Microwave Pre-Treatment for Efficient Zinc Recovery via Acid Leaching
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Analytical Techniques
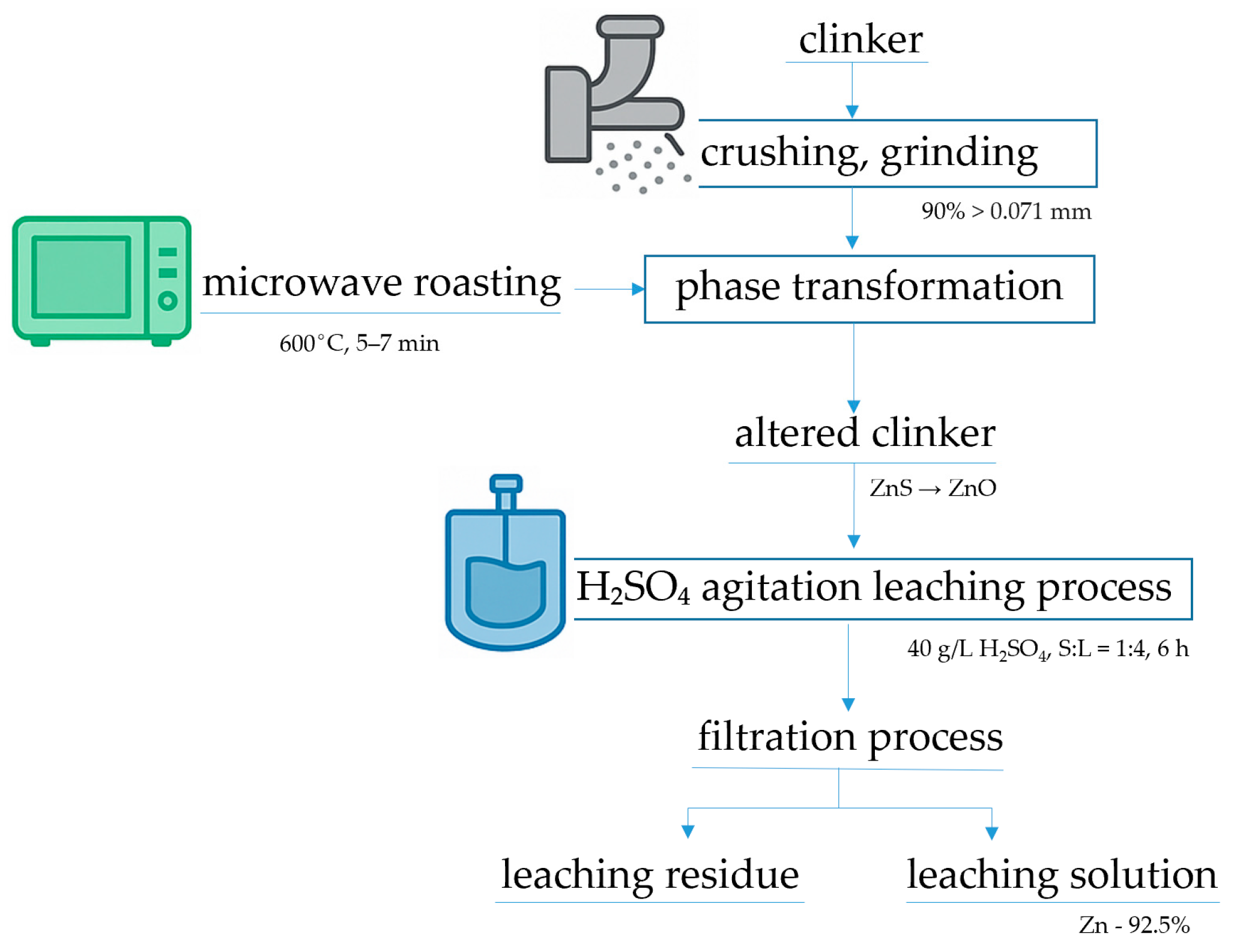
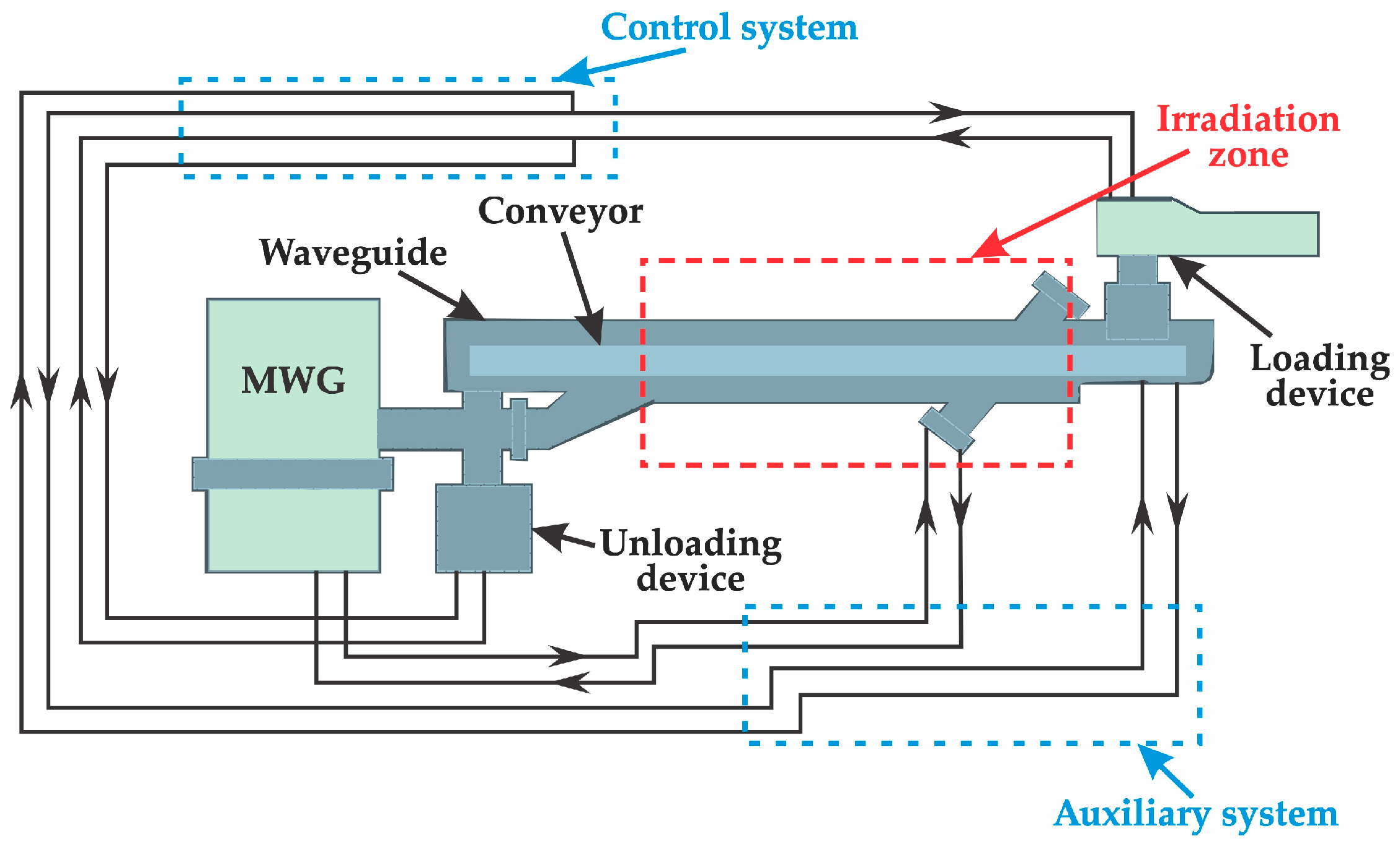
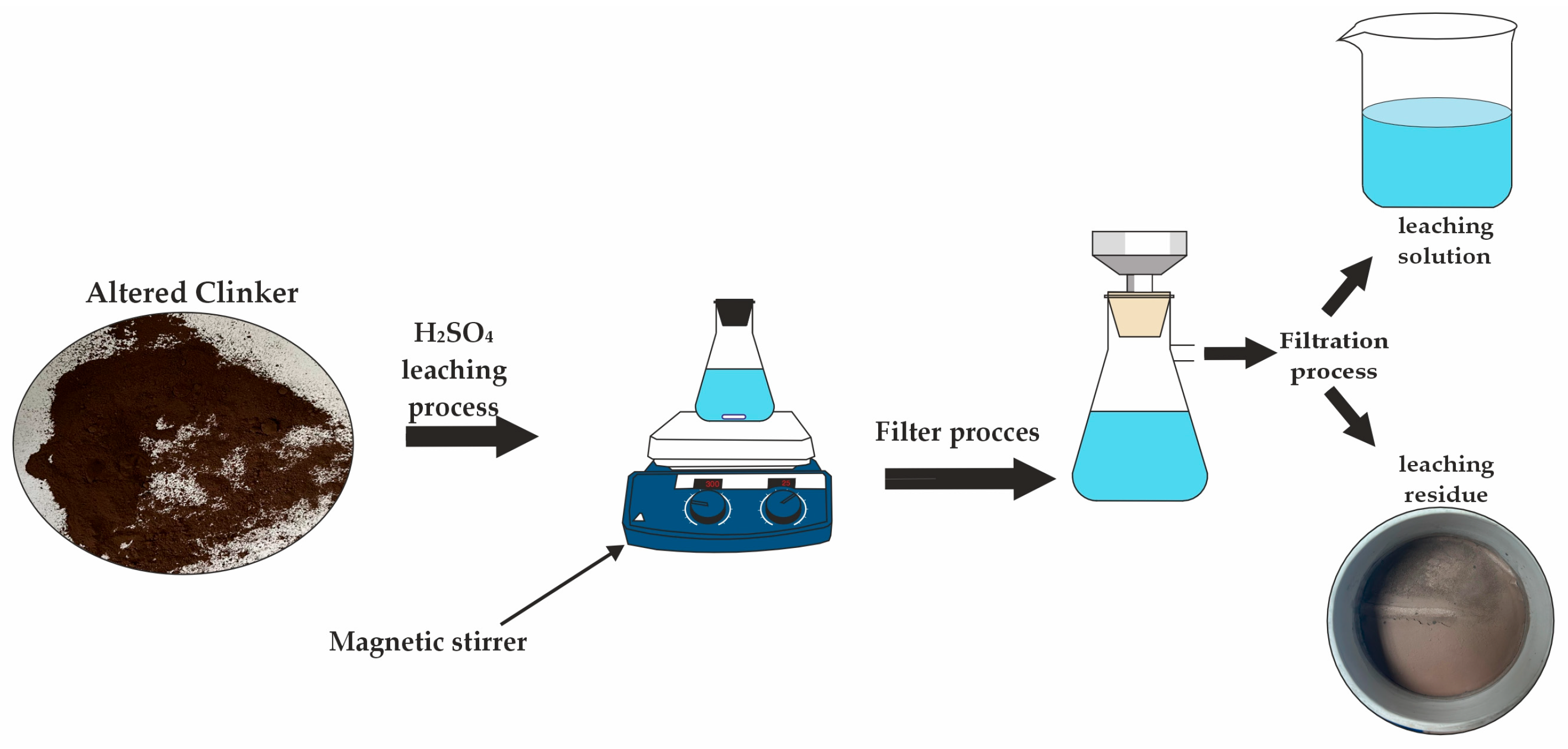
2.3. Experimental Method
3. Results and Discussion
3.1. Phase Analysis of the Initial Clinker
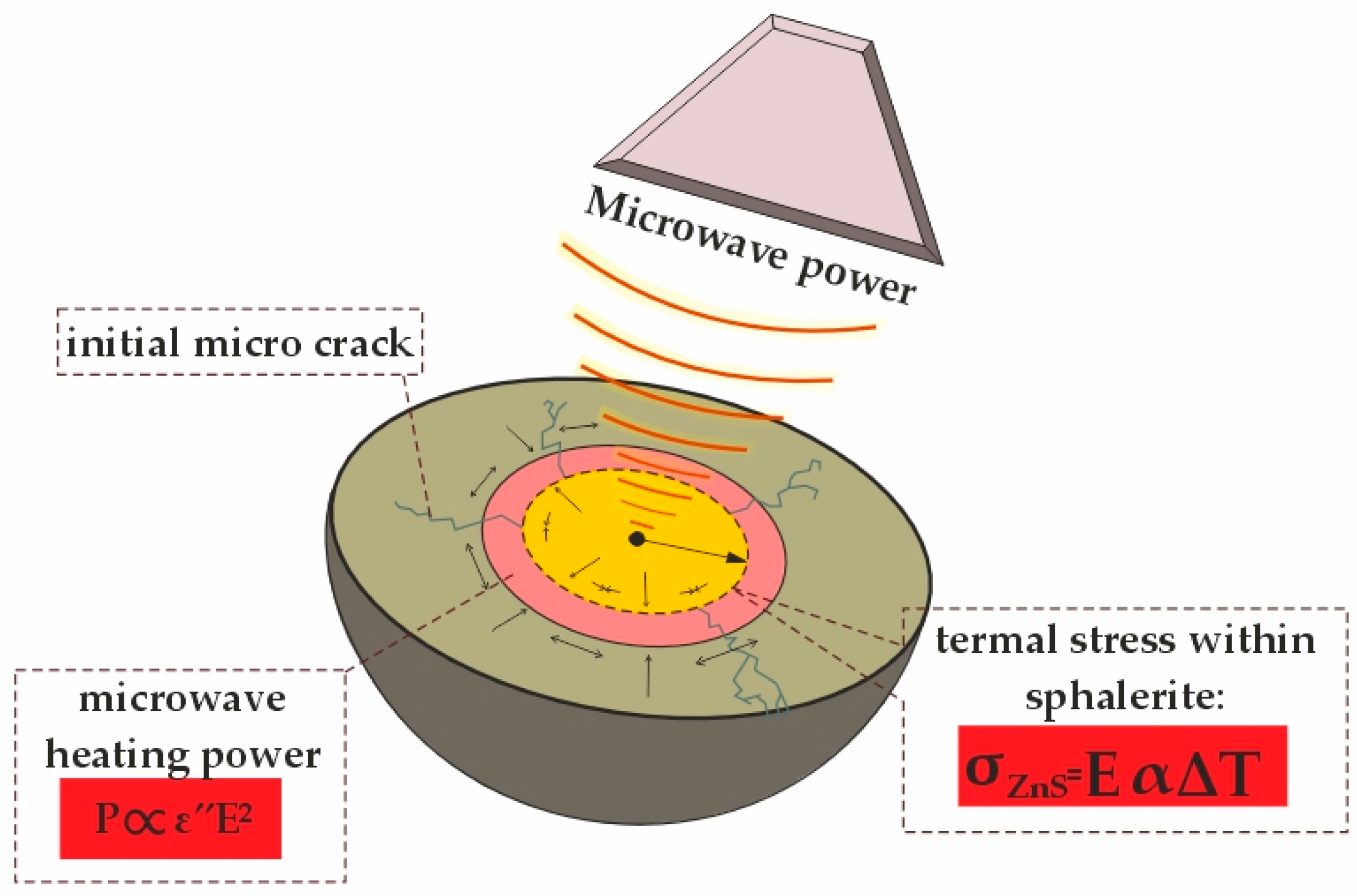
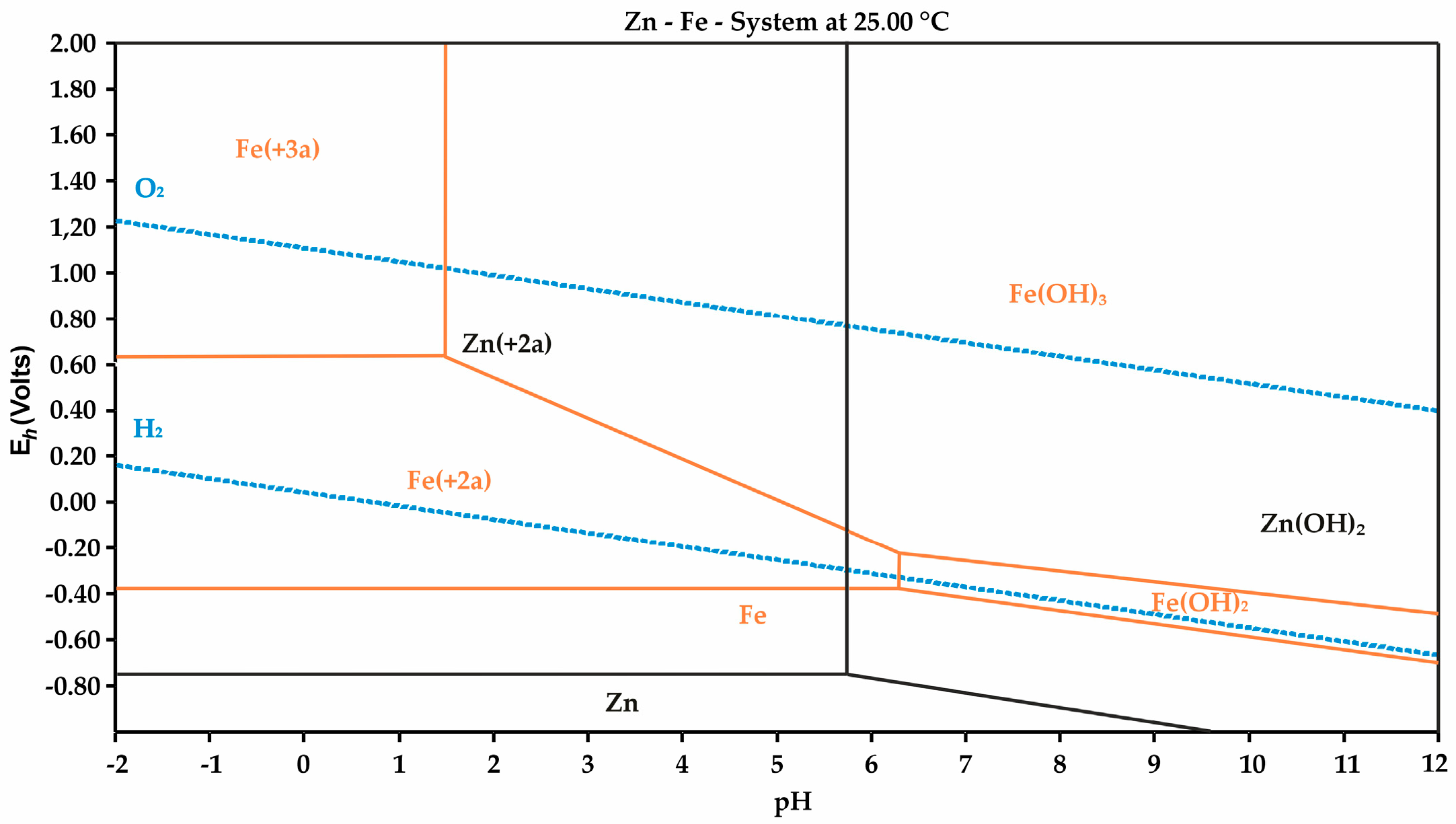
3.2. Effects of Microwave Irradiation on Phase Transformations and Thermal Stress in Clinker
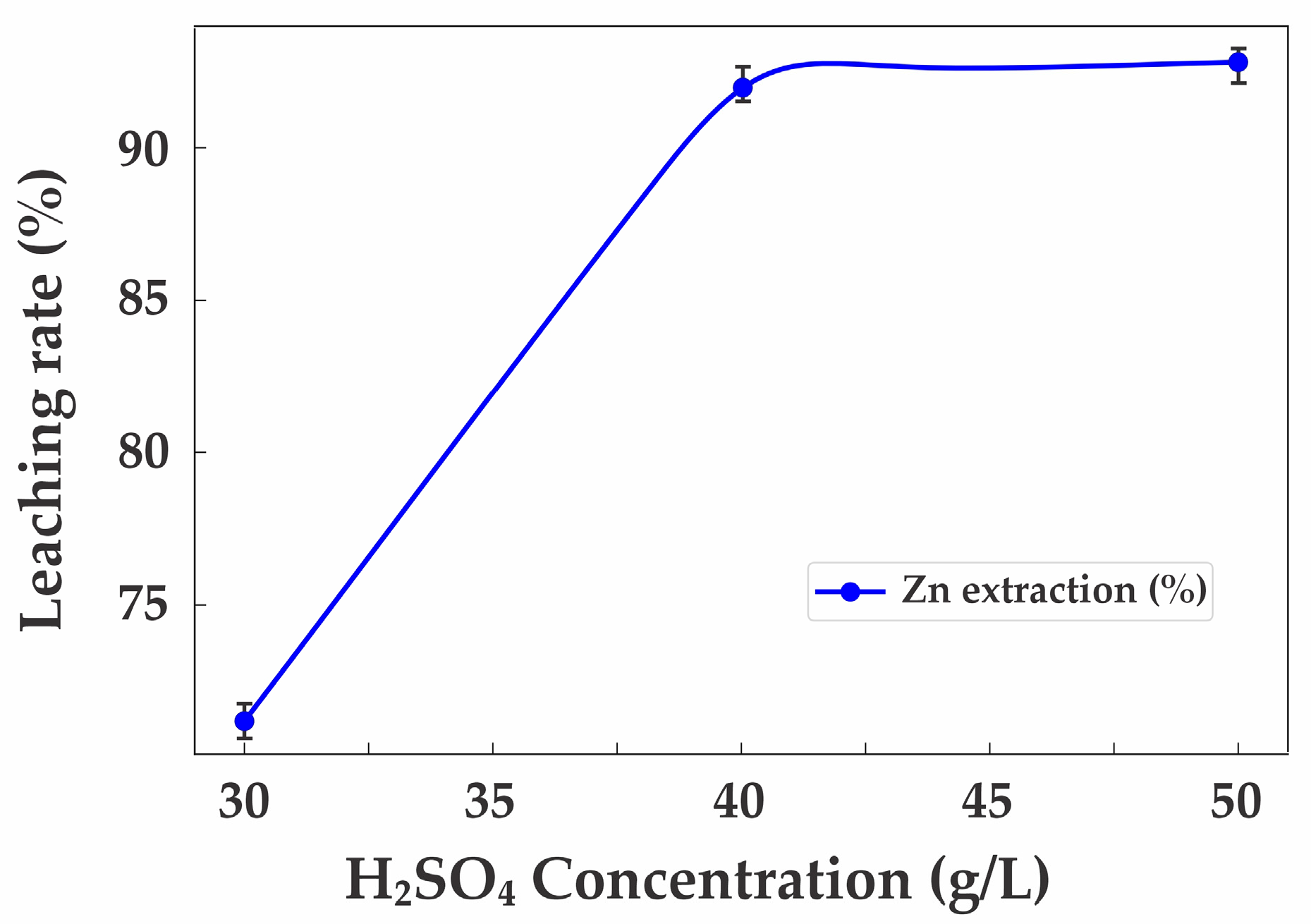
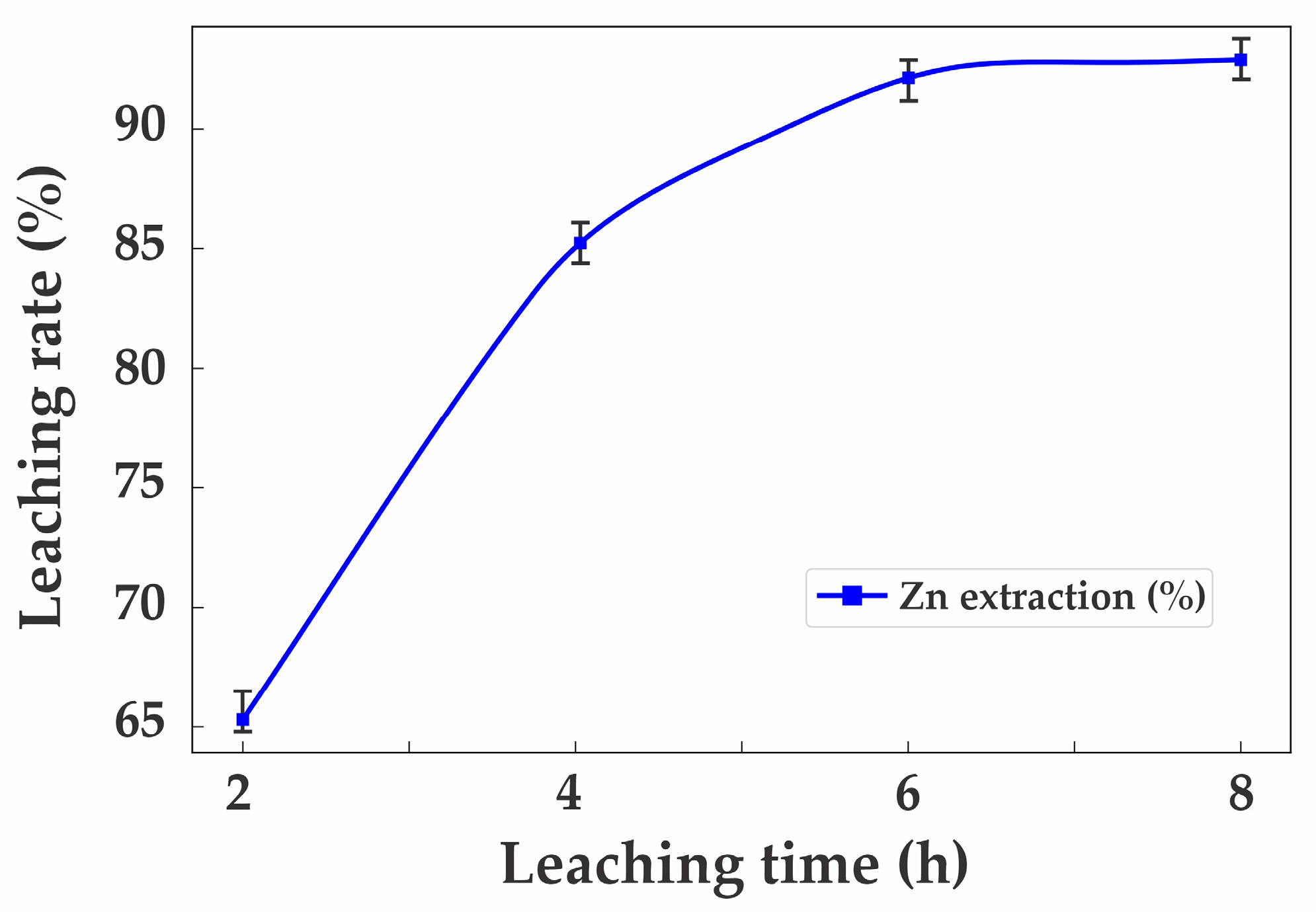
3.3. Leaching Experiments
3.4. Comparison of Conventional and Microwave-Assisted Leaching
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, W.P.; Wang, Z.X.; Wang, X.M.; Miller, J.D. Smithsonite flotation with lauryl phosphate. Miner. Eng. 2020, 147, 106155. [Google Scholar] [CrossRef]
- Langer, E.; Zubielewicz, M.; Królikowska, A.; Komorowski, L.; Krawczyk, K.; Wanner, M.; Aktas, L.; Hilt, M. Zinc-Reduced Anticorrosive Primers—Water-Based Versus Solvent-Based. Coatings 2025, 15, 64. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, J.; Li, X.; Peng, Y. Corrosion resistance of MWCNTs-nanoZnO composite reinforced chromium-free Zn-Al coating. Anti-Corrosion Methods Mater. 2024, 71, 640–649. [Google Scholar] [CrossRef]
- He, X.; Zhou, X.; Shang, T.; Liu, W.; Jiang, G.; Liu, C.; Cheng, X.; Li, X. Influencial mechanisms of elements and alloy phases on corrosion resistance of Zn-Al-Mg coated steel in atmospheric environments: A review. Corros. Commun. 2024, 13, 49–59. [Google Scholar] [CrossRef]
- Wojcik, P. Zinc Supply and Influences from Geology; Irish Association for Economic Geology: Dublin, Ireland, 2023. [Google Scholar] [CrossRef]
- Clark, S. Zinc Systems|Introduction, History, and Overview of Zinc-Based Batteries; Elsevier BV: Amsterdam, The Netherlands, 2023. [Google Scholar]
