High-Performance Vacuum-Free Processed Organic Solar Cells with Gallium-Based Liquid Metal Top Electrodes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Device Fabrication
2.3. Characterizations
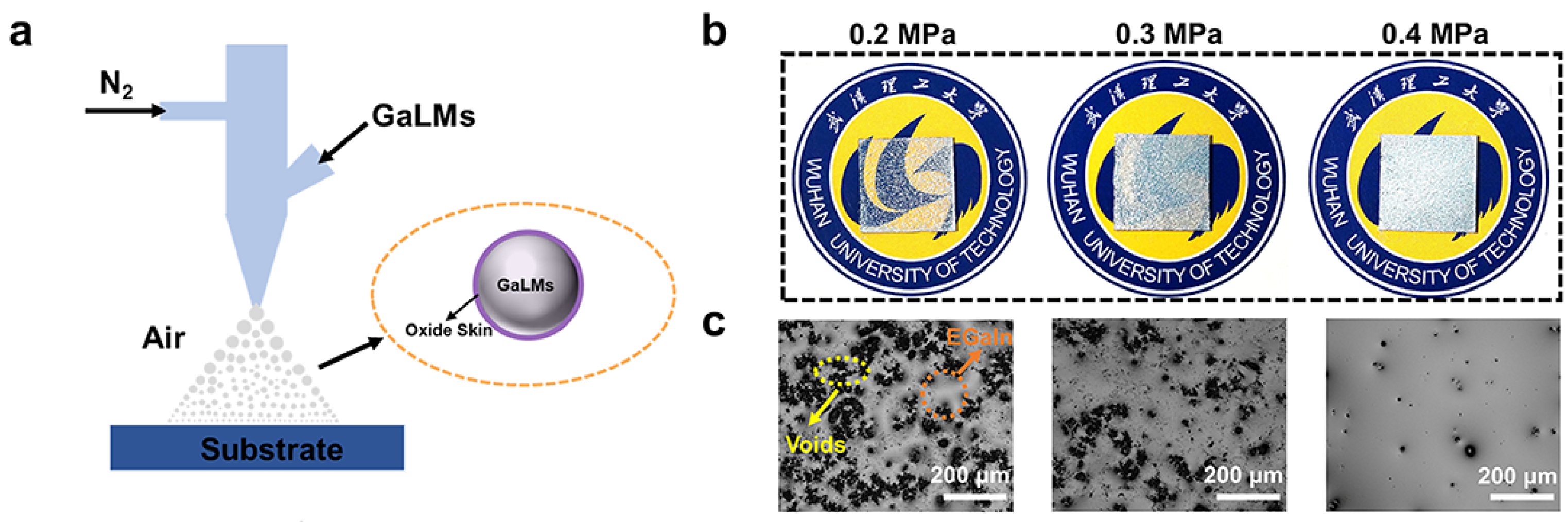
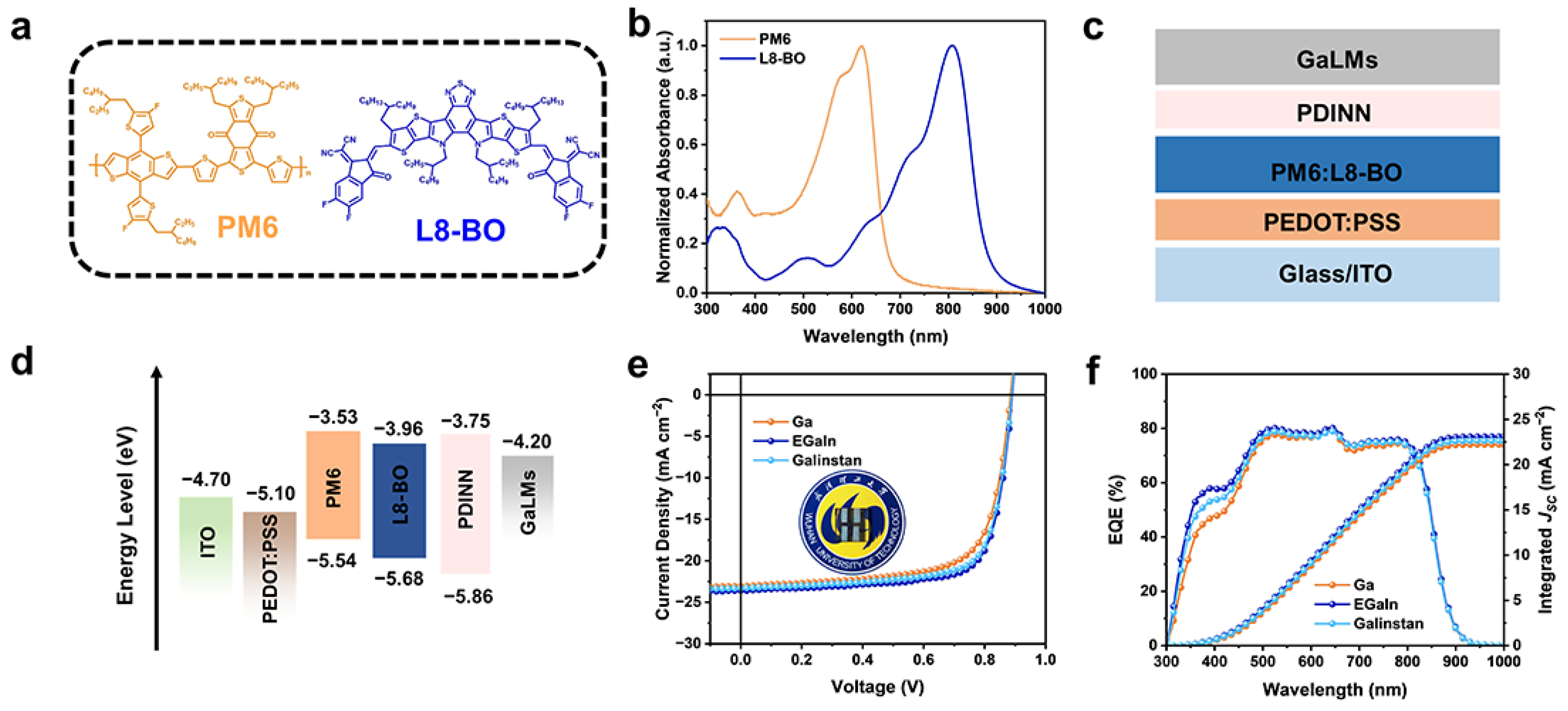
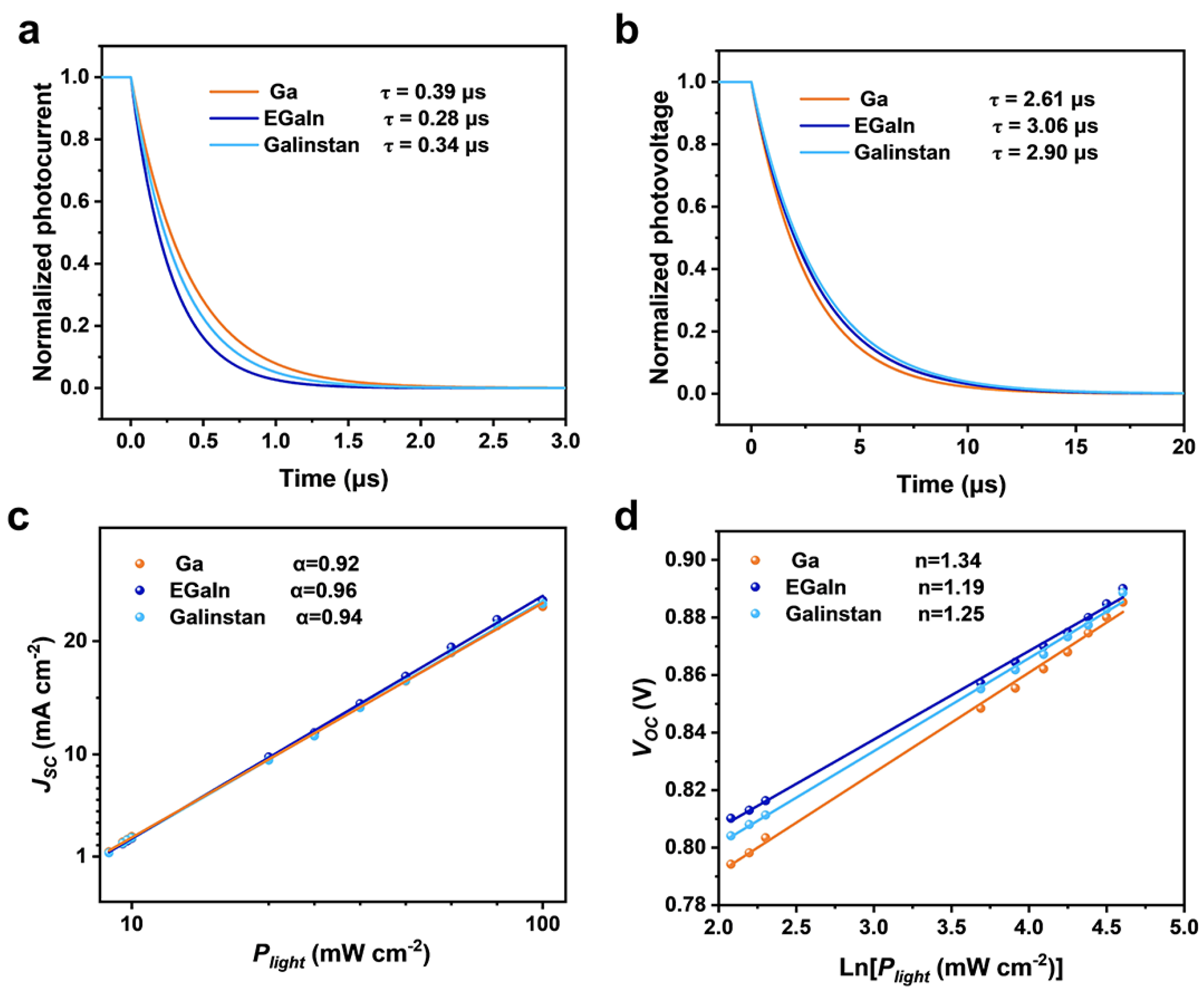
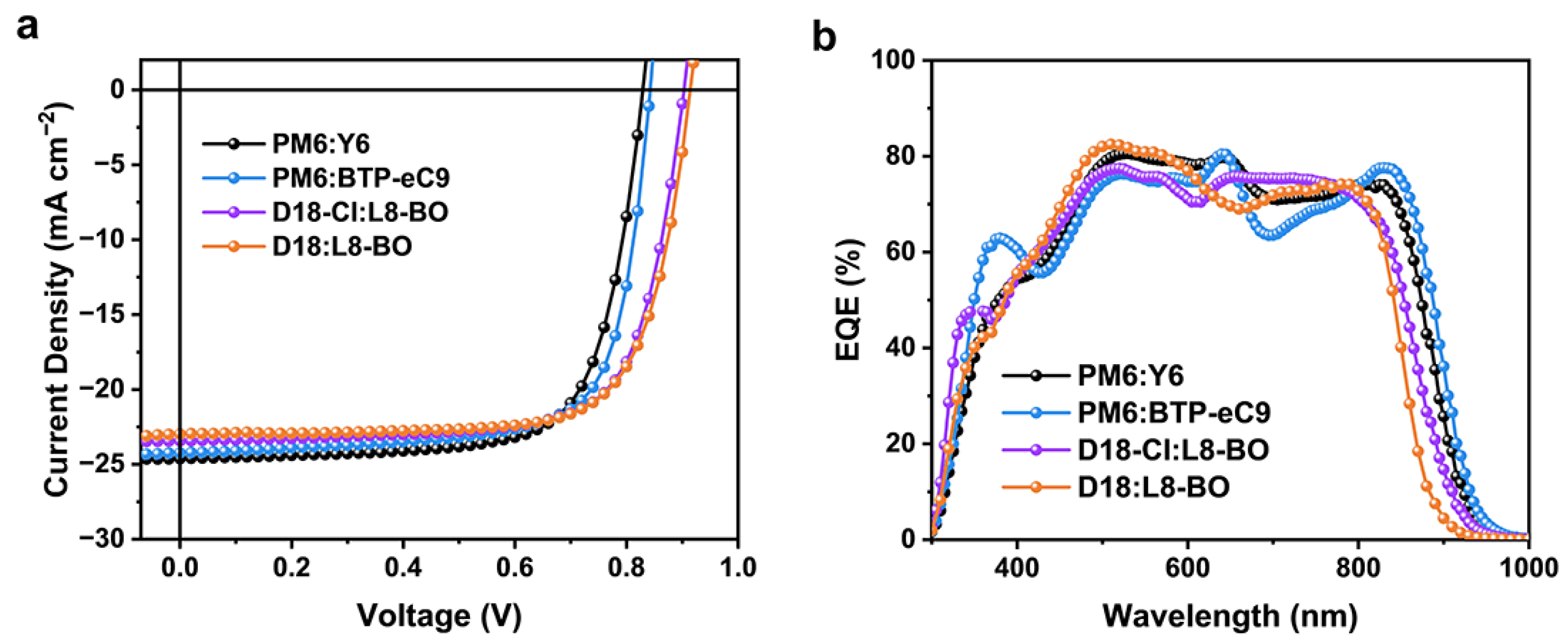
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.-G.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Andersen, T.R.; Larsen-Olsen, T.T.; Andreasen, B.; Böttiger, A.P.L.; Carlé, J.E.; Helgesen, M.; Bundgaard, E.; Norrman, K.; Andreasen, J.W.; Jørgensen, M.; et al. Aqueous Processing of Low-Band-Gap Polymer Solar Cells Using Roll-to-Roll Methods. ACS Nano 2011, 5, 4188–4196. [Google Scholar] [CrossRef]
- Hashemi, S.A.; Ramakrishna, S.; Aberle, A.G. Recent Progress in Flexible–Wearable Solar Cells for Self-Powered Electronic Devices. Energy Environ. Sci. 2020, 13, 685–743. [Google Scholar] [CrossRef]
- Zhang, D.; Ji, Y.; Cheng, Y.; Liu, X.; Xia, Z.; Liu, X.; Liu, X.; Yang, X.; Huang, W. High-Efficiency Ultrathin Flexible Organic Solar Cells with a Bilayer Hole Transport Layer. J. Mater. Chem. A 2024, 12, 15099–15105. [Google Scholar] [CrossRef]
- Chen, C.; Wang, L.; Xia, W.; Qiu, K.; Guo, C.; Gan, Z.; Zhou, J.; Sun, Y.; Liu, D.; Li, W.; et al. Molecular Interaction Induced Dual Fibrils towards Organic Solar Cells with Certified Efficiency over 20%. Nat. Commun. 2024, 15, 6865. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, M.; Zhou, G.; Wang, Z.; Zhong, W.; Zhuang, J.; Zhou, Z.; Gao, X.; Kan, L.; Hao, B.; et al. Achieving 20.8% Organic Solar Cells via Additive-Assisted Layer-by-Layer Fabrication with Bulk p-i-n Structure and Improved Optical Management. Joule 2024, 8, 3153–3168. [Google Scholar] [CrossRef]
