Enhanced Phosphorus Removal from Metallurgical Grade Silicon by the Combined Process of Si-Cu Solvent Refining and CaO-CaF2-CaCl2 Slag Treatment
Abstract
1. Introduction
2. Materials and Experimental
3. Results and Discussion
3.1. Morphology Characteristics of Si-Cu Alloy Treated by CaO-CaF2-CaCl2 Slag
3.2. Removal of P Impurity
3.2.1. Effect of CaO Content in Slag
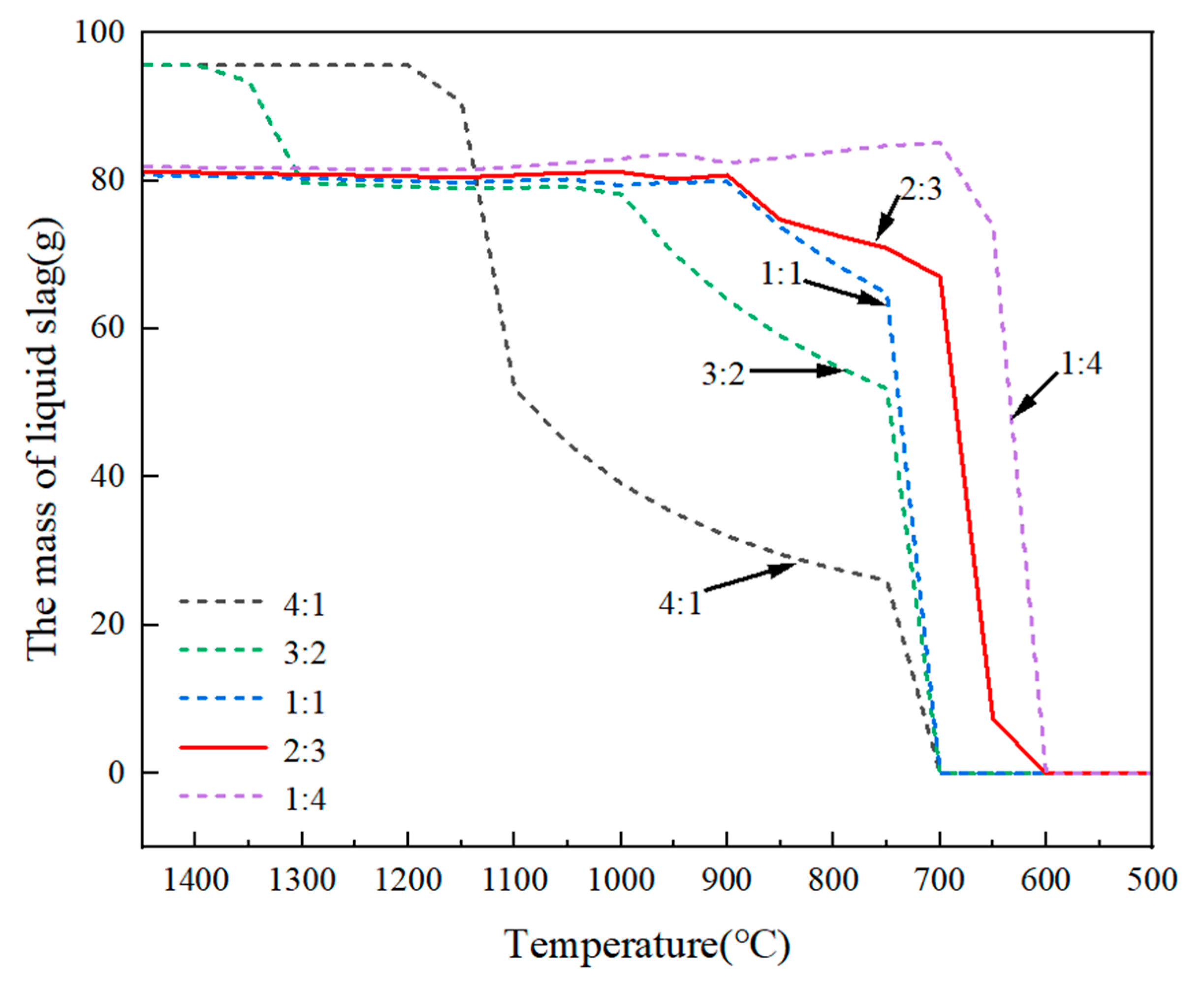
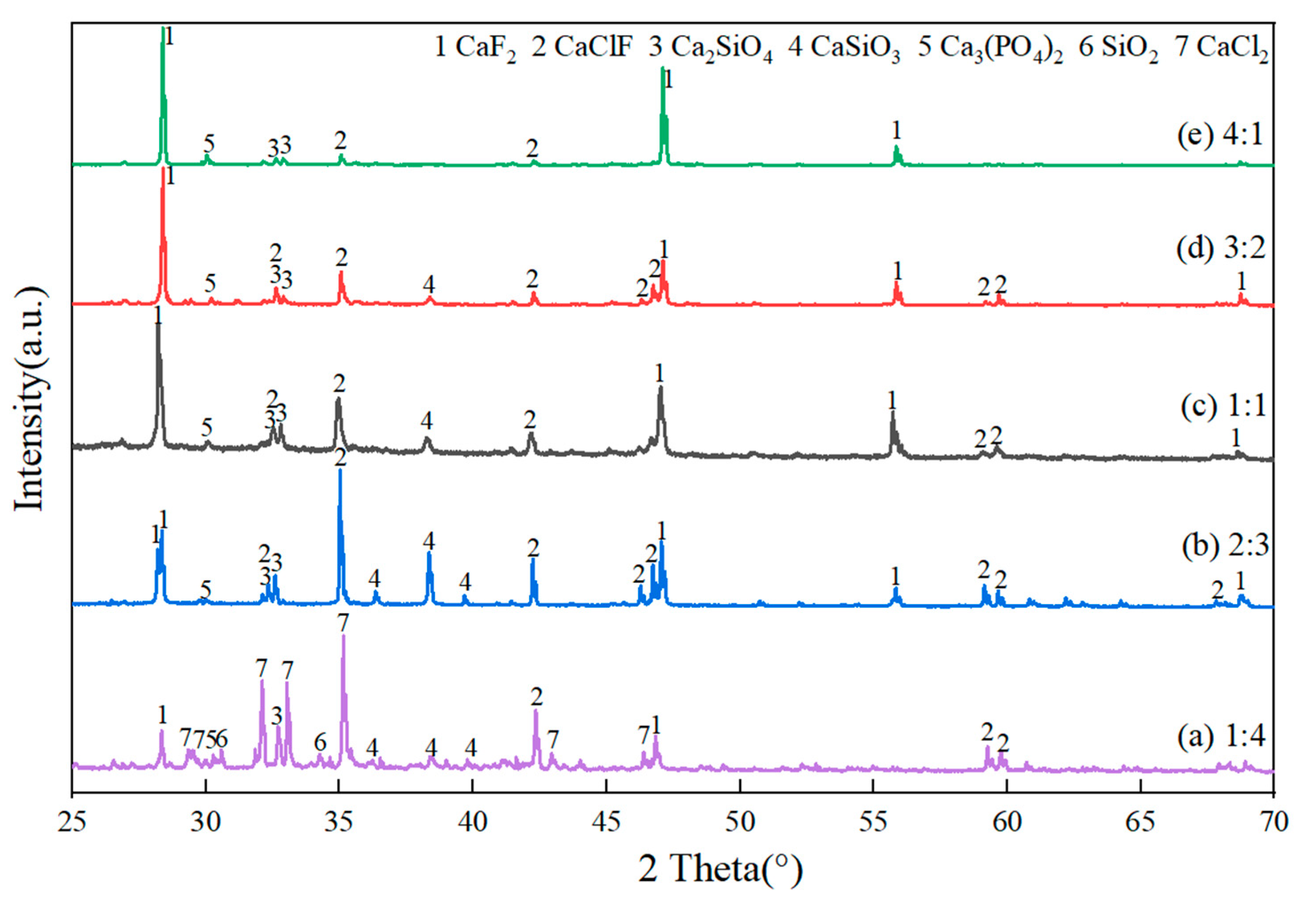
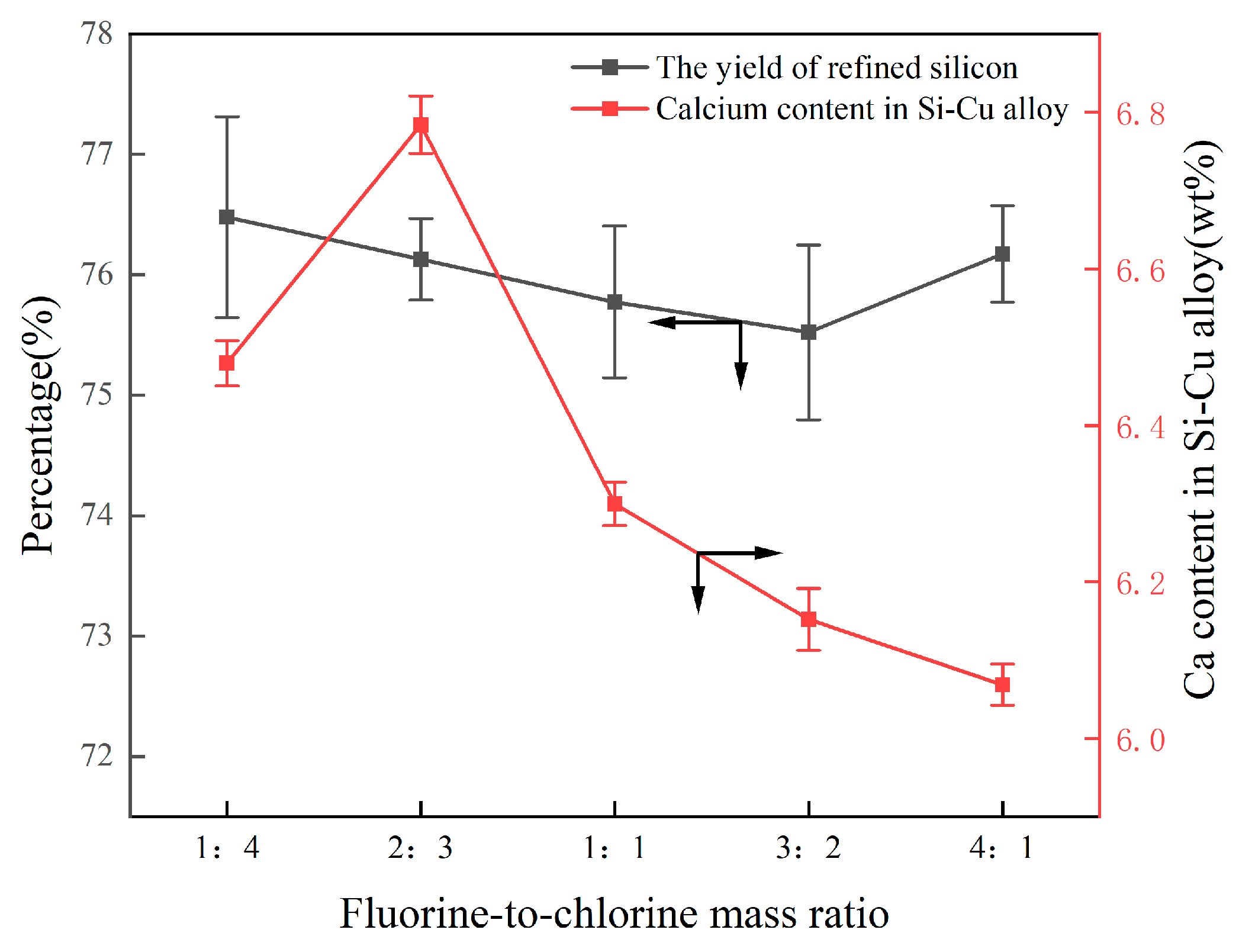
3.2.2. Effect of Fluorine-to-Chlorine Ratios in Slag
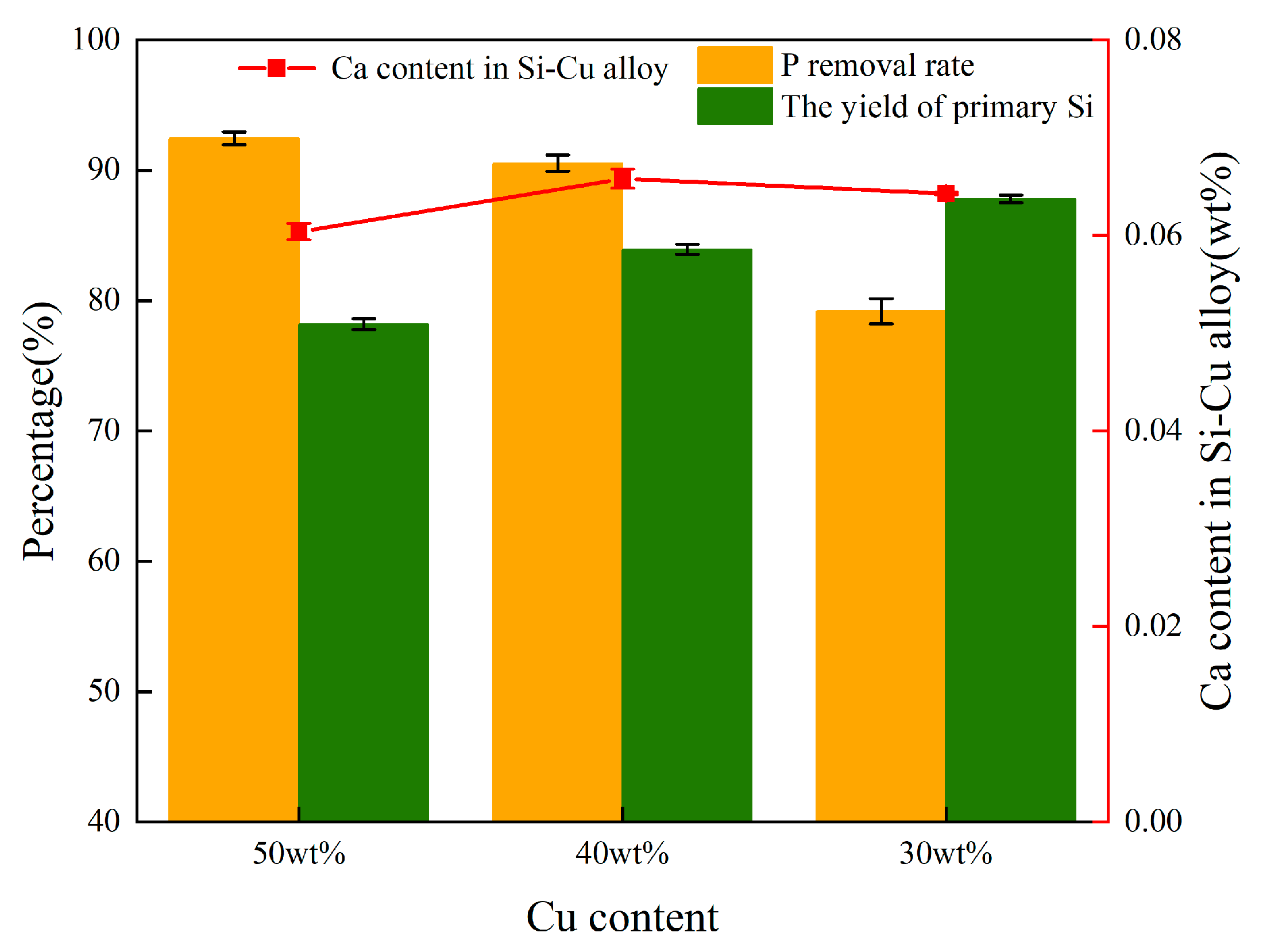
3.2.3. Effect of Alloy Composition
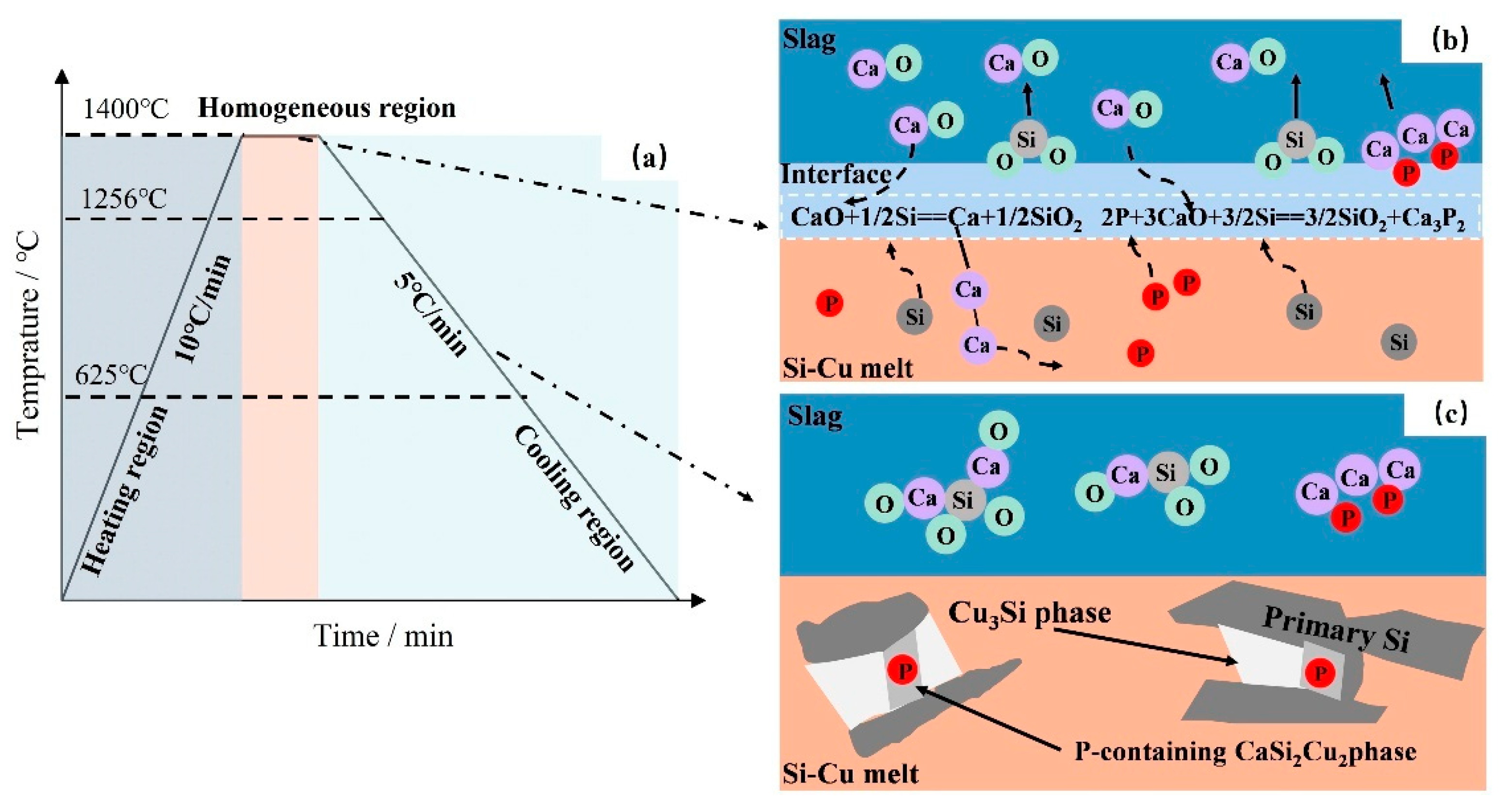
3.3. Mechanism of P Removal
3.4. Recovery of Refined Slag and Cu
4. Conclusions
- In the refining process, impurity P was found to simultaneously concentrate in the P-rich phases of Ca3P2 in the slag and CaCu2Si2 in the alloy. The Ca3P2 tends to be oxidized to calcium phosphate Ca3(PO4)2 when exposed to air, while CaCu2Si2 could be removed by selective acid leaching.