- Funk, J.A.; Mauk, J.L.; Karl, N. The Importance of Zinc in Combating Climate Change and How U.S. Resources Could Contribute; Geological Society of America Abstracts with Programs: Boulder, CO, USA, 2022. [Google Scholar]
- Jia, K.; Lu, Y.; Liu, J.; Cheng, S.; Liu, S.; Cao, Y.; Li, G. Selective flotation separation of hemimorphite from quartz using the biosurfactant sodium N-lauroylsarcosinate as a novel collector. Miner. Eng. 2023, 198, 108073. [Google Scholar] [CrossRef]
- Engin, A.B. Zinc and Zinc Compounds. In Patty’s Toxicology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2023; pp. 1–33. [Google Scholar]
- Kenzhaliyev, B.; Surkova, T.; Berkinbayeva, A.; Baltabekova, Z.; Smailov, K. Harnessing Microwave Technology for Enhanced Recovery of Zinc from Industrial Clinker. Metals 2024, 14, 699. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Surkova, T.; Berkinbayeva, A.; Baltabekova, Z.; Smailov, K.; Abikak, Y.; Saulebekkyzy, S.; Tolegenova, N.; Omirbek, T.; Dosymbaeva, Z. Innovative Methods for Intensifying the Processing of Zinc Clinker: Synergy of Microwave Treatment and Ultrasonic Leaching. Metals 2025, 15, 246. [Google Scholar] [CrossRef]
- Zhanikulov, N.N.; Sapargaliyeva, B.; Agabekova, A.B.; Alfereva, Y.O.; Syrlybekkyzy, S.; Nurshakhanova, L.K.; Nurbayeva, F.K.; Sabyrbaeva, G.S.; Kozlov, P.T.; Kolesnikova, O.G. Studies of Utilization of Technogenic Raw Materials in the Synthesis of Cement Clinker from It and Further Production of Portland Cement. J. Compos. Sci. 2023, 7, 226. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Ultarakova, A.; Lokhova, N.; Mukangaliyeva, A.; Kassymzhanov, K. Optimized Hydrometallurgical Extraction of Molybdenum via Mechanoactivation and Nitric–Sulfuric Leaching. Processes 2025, 13, 1486. [Google Scholar] [CrossRef]
