Advancements in the Research on the Preparation of Isoamyl Acetate Catalyzed by Immobilized Lipase
Highlights
- Enzymatic synthesis of isoamyl acetate has broad application prospects.
- Enzyme immobilization enhanced the synthesis efficiency of isoamyl acetate.
- Novel immobilized technology is the direction of preparation of isoamyl acetate.
- Solvent engineering further improved the synthesis efficiency of isoamyl acetate.
Abstract
1. Introduction
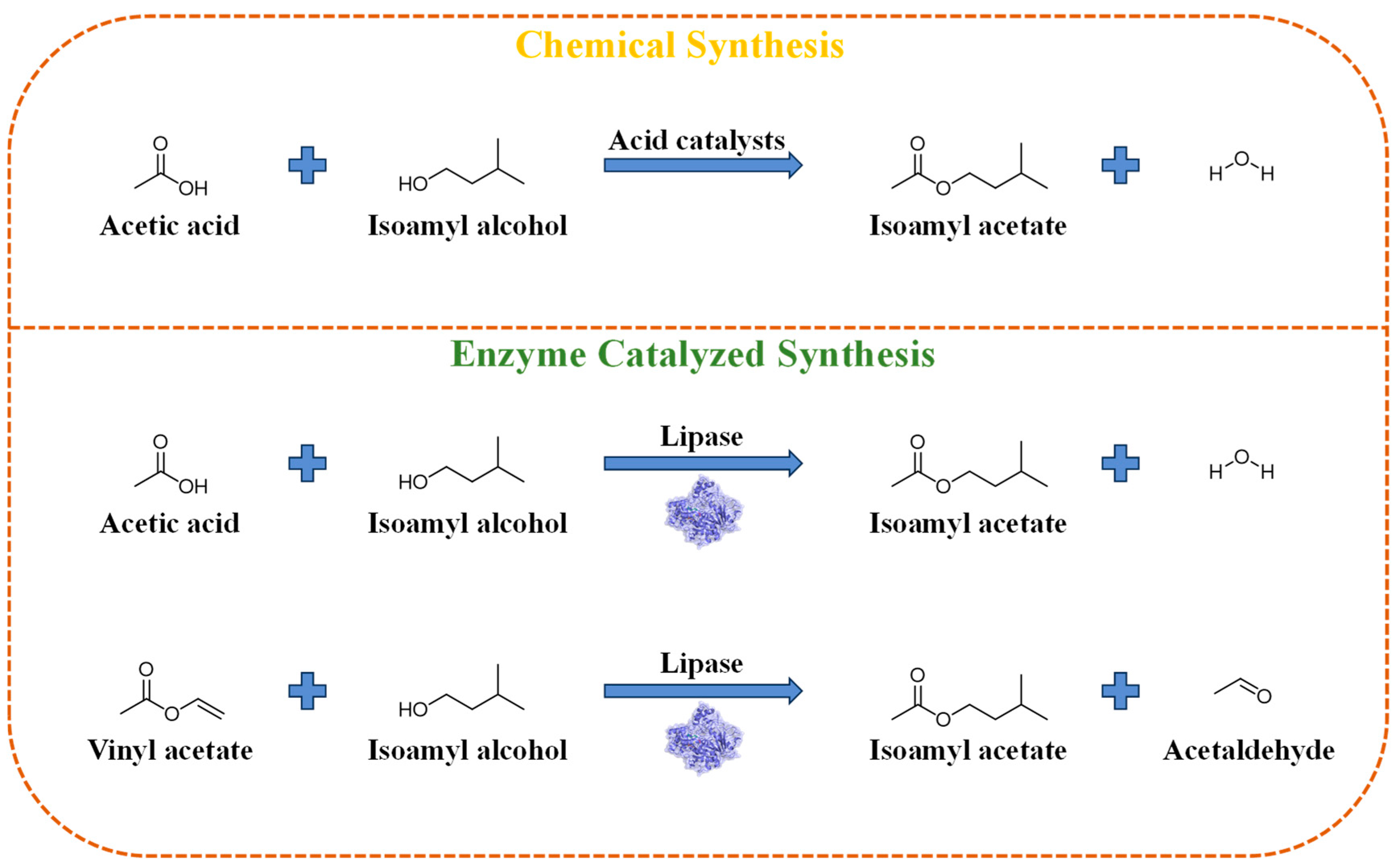
2. Preparation Method of Isoamyl Acetate
3. Research Progress on Enzymatic Synthesis of Isoamyl Acetate
3.1. Overview of Lipase
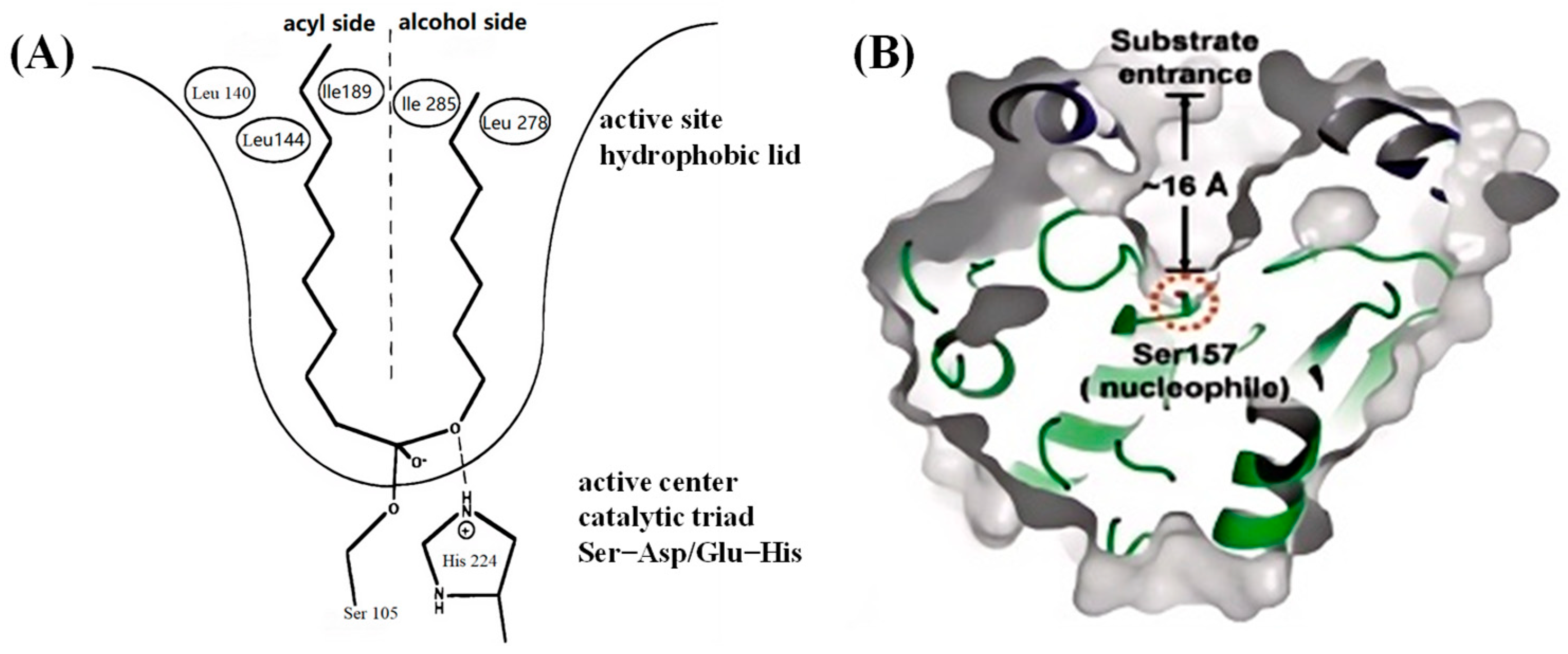
3.2. The Catalytic Mechanism of Lipase
3.3. Present Situation of Lipase Used in Synthesis of Ester
3.4. Immobilization Method of Lipase
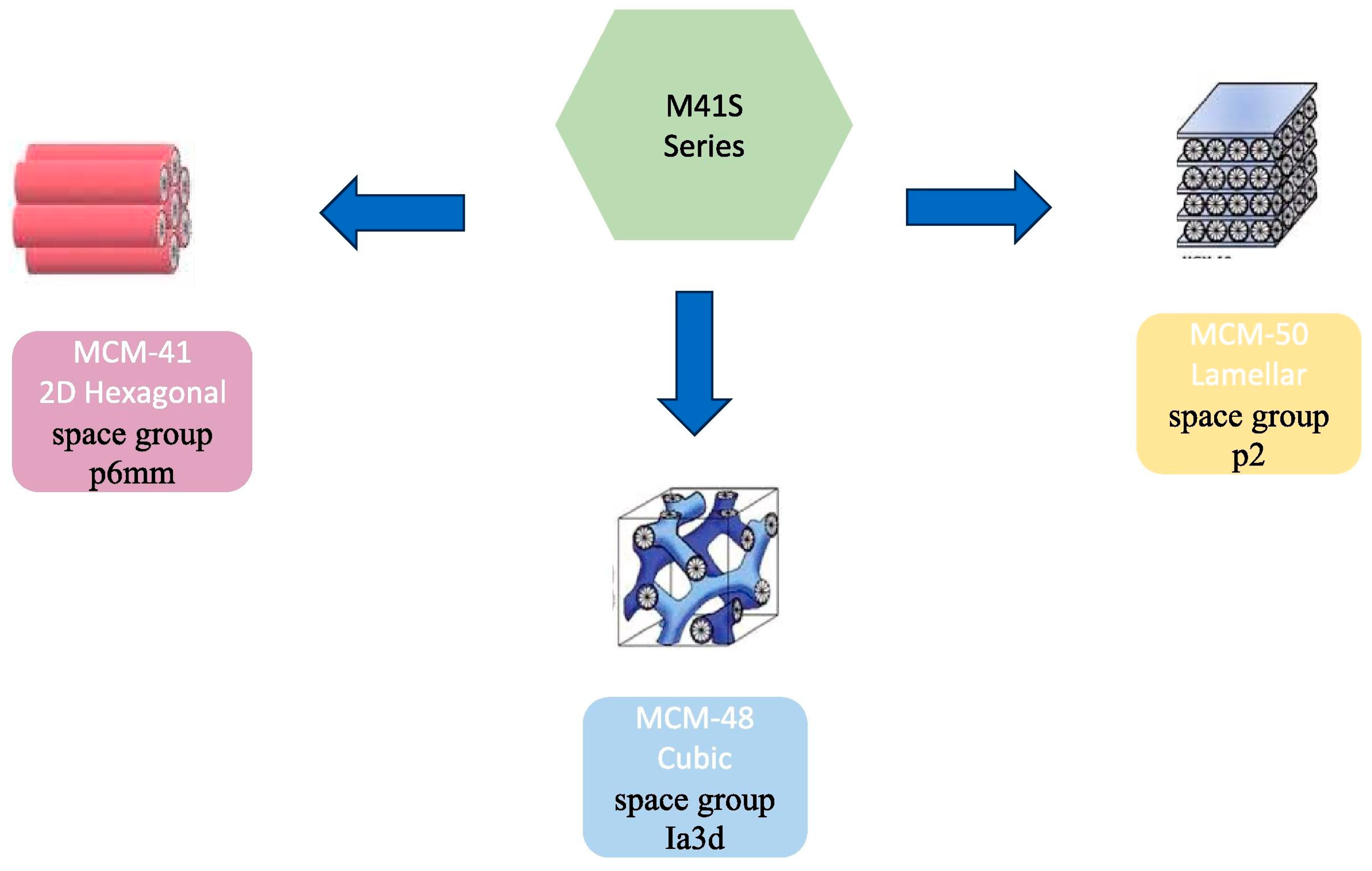
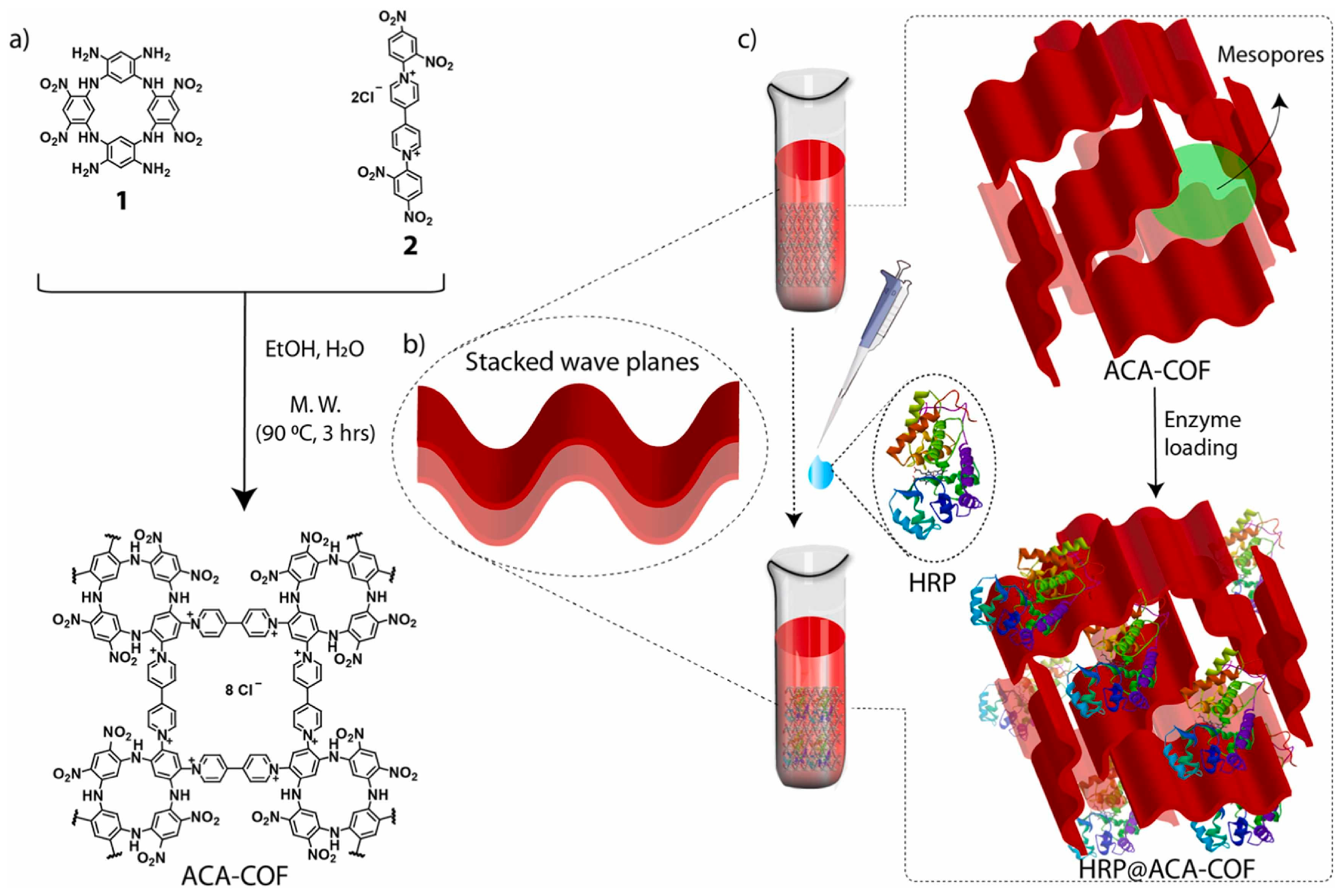
3.5. Selection of New Immobilized Lipase Carrier
3.6. Research Progress on Synthesis of Isoamyl Acetate Catalyzed by Immobilized Lipase
4. Prospect of Solvent Engineering to Enhance the Enzyme Catalytic Process
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.Y.; Feng, T.; Ni, X.; Xia, J.J.; Suo, H.B.; Yan, L.S.; Zou, B. Immobilized lipase based on SBA-15 adsorption and gel embedding for catalytic synthesis of isoamyl acetate. Food Biosci. 2024, 60, 104427. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Xiao, L.L.; Li, X.; Hu, M. Effect of fermentation parameters and their optimization on the phytochemical properties of lactic-acid-fermented mulberry juice. J. Food Meas. Charact. 2017, 11, 1462–1473. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Xiao, X.; Dong, Y.; Xu, T.; Wu, F. Dietary supplementation with Lactobacillus plantarum dy-1 fermented barley suppresses body weight gain in high-fat diet-induced obese rats. J. Sci. Food Agric. 2016, 96, 4907–4917. [Google Scholar] [CrossRef] [PubMed]
- He, W.S.; Hu, D.; Wang, Y.; Chen, X.Y.; Jia, C.S.; Ma, H.L.; Feng, B.A. A novel chemo-enzymatic synthesis of hydrophilic phytosterol derivatives. Food Chem. 2016, 192, 557–565. [Google Scholar] [CrossRef] [PubMed]
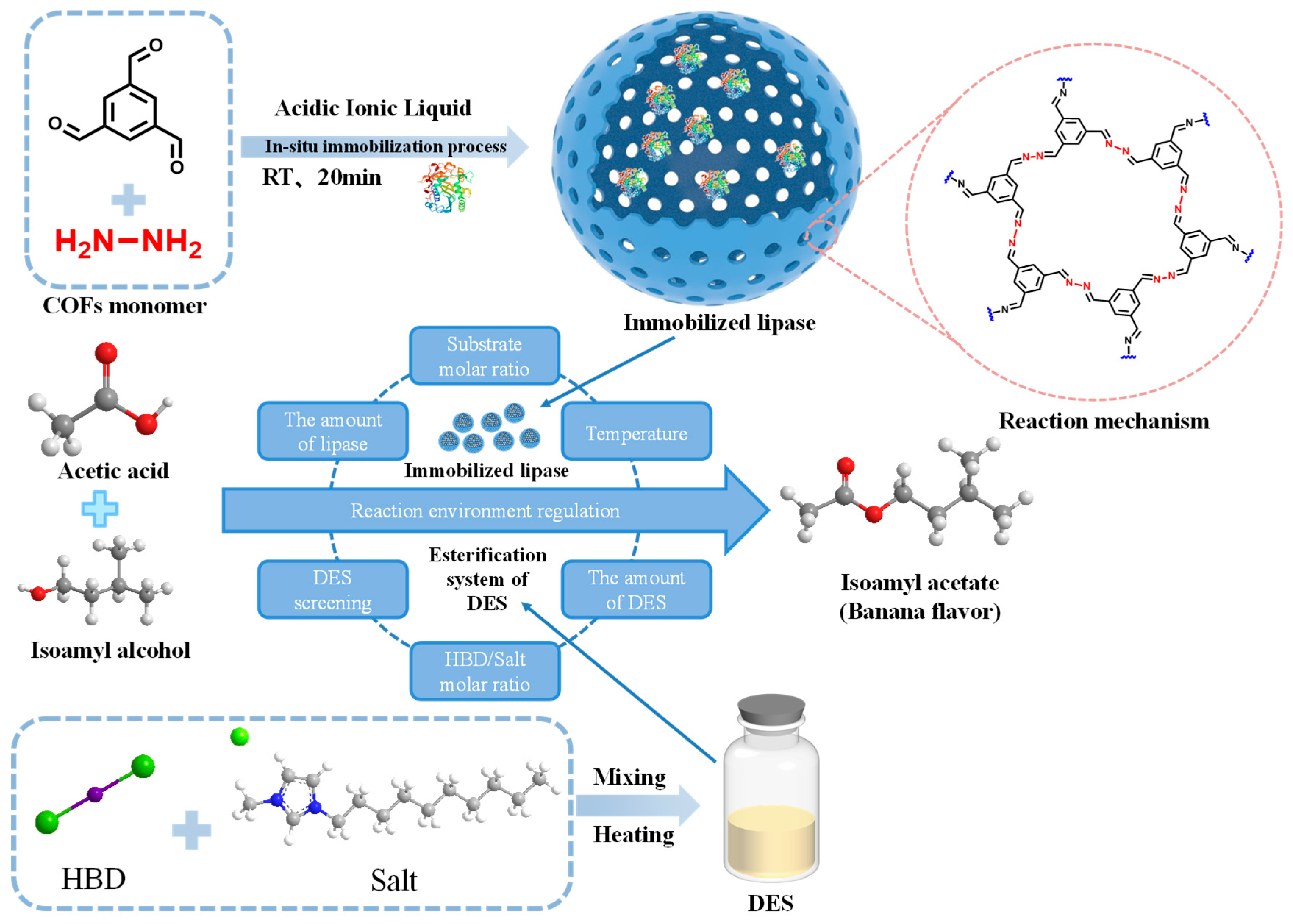
- Zhang, Y.Y.; Gu, X.; Lin, Z.Y.; Suo, H.B.; Xia, J.J.; Zou, B. In-situ aqueous phase encapsulation immobilized lipase assisted by acidic ionic liquids for the synthesis of isoamyl acetate. Food Biosci. 2025, 63, 105607. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Wang, Q.J.; Lin, Z.Y.; Xia, J.J.; Suo, H.B.; Huang, R.B.; Zou, B. Efficient synthesis of isoamyl acetate in deep eutectic solvent utilizing in-situ aqueous phase immobilized lipase biocatalyst. Food Biosci. 2025, 64, 105963. [Google Scholar] [CrossRef]
- Wang, Y.C.; Hu, Z.L.; Wang, B.; Yang, D.M.; Liao, J.Y.; Zhang, M. Effect of high-voltage electrospray on the inactivation, induced damage and growth of microorganisms and flavour components of honey raspberry wine. Int. J. Food Microbiol. 2023, 388, 110060. [Google Scholar] [CrossRef]
- Lin, H.; Kang, W.C.; Jin, H.J.; Man, Z.X.; Chen, Q.S. Discrimination of Chinese Baijiu grades based on colorimetric sensor arrays. Food Sci. Biotechnol. 2020, 29, 1037–1043. [Google Scholar] [CrossRef]
- Hao, J.; Xu, H.N.; Yan, P.F.; Yang, M.Y.; Mintah, B.K.; Dai, C.H.; Zhang, R.; Ma, H.L.; He, R.H. Application of fixed-frequency ultrasound in the cultivation of Saccharomyces cerevisiae for rice wine fermentation. J. Sci. Food Agric. 2024, 104, 6417–6430. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, Q.Y.; Solairaj, D.; Sallam, N.M.A.; Zhu, M.R.; You, S.Y.; Zhang, H.Y. Volatile organic compounds of wickerhamomyces anomalus prevent postharvest black spot disease in tomato. Foods 2024, 13, 1949. [Google Scholar] [CrossRef]
- Hu, W.; Li, C.Z.; Dai, J.M.; Cui, H.Y.; Lin, L. Antibacterial activity and mechanism of Litsea cubeba essential oil against methicillin-resistant Staphylococcus aureus (MRSA). Ind. Crops Prod. 2019, 130, 34–41. [Google Scholar] [CrossRef]
- Gao, R.C.; Yuan, L.; Yu, M.S.; Liu, W.M. Effects of heat pump drying parameters on the volatile flavor compounds in silver carp. J. Aquat. Food Prod. Technol. 2016, 25, 735–744. [Google Scholar] [CrossRef]
- Wang, L.; Gao, E.Y.; Hu, M.; Oladejo, A.; Gong, X.F.; Wang, J.Y.; Zhong, H. Isolation, identification and screening of high-quality yeast strains for the production of milk beer. Int. J. Dairy Technol. 2018, 71, 944–953. [Google Scholar] [CrossRef]
- Yin, H.; Hu, X.T.; Huang, X.W.; Zou, X.B.; Xu, Y.W.; Shi, J.Y.; Yang, M. Rapid discrimination of beer flavors using ion-selective electrode array system combined with chemometrics. Food Anal. Methods 2021, 14, 1836–1842. [Google Scholar] [CrossRef]
