Insight into Y Content on Microstructure and Mechanical Properties of Mg-Gd-Y-Zr Alloy
Abstract
1. Introduction
2. Experiments
3. Results
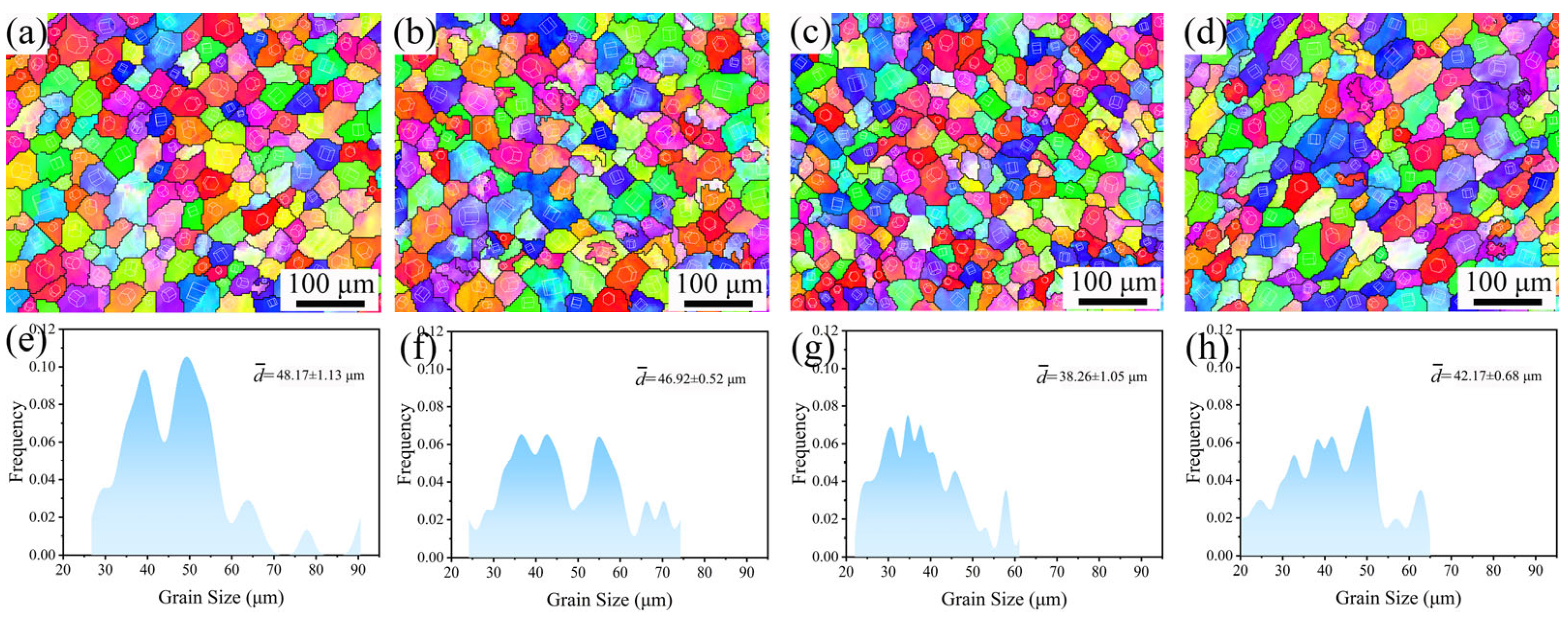
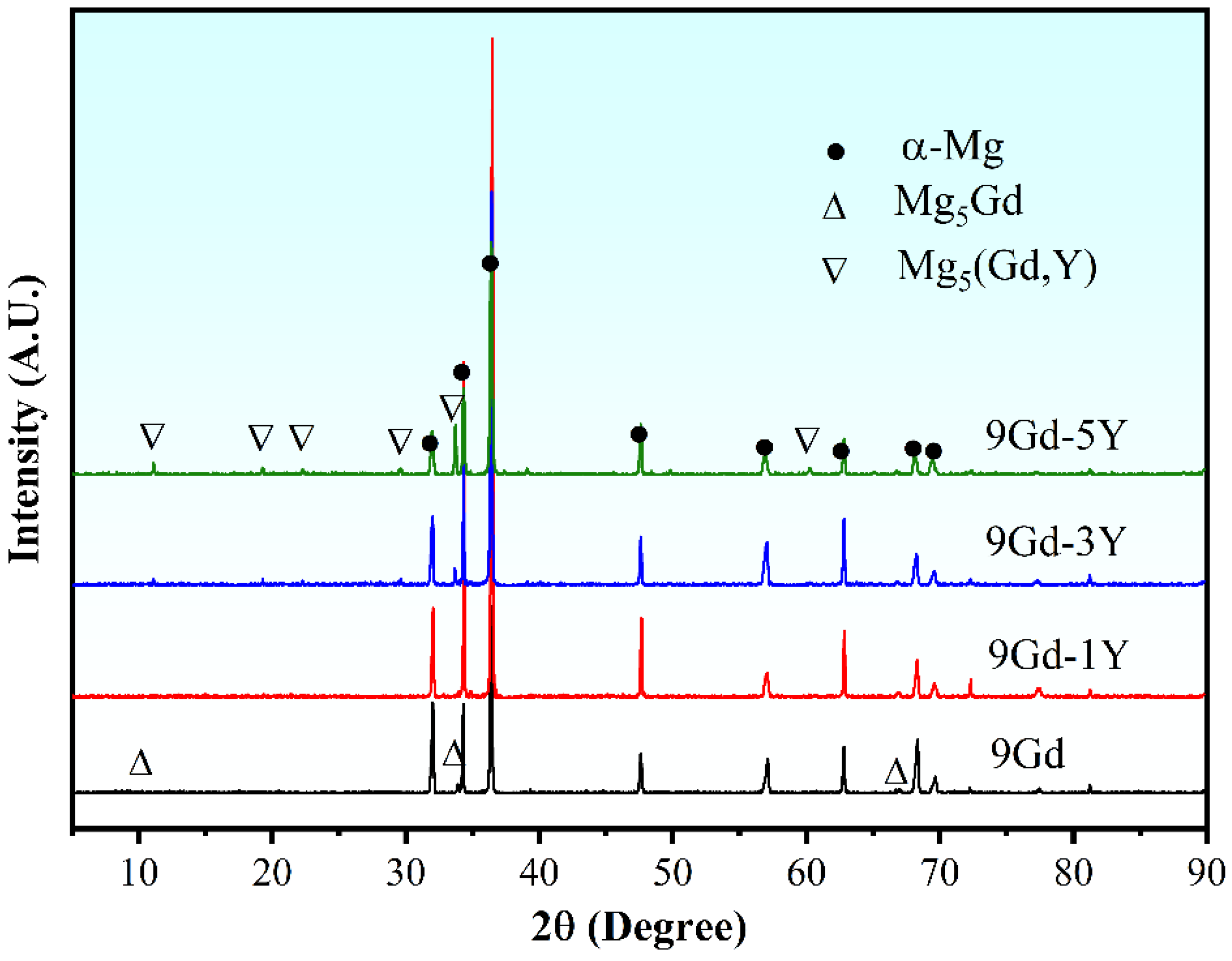
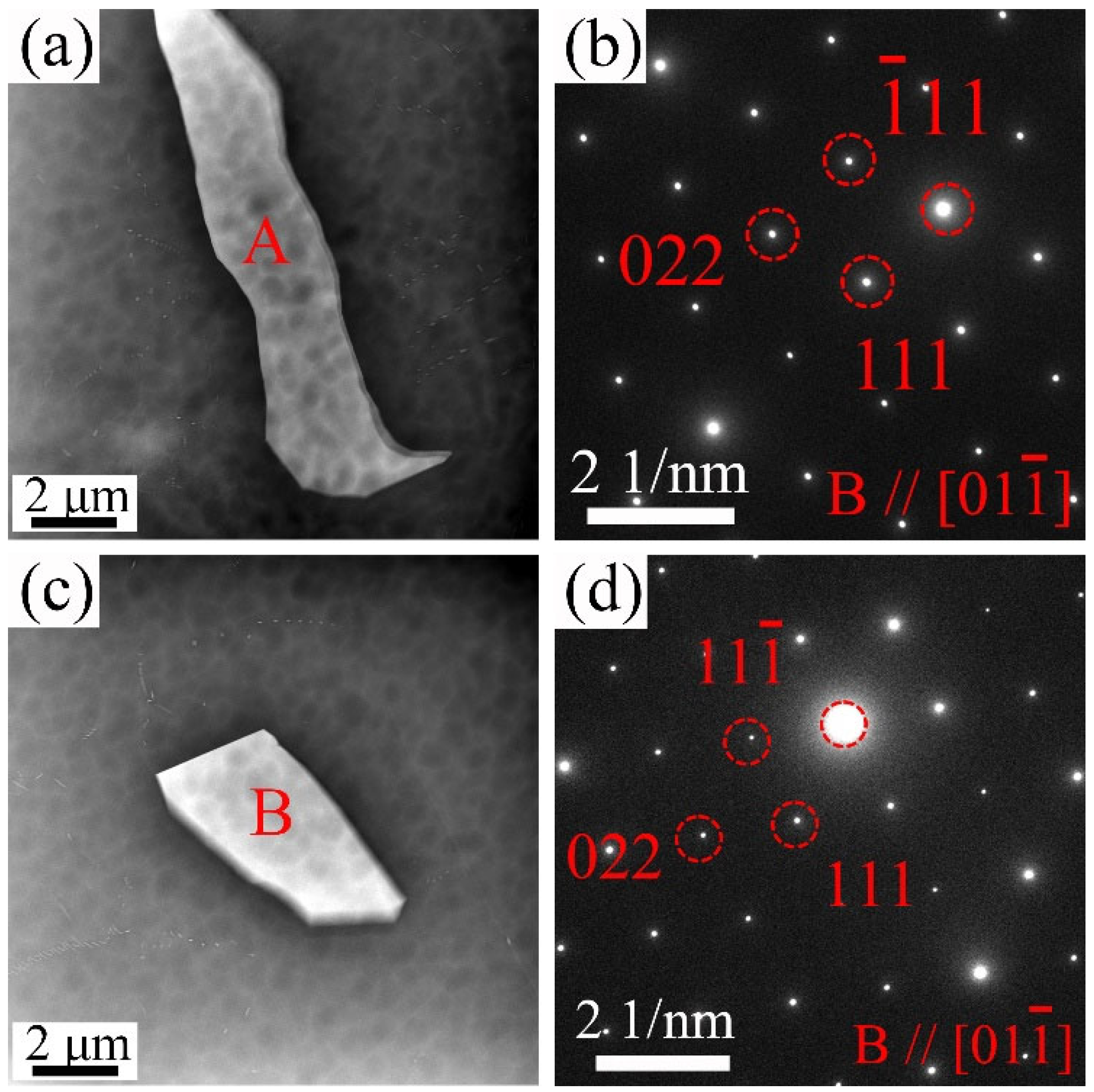
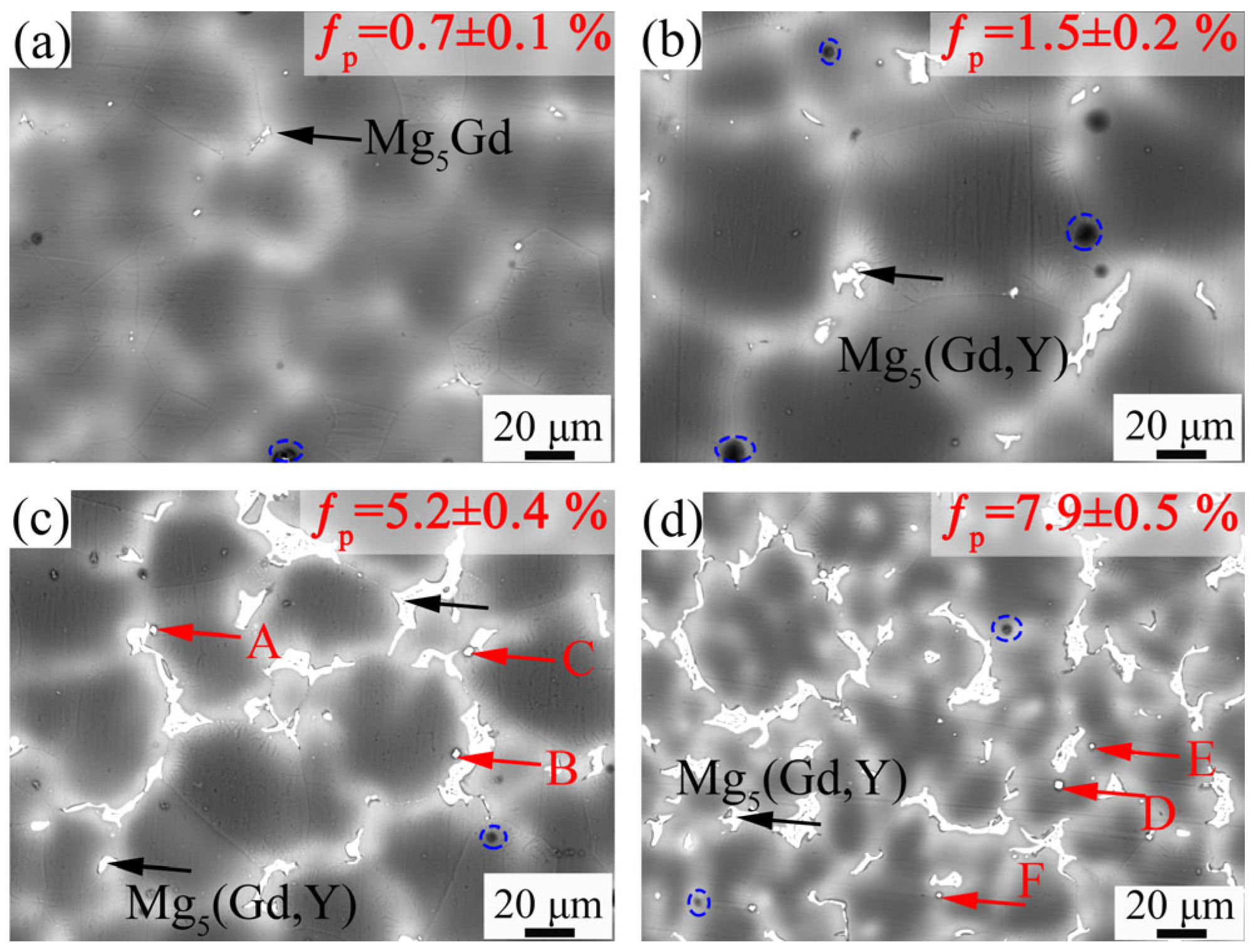
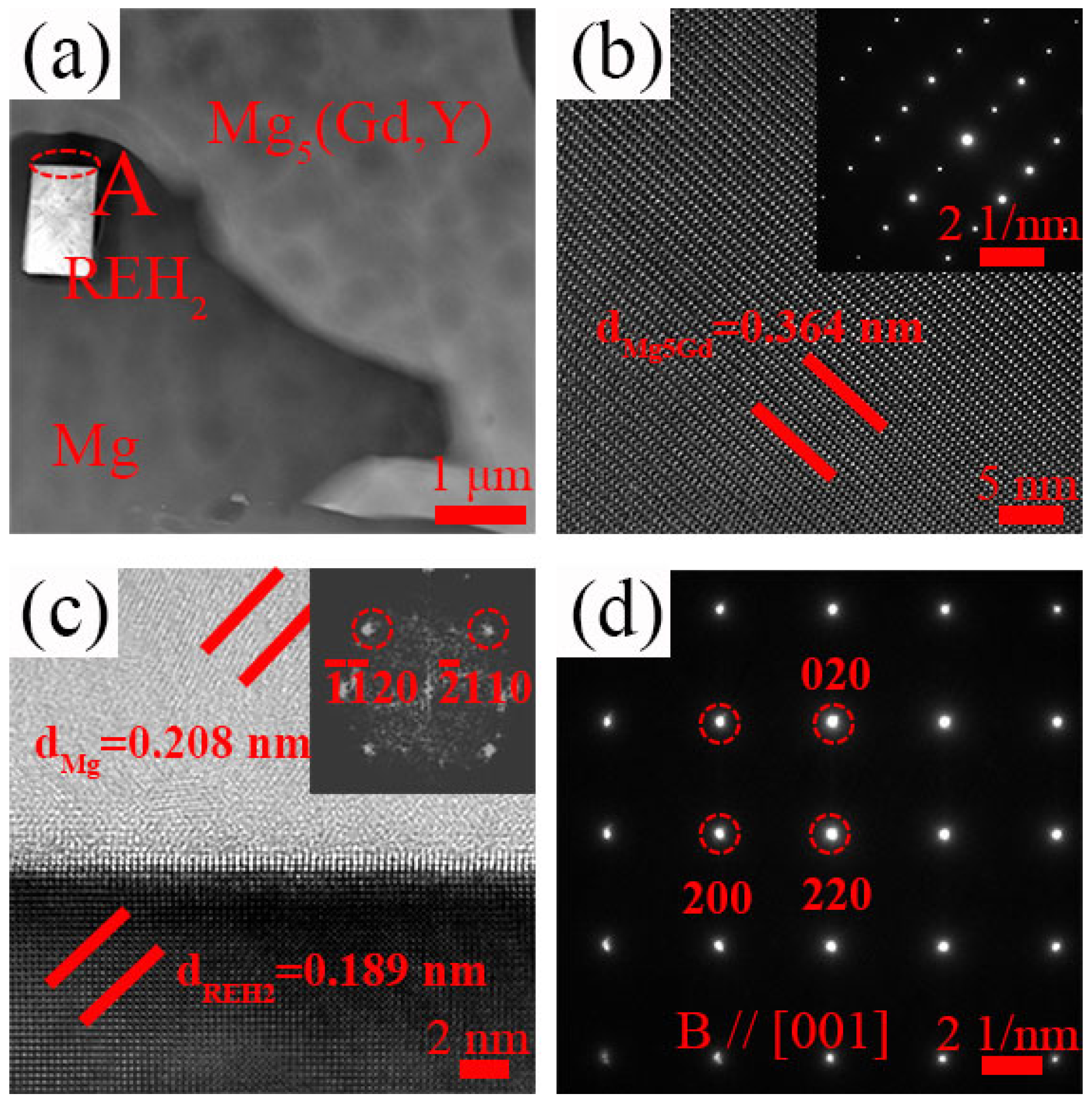
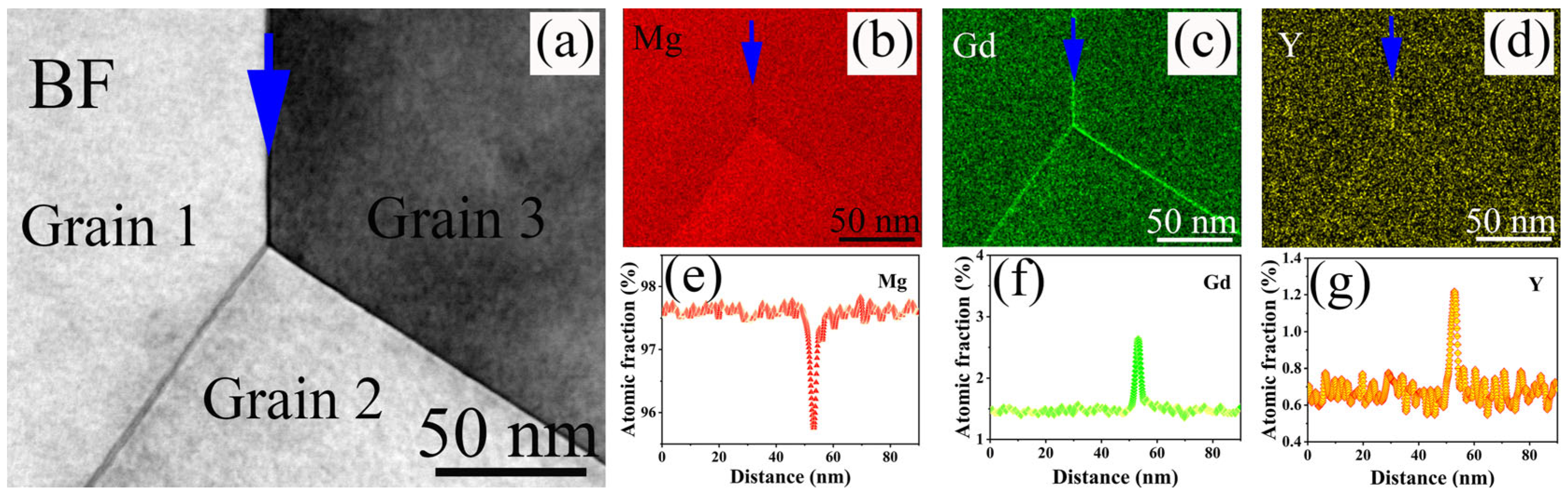
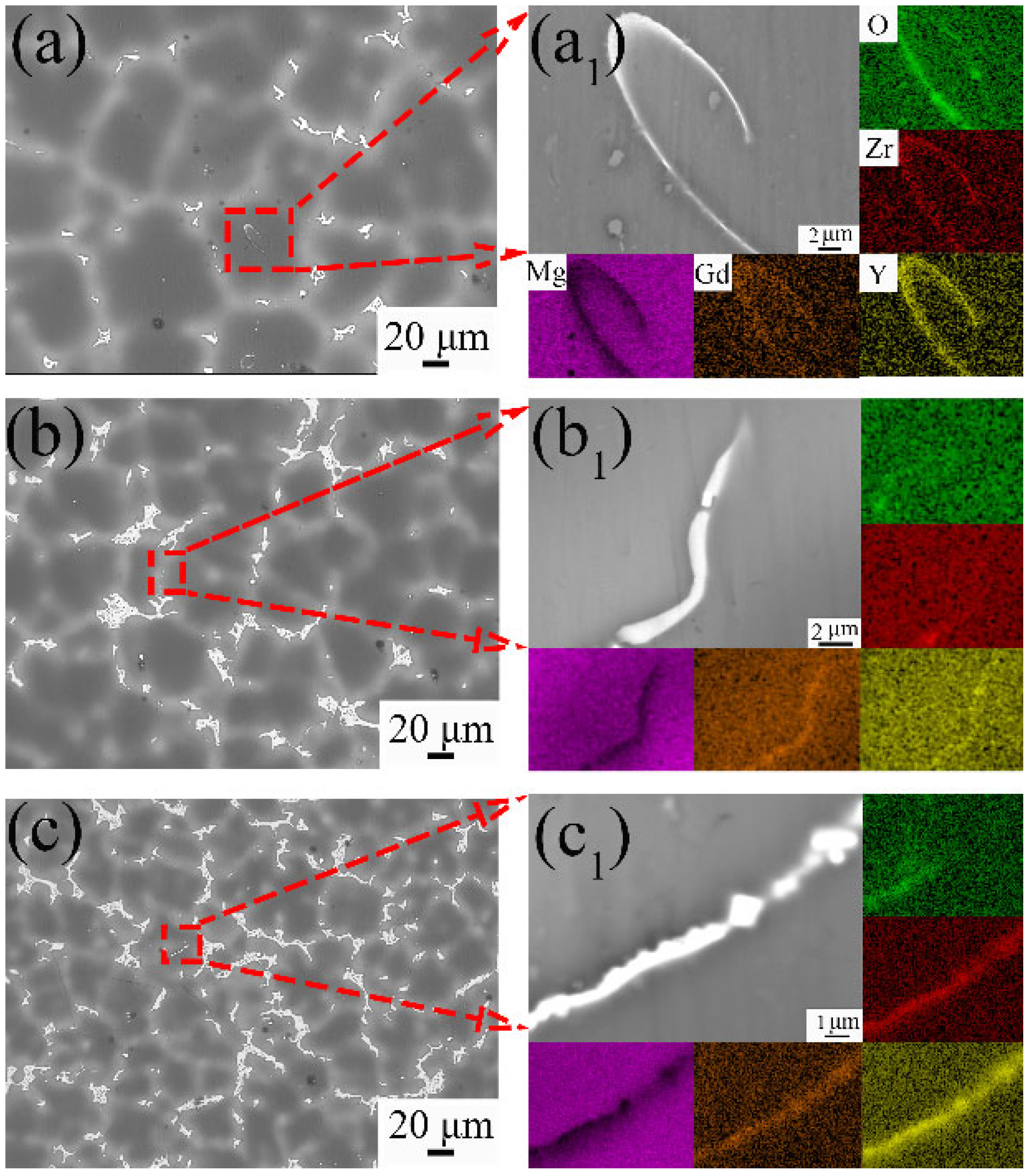
3.1. Microstructure Evolution
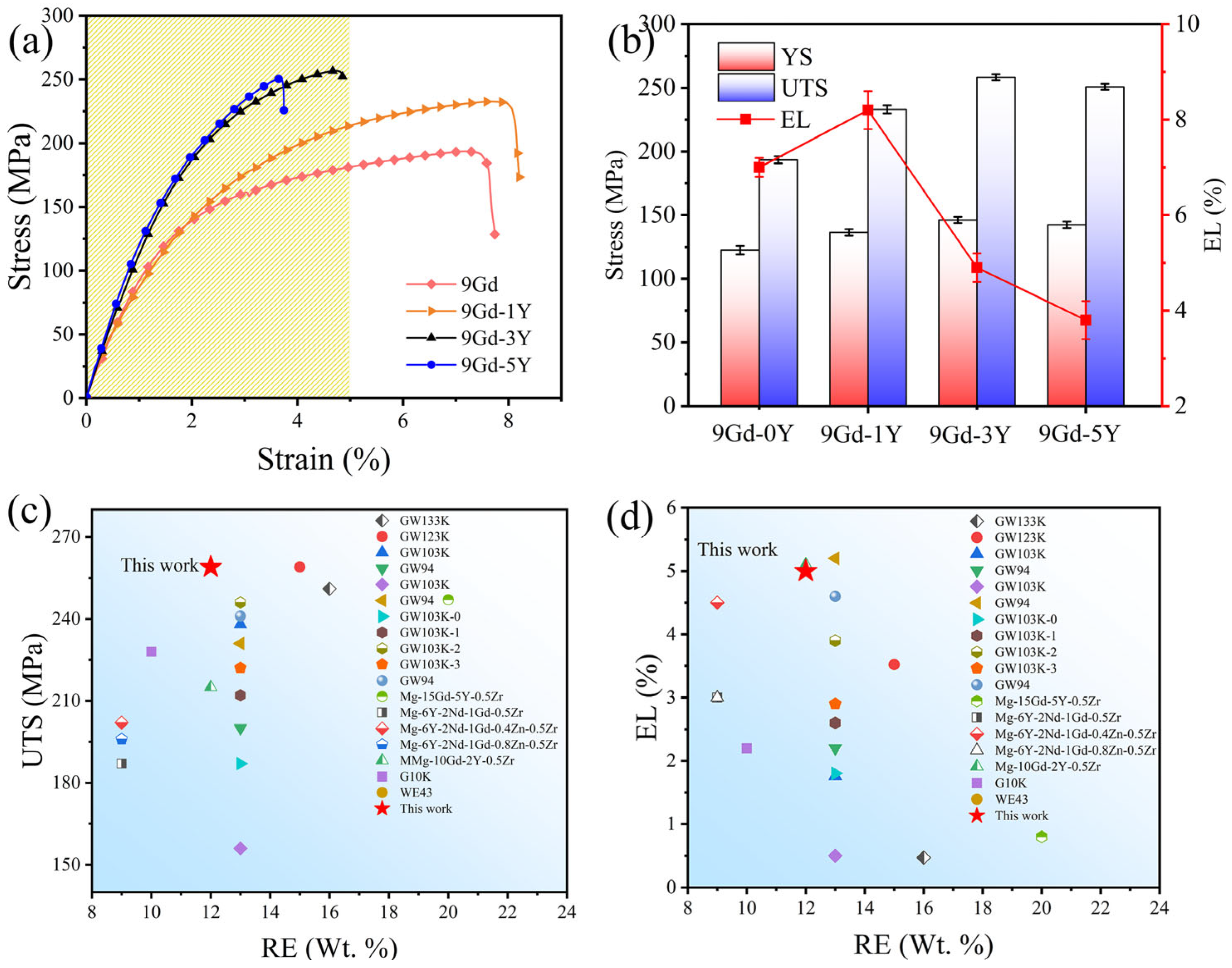
3.2. Tensile Mechanical Properties
4. Discussion
4.1. Effect of Y Content on Microstructure of the Mg-Gd-Y-Zr Alloys
4.2. Effect of Y Content on Mechanical Properties of the Mg-Gd-Y-Zr Alloys
4.3. Strengthening Mechanism of Mg-Gd-Y-Zr Alloy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Agnew, S.R.; Nie, J.F. Preface to the viewpoint set on: The current state of magnesium alloy science and technology. Scr. Mater. 2010, 63, 671–673. [Google Scholar] [CrossRef]
- Zeng, Z.; Stanford, N.; Davies, C.H.J.; Nie, J.-F.; Birbilis, N. Magnesium extrusion alloys: A review of developments and prospects. Int. Mater. Rev. 2019, 64, 27–62. [Google Scholar] [CrossRef]
- Peng, Z.Z.; Shao, X.H.; Liang, Z.M.; Wang, D.L.; Wang, L.W.; Ma, X.L. Synergetic deformation mechanisms in an Mg-Zn-Y-Zr alloy with intragranular LPSO structures. J. Magnes. Alloy 2023, 11, 1754–1768. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Z.; Zhu, Y.; Qin, L.; Zou, J.; Ma, L. Study of Corrosion Behavior of Mg–Gd-Based Soluble Magnesium Alloys. Metals 2025, 15, 35. [Google Scholar] [CrossRef]
- Chen, W.; Yang, J.; Yu, W.; Su, Y.; Zhang, A.; Ma, C.; Ma, Y. One developed finite element model used in nano-layered flaky Ti2AlC MAX ceramic particles reinforced magnesium composite. J. Magnes. Alloy 2024, 12, 4219–4228. [Google Scholar] [CrossRef]
