A One-Phase Injection Method with Dual Inhibition for Improving the Strength and Uniformity of MICP
Abstract
1. Introduction
2. Materials and Methods
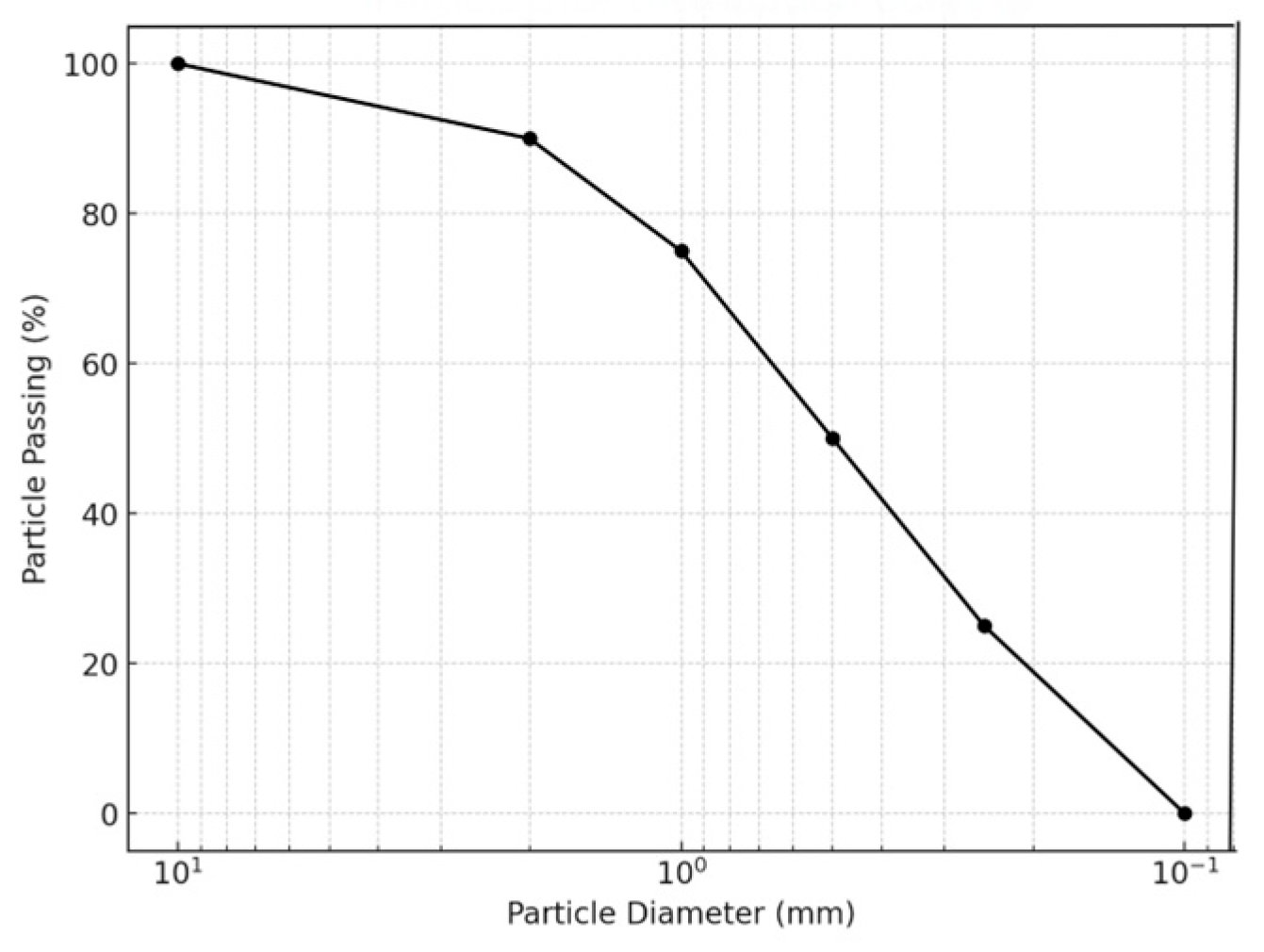
2.1. Experiment Materials
2.2. Experimental Methods
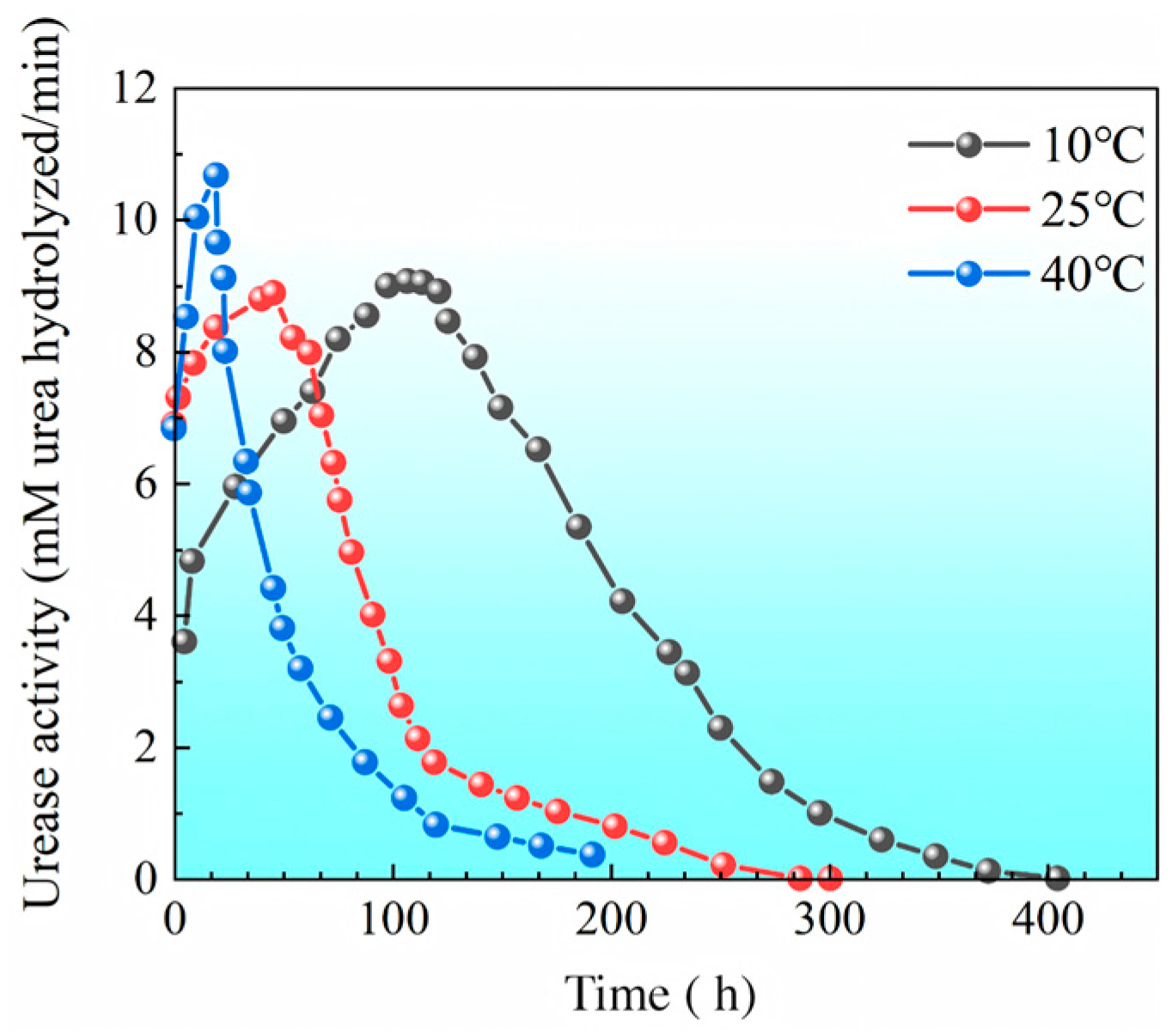
2.2.1. Bacterial Concentration and Urease Activity
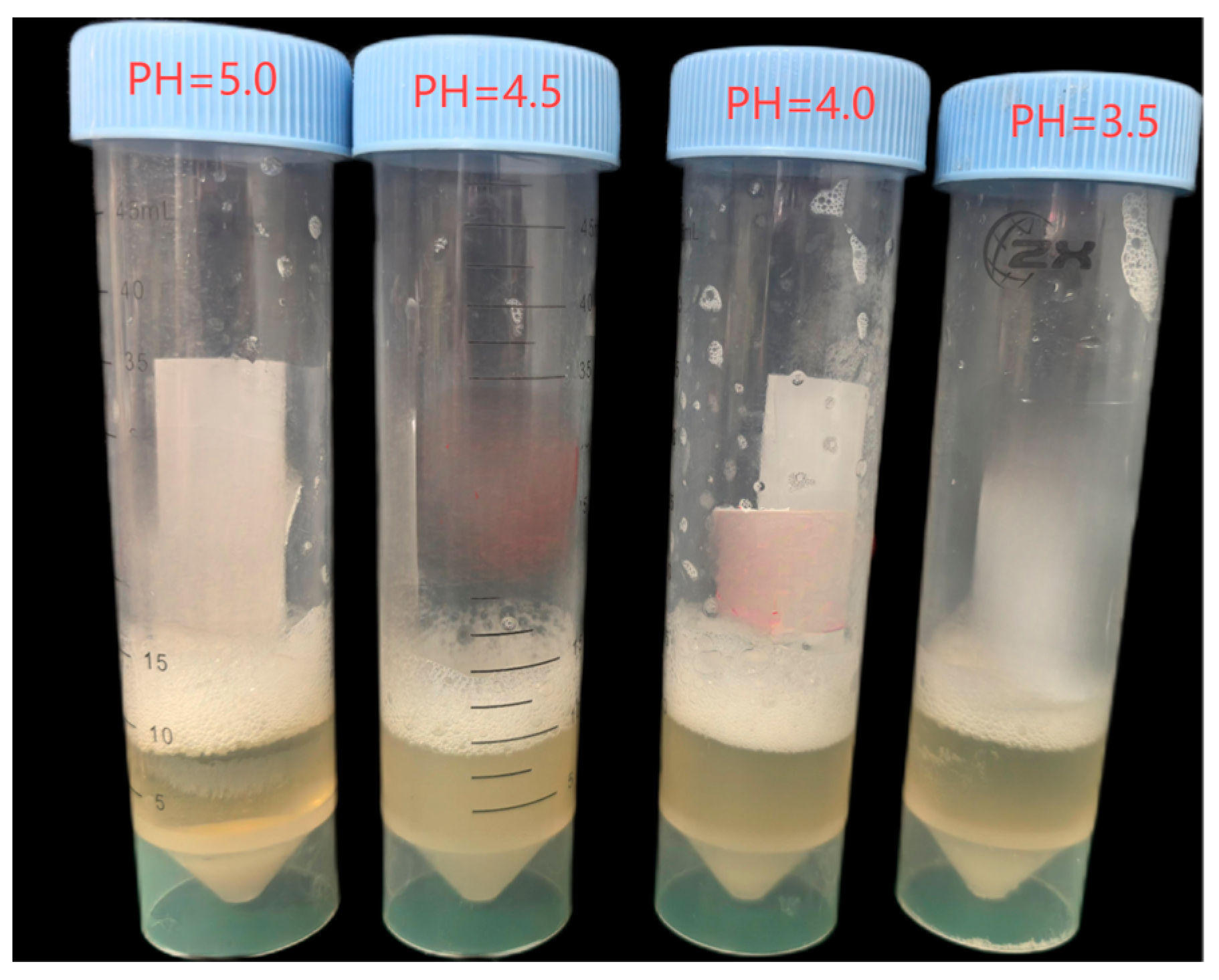
2.2.2. Flocculent Precipitate Testing Method