- Wei, N.; Chen, J.; Cheng, Y.; Bian, Z.; Liu, W.; Song, H.; Guo, Y.; Zhang, W.; Liu, Y.; Lu, H.; et al. Constructing Multiscale Fibrous Morphology to Achieve 20% Efficiency Organic Solar Cells by Mixing High and Low Molecular Weight D18. Adv. Mater. 2024, 36, 2408934. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, L.; Guo, C.; Xiao, J.; Liu, C.; Chen, C.; Xia, W.; Gan, Z.; Cheng, J.; Zhou, J.; et al. π-Extended Nonfullerene Acceptor for Compressed Molecular Packing in Organic Solar Cells to Achieve over 20% Efficiency. J. Am. Chem. Soc. 2024, 146, 12011–12019. [Google Scholar] [CrossRef]
- Kaltenbrunner, M.; White, M.S.; Głowacki, E.D.; Sekitani, T.; Someya, T.; Sariciftci, N.S.; Bauer, S. Ultrathin and Lightweight Organic Solar Cells with High Flexibility. Nat. Commun. 2012, 3, 770. [Google Scholar] [CrossRef]
- Jinno, H.; Yokota, T.; Koizumi, M.; Yukita, W.; Saito, M.; Osaka, I.; Fukuda, K.; Someya, T. Self-Powered Ultraflexible Photonic Skin for Continuous Bio-Signal Detection via Air-Operation-Stable Polymer Light-Emitting Diodes. Nat. Commun. 2021, 12, 2234. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ochiai, Y.; Wu, N.; Adachi, K.; Inoue, D.; Hashizume, D.; Kong, D.; Matsuhisa, N.; Yokota, T.; Wu, Q.; et al. Intrinsically Stretchable Organic Photovoltaics by Redistributing Strain To PEDOT:PSS with Enhanced Stretchability and Interfacial Adhesion. Nat. Commun. 2024, 15, 4902. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Kee, S.; Lee, S.H.; Lee, B.H.; Kahng, Y.H.; Jo, Y.