- Caceres, C.H. Economical and Environmental Factors in Light Alloys Automotive Applications. Metall. Mater. Trans. A 2007, 38, 1649–1662. [Google Scholar] [CrossRef]
- Zhao, T.; Hu, Y.; He, B.; Zhang, C.; Zheng, T.; Pan, F. Effect of manganese on microstructure and properties of Mg-2Gd magnesium alloy. Mater. Sci. Eng., A 2019, 765, 138292. [Google Scholar] [CrossRef]
- Wang, G.G.; Weiler, J.P. Recent developments in high-pressure die-cast magnesium alloys for automotive and future applications. J. Magnes. Alloy 2023, 11, 78–87. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, N.; Chu, Z.; Niu, Y.; Ma, L.; Qin, L. Study of the corrosion mechanism of Mg–Gd based soluble magnesium alloys with different initial texture states. Corros. Rev. 2023, 41, 659–672. [Google Scholar] [CrossRef]
- Zhu, Y.; Hao, C.; Li, Z.; Zou, J.; Qin, L.; Niu, Y.; Ma, L.; Zhou, J. First-principles study of binary and ternary phases in Mg-Gd-Ni alloys. Phys. B Condens. Matter 2024, 685, 416065. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Q.; Huang, Z.; Qin, L.; Li, Z.; Ma, L. Strain Hardening Exponent and Strain Rate Sensitivity Exponent of Cast AZ31B Magnesium Alloy. Metals 2022, 12, 1942. [Google Scholar] [CrossRef]
- Qin, L.; Du, W.; Cipiccia, S.; Bodey, A.J.; Rau, C.; Mi, J. Synchrotron X-ray operando study and multiphysics modelling of the solidification dynamics of intermetallic phases under electromagnetic pulses. Acta Mater. 2024, 265, 119593. [Google Scholar] [CrossRef]
- Guo, W.; Wang, Q.D.; Ye, B.; Liu, M.P.; Peng, T.; Liu, X.T.; Zhou, H. Enhanced microstructure homogeneity and mechanical properties of AZ31 magnesium alloy by repetitive upsetting. Mater. Sci. Eng. A 2012, 540, 115–122. [Google Scholar] [CrossRef]
- Zou, J.; Ma, L.; Zhu, Y.; Qin, L.; Yuan, Y. Gradient microstructure and superior strength–ductility synergy of AZ61 magnesium alloy bars processed by radial forging with different deformation temperatures. J. Mater. Sci. Technol. 2024, 170, 65–77. [Google Scholar] [CrossRef]