- Kolesnikov, A.; Fediuk, R.; Kolesnikova, O.; Zhanikulov, N.; Zhakipbayev, B.; Kuraev, R.; Akhmetova, E.; Shal, A. Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc. Materials 2022, 15, 324. [Google Scholar] [CrossRef]
- Rahmati, S.; Adavodi, R.; Hosseini, M.R.; Veglio’, F. Efficient Metal Extraction from Dilute Solutions: A Review of Novel Selective Separation Methods and Their Applications. Metals 2024, 14, 605. [Google Scholar] [CrossRef]
- Feng, Q.C.; Wen, S.M.; Cao, Q.B.; Lu, C. A novel method for improving cerussite sulfidization. Int. J. Miner. Metall. Mater. 2016, 23, 609–617. [Google Scholar] [CrossRef]
- Abdulvaliev, R.A.; Surkova, T.Y.; Baltabekova, Z.; Yessimova, D.M.; Stachowicz, M.; Smailov, K.M.; Dossymbayeva, Z.D.; Ainur, B. Effect of Amino Acids on the Extraction of Copper from Sub-Conditional Raw Materials. Complex Use Miner. Resour. 2024, 335, 50–58. [Google Scholar] [CrossRef]
- Gamutan, J.; Koide, S.; Sasaki, Y.; Nagasaka, T. Selective dissolution and kinetics of leaching zinc from lime treated electric arc furnace dust by alkaline media. J. Environ. Chem. Eng. 2024, 12, 111789. [Google Scholar] [CrossRef]
- Ye, F.; Li, M.; Su, S.; Xia, H.; Wei, C.; Li, X.; Deng, Z. Separation and recovery of zinc from blast furnace dust via coordination leaching of Cl− and hydrolysis of NH4+. Sep. Purif. Technol. 2024, 330, 125361. [Google Scholar] [CrossRef]
- Nath, S.; Singh, K.K.; Tangjang, S.; Das, S. Industrial Solid Wastes and Environment: An Overview on Global Generation, Implications, and Available Management Options. In Springer Water; Springer: Berlin/Heidelberg, Germany, 2024; pp. 221–246. [Google Scholar]
- Varjani, S. Trends in Mitigation of Industrial Waste: Global Health Hazards, Environmental Implications and Waste Derived Economy for Environmental Sustainability. Sci. Total Environ. 2022, 811, 152357. [Google Scholar]
