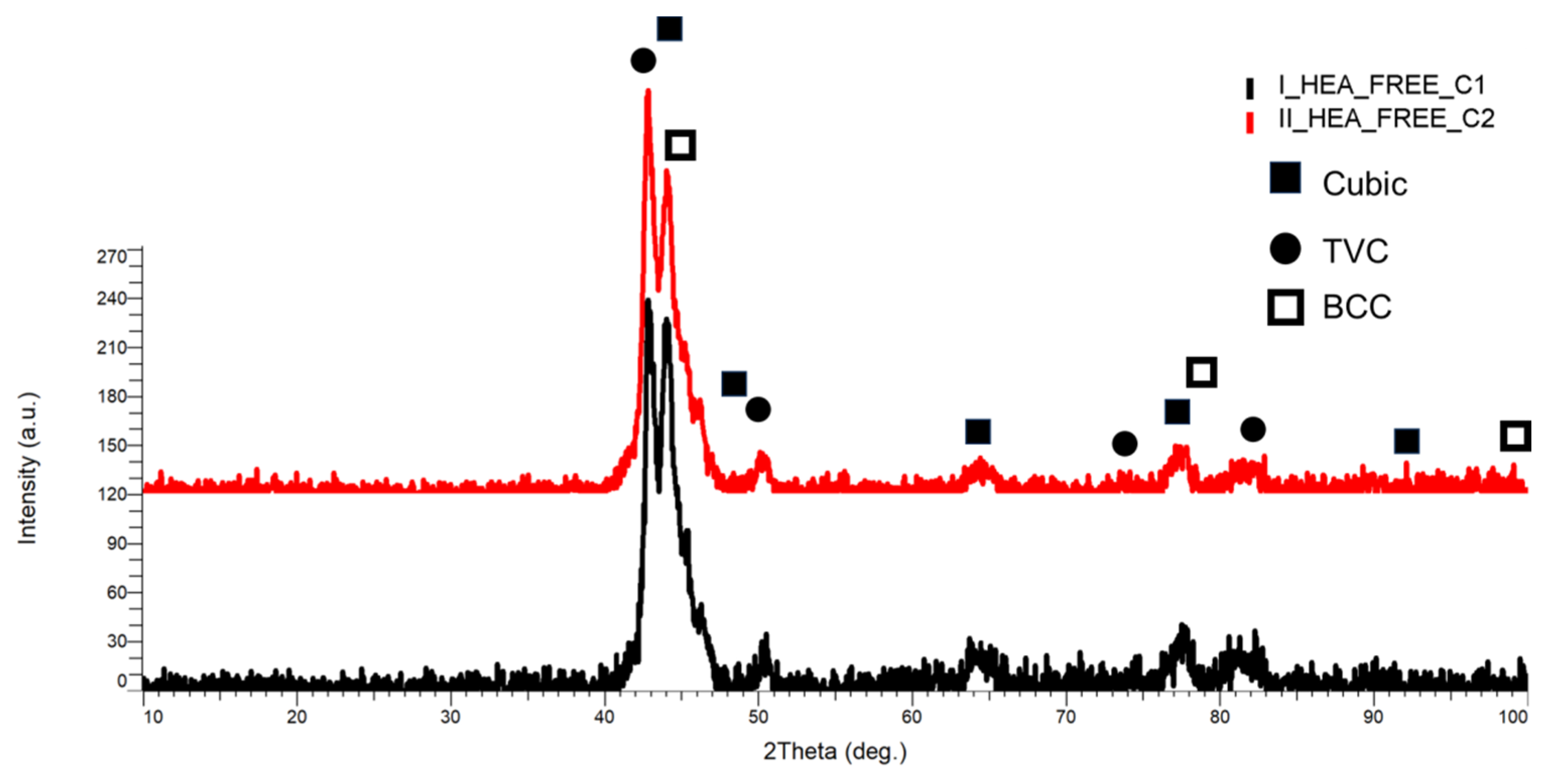
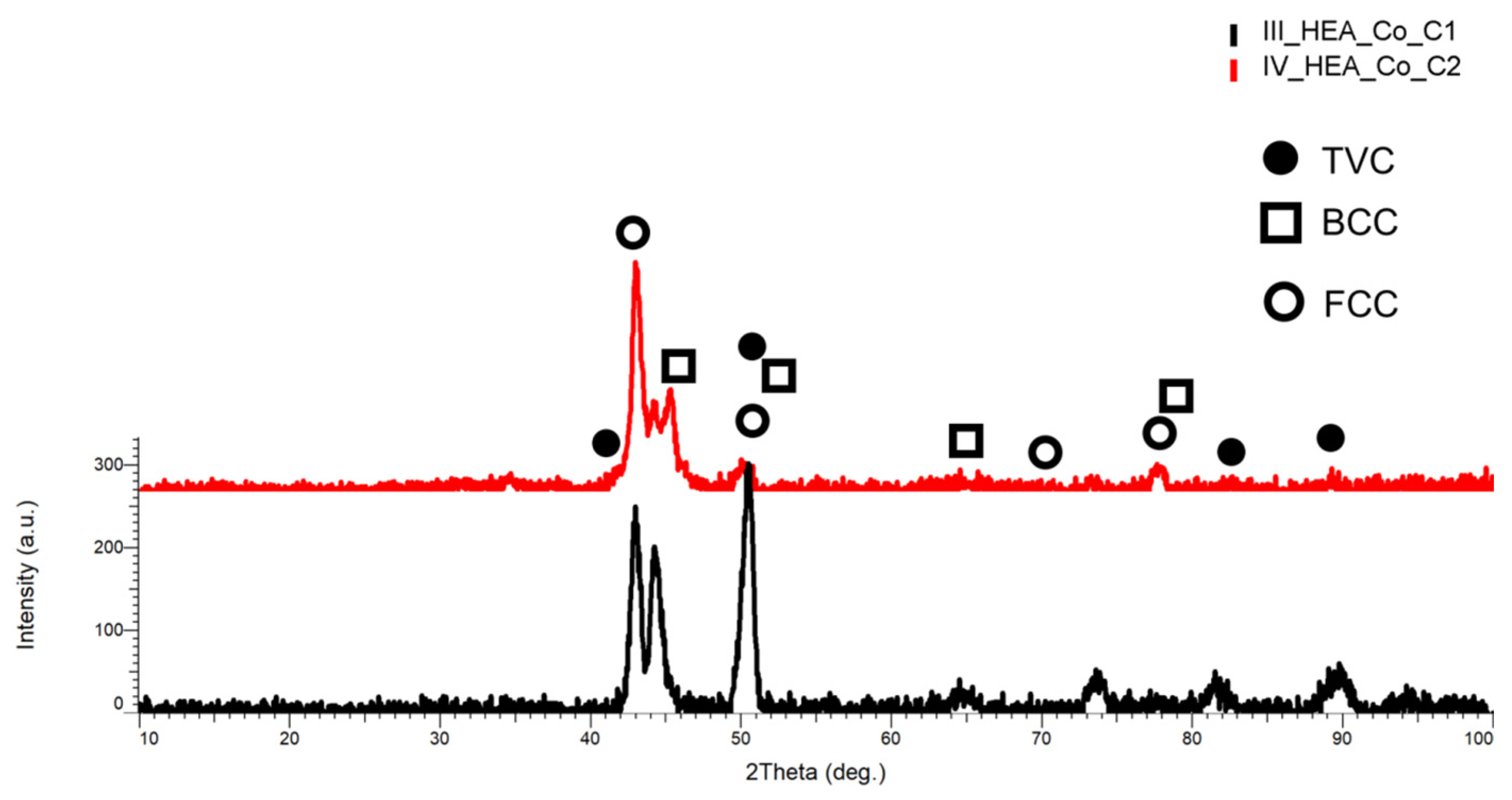
3.1.1. CrFeNiMo
3.1.2. Co0.5CrFeNiMo
3.1.3. Al0.5CrFeNiMo
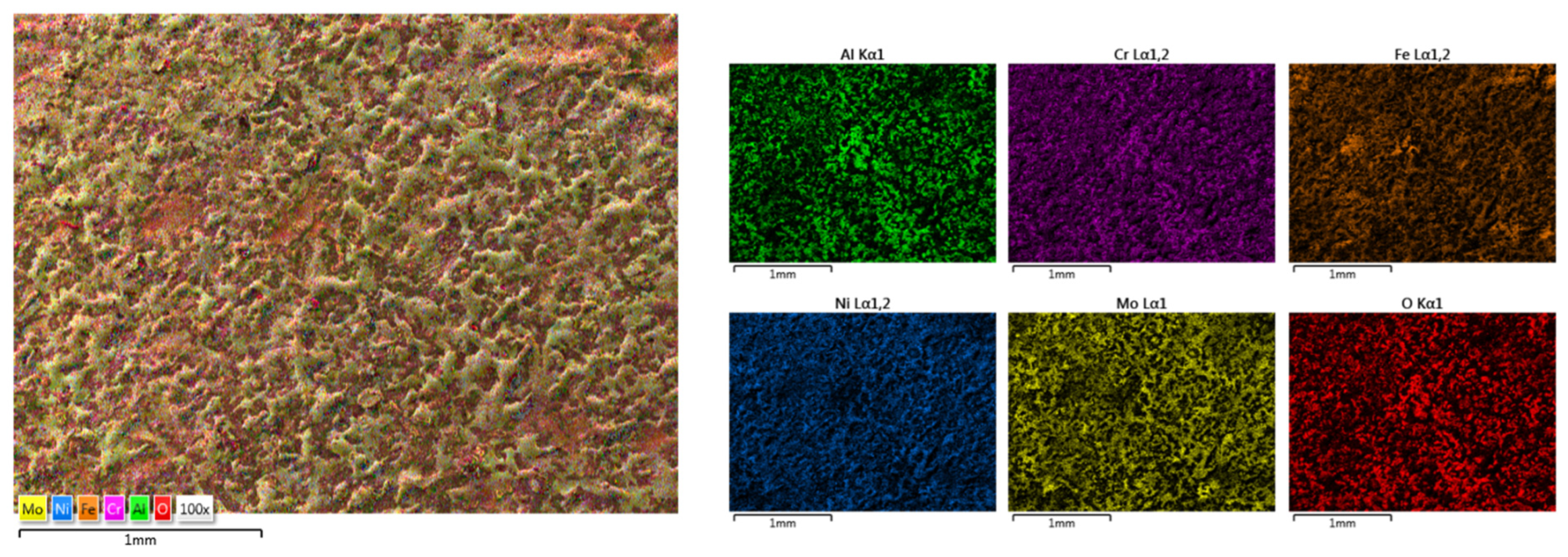
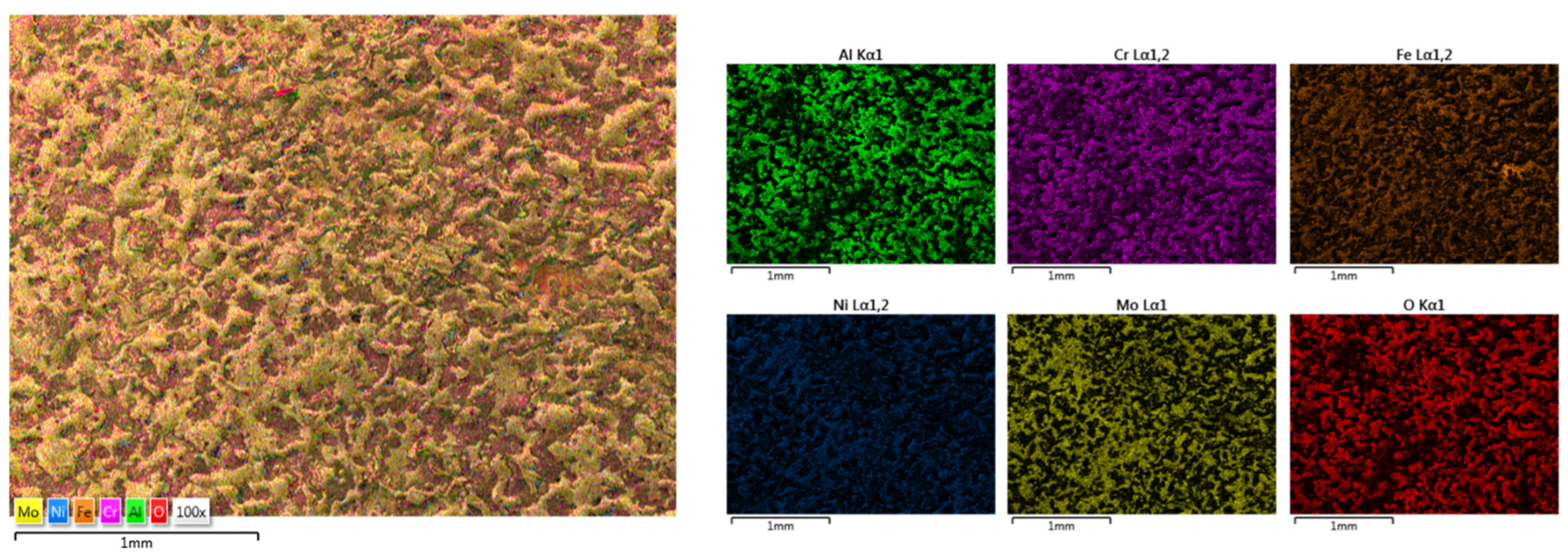
3.2. HEA Coatings Obtained by ElectroSpark Deposition (ESD)
3.2.1. CrFeNiMo
3.2.2. Co0.5CrFeNiMo
3.2.3. Al0.5CrFeNiMo
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campanelli, S.L.; Angelastro, A.; Signorile, C.G.; Casalino, G. Investigation on direct laser powder deposition of 18 Ni (300) marage steel using mathematical model and experimental characterization. J. Adv. Manuf. Technol. 2017, 89, 885–895. [Google Scholar] [CrossRef]
- Angelastro, A.; Campanelli, S.L.; Casalino, G. Statistical analysis and optimization of direct metal laser deposition of 227-F Colmonoy nickel alloy. Opt. Laser Technol. 2017, 94, 138–145. [Google Scholar] [CrossRef]
- Frangini, S.; Masci, A. A study on the effect of a dynamic contact force control for improving electrospark coating properties. Surf. Coat. Technol. 2010, 204, 2613–2623. [Google Scholar] [CrossRef]
- Rakhadilov, B.; Magazov, N.; Kakimzhanov, D.; Apsezhanova, A.; Molbossynov, Y.; Kengesbekov, A. Influence of Spraying Process Parameters on the Characteristics of Steel Coatings Produced by Arc Spraying Method. Coatings 2024, 14, 1145. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, Y.; Chen, S.; Duan, J.; Li, J. Research on Film Formation Characteristics by Spraying on Unidiameter Vertical Interpenetrating Cylindrical Surfaces. Coatings 2024, 14, 847. [Google Scholar] [CrossRef]
- Aristia, G.; Hoa, L.Q.; Bäßler, R. Corrosion of Carbon Steel in Artificial Geothermal Brine: Influence of Carbon Dioxide at 70 °C and 150 °C. Materials 2019, 12, 3801. [Google Scholar] [CrossRef]
- Thorhallsson, S. Common problems faced in geothermal generation and how to deal with them. In Workshop for Decision Makers on Geothermal Projects and Management Proceedings; UNU-GTP: Naivasha, Kenya, 2005. [Google Scholar]
- Li, Z.; Sun, M.; Zhang, H.; Xu, W.; Wu, L. Corrosion fatigue performance of modified 17-4PH in simulated geothermal environment. Front. Mater. 2024, 11, 1342276. [Google Scholar] [CrossRef]
- Ghaini, M.F.; Sheikhi, M.; Torkamany, M.J.; Sabbaghzadeh, J. The relation between liquation and solidification cracks in pulsed laser welding of 2024 aluminium alloy. Mater. Sci. Eng. A 2009, 519, 167–171. [Google Scholar] [CrossRef]
- Yan, F.; Yan, J.; Linder, D. Understanding Hot Cracking of Steels during Rapid Solidification: An ICME Approach. Mater. Proc. 2021, 3, 30. [Google Scholar]
- Del Guercio, G.; Faron, S.; McCartney, D.G.; Robertson, S.; Aboulkhair, N.T.; Tuck, C.; Simonelli, M. Experimental and computational studies on hot cracking in single laser tracks of aluminium alloy AA2024 and related implications for laser powder bed fusion. Discov. Mater. 2024, 4, 83. [Google Scholar] [CrossRef]
- Verbitchi, V.; Ciuca, C.; Cojocaru, R. Electro-Spark Coating with Special Materials. Nonconv. Technol. Rev. 2011, 1, 57–62. [Google Scholar]
- Barile, C.; Casavola, C.; Pappalettera, G.; Renna, G. Advancements in Electrospark Deposition (ESD) Technique: A Short Review. Coatings 2022, 12, 1536. [Google Scholar] [CrossRef]
- Kostadinov, G.; Penyashki, T.; Nikolov, A.; Vencl, A. Improving the Surface Quality and Tribological Characteristics of 3D-Printed Titanium Parts through Reactive Electro-Spark Deposition. Materials 2024, 17, 382. [Google Scholar] [CrossRef] [PubMed]
- Nikolov, A.; Kostadinov, G.; Penyashki, T. Possibilities for Improving Surface Defects of 3D Printed Titanium Products Through Reactive Electro-Spark Processing. Mater. Methods Technol. 2023, 17, 1–13. [Google Scholar] [CrossRef]
- Brochu, M.; Portillo, J.G.; Milligan, J.; Head, D.W. Development of Metastable Solidification Structures Using the Electrospark Deposition Process. Open Surf. Sci. J. 2011, 3, 105–114. [Google Scholar] [CrossRef]
- Perju, M.C.; Vizureanu, P.; Nejneru, C. The study of energy transfer on thin layers achieved by electro-spark deposition with tic electrode. In Proceedings of the International Conference of Scientific Paper Afases, Brasov, Romania, 22–24 May 2014. [Google Scholar]
- Radek, N.; Pietraszek, J.; Klimecka-Tatar, D. Production of Zinc Coatings By Electro-Spark Deposition. Syst. Saf. Hum. Tech. Facil. Environ. 2020, 2, 253–258. [Google Scholar]
- Wang, J.; Zhang, M.; Dai, S.; Zhu, L. Research Progress in Electrospark Deposition Coatings on Titanium Alloy Surfaces: A Short Review. Coatings 2023, 13, 1473. [Google Scholar] [CrossRef]
- Geng, C.; Zhang, H.; Li, X.; Geng, H. Elevated temperature tensile behaviour of 5183 aluminium alloy made by electrospark deposition additive manufacturing. Mater. Sci. Eng. A 2023, 868, 144746. [Google Scholar] [CrossRef]
- Cadney, S.; Brochu, M. Formation of amorphous Zr41.2Ti13.8Ni10Cu12.5Be22.5 coatings via the ElectroSpark Deposition process. Intermetallics 2008, 16, 518–523. [Google Scholar] [CrossRef]
- Boakye, G.O.; Geambazu, L.E.; Ormsdottir, A.M.; Gunnarsson, B.G.; Csaki, I.; Fanicchia, F.; Kovalov, S.; Karlsdóttir, S.N. Microstructural Properties and Wear Resistance of Fe-Cr-Co-Ni-Mo-Based High Entropy Alloy Coatings Deposited with Different Coating Techniques. Appl. Sci. 2022, 12, 3156. [Google Scholar] [CrossRef]