2.2.3. Calcium Carbonate Content
2.2.4. Unconfined Strength
2.2.5. Microscopic Analysis
2.3. Test Scheme
2.3.1. Test Tube Test
- (1)
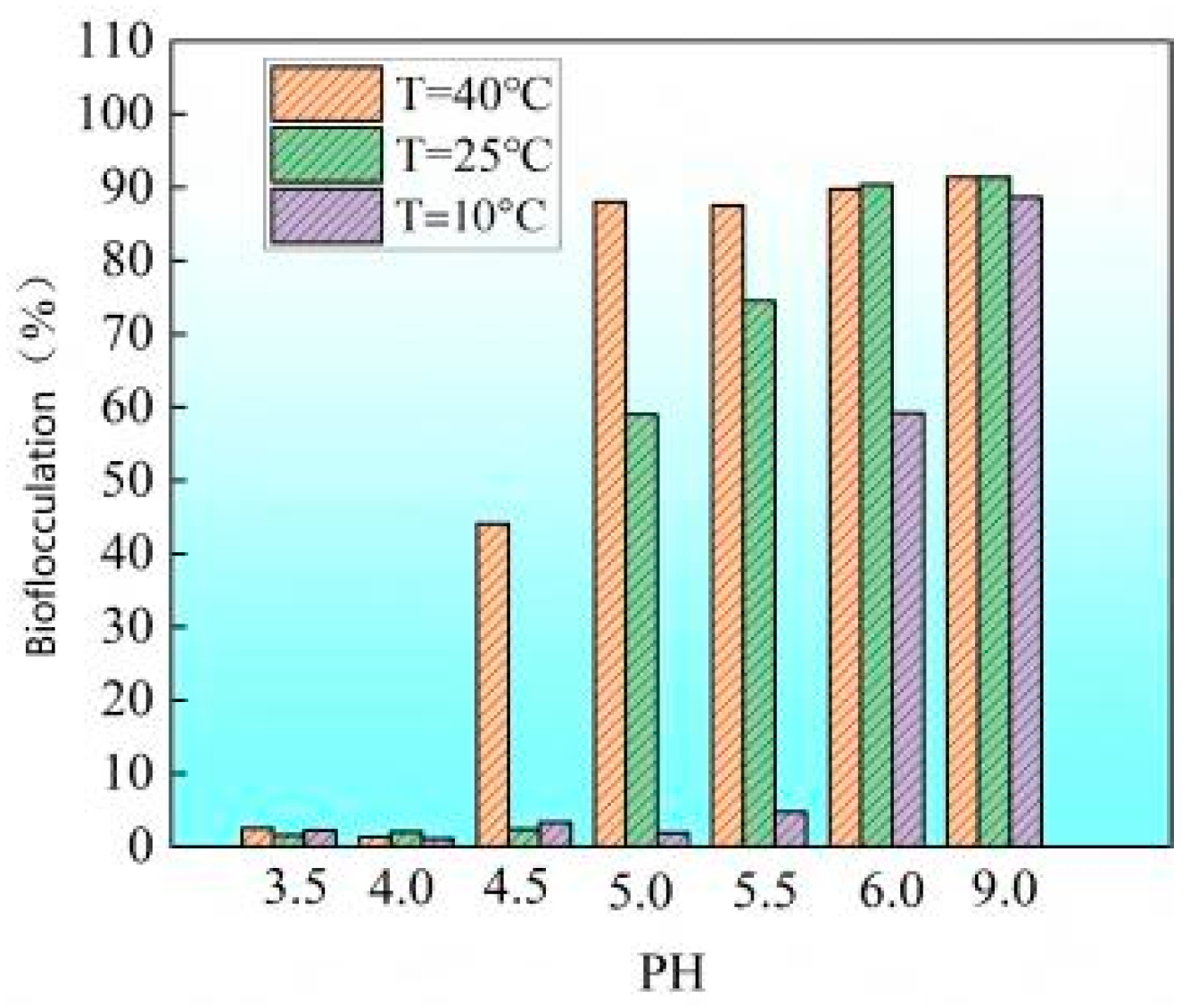
- Ca2⁺-induced flocculation characteristics under different pH conditions
- (2)
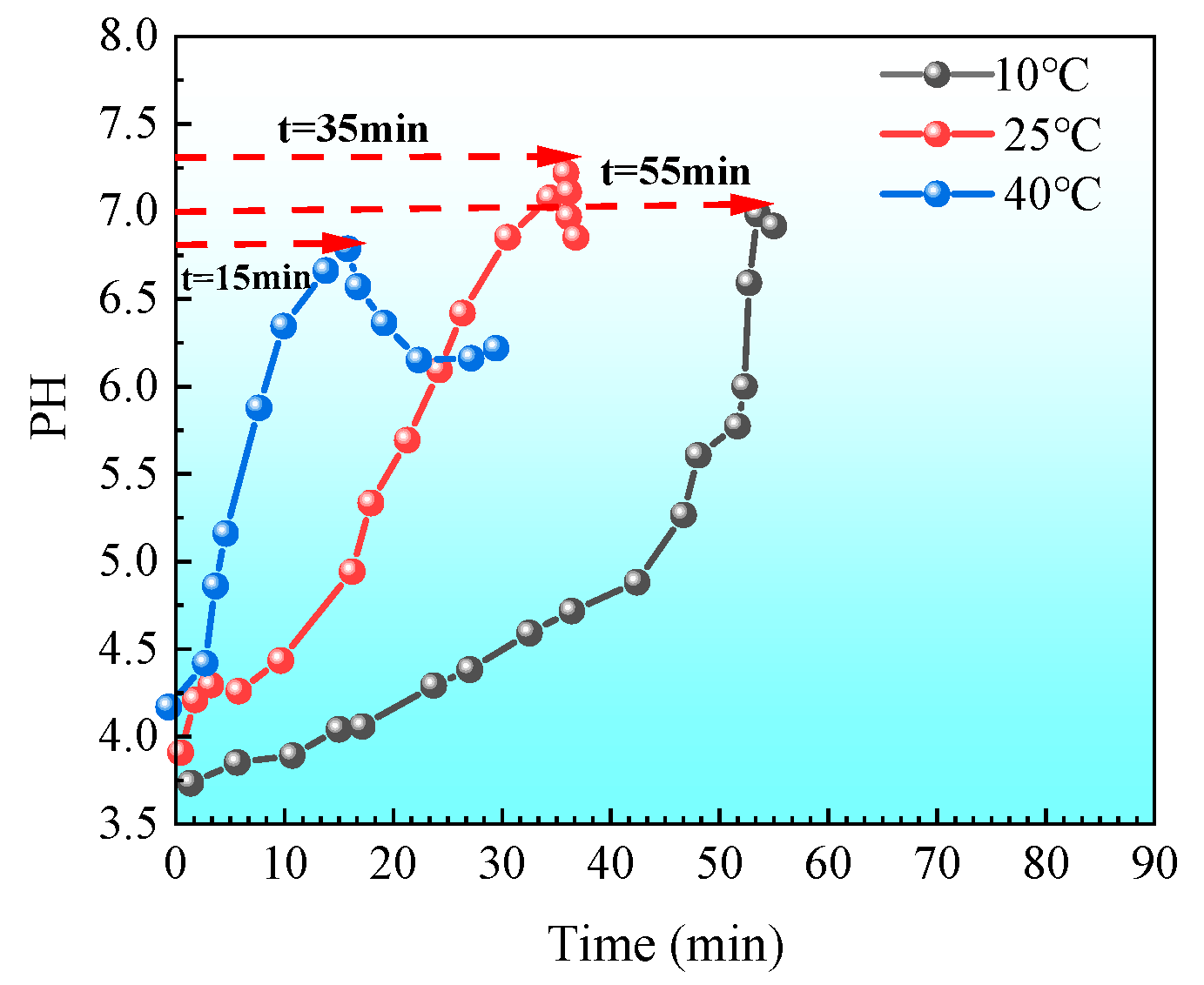
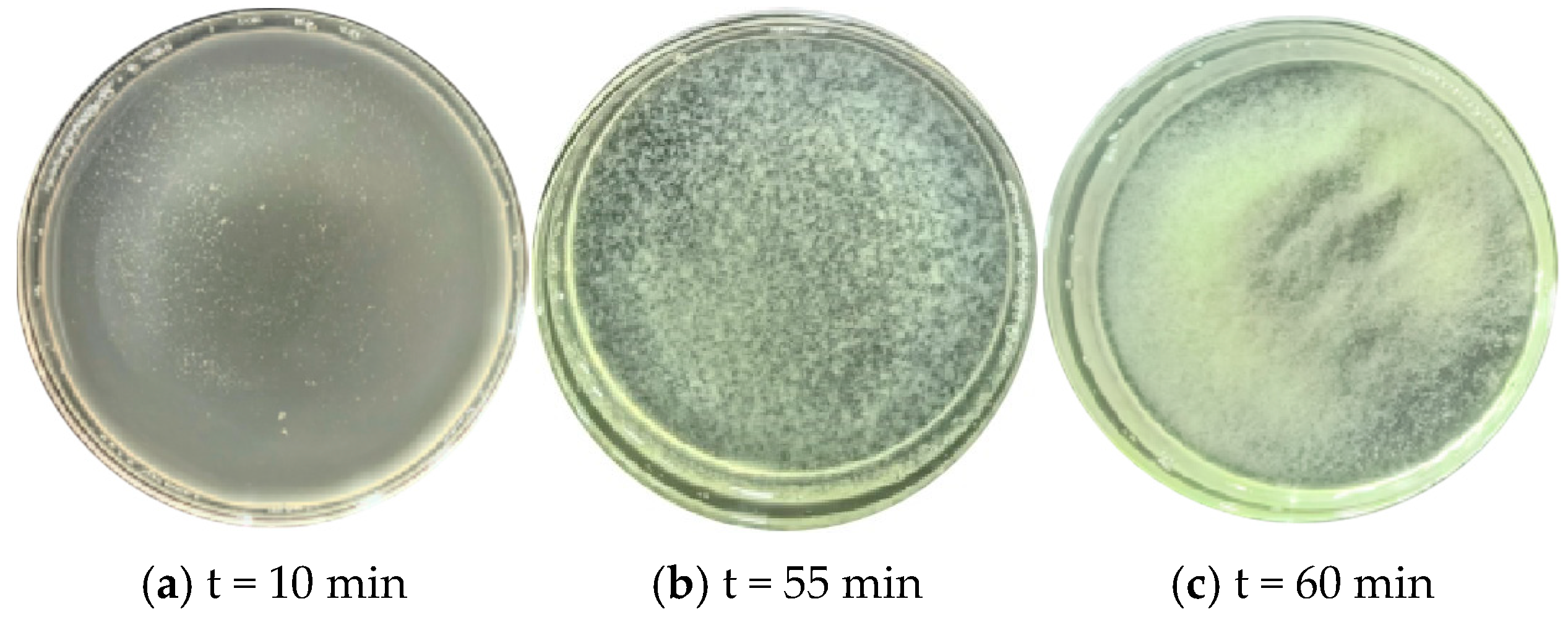
- Determination of hysteresis period under low pH and low temperature conditions
