Unveiling the Influence and Mechanisms of Enhancing Ferrite-Phase Composition on the Properties of Calcium Sulfoaluminate Cement
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Clinker Design Methodology
- (a)
- Target Composition Design
- (b) Stoichiometric Batching Calculation
- (c) Raw Meal Proportioning
- (d) Theoretical Clinker Yield and Basicity Coefficient Validation
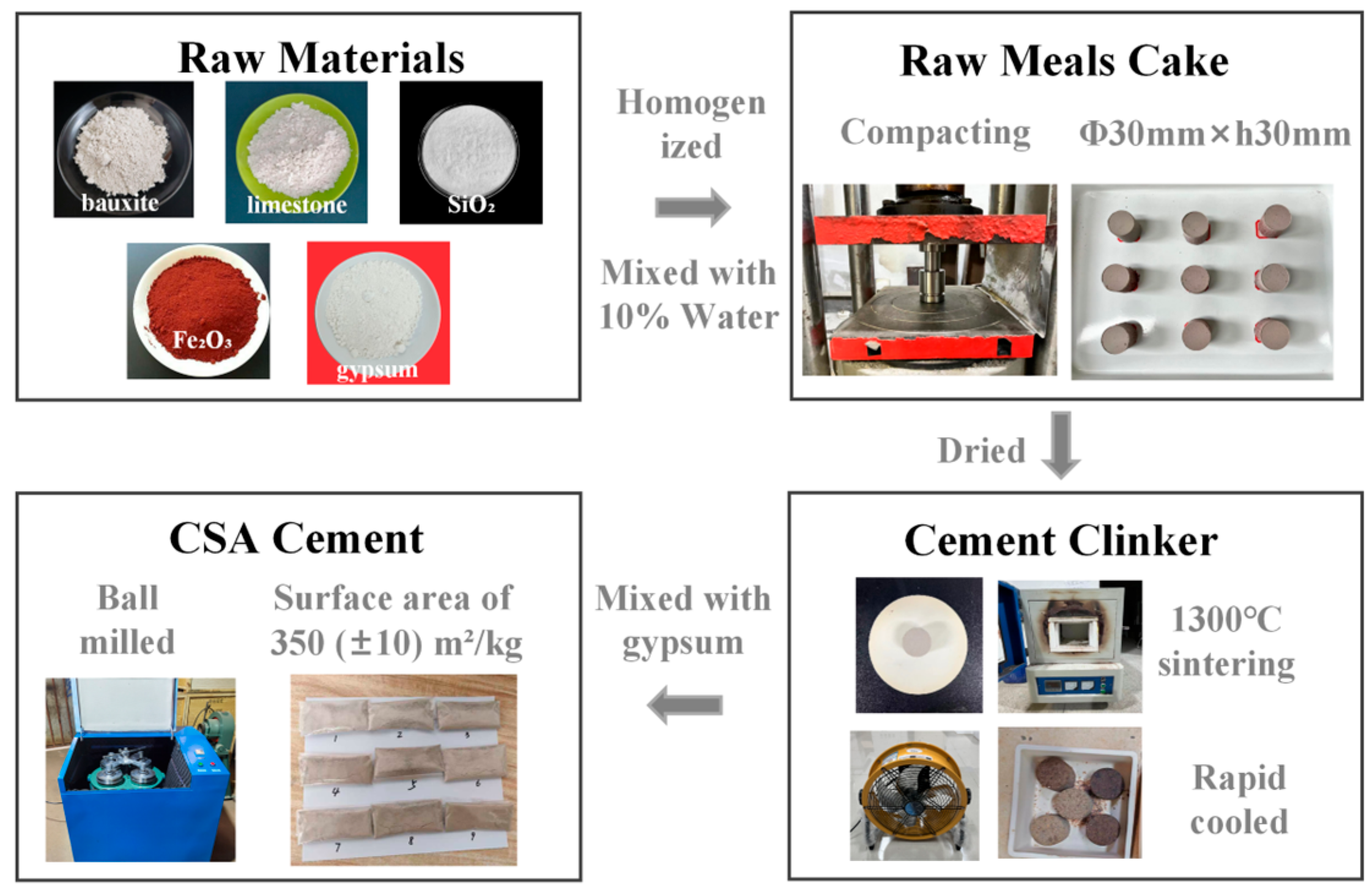
2.3. CSA Cement Production
2.4. Cement Hydration Heat Testing
2.5. Hardened Cement Performance Testing
- (a)
- Compressive Strength