-R.; Kim, B.-J.; Lee, K. Highly Conductive PEDOT:PSS Nanofibrils Induced by Solution-Processed Crystallization. Adv. Mater. 2014, 26, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; Wang, S.; Jia, Y.; Bian, Z.; Wu, D.; Zhang, L.; Cao, A.; Huang, C. Infrared-Transparent Polymer Solar Cells. J. Mater. Chem. 2010, 20, 8478–8482. [Google Scholar] [CrossRef]
- Liu, Z.; You, P.; Liu, S.; Yan, F. Neutral-Color Semitransparent Organic Solar Cells with All-Graphene Electrodes. ACS Nano 2015, 9, 12026–12034. [Google Scholar] [CrossRef]
- Jeon, I.; Delacou, C.; Kaskela, A.; Kauppinen, E.I.; Maruyama, S.; Matsuo, Y. Metal-Electrode-Free Window-Like Organic Solar Cells with P-Doped Carbon Nanotube Thin-film Electrodes. Sci. Rep. 2016, 6, 31348. [Google Scholar] [CrossRef]
- Jeon, I.; Xiang, R.; Shawky, A.; Matsuo, Y.; Maruyama, S. Single-Walled Carbon Nanotubes in Emerging Solar Cells: Synthesis and Electrode Applications. Adv. Energy Mater. 2019, 9, 1801312. [Google Scholar] [CrossRef]
- Lee, C.; Oh, Y.; Yoon, I.S.; Kim, S.H.; Ju, B.-K.; Hong, J.-M. Flash-Induced Nanowelding of Silver Nanowire Networks for Transparent Stretchable Electrochromic Devices. Sci. Rep. 2018, 8, 2763. [Google Scholar] [CrossRef]
- Fan, X.; Wen, R.J.; Xia, Y.G.; Wang, J.Z.; Liu, X.H.; Huang, H.H.; Li, Y.; Zhu, W.Y.; Cheng, Y.J.; Ma, L.J.; et al. Vacuum-Free, All-Solution, and All-Air Processed Organic Photovoltaics with over 11% Efficiency and Promoted Stability Using Layer-by-Layer Codoped Polymeric Electrodes. Sol. RRL 2020, 4, 1900543. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.-H.; Lee, S.; Jeong, S.Y.; Choi, J.W.; Lee, C.-L.; Kim, J.-H.; Lee, K. Retarding Ion Exchange between Conducting Polymers and Ionic Liquids for Printable Top Electrodes in Semitransparent Organic Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 2276–2284. [Google Scholar] [CrossRef]
