1. Introduction
Electrochemical sensors have emerged as indispensable tools in various fields due to their exceptional advantages, including high sensitivity, excellent selectivity, rapid detection, and low cost. These attributes have facilitated their widespread application in areas such as clinical diagnostics, industrial processes, environmental monitoring, and agricultural analysis [
1]. Enhancing the sensitivity of electrochemical sensors remains a critical research focus, with significant efforts dedicated to improving electrode materials. In particular, catalytic electrodes incorporating small-sized noble metal particles—renowned for their high catalytic activity—supported on polyaniline (PANI) have demonstrated significant enhancements in catalytic performance for the electrochemical oxidation of organic compounds, including ethanol, methanol, propanol, and glucose [
2,
3,
4,
5,
6].
Polyaniline (PANI) stands out as an excellent support material for catalytic clusters due to its high active surface area, decent electrical conductivity, excellent stability, and simple preparation process [
7]. The integration of titanium dioxide (TiO
2) decorated with gold nanoparticles (AuNPs) and PANI as a support material has been shown to ensure the uniform distribution of AuNPs on TiO
2 and the homogeneous deposition of the resulting AuNP/TiO
2 composite particles on PANI. This configuration has been demonstrated to further enhance catalytic activity, particularly for glucose oxidation [
8]. Palladium has also emerged as a promising catalytic material. For instance, palladium/poly(3,4-ethylenedioxythiophene) (Pd/PEDOT) composite-decorated glassy carbon electrodes (GCEs) have exhibited significantly enhanced catalytic activity for H
2O
2 sensing [
9].
A key strategy for improving catalytic activity is reducing the size of catalytic materials [
2,
7,
9,
10,
11]. For example, studies on platinum nanoparticles supported on PANI have revealed that the combination of PANI and platinum nanoparticles exhibits superior catalytic performance [
11]. Additionally, comparisons between bulk-sized gold and atomic-level gold have shown that, while bulk-sized gold is catalytically inert in propanol oxidation, atomic-level gold exhibits notable catalytic activity [
2]. These findings highlight the potential of reducing the size of Pt clusters supported on PANI to achieve exceptional catalytic properties.
The size effects of noble metals are particularly significant at the atomic scale, where the smallest theoretical size corresponds to the atomic state. Controlling noble metals at the atomic scale offers the potential to develop materials with unique and exceptional catalytic properties for sensor and catalytic applications. A previous study [
10] reported the fabrication of atomic-level Au/PANI composite electrodes using a cyclic atomic-metal electrodeposition method. In this method, tetrachloroaurate(III) ions serve as the source of atomic-level Au clusters. The interactions between the iminium groups in emeraldine salt PANI and the gold chloride complexes enable the decoration of atomic-level Au clusters. Specifically, clusters composed of single gold atoms are deposited on the PANI in one cycle, while clusters consisting of two gold atoms are deposited after the second cycle. Interestingly, the catalytic activity of these atomic-level Au clusters for alcohol oxidation revealed a distinct “even–odd effect”, wherein clusters with an even number of gold atoms exhibited higher catalytic activity than those with an odd number [
2,
5,
10]. Similarly, palladium has also demonstrated enhanced catalytic activity when arranged in even-numbered atomic-level metal clusters [
12].
Furthermore, the catalytic activity of bi-metallic catalysts differs significantly from that of mono-metallic catalysts [
12,
13]. Simulation studies have shown that the physical properties of atomic-level bimetallic clusters are highly dependent on their composition [
13]. Experimental studies have further demonstrated that combining gold and palladium results in substantial changes in catalytic activity [
12]. Notably, even when the ratio of the two metal elements in atomic-level bimetallic clusters is identical, the sequence in which the atoms are deposited has a significant impact on catalytic performance. These findings suggest that mixing different noble metal atoms can yield catalytic activity trends distinct from those observed in mono-metallic systems.
In this study, we investigate the catalytic properties of PANI-supported atomic-level clusters for propanol oxidation. Specifically, PANI-PtN clusters (where N corresponds to the cycle numbers of the cyclic atomic-metal electrodeposition process conducted and is suggested to represent the atomic size of the cluster, with N = 1–6), consisting of atomic-level Pt clusters supported on PANI, and PANI-PtyAuz clusters (where y and z correspond to the cycle numbers of the atomic electrodeposition process with K2PtCl4 and KAuCl, respectively, with 1 ≤ y + z ≤ 4), composed of atomic-level Pt-Au clusters supported on PANI, were fabricated using the cyclic atomic-metal electrodeposition method. The effects of cluster size and deposition sequence on the catalytic activity for propanol oxidation were systematically examined to evaluate their potential as catalytic electrodes in electrochemical sensors.
2. Materials and Methods
Aniline (C6H5NH2, 99.5%), perchloric acid (HClO4, 70%), tetrafluoroboric acid (HBF4, 48%), propanol (C3H8O, 99.5%), potassium tetrachloroaurate(III) (KAuCl4, 99.99%), dipotassium tetrachloroplatinate(II) (K2PtCl4, 99.99%), and phosphate-buffered saline (PBS, P5493) were purchased from Sigma-Aldrich (Tokyo, Japan). KAuCl4 and K2PtCl4 were used to form the gold chloride complex and platinum chloride complex, respectively. Potassium chloride (KCl, 99.5+%) and potassium hydroxide (KOH, 86+%) were obtained from Kanto Chemical Co. (Tokyo, Japan).
A platinum disk electrode with polyether ether ketone (PEEK) isolation and a diameter of 3 mm (PTE Platinum Electrode, No. 002422, ALS Co., Sagamihara, Japan) served as the working electrode (WE), while a platinum plate with a surface area of 2 cm2 was used as the counter electrode (CE). A Ag/AgCl/Sat. KCl electrode (Ag/AgCl, RE-1S, ALS Co., Tokyo, Japan) was employed as the reference electrode (RE).