- Manea, C.A.; Sohaciu, M.; Stefănoiu, R.; Petrescu, I.M.; Lungu, M.V.; Csaki, I. New HfNbTaTiZr high entropy alloy coatings produced by electro spark deposition with high corrosion resistance. Materials 2021, 14, 4333. [Google Scholar] [CrossRef] [PubMed]
- Kuptsov, K.A.; Antonyuk, M.N.; Sheveyko, A.N.; Bondarev, A.V.; Ignatov, S.G.; Slukin, P.V.; Dwivedi, P.; Fraile, A.; Polcar, T.; Shtansky, D.V. High-entropy Fe-Cr-Ni-Co-(Cu) coatings produced by vacuum electro-spark deposition for marine and coastal applications. Surf. Coat. Technol. 2023, 453, 129136. [Google Scholar] [CrossRef]
- Chandrakant; Reddy, N.S.; Panigrahi, B.B. Electro spark coating of AlCoCrFeNi high entropy alloy on AISI410 stainless steel. Mater. Lett. 2021, 304, 130580. [Google Scholar] [CrossRef]
- Yeh, J.W. Recent progress in high-entropy alloys. Eur. J. Control 2006, 31, 633–648. [Google Scholar] [CrossRef]
- Zhang, G.; Ming, K.; Kang, J.; Huang, Q.; Zhang, Z.; Zheng, X.; Bi, X. High entropy alloy as a highly active and stable electrocatalyst for hydrogen evolution reaction. Electrochim. Acta 2018, 279, 19–23. [Google Scholar] [CrossRef]
- Gao, M. Development of new high entropy alloys for brazing of Ni-base superalloys. In 2017-Mines Masters Theses & Dissertations; Colorado School of Mines: Golden, CO, USA, 2017. [Google Scholar]
- Bridges, D. Nanobrazing of Inconel 718 and Ti-6Al-4V. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 2019. [Google Scholar]
- Jiang, N.; Bian, H.; Song, X.; Wang, M.; Lin, D.; Long, W.; Zhong, S.; Jia, L. Contact-reactive brazing of CoCrFeMnNi high-entropy alloy to Zr alloys using Cu interlayer. Mater. Charact. 2023, 204, 113186. [Google Scholar] [CrossRef]
- Fieser, D.; Lan, Y.; Gulino, A.; Compagnini, G.; Aaron, D.; Mench, M.; Bridges, D.; Shortt, H.; Liaw, P.; Hu, A. Synthesis and Unique Behaviors of High-Purity HEA Nanoparticles Using Femtosecond Laser Ablation. Nanomaterials 2024, 14, 554. [Google Scholar] [CrossRef]
- Wang, G.; Sheng, G.; Yu, Q.; Yuan, X.; Sun, J.; Jiao, Y.; Zhang, Y. Investigation of intergranular penetration behavior in CrMnFeCoNi HEA/304 SS dissimilar brazing joints. Intermetallics 2020, 126, 106940. [Google Scholar] [CrossRef]
- Li, G.; Meng, X.; Geng, C.; Wang, C.; Ren, H.; Guo, X.; Li, S.; Tao, Y. Microstructure and Properties of AlxCr1−xCoFeNi High-Entropy Alloys Prepared by Spark Plasma Sintering. Materials 2025, 18, 755. [Google Scholar] [CrossRef]
- Yan, J.; Chan, K.; Peng, P. Fabrication of High-Entropy Alloy Coatings Using Electro-spark Deposition. In Proceedings of the 62nd Conference of Metallurgists, Toronto, ON, Canada, 21–24 August 2023; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Yan, J.; Lopes, J.G.; Chan, K.; Scotchmer, N.; Oliveira, J.P.; Zhou, Y.N.; Peng, P. Microstructure characterization of AlCrFeCoNi high-entropy alloy coating on Inconel 718 by electrospark deposition. Mater. Charact. 2025, 225, 115139. [Google Scholar] [CrossRef]
- Ron, T.; Shirizly, A.; Aghion, E. Additive Manufacturing Technologies of High Entropy Alloys (HEA): Review and Prospects. Materials 2023, 16, 2454. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Fu, Z.; Wang, W.; Wang, H.; Zhang, J.; Wang, Y.; Zhang, F. Mechanical alloying synthesis and spark plasma sintering consolidation of CoCrFeNiAl high-entropy alloy. J. Alloys Compd. 2014, 589, 61–66. [Google Scholar] [CrossRef]
- Arun, S.; Radhika, N.; Saleh, B. Advances in vacuum arc melting for high entropy alloys: A review. Vacuum 2024, 226, 113314. [Google Scholar] [CrossRef]
- Guillon, O.; Gonzalez-Julian, J.; Dargatz, B.; Kessel, T.; Schierning, G.; Räthel, J.; Herrmann, M. Field-assisted sintering technology/spark plasma sintering: Mechanisms, materials, and technology developments. Adv. Eng. Mater. 2014, 16, 830–849. [Google Scholar] [CrossRef]
- Ujah, C.O.; Von Kallon, D.V.; Aigbodion, V.S. High entropy alloys prepared by spark plasma sintering: Mechanical and thermal properties. Mater. Today Sustain. 2024, 25, 100639. [Google Scholar] [CrossRef]
- Hu, Z.Y.; Zhang, Z.H.; Cheng, X.W.; Wang, F.C.; Zhang, Y.F.; Li, S.L. A review of multi-physical fields induced phenomena and effects in spark plasma sintering: Fundamentals and applications. Mater. Des. 2020, 191, 108662. [Google Scholar] [CrossRef]
- Ma, S.; Yang, Y.; Li, A.; Zhou, S.; Shi, L.; Wang, S.; Liu, M. Effects of temperature on microstructure and mechanical properties of IN718 reinforced by reduced graphene oxide through spark plasma sintering. J. Alloys Compd. 2018, 767, 675–681. [Google Scholar] [CrossRef]
- Choi, J.; Sung, H.M.; Roh, K.B.; Hong, S.H.; Kim, G.H.; Han, H.N. Fabrication of sintered tungsten by spark plasma sintering and investigation of thermal stability. Int. J. Refract. Met. Hard Mater. 2017, 69, 164–169. [Google Scholar] [CrossRef]
- Geambazu, L.E.; Manea, C.A.; Mateș, I.M.; Pătroi, D.; Sbârcea, G.B.; Manta, E.; Semenescu, A. Effect of Co and Al Content on CrFeNiMo-System High Entropy Alloys Produced by Mechanical Alloying. Materials 2025, 18, 1936. [Google Scholar] [CrossRef]
- EN ISO 6507-1:2023; Metallic Materials—Vickers Hardness Test—Part 1: Test Method. CEN: Brussels, Belgium, 2023.