- Kang, M.H.; Cheon, T.; Kim, H. Fully Vacuum-Free Large-Area Organic Solar Cell Fabrication from Polymer Top Electrode. Solid-State Electron. 2021, 186, 108192. [Google Scholar] [CrossRef]
- Zeng, G.; Chen, W.; Chen, X.; Hu, Y.; Chen, Y.; Zhang, B.; Chen, H.; Sun, W.; Shen, Y.; Li, Y.; et al. Realizing 17.5% Efficiency Flexible Organic Solar Cells via Atomic-Level Chemical Welding of Silver Nanowire Electrodes. J. Am. Chem. Soc. 2022, 144, 8658–8668. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Lei, X.; Hu, Y.; Liu, X.; Ji, Y.; Zhang, D.; Cheng, Y.; Liu, X.; Xu, Z.; Yang, X.; et al. Ultrathin Flexible Indium Tin Oxide-Free Organic Solar Cells with Gradient Bilayer Electron Transport Materials. Sol. RRL 2024, 8, 2400230. [Google Scholar] [CrossRef]
- Yu, B.; Liu, L.; Liu, B.; Zhao, X.; Deng, W. Printing of Low-Melting-Point Alloy as Top Electrode for Organic Solar Cells. Adv. Opt. Mater. 2023, 11, 2201977. [Google Scholar] [CrossRef]
- Wan, Q.; Seo, S.; Lee, S.-W.; Lee, J.; Jeon, H.; Kim, T.-S.; Kim, B.J.; Thompson, B.C. High-Performance Intrinsically Stretchable Polymer Solar Cell with Record Efficiency and Stretchability Enabled by Thymine-Functionalized Terpolymer. J. Am. Chem. Soc. 2023, 145, 11914–11920. [Google Scholar] [CrossRef]