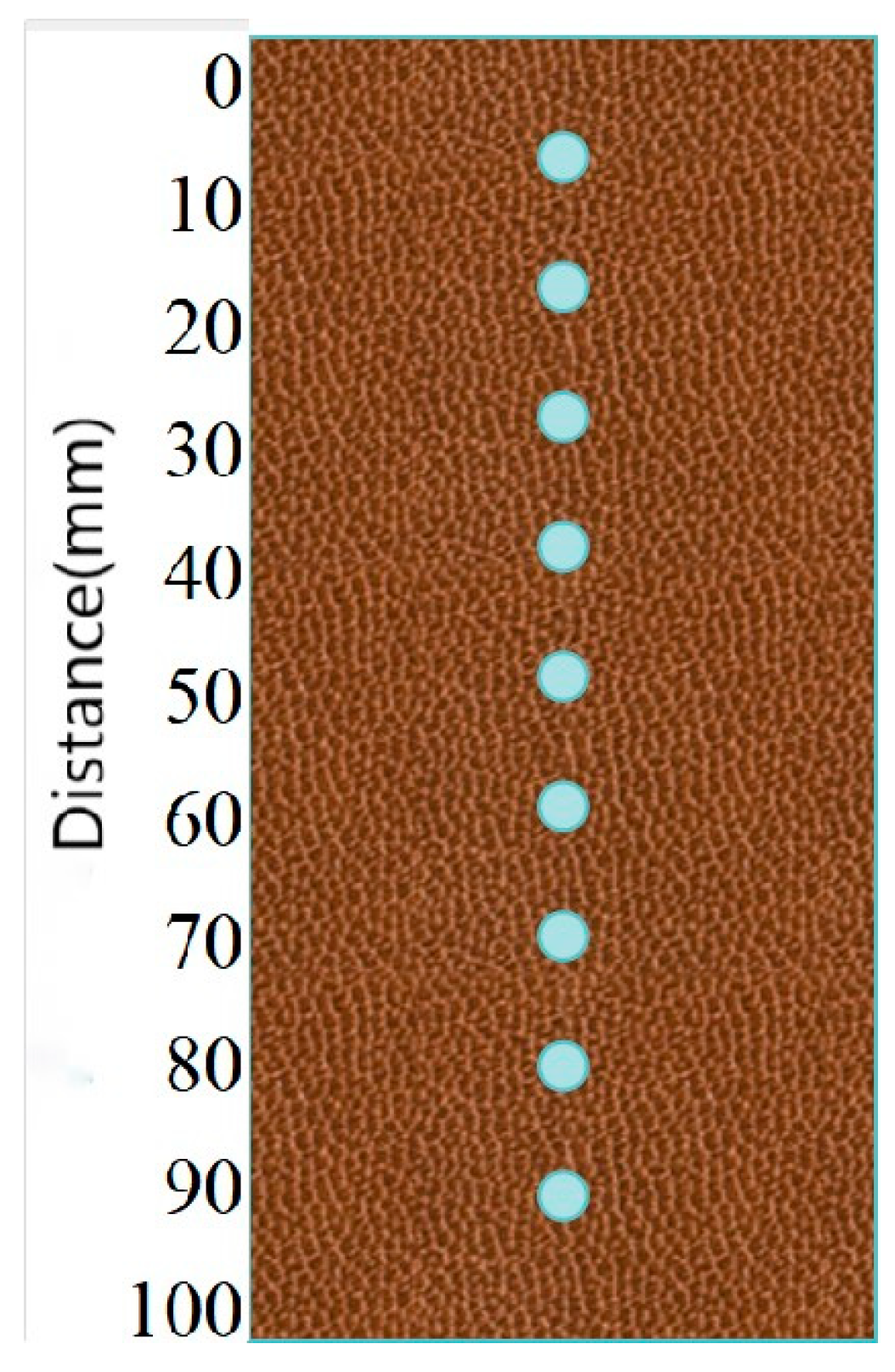
2.3.2. Sand Column Test
- (1)
- Distribution of bacterial solutions in the sand column under different temperature conditions
- (2)
- Uniformity and strength of sand column reinforcement under different temperature conditions
3. Results and Discussion
3.1. Effect of Dual Inhibition on Calcium Carbonate Precipitation (Aqueous Solution Test)