- Li, T.; Song, J.; Zhang, A.; You, G.; Yang, Y.; Jiang, B.; Qin, X.; Xu, C.; Pan, F. Progress and prospects in Mg-alloy super-sized high pressure die casting for automotive structural components. J. Magnes. Alloy 2023, 11, 4166–4180. [Google Scholar] [CrossRef]
- Xu, J.; Liu, W.; Jiang, B.; Yang, H.; Li, X.; Kang, Y.; Zhou, N.; Zhang, W.; Zheng, K.; Pan, F. Forming novel texture and enhancing the formability in Mg–3Al–Zn alloy sheets fabricated by transverse gradient extrusion. J. Mater. Res. Technol. 2022, 18, 3143–3149. [Google Scholar] [CrossRef]
- Zhang, Z.; Huo, Q.; Zhang, Y.; Zhao, G.; Nagaumi, H.; Yang, X. Enhanced creep resistance in Mg-Y-Nd alloy via regulating prior thermo-mechanical treatment. J. Magnes. Alloy 2023, 12, 2766–2782. [Google Scholar] [CrossRef]
- Wang, L.; Liu, D.-R.; Chen, T.; Wang, S.; Cao, Y. Grain refinement and high thermal stability in laser surface remelted Mg-4Y-3Nd-1.5Al alloy. Scr. Mater. 2023, 222, 115000. [Google Scholar] [CrossRef]
- Wei, K.; Xiao, L.; Gao, B.; Mao, Q.; Liu, Y.; Li, L.; Sun, W.; Sui, Y.; Zhou, H.; Zhao, Y. Effect of aging temperature on the hardening behavior and precipitation evolution of Mg-10Gd alloy. Mater. Charact. 2023, 196, 112580. [Google Scholar] [CrossRef]
- Li, Y.; Wang, J.; Ma, H.; Zhen, Z.; Liu, Y.; Zhang, H.; Jin, P. An investigation on the precipitates in T5 treated high vacuum die-casting AE44-2 magnesium alloy. Mater. Res. Express 2022, 9, 020005. [Google Scholar] [CrossRef]
- Fan, Y.; Xiang, Y.; Shen, H.-S. Temperature-Dependent Mechanical Properties of Graphene/Cu Nanocomposites with In-Plane Negative Poisson’s Ratios. Research 2020, 2020, 5618021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xie, H.; Ma, Z.; Zhao, H.; Ren, L. Graphene Oxide-Induced Substantial Strengthening of High-Entropy Alloy Revealed by Micropillar Compression and Molecular Dynamics Simulation. Research 2022, 2022, 9839403. [Google Scholar] [CrossRef]