Prior to PANI deposition, the platinum WE was pretreated in a saturated KCl solution. During the pretreatment, a potential of +1.5 V vs. Ag/AgCl was applied for 180 s while stirring at 400 rpm using a magnetic stirrer. The WE surface was cleaned by rinsing with ultrapure water to ensure complete removal of the residual electrolyte.
PANI was deposited via electropolymerization using an electrolytic solution containing 0.1 M aniline and 2 M HBF
4, as reported in a previous study [
4]. The electropolymerization process consisted of two steps: (1) sweeping the applied potential from 0 to +1.5 V vs. Ag/AgCl at a scan rate of 50 mV/s, followed by (2) applying a constant current of 0.05 mA for 260 s.
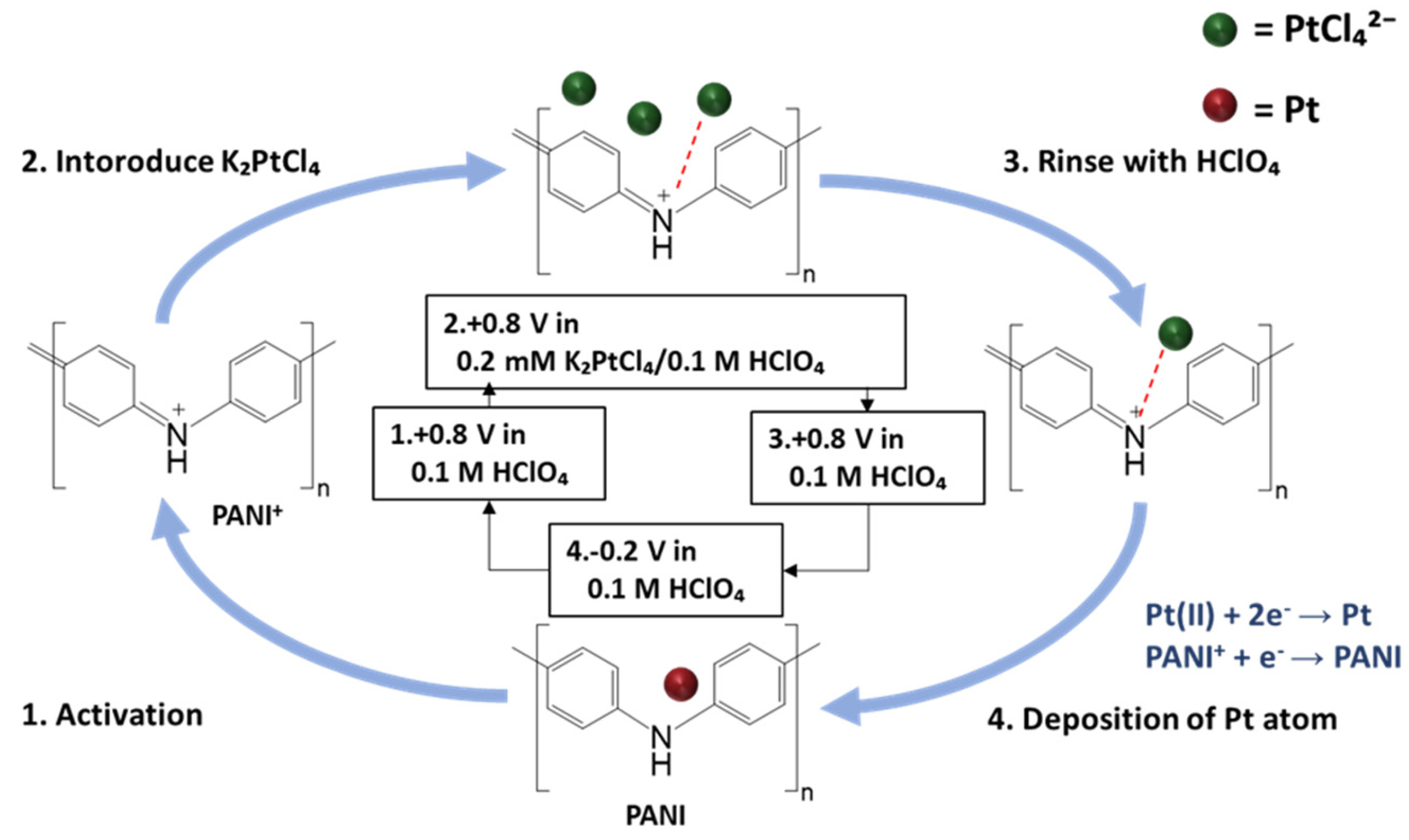
The cyclic atomic-metal electrodeposition process was performed in a three-electrode system, with the PANI-coated Pt electrode as the WE, a Pt foil as the CE, and an Ag/AgCl electrode serving as the RE.
Figure 1 illustrates one cycle of the cyclic atomic-metal electrodeposition process, which consisted of the following four steps:
Activation step: The base solution was 0.1 M HClO4. The base solution was introduced into the flow cell, and the potential was swept from −0.2 V to +0.8 V to activate PANI into its emeraldine salt state.
PANI–metal chloride complex formation step: The PtCl42− (or AuCl4−) was introduced into the flow cell by injecting 0.2 mM K2PtCl4 (or KAuCl4) in the base solution into the flow cell, while maintaining the potential at +0.8 V. During this step, the metal chloride complexes in the base solution were attracted to the iminium groups.
Rinsing step: The WE surface was rinsed with the base solution to remove any excess metal chloride complexes that were not bound to the iminium groups, while maintaining the potential at +0.8 V.
Reduction step: The potential was swept from +0.8 V to −0.2 V to reduce the metal chloride complexes.
The reduction of one metal chloride complex attached to the iminium group would produce one metal atom. The cycle was repeated to increase the size of the atomic-level metal clusters deposited on the PANI electrode. To fabricate atomic-level bimetallic clusters, the solution introduced into the flow cell was alternated between 0.2 mM KAuCl4 and 0.2 mM K2PtCl4 in the base solution.