- Foremny, K.; Nagels, S.; Kreienmeyer, M.; Doll, T.; Deferme, W. Biocompatibility Testing of Liquid Metal as an Interconnection Material for Flexible Implant Technology. Nanomaterials 2021, 11, 3251. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, H.; Li, G.; Peng, M.; Ma, X.; Li, M.; Yan, S. Construction of Liquid Metal-Based Soft Microfluidic Sensors via Soft Lithography. J. Nanobiotechnol. 2022, 20, 246. [Google Scholar] [CrossRef]
- Jiao, C.; Li, L.; Lu, B.; Wang, Q.; Hong, W.; Chen, X.; Chang, L.; Wang, X.; Wang, Y.; Sun, K.; et al. Giant Electrical Conductivity Difference Enabled Liquid Metal-Hydrogel Hybrid Printed Circuits for Soft Bioelectronics. Chem. Eng. J. 2024, 482, 148951. [Google Scholar] [CrossRef]
- Xu, X.; Shen, Y.; Xing, R.; Kong, J.; Su, R.; Huang, R.; Qi, W. Galvanic Replacement Synthesis of VOx@EGaIn–PEG Core–Shell Nanohybrids for Peroxidase Mimics. ACS Appl. Mater. Interfaces 2024, 16, 21975–21986. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, S.; Dickey, M.D.; So, J.-H.; Koo, H.-J. Interface of Gallium-Based Liquid Metals: Oxide Skin, Wetting, and Applications. Nanoscale Horiz. 2024, 9, 1099–1119. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Zhang, J.; Jiang, X.; Zhu, Z.; Liu, Q. Interface Engineering Based on Liquid Metal for Compact-Layer-free, Fully Printable Mesoscopic Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2018, 10, 15616–15623. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, Y.; Hoang, M.T.; Chiu, W.-H.; Pang, L.; O’Mullane, A.P.; Wang, H. Incorporation of Liquid Metal Gallium into Carbon Electrode for Efficient Charge Transportation in Planar Perovskite Solar Cells. Sol. RRL 2024, 8, 2300732. [Google Scholar] [CrossRef]