- Jin, X.; Xu, W.; Yang, Z.; Yuan, C.; Shan, D.; Teng, B.; Jin, B.C. Analysis of abnormal texture formation and strengthening mechanism in an extruded Mg-Gd-Y-Zn-Zr alloy. J. Mater. Sci. Technol. 2020, 45, 133–145. [Google Scholar] [CrossRef]
- Jin, Z.-Z.; Zha, M.; Wang, S.-Q.; Wang, S.-C.; Wang, C.; Jia, H.-L.; Wang, H.-Y. Alloying design and microstructural control strategies towards developing Mg alloys with enhanced ductility. J. Magnes. Alloy 2022, 10, 1191–1206. [Google Scholar] [CrossRef]
- Sha, G.; Li, J.H.; Xu, W.; Xia, K.; Jie, W.Q.; Ringer, S.P. Hardening and microstructural reactions in high-temperature equal-channel angular pressed Mg–Nd–Gd–Zn–Zr alloy. Mater. Sci. Eng. A 2010, 527, 5092–5099. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, A.; Li, C.; Xie, H.; Jiang, B.; Dong, Z.; Jin, P.; Pan, F. Recent advances of high strength Mg-RE alloys: Alloy development, forming and application. J. Mater. Res. Technol. 2023, 26, 2919–2940. [Google Scholar] [CrossRef]
- Hu, G.-s.; Zhang, D.-f.; Zhao, D.-z.; Shen, X.; Jiang, L.-y.; Pan, F.-s. Microstructures and mechanical properties of extruded and aged Mg–Zn–Mn–Sn–Y alloys. Trans. Nonferrous Met. Soc. China 2014, 24, 3070–3075. [Google Scholar] [CrossRef]
- Zhou, H.; Hou, R.; Yang, J.; Sheng, Y.; Li, Z.; Chen, L.; Li, W.; Wang, X. Influence of Zirconium (Zr) on the microstructure, mechanical properties and corrosion behavior of biodegradable zinc-magnesium alloys. J. Alloys Compd. 2020, 840, 155792. [Google Scholar] [CrossRef]