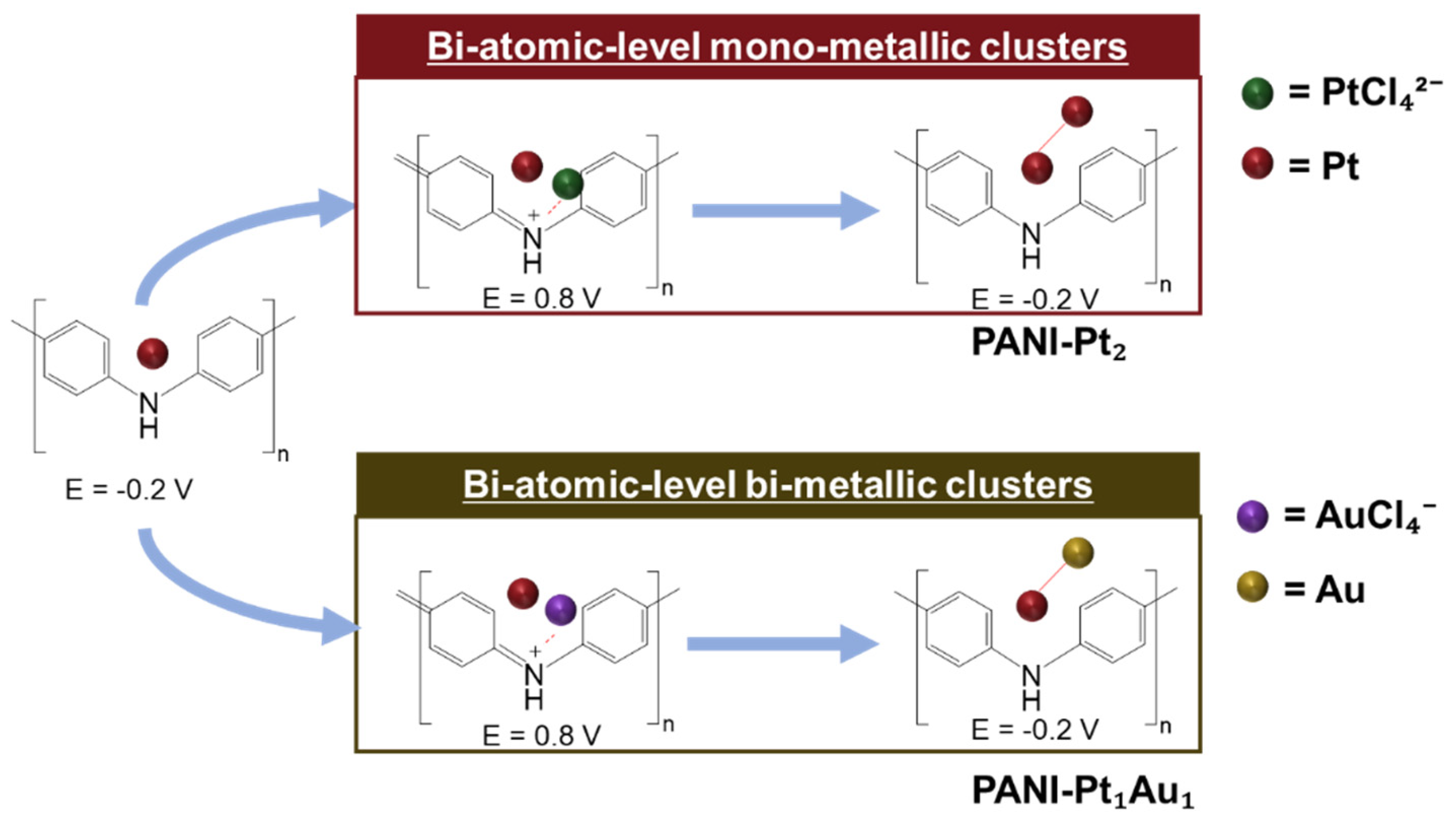
Figure 2 schematically illustrates the procedure for decorating atomic-level Pt clusters (e.g., PANI-Pt
2: Pt deposited in both the first and second cycles) and PANI-Pt
1Au
1 clusters (Pt deposited in the first cycle and Au in the second cycle) on the PANI electrode. The naming of the atomic-level metal clusters was based on the sequence of decoration. For example, PANI-Au
1Pt
1 refers to bi-atomic-level bi-metallic clusters where Au was deposited in the first cycle and Pt in the second cycle, while PANI-Pt
2Au
1 refers to tri-atomic-level bi-metallic clusters where Pt was deposited in the first two cycles and Au in the third cycle.
Cyclic voltammetry (CV) was conducted using a three-electrode system with the atomic-level metal cluster-decorated PANI, a Pt foil, and an Ag/AgCl electrode as the We, CE, and RE, respectively, to evaluate the catalytic activity for propanol oxidation. Prior to the CV, an activation step was performed by subjecting the WE to 10 CV cycles in 1 M KOH within a potential range of −0.6 V to +0.4 V at a scan rate of 100 mV/s. For catalytic activity measurements, an aqueous solution containing 1.3 M propanol and 1 M KOH was used. For the CV, the potential range was from −0.6 V to +0.4 V at a scan rate of 50 mV/s for 20 cycles. The CV curve from the final cycle was used as the result in this study.