- Wang, J.; Fukuda, K.; Inoue, D.; Hashizume, D.; Sun, L.; Xiong, S.; Yokota, T.; Someya, T. Solution-Processed Electron-Transport Layer-free Organic Photovoltaics with Liquid Metal Cathodes. ACS Appl. Mater. Interfaces 2022, 14, 14165–14173. [Google Scholar] [CrossRef]
- Noh, J.; Kim, G.-U.; Han, S.; Oh, S.J.; Jeon, Y.; Jeong, D.; Kim, S.W.; Kim, T.-S.; Kim, B.J.; Lee, J.-Y. Intrinsically Stretchable Organic Solar Cells with Efficiencies of over 11%. ACS Energy Lett. 2021, 6, 2512–2518. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Xu, M.; Liu, J.; He, J.; Yang, L.; Li, Z.; Gao, Y.; Shao, M. Intrinsically Stretchable Organic Solar Cells with Simultaneously Improved Mechanical Robustness and Morphological Stability Enabled by a Universal Crosslinking Strategy. Small 2022, 18, 2201589. [Google Scholar] [CrossRef]
- Seo, S.; Lee, J.-W.; Kim, D.J.; Lee, D.; Phan, T.N.-L.; Park, J.; Tan, Z.; Cho, S.; Kim, T.-S.; Kim, B.J. Poly(dimethylsiloxane)-block-PM6 Polymer Donors for High-Performance and Mechanically Robust Polymer Solar Cells. Adv. Mater. 2023, 35, 2300230. [Google Scholar] [CrossRef]
- Lee, J.-W.; Sun, C.; Lee, S.; Kim, D.J.; Oh, E.S.; Phan, T.N.-L.; Nguyen, T.H.-Q.; Seo, S.; Tan, Z.; Lee, M.J.; et al. High-performance Intrinsically Stretchable Organic Solar Cells Based on Flexible Spacer Incorporated Dimerized Small-Molecule Acceptors. Nano Energy 2024, 125, 109541. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, D.; Yang, L.; Allam, O.; Gao, Y.; Su, Y.; Xu, M.; Mo, S.; Wu, Q.; Wang, Z.; et al. Mechanically Robust and Stretchable Organic Solar Cells Plasticized by Small-Molecule Acceptors. Science 2025, 387, 381–387. [Google Scholar] [CrossRef]