- Wei, X.; Jin, L.; Wang, F.; Li, J.; Ye, N.; Zhang, Z.; Dong, J. High strength and ductility Mg-8Gd-3Y-0.5Zr alloy with bimodal structure and nano-precipitates. J. Mater. Sci. Technol. 2020, 44, 19–23. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Gu, J.; Zhao, Y.; Tong, Y.; Kaneda, J. Effects of heat treatments on microstructure and mechanical properties of Mg-15Gd-5Y-0.5Zr alloy. J. Rare Earths 2008, 26, 298–302. [Google Scholar] [CrossRef]
- Jiang, R.; Qian, S.; Dong, C.; Qin, Y.; Wu, Y.; Zou, J.; Zeng, X. Composition optimization of high-strength Mg-Gd-Y-Zr alloys based on the structural unit of Mg-Gd solid solution. J. Mater. Sci. Technol. 2021, 72, 104–113. [Google Scholar] [CrossRef]
- Li, J.-L.; Zhang, N.; Wang, X.-X.; Wu, D.; Chen, R.-S. Effect of Solution Treatment on the Microstructure and Mechanical Properties of Sand-Cast Mg–9Gd–4Y–0.5Zr Alloy. Acta Metall. Sin. (Engl. Lett.) 2018, 31, 189–198. [Google Scholar] [CrossRef]
- Zhang, Z.; Peng, L.; Zeng, X.; Fu, P.; Ding, W. Characterization of phases in a Mg–6Gd–4Sm–0.4Zr (wt.%) alloy during solution treatment. Mater. Charact. 2009, 60, 555–559. [Google Scholar] [CrossRef]
- Deng, Q.; Chang, Z.; Su, N.; Luo, J.; Liang, Y.; Jin, Y.; Wu, Y.; Peng, L.; Ding, W. Developing a novel high-strength Mg-Gd-Y-Zn-Mn alloy for laser powder bed fusion additive manufacturing process. J. Magnes. Alloy, 2023; in press. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, Y.; Zhu, W.; Chen, K.; Liu, D.; Peng, L.; Ding, W. Effect of heat treatment on microstructure evolution and mechanical properties of selective laser melted Mg-11Gd-2Zn-0.4Zr alloy. Mater. Sci. Eng. A 2022, 829, 142139. [Google Scholar] [CrossRef]
- Qian, X.; Dong, Z.; Jiang, B.; Lei, B.; Yang, H.; He, C.; Liu, L.; Wang, C.; Yuan, M.; Yang, H.; et al. Influence of alloying element segregation at grain boundary on the microstructure and mechanical properties of Mg-Zn alloy. Mater. Des. 2022, 224, 111322. [Google Scholar] [CrossRef]
