Dual Effects of Ag Doping and S Vacancies on H2 Detection Using SnS2-Based Photo-Induced Gas Sensor at Room Temperature
Abstract
1. Introduction
2. Materials and Methods
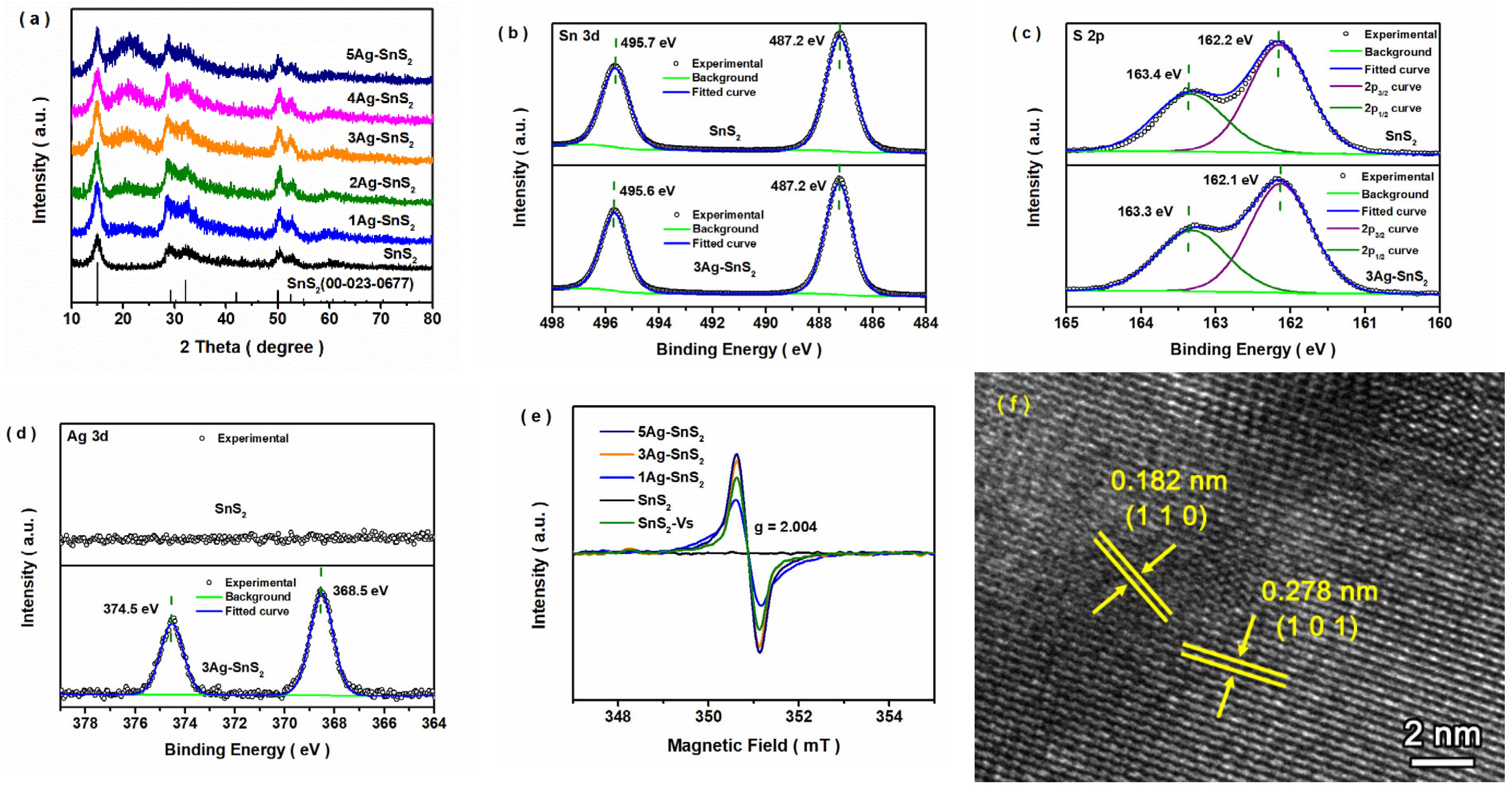
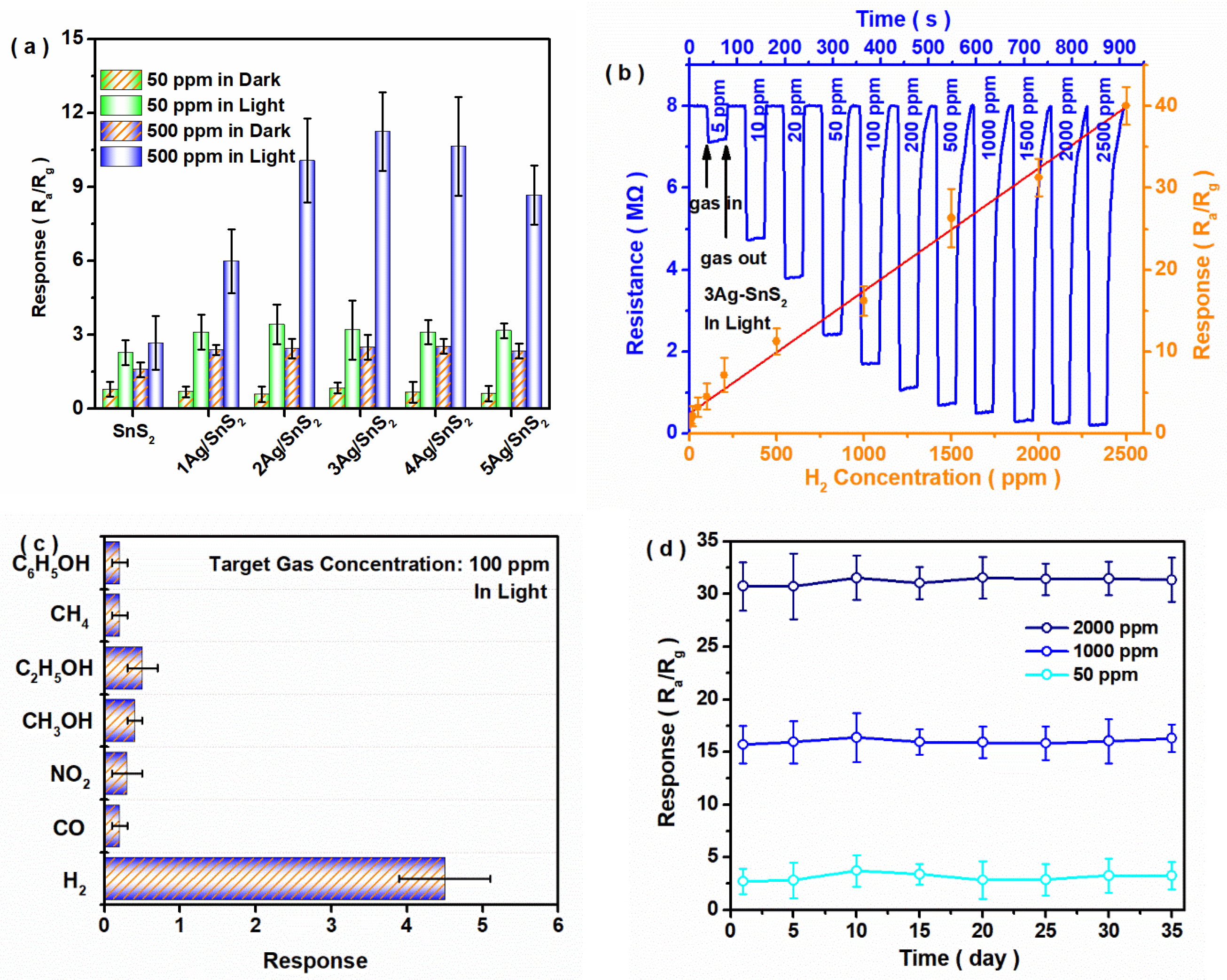
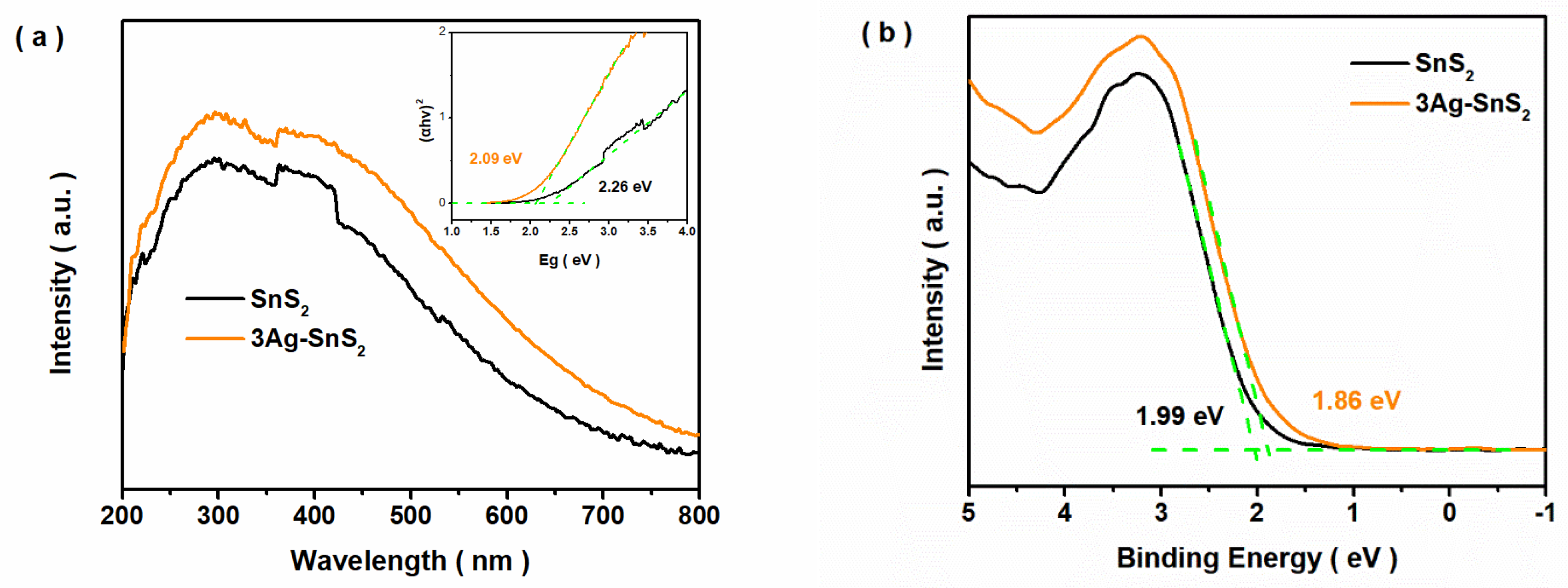
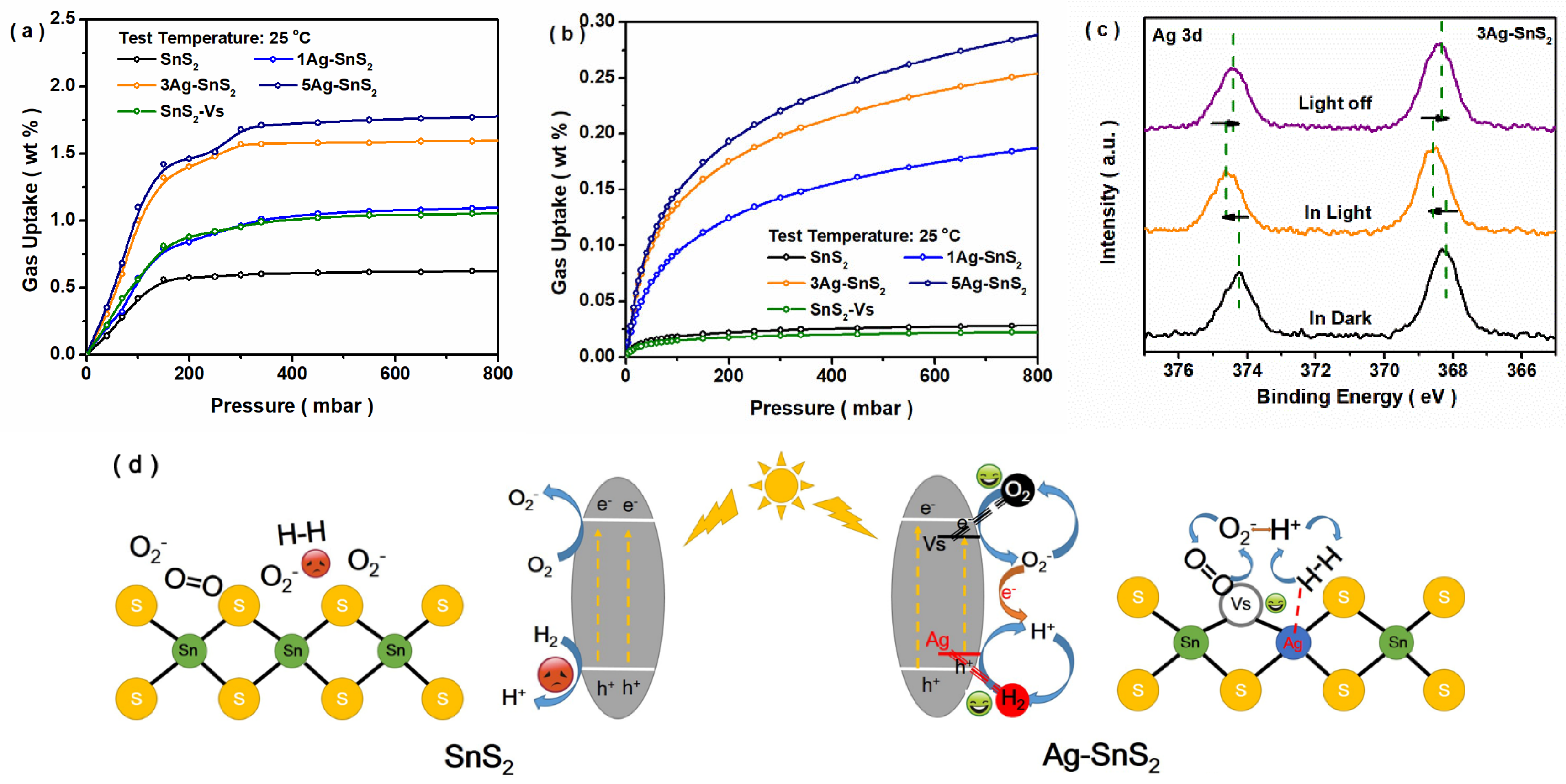
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hjiri, M.; Dhahri, R.; Benmansour, N.; Neri, G. Laser irradiated gas sensors: A review. Micro Nanostruct. 2025, 204, 208157. [Google Scholar] [CrossRef]
- Shooshtari, M.; Salehi, A. An electronic nose based on carbon nanotube -titanium dioxide hybrid nanostructures for detection and discrimination of volatile organic compounds. Sens. Actuators B 2022, 357, 131418. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, S.; Xiao, B.; Chu, J.; Wang, H.; Chen, Y.; Yao, T.; Yang, A.; Han, X.; Rong, M.; et al. Ultra-large Sn3O4 nanosheets with Sn2+ defect for highly efficient hydrogen sensing. Sens. Actuators B 2024, 401, 135025. [Google Scholar] [CrossRef]
- Yin, X.-T.; Wu, S.-S.; Dastan, D.; Nie, S.; Liu, Y.; Li, Z.-G.; Zhou, Y.-W.; Li, J.; Faik, A.; Shan, K.; et al. Sensing selectivity of SnO2-Mn3O4 nanocomposite sensors for the detection of H2 and CO gases. Surf. Interfaces 2021, 25, 101190. [Google Scholar] [CrossRef]
- Meng, X.; Bi, M.; Xiao, Q.; Gao, W. Ultrasensitive gas sensor based on Pd/SnS2/SnO2 nanocomposites for rapid detection of H2. Sens. Actuators B 2022, 359, 131612. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, H.; Zhang, S.; Yao, H.; Shi, X.; Xu, S. Chemoresistive gas sensors based on noble-metal-decorated metal oxide semiconductors for H2 detection. Materials 2025, 18, 451. [Google Scholar] [CrossRef]