- The P removal rate and LP were linearly increased to the content of CaO; the increase in the mass ratios of fluorine-to-chlorine in the slag system had a negligible effect on Ca migration and the yield of refined Si; the removal efficiency of P in the alloy significantly increased as the Cu content increased from 30 wt.% to 50 wt.%, but the yield of Si decreased from 87.59% to 77.89%.
- The reaction model of CaO-CaF2-CaCl2 slag and Si-Cu alloy for P removal was established. During the refining process, a portion of the P in the silicon was reduced to P3− at the slag–metal interface and then entered the slag phase in the form of phosphide Ca3P2. At the same time, a silicothermal reduction reaction occurred between CaO and Si, resulting in the migration of Ca into the alloy and the precipitation of P-rich CaCu2Si2 in the Si-Cu alloy. When the Si-40 wt.% Cu alloy was treated with 20 wt.% CaO-32 wt.% CaF2-48 wt.% CaCl2 slag for 60 min at 1400 °C, the P removal rate of refined silicon reached 90.1%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xi, F.S.; Li, S.Y.; Ma, W.H.; Chen, Z.J.; Wei, K.X.; Wu, J.J. A review of hydrometallurgy techniques for the removal of impurities from metallurgical-grade silicon. Hydrometallurgy 2021, 201, 105553. [Google Scholar] [CrossRef]
- Cheng, H.R.; Zheng, S.S.; Chen, C. The behavior of Ca and its compounds in Si during the slag refining with CaO-SiO2-CaF2 system under air atmosphere. Sep. Purif. Technol. 2018, 201, 60–70. [Google Scholar] [CrossRef]
- Liang, J.S.; Zhang, Y.Z.; Huang, X.P.; Li, J.C.; Zhao, Q.; Li, Y.B.; Li, J.W. Low-temperature enhancement in the extraction of phosphorus from metallurgical-grade silicon by simultaneously re-constructing phosphorus-rich phases of Ca3P2 and CaAl2Si2-P. Metall. Res. Technol. 2024, 121, 317. [Google Scholar] [CrossRef]
- Lei, Y.; Ma, W.; Wu, J.; Wei, K.; Lv, G.Q.; Li, S.; Morita, K. Purification of metallurgical-grade silicon using Si-Sn alloy in presence of Hf, Zr, or Ti. Mater. Sci. Semicond. Process. 2018, 88, 97–103. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.W.; Song, W.F.; Jian, X.S.; Ding, J.X.; Zhu, X.X.; Chen, J.; Ban, B.Y. Enhancing P removal from primary Si by P-containing Al2Si2Sr phase during Al-Si solvent refining. Mater. Sci. Semicond. Process. 2022, 147, 106769. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Yue, S.Y.; Tang, K.; Safarian, J. New insights into silicon purification by alloying-leaching refining: A comparative study of Mg-Si, Ca-Si, and Ca-Mg-Si systems. ACS Sustain. Chem. Eng. 2020, 8, 15953–15966. [Google Scholar] [CrossRef]