- Moravcik, I.; Cizek, J.; Zapletal, J.; Kovacova, Z.; Vesely, J.; Minarik, P.; Kitzmantel, M.; Neubauer, E.; Dlouhy, I. Microstructure and mechanical properties of Ni1.5Co1.5CrFeTi0.5 high entropy alloy fabricated by mechanical alloying and spark plasma sintering. Mater. Des. 2017, 119, 141–150. [Google Scholar] [CrossRef]
- Barry, C.C.; Norton, M.G. Ceramic Materials: Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 978-0387462707. [Google Scholar]
- Schneider, S.J. Engineered Materials Handbook; Ceramics and Glasses; ASM International: Materials Park, OH, USA, 1991; Volume 4, ISBN 978-0871702823. [Google Scholar]
- Bogdanov, R.I.; Vorkel, V.A.; Ignatenko, V.E.; Gavrushina, M.A.; Voennov, A.V.; Teplyakova, S.N.; Bachurina, D.M.; Sevrukov, O.N.; Marshakov, A.I. Corrosion and electrochemical behavior of CoCrFeNiMo high-entropy alloy in acidic oxidizing and neutral chloride solutions. Mater. Chem. Phys. 2023, 295, 127123. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, M.; Wang, Q.; Qi, F.; Kong, M.; Han, B. Investigation of the Microstructure and Properties of CoCrFeNiMo High-Entropy Alloy Coating Prepared through High-Speed Laser Cladding. Coatings 2023, 13, 1211. [Google Scholar] [CrossRef]