- Mishra, R.K.; Choi, H.J.; Ryu, J.W.; Choi, G.J.; Kumar, V.; Kumar, P.; Singh, J.; Kumar, S.; Gwag, J.S. Recent progress in gas sensing based on 2D SnS2 and its heterostructure platforms: A review. Sens. Actuators A 2024, 365, 114860. [Google Scholar] [CrossRef]
- Saggu, I.S.; Singh, S.; Chen, K.; Xuan, Z.; Swihart, M.T.; Sharma, S. Ultrasensitive room-temperature NO2 detection using SnS2/MWCNT composites and accelerated recovery kinetics by UV activation. ACS Sens. 2023, 8, 243–253. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, Y.J.; Park, S.J.; Cheon, W.S.; Kim, J.; Nam, G.B.; Kim, Y.; Jang, H.W. In-situ growth of 2D MOFs as a molecular sieving layer on SnS2 nanoflakes for realizing ultraselective H2S detection. Adv. Funct. Mater. 2025, 35, 2417019. [Google Scholar] [CrossRef]
- Qiu, P.; Qin, Y.; Wang, X. S-vacancies and Ag nanoparticles in SnS2 nanoflakes for ethanol sensing: A combined experimental and theoretical investigation. ACS Appl. Nano Mater. 2022, 5, 10839–10847. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, J.; Wang, W.; Fan, Y.; Liu, C.; Zhou, J.; Liu, D.; Ruan, S. Au-Pd modified SnS2 nanosheets for conductometric detection of xylene gas. Sens. Actuators B 2022, 351, 130907. [Google Scholar] [CrossRef]
- Maria, K.H.; Sakhuja, N.; Jha, R.K.; Bhat, N. Ultra-sonication assisted synthesis of 2D SnS2 nanoflakes for room-temperature NO gas detection. IEEE Sens. J. 2021, 21, 10420–10427. [Google Scholar] [CrossRef]