3. Results and Discussion
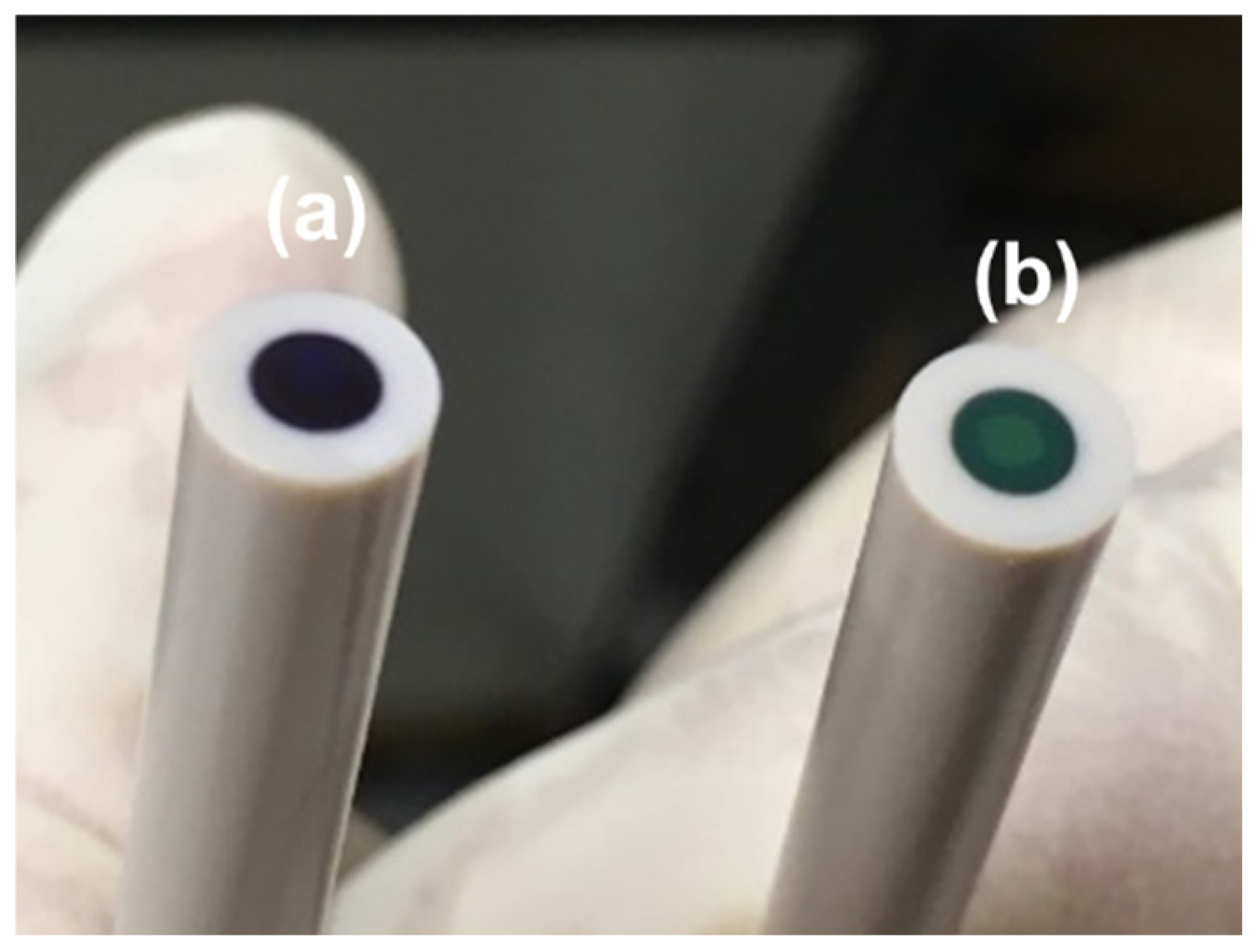
The as-fabricated PANI on the Pt electrode exhibited a dark color, as shown in
Figure 3a. The electrolyte used during the electropolymerization process contained 2 M HBF
4, and a highly positive potential was applied at the end of the process. Therefore, the dark-colored PANI is likely in the fully oxidized pernigraniline salt form. After undergoing the cyclic atomic-metal electrodeposition process, the color changed to emerald green, as illustrated in
Figure 3b. During this process, a 0.1 M HClO
4 solution was used as the electrolyte, and a potential of −0.2 V was applied at the end of the process. As a result, the PANI transitioned to the emeraldine salt form.
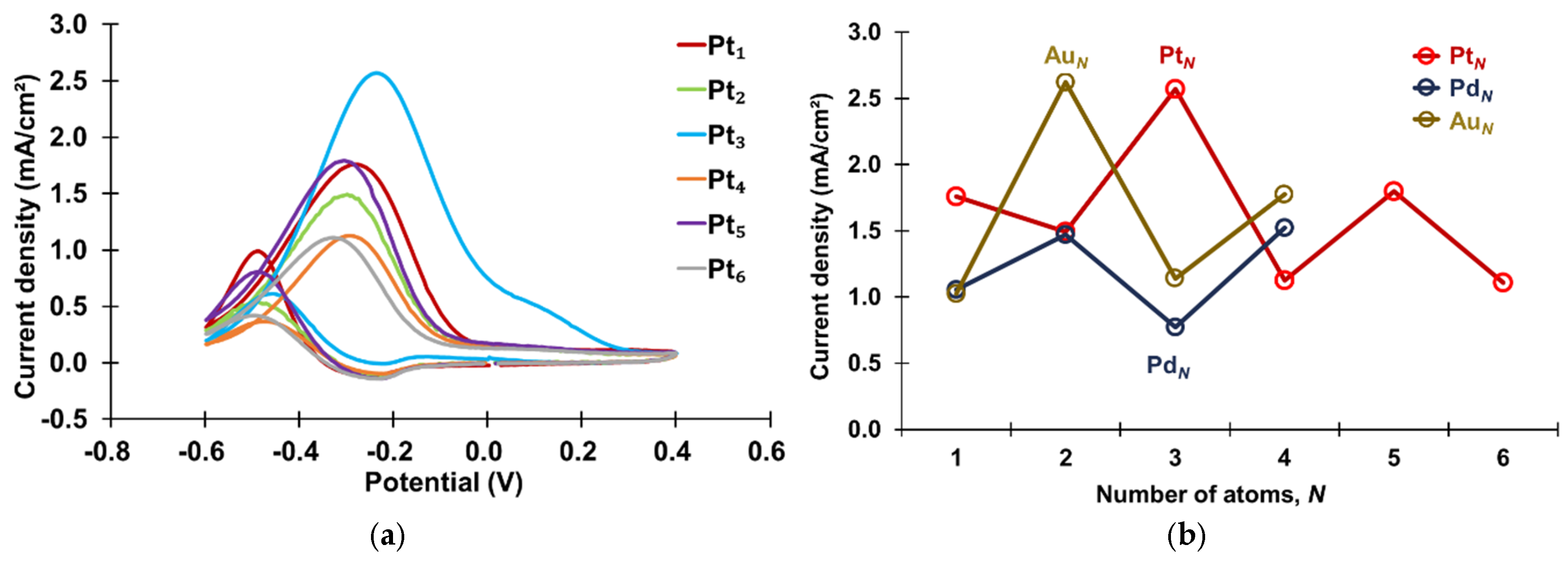
The CVs of atomic-level Pt clusters, PANI-Pt
N (
N = 1–6), in 1.3 M propanol and 1 M KOH solution are presented in
Figure 4a. The oxidation current response at approximately −0.3 V during the forward scan corresponds to the oxidation of propanol [
14]. Generally, two intermediate reactions are involved in the oxidation of propanol. In the forward scan of the CVs, the first peak corresponds to the oxidation of propanol, with the product suggested to be propionaldehyde. The second peak corresponds to the subsequent oxidation reaction, attributed to the oxidation of propionaldehyde to propionic acid. The peak oxidation current density at approximately −0.3 V was used as an indicator of catalytic activity, with higher values signifying enhanced catalytic performance. The current densities for PANI-Pt
1, PANI-Pt
3, and PANI-Pt
5 were 1.76 mA/cm
2, 2.57 mA/cm
2, and 1.80 mA/cm
2, respectively, whereas those for PANI-Pt
2, PANI-Pt
4, and PANI-Pt
6 were 1.50 mA/cm
2, 1.13 mA/cm
2, and 1.11 mA/cm