- Karlsdottir, S.N.; Geambazu, L.E.; Csaki, I.; Thorhallsson, A.I.; Stefanoiu, R.; Magnus, F.; Cotrut, C. Phase evolution and microstructure analysis of CoCrFeNiMo high-entropy alloy for electro-spark-deposited coatings for geothermal environment. Coatings 2019, 9, 406. [Google Scholar] [CrossRef]
- The International Renewable Energy Agency (IRENA). Geopolitics of the Energy Transition: Critical Materials; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2023. [Google Scholar]
- You, X.; Xing, Z.; Jiang, S.; Zhu, Y.; Lin, Y.; Qiu, H.; Nie, R.; Yang, J.; Hui, D.; Chen, W.; et al. A review of research on aluminum alloy materials in structural engineering. Dev. Built Environ. 2024, 17, 100319. [Google Scholar] [CrossRef]
- Alleg, S.; Bekhouche, A.; Hachache, H.; Sunol, J.J. Effect of Aluminum Addition on the Microstructure, Magnetic, and Mechanical Properties of FeCrCoNiMn High-Entropy Alloy. Crystals 2023, 13, 1483. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Nosova, G.I. FCC → FCT martensitic transformation in two-phase Mn–Cu alloys. Metally 2017, 3, 216–220. [Google Scholar] [CrossRef]