- Wang, L.; Gu, Y.C.; Zheng, X.Y.; Zhang, Y.; Deng, K.W.; Wu, T.; Cheng, H. Analysis of physicochemical properties of exopolysaccharide from Leuconostoc mesenteroides strain XR1 and its application in fermented milk. LWT Food Sci. Technol. 2021, 146, 111449. [Google Scholar] [CrossRef]
- Tchabo, W.; Ma, Y.K.; Kwaw, E.; Zhang, H.N.; Li, X. Influence of fermentation parameters on phytochemical profile and volatile properties of mulberry (Morus nigra) wine. J. Inst. Brew. 2017, 123, 151–158. [Google Scholar] [CrossRef]
- Dai, C.X.; Huang, X.Y.; Lv, R.Q.; Zhang, Z.C.; Sun, J.; Aheto, J.H. Analysis of volatile compounds of Tremella aurantialba fermentation via electronic nose and HS-SPME-GC-MS. J. Food Saf. 2018, 38, e12555. [Google Scholar] [CrossRef]
- Li, M.; Jiang, H.N.; Zhang, L.; Yu, X.J.; Liu, H.; Yagoub, A.A.; Zhou, C.S. Synthesis of 5-HMF from an ultrasound-ionic liquid pretreated sugarcane bagasse by using a microwave-solid acid/ionic liquid system. Ind. Crops Prod. 2020, 149, 112361. [Google Scholar] [CrossRef]
- Wang Li, X.; Liu Shu, H.; Yuan, H.; Guo Lin, L. Catalytic Synthesis of Isoamyl Acetate by Ion Exchange Resin-supported (NH4)6[MnMo9O32]•8H2O with Waugh Structure. Adv. Mater. Res. 2013, 750, 1231–1234. [Google Scholar] [CrossRef]
- Ni, X.; Feng, T.; Zhang, Y.Y.; Lin, Z.Y.; Kong, F.Z.; Zhang, X.; Lu, Q.Y.; Zhao, Y.N.; Zou, B. Application progress of immobilized enzymes in the catalytic synthesis of 1,3-dioleoyl-2-palmitoyltriglyceride structured lipids. Foods 2025, 14, 475. [Google Scholar] [CrossRef]
- He, Y.F.; Liu, T.; Larsen, D.S.; Lei, Y.X.; Huang, M.C.; Zhu, L.; Daglia, M.; Xiao, X. Barley fermentation on nutritional constituents: Structural changes and structure-function correlations. Crit. Rev. Food Sci. Nutr. 2025. [Google Scholar] [CrossRef] [PubMed]
- Mansor, N.; Singaravelan, R.; Abd Shukor, S.R. Parameters study on the production of isoamyl acetate via milli-reactor in a solvent-free system. IOP Conf. Ser. Mater. Sci. Eng. 2020, 778, 012067. [Google Scholar] [CrossRef]
- Li, Y.X.; Qaria, M.A.; Sivasamy, S.; Sun, J.Z.; Zhu, D.C. Curcumin production and bioavailability: A comprehensive review of curcumin extraction, synthesis, biotransformation and delivery systems. Ind. Crops Prod. 2021, 172, 114050. [Google Scholar] [CrossRef]
- Duan, N.; Chang, B.Y.; Zhang, H.; Wang, Z.P.; Wu, S.J. Salmonella typhimurium detection using a surface-enhanced Raman scattering-based aptasensor. Int. J. Food Microbiol. 2016, 218, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.Y.; Wu, J.; Li, C.Z.; Lin, L. Promoting anti-listeria activity of lemongrass oil on pork loin by cold nitrogen plasma assist. J. Food Saf. 2017, 37, e12316. [Google Scholar] [CrossRef]
- Narwal, S.K.; Saun, N.K.; Dogra, P.; Gupta, R. Green synthesis of isoamyl acetate via silica immobilized novel thermophilic lipase from Bacillus aerius. Russ. J. Bioorg. Chem. 2016, 42, 69–73. [Google Scholar] [CrossRef]
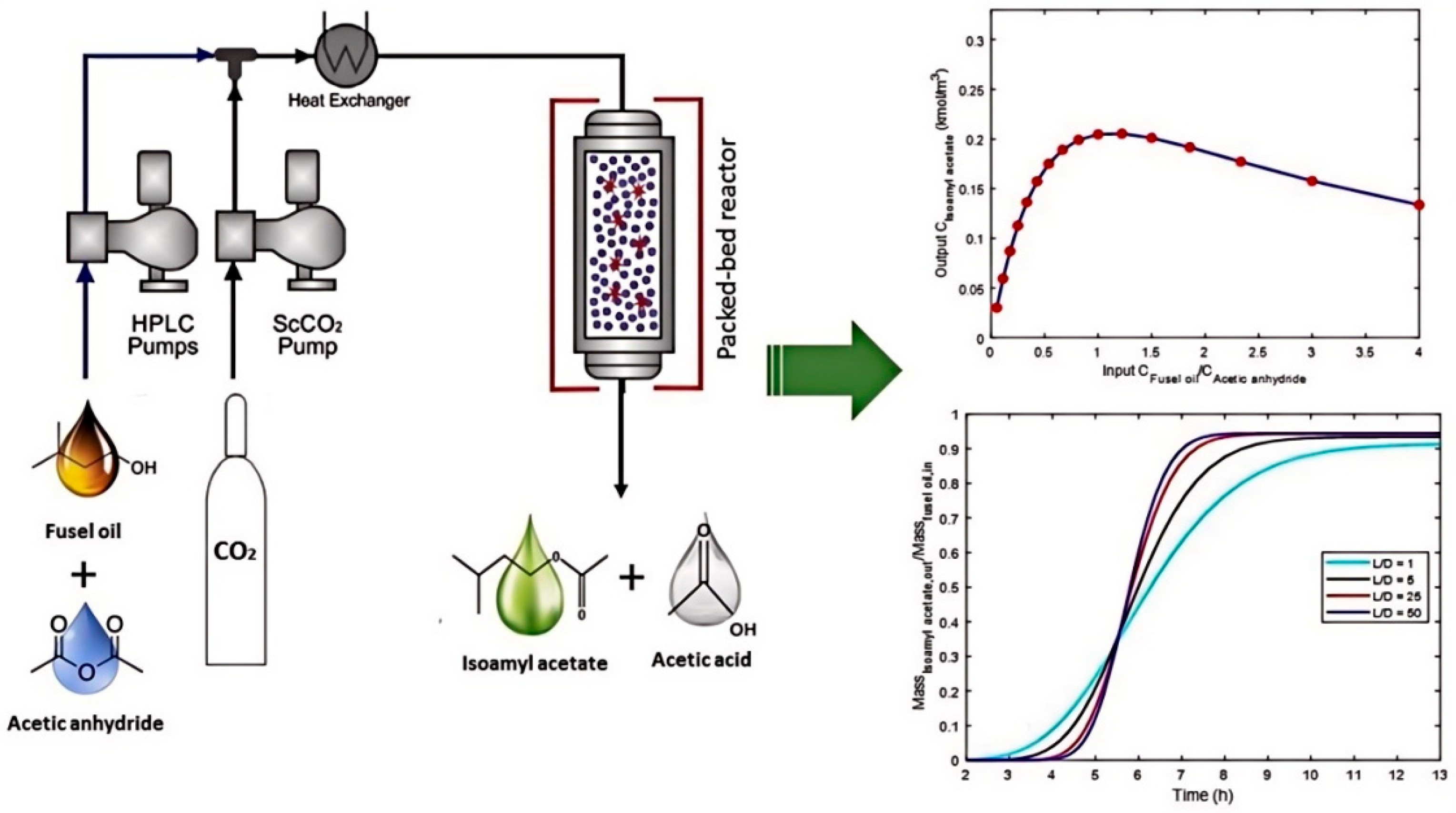
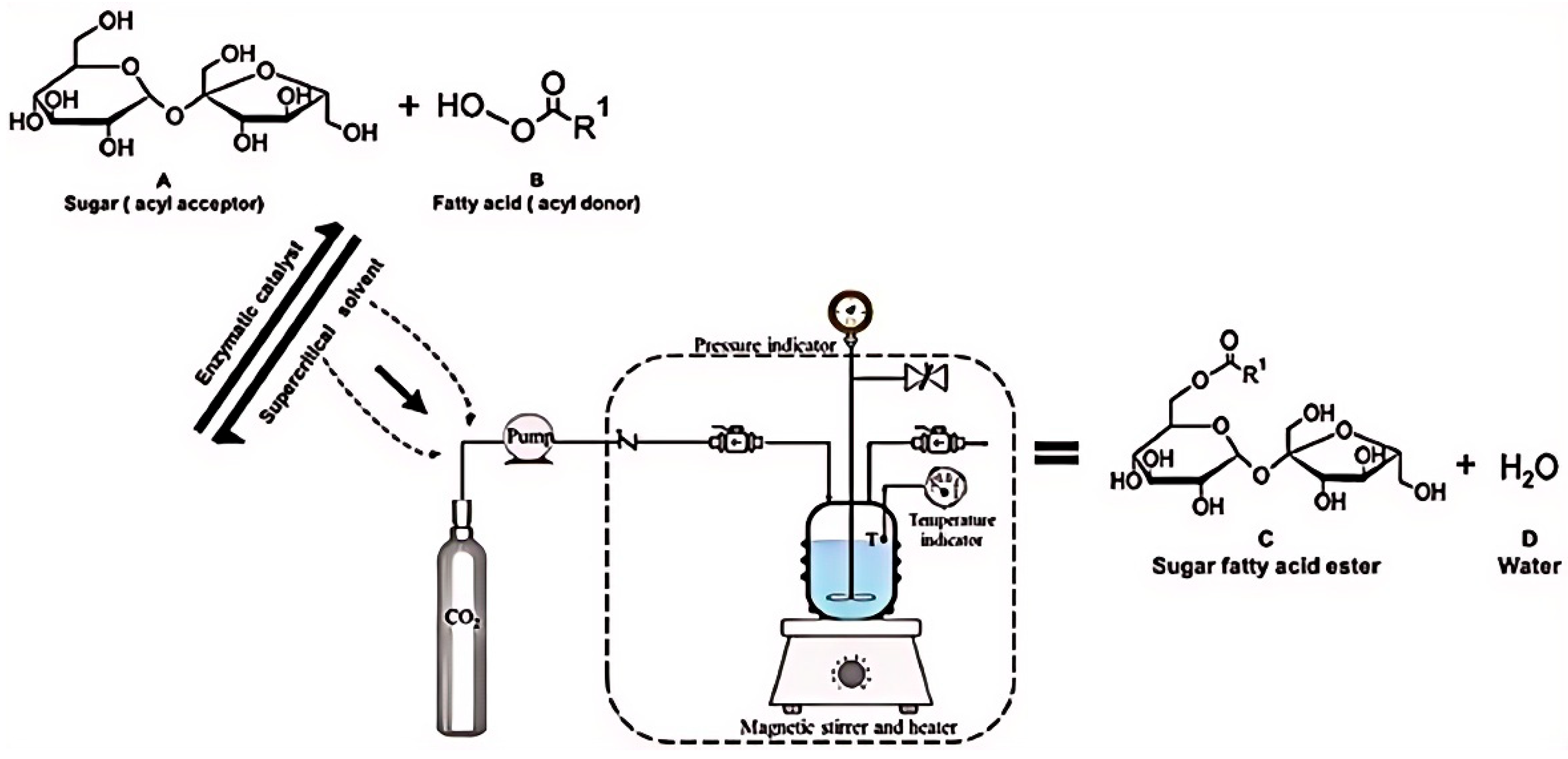
- dos Santos, P.; Meireles, M.A.A.; Martínez, J. Production of isoamyl acetate by enzymatic reactions in batch and packed bed reactors with supercritical CO2. J. Supercrit. Fluids 2017, 127, 71–80. [Google Scholar] [CrossRef]
- Zare, M.; Golmakani, M.T.; Niakousari, M. Lipase synthesis of isoamyl acetate using different acyl donors: Comparison of novel esterification techniques. LWT Food Sci. Technol. 2019, 101, 214–219. [Google Scholar] [CrossRef]
- He, W.S.; Cu, D.D.; Zhang, Y.L.; Liu, Y.; Yin, J.; Chen, G.; Jia, C.S.; Feng, B. Highly efficient synthesis of phytosterol linolenate catalyzed by Candida rugosa lipase through transesterification. Food Sci. Technol. Res. 2017, 23, 525–533. [Google Scholar] [CrossRef]
- He, W.S.; Zhu, H.Y.; Chen, Z.Y. Plant sterols: Chemical and enzymatic structural modifications and effects on their cholesterol-lowering activity. J. Agric. Food. Chem. 2018, 66, 3047–3062. [Google Scholar] [CrossRef]
- Xiao, Y.R.; Nie, M.M.; Zhao, H.W.; Li, D.J.; Gao, R.C.; Zhou, C.N.; Xu, Y.Y.; Dai, Z.Q.; Zhang, Z.Y. Citrus flavanones enhance the bioaccessibility of 8-carotene by improving lipid lipolysis and incorporation into mixed micelles. J. Funct. Foods 2021, 87, 104792. [Google Scholar] [CrossRef]
- He, W.S.; Li, L.L.; Zhao, J.; Xu, H.S.; Rui, J.X.; Cui, D.D.; Li, H.; Zhang, H.J.; Liu, X.Q. Candida sp. 99-125 lipase-catalyzed synthesis of ergosterol linolenate and its characterization. Food Chem. 2019, 280, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Ahima, J.; Zhang, H.Y.; Apaliya, M.T.; Zhang, X.Y.; Yang, Q.Y.; Zhao, L.N. The effect of Rhodotorula mucilaginosa on degradation of citrinin production by Penicillium digitatum and its toxin in vitro. J. Food Meas. Charact. 2019, 13, 2998–3004. [Google Scholar] [CrossRef]