2, respectively. These results reveal an even–odd effect in the catalytic activity of atomic-level Pt clusters decorated on PANI, with odd-numbered atomic-level Pt clusters demonstrating higher activity than even-numbered ones. This trend contrasts with the even–odd effect observed for atomic-level Au and Pd clusters in previous studies, where even-numbered mono-metallic clusters exhibited greater catalytic activity [
10,
12,
15]. A comparative analysis of these findings is illustrated in
Figure 4b.
The superior catalytic activity of odd-numbered atomic-level Pt clusters is attributed to changes in their electronic structure. Prior studies have shown that the HOMO-LUMO band gap of atomic-level Pt clusters varies quantumly with the number of atoms, with odd-numbered clusters exhibiting a larger band gap and, consequently, higher catalytic activity [
16]. Similar trends in HOMO-LUMO band gaps have been reported for Au and Pd atomic clusters, where even-numbered clusters displayed larger band gaps and higher activity [
17]. These quantum variations in the HOMO-LUMO band gap likely account for the observed odd–even effect in catalytic activity.
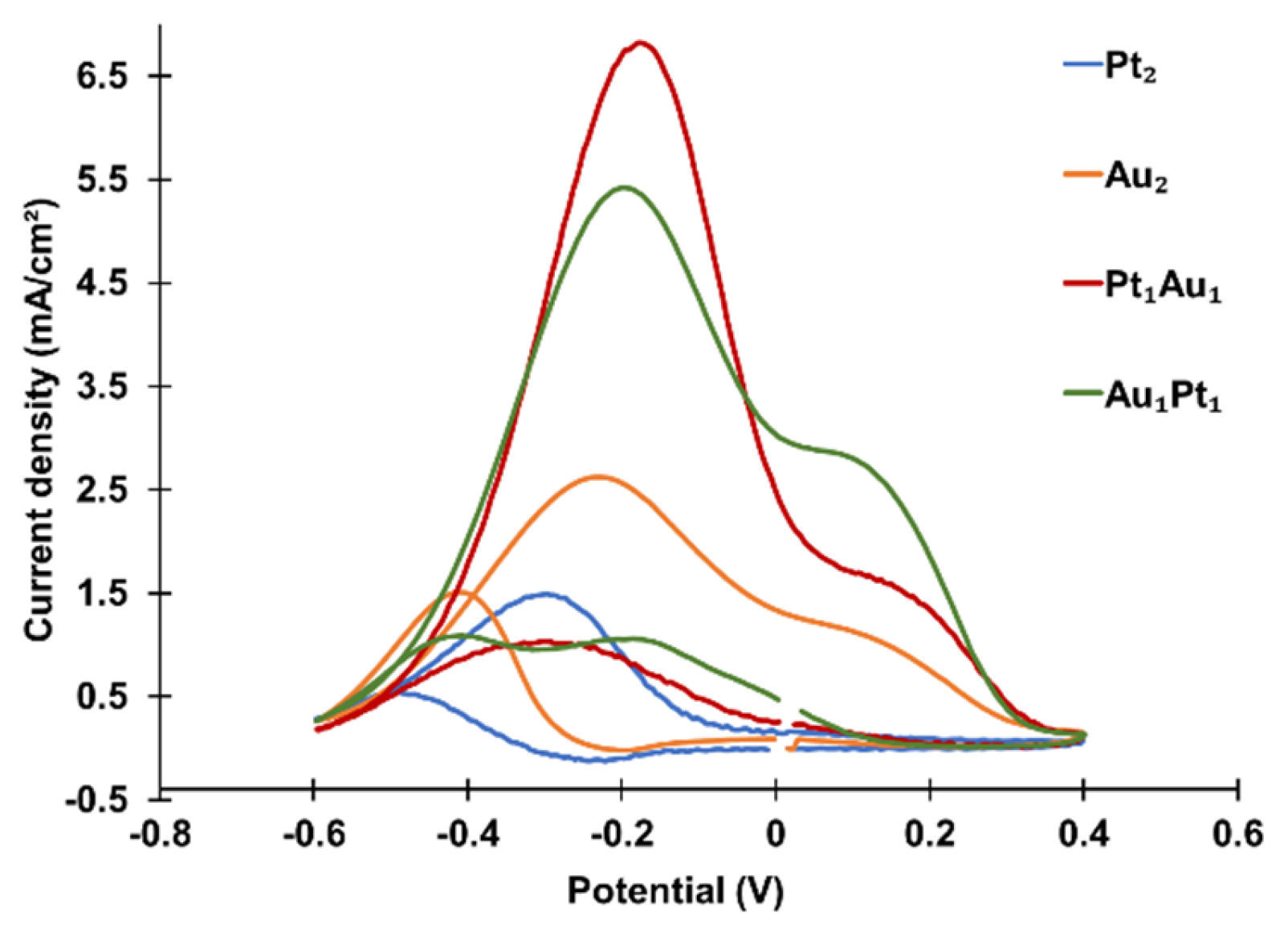
Four combinations of bi-atomic-level metal clusters were prepared: PANI-Pt
2, PANI-Au
2, PANI-Pt
1Au
1 (Pt deposited first), and PANI-Au
1Pt
1 (Au deposited first). The CVs of these clusters are shown in
Figure 5, and the peak oxidation current densities are summarized in
Table 1. Among these, PANI-Pt
1Au
1 exhibited the highest catalytic activity, followed by PANI-Au
1Pt
1, PANI-Au
2, and PANI-Pt
2. The results indicate that bi-atomic-level bi-metallic clusters (PANI-Pt
1Au
1 and PANI-Au
1Pt
1) exhibit higher catalytic activity than bi-atomic-level mono-metallic clusters (PANI-Pt
2 and PANI-Au
2). Regarding the effects of the deposition sequence, PANI-Pt
1Au
1 exhibited higher catalytic activity than PANI-Au
1Pt
1, suggesting that depositing Pt before Au is beneficial for improving catalytic performance.