3.2. Effect of Low Temperature and Low pH Single-Phase Method on MICP Cementation (Sand Column Test)
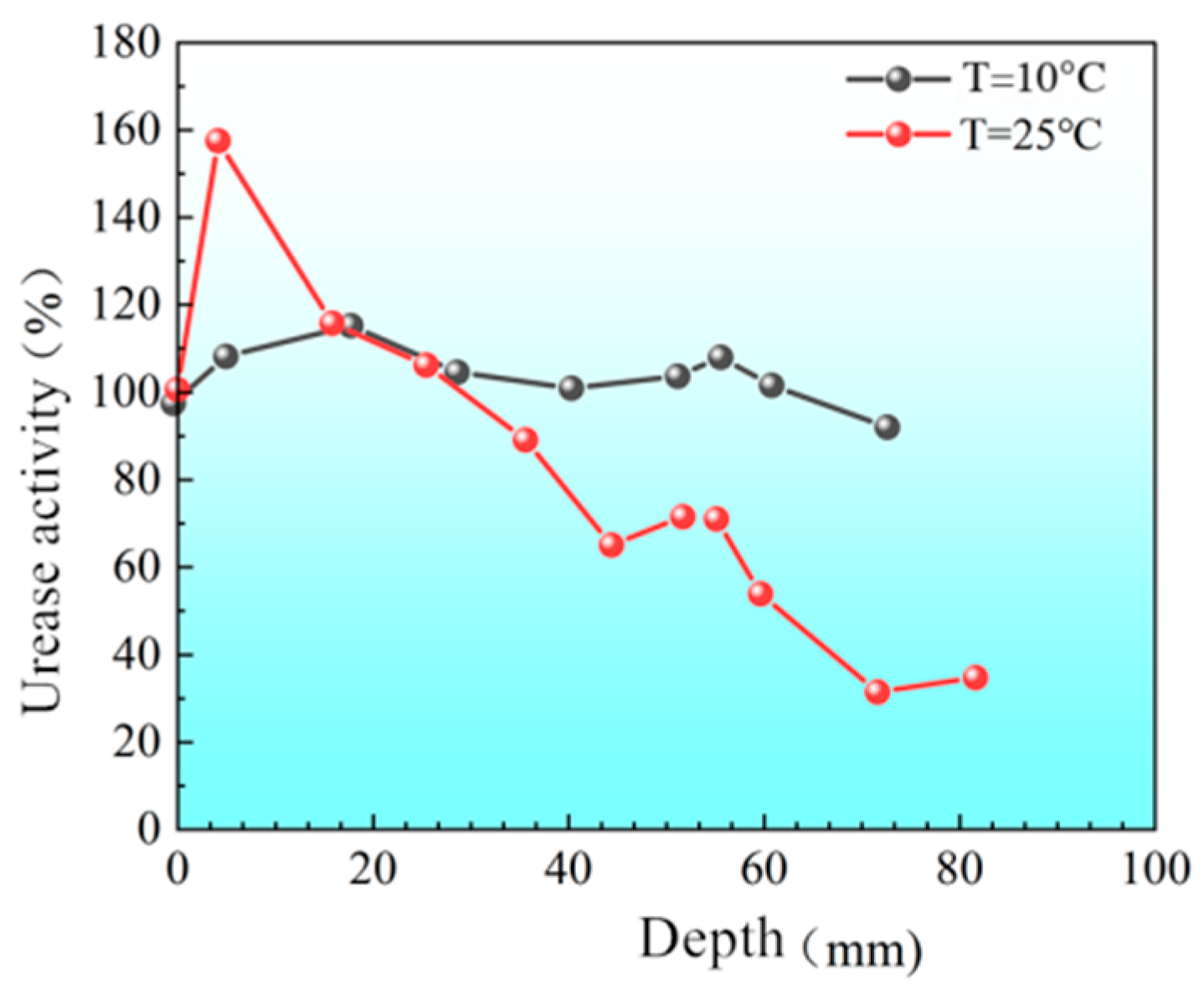
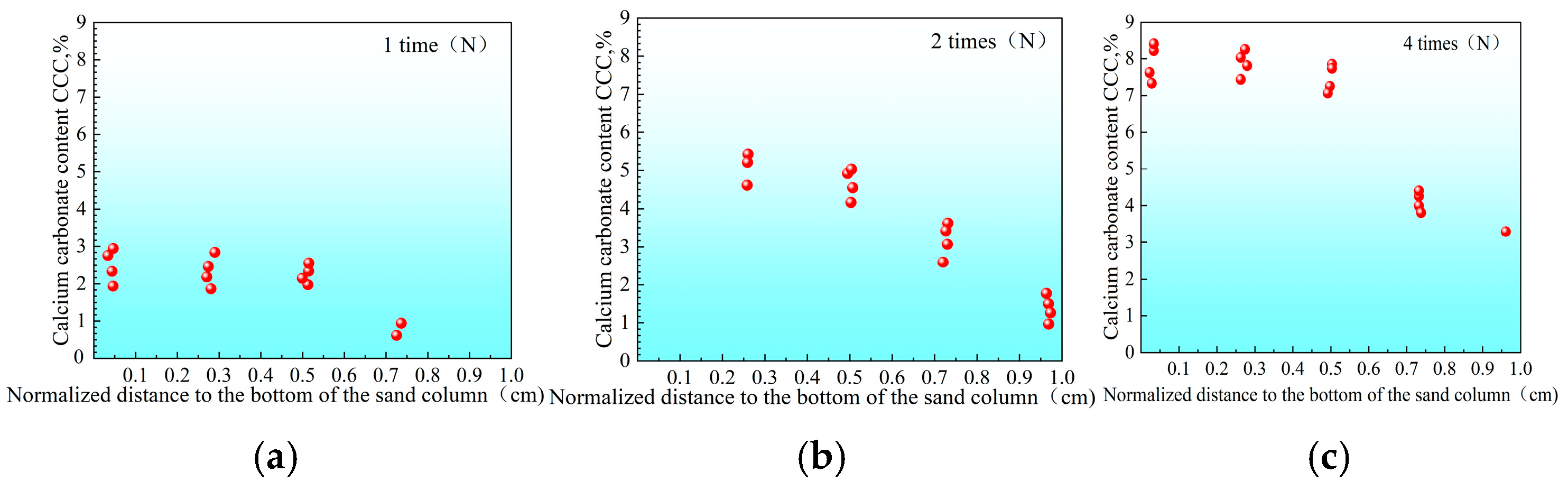
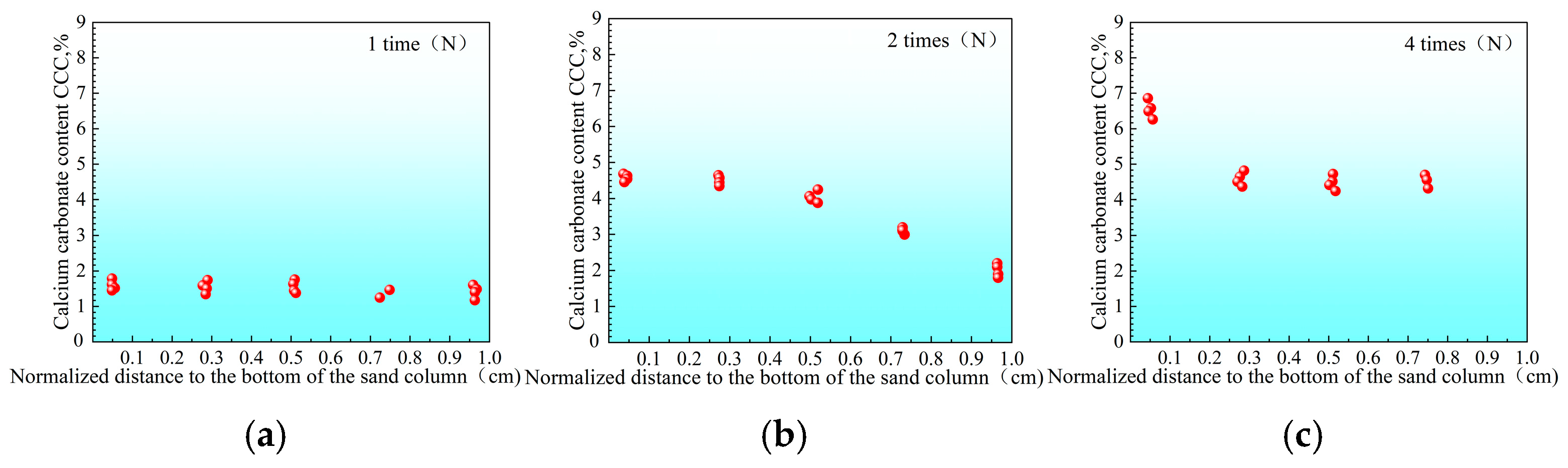
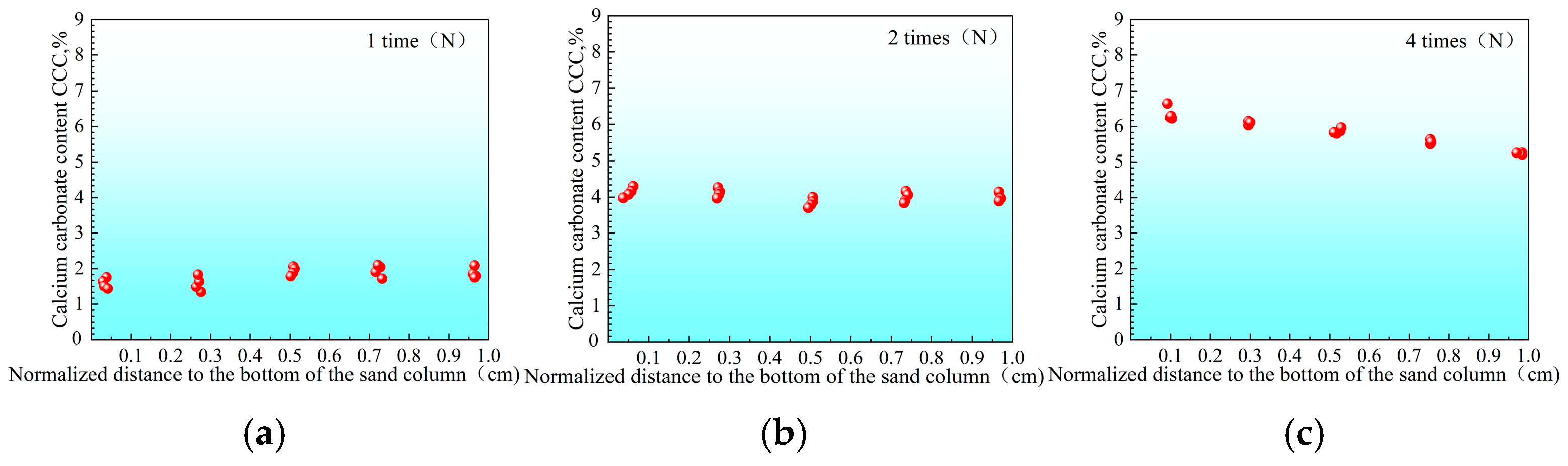
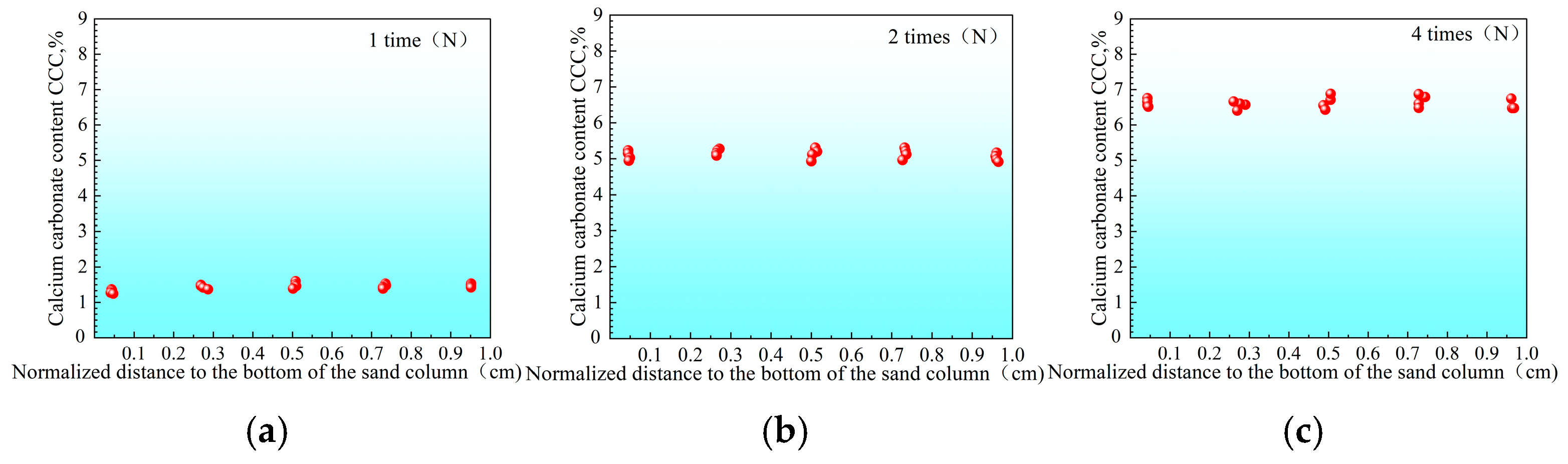
3.2.1. Distribution of Urease Activity in Sand Columns
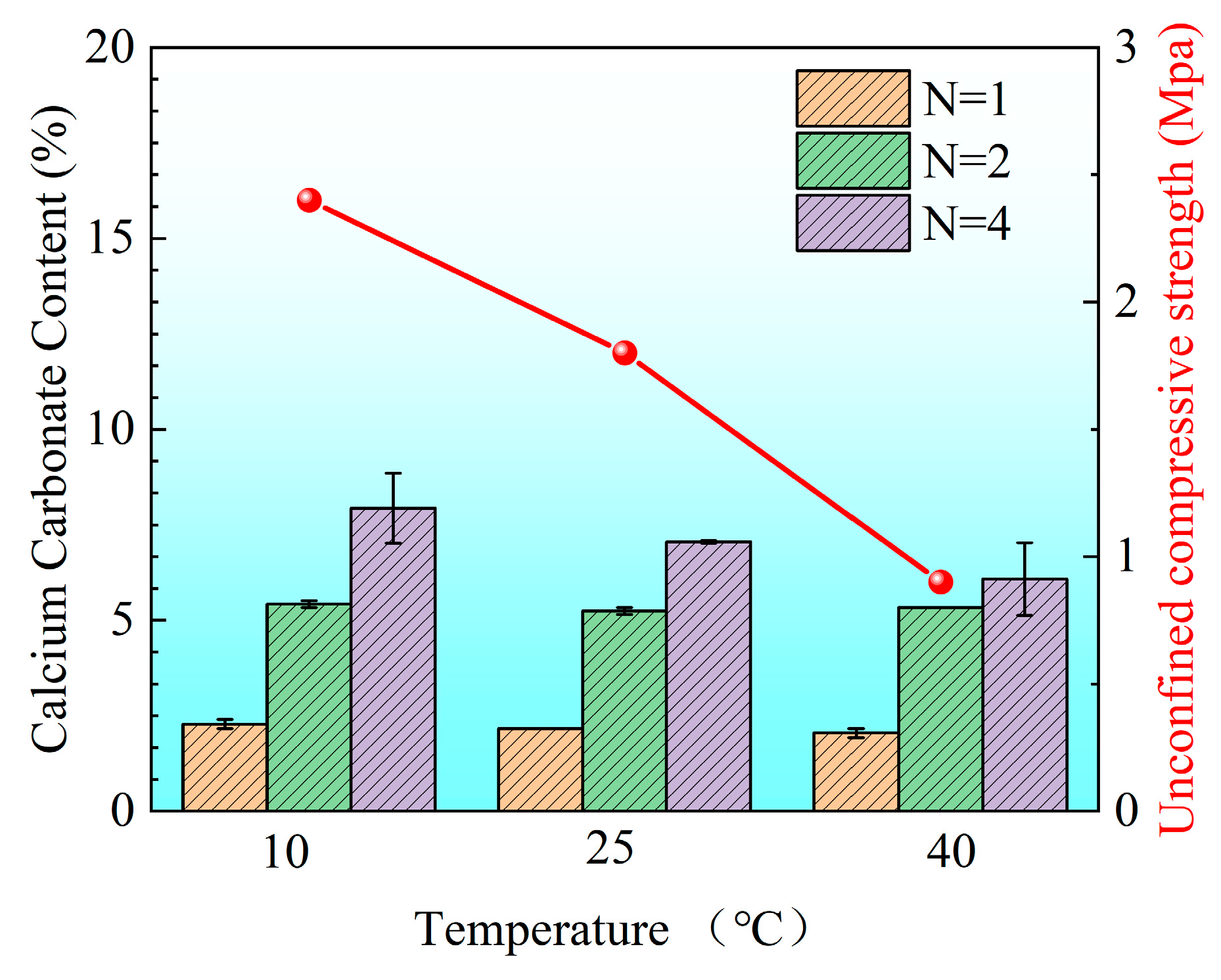
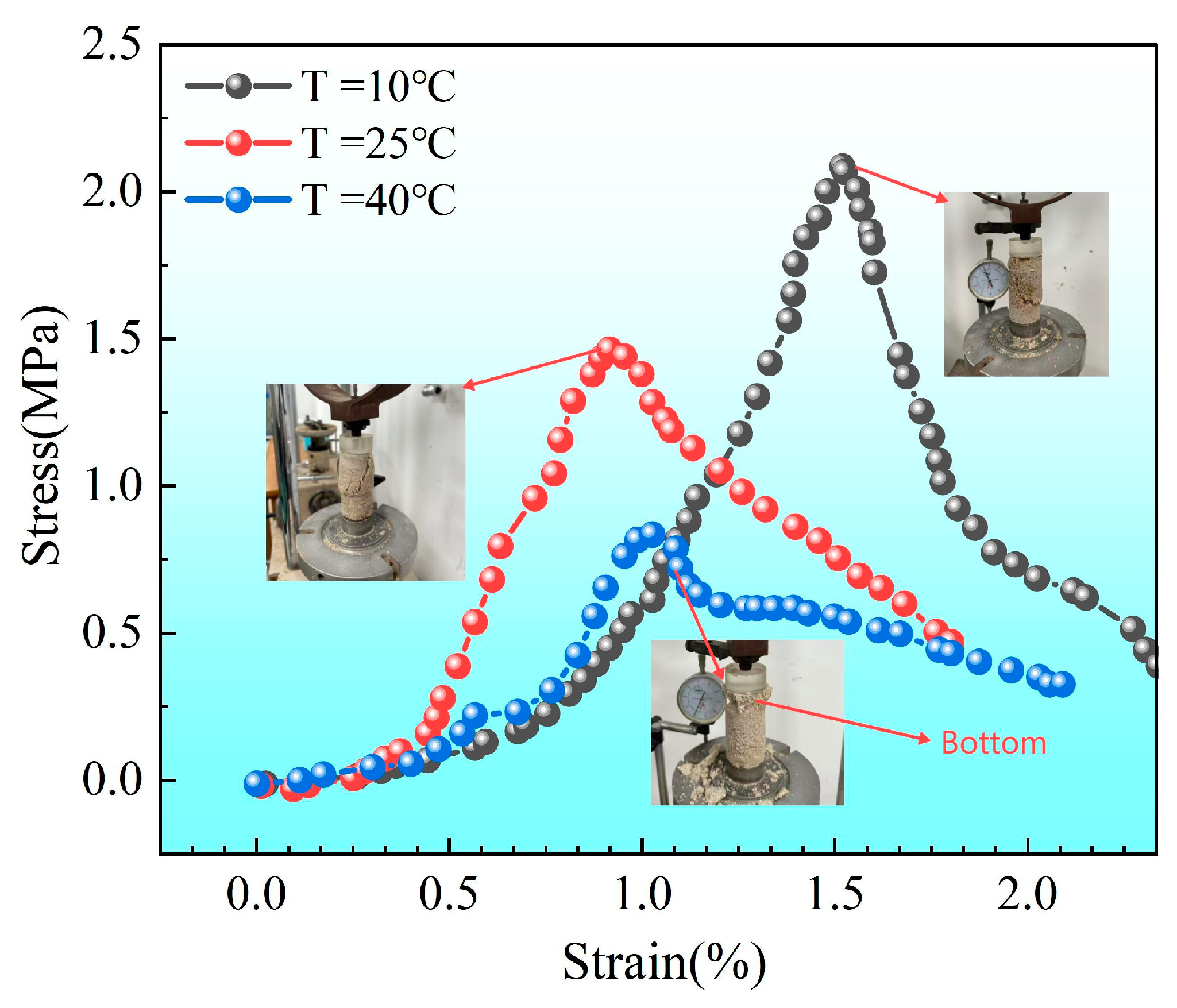
3.2.2. Calcium Carbonate Content and Unconfined Strength of Sand Columns
- From the perspective of the uneven characteristic index () of the spatial distribution of calcium carbonate, the values () of each group (respectively, 1.0, 0.25, and 0.065) are all greater than zero, which shows that although the crystal content has gradually increased, the spatial distribution of calcium carbonate has not reached a completely uniform state as a whole.