- Hardy, S.C. The Surface Tension of Liquid Gallium. J. Cryst. Growth 1985, 71, 602–606. [Google Scholar] [CrossRef]
- Zrnic, D.; Swatik, D.S. On the Resistivity and Surface Tension of the Eutectic Alloy of Gallium and Indium. J. Less Common Met. 1969, 18, 67–68. [Google Scholar] [CrossRef]
- Prokhorenko, V.Y.; Roshchupkin, V.V.; Pokrasin, M.A.; Prokhorenko, S.V.; Kotov, V.V. Liquid Gallium: Potential Uses as a Heat-Transfer Agent. High Temp. 2000, 38, 954–968. [Google Scholar] [CrossRef]
- Neupane, S.; Li, D.-B.; Jamarkattel, M.K.; Abudulimu, A.; Jiang, C.-S.; Bista, S.S.; Adhikari, A.; Budhathoki, S.; Sharif, H.; Lamichhane, K.; et al. Evaporated CdSe for Efficient Polycrystalline CdSeTe Thin-Film Solar Cells. ACS Energy Lett. 2024, 9, 6233–6237. [Google Scholar] [CrossRef]
- Mortadi, A.; El Hafidi, E.; Nasrellah, H.; Monkade, M.; El Moznine, R. Analysis and Optimization of Lead-Free Perovskite Solar Cells: Investigating Performance and Electrical Characteristics. Mater. Renew. Sustain. Energy 2024, 13, 219–232. [Google Scholar] [CrossRef]
- Mortadi, A.; Tabbai, Y.; El Hafidi, E.; Nasrellah, H.; Chahid, E.; Monkade, M.; El Moznine, R. Investigating Temperature Effects on Perovskite Solar Cell Performance via SCAPS-1D and Impedance Spectroscopy. Clean. Eng. Technol. 2025, 24, 100876. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, D.-H.; Park, N.-G.; Ko, M.J.; Cho, J.; Koo, H.J. Liquid Metal-Based Perovskite Solar Cells: In Situ Formed Gallium Oxide Interlayer Improves Stability and Efficiency. Adv. Funct. Mater. 2024, 34, 2311597. [Google Scholar] [CrossRef]

| Top Electrodes | VOC (V) | JSC (mA cm−2) | Cal.JSC (mA cm−2) | FF (%) | PCEmax (%) | PCEavg ± SD (%) |