- Ren, Y.S.; Chen, H.; Mizutani, T.; Ma, W.H.; Zeng, Y.; Morita, K. Efficient separation of bulk Si and enhanced B removal by Si-Sn-Cu ternary solvent refining with Zr addition. Sep. Purif. Technol. 2021, 275, 119242. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.W.; Zuo, Q.X.; Ban, B.Y.; Chen, J. Simultaneously removal of P and B from Si by Sr and Zr co-addition during Al-Si low-temperature solvent refining. Waste Manag. Res. 2023, 30, 365–377. [Google Scholar] [CrossRef]
- Ebrahimfar, F.; Ahmadian, M. Purification of metallurgical-grade silicon by acid leaching. Silicon 2019, 11, 1979–1987. [Google Scholar] [CrossRef]
- Hoseinpur, A.; Safarian, J. Vacuum refining of silicon at ultrahigh temperatures. Vacuum 2021, 184, 109924. [Google Scholar] [CrossRef]
- Tan, Y.; Ren, S.Q.; Shi, S.; Wen, S.T.; Jiang, D.C.; Dong, W.; Ji, M.; Sun, S.H. Removal of aluminum and calcium in multicrystalline silicon by vacuum induction melting and directional solidification. Vacuum 2014, 99, 272–276. [Google Scholar] [CrossRef]
- Shi, S.; Dong, W.; Peng, X.; Jiang, D.C.; Tan, Y. Evaporation and removal mechanism of phosphorus from the surface of silicon melt during electron beam melting. Appl. Surf. Sci. 2013, 266, 344–349. [Google Scholar] [CrossRef]
- Qian, G.; Sun, L.; Chen, H.; Wang, Z.; Wei, K.; Ma, W. Enhancing impurities removal from Si by controlling crystal growth in directional solidification refining with Al-Si alloy. J. Alloys Compd. 2020, 820, 153300. [Google Scholar] [CrossRef]
- He, Y.F.; Ma, W.H.; Xing, A.M.; Hu, M.S. A review of the process on the purification of metallurgical grade silicon by solvent refining. Mater. Sci. Semicond. Process. 2022, 141, 106438. [Google Scholar] [CrossRef]
- Mitrašinović, A.M.; Utigard, T.A. Trace elements distribution in Cu–Si alloys. Chem. Phys. Lett. 2011, 515, 72–77. [Google Scholar] [CrossRef]
- Zhang, C.T.; Huang, L.Q.; Danaei, A.; Chen, G.Y.; Luo, X.T. Effect of impurity trappers on the distribution and removal of phosphorus from Si with Si-Cu solvent. Mater. Lett. 2022, 308, 131126. [Google Scholar] [CrossRef]