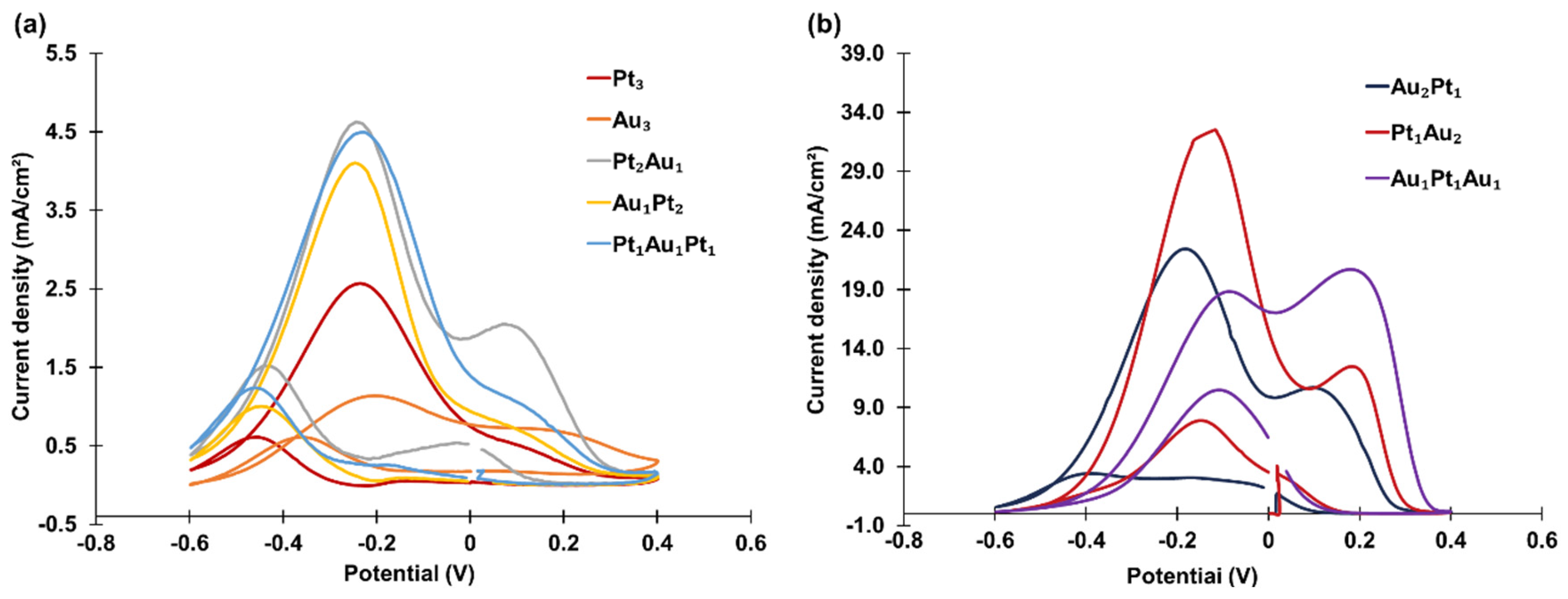
Eight tri-atomic-level metal cluster combinations were investigated, including two mono-metallic clusters (PANI-Pt
3 and PANI-Au
3) and six bi-metallic clusters: PANI-Pt
1Au
2, PANI-Au
1Pt
1Au
1, PANI-Au
2Pt
1, PANI-Pt
2Au
1, PANI-Pt
1Au
1Pt
1, and PANI-Au
1Pt
2. The CVs of these clusters are divided into two groups and presented in
Figure 6. In
Figure 6a, the tri-atomic-level metal clusters contain zero or more than two Pt atoms, while in
Figure 6b, the clusters contain one Pt atom. The corresponding peak oxidation current densities are summarized in
Table 2.
Again, the bi-metallic clusters showed higher catalytic activity than the mono-metallic clusters. Comparisons between
Figure 6a,b revealed that the catalytic activity was influenced by the composition, with the three tri-atomic-level metal clusters containing one Pt atom exhibiting much higher catalytic activity than the other five tri-atomic-level metal clusters.