- (b) X-Ray Diffraction (XRD)
- (c) Mercury Intrusion Porosimetry (MIP)
- (d) Scanning Electron Microscopy (SEM)
3. Results and Discussion
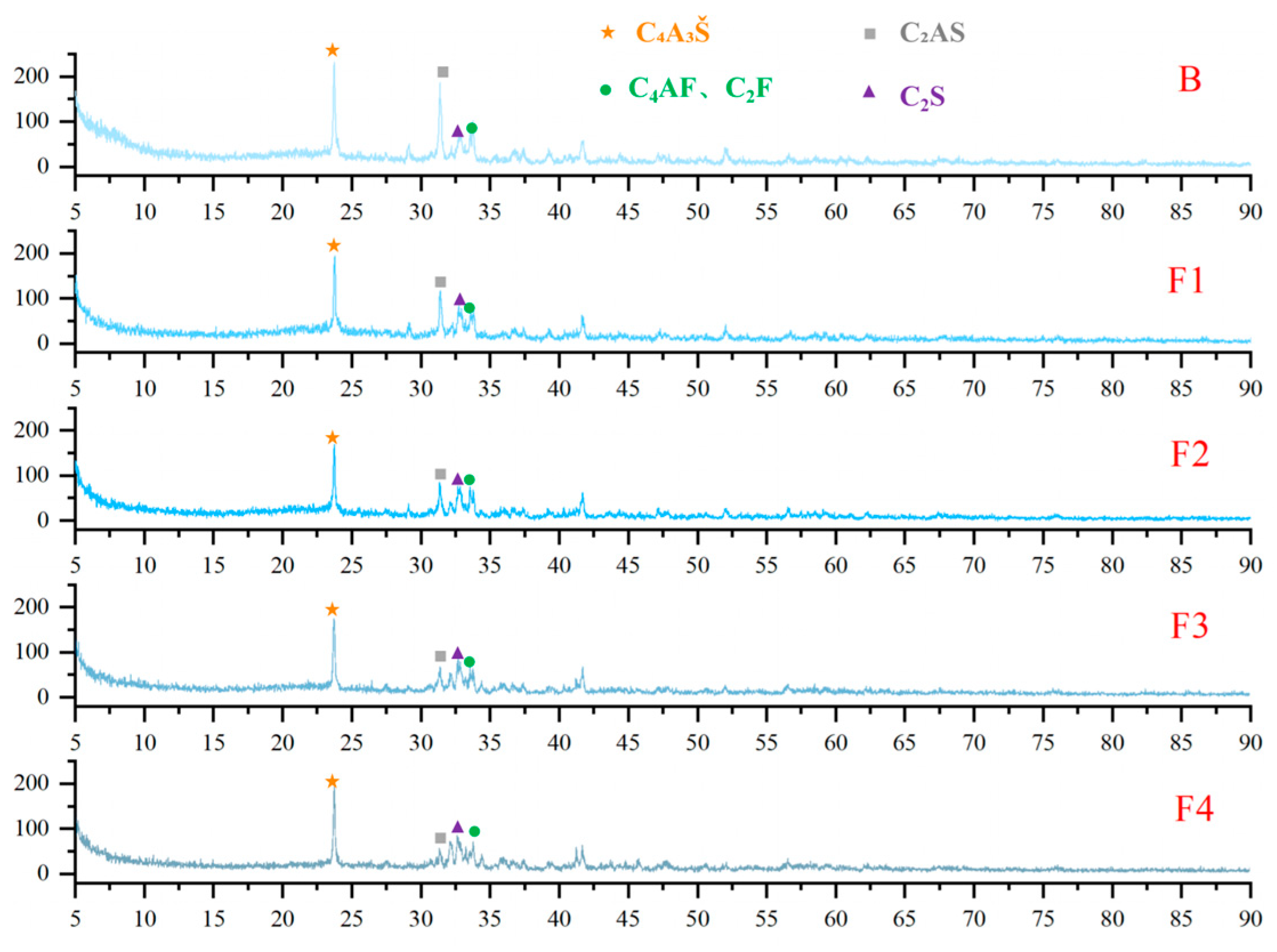