- Huang, L.Q.; Lai, H.X.; Lu, C.H.; Fang, M.; Ma, W.H. Enhancement in extraction of boron and phosphorus from metallurgical grade silicon by copper alloying and aqua regia leaching. Hydrometallurgy 2016, 161, 14–21. [Google Scholar] [CrossRef]
- Huang, L.Q.; Danaei, A.; Thomas, S.; Xing, P.F.; Li, J.T.; Luo, X.T.; Barati, M. Solvent extraction of phosphorus from Si-Cu refining system with calcium addition. Sep. Purif. Technol. 2018, 204, 205–212. [Google Scholar] [CrossRef]
- He, F.L.; Zheng, S.S.; Chen, C. The Effect of Calcium Oxide Addition on the Removal of Metal Impurities from Metallurgical-Grade Silicon by Acid Leaching. Metall. Mater. Trans. B 2012, 43, 1011–1018. [Google Scholar] [CrossRef][Green Version]
- Kawamura, H.; Yanaba, Y.; Yoshikawa, T. Reductive removal of phosphorus in silicon using CaO-CaF2 slag. Mater. Sci. Forum 2013, 750, 284–287. [Google Scholar] [CrossRef]
- Shin, J.H.; Park, J.H. Thermodynamics of reducing refining of phosphorus from Si-Mn alloy using CaO-CaF2 slag. Metall. Mater. Trans. B 2012, 43, 1243–1246. [Google Scholar] [CrossRef]
- Huang, L.Q.; Lai, H.X.; Gan, C.H.; Xiong, H.Q.; Xing, P.F.; Luo, X.T. Separation of boron and phosphorus from Cu-alloyed metallurgical grade silicon by CaO-SiO2-CaCl2 slag treatment. Sep. Purif. Technol. 2016, 170, 408–416. [Google Scholar] [CrossRef]
- Zhang, C.T.; Lai, H.X.; Zhang, Y.H.; Sheng, Z.L.; Luo, X.T. Extraction of phosphorus from metallurgical grade silicon using a combined process of Si-Al-Ca solvent refining and CaO-CaF2 slag treatment. Sep. Purif. Technol. 2019, 232, 115954. [Google Scholar] [CrossRef]
- Mitrašinović, A.M.; Utigard, T.A. Refining silicon for solar cell application by copper alloying. Silicon 2009, 1, 239–248. [Google Scholar] [CrossRef]
- Fang, M.; Lu, C.H.; Huang, L.Q.; Lai, H.X.; Chen, J. Effect of calcium-based slag treatment on hydrometallurgical purification of metallurgical-grade silicon. Ind. Eng. Chem. Res. 2014, 53, 972–979. [Google Scholar] [CrossRef]
- Li, M.; Utigard, T.; Barati, M. Removal of Boron and Phosphorus from Silicon Using CaO-SiO2-Na2O-Al2O3 Flux. Metall. Mater. Trans. B 2014, 45, 221–228. [Google Scholar] [CrossRef]
- Teixeira, L.A.V.; Morita, K. Removal of Boron from Molten Silicon Using CaO-SiO2 Based Slags. ISIJ Int. 2009, 49, 783–787. [Google Scholar] [CrossRef]
- Park, J.H.; Min, D.J.; Song, H.S. The Effect of CaF2 on the viscosities and structures of CaO-SiO2(-MgO)-CaF2 slags. Metall. Mater. Trans. B 2002, 33, 723–729. [Google Scholar] [CrossRef]