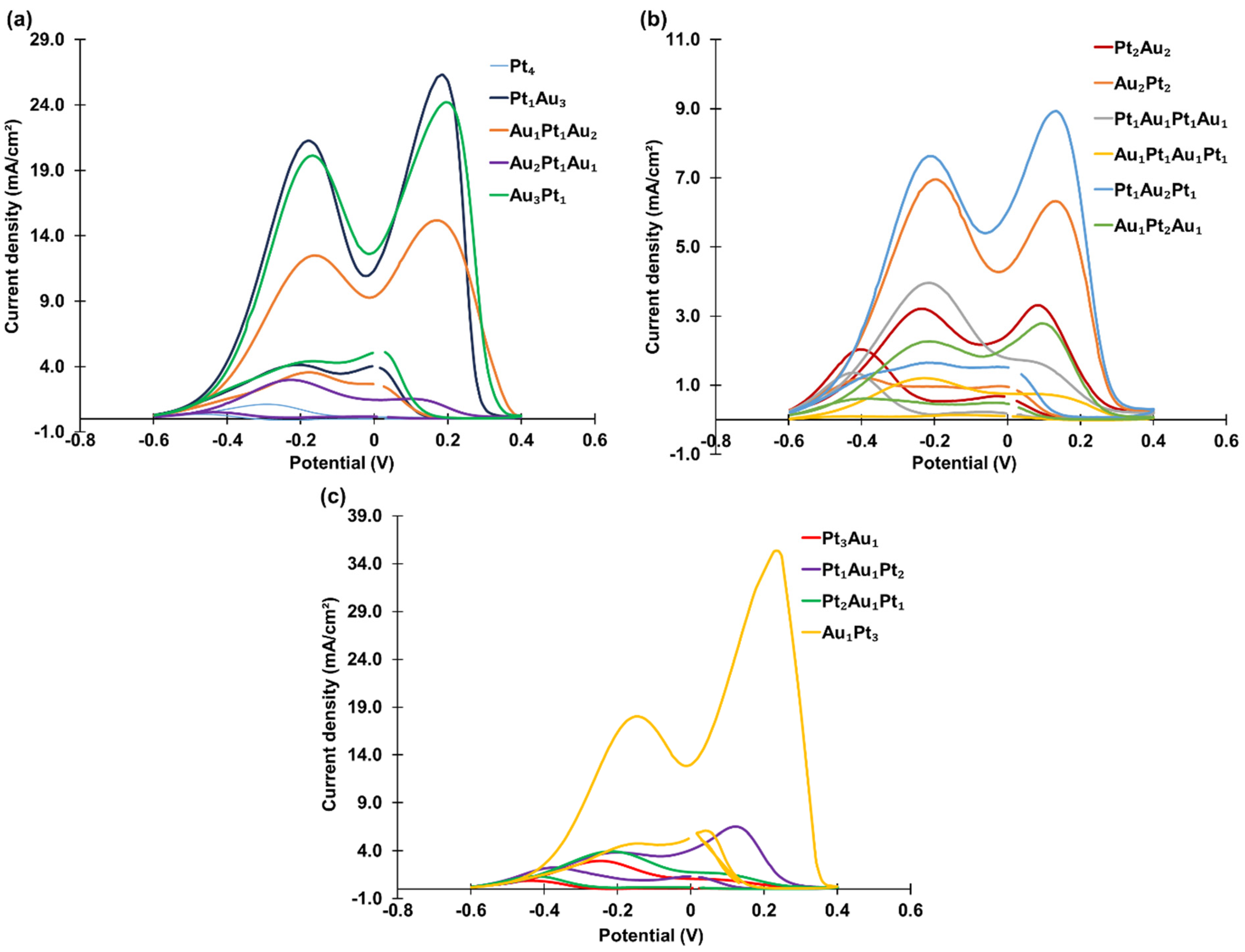
Sixteen tetra-atomic-level metal cluster combinations were studied, including the two mono-metallic clusters (PANI-Pt
4 and PANI-Au
4), and bi-metallic clusters such as PANI-Pt
1Au
3, PANI-Au
1Pt
1Au
2, PANI-Au
2Pt
1Au
1, and PANI-Au
3Pt
1 (one Pt and three Au atoms), as well as PANI-Pt
2Au
2, PANI-Au
2Pt
2, PANI-Pt
1Au
2Pt
1, and others. The CVs of these clusters are shown in
Figure 7, and the current densities are summarized in
Table 3.
Among the tetra-atomic-level metal clusters, PANI-Au1Pt3 exhibited the highest catalytic activity, with a peak current density of 35.34 mA/cm2. Bi-metallic clusters consistently demonstrated higher activity than mono-metallic clusters. For instance, PANI-Pt1Au3 achieved a propanol oxidation current density of 26.32 mA/cm2, outperforming both PANI-Pt4 and PANI-Au4. Among the fourteen tetra-atomic-level bi-metallic clusters, the four clusters composed of only one Pt atom generally showed better catalytic activity, except for PANI-Au2Pt1Au1, which had a current density of only 2.99 mA/cm2. The effect of the deposition sequence was not significant as the cluster size increased to four atoms.
4. Conclusions
Polyaniline-supported atomic-level Pt and Pt-Au clusters were successfully fabricated as catalytic electrodes for propanol oxidation using cyclic atomic-metal electrodeposition. The precise control over cluster size, composition, and deposition sequence was achieved through the number of electrodeposition cycles and the selection of metal chloride complexes, enabling a systematic evaluation of their catalytic performance.
The study revealed a distinct even–odd effect in the catalytic activity of atomic-level mono-metallic Pt clusters, where odd-numbered clusters (e.g., PANI-Pt1, PANI-Pt3, PANI-Pt5) demonstrated higher catalytic activity compared to even-numbered clusters (e.g., PANI-Pt2, PANI-Pt4, PANI-Pt6). This trend contrasts with the previously reported odd–even effect observed for atomic-level mono-metallic Au and Pd clusters.
For atomic-level bi-metallic Pt-Au clusters, the catalytic activity was significantly enhanced compared to mono-metallic clusters, with the highest current density for propanol oxidation, 35.34 mA/cm2, achieved by PANI-Au1Pt3. The results further showed that bi-metallic clusters containing only one Pt atom generally exhibited superior catalytic activity.