- Kiryukhantsev-Korneev, P.V.; Sheveyko, A.N.; Shvindina, N.V.; Levashov, E.A.; Shtansky, D.V. Comparative study of Ti-C-Ni-Al, Ti-C-Ni-Fe, and Ti-C-Ni-Al/Ti-C-Ni-Fe coatings produced by magnetron sputtering, electro-spark deposition, and a combined two-step process. Ceram. Int. 2018, 44, 7637–7646. [Google Scholar] [CrossRef]
- Geambazu, L.E.; Cotrut, C.M.; Miculescu, F.; Csaki, I. Mechanically Alloyed CoCrFeNiMo0.85 High-Entropy Alloy for Corrosion Resistance Coatings. Materials 2021, 14, 3802. [Google Scholar] [CrossRef]
- Manea, C.A.; Ciocoiu, R.; Gheorghe, D.; Bibiş, A.; Ştefănoiu, R.; Csaki, I. Electro Spark Deposition of HfNbTaTiZr High Entropy Alloy Processed In Solid State And Experimental Adhesive Testing. U.P.B. Sci. Bull. Ser. B 2021, 83, 201–208. [Google Scholar]
- Geambazu, L.E.; Ciocoiu, R.; Miculescu, F.; Csaki, I. Coatings of CoxCrFeMoNi High Entropy Alloy Produced By Electro Spark Deposition Technique. U.P.B. Sci. Bull. Ser. B 2021, 83, 165–174. [Google Scholar]
- Wang, X.; Ju, P.; Lu, X.; Chen, Y.; Wang, F. Influence of Cr2O3 particles on corrosion, mechanical and thermal control properties of green PEO coatings on Mg alloy. Ceram. Int. 2022, 48, 3615–3627. [Google Scholar] [CrossRef]

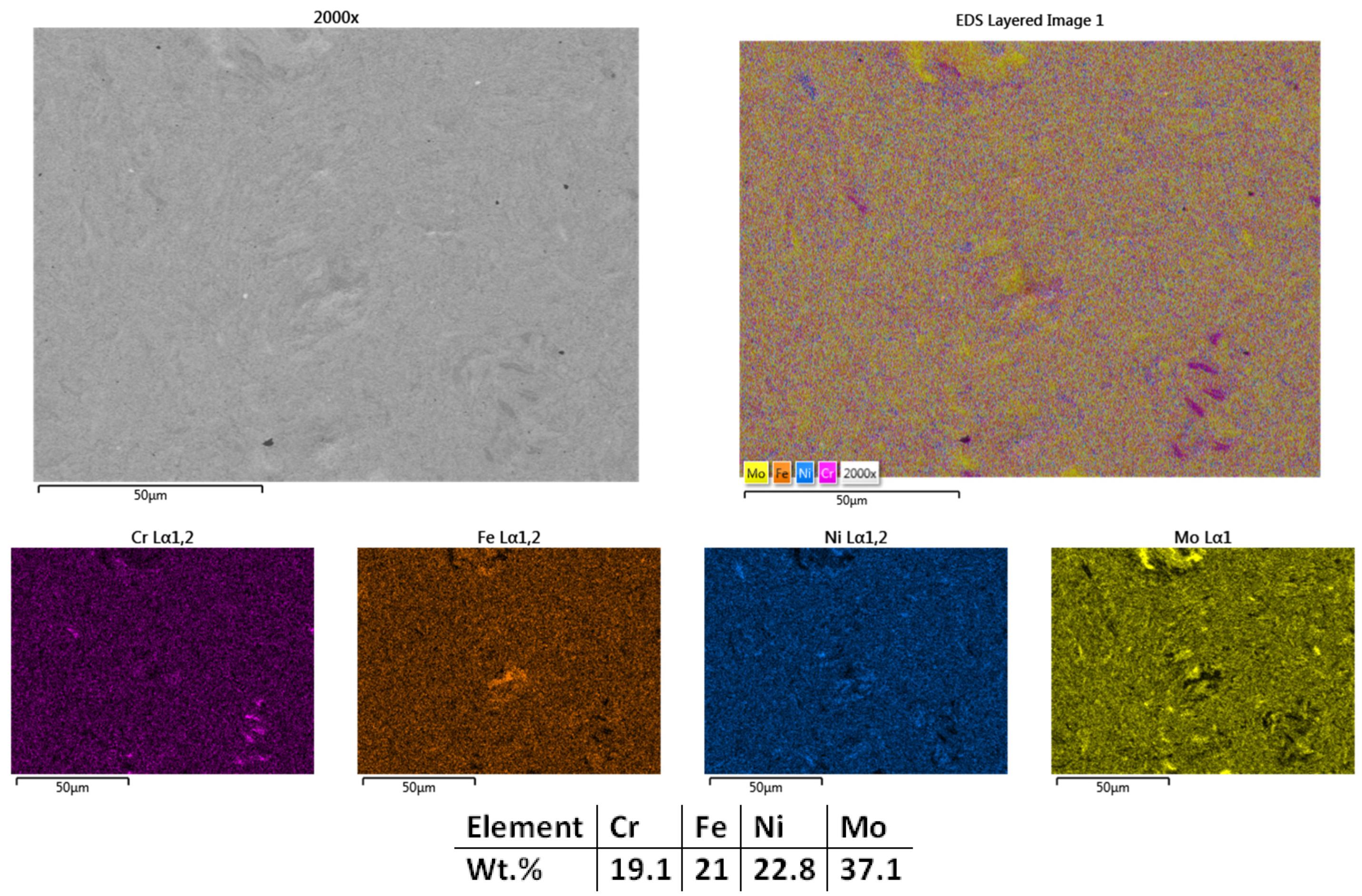
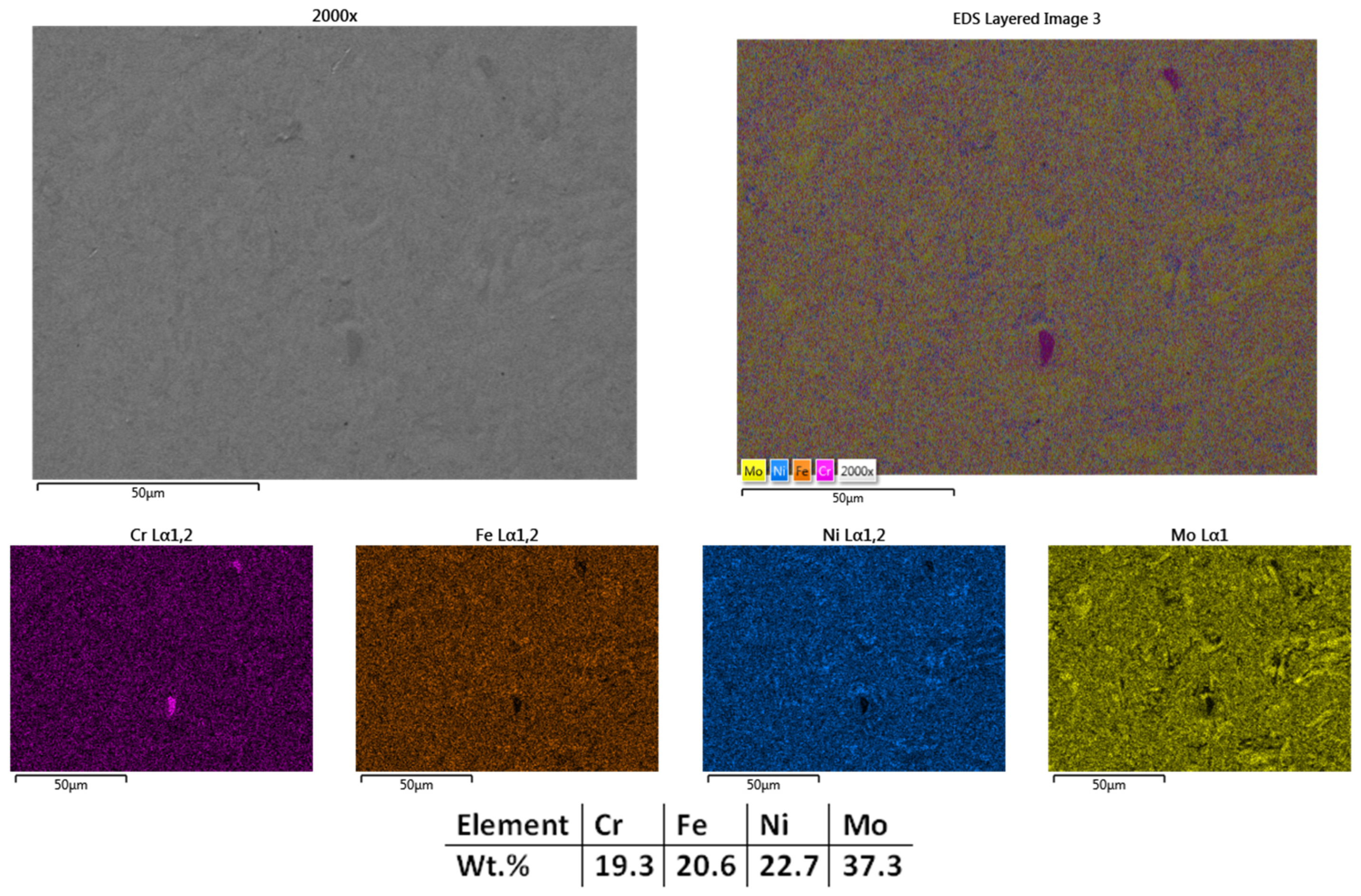
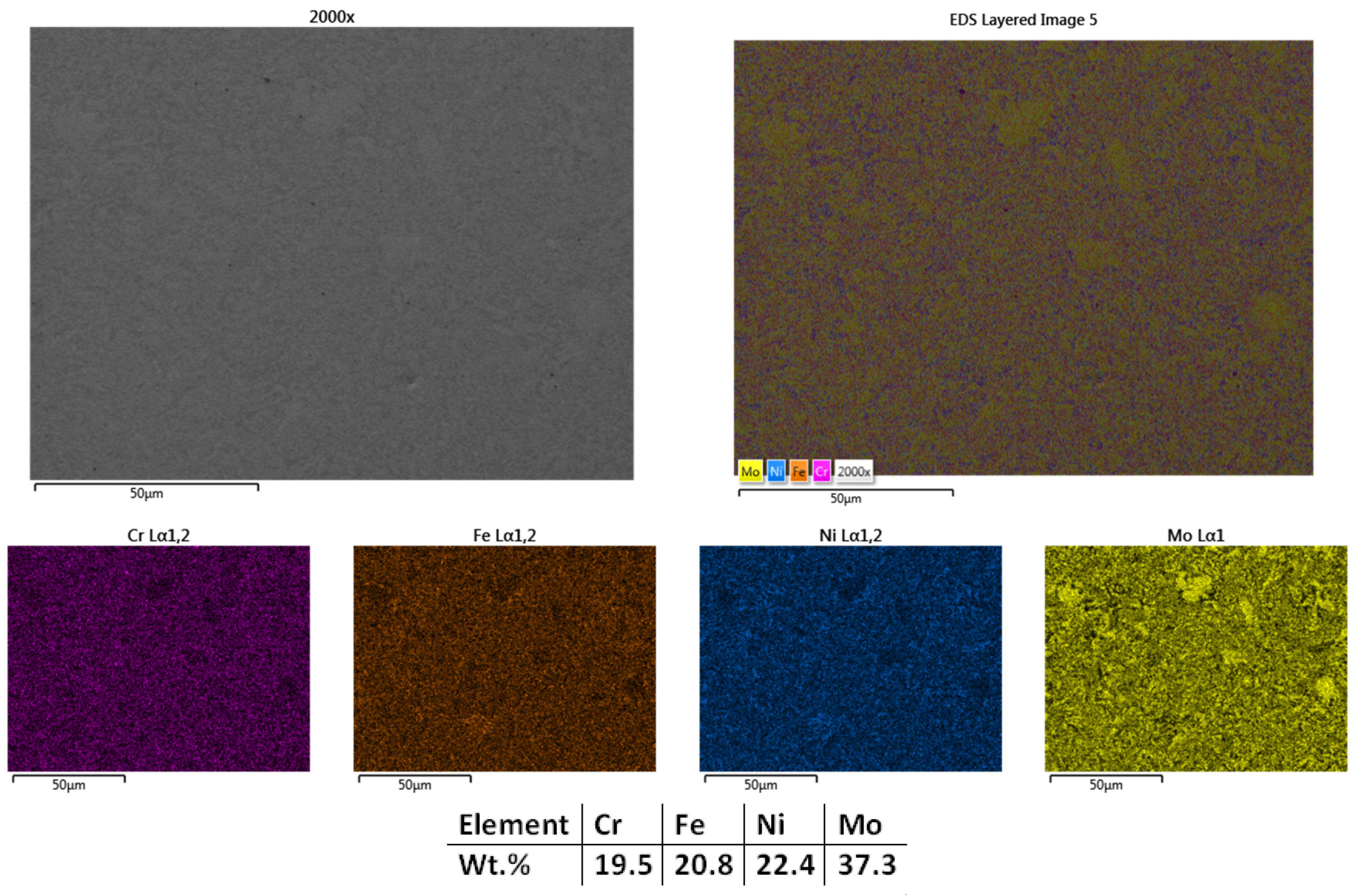