- For different spatial distributions of calcium carbonate (different ), the structural strength shows obvious differences. Under the premise that the average % of calcium carbonate content is similar, as the uniformity of calcium carbonate distribution increases ( becomes smaller), the structural strength increases significantly. For example, when it decreases from 1.0 to 0.25 and further to 0.065, the average content of calcium carbonate increases from 5.93% to 6.50%, and the strength of the corresponding structure increases significantly (from 1.3 MPa to 2.5 MPa). This shows that the more uniform (the smaller) the spatial distribution of calcium carbonate is, the more uniform the cementation effect of the structure is, which is conducive to significantly improving the overall strength of the structure.
- From the perspective of calcium carbonate content, under certain conditions (the degree of spatial distribution uniformity is close), increasing the calcium carbonate content is obviously conducive to improving the structural strength. For example, when the decreases from 0.25 to 0.065, the average content of calcium carbonate only increases from 6.25% to 6.50%, but the structural strength increases from 1.7 MPa to 2.5 MPa, which shows that even if the difference is not large, a small increase in the content of calcium carbonate can still significantly enhance the bearing capacity of the structure. This is consistent with the principle that after the calcium carbonate content increases, the internal bonding and filling effect of the structure are enhanced, the integrity of the structure is improved, and thus the structural strength is improved.
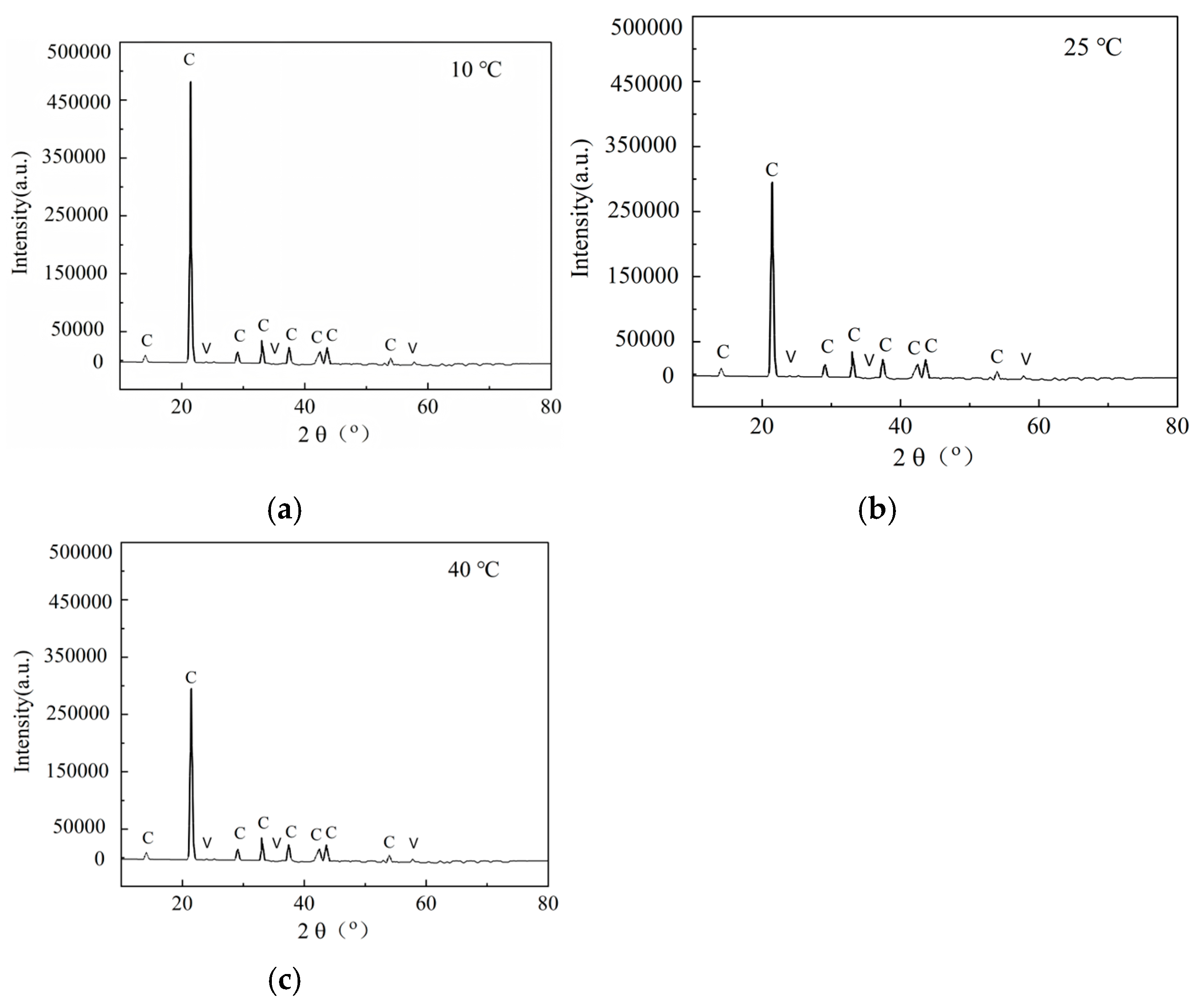
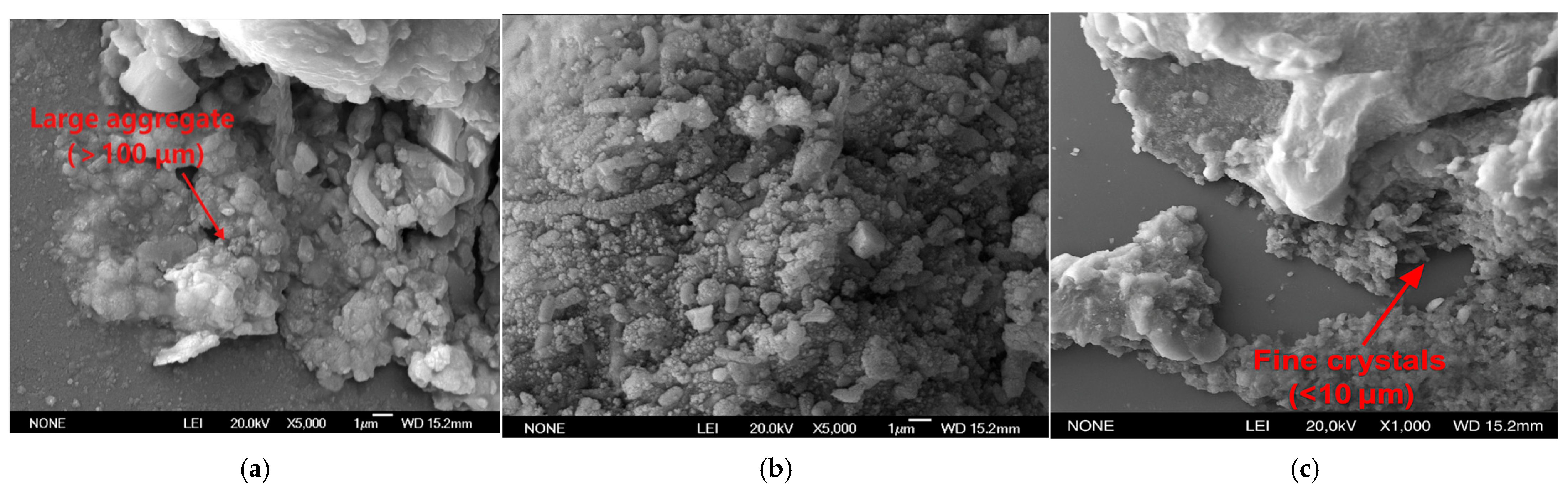
3.3. Microstructural Characteristics and XRD Analysis
3.4. Practical Considerations for Field Implementation
4. Conclusions
- “Double inhibition” significantly improves precipitation uniformity: The double inhibition of low temperature (10 °C) and low pH (pH = 4) extends the lag period of the MICP reaction to about 60 min, promotes the uniform penetration of bacteria and cementing fluid in the sand column, and reduces accumulation deposition at the injection port. After double inhibition treatment, the coefficient of variation in the Ca CO3 content in the sand column along the height decreased from 0.12 at 40 °C to 0.03 at 10 °C, and the precipitation uniformity increased by about four times.