- Eom, T.H.; Cho, S.H.; Suh, J.M.; Kim, T.; Lee, T.H.; Jun, S.E.; Yang, J.W.; Lee, J.; Hong, S.-H.; Jang, H.W. Substantially improved room temperature NO2 sensing in 2-dimensional SnS2 nanoflowers enabled by visible light illumination. J. Mater. Chem. A 2021, 9, 11168–11178. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Ajitha, B.; Ashok Kumar Reddy, Y. Review on metal sulfide-based nanostructures for photodetectors: From ultraviolet to infrared regions. Sens. Actuators A 2023, 349, 114051. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Li, H.; Guo, X. Light-activated gas sensors. Chin. Sci. Bull. 2022, 67, 1837–1850. [Google Scholar] [CrossRef]
- Arain, S.; Usman, M.; Saeed, F.; Feng, S.; Rehman, W.; Liu, X.; Dai, H. Microemulsion-based synthesis of highly efficient Ag-doped fibrous SiO2-TiO2 photoanodes for photoelectrochemical water splitting. Catalysts 2025, 15, 66. [Google Scholar] [CrossRef]
- An, Q.; Li, J.; Peng, J.; Hu, L. DFT study of small gas molecules (C2H2, CH4, CO and H2) adsorbed on Au, Ag-doped ZnO monolayer. Chem. Phys. Lett. 2025, 869, 142043. [Google Scholar] [CrossRef]
- Yang, H.; Du, Z.; Yang, Y.; Li, X.; Wu, Q.; Tang, J.; Wang, X.; Zeng, D. Ag intercalated SnS2 with S vacancy and expanded interlayer for enhancing NO2 sensing. Sens. Actuators B 2023, 393, 134140. [Google Scholar] [CrossRef]
- Wang, J.-C.; Ma, H.; Shi, W.; Li, W.; Zhang, Z.; Hou, Y.; Zhang, W.; Chen, J. Designed synthesized step-scheme heterojunction of Bi2WO6 nanosheet supported on CuBi2O4 nanorod with remarkable photo-assisted gas sensing for N-butyl alcohol. J. Environ. Chem. Eng. 2024, 12, 112698. [Google Scholar] [CrossRef]