- Vo, N.Q.; Schäfer, J.; Averback, R.S.; Albe, K.; Ashkenazy, Y.; Bellon, P. Reaching theoretical strengths in nanocrystalline Cu by grain boundary doping. Scr. Mater. 2011, 65, 660–663. [Google Scholar] [CrossRef]
- Chang, K.I.; Hong, S.I. Effect of sulphur on the strengthening of a Zr–Nb alloy. J. Nucl. Mater. 2008, 373, 16–21. [Google Scholar] [CrossRef]
- Tao, J.Q.; Huang, S.H.; Zhao, G.Z.; Huang, Z.W.; Wu, Y.; Wan, Y.Y.; Li, M. Effect of Zn on the Mechanical Properties and Microstructure of as-Cast and Solution-Treated Mg–6Y–2Nd–1Gd–0.5Zr Alloys. Strength. Mater. 2019, 51, 609–615. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, K.; Li, H.; Zhen, Y.; Han, F.; Chen, X.; Jiang, H.; Wang, Q.; Ding, W. Effects of novel Mg-RE master alloys prepared via ultrasonic assisted cast on the microstructure and properties of Mg-Gd-Y alloy. Mater. Sci. Eng. A 2024, 895, 146222. [Google Scholar] [CrossRef]
- Deng, Q.; Wu, Y.; Su, N.; Chang, Z.; Chen, J.; Peng, L.; Ding, W. Influence of friction stir processing and aging heat treatment on microstructure and mechanical properties of selective laser melted Mg-Gd-Zr alloy. Addit. Manuf. 2021, 44, 102036. [Google Scholar] [CrossRef]
- Gao, L.; Chen, R.S.; Han, E.H. Microstructure and strengthening mechanisms of a cast Mg–1.48Gd–1.13Y–0.16Zr (at.%) alloy. J. Mater. Sci. 2009, 44, 4443–4454. [Google Scholar] [CrossRef]
- Gao, L.; Chen, R.-s.; Han, E.-h. Fracture behavior of high strength Mg-Gd-Y-Zr magnesium alloy. Trans. Nonferrous Met. Soc. China 2010, 20, 1217–1221. [Google Scholar] [CrossRef]
- He, S.M.; Zeng, X.Q.; Peng, L.M.; Gao, X.; Nie, J.F.; Ding, W.J. Microstructure and strengthening mechanism of high strength Mg–10Gd–2Y–0.5Zr alloy. J. Alloys Compd. 2007, 427, 316–323. [Google Scholar] [CrossRef]
- Hyer, H.; Zhou, L.; Benson, G.; McWilliams, B.; Cho, K.; Sohn, Y. Additive manufacturing of dense WE43 Mg alloy by laser powder bed fusion. Addit. Manuf. 2020, 33, 101123. [Google Scholar] [CrossRef]