- Dynamic transformation of the relationship between strength and number of treatments: The compressive strength of the sand column increased significantly with the increase in the number of treatments (the peak strength was about 2.4 M Pa after four treatments). The initial strength was high at a high temperature of 40 °C, but after four treatments, the strength of the sand column was higher at low temperature (10 °C) and room temperature (25 °C). This shows that with the increase in the number of treatments, the dominant factor affecting the strength of the sand column changes from the amount of Ca CO3 precipitation to the uniformity of precipitation distribution: the early reinforcement strength is mainly affected by the amount of generation, while the spatial consistency of precipitation after multiple reinforcements contributes more significantly to the strength.
- Crystal morphology and environmental conditions: XRD analysis shows that the cement generated under various temperature conditions is mainly calcite as the main crystal form, accompanied by a small amount of vaterite. The crystal morphology of CaCO3 crystals is less affected by temperature, and the crystal phase composition remains consistent at different temperatures. However, the temperature has a significant effect on crystal size: some large-sized calcite crystals are formed under low-temperature conditions, while the precipitation under high-temperature conditions is mainly fine grains, but these differences do not change the cementing effect of precipitation on sand particles.
- Economy and seasonal adaptability: In low-temperature winter environments, by adjusting the pH and temperature of the bacterial solution, the required minimum pH threshold can be increased to about 6 to obtain a sufficient lag period and reduce the risk of uneven reinforcement; in high-temperature summer environments (such as T = 40 °C), the pH threshold should be reduced to about 4.5 to ensure the required lag period. This strategy of flexibly adjusting parameters according to seasonal temperature changes can save time and reagent costs while ensuring reinforcement effects, providing higher economy and adaptability for field applications of MICP technology.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019, 14, 615–626. [Google Scholar] [CrossRef]
- Al Qabany, A.; Soga, K. Effect of chemical treatment used in MICP on engineering properties of cemented soils. Géotechnique 2013, 63, 331–339. [Google Scholar] [CrossRef]
- DeJong, J.T.; Soga, K.; Kavazanjian, E.; Burns, S.; Van Paassen, L.A.; Al Qabany, A.; Aydilek, A.; Bang, S.S.; Burbank, M.; Caslake, L.F.; et al. Biogeochemical processes and geotechnical applications: Progress, opportunities and challenges. Géotechnique 2020, 70, 574–609. [Google Scholar]
- Xiao, Y.; Cui, H.; Zaman, M.; Shi, J.; Wu, H. Constitutive modeling for biocemented calcareous sands. Int. J. Geomech. 2024, 24, 04024088. [Google Scholar] [CrossRef]
- Wang, Y.; Konstantinou, C.; Tang, S.; Chen, H. Applications of microbial-induced carbonate precipitation: A state-of-the-art review. Biogeotechnics 2023, 1, 100008. [Google Scholar] [CrossRef]
- Hammes, F.; Seka, A.; de Knijf, S.; Verstraete, W. A novel approach to calcium removal from calcium-rich industrial wastewater. Water Res. 2003, 37, 699–704. [Google Scholar] [CrossRef]
- Chen, F.; Deng, C.; Song, W.; Zhang, D.; Al-Misned, F.A.; Mortuza, M.G.; Gadd, G.M.; Pan, X. Biostabilization of desert sands using bacterially induced calcite precipitation. Geomicrobiol. J. 2016, 33, 243–249. [Google Scholar] [CrossRef]
- Bang, S.S.; Galinat, J.K.; Ramakrishnan, V. Calcite precipitation induced by polyurethane-immobilized Bacillus pasteurii. Enzym. Microb. Technol. 2001, 28, 404–409. [Google Scholar] [CrossRef]
- Choi, S.G.; Chang, I.; Lee, M.; Lee, J.H.; Han, J.T.; Kwon, T.H. Review on geotechnical engineering properties of sands treated by microbially induced calcium carbonate precipitation (MICP) and biopolymers. Constr. Build. Mater. 2020, 246, 118415. [Google Scholar] [CrossRef]
- Feng, D.; Wang, Y.; Chen, D.; Liang, S. Experimental study on the influence mechanism of clay particles on the microbial treatment of granite residual soil. Constr. Build. Mater. 2024, 411, 134659. [Google Scholar] [CrossRef]
- Fu, T.; Saracho, A.C.; Haigh, S.K. Microbially induced carbonate precipitation (MICP) for soil strengthening: A comprehensive review. Biogeotechnics 2023, 1, 100002. [Google Scholar] [CrossRef]
- Liu, S.; Wang, R.; Yu, J.; Peng, X.; Cai, Y.; Tu, B. Effectiveness of the anti-erosion of an MICP coating on the surfaces of ancient clay roof tiles. Constr. Build. Mater. 2020, 243, 118202. [Google Scholar] [CrossRef]
- Xiao, Y.; He, X.; Zaman, M.; Ma, G.; Zhao, C. Review of strength improvements of biocemented soils. Int. J. Geomech. 2022, 22, 04022221. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Z.; Stuedlein, A.W.; Evans, T.M. Liquefaction modeling for biocemented calcareous sand. J. Geotech. Geoenviron. Eng. 2021, 147, 04021113. [Google Scholar] [CrossRef]
- Yu, K.; Ran, Y.; Shi, J.; Duan, M.; Ouyang, Z. Physical property of MICP-treated calcareous sand under seawater conditions by CPTU. Biogeotechnics 2025, 3, 100131. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, Y.; Liu, H.; Chu, J. Role of bacteria on bio-induced calcium carbonate formation: Insights from droplet microfluidic experiments. Géotechnique 2024, 74, 1–35. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Wang, Y.; Wang, J.; Cao, J.; Zhang, G. Improved methods, properties, applications and prospects of microbial induced carbonate precipitation (MICP) treated soil: A review. Biogeotechnics 2024, 3, 100123. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Sun, X.; Zeng, W.; Xing, H.; Lin, J.; Kang, S.; Yu, L. Application of microbially induced calcium carbonate precipitation (MICP) technique in concrete crack repair: A review. Constr. Build. Mater. 2024, 411, 134313. [Google Scholar] [CrossRef]
- Cheng, L.; Kobayashi, T.; Shahin, M.A. Microbially induced calcite precipitation for production of “bio-bricks” treated at partial saturation condition. Constr. Build. Mater. 2020, 231, 117095. [Google Scholar] [CrossRef]
- DeJong, J.T.; Fritzges, M.B.; Nüsslein, K. Microbially induced cementation to control sand response to undrained shear. J. Geotech. Geoenviron. Eng. 2006, 132, 1381–1392. [Google Scholar] [CrossRef]
- Ivanov, V.; Chu, J. Applications of microorganisms to geotechnical engineering for bioclogging and biocementation of soil in situ. Rev. Environ. Sci. Biotechnol. 2008, 7, 139–153. [Google Scholar] [CrossRef]
- Nemati, M.; Voordouw, G. Modification of porous media permeability using calcium carbonate produced enzymatically in situ. Enzym. Microb. Technol. 2003, 33, 635–642. [Google Scholar] [CrossRef]
- Wu, C.; Chu, J.; Cheng, L.; Wu, S. Biogrouting of aggregates using premixed injection method with or without pH adjustment. J. Mater. Civ. Eng. 2019, 31, 06019008. [Google Scholar] [CrossRef]
- Ma, G.; He, X.; Xiao, Y.; Chu, J.; Liu, H.; Stuedlein, A.W.; Evans, T.M. Spatiotemporal evolution of biomineralization in heterogeneous pore structure. Can. Geotech. J. 2023, 61, 447–468. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, Y.; Shi, W.; Bian, H.; Shi, L.; Wu, L.; Han, Z.; Zheng, J.; He, X. Strength and uniformity of EICP-treated sand under multi-factor coupling effects. Biogeotechnics 2023, 1, 100007. [Google Scholar] [CrossRef]
- Zheng, J.; Lai, H.; Cui, M.; Ding, X.; Weng, Y.; Zhang, J. Biogrouting technologies for enhancing uniformity of biocementation: A review. Biogeotechnics 2023, 1, 100033. [Google Scholar] [CrossRef]
- Martinez, B.C.; DeJong, J.T.; Ginn, T.R.; Montoya, B.M.; Barkouki, T.H.; Hunt, C.; Tanyu, B.; Major, D. Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J. Geotech. Geoenviron. Eng. 2013, 139, 587–598. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Ong, D.E.L.; Li, P.Y.; Senian, N.; Hei, N.L.; Esnault-Filet, A.; Muda, K.; Nissom, P.M. Effects of push-pull injection–suction spacing on sand biocementation treatment. Geotech. Res. 2024, 11, 28–38. [Google Scholar] [CrossRef]
- Zhao, C.; Xiao, Y.; He, X.; Liu, H.; Liu, Y.; Chu, J. Influence of injection methods on bio-mediated precipitation of carbonates in fracture-mimicking microfluidic chip. Géotechnique 2025, 75, 153–165. [Google Scholar]