- Wang, J.-C.; Shi, W.; Sun, X.-Q.; Wu, F.-Y.; Li, Y.; Hou, Y. Enhanced photo-assisted acetone gas sensor and efficient photocatalytic degradation ssing Fe-doped hexagonal and monoclinic WO3 phase−junction. Nanomaterials 2020, 10, 398. [Google Scholar] [CrossRef]
- Yang, J.; Sun, R.; Bao, X.; Liu, J.; Ng, J.W.; Tang, B.; Liu, Z. Enhancing selectivity of two-dimensional materials-based gas sensors. Adv. Funct. Mater. 2025, 35, 2420393. [Google Scholar] [CrossRef]
- Qiao, X.; Xu, Y.; Yang, K.; Ma, J.; Li, C.; Wang, H.; Jia, L. Mo doped BiVO4 gas sensor with high sensitivity and selectivity towards H2S. Chem. Eng. J. 2020, 395, 125144. [Google Scholar] [CrossRef]
- Venkatesan, A.; Ryu, H.; Devnath, A.; Yoo, H.; Lee, S. Air-stable, all-dry transferred ReS2/GaSe heterostructure-based NO2 gas sensor. J. Mater. Sci. Technol. 2024, 168, 79–87. [Google Scholar] [CrossRef]
- Wu, K.; Chai, H.; Xu, K.; Debliquy, M.; Zhang, C. Effect of {010} crystal facets of Bi2MoO6 and 1D/2D heterostructures for conductometric room temperature NH3 gas sensors. Sens. Actuators B 2023, 376, 132983. [Google Scholar] [CrossRef]
- Tian, W.; Han, J.; Wan, L.; Li, N.; Chen, D.; Xu, Q.; Li, H.; Lu, J. Enhanced piezocatalytic activity in ion-doped SnS2 via lattice distortion engineering for BPA degradation and hydrogen production. Nano Energy 2023, 107, 108165. [Google Scholar] [CrossRef]
- Ageeva, T.A.; Bush, A.A.; Golubev, D.V.; Gorshkova, A.S.; Mozhchil, R.N.; Koifman, O.I.; Kozlov, V.S.; Matis, M.E.; Rumyantseva, V.D.; Sigov, A.S.; et al. Porphyrin complexes of transition metals with a large dipole moment as active components of new film electret materials. Russ. Chem. Bull. 2023, 72, 2070–2082. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Zhou, X.; Jin, X.; Li, B.; Liu, J.; Chen, G. Cu doped SnS2 nanostructure induced sulfur vacancy towards boosted photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 407, 127180. [Google Scholar] [CrossRef]