- Chen, G.; Khan, I.M.; He, W.S.; Li, Y.X.; Jin, P.; Campanella, O.H.; Zhang, H.H.; Huo, Y.R.; Chen, Y.; Yang, H.Q.; et al. Rebuilding the lid region from conformational and dynamic features to engineering applications of lipase in foods: Current status and future prospects. Compr. Rev. Food Sci. Food Saf. 2022, 21, 2688–2714. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.Q.; Wang, G.; Hou, M.X.; Du, S.L.; Han, J.; Yu, Y.G.; Gao, H.X.; He, D.; Shi, J.R.; Lee, Y.W.; et al. New Hydrolase from Aeromicrobium sp. HA for the Biodegradation of Zearalenone: Identification, Mechanism, and Application. J. Agric. Food Chem. 2023, 71, 2411–2420. [Google Scholar] [CrossRef]
- Gao, S.; Qi, X.H.; Hart, D.J.; Gao, H.R.; An, Y.F. Expression and Characterization of Levansucrase from Clostridium acetobutylicum. J. Agric. Food Chem. 2017, 65, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Urrutia, C.; Sangaletti-Gerhard, N.; Cea, M.; Suazo, A.; Aliberti, A.; Navia, R. Two step esterification-transesterification process of wet greasy sewage sludge for biodiesel production. Bioresour. Technol. 2016, 200, 1044–1049. [Google Scholar] [CrossRef]
- Bayramoglu, G.; Celikbicak, O.; Kilic, M.; Arica, M.Y. Immobilization of Candida rugosa lipase on magnetic chitosan beads and application in flavor esters synthesis. Food Chem. 2022, 366, 130699. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Oveisi, H.; Meshkini, A. Functionalized ZnFe2O4@Mesoporous silica nano-support for lipase enzyme immobilization: Enhanced biocatalysis and antibacterial activity for food industry applications. Food Biosci. 2024, 61, 104985. [Google Scholar] [CrossRef]
- Ozyilmaz, G.; Yagiz, E. Isoamylacetate production by entrapped and covalently bound Candida rugosa and porcine pancreatic lipases. Food Chem. 2012, 135, 2326–2332. [Google Scholar] [CrossRef]
- Türk, M. Synthesis of isoamyl acetate using protein-coated microcrystals of different lipases. Pol. J. Chem. Technol. 2023, 25, 15–20. [Google Scholar] [CrossRef]
- Qu, W.J.; Sehemu, R.M.; Zhang, T.; Song, B.J.; Yang, L.; Ren, X.F.; Ma, H.L. Immobilized enzymolysis of corn gluten meal under triple-frequency ultrasound. Int. J. Food Eng. 2018, 14, 20170347. [Google Scholar] [CrossRef]
- Rong, J.H.; Zhou, Z.J.; Wang, Y.; Han, J.; Li, C.M.; Zhang, W.L.; Ni, L. Immobilization of Horseradish Peroxidase on Multi-Armed Magnetic Graphene Oxide Composite: Improvement of Loading Amount and Catalytic Activity. Food Technol. Biotech. 2019, 57, 260–271. [Google Scholar] [CrossRef]
- Shouket, S.; Khurshid, S.; Khan, J.; Batool, R.; Sarwar, A.; Aziz, T.; Alhomrani, M.; Alamri, A.S.; Sameeh, M.Y.; Filimban, F.Z. Enhancement of shelf-life of food items via immobilized enzyme nanoparticles on varied supports. A sustainable approach towards food safety and sustainability. Food Res. Int. 2023, 169, 112940. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, H.; Wang, M.M.; Yu, X.L.; Cui, Y.; Xu, L.; Ma, A.Z.; Ding, Z.Y.; Huo, S.H.; Zou, B.; et al. Application of Immobilized Enzymes in Juice Clarification. Foods 2023, 12, 4258. [Google Scholar] [CrossRef]
- Yan, M.C.; Chen, Y.; Feng, Y.; Saeed, M.; Fang, Z.; Zhen, W.; Ni, Z.; Chen, H.Y. Perspective on Agricultural Industrialization: Modification Strategies for Enhancing the Catalytic Capacity of Keratinase. J. Agric. Food. Chem. 2024, 72, 12915–12929. [Google Scholar] [CrossRef]
- Wang, F.; Wang, M.T.; Wang, M.M.; Xu, L.; Qian, J.Y.; Guan, G.Q.; Xu, B.G. Clarification of Sugarcane Juice Catalyzed by Magnetic Immobilized Laccase Intensified by Alternating Magnetic Field. Foods 2025, 14, 444. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Sehgal, R.; Sharma, D.; Gupta, R. Polyhydroxyalkanoates as matrices for enzyme immobilization: In vivo and In vitro approaches. Process Biochem. 2024, 147, 530–542. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Liu, C.; Yang, H.; Jia, L.; Zhao, L.; Gong, F.; Tan, C.; Tao, H.; He, W.-S. Improved antioxidant activity of rutin via lipase-mediated esterification with oleic acid. J. Sci. Food Agric. 2023, 103, 3489–3500. [Google Scholar] [CrossRef]
- Novak, U.; Lavric, D.; Znidarsic-Plazl, P. Continuous Lipase B-Catalyzed Isoamyl Acetate Synthesis in a Two-Liquid Phase System Using Corning® AFR™ Module Coupled with a Membrane Separator Enabling Biocatalyst Recycle. J. Flow Chem. 2016, 6, 33–38. [Google Scholar] [CrossRef]