- Ma, G.; He, X.; Jiang, X.; Liu, H.; Chu, J.; Xiao, Y. Strength and permeability of bentonite-assisted biocemented coarse sand. Can. Geotech. J. 2021, 58, 969–981. [Google Scholar] [CrossRef]
- Xiao, Y.; He, X.; Evans, T.M.; Stuedlein, A.W.; Liu, H. Unconfined compressive and splitting tensile strength of basalt fiber-reinforced biocemented sand. J. Geotech. Geoenviron. Eng. 2019, 145, 04019048. [Google Scholar] [CrossRef]
- Xu, X.; Guo, H.; Li, M.; Fu, H. Improving microbially induced calcium carbonate precipitation effects by nacre extractions. Geotech. Lett. 2022, 12, 20–26. [Google Scholar] [CrossRef]
- Cui, M.-J.; Chu, J.; Lai, H.-J. Optimization of one-phase-low-pH enzyme-induced carbonate precipitation method for soil improvement. Acta Geotech. 2024, 19, 1067–1079. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Wang, L.; Yang, H. A coupled bio-chemo-hydro-wave model and multi-stages for MICP in the seabed. Ocean Eng. 2023, 283, 114667. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Z.; Wang, L.; Zhu, Y.; Rui, S. Field implementation to resist coastal erosion of sandy slope by eco-friendly methods. Coast. Eng. 2024, 189, 104489. [Google Scholar] [CrossRef]
- Safdar, M.U.; Mavroulidou, M.; Gunn, M.J.; Garelick, J.; Payne, I.; Purchase, D. Innovative methods of ground improvement for railway embankment peat fens foundation soil. Géotechnique 2021, 71, 985–998. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. In situ soil cementation with ureolytic bacteria by surface percolation. Ecol. Eng. 2012, 42, 64–72. [Google Scholar] [CrossRef]
- Ghasemi, P.; Montoya, B.M. Field application of the microbially induced calcium carbonate precipitation on a coastal sandy slope. In Geo-Congress 2020; ASCE: Minneapolis, MN, USA, 2020. [Google Scholar]
- Tang, Y.; Lian, J.; Xu, G.; Yan, Y.; Xu, H. Effect of cementation on calcium carbonate precipitation of loose sand resulting from microbial treatment. Trans. Tianjin Univ. 2017, 23, 547–554. [Google Scholar] [CrossRef]
- Yang, Y.; Chu, J.; Liu, H.; Cheng, L. Improvement of uniformity of biocemented sand column using CH₃COOH-buffered one-phase-low-pH injection method. Acta Geotech. 2023, 18, 413–428. [Google Scholar] [CrossRef]
- Lai, H.-J.; Cui, M.-J.; Chu, J. Effect of pH on soil improvement using one-phase-low-pH MICP or EICP biocementation method. Acta Geotech. 2023, 18, 3259–3272. [Google Scholar] [CrossRef]
- Cui, M.-J.; Lai, H.-J.; Hoang, T.; Chu, J. One-phase-low-pH enzyme induced carbonate precipitation (EICP) method for soil improvement. Acta Geotech. 2021, 16, 481–489. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, Y.; Wang, S.; Evans, T.M.; Stuedlein, A.W.; Chu, J.; Zhao, C.; Wu, H.; Liu, H. Homogeneity and mechanical behaviors of sands improved by a temperature-controlled one-phase MICP method. Acta Geotech. 2021, 16, 1417–1427. [Google Scholar] [CrossRef]
- Do, J. Linguistic Markers of Maternal Focus Within Emotional Conversations: The Role of Depressive Symptoms and Maltreatment. Ph.D. Dissertation, North Carolina State University, Raleigh, NC, USA, 2019. [Google Scholar]
- Karimian, A.; Hassanlourad, M. Mechanical behaviour of MICP-treated silty sand. Bull. Eng. Geol. Environ. 2022, 81, 308. [Google Scholar] [CrossRef]
- Najafian Jazi, F.; Hosseini, S.M.M.; Ghasemi-Fare, O.; Rockaway, T.D. Volume-change behavior of lime-stabilized expansive soils after experiencing different loading conditions. In Proceedings of the IFCEE 2024, Dallas, TX, USA, 5–10 May 2024. [Google Scholar]
- Jazi, F.N.; Hosseini, S.M.M.M.; Ghasemi-Fare, O.; Rockaway, T.D. Experimental evaluation of stress history effect on compressibility characteristics of lime-stabilized expansive soils. Géoméch. Geoengin. 2024, 20, 21–34. [Google Scholar] [CrossRef]
- Harkes, M.P.; Van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Guan, D.; Zhou, Y.; Shahin, M.A.; Tirkolaei, H.K.; Cheng, L. Assessment of urease enzyme extraction for superior and economic bio-cementation of granular materials using enzyme-induced carbonate precipitation. Acta Geotech. 2023, 18, 2263–2279. [Google Scholar] [CrossRef]
- Rabbani, M.; Werner, J.; Fahimi, A.; Vahidi, E. Innovative pilot-scale process for sustainable rare earth oxide production from coal byproducts: A comprehensive environmental impact assessment. J. Rare Earths 2025, 43, 397–404. [Google Scholar] [CrossRef]
- Zhu, T.; He, R.; Hosseini, S.M.J.; He, S.; Cheng, L.; Guo, Y.; Guo, Z. Influence of precast microbial reinforcement on lateral responses of monopiles. Ocean Eng. 2024, 307, 118211. [Google Scholar] [CrossRef]
- ASTM D2166/D2166M-16; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. ASTM International: West Conshohocken, PA, USA, 2016.
- Peng, J.; Liu, Z. Influence of temperature on microbially induced calcium carbonate precipitation for soil treatment. PLoS ONE 2019, 14, e0218396. [Google Scholar] [CrossRef]
- Hosseini, S.M.J.; Guan, D.; Cheng, L. Modification of All-in-One Solution for Improvement of 1m Sand Columns. Geomicrobiol. J. 2024, 41, 870–882. [Google Scholar] [CrossRef]
- Kooban, F.; Zadeh, S.S.; Birgani, S.A.; Khorshidi, M. Concrete surface crack detection with convolutional-based deep learning models. ESS Open Arch. Eprints 2024, 603, 60367338. [Google Scholar]
- Zhu, Y.-Q.; Li, Y.-J.; Sun, X.-Y.; Guo, Z.; Rui, S.-J. A one-phase injection method to improve the strength and uniformity in MICP with polycarboxylic acid added. Acta Geotech. 2025, 20, 2279–2291. [Google Scholar] [CrossRef]
- Liu, H.; Wei, Z.; He, X.; Wang, C. Effect of skim milk powder and injection method on efficiency and uniformity of bio-treated 0.5 m-scale sand column. Sci. China Technol. Sci. 2025, 68, 1520203. [Google Scholar] [CrossRef]
- Wang, Y.; Soga, K.; DeJong, J.T.; Kabla, A.J. Microscale visualization of microbial-induced calcium carbonate precipitation processes. J. Geotech. Geoenviron. Eng. 2019, 145, 04019045. [Google Scholar] [CrossRef]
- Benini, S.; Rypniewski, W.R.; Wilson, K.S.; Miletti, S.; Ciurli, S.; Mangani, S. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: Why urea hydrolysis costs two nickels. Structure 1999, 7, 205–217. [Google Scholar] [CrossRef]
- Dadda, A.; Geindreau, C.; Emeriault, F.; Du Roscoat, S.R.; Garandet, A.; Sapin, L.; Filet, A.E. Characterization of microstructural and physical properties changes in biocemented sand using 3D X-ray microtomography. Acta Geotech. 2017, 12, 955–970. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D. Synthesis Methods and Favorable Conditions for Spherical Vaterite Precipitation: A Review. Curr. Comput. Aided Drug Des. 2019, 9, 223. [Google Scholar] [CrossRef]
- Meldrum, F.C.; Hyde, S.T. Morphological Influence of Magnesium and Organic Additives on the Precipitation of Calcite. J. Cryst. Growth 2001, 231, 544–558. [Google Scholar] [CrossRef]
| Sand Column A | Sand Column B | ||
|---|---|---|---|
| 0.6 PV bacterial solution (PH = 4) | 0.6 PV urea (2 M) | 0.6 PV bacterial solution (PH = 4) | 0.6 PV urea (2 M) |
| T = 25 °C | T = 25 °C | T = 10 °C | T = 10 °C |
| Serial Number | CS (M) | PH | Temperature (°C) | Treatment Cycles (N) | ||
|---|---|---|---|---|---|---|
| 1# | 2.0 | 4 | 10 | 1 | 2 | 4 |
| 2# | 2.0 | 4 | 25 | 1 | 2 | 4 |
| 3# | 2.0 | 4 | 40 | 1 | 2 | 4 |
| UCS (MPa) | 1.3 MPa | 1.7 MPa | 2.5 Mpa |
|---|---|---|---|
| Inhomogeneous characteristic index | 1.0 | 0.25 | 0.065 |
| Calcium carbonate content CCC% | 5.93 | 6.25 | 6.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Liu, F.; Zhang, X. A One-Phase Injection Method with Dual Inhibition for Improving the Strength and Uniformity of MICP. Materials 2025, 18, 2514. https://doi.org/10.3390/ma18112514
Huang Y, Liu F, Zhang X. A One-Phase Injection Method with Dual Inhibition for Improving the Strength and Uniformity of MICP. Materials. 2025; 18(11):2514. https://doi.org/10.3390/ma18112514
Chicago/Turabian StyleHuang, Yanni, Fengyin Liu, and Xiangtong Zhang. 2025. "A One-Phase Injection Method with Dual Inhibition for Improving the Strength and Uniformity of MICP" Materials 18, no. 11: 2514. https://doi.org/10.3390/ma18112514
APA StyleHuang, Y., Liu, F., & Zhang, X. (2025). A One-Phase Injection Method with Dual Inhibition for Improving the Strength and Uniformity of MICP. Materials, 18(11), 2514. https://doi.org/10.3390/ma18112514