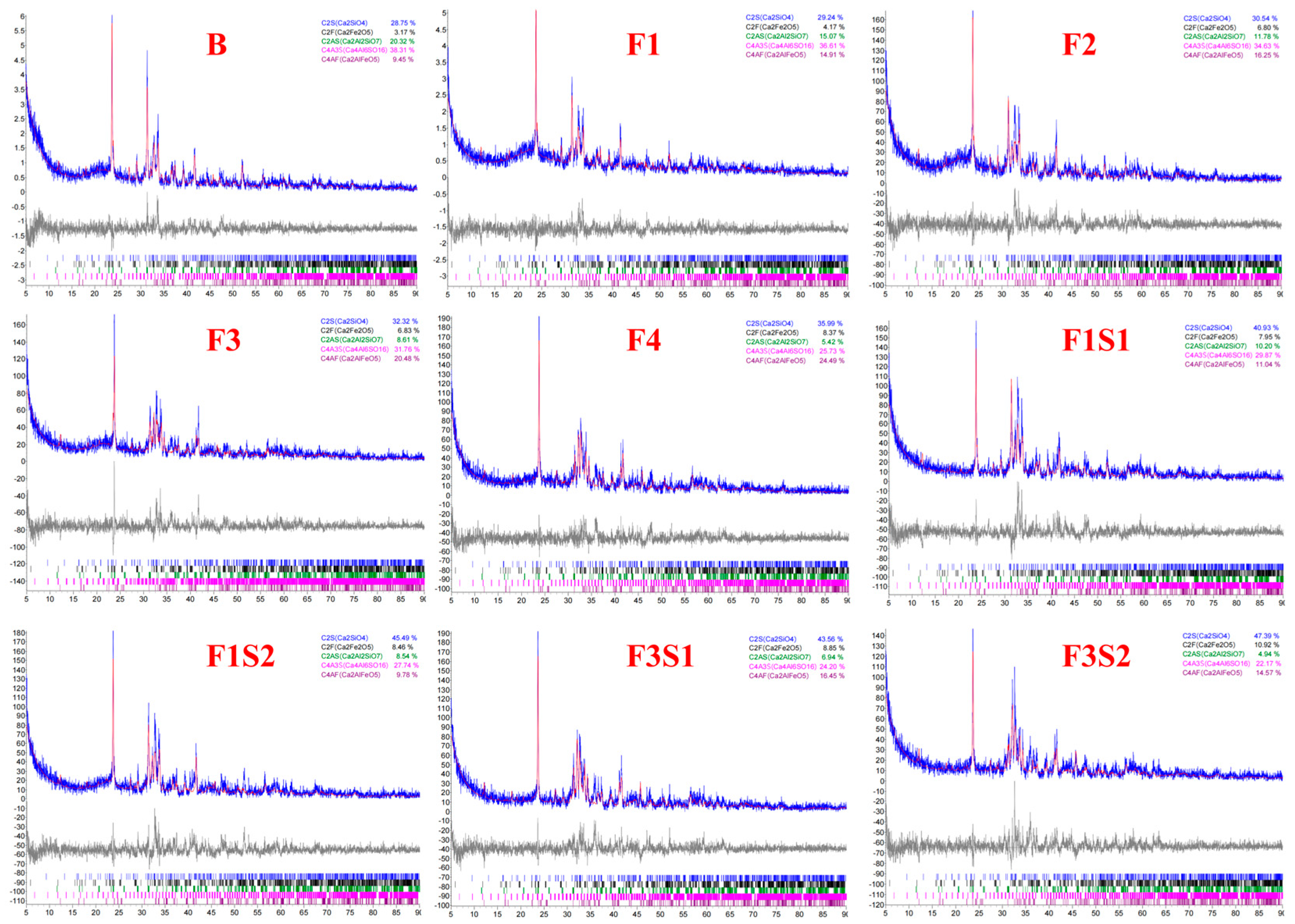
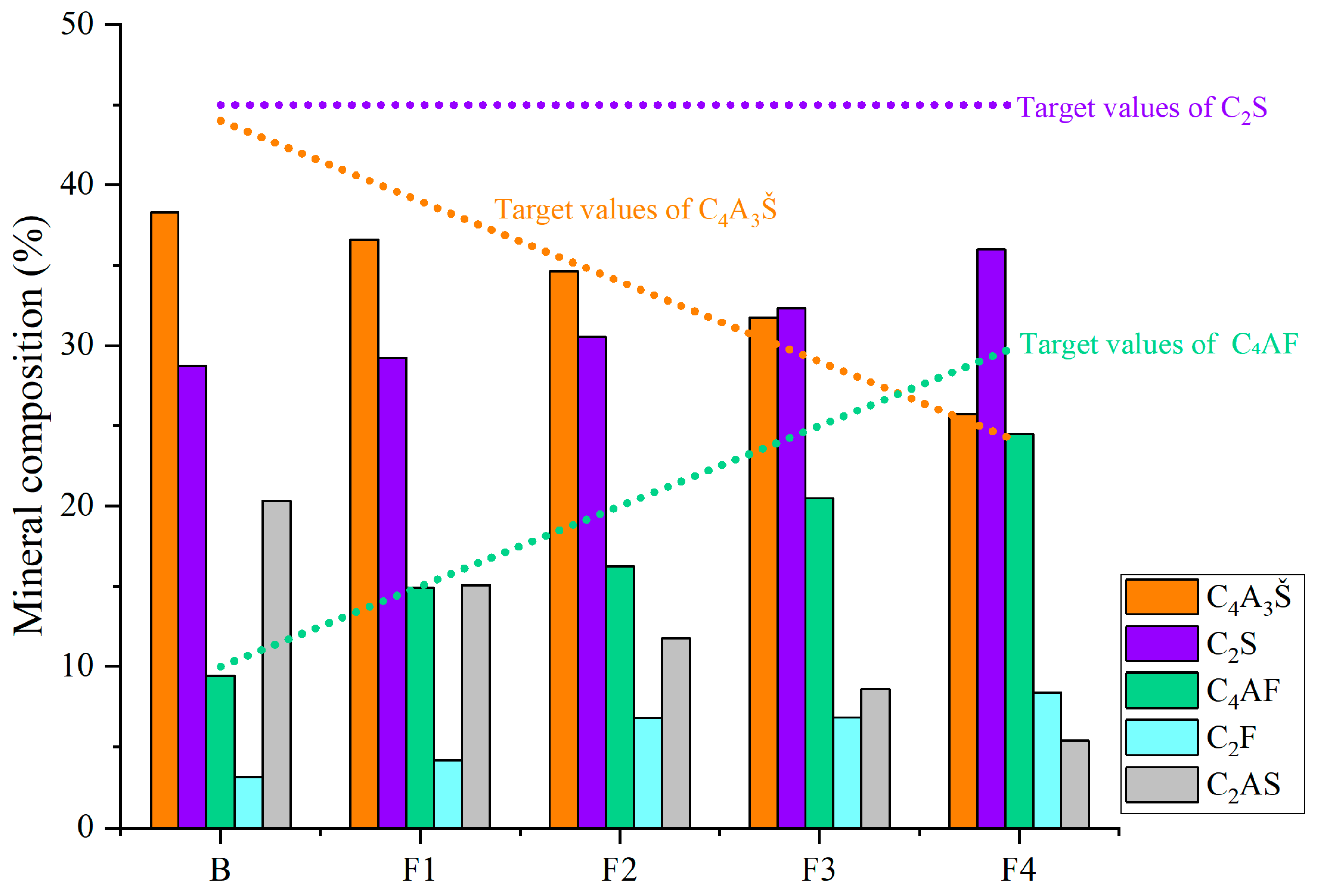
3.1. Clinker Composition
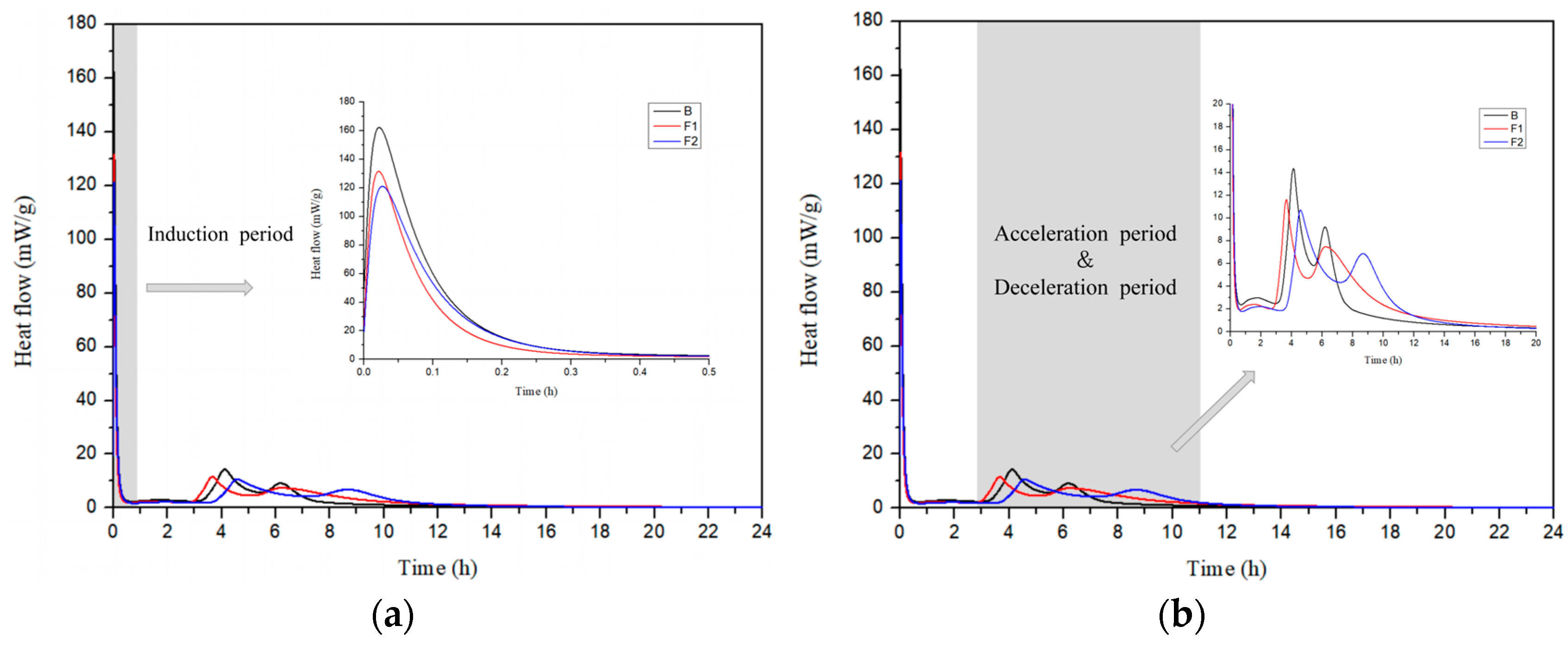
3.2. Hydration Characteristics