- Visnovec, K.; Variawa, C.; Utigard, T.; Mitrasinovic, A. Elimination of impurities from the surface of silicon using hydrochloric and nitric acid. Mater. Sci. Semicond. Process. 2013, 16, 106–110. [Google Scholar] [CrossRef]
- Yin, Z.; Oliazadeh, A.; Esfahani, S.; Johnston, M.; Barati, M. Solvent refining of silicon using nickel as impurity getter. Can. Metall. Q. 2011, 50, 166–172. [Google Scholar] [CrossRef]
- Sun, L.Y.; Wang, Z.; Chen, H.; Wang, D.; Qian, G.Y. Removal of phosphorus in silicon by the formation of CaAl₂Si₂ phase at the solidification interface. Metall. Mater. Trans. B 2016, 48, 420–428. [Google Scholar] [CrossRef]
- Zhu, M.Y.; Yue, S.Y.; Wu, G.X.; Tang, K.; Xu, Y.J.; Safarian, J. P removal from Si by Si-Ca-Al alloying-leaching refining: Effect of Al and the CaAl₂Si₂ phase. Sep. Purif. Technol. 2021, 271, 118675. [Google Scholar] [CrossRef]
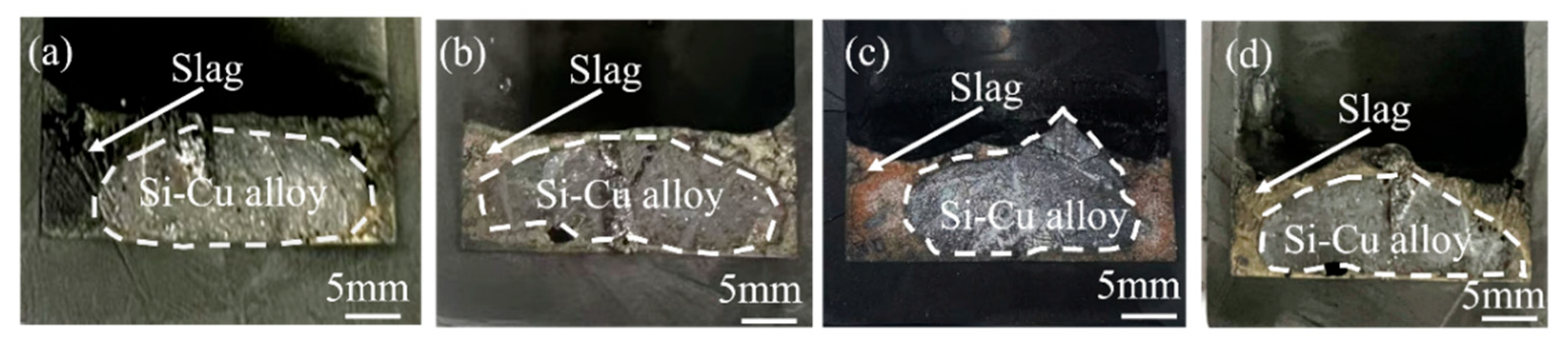
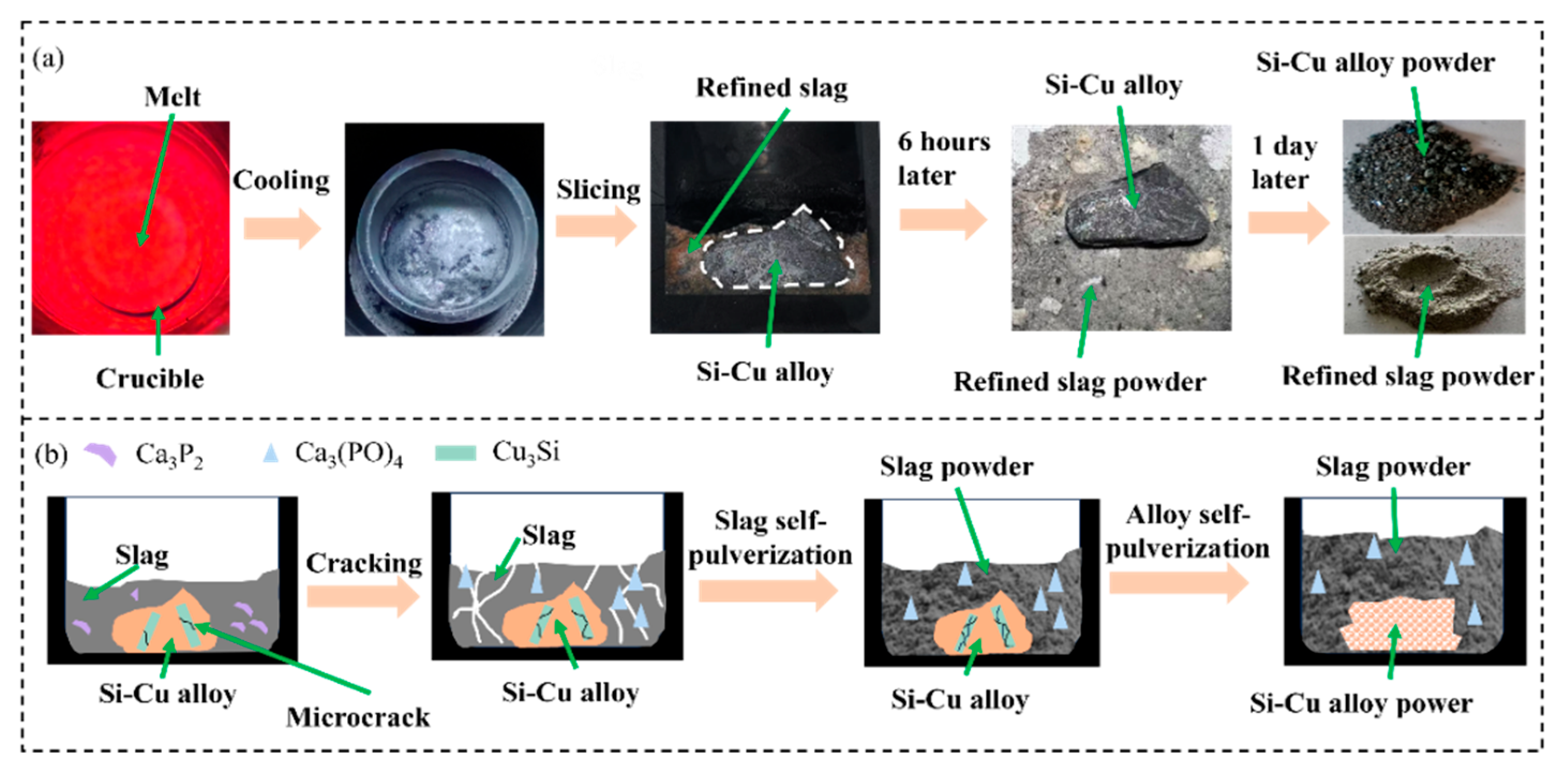

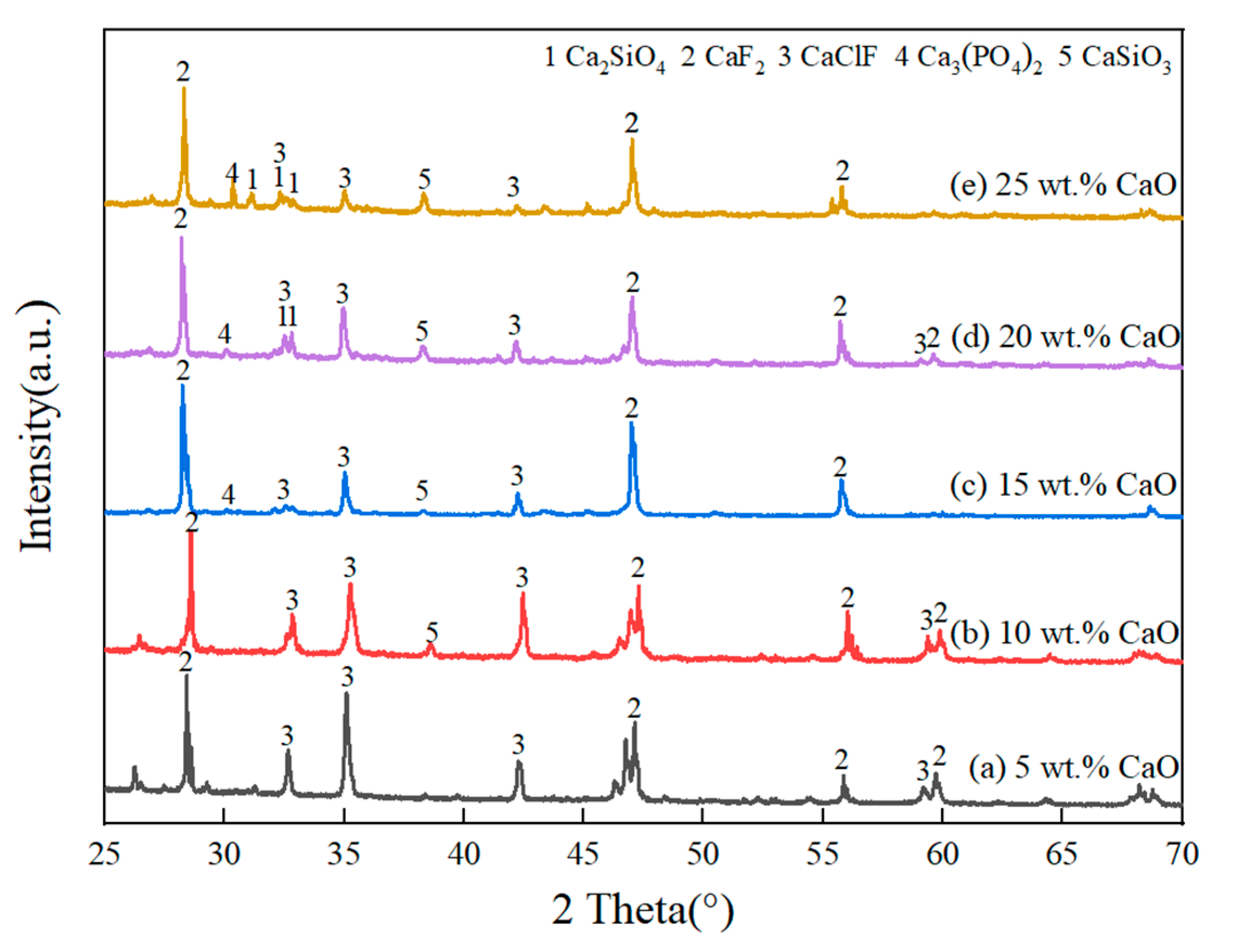
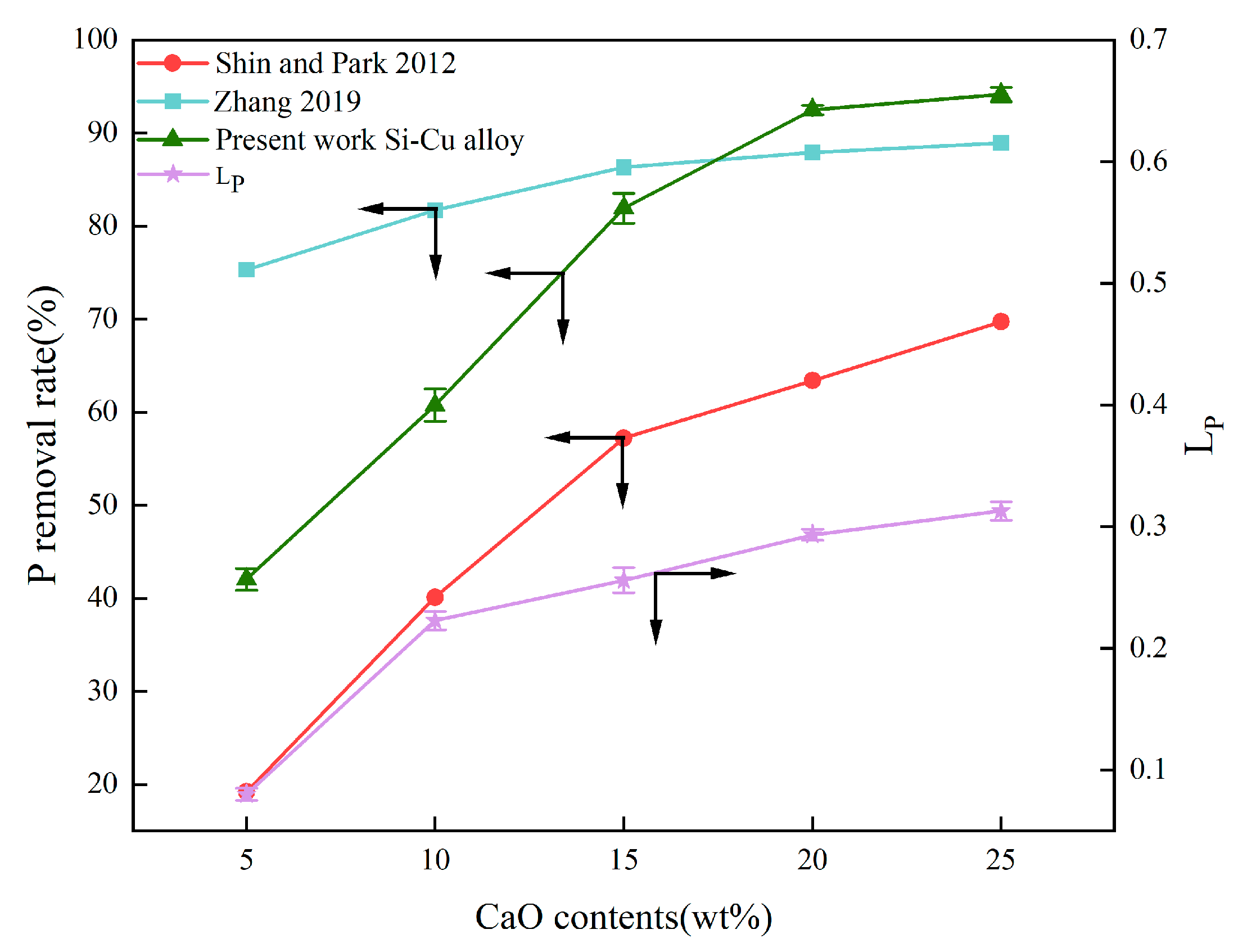


| Method | Key Findings | Limitations | References |
|---|---|---|---|
| Si-Cu Alloy | Impurity P concentrated in Cu3Si phase | Relatively low removal rate of impurity P (23–42%) | [15,17] |
| Si-Cu-Ca Alloy | Impurity P concentrated in CaCu2Si2 phase with the P removal rate up to 82% | Difficulty in obtaining “Ca” in industry | [16,18] |
| CaO-based Slag | CaO could be reduced to Ca by Si, enhancing P removal | The mechanism of P removal was uncovered | [2,20,21] |
| Si-Cu alloy coupled with CaO-SiO2-CaCl2 slag | Impurity P concentrated in both Cu3Si phase in the alloy and oxidized to P2O5 in the slag. | Difficult for P to be oxidized in comparison with impurities of Al, Ca, Mg, and B in MG-Si, and relatively low removal rate of impurity P (32.7–57.6%). | [22] |
| No. | Composition of Alloy (wt.%) | Total Mass of Alloy (g) | Composition of Slag (wt.%) | Total Slag Mass (g) | ||||
|---|---|---|---|---|---|---|---|---|
| Si | Cu | P | CaCl2 | CaO | CaF2 | |||
| E0 | 49 | 50 | 1 | 10 | 0 | 0 | 0 | 0 |
| E1 | 49 | 50 | 1 | 10 | 47.5 | 5 | 47.5 | 10 |
| E2 | 49 | 50 | 1 | 10 | 45 | 10 | 45 | 10 |
| E3 | 49 | 50 | 1 | 10 | 42.5 | 15 | 42.5 | 10 |
| E4 | 49 | 50 | 1 | 10 | 40 | 20 | 40 | 10 |
| E5 | 49 | 50 | 1 | 10 | 37.5 | 25 | 37.5 | 10 |
| E6 | 49 | 50 | 1 | 10 | 64 | 20 | 16 | 10 |
| E7 | 49 | 50 | 1 | 10 | 48 | 20 | 32 | 10 |