- Abdulvaliyev, R.; Ultarakova, A.; Mukangaliyeva, A.; Lokhova, N.; Kassymzhanov, K. Comparative Analysis of Acid Leaching for the Efficient Recovery of Lanthanum and Cerium from Phosphate. Separations 2024, 11, 288. [Google Scholar] [CrossRef]
- Yessengaziyev, A.; Mukangaliyeva, A.; Toishybek, A.; Karshyga, Z.; Abdyldayev, N.; Yersaiynova, A.; Bakhytuly, N. Studies the crucial role of selection of extractant to extract niobium from sulfuryl fluoride solution and optimization of extraction conditions. Acta Metall. Slovaca 2024, 30, 120–126. [Google Scholar] [CrossRef]
- Toshkodirova, R.E.; Abdurakhmonov, S. Processing of Clinker—Technogenic Waste of Zinc Production. Univers. Tech. Sci. 2020, 11, 78–81. [Google Scholar]
- Kenzhaliyev, B.; Berkinbayeva, A.; Baltabekova, Z.; Moldabayeva, G.; Smailov, K.; Saulebekkyzy, S.; Tolegenova, N.; Karim, D.; Omirbek, T. Investigation of Phase Transformations in Technogenic Raw Materials Under Microwave Treatment for Enhanced Zinc Leaching. Processes 2025, 13, 1099. [Google Scholar] [CrossRef]
- Klein, S.E.; Kozlov, P.A.; Naboychenko, S.S. Extraction of Zinc from Ore Raw Materials; Ural State Mining University (USMU): Yekaterinburg, Russia, 2009; 492p. [Google Scholar]
- Diaz, F.; Sommerfeld, M.; Hovestadt, G.; Latacz, D.; Friedrich, B. Lessons Learned from Attempts at Minimising CO2 Emissions in Process Metallurgy—Pyrolysed Secondary Raw Materials, Bio-Coke, and Hydrogen as Alternative Reducing Agents; Proceedings; AusIMM: Carlton, VIC, Australia, 2024. [Google Scholar]
- Yang, K.; Sun, C.; Qu, H.; Shuo, L.; Luo, Y.; Zhang, L.; Ma, A. Recovery of Zinc from Oxide-Sulphide Zinc Ore Through Oxidation and Chelation. In Materials Engineering—From Ideas to Practice: An EPD Symposium in Honor of Jiann-Yang Hwang; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 161–169. [Google Scholar]
- Kuma, C.; Obut, A. Effect of heating on structure and leaching characteristics of a zinc carbonate ore. Physicochem. Probl. Miner. Process 2021, 57, 23–32. [Google Scholar] [CrossRef]