- Wang, C.; Wu, G.; Lavernia, E.J.; Ding, W. Influences of heat treatment on microstructural evolution and tensile behavior of squeeze-cast Mg–Gd–Y–Zr alloy. J. Mater. Sci. 2017, 52, 1831–1846. [Google Scholar] [CrossRef]
- Guo, Y.; Li, J.; Li, J.; Yang, Z.; Zhao, J.; Xia, F.; Liang, M. Mg–Gd–Y system phase diagram calculation and experimental clarification. J. Alloys Compd. 2008, 450, 446–451. [Google Scholar] [CrossRef]
- Bai, Y.; Ye, B.; Guo, J.; Wang, L.; Kong, X.; Ding, W. Mechanical properties and yield asymmetry of Mg-Y-Zn alloys: Competitive behavior of second phases. Mater. Charact. 2020, 164, 110301. [Google Scholar] [CrossRef]
- Lei, B.; Wang, C.; Jiang, B.; Bai, S.; Dong, Z.; Qian, X.; He, C.; Xu, J.; Yang, H.; Wang, Q.; et al. Role of Y on the microstructure and mechanical properties of Mg-Gd-Zr alloy. Mater. Sci. Eng., A 2022, 861, 144371. [Google Scholar] [CrossRef]
- Wang, C.; Dong, Z.; Jiang, B.; Zheng, Z.; Wu, S.; Song, J.; Zhang, A.; Xu, J.; Yang, B.; Zheng, C.; et al. Lowering thermal expansion of Mg with the enhanced strength by Ca alloying. J. Mater. Res. Technol. 2023, 24, 1293–1303. [Google Scholar] [CrossRef]
- Gao, L.; Chen, R.S.; Han, E.H. Effects of rare-earth elements Gd and Y on the solid solution strengthening of Mg alloys. J. Alloys Compd. 2009, 481, 379–384. [Google Scholar] [CrossRef]
- Sun, W.T.; Qiao, X.G.; Zheng, M.Y.; Xu, C.; Gao, N.; Starink, M.J. Microstructure and mechanical properties of a nanostructured Mg-8.2Gd-3.8Y-1.0Zn-0.4Zr supersaturated solid solution prepared by high pressure torsion. Mater. Des. 2017, 135, 366–376. [Google Scholar] [CrossRef]
- Ning, J.; Gao, B.; Yuan, X.; Zhou, J.; Tang, G.; Chen, L. Strength and ductility improvement in a heterostructured Mg-Gd-Y alloy with inversely-gradient hardness distribution. J. Mater. Res. Technol. 2024, 28, 3781–3793. [Google Scholar] [CrossRef]