| E8 | 49 | 50 | 1 | 10 | 32 | 20 | 48 | 10 |
| E9 | 49 | 50 | 1 | 10 | 16 | 20 | 64 | 10 |
| E10 | 59 | 40 | 1 | 10 | 48 | 20 | 32 | 10 |
| E11 | 69 | 30 | 1 | 10 | 48 | 20 | 32 | 10 |
| EDS Analysis | Element (at.%) | Potential Formula | |||
|---|---|---|---|---|---|
| Si | Cu | Ca | P | ||
| #1 | 100 | - | - | - | Si |
| #2 | 24.97 | 72.98 | - | 2.05 | Cu3Si |
| #3 | 39.06 | 35.14 | 22.77 | 3.03 | CaCu2Si2 |
| #4 | 40.67 | 34.88 | 21.72 | 2.74 | CaCu2Si2 |
| #5 | 20.09 | 76.68 | - | 1.23 | Cu3Si |
| #6 | 19.80 | 74.88 | - | 0.98 | Cu3Si |
| #7 | 100 | - | - | - | Si |
| No. | Si | Cu | P | Ca |
|---|---|---|---|---|
| E1 | 47.87 | 49.45 | 0.99 | 1.69 |
| E2 | 46.87 | 48.93 | 0.91 | 3.29 |
| E3 | 45.77 | 48.54 | 0.87 | 4.82 |
| E4 | 44.91 | 47.95 | 0.82 | 6.32 |
| E5 | 43.81 | 47.63 | 0.79 | 7.77 |
| Researcher | System | Temperature | Removal Efficiency of P | The State of P in the Alloy |
|---|---|---|---|---|
| Sun [31] | Si-Al-Ca | T = 1723 K | P: 23→5 ppmw P removed = 78.26% | Dissolved in CaAl2Si2 phase |
| Zhu [32] | Si-Al-Ca | T = 1723 K | P:12.3→1.722 ppmw P removed = 86% | Dissolved in CaAl2Si2 phase |
| Chen [5] | Si-Al-Sr | T = 1323 K | P: 100→5.34 ppmw P removed = 94.66% | Dissolved in Al2Si2Sr phase |
| Huang [18] | Si-Cu-Ca | T = 1823 K | P: 1622→292 ppmw P removed = 82% | Dissolved in CaCu2Si2 phase |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, X.; Zhao, Q.; Li, J.; Li, J. Enhanced Phosphorus Removal from Metallurgical Grade Silicon by the Combined Process of Si-Cu Solvent Refining and CaO-CaF2-CaCl2 Slag Treatment. Materials 2025, 18, 2502. https://doi.org/10.3390/ma18112502
Wei X, Zhao Q, Li J, Li J. Enhanced Phosphorus Removal from Metallurgical Grade Silicon by the Combined Process of Si-Cu Solvent Refining and CaO-CaF2-CaCl2 Slag Treatment. Materials. 2025; 18(11):2502. https://doi.org/10.3390/ma18112502
Chicago/Turabian StyleWei, Xinlin, Qing Zhao, Juncheng Li, and Jingwei Li. 2025. "Enhanced Phosphorus Removal from Metallurgical Grade Silicon by the Combined Process of Si-Cu Solvent Refining and CaO-CaF2-CaCl2 Slag Treatment" Materials 18, no. 11: 2502. https://doi.org/10.3390/ma18112502
APA StyleWei, X., Zhao, Q., Li, J., & Li, J. (2025). Enhanced Phosphorus Removal from Metallurgical Grade Silicon by the Combined Process of Si-Cu Solvent Refining and CaO-CaF2-CaCl2 Slag Treatment. Materials, 18(11), 2502. https://doi.org/10.3390/ma18112502