- Soltani, F.; Darabi, H.; Aram, R.; Ghadiri, M. Leaching and solvent extraction purification of zinc from Mehdiabad complex oxide ore. Sci. Rep. 2021, 11, 1566. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Zhang, Q.W.; He, X.M.; Hu, H.M.; Liu, Y.C. Mechanochemical leaching of Zn from low-grade smithsonite using Fe2(SO4)3 solution. Hydrometallurgy 2020, 198, 105497. [Google Scholar] [CrossRef]
- Shen, X.Y.; Shao, H.M.; Gu, H.M. Reaction mechanism analysis of roasting Zn2SiO4 using NaOH. Trans. Nonferrous Met. Soc. China 2018, 28, 1878–1886. [Google Scholar] [CrossRef]
- Wang, S.; Gao, F.; Li, B.; Liu, Y.; Deng, T.; Zhang, Y.; Chen, W. Clinkerization of Carbonatable Belite–Melilite Clinker Using Solid Waste at Low Temperature. Constr. Build. Mater. 2024, 418, 135357. [Google Scholar] [CrossRef]
- Zhidebekkyzy, A.; Temerbulatova, Z.; Amangeldiyeva, B.; Sakhariyeva, A. Towards a Circular Economy: An Analysis of Kazakhstani Case. J. Econ. Res. Bus. Adm. 2023, 143, 16–32. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, G. Recent Status of Production, Administration Policies, and Low-Carbon Technology Development of China’s Steel Industry. Metals 2024, 14, 480. [Google Scholar] [CrossRef]
- Harvey, J.-P.; Courchesne, W.; Vo, M.D.; Oishi, K.; Robelin, C.; Mahue, U.; Leclerc, P.; Al-Haiek, A. Greener Reactants, Renewable Energies and Environmental Impact Mitigation Strategies in Pyrometallurgical Processes: A Review. MRS Energy Sustain. 2022, 9, 212–247. [Google Scholar] [CrossRef]
- Yang, K.; Li, S.; Zhang, L.; Peng, J.; Chen, W.; Xie, F.; Ma, A. Microwave roasting and leaching of an oxide-sulphide zinc ore. Hydrometallurgy 2016, 166, 243–251. [Google Scholar] [CrossRef]
- Hamidi, A.; Nazari, P.; Shakibania, S.; Rashchi, F. Microwave irradiation for the recovery enhancement of fly ash components: Thermodynamic and kinetic aspects. Chem. Eng. Process. 2023, 191, 109472. [Google Scholar] [CrossRef]