- Qian, J.; Shi, B.; Li, Q.; Gou, L.; Zhao, C.; Huang, A. Synthesis of sucrose 6-acetate by immobilized aspergillus Niger lipase imprinted with oleic acid and sorbitol. Food Chem. 2025, 468, 142231. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, X.; Qin, X.; Liu, M.; Wang, Q.; Zhong, J. Improved lipase performance by covalent immobilization of Candida antarctica lipase B on amino acid modified microcrystalline cellulose as green renewable support. Colloids Surf. B 2024, 235, 113764. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Jana, A.K.; Jana, M.M. Immobilization of Candida rugosa lipase on optimized polyamidoamine dendrimer functionalized magnetic multiwalled carbon nanotubes for green manufacture of butyl butyrate ester. Mol. Catal. 2024, 553, 113779. [Google Scholar] [CrossRef]
- Xia, S.; Shen, C.; Lin, J.; Tu, M.; Tan, C.-P.; Cheong, L.-Z. Enhanced methanol tolerance of ZIF-8-immobilized Aspergillus oryzae lipase for biodiesel production from used cooking oil. Renew. Energ. 2025, 239, 122122. [Google Scholar] [CrossRef]
- Dias, A.L.B.; Ubeyitogullari, A.; Hatami, T.; Martínez, J.; Ciftci, O.N. Continuous production of isoamyl acetate from fusel oil under supercritical CO2: A mass transfer approach. Chem. Eng. Res. Des. 2021, 176, 23–33. [Google Scholar] [CrossRef]
- Jegannathan, K.R.; Abang, S.; Poncelet, D.; Chan, E.S.; Ravindra, P. Production of Biodiesel Using Immobilized LipaseA Critical Review. Crit. Rev. Biotechnol. 2008, 28, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Hu, Y.; Yu, D.H.; Xia, J.J.; Tang, S.S.; Liu, W.M.; Huang, H. Immobilization of porcine pancreatic lipase onto ionic liquid modified mesoporous silica SBA-15. Biochem. Eng. J. 2010, 53, 150–153. [Google Scholar] [CrossRef]
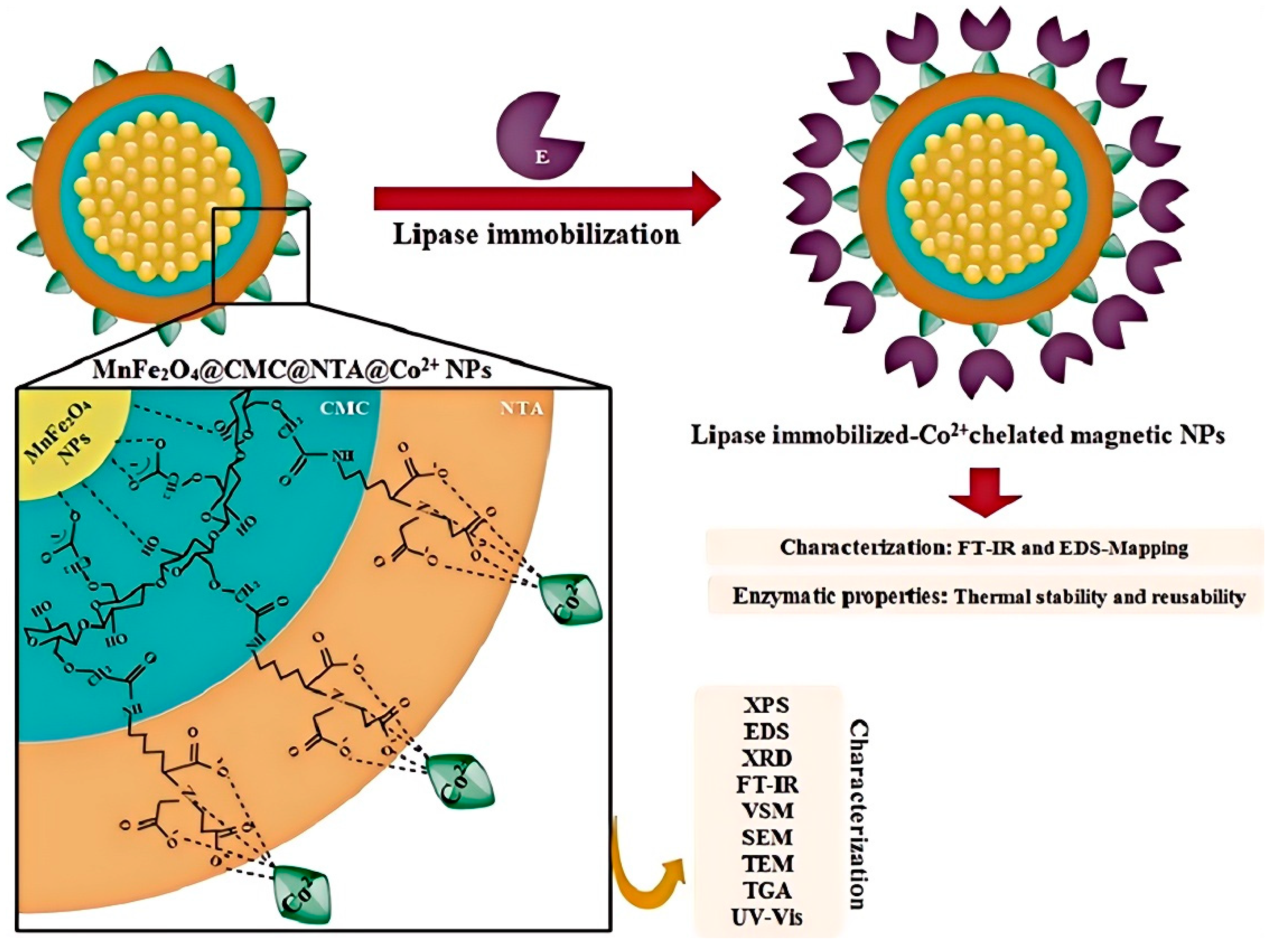
- Akhlaghi, N.; Najafpour-Darzi, G. Preparation of immobilized lipase on Co2+-chelated carboxymethyl cellulose based MnFe2O4 magnetic nanocomposite particles. Mol. Catal. 2022, 519, 112118. [Google Scholar] [CrossRef]
- Chronopoulou, L.; Kamel, G.; Sparago, C.; Bordi, F.; Lupi, S.; Diociaiuti, M.; Palocci, C. Structure–activity relationships of Candida rugosa lipase immobilized on polylactic acid nanoparticles. Soft Matter 2011, 7, 2653–2662. [Google Scholar] [CrossRef]
- Khoshnevisan, K.; Bordbar, A.K.; Zare, D.; Davoodi, D.; Noruzi, M.; Barkhi, M.; Tabatabaei, M. Immobilization of cellulase enzyme on superparamagnetic nanoparticles and determination of its activity and stability. Chem. Eng. J. 2011, 171, 669–673. [Google Scholar] [CrossRef]
- Cunha, A.G.; Fernández-Lorente, G.; Bevilaqua, J.V.; Destain, J.; Paiva, L.M.C.; Freire, D.M.G.; Fernández-Lafuente, R.; Guisán, J.M. Immobilization of Yarrowia lipolytica Lipase—A Comparison of Stability of Physical Adsorption and Covalent Attachment Techniques. Appl. Biochem. Biotechnol. 2008, 146, 49–56. [Google Scholar] [CrossRef]
- Silva, A.R.d.M.; Gonçalves, L.R.B.; da Silva, I.J. Innovations in packed-bed reactors utilizing immobilized lipase catalysts: A comprehensive technical and scientific review. Mol. Catal. 2025, 573, 114814. [Google Scholar] [CrossRef]
- Sampaio, C.S.; Angelotti, J.A.F.; Fernandez-Lafuente, R.; Hirata, D.B. Lipase immobilization via cross-linked enzyme aggregates: Problems and prospects—A review. Int. J. Biol. Macromol. 2022, 215, 434–449. [Google Scholar] [CrossRef]
- Pinto Brito, M.J.; Bauer, L.C.; Flores Santos, M.P.; Santos, L.S.; Ferreira Bonomo, R.C.; da Costa Ilhéu Fontan, R.; Veloso, C.M. Lipase immobilization on activated and functionalized carbon for the aroma ester synthesis. Microporous Mesoporous Mater. 2020, 309, 110576. [Google Scholar] [CrossRef]
- Ghamgui, H.; Karra-Chaâbouni, M.; Bezzine, S.; Miled, N.; Gargouri, Y. Production of isoamyl acetate with immobilized Staphylococcus simulans lipase in a solvent-free system. Enzyme Microb. Technol. 2006, 38, 788–794. [Google Scholar] [CrossRef]
- Cui, H.Y.; Lu, J.Y.; Li, C.Z.; Rashed, M.M.A.; Lin, L. Antibacterial and physical effects of cationic starch nanofibers containing carvacrol@casein nanoparticles against Bacillus cereus in soy products. Int. J. Food Microbiol. 2022, 364, 109530. [Google Scholar] [CrossRef]
- Liu, X.; Qi, X.H.; Zhu, S.; Jiang, B.; Gao, S.; Zhang, Y.F.; Wang, H.L.; Xu, S.M.; Liu, Y.F.; An, Y.F. Embedding inulin fructotransferase from Arthrobacter aurescens into novel curdlan-based mesoporous silica microspheres for efficient production of Difructose Anhydride III. Food Chem. 2019, 299, 125128. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Chen, W.Q.; Li, C.Z.; Ye, Y.; Cui, H.Y.; Lin, L. Preparation and physicochemical effects of zein nanofiber membrane encapsulated with citral/HP-β-CD inclusion complex and its application on cheese. Food Biosci. 2022, 50, 101990. [Google Scholar] [CrossRef]