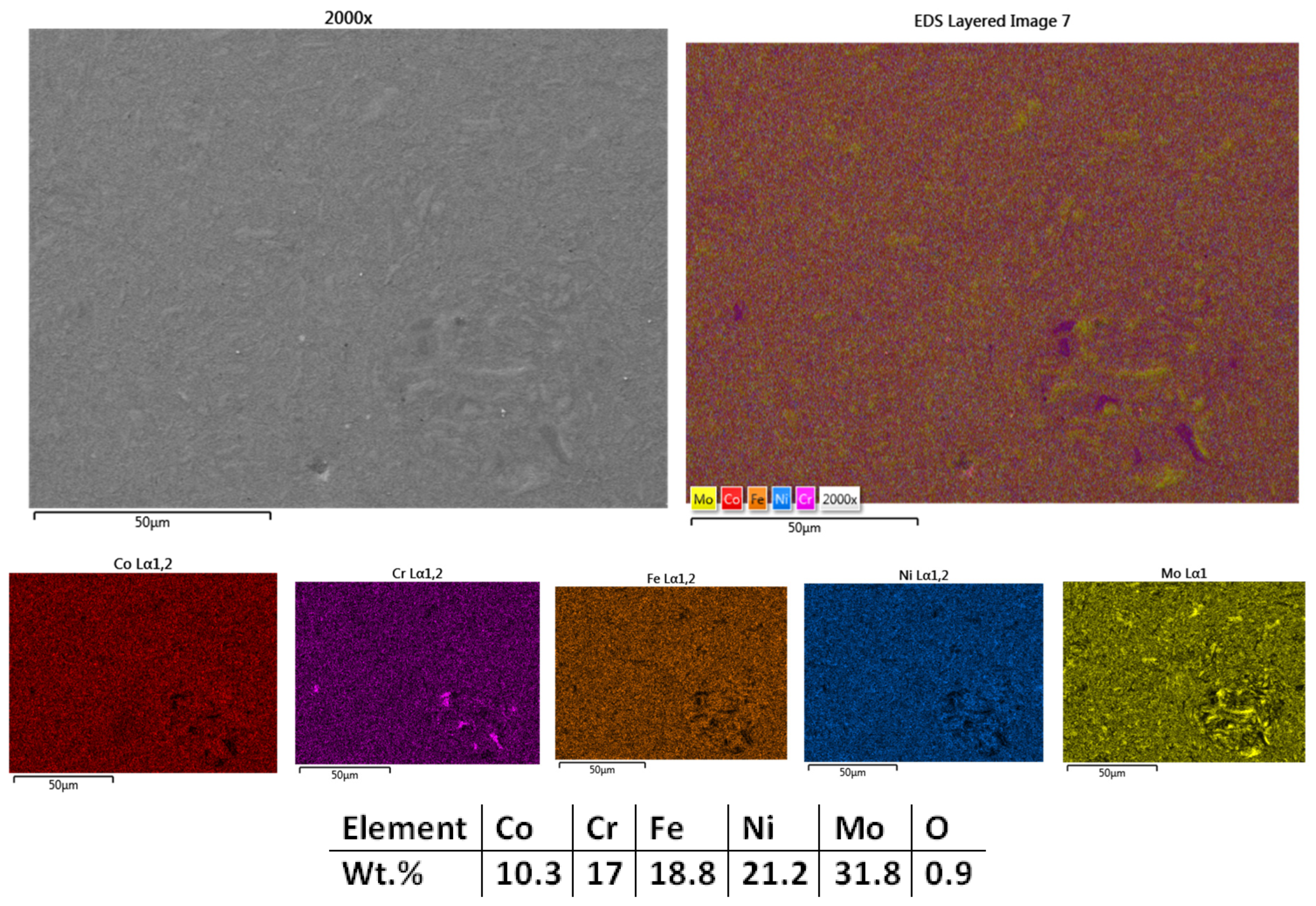
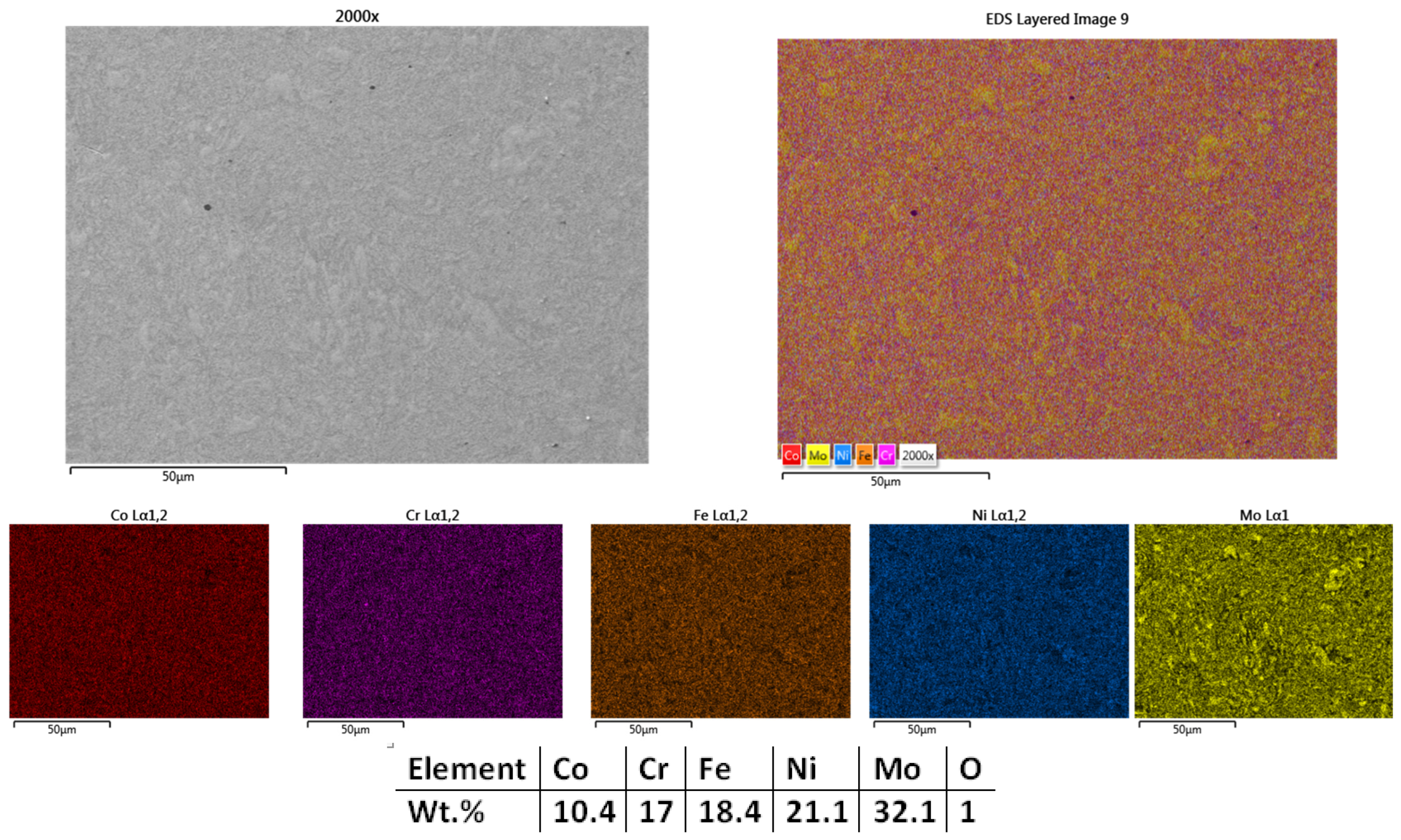
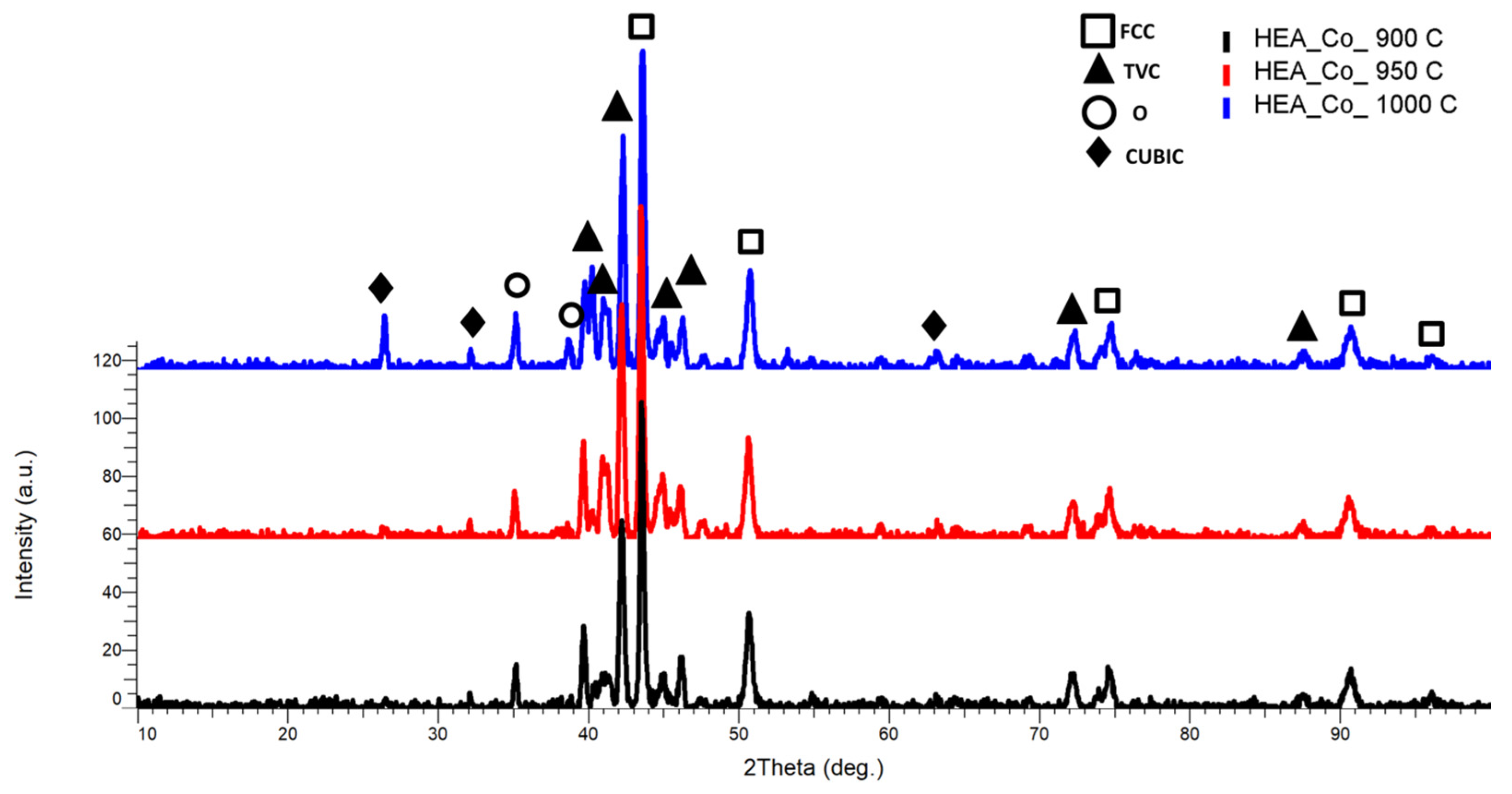

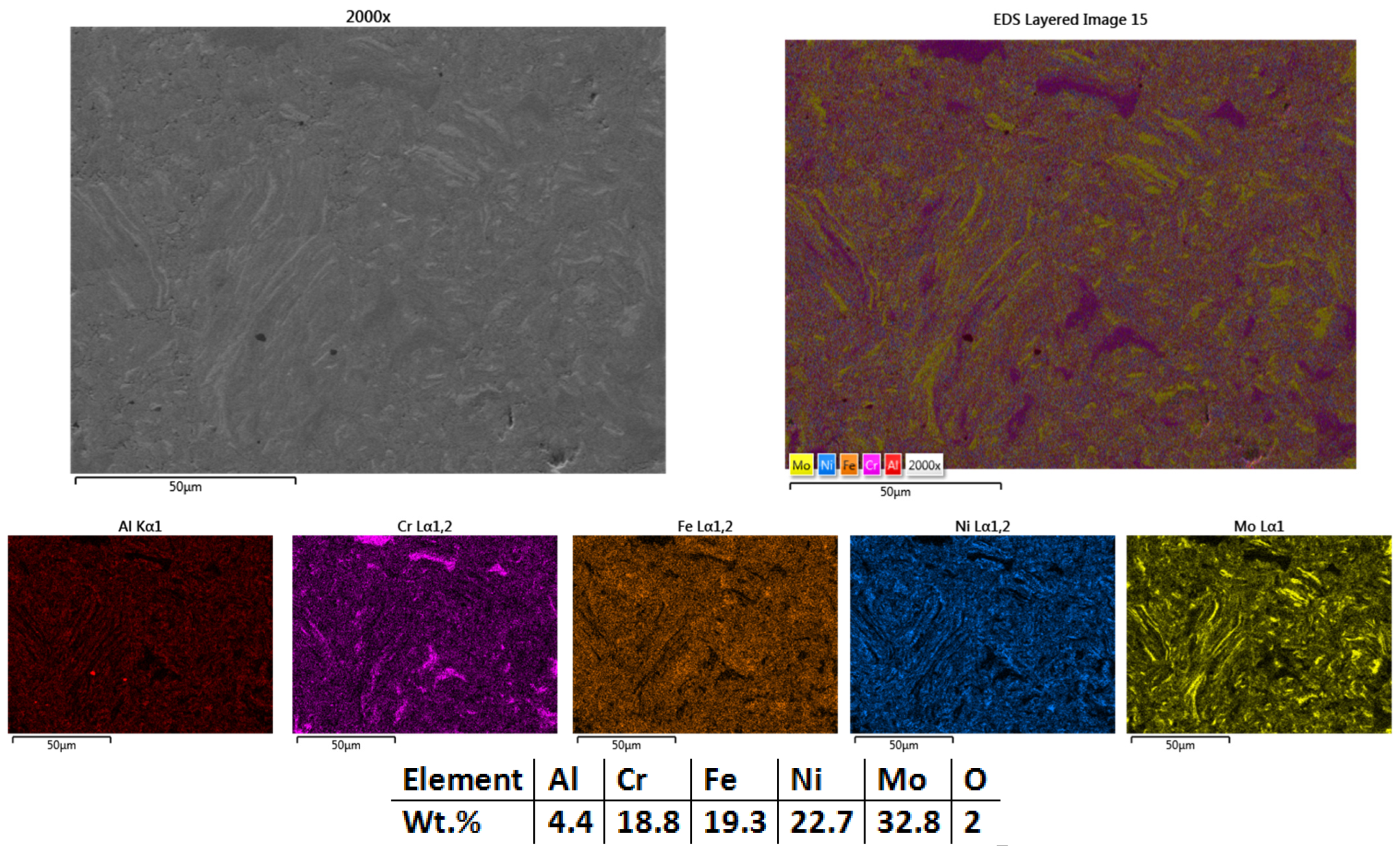
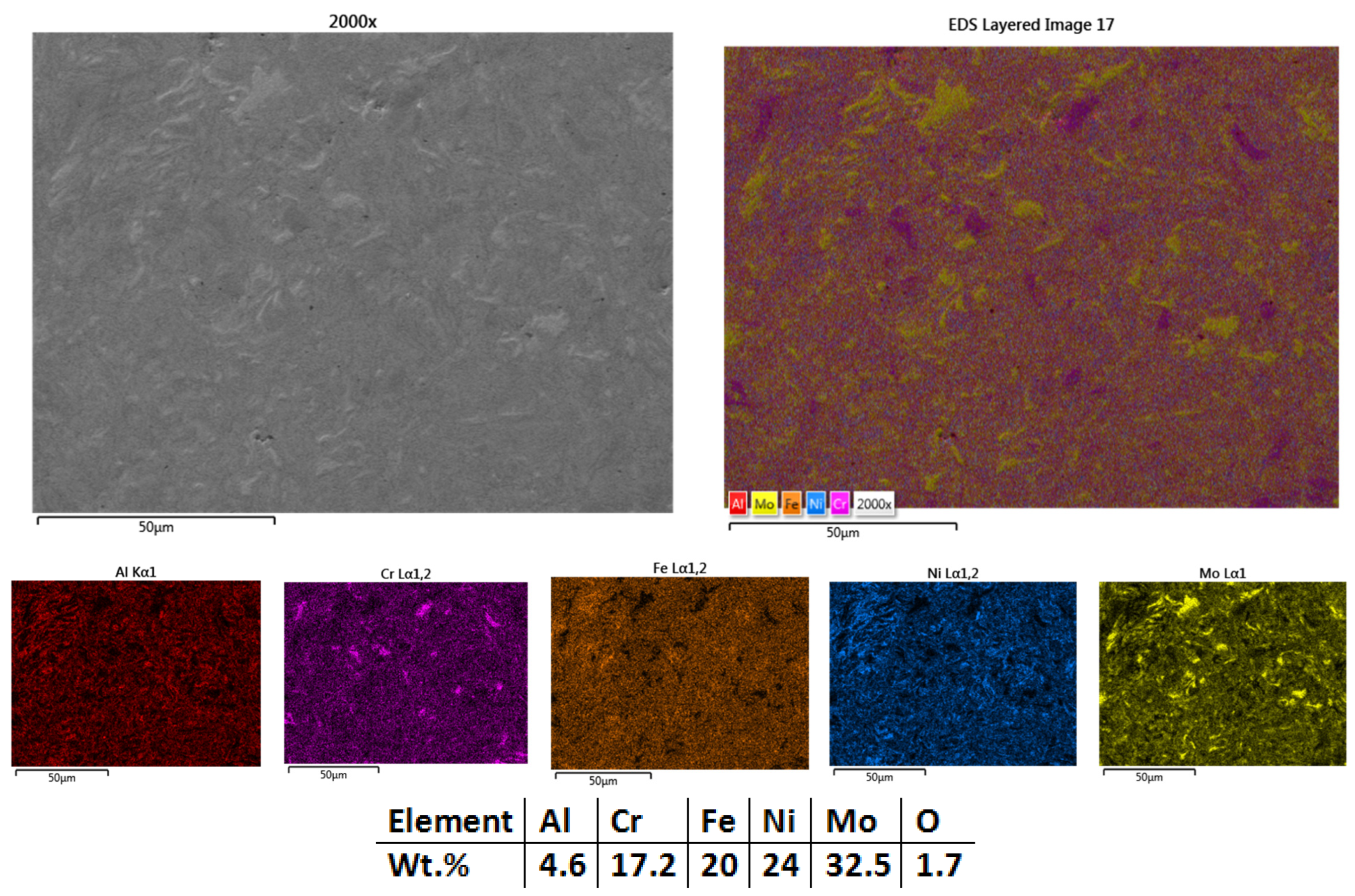
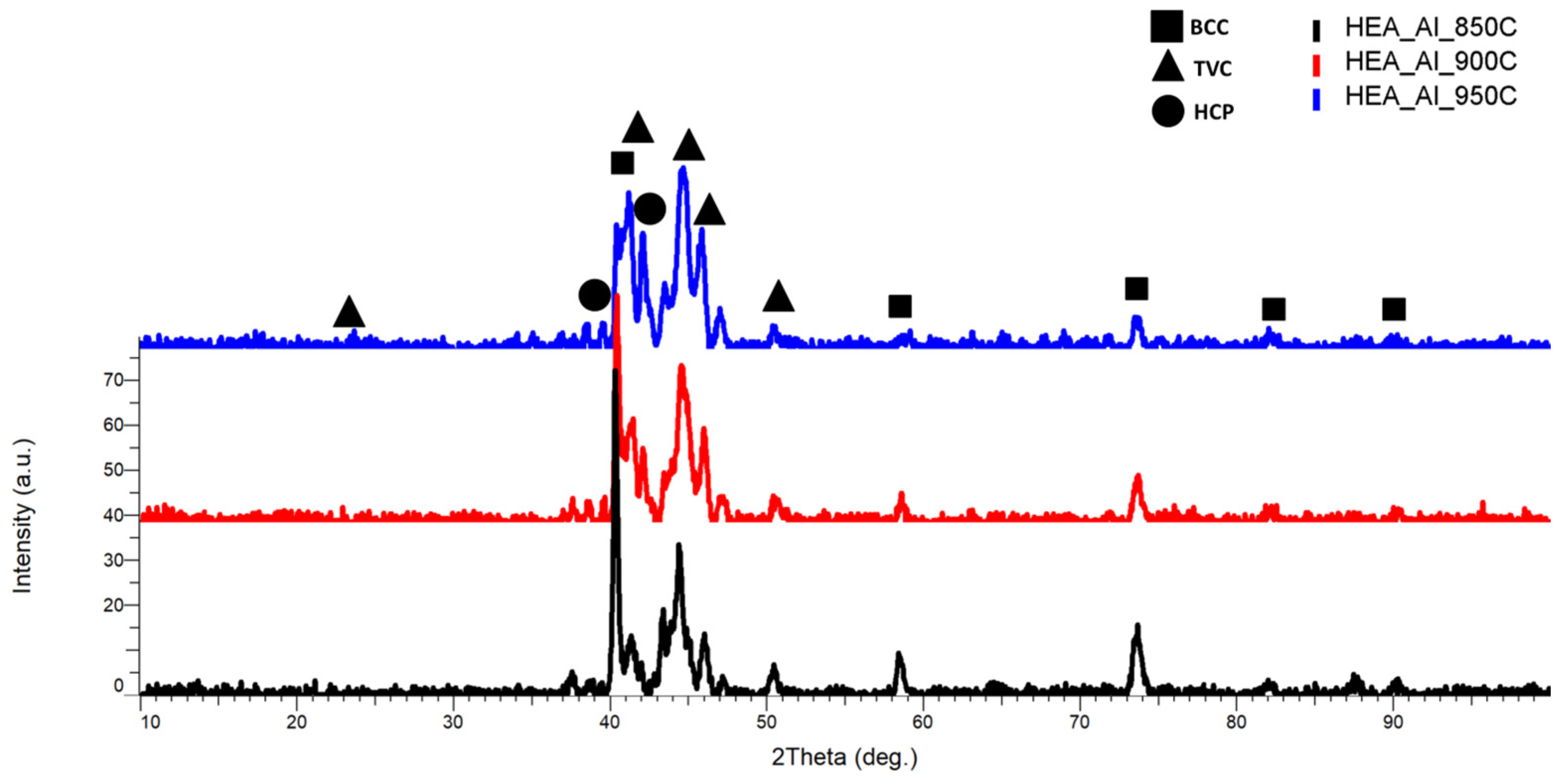

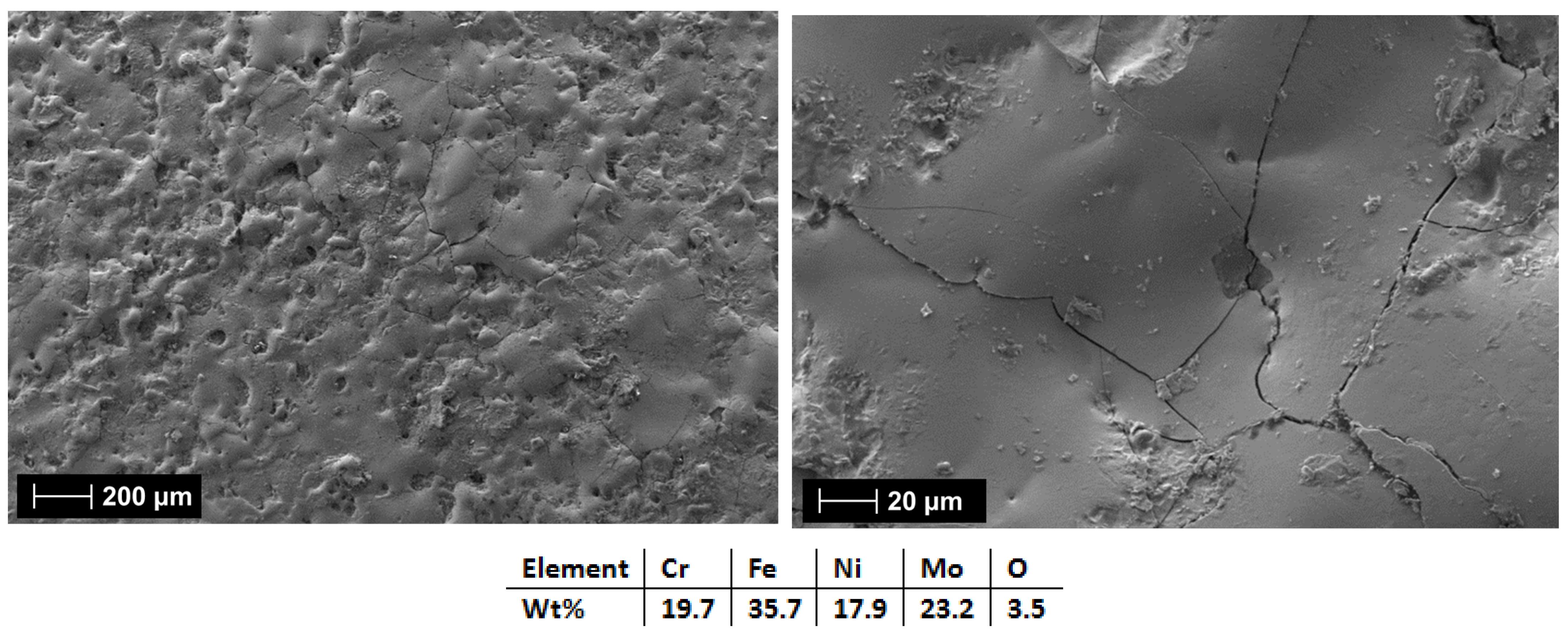
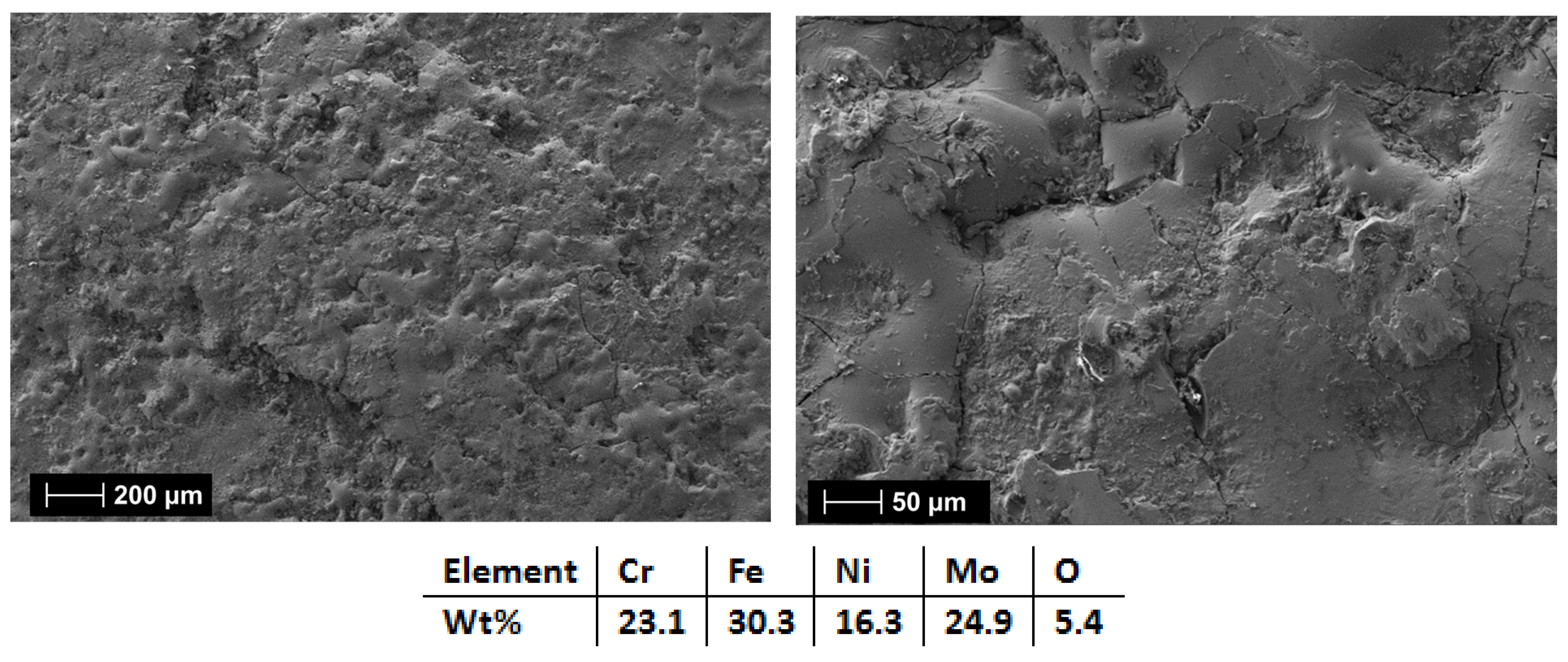
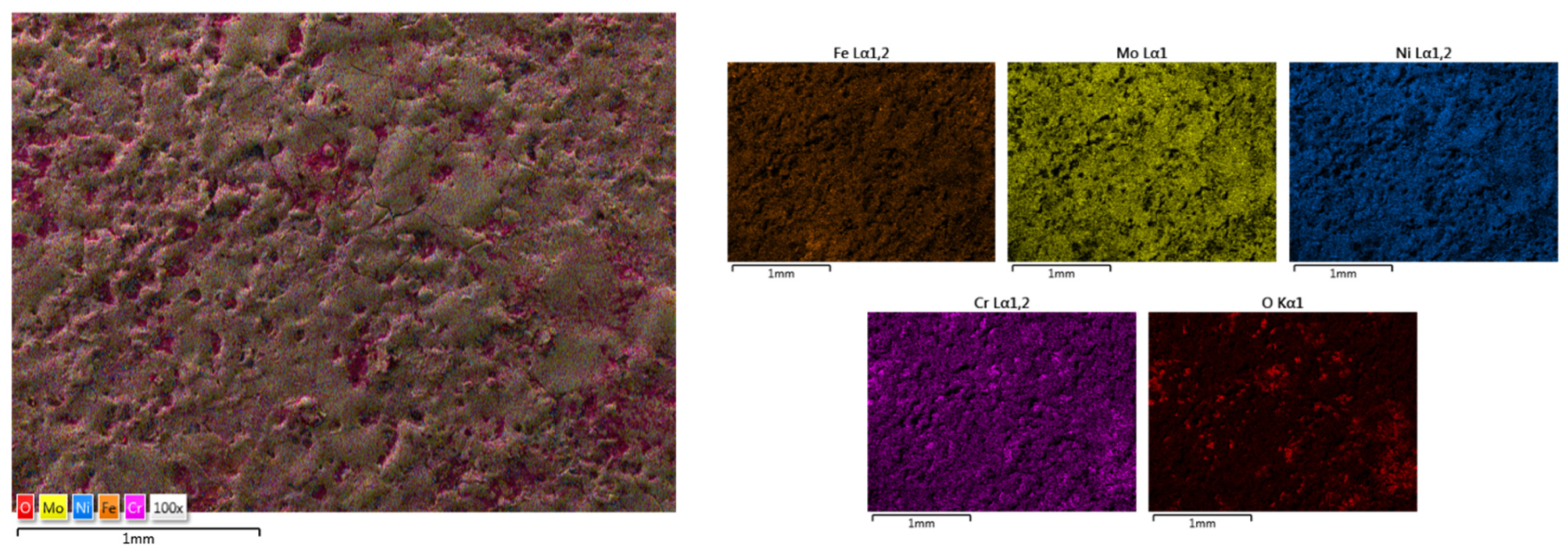
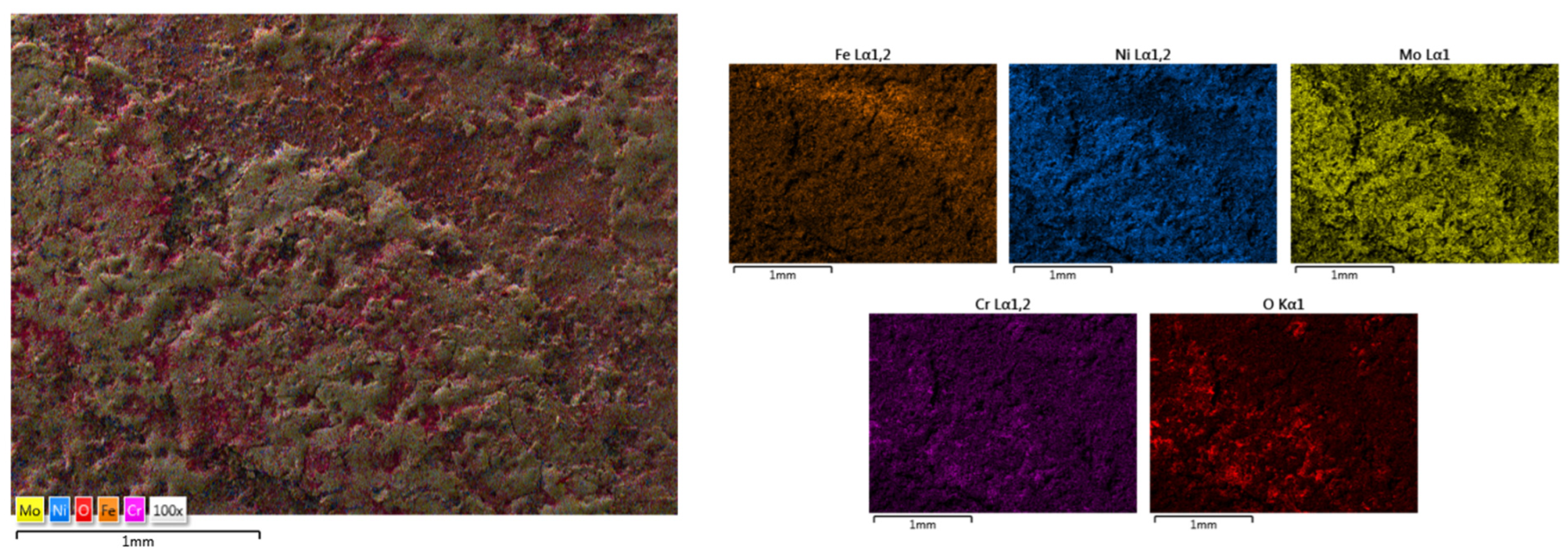
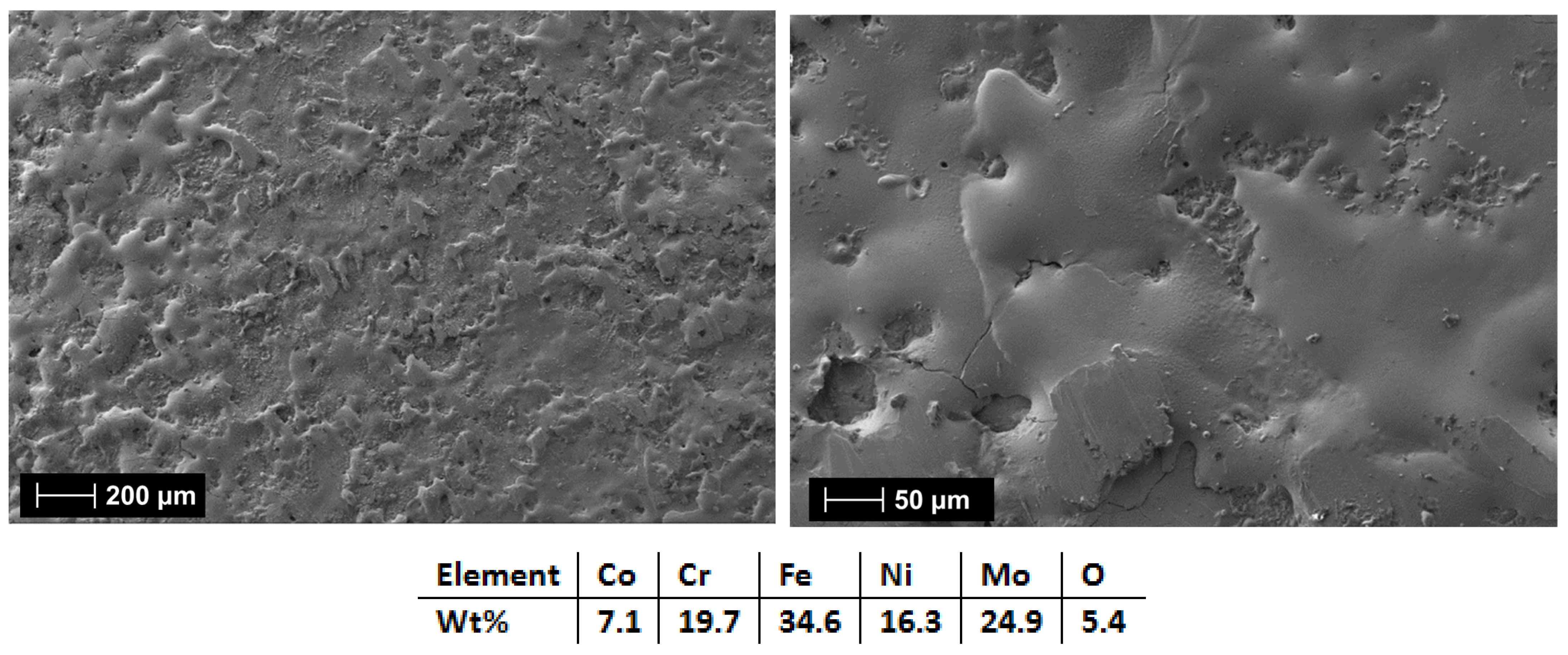
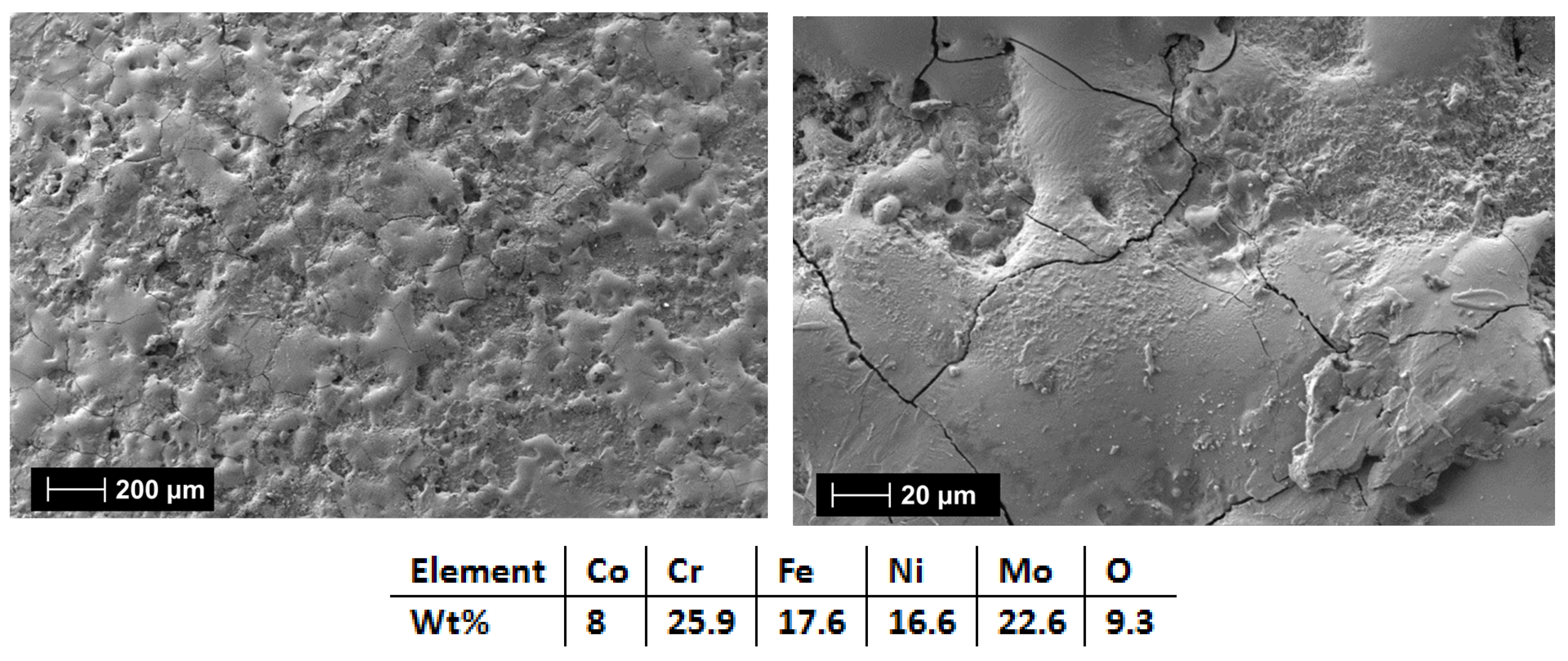
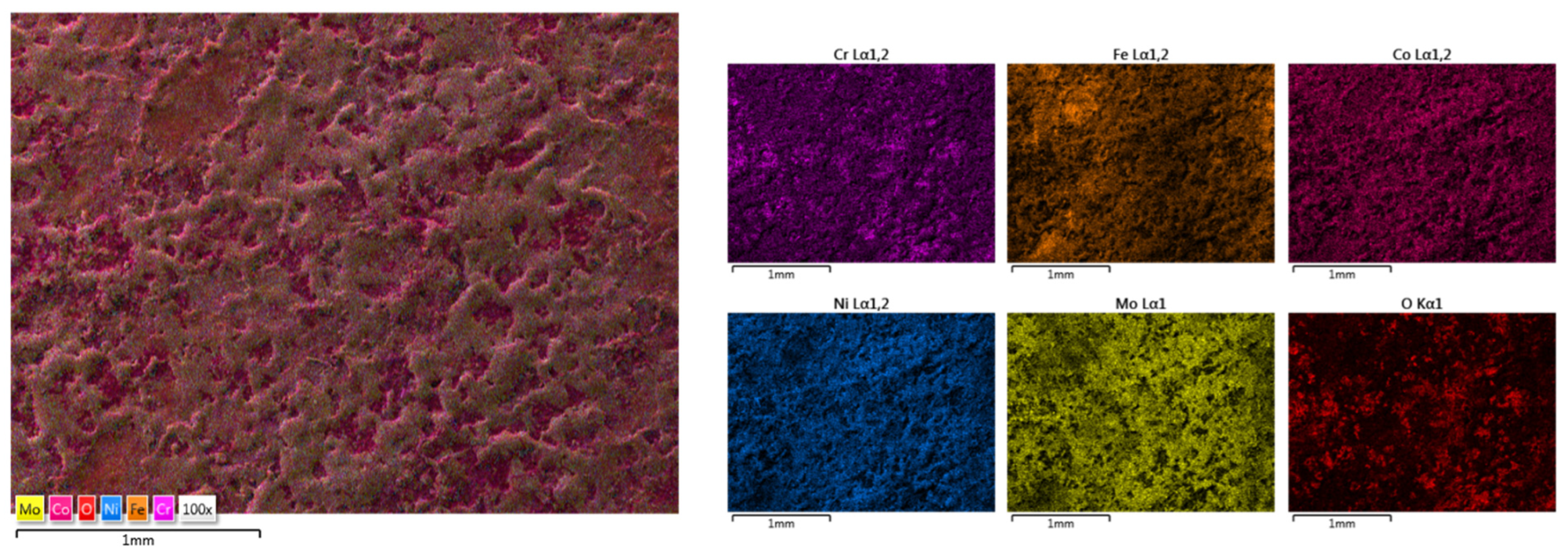
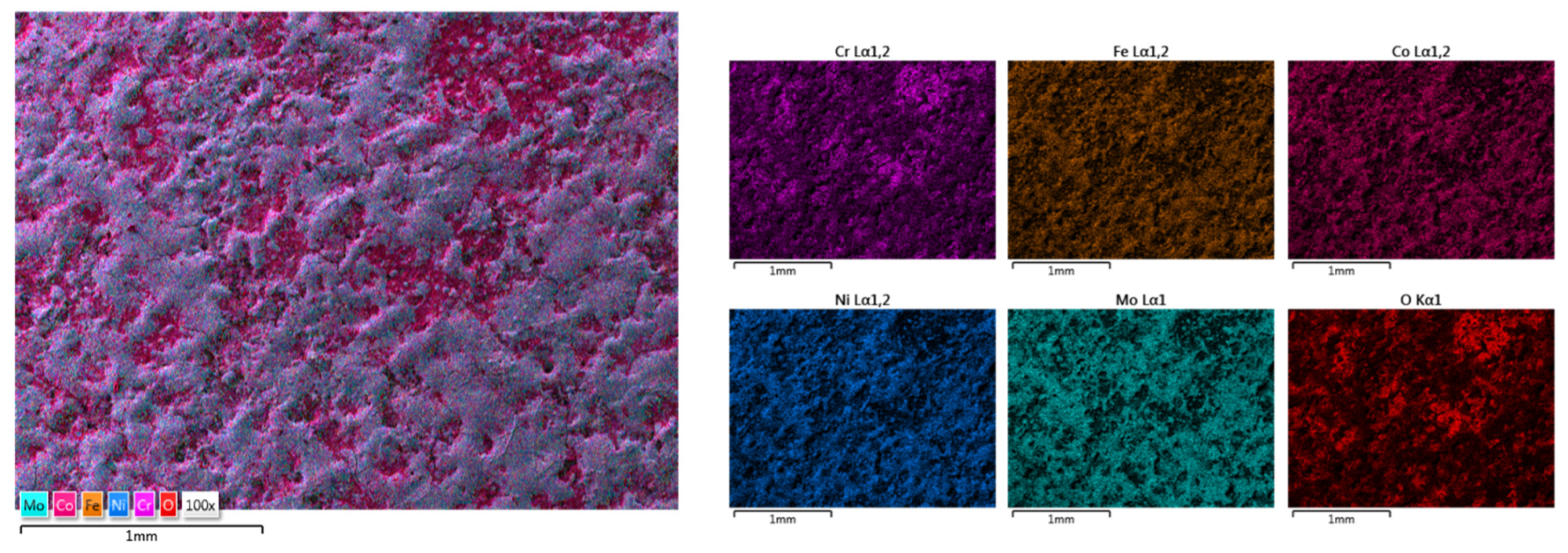
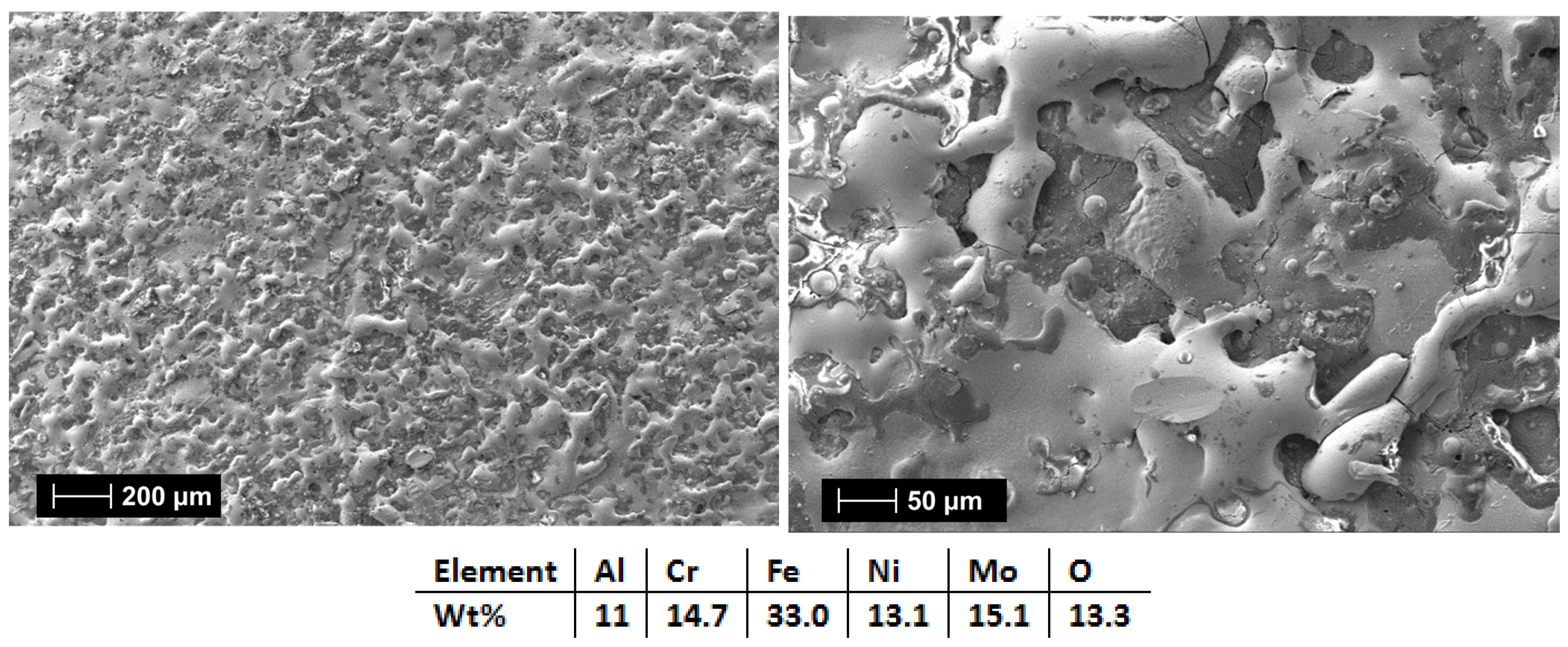
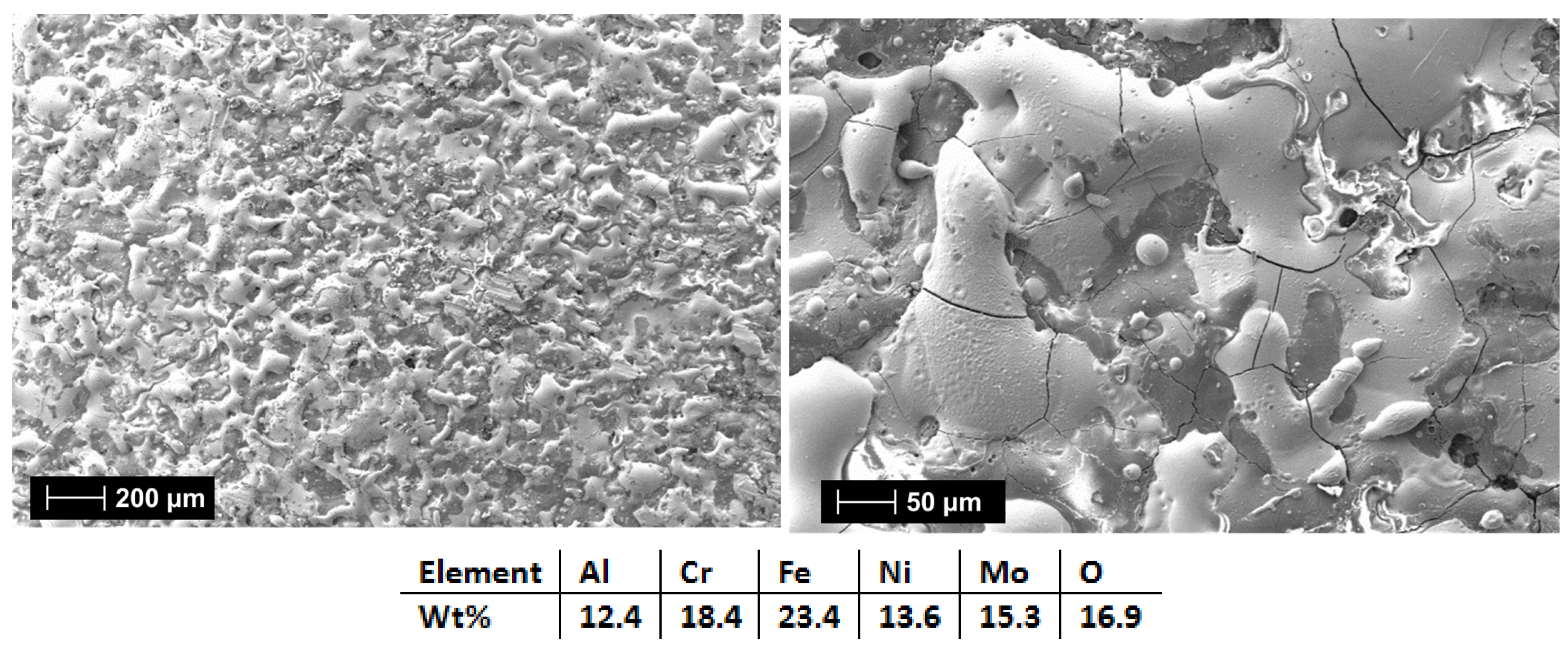

| HEA Composition | Sintered Sample Codification | Pressure (MPa) | Heating Rate (°C/min) | Dwell Time (min) | Cooling Rate (°C/min) | Sintering Temperature (°C) |
|---|---|---|---|---|---|---|
| CrFeNiMo | HEA-FREE-S1-950 | 50 | 50 | 5 | 30 | 950 |
| HEA-FREE-S2-1000 | 50 | 50 | 5 | 30 | 1000 | |
| HEA-FREE-S3-1050 | 50 | 50 | 5 | 30 | 1050 | |
| Co0.5CrFeNiMo | HEA-Co-S1-900 | 50 | 50 | 5 | 30 | 900 |
| HEA-Co-S2-950 | 50 | 50 | 5 | 30 | 950 | |
| HEA-Co-S3-1000 | 50 | 50 | 5 | 30 | 1000 | |
| Al0.5CrFeNiMo | HEA-Al-S1-850 | 50 | 50 | 5 | 30 | 850 |
| HEA-Al-S2-900 | 50 | 50 | 5 | 30 | 900 | |