- Kumar, P.; Ingle, A.; Jhavar, S. Parametric review of microwave-based materials processing and its applications. J. Mater. Res. Technol. 2019, 8, 3306–3326. [Google Scholar]
- Ma, A.; Zheng, X.; Gao, L.; Li, K.; Omran, M.; Chen, G. Enhanced Leaching of Zinc from Zinc-Containing Metallurgical Residues via Microwave Calcium Activation Pretreatment. Metals 2021, 11, 1922. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Zhang, L.; Yang, K. Application of the Microwave and Ultrasonic Combined Technique in the Extraction of Refractory Complex Zinc Ore. Metals 2023, 13, 356. [Google Scholar] [CrossRef]
- Erans, M.; Durán-Jimenez, G.; Rodríguez, J.M.; Stevens, L.; Dodds, C. Microwave Thermal Pre-treatment and Calcination of Biomineralised Sorbents for Calcium Looping. J. CO2 Util. 2024, 83, 102794. [Google Scholar] [CrossRef]
- Ospanov, K.; Smailov, K.; Nuruly, Y. Patterns of Non-Traditional Thermodynamic Functions ΔrG0/n and ΔfG0(Averaged) Changes for Cobalt Minerals. Chem. Bull. Kazakh Natl. Univ. 2020, 96, 22–30. [Google Scholar] [CrossRef]
- MAl-Harahsheh, S.W. Kingman, Microwave-assisted leaching—A review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Kobusheshe, J. Microwave Enhanced Processing of Ores. Ph.D. Thesis, University of Nottingham, Nottingham, UK, June 2010. [Google Scholar]
- Beisembaev, B.B.; Kenzhaliyev, B.K.; Gorkun, V.I.; Govyadovskaya, O.U.; Ignatyev, M.M. Deep Processing of Lead-Zinc Ores and Intermediary Products with Receiving of Products with Increased Marketability. Almaty 2002, 3, 220. [Google Scholar]
- Kenzhaliyev, B.; Koizhanova, A.; Fischer, D.; Magomedov, D.; Yerdenova, M.; Smailov, K.; Abdyldayev, N. Study of efficiency of organic activator application to process difficult-to-beneficiate polymetallic ridder ores. Transit. Met. Chem. 2025. [Google Scholar] [CrossRef]