- Zhong, L.; Feng, Y.; Wang, G.; Wang, Z.; Bilal, M.; Lv, H.; Jia, S.; Cui, J. Production and use of immobilized lipases in/on nanomaterials: A review from the waste to biodiesel production. Int. J. Biol. Macromol. 2020, 152, 207–222. [Google Scholar] [CrossRef]
- Sun, H.H.; Scharff-Poulsen, A.M.; Gu, H.; Jakobsen, I.; Kossmann, J.M.; Frommer, W.B.; Almdal, K. Phosphate sensing by fluorescent reporter proteins embedded in polyacrylamide nanoparticles. ACS Nano 2008, 2, 19–24. [Google Scholar] [CrossRef]
- Huang, L.R.; Yan, Y.H.; Qu, L.L.; Li, F.; Chen, L.H.; Li, Y.L. Structure, emulsifying and embedding characteristics of soy protein isolate induced by the complexation of carrageenan with different charge groups. Food Hydrocolloids 2024, 157, 110455. [Google Scholar] [CrossRef]
- Zhang, F.; Zheng, L.L.; Cao, H.; Cheng, L.F.; Li, C. Preparation and Characterization of an Inner Flower Gel Microsphere Double-embedded with the Activated-lipase. Chem. J. Chin. U. 2023, 44, 20220699. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Zhang, Y.; Wang, C.; Liu, X.J.; El-Seedi, H.R.; Gomez, P.L.; Alzamora, S.M.; Zou, X.B.; Guo, Z.M. Enhanced composite Co-MOF-derived sodium carboxymethyl cellulose visual films for real-time and in situ monitoring fresh-cut apple freshness. Food Hydrocoll. 2024, 157, 110475. [Google Scholar] [CrossRef]
- Cai, M.H.; Zhang, G.; Wang, J.; Li, C.Z.; Cui, H.Y.; Lin, L. Application of glycyrrhiza polysaccharide nanofibers loaded with tea tree essential oil/gliadin nanoparticles in meat preservation. Food Biosci. 2021, 43, 101270. [Google Scholar] [CrossRef]
- Lin, L.; Gu, Y.L.; Cui, H.Y. Moringa oil/chitosan nanoparticles embedded gelatin nanofibers for food packaging against Listeria monocytogenes and Staphylococcus aureus on cheese. Food Packag. Shelf Life 2019, 19, 86–93. [Google Scholar] [CrossRef]
- Lee, S.H.; Doan, T.T.N.; Won, K.; Ha, S.H.; Koo, Y.-M. Immobilization of lipase within carbon nanotube–silica composites for non-aqueous reaction systems. J. Mol. Catal. B Enzym. 2010, 62, 169–172. [Google Scholar] [CrossRef]
- Kanwar, L.; Goswami, P. Isolation of a Pseudomonas lipase produced in pure hydrocarbon substrate and its application in the synthesis of isoamyl acetate using membrane-immobilised lipase. Enzyme Microb. Technol. 2002, 31, 727–735. [Google Scholar] [CrossRef]
- Soleimani, M.; Khani, A.; Najafzadeh, K. α-Amylase immobilization on the silica nanoparticles for cleaning performance towards starch soils in laundry detergents. J. Mol. Catal. B Enzym. 2012, 74, 1–5. [Google Scholar] [CrossRef]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotec. Eq. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Ispas, C.; Sokolov, I.; Andreescu, S. Enzyme-functionalized mesoporous silica for bioanalytical applications. Anal. Bioanal. Chem. 2009, 393, 543–554. [Google Scholar] [CrossRef]
- Zhai, R.; Zhang, B.; Wan, Y.; Li, C.; Wang, J.; Liu, J. Chitosan–halloysite hybrid-nanotubes: Horseradish peroxidase immobilization and applications in phenol removal. Chem. Eng. J. 2013, 214, 304–309. [Google Scholar] [CrossRef]
- Wang, B.; Zhou, J.; Zhang, X.Y.; Yang, Y.S.; Liu, C.H.; Zhu, H.L.; Jiao, Q.C. Covalently immobilize crude D-amino acid transaminase onto UiO-66-NH2 surface for D-Ala biosynthesis. Int. J. Biol. Macromol. 2021, 175, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Liu, D.; Wang, Z.; Li, C.Y.; Huang, W.Y.; Liu, S.B.; Li, Y. Interpenetrating network hydrogels loaded with nanostructured lipid carriers for curcumin delivery: Impact of dual crosslinking with genipin and calcium ions. Food Res. Int. 2025, 202, 115704. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A. Cross-linked enzyme aggregates (CLEA®s): Stable and recyclable biocatalysts. Biochem. Soc. Trans. 2007, 35, 1583–1587. [Google Scholar] [CrossRef]
- Wei, B.X.; Zou, J.; Pu, Q.Q.; Shi, K.; Xu, B.G.; Ma, Y.K. One-step preparation of hydrogel based on different molecular weights of chitosan with citric acid. J. Sci. Food Agric. 2022, 102, 3826–3834. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, X.X.; Shi, X.M.; Han, Y.F.; Guo, Z.M.; Liu, Y. Development of Carbon Quantum Dot-Labeled Antibody Fluorescence Immunoassays for the Detection of Morphine in Hot Pot Soup Base. Food Anal. Methods 2020, 13, 1042–1049. [Google Scholar] [CrossRef]
- Luo, C.; Hu, Y.; Xing, S.; Xie, W.; Li, C.; He, L.; Wang, X.; Zeng, X. Adsorption–precipitation–cross-linking immobilization of GDSL-type esterase from Aspergillus niger GZUF36 by polydopamine-modified magnetic clarity tetroxide nanocouplings and its enzymatic characterization. Int. J. Biol. Macromol. 2023, 245, 125533. [Google Scholar] [CrossRef]