- Park, S.J.; Lee, S.M.; Lee, J.; Choi, S.; Nam, G.B.; Jo, Y.K.; Hwang, I.-S.; Jang, H.W. Pd-W18O49 nanowire MEMS gas sensor for ultraselective dual detection of hydrogen and ammonia. Small 2025, 21, 2405809. [Google Scholar] [CrossRef]
- Lv, Y.-R.; Wang, Z.-L.; Yang, Y.-X.; Luo, Y.; Yang, S.-Y.; Xu, Y.-H. Tin bisulfide nanoplates anchored onto flower-like bismuth tungstate nanosheets for enhancement in the photocatalytic degradation of organic pollutant. J. Hazard. Mater. 2022, 432, 128665. [Google Scholar] [CrossRef]
- Lei, Z.; Wang, W.; Sun, T.; Liu, E.; Gao, T. Efficient photocatalytic H2 evolution over SnS2/twinned Mn0.5Cd0.5S hetero-homojunction with double S-scheme charge transfer routes. J. Mater. Sci. Technol. 2025, 216, 81–92. [Google Scholar] [CrossRef]
- Mamo, T.T.; Qorbani, M.; Hailemariam, A.G.; Putikam, R.; Chu, C.-M.; Ko, T.-R.; Sabbah, A.; Huang, C.-Y.; Kholimatussadiah, S.; Billo, T.; et al. Enhanced CO2 photoreduction to CH4 via *COOH and *CHO intermediates stabilization by synergistic effect of implanted P and S vacancy in thin-film SnS2. Nano Energy 2024, 128, 109863. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Y.; Qiu, P.; Lei, S. SnO2-Co3O4 nanocomposite sensor: Achieving ultra-selective hydrogen detection in mixed gas environments. Sens. Actuators B 2025, 422, 136521. [Google Scholar] [CrossRef]