- Kenzhaliyev, B.; Fischer, D.; Temirova, S.; Ultarakova, A.; Baltabekova, Z.; Bakhytuly, N.; Smailov, K. Removal of Hexavalent Chromium Ions from Industrial Effluents Using Natural and Modified Diatomite, Taurite, Lewatit M500, and Activated Carbon. Processes 2025, 13, 997. [Google Scholar] [CrossRef]
- Wang, X.; Zhong, Y.W.; Kang, Y.Z.; Gao, J.T.; Guo, Z.C. Promoted acid leaching of Zn from hazardous zinc-containing metallurgical dusts: Focusing on transformation of Zn phases in selective reduction roasting. Process. Saf. Environ. 2022, 163, 353–361. [Google Scholar] [CrossRef]
- Stefánsson, A. Iron(III) Hydrolysis and Solubility at 25 °C. Environ. Sci. Technol. 2007, 41, 6117–6123. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Wu, J.; Zhang, H. Degradation of bisphenol a in aqueous solution by a novel electro/Fe3+/peroxydisulfate process. Sep. Purif. Technol. 2013, 117, 18–23. [Google Scholar] [CrossRef]
- Chiou, C.S.; Chen, Y.H.; Chang, C.T.; Chang, C.Y.; Shie, J.L.; Li, Y.S. Photochemical mineralization of di-n-butyl phthalate with H2O2/Fe3+. J. Hazard. Mater. 2006, 135, 344–349. [Google Scholar] [CrossRef] [PubMed]
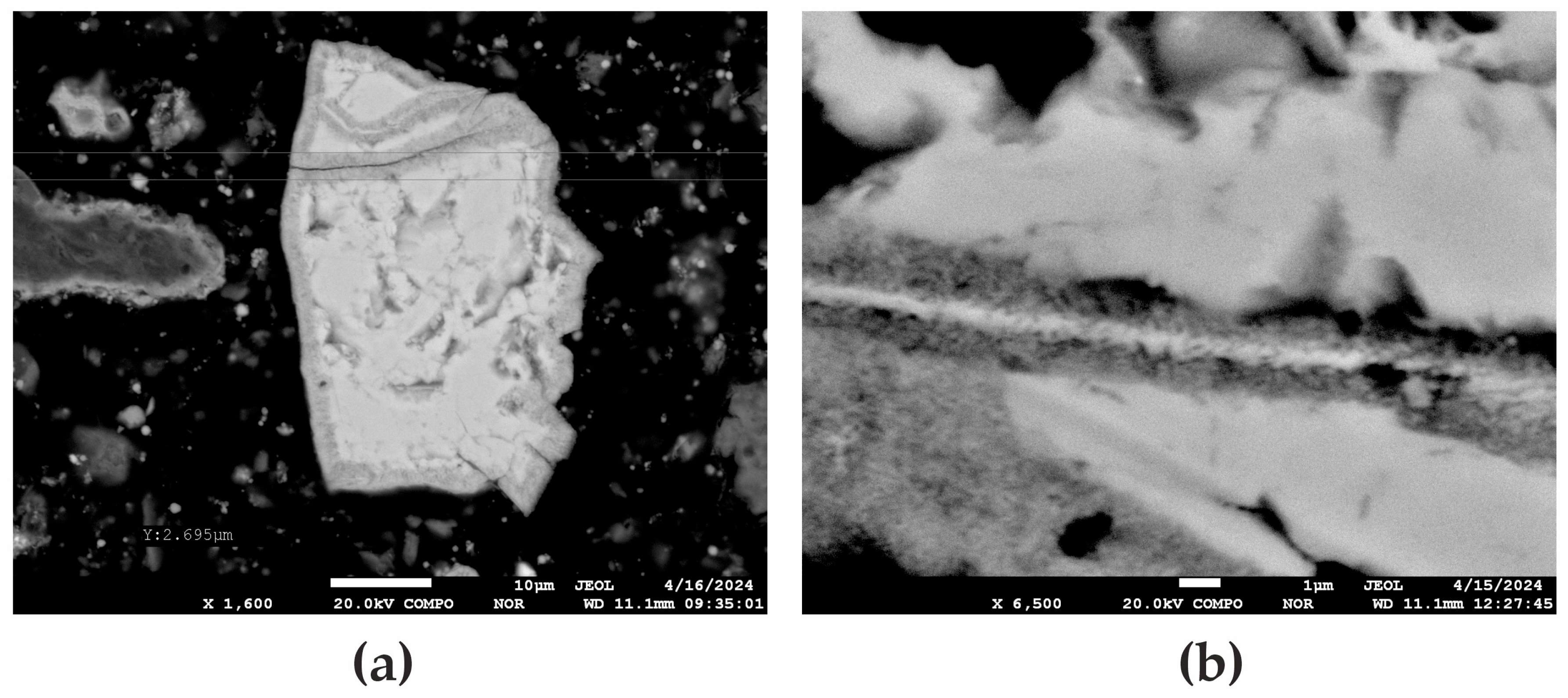
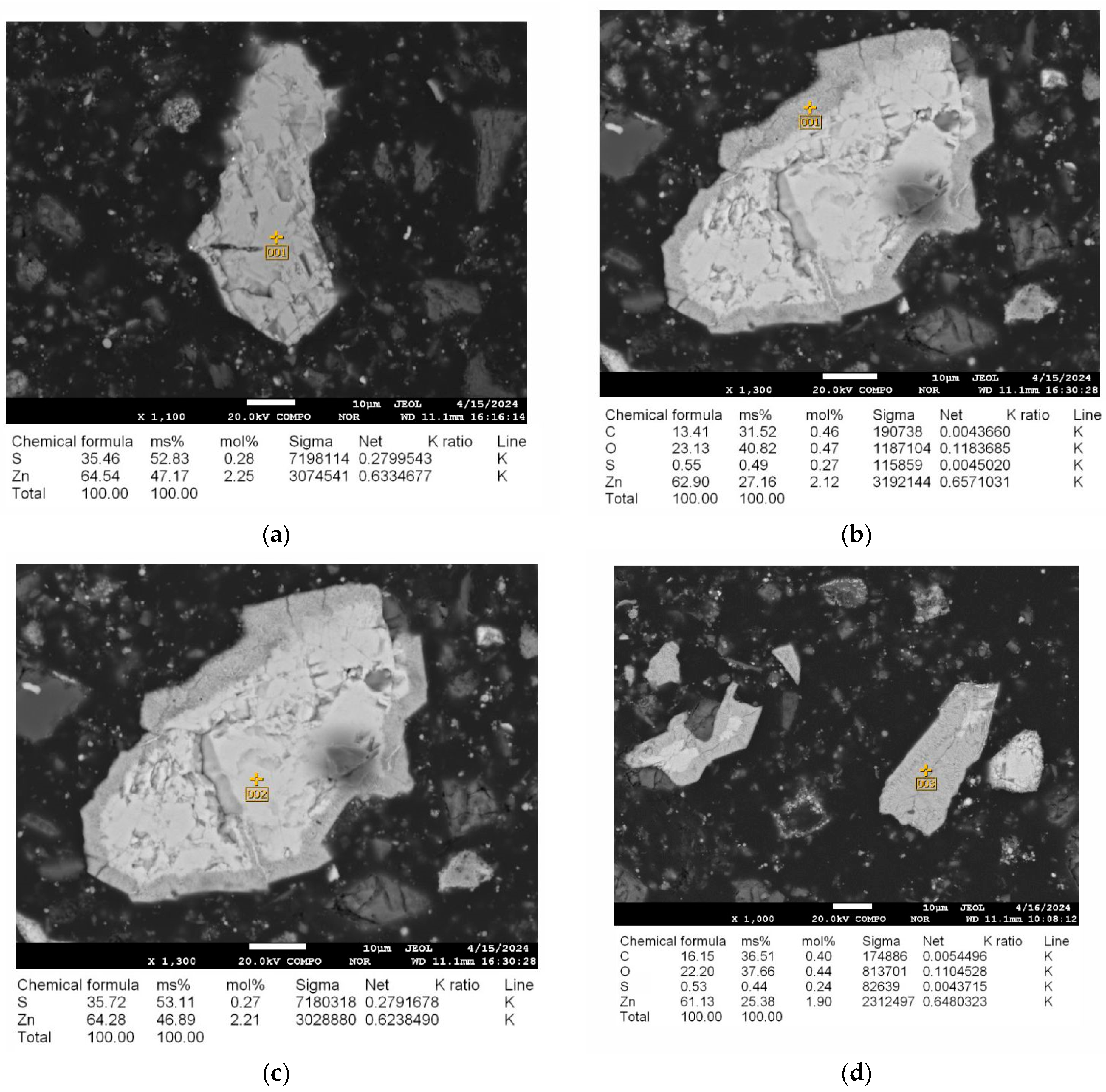

| Elemental Content, % | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| O | Mg | Al | Si | P | S | K | Ca | Ti | Cr |
| 31.268 | 0.752 | 3.062 | 9.592 | 0.075 | 4.427 | 0.320 | 5.268 | 0.403 | 0.057 |
| Mn | Fe | Ni | Cu | Zn | As | Sr | Mo | Ba | Pb |
| 1.191 | 20.980 | 0.023 | 3.207 | 3.468 | 0.121 | 0.077 | 0.015 | 1.855 | 2.579 |
| Name | Content, % | |
|---|---|---|
| Before Microwave Treatment | After Microwave Treatment | |
| Quartz SiO2 | 19.8 | 19.5 |
| Magnetite Fe3O4 | 15.1 | 15.1 |
| Feldspar (Anorthite) CaAl2Si2O8 | 10.4 | 10.5 |
| Troilite FeS | 10.3 | 4.5 |
| Chalcopyrite CuFeS2 | 8.4 | 6.5 |
| Gypsum CaSO4·2H2O | 8.2 | 4.5 |
| Goethite FeO(OH) | 8.1 | 1.5 |