- Sóti, P.L.; Weiser, D.; Vigh, T.; Nagy, Z.K.; Poppe, L.; Marosi, G. Electrospun polylactic acid and polyvinyl alcohol fibers as efficient and stable nanomaterials for immobilization of lipases. Bioprocess. Biosyst. Eng. 2016, 39, 449–459. [Google Scholar] [CrossRef]
- Oliveira, F.L.; de SFrança, A.; de Castro, A.M.; Alves de Souza, R.O.M.; Esteves, P.M.; Gonçalves, R.S.B. Enzyme Immobilization in Covalent Organic Frameworks: Strategies and Applications in Biocatalysis. ChemPlusChem 2020, 85, 2051–2066. [Google Scholar] [CrossRef]
- Bullo, G.T.; Marasca, N.; Almeida, F.L.C.; Forte, M.B.S. Lipases: Market study and potential applications of immobilized derivatives. Biofuel. Bioprod. Bior. 2024, 18, 1676–1689. [Google Scholar] [CrossRef]
- Sun, J.; Xu, B.; Mu, Y.Y.; Ma, H.L.; Qu, W.J. Functional Magnetic Nanoparticles for Highly Efficient Cholesterol Removal. J. Food Sci. 2018, 83, 122–128. [Google Scholar] [CrossRef]
- Han, J.; Wang, L.; Wang, L.; Li, C.M.; Mao, Y.L.; Wang, Y. Fabrication of a core-shell-shell magnetic polymeric microsphere with excellent performance for separation and purification of bromelain. Food Chem. 2019, 283, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Kutsanedzie, F.Y.H.; Hassan, M.; Zhu, J.J.; Ahmad, W.; Li, H.H.; Chen, Q.S. Mesoporous silica supported orderly-spaced gold nanoparticles SERS-based sensor for pesticides detection in food. Food Chem. 2020, 315, 126300. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater. 2005, 17, 4577–4593. [Google Scholar] [CrossRef]
- Kausar, S.; Yousaf, M.; Mir, S.; Awwad, N.S.; Alturaifi, H.A.; Riaz, F. Mesoporous Materials: Synthesis and electrochemical applications. Electrochem. Commun. 2024, 169, 107836. [Google Scholar] [CrossRef]
- Carteret, C.; Jacoby, J.; Blin, J.L. Using factorial experimental design to optimize biocatalytic biodiesel production from Mucor Miehei Lipase immobilized onto ordered mesoporous materials. Microporous Mesoporous Mater. 2018, 268, 39–45. [Google Scholar] [CrossRef]
- Pedro, K.C.N.R.; da Silva, G.A.R.; da Silva, M.A.P.; Henriques, C.A.; Langone, M.A.P. Immobilization of lipase on zeolite, silica, and silica-aluminas and its use in hydrolysis, esterification, and transesterification reactions. Catal. Today 2025, 447, 115141. [Google Scholar] [CrossRef]
- Xia, J.J.; Liu, F.; Yan, L.S.; Suo, H.B.; Qian, J.Y.; Zou, B. Simultaneous determination of tert-butylhydroquinone, butylated hydroxyanisole and phenol in plant oil by metalloporphyrin-based covalent organic framework electrochemical sensor. J. Food Compos. Anal. 2023, 122, 105486. [Google Scholar] [CrossRef]
- Elmerhi, N.; Al-Maqdi, K.; Athamneh, K.; Mohammed, A.K.; Skorjanc, T.; Gándara, F.; Raya, J.; Pascal, S.; Siri, O.; Trabolsi, A.; et al. Enzyme-immobilized hierarchically porous covalent organic framework biocomposite for catalytic degradation of broad-range emerging pollutants in water. J. Hazard. Mater. 2023, 459, 132261. [Google Scholar] [CrossRef]
- Wang, H.; Jiao, F.; Gao, F.; Zhao, X.; Zhao, Y.; Shen, Y.; Zhang, Y.; Qian, X. Covalent organic framework-coated magnetic graphene as a novel support for trypsin immobilization. Anal. Bioanal. Chem. 2017, 409, 2179–2187. [Google Scholar] [CrossRef]
- Aghaei, H.; Yasinian, A.; Taghizadeh, A. Covalent immobilization of lipase from Candida rugosa on epoxy-activated cloisite 30B as a new heterofunctional carrier and its application in the synthesis of banana flavor and production of biodiesel. Int. J. Biol. Macromol. 2021, 178, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Asmat, S.; Anwer, A.H.; Husain, Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int. J. Biol. Macromol. 2019, 140, 484–495. [Google Scholar] [CrossRef] [PubMed]
- Suo, H.B.; Geng, X.Y.; Sun, Y.H.; Zhang, L.; Yang, J.; Yang, F.; Yan, H.; Hu, Y.; Xu, L.L. Surface Modification of Magnetic ZIF-90 Nanoparticles Improves the Microenvironment of Immobilized Lipase and Its Application in Esterification. Langmuir 2022, 38, 15384–15393. [Google Scholar] [CrossRef]
- Suo, H.B.; Geng, H.N.; Zhang, L.; Liu, G.Y.; Yan, H.; Cao, R.; Zhu, J.H.; Hu, Y.; Xu, L.L. Covalent immobilization of lipase on an ionic liquid-functionalized magnetic Cu-based metal-organic framework with boosted catalytic performance in flavor ester synthesis. J. Mater. Chem. B 2023, 11, 1302–1311. [Google Scholar] [CrossRef]
- de Oliveira, T.P.; Santos, M.P.F.; Brito, M.J.P.; Veloso, C.M. Incorporation of metallic particles in activated carbon used in lipase immobilization for production of isoamyl acetate. J. Chem. Technol. Biotechnol. 2022, 97, 1736–1746. [Google Scholar] [CrossRef]