|---|---|---|---|---|---|---|
| Ga | 0.885 | 23.1 | 22.3 | 70.2 | 14.4 | 14.0 ± 0.4 |
| EGaIn | 0.890 | 23.6 | 23.0 | 74.5 | 15.6 | 15.4 ± 0.2 |
| Galinstan | 0.889 | 23.3 | 22.6 | 73.6 | 15.3 | 15.0 ± 0.3 |
| Active Layer | VOC (V) | JSC (mA cm−2) | FF (%) | PCEmax (%) | PCEavg ± SD (%) |
|---|---|---|---|---|---|
| PM6:Y6 | 0.829 | 24.6 | 72.3 | 14.7 | 14.3 ± 0.4 |
| PM6:BTP-eC9 | 0.842 | 24.5 | 73.1 | 15.1 | 14.6 ± 0.5 |
| D18:L8-BO | 0.914 | 23.0 | 73.6 | 15.5 | 15.1 ± 0.4 |
| D18-Cl:L8-BO | 0.903 | 23.4 | 72.9 | 15.4 | 15.0 ± 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, R.; Xie, D.; Jin, Y.; Ren, X.; Huang, X.; Ji, Y.; Liu, X.; Yang, X.; Huang, W. High-Performance Vacuum-Free Processed Organic Solar Cells with Gallium-Based Liquid Metal Top Electrodes. Materials 2025, 18, 2675. https://doi.org/10.3390/ma18122675
Hu R, Xie D, Jin Y, Ren X, Huang X, Ji Y, Liu X, Yang X, Huang W. High-Performance Vacuum-Free Processed Organic Solar Cells with Gallium-Based Liquid Metal Top Electrodes. Materials. 2025; 18(12):2675. https://doi.org/10.3390/ma18122675
Chicago/Turabian StyleHu, Rui, Di Xie, Yi Jin, Xiaojie Ren, Xiang Huang, Yitong Ji, Xiaotong Liu, Xueyuan Yang, and Wenchao Huang. 2025. "High-Performance Vacuum-Free Processed Organic Solar Cells with Gallium-Based Liquid Metal Top Electrodes" Materials 18, no. 12: 2675. https://doi.org/10.3390/ma18122675
APA StyleHu, R., Xie, D., Jin, Y., Ren, X., Huang, X., Ji, Y., Liu, X., Yang, X., & Huang, W. (2025). High-Performance Vacuum-Free Processed Organic Solar Cells with Gallium-Based Liquid Metal Top Electrodes. Materials, 18(12), 2675. https://doi.org/10.3390/ma18122675