These findings provide valuable insights into the design of high-performance catalytic materials by leveraging atomic-level control of cluster composition, size, and structure. The enhanced catalytic activity of these atomic-level clusters directly translates into improved sensor performance, as higher current densities enable more sensitive detection of target analytes. Furthermore, the ability to fine-tune the composition and deposition sequence of bi-metallic clusters allows for the optimization of sensor response and efficiency. By improving the catalytic efficiency of the electrode materials, this study paves the way for the development of next-generation electrochemical sensors with increased sensitivity, faster response times, and greater reliability, particularly for applications in clinical diagnostics and environmental monitoring systems.
Author Contributions
Conceptualization, T.K. and T.-F.M.C.; methodology, K.W.; software, K.W. and T.-F.M.C.; validation, T.K., C.-Y.C., C.-H.Y., Y.-J.H., T.N., M.S. and T.-F.M.C.; formal analysis, K.W.; investigation, K.W.; resources, T.N., M.S. and T.-F.M.C.; data curation, K.W., K.O. H.K. and S.Y.; writing—original draft preparation, K.W.; writing—review and editing, K.W. and T.-F.M.C.; visualization, K.W. and T.-F.M.C.; supervision, M.S. and T.-F.M.C.; project administration, T.K., M.S. and T.-F.M.C.; funding acquisition, T.K., C.-Y.C., M.S. and T.-F.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI grant numbers JP21K04827, JP22K20475, JP23K13557, and JP23K04369, and the Cooperative Research Project of the Research Center for Biomedical Engineering, Institute of Science, Tokyo.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Stradiotto, N.R.; Yamanaka, H.; Zanoni, M.V.B. Electrochemical sensors: A powerful tool in analytical chemistry. J. Braz. Chem. Soc. 2003, 14, 159–173. [Google Scholar] [CrossRef]
- Schwartz, I.; Jonke, A.P.; Josowicz, M.; Janata, J. Polyaniline-Supported Atomic Gold Electrodes: Comparison with Macro Electrodes. Catal. Lett. 2012, 142, 1344–1351. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, H.; Zhang, J.; Li, G. Electrochemical Sensors for Clinic Analysis. Sensors 2008, 8, 2043–2081. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Diaz, M.T. Electrochemical Sensors. Anal. Chem. 2002, 74, 2781–2800. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, I.T.; Jonke, A.P.; Josowicz, M.; Janata, J. Effect of Structured Atomic Gold on Electrooxidation. Catal. Lett. 2013, 143, 772–782. [Google Scholar] [CrossRef]
- Hatchett, D.W.; Quy, T.; Goodwin, N.; Millick, N.M. In-situ Reduction of Au, Pd and Pt Metal Precursors in Polyaniline: Electrochemistry of Variable Content Polymer/Metal Composites in Alkaline Solution. Electrochim. Acta 2017, 251, 669–709. [Google Scholar] [CrossRef]
- Nodehi, Z.; Rafati, A.A.; Ghaffarinejad, A. Palladium-silver polyaniline composite as an efficient catalyst for ethanol oxidation. Appl. Catal. A Gen. 2018, 554, 24–34. [Google Scholar] [CrossRef]
- Chiu, W.T.; Chang, T.F.M.; Sone, M.; Mita, A.T.; Toshiyoshi, H. Roles of TiO2 in the highly robust Au nanoparticles-TiO₂ modified polyaniline electrode towards non-enzymatic sensing of glucose. Talanta 2020, 212, 120780. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Yue, R.; Du, Y.; Xu, J.; Yang, P. A one-pot ‘green’ synthesis of Pd-decorated PEDOT nanospheres for nonenzymatic hydrogen peroxide sensing. Biosens. Bioelectron. 2013, 44, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Chang, T.F.M.; Chien, Y.A.; Chen, C.Y.; Chakraborty, P.; Nakamoto, T.; Sone, M. Catalytic activity of atomic gold-decorated polyaniline support in glucose oxidation. Electrochem 2020, 1, 394–399. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. Polyaniline/Pt hybrid nanofibers: High-efficiency nanoelectrocatalysts for electrochemical devices. Small 2009, 5, 1869–1876. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Okamoto, K.; Kurioka, T.; Chen, C.Y.; Chakraborty, P.; Lin, Y.F.; Hsu, Y.J.; Nakamoto, N.; Sone, M.; Chang, T.F.M. Decoration of atomic PdxAuy (1 ≤ x + y ≤ 4) clusters on polyaniline in electrochemical sensing of 1-propanol. Surf. Coat. Technol 2024, 483, 130762. [Google Scholar] [CrossRef]
- Yuan, D.W.; Wang, Y.; Zeng, Z. Geometric, electronic, and bonding properties of AuNM (N = 1–7, M = Ni, Pd, Pt) clusters. J. Chem. Phys. 2005, 122, 114310. [Google Scholar] [CrossRef] [PubMed]
- Schnaidt, J.; Heinen, M.; Jusys, Z.; Behm, R.J. Oxidation of 1-propanol on a Pt film electrode studied by combined electrochemical, in situ IR spectroscopy and online mass spectrometry measurements. Electrochim. Acta 2013, 104, 505–517. [Google Scholar] [CrossRef]
- Chakraborty, P.; Chien, Y.A.; Chiu, W.T.; Chang, T.F.M.; Sone, M.; Nakamoto, T.; Josowicz, M.; Janata, J. Design and development of amperometric gas sensor with atomic Au–polyaniline/Pt composite. IEEE Sens. J. 2020, 20, 12479–12487. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, L. Structures of platinum clusters: Planar or spherical? J. Phys. Chem. A 2004, 108, 8605–8614. [Google Scholar] [CrossRef]
- Guo, J.J.; Yang, J.X.; Die, D. Quantum-Mechanical Study of Small Au2Pdn (n = 1~4) Clusters. Commun. Theor. Phys. 2006, 46, 155. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).