- Li, A.; Zhao, S.; Bai, J.; Xiao, H.; Gao, S.; Shen, Y.; Yuan, Z.; Meng, F. The role of AuSn alloys in optimizing SnO2 nanospheres for chemoresistive hydrogen sensing. Sens. Actuators B 2025, 427, 137214. [Google Scholar] [CrossRef]
- Geethukrishnan; Apte, O.; Tadi, K.K. Geethukrishnan; Apte, O.; Tadi, K.K. Electrochemical sensor for dopamine detection based on multiwalled carbon nanotube/molybdenum disulfide quantum dots with machine learning integration and anti-interference capability. Tungsten 2025, 7, 137–151. [Google Scholar] [CrossRef]
- Liu, W.; Zou, J.; Li, S.; Li, J.; Li, F.; Zhan, Z.; Zhang, Y. Pd/In2O3-based bilayer H2 sensor with high resistance to silicone toxicity and ultra-fast response. Int. J. Hydrogen Energy 2023, 48, 5743–5753. [Google Scholar] [CrossRef]
- Murali, A.S.; Sreelekshmi; Saraswathyamma, B. The role of engineered MoS2 and MoSe2 transition metal dichalcogenides in electrochemical sensors and batteries: A review. Tungsten 2025, 7, 215–242. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Liu, J.; Li, H.-Y.; Hu, Z.; Luo, X.; Gao, N.; Zhang, B.; Jiang, J.; Zhong, A.; et al. Sensitive H2 gas sensors based on SnO2 nanowires. Sens. Actuators B 2021, 345, 130334. [Google Scholar] [CrossRef]
- Tang, C.; Jin, W.; Xiao, X.; Qi, X.; Ma, Y.; Ma, L. Graphene-based chemiresistive hydrogen sensor for room temperature operation. Sens. Actuators B 2025, 424, 136889. [Google Scholar] [CrossRef]
- Kumar, G.; Li, X.; Du, Y.; Geng, Y.; Hong, X. UV-light enhanced high sensitive hydrogen (H2) sensor based on spherical Au nanoparticles on ZnO nano-structured thin films. J. Alloys Compd. 2019, 798, 467–477. [Google Scholar] [CrossRef]