| Lead Oxide Sulfate Pb3O2SO4 | 6.1 | 5.3 |
| Barium Sulfate BaSO4 | 4.6 | 4.8 |
| Sphalerite ZnS | 4.2 | - |
| Magnesite MgCO3 | 3.6 | 2.3 |
| Marmatite Zn0.66Fe0.34S | 2.4 | - |
| Hematite Fe2O3 | - | 12.4 |
| Zincite ZnO | - | 6.6 |
| Anhydrite CaSO4 | - | 3.5 |
| Copper Oxides CuO/Cu2O | - | 1.5 |
| Magnesium Oxide MgO | - | 1.3 |
| Lead Oxide PbO | - | 0.8 |
| Reaction | T, K | ΔH, kJ | ΔS, J/K | ΔG, kJ | K | Log K |
|---|---|---|---|---|---|---|
| ZnS(s) + 4H2SO4(aq) → ZnSO4(aq) + 4SO2(g) + 4H2O(l) | 298 | 148.283 | 696.902 | −59.498 | 2.659 × 1010 | 10.425 |
| ZnO(s) +H2SO4(aq) → ZnSO4(aq) +H2O(l) | −103.665 | −19.614 | −97.817 | 1.376 × 1017 | 17.139 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenzhaliyev, B.; Berkinbayeva, A.; Smailov, K.; Baltabekova, Z.; Saulebekkyzy, S.; Tolegenova, N.; Yessengaziyev, A.; Bakhytuly, N.; Tugambay, S. Microwave Pre-Treatment for Efficient Zinc Recovery via Acid Leaching. Materials 2025, 18, 2496. https://doi.org/10.3390/ma18112496
Kenzhaliyev B, Berkinbayeva A, Smailov K, Baltabekova Z, Saulebekkyzy S, Tolegenova N, Yessengaziyev A, Bakhytuly N, Tugambay S. Microwave Pre-Treatment for Efficient Zinc Recovery via Acid Leaching. Materials. 2025; 18(11):2496. https://doi.org/10.3390/ma18112496
Chicago/Turabian StyleKenzhaliyev, Bagdaulet, Ainur Berkinbayeva, Kenzhegali Smailov, Zhazira Baltabekova, Shynar Saulebekkyzy, Nazerke Tolegenova, Azamat Yessengaziyev, Nauryzbek Bakhytuly, and Symbat Tugambay. 2025. "Microwave Pre-Treatment for Efficient Zinc Recovery via Acid Leaching" Materials 18, no. 11: 2496. https://doi.org/10.3390/ma18112496
APA StyleKenzhaliyev, B., Berkinbayeva, A., Smailov, K., Baltabekova, Z., Saulebekkyzy, S., Tolegenova, N., Yessengaziyev, A., Bakhytuly, N., & Tugambay, S. (2025). Microwave Pre-Treatment for Efficient Zinc Recovery via Acid Leaching. Materials, 18(11), 2496. https://doi.org/10.3390/ma18112496