| HEA-Al-S3-950 | 50 | 50 | 5 | 30 | 950 |
| Electrode Chemical Composition | Output (μF) | Power (V) | Frequency (Hz) | Ar Atm. (L/min) | Deposition Rate (mm/s) |
|---|---|---|---|---|---|
| CrFeNiMo | 30 | 100 | 360 | 5 | 1.86 |
| Co0.5CrFeNiMo | 20 | 100 | 850 | 5 | 2.62 |
| Al0.5CrFeNiMo | 20 | 100 | 360 | 5 | 2.71 |
| HEA Sample | Avg. Dens. ± Std. Dev. (g/cm3) | Theor. Dens. (g/cm3) | Densification (%) | Porosity (%) | Avg. Hardness HV2/15 ± Std. Dev. |
|---|---|---|---|---|---|
| HEA-FREE-S1-950 | 8.192 ± 0.007 | 8.65 | 94.703 | 5.297 | 835.5 ± 21.68 |
| HEA-FREE-S2-1000 | 8.389 ± 0.006 | 8.65 | 96.983 | 3.017 | 923.34 ± 2.70 |
| HEA-FREE-S3-1050 | 8.380 ± 0.003 | 8.65 | 96.876 | 3.124 | 891.42 ± 8.89 |
| HEA-Co-S1-900 | 7.624 ± 0.009 | 8.67 | 87.933 | 12.067 | 493.95 ± 23.17 |
| HEA-Co-S2-950 | 8.240 ± 0.006 | 8.67 | 95.045 | 4.955 | 757.7± 5.97 |
| HEA-Co-S3-1000 | 8.387 ± 0.014 | 8.67 | 96.736 | 3.264 | 790.74± 6.22 |
| HEA-Al-S1-850 | 6.195 ± 0.089 | 7.81 | 79.325 | 20.675 | 192.04 ± 11.11 |
| HEA-Al-S2-900 | 6.447 ± 0.057 | 7.81 | 82.548 | 17.452 | 307.68 ± 32.20 |