- de Lima, L.N.; Mendes, A.A.; Fernandez-Lafuente, R.; Tardioli, P.W.; Giordano, R.D.C. Performance of Different Immobilized Lipases in the Syntheses of Short- and Long-Chain Carboxylic Acid Esters by Esterification Reactions in Organic Media. Molecules 2018, 23, 766. [Google Scholar] [CrossRef]
- Carrillo, G.; Vaschetto, E.G.; Ferrero, G.O.; Eimer, G.A. Development of mesoporous biocatalysts synthesized from a biomass surfactant for flavorings production. Microporous Mesoporous Mater. 2025, 387, 113536. [Google Scholar] [CrossRef]
- Sarno, M.; Iuliano, M.; Polichetti, M.; Ciambelli, P. High activity and selectivity immobilized lipase on Fe3O4 nanoparticles for banana flavour synthesis. Process Biochem. 2017, 56, 98–108. [Google Scholar] [CrossRef]
- Yildirim, D.; Baran, E.; Ates, S.; Yazici, B.; Tukel, S.S. Improvement of activity and stability of Rhizomucor miehei lipase by immobilization on nanoporous aluminium oxide and potassium sulfate microcrystals and their applications in the synthesis of aroma esters. Biocatal. Biotransform. 2019, 37, 210–223. [Google Scholar] [CrossRef]
- Corovic, M.; Mihailovic, M.; Banjanac, K.; Carevic, M.; Milivojevic, A.; Milosavic, N.; Bezbradica, D. Immobilization of Candida antarctica lipase B onto Purolite® MN102 and its application in solvent-free and organic media esterification. Bioprocess. Biosyst. Eng. 2017, 40, 23–34. [Google Scholar] [CrossRef]
- Kirdi, R.; Ben Akacha, N.; Messaoudi, Y.; Gargouri, M. Enhanced synthesis of isoamyl acetate using liquid-gas biphasic system by the transesterification reaction of isoamyl alcohol obtained from fusel oil. Biotechnol. Bioprocess Eng. 2017, 22, 413–422. [Google Scholar] [CrossRef]
- Padilha, G.S.; Tambourgi, E.B.; Alegre, R.M. Evaluation of lipase from Burkholderia cepacia immobilized in alginate beads and application in the synthesis of banana flavor (isoamyl acetate). Chem. Eng. Commun. 2018, 205, 23–33. [Google Scholar] [CrossRef]
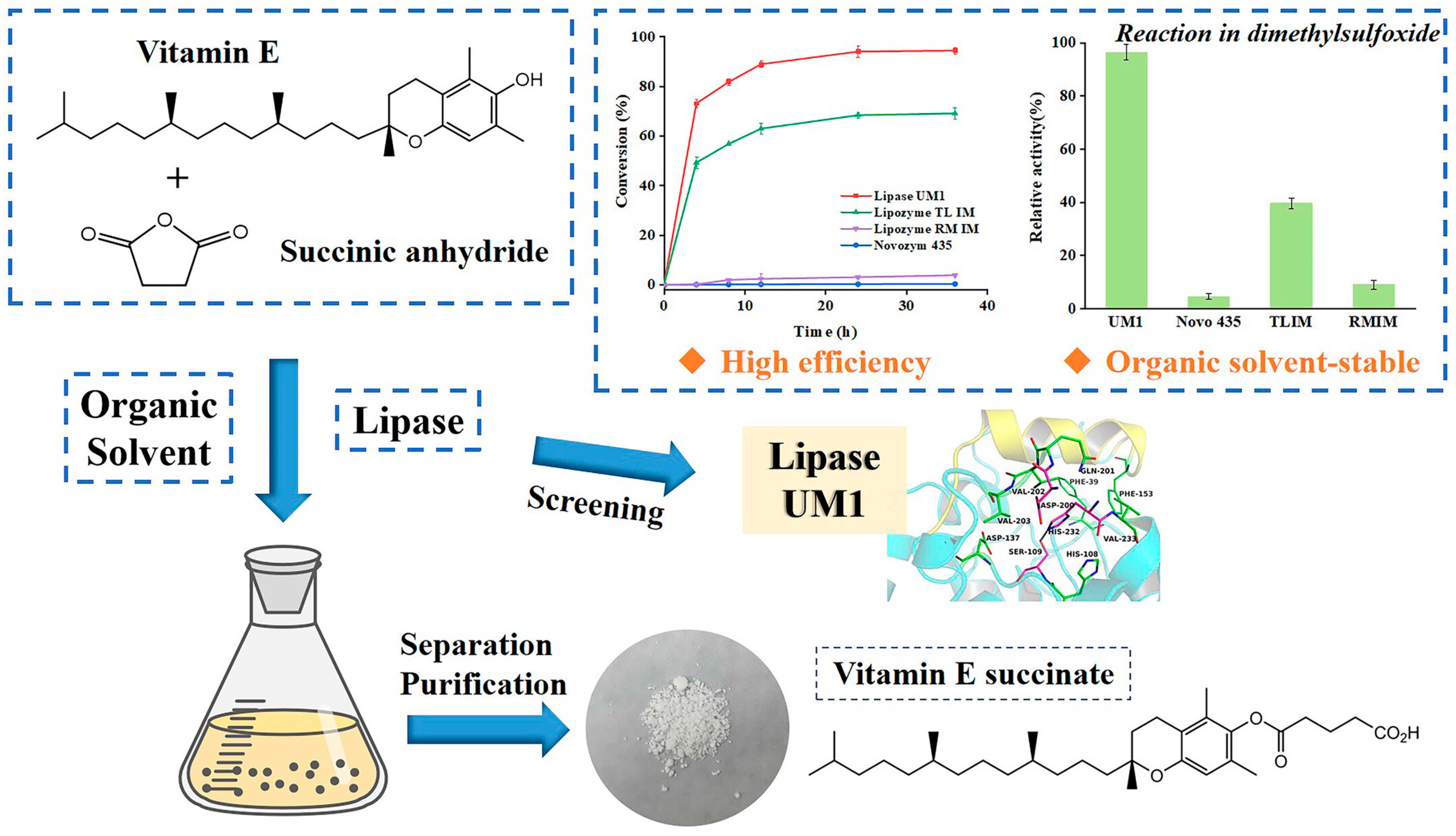
- Li, W.L.; Lin, S.; Lan, D.M.; Wang, Y.H. Efficient enzymatic synthesis of vitamin E succinate using an organic solvent-stable immobilized lipase. J. Am. Oil Chem. Soc. 2024, 101, 1357–1366. [Google Scholar] [CrossRef]
- Enayati, M.; Gong, Y.; Goddard, J.M.; Abbaspourrad, A. Synthesis and characterization of lactose fatty acid ester biosurfactants using free and immobilized lipases in organic solvents. Food Chem. 2018, 266, 508–513. [Google Scholar] [CrossRef]
- Riaz, T.; Iqbal, M.W.; Mahmood, S.; Yasmin, I.; Leghari, A.A.; Rehman, A.; Mushtaq, A.; Ali, K.; Azam, M.; Bilal, M. Cottonseed oil: A review of extraction techniques, physicochemical, functional, and nutritional properties. Crit. Rev. Food Sci. Nutr. 2023, 63, 1219–1237. [Google Scholar] [CrossRef]
- Kumar, P.; Kermanshahi-Pour, A.; Brar, S.K.; He, Q.S.; Rainey, J.K. Influence of elevated pressure and pressurized fluids on microenvironment and activity of enzymes. Biotechnol. Adv. 2023, 68, 108219. [Google Scholar] [CrossRef]
- Barros, E.L.S.; Rebelatto, E.A.; Mayer, D.A.; Wancura, J.H.C.; Oliveira, J.V. Lipase-catalyzed Production of Sugar Esters in Pressurized Fluid Media: A Review. Chem. Eng. Process. 2023, 191, 109480. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, S.B.; Yoo, H.Y.; Lee, J.H.; Park, C.; Han, S.O.; Kim, S.W. Kinetic modeling of biodiesel production by mixed immobilized and co-immobilized lipase systems under two pressure conditions. Korean J. Chem. Eng. 2013, 30, 1272–1276. [Google Scholar] [CrossRef]
- He, W.S.; Liu, Q.; Yu, H.; Si, X.J.; Zhang, J.K. Efficient Synthesis of Octacosanol Linoleate Catalyzed by Ionic Liquid and Its Structure Characterization. J. Am. Oil Chem. Soc. 2016, 93, 509–517. [Google Scholar] [CrossRef]
- Ji, Q.H.; Yu, X.J.; Yagoub, A.G.A.; Chen, L.; Zhou, C.S. Efficient removal of lignin from vegetable wastes by ultrasonic and microwave-assisted treatment with ternary deep eutectic solvent. Ind. Crops Prod. 2020, 149, 112357. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, C.; Liu, C.-Z. Efficient Kinetic Resolution of (R,S)-2-Octanol Catalyzed by Magnetite-Immobilized Yarrowia lipolytica Lipase in Mixed Ionic Liquids. Catal. Lett. 2014, 144, 1552–1556. [Google Scholar] [CrossRef]
- Papadopoulou, A.A.; Tzani, A.; Polydera, A.C.; Katapodis, P.; Voutsas, E.; Detsi, A.; Stamatis, H. Green biotransformations catalysed by enzyme-inorganic hybrid nanoflowers in environmentally friendly ionic solvents. Environ. Sci. Pollut. Res. 2018, 25, 26707–26714. [Google Scholar] [CrossRef]