- Yu, Z.; Xu, X.; Shi, K.; Du, B.; Han, X.; Xiao, T.; Li, S.; Liu, K.; Du, W. Development and characteristics of a low rare-earth containing magnesium alloy with high strength-ductility synergy. J. Magnes. Alloy 2023, 11, 1629–1642. [Google Scholar] [CrossRef]






| Alloys | Gd | Y | Zr | Mg |
|---|---|---|---|---|
| Mg-9Gd-0.5Zr | 8.99 | - | 0.51 | Bal. |
| Mg-9Gd-1Y-0.5Zr | 8.97 | 1.03 | 0.49 | Bal. |
| Mg-9Gd-3Y-0.5Zr | 8.96 | 2.99 | 0.48 | Bal. |
| Mg-9Gd-5Y-0.5Zr | 9.04 | 5.03 | 0.51 | Bal. |
| Points | Mg | Gd | Y | Zr |
|---|---|---|---|---|
| A | 75.3 | 9.0 | - | 15.7 |
| B | 98.2 | 1.4 | 0.3 | 0.1 |
| C | 94.0 | 3.9 | 1.5 | 0.6 |
| D | 36.3 | 23.1 | 40.5 | 0.1 |
| Experimental Value (MPa) | Calculated Value (MPa) | |||
|---|---|---|---|---|
| 23.6 | 29.5 | 3.3 | 24.4 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zhang, A.; Li, C.; Xie, H.; Hu, H.; Gao, Y.; Cui, Y.; Dong, Z.; Li, T.; Jiang, B. Insight into Y Content on Microstructure and Mechanical Properties of Mg-Gd-Y-Zr Alloy. Materials 2025, 18, 2475. https://doi.org/10.3390/ma18112475
Li Y, Zhang A, Li C, Xie H, Hu H, Gao Y, Cui Y, Dong Z, Li T, Jiang B. Insight into Y Content on Microstructure and Mechanical Properties of Mg-Gd-Y-Zr Alloy. Materials. 2025; 18(11):2475. https://doi.org/10.3390/ma18112475
Chicago/Turabian StyleLi, Yongfeng, Ang Zhang, Chuangming Li, Hecong Xie, Hengrui Hu, Yuyang Gao, Yuhong Cui, Zhihua Dong, Tian Li, and Bin Jiang. 2025. "Insight into Y Content on Microstructure and Mechanical Properties of Mg-Gd-Y-Zr Alloy" Materials 18, no. 11: 2475. https://doi.org/10.3390/ma18112475
APA StyleLi, Y., Zhang, A., Li, C., Xie, H., Hu, H., Gao, Y., Cui, Y., Dong, Z., Li, T., & Jiang, B. (2025). Insight into Y Content on Microstructure and Mechanical Properties of Mg-Gd-Y-Zr Alloy. Materials, 18(11), 2475. https://doi.org/10.3390/ma18112475