| HEA-Al-S3-950 | 6.952 ± 0.010 | 7.81 | 89.014 | 10.986 | 530 ± 28.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manea, C.A.; Geambazu, L.E.; Mateș, I.M.; Pătroi, D.; Sbârcea, G.B.; Tălpeanu, D.; Přikryl, J.; Oppong, G.B.; Semenescu, A. Spark Plasma Sintering and Electrospark Deposition of High Entropy Alloys with Elemental Variation. Materials 2025, 18, 2799. https://doi.org/10.3390/ma18122799
Manea CA, Geambazu LE, Mateș IM, Pătroi D, Sbârcea GB, Tălpeanu D, Přikryl J, Oppong GB, Semenescu A. Spark Plasma Sintering and Electrospark Deposition of High Entropy Alloys with Elemental Variation. Materials. 2025; 18(12):2799. https://doi.org/10.3390/ma18122799
Chicago/Turabian StyleManea, Ciprian Alexandru, Laura Elena Geambazu, Ileana Mariana Mateș, Delia Pătroi, Gabriela Beatrice Sbârcea, Dorinel Tălpeanu, Jan Přikryl, Gifty B. Oppong, and Augustin Semenescu. 2025. "Spark Plasma Sintering and Electrospark Deposition of High Entropy Alloys with Elemental Variation" Materials 18, no. 12: 2799. https://doi.org/10.3390/ma18122799
APA StyleManea, C. A., Geambazu, L. E., Mateș, I. M., Pătroi, D., Sbârcea, G. B., Tălpeanu, D., Přikryl, J., Oppong, G. B., & Semenescu, A. (2025). Spark Plasma Sintering and Electrospark Deposition of High Entropy Alloys with Elemental Variation. Materials, 18(12), 2799. https://doi.org/10.3390/ma18122799