- Wang, F.; Hu, K.; Liu, H.; Zhao, Q.; Wang, K.; Zhang, Y. Low temperature and fast response hydrogen gas sensor with Pd coated SnO2 nanofiber rods. Int. J. Hydrogen Energy 2020, 45, 7234–7242. [Google Scholar] [CrossRef]
- Pandey, G.; Lawaniya, S.D.; Kumar, S.; Dwivedi, P.K.; Awasthi, K. A highly selective, efficient hydrogen gas sensor based on bimetallic (Pd–Au) alloy nanoparticle (NP)-decorated SnO2 nanorods. J. Mater. Chem. A 2023, 11, 26687–26697. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, B.; Zhang, L.; Yu, J.; Tan, H. BiOBr/NiO S-scheme heterojunction photocatalyst for CO2 photoreduction. Sol. RRL 2022, 6, 2100587. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Shi, X.; Fang, N.; Ma, H.; Wang, J. Dual Effects of Ag Doping and S Vacancies on H2 Detection Using SnS2-Based Photo-Induced Gas Sensor at Room Temperature. Materials 2025, 18, 2687. https://doi.org/10.3390/ma18122687
Wang S, Shi X, Fang N, Ma H, Wang J. Dual Effects of Ag Doping and S Vacancies on H2 Detection Using SnS2-Based Photo-Induced Gas Sensor at Room Temperature. Materials. 2025; 18(12):2687. https://doi.org/10.3390/ma18122687
Chicago/Turabian StyleWang, Shaoling, Xianju Shi, Na Fang, Haoran Ma, and Jichao Wang. 2025. "Dual Effects of Ag Doping and S Vacancies on H2 Detection Using SnS2-Based Photo-Induced Gas Sensor at Room Temperature" Materials 18, no. 12: 2687. https://doi.org/10.3390/ma18122687
APA StyleWang, S., Shi, X., Fang, N., Ma, H., & Wang, J. (2025). Dual Effects of Ag Doping and S Vacancies on H2 Detection Using SnS2-Based Photo-Induced Gas Sensor at Room Temperature. Materials, 18(12), 2687. https://doi.org/10.3390/ma18122687