- Attri, P.; Venkatesu, P.; Kumar, A. Activity and stability of α-chymotrypsin in biocompatible ionic liquids: Enzyme refolding by triethyl ammonium acetate. PCCP 2011, 13, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Deive, F.J.; Ruivo, D.; Rodrigues, J.V.; Gomes, C.M.; Sanroman, M.A.; Rebelo, L.P.N.; Esperança, J.; Rodríguez, A. On the hunt for truly biocompatible ionic liquids for lipase-catalyzed reactions. RSC Adv. 2015, 5, 3386–3389. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Papadopoulou, A.A.; Efstathiadou, E.; Patila, M.; Polydera, A.C.; Stamatis, H. Deep Eutectic Solvents as Media for Peroxidation Reactions Catalyzed by Heme-Dependent Biocatalysts. Ind. Eng. Chem. Res. 2016, 55, 5145–5151. [Google Scholar] [CrossRef]


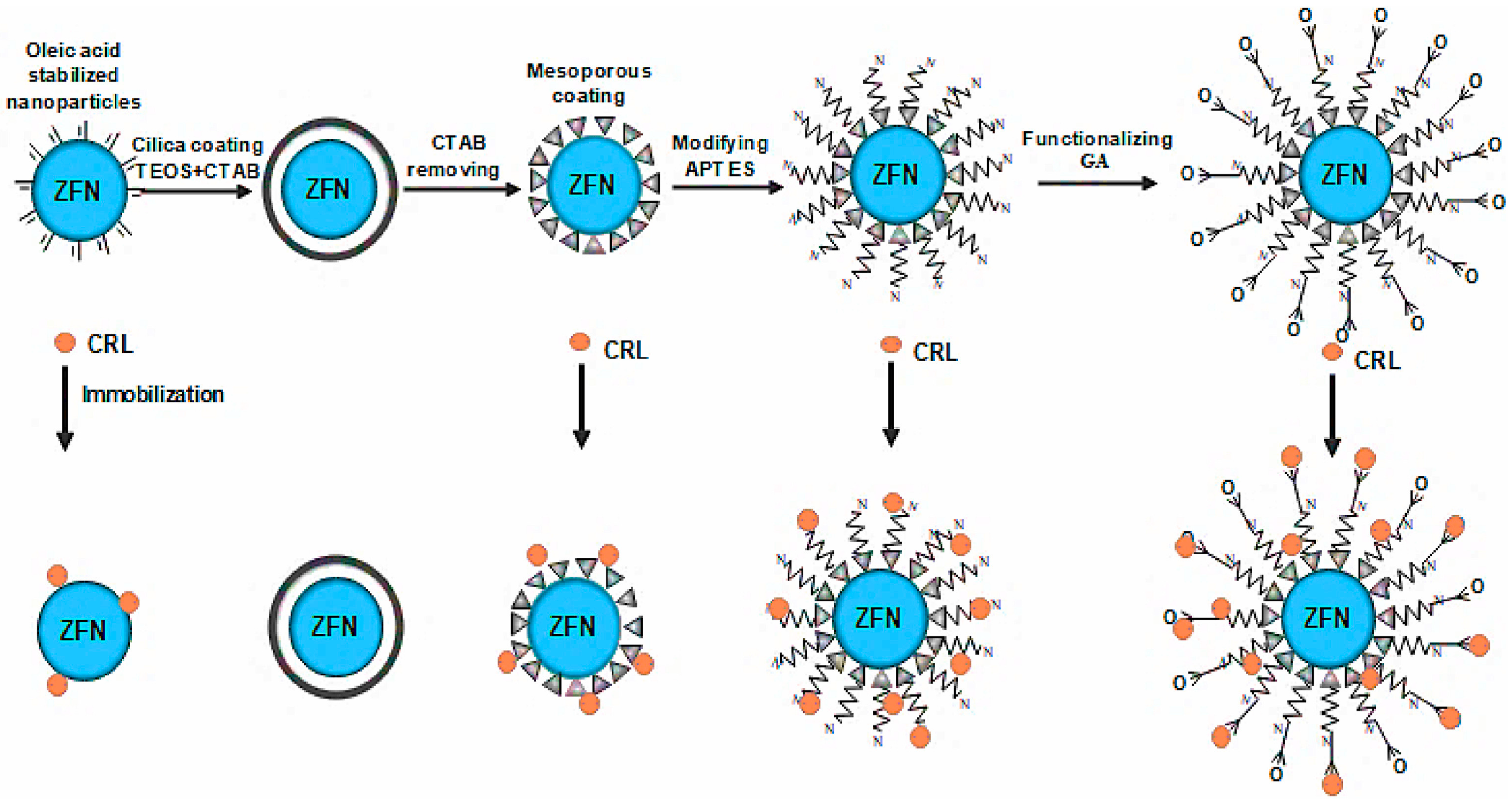
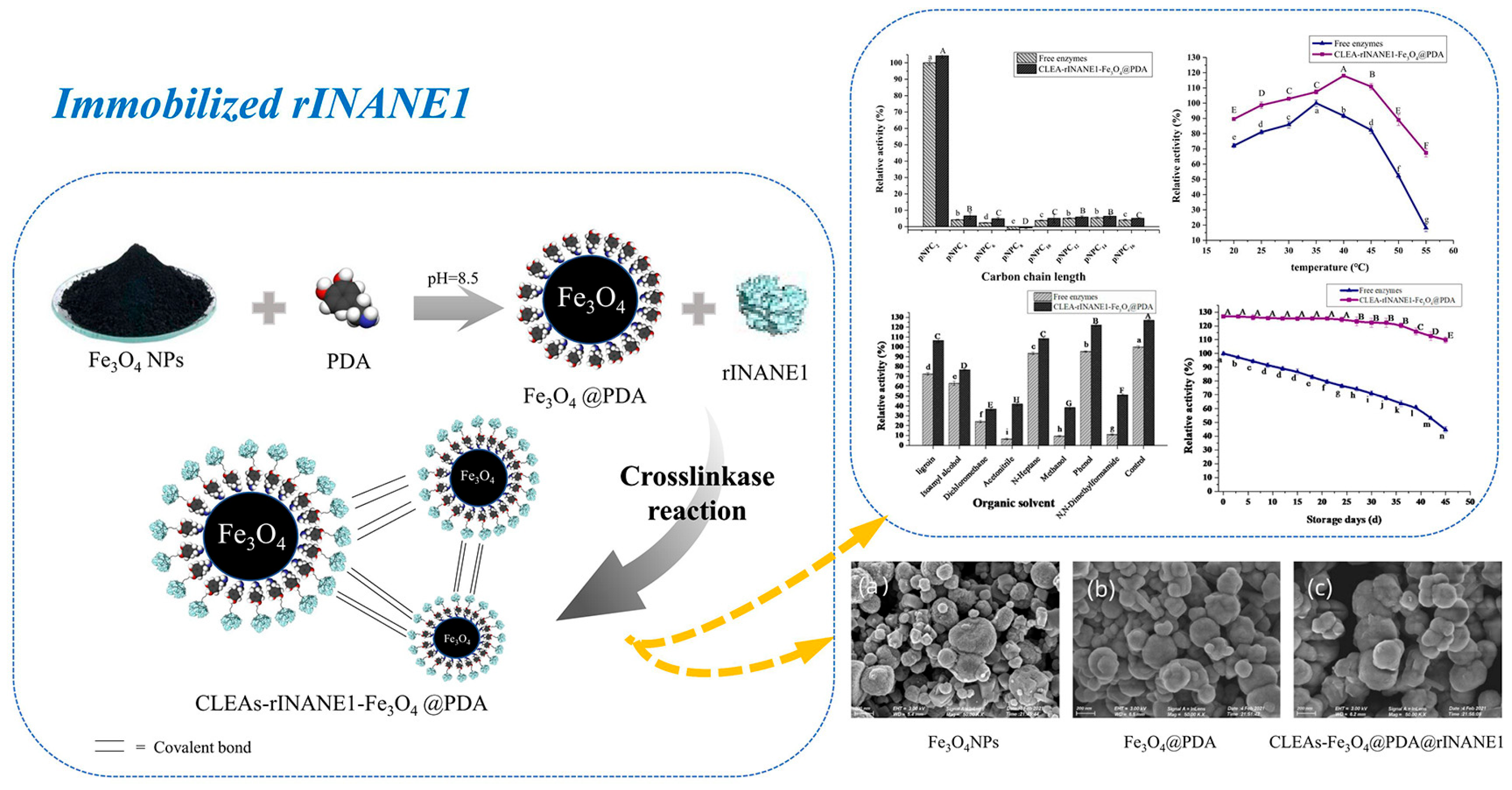
| Classification | Adsorption | Embedding | Covalent Bonding | Crosslinking |
|---|---|---|---|---|
| Advantages | Simple method, little loss of activity, cheap and fast | Large amount of immobilized enzyme, no need for extraction or purification, low loss of activity | Strong bonding properties, excellent stability | Strongly binds to lipase, good stability in aqueous solution |
| Disadvantages | Leaks easily, binds non-specifically | Methodological complexity, mass transfer limitations, leakage | Increased cost, decreased activity | May be inactive, lack of mechanical properties, difficult to control size |
| Lipase | Carrier (Immobilization Method) | Reaction Method | Temperature (°C) | Time (h) | Yield (%) | Reference |
|---|---|---|---|---|---|---|
| Candida rugosa lipase | COFs (embedding) | Esterification | 50 | 7 | 86.94 | [5] |
| Candida rugosa lipase | SBA-15, calcium alginate gel (adsorption, embedding) | Esterification | 50 | 8 | 85.19 | [1] |
| Candida rugosa lipase | Magnetic chitosan beads (covalent bonding) | Esterification | 35 | 24 | 98.4 | [38] |
| Candida rugosa lipase | COFs (embedding) | Esterification | 50 | 7 | 98.26 | [6] |
| Candida rugosa lipase | Epoxy-activated cloisite 30B (covalent bonding) | Esterification | 50 | 4 | 91.6 | [101] |
| Candida rugosa lipase | ZnFe2O4@MS (covalent bonding) | Esterification | 45 | 4 | 64 | [39] |
| Candida rugosa lipase | PDA@Co-MWCNT (covalent bonding) | Esterification | 45 | 24 | 75 | [102] |
| Porcine pancreatic lipase | ILs/MZIF-90 (adsorption) | Esterification | 45 | 9 | 85.5 | [103] |
| Porcine pancreatic lipase | ILs/Fe3O4@MOF (covalent bonding) | Esterification | 45 | 24 | 75.2 | [104] |
| Porcine pancreas lipase | Activated carbon (adsorption) | Esterification | 40 | 3 | 93 | [64] |
| Porcine pancreas lipase | Metallized activated carbon (adsorption) | Esterification | 40 | 4 | 96.62 | [105] |
| Pseudomonas fluorescens lipase | Octyl-silica (adsorption) | Esterification | 37 | 24 | 96.9 | [106] |
| Pseudomonas fluorescens lipase | Mesoporous silica matrix (adsorption) | Transesterification | 40 | 20 | 62 | [107] |
| Thermomyces lanuginosus lipase | K2SO4 crystal (adsorption) | Transesterification | 50 | 6 | 95 | [41] |
| Thermomyces lanuginosus lipase | Fe3O4 (adsorption) | Transesterification | 30 | 7 | 61 | [108] |
| Rhizomucor miehei lipase | Al2O3-NP (adsorption) | Transesterification | 50 | 1 | 15.4 | [109] |
| Candida antarctica lipase B | Purolite® MN102 (adsorption) | Transesterification | 75 | 6 | 100 | [110] |
| Aspergillus oryzae lipase | Calcium alginate gel (embedding) | Transesterification | 68.5 | 6 | 89.55 | [111] |
| Bacillus aerius lipase | Silica gel matrix (covalent bonding) | Esterification | 55 | 10 | 68.38 | [26] |
| Burkholderia cepacian lipase | Calcium alginate gel (embedding) | Esterification | 37 | 120 | 92 | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, G.; Zhang, Y.; Qian, J.; Wang, F.; Qu, L.; Zou, B. Advancements in the Research on the Preparation of Isoamyl Acetate Catalyzed by Immobilized Lipase. Materials 2025, 18, 2476. https://doi.org/10.3390/ma18112476
Guan G, Zhang Y, Qian J, Wang F, Qu L, Zou B. Advancements in the Research on the Preparation of Isoamyl Acetate Catalyzed by Immobilized Lipase. Materials. 2025; 18(11):2476. https://doi.org/10.3390/ma18112476
Chicago/Turabian StyleGuan, Guoqiang, Yuyang Zhang, Jingya Qian, Feng Wang, Liang Qu, and Bin Zou. 2025. "Advancements in the Research on the Preparation of Isoamyl Acetate Catalyzed by Immobilized Lipase" Materials 18, no. 11: 2476. https://doi.org/10.3390/ma18112476
APA StyleGuan, G., Zhang, Y., Qian, J., Wang, F., Qu, L., & Zou, B. (2025). Advancements in the Research on the Preparation of Isoamyl Acetate Catalyzed by Immobilized Lipase. Materials, 18(11), 2476. https://doi.org/10.3390/ma18112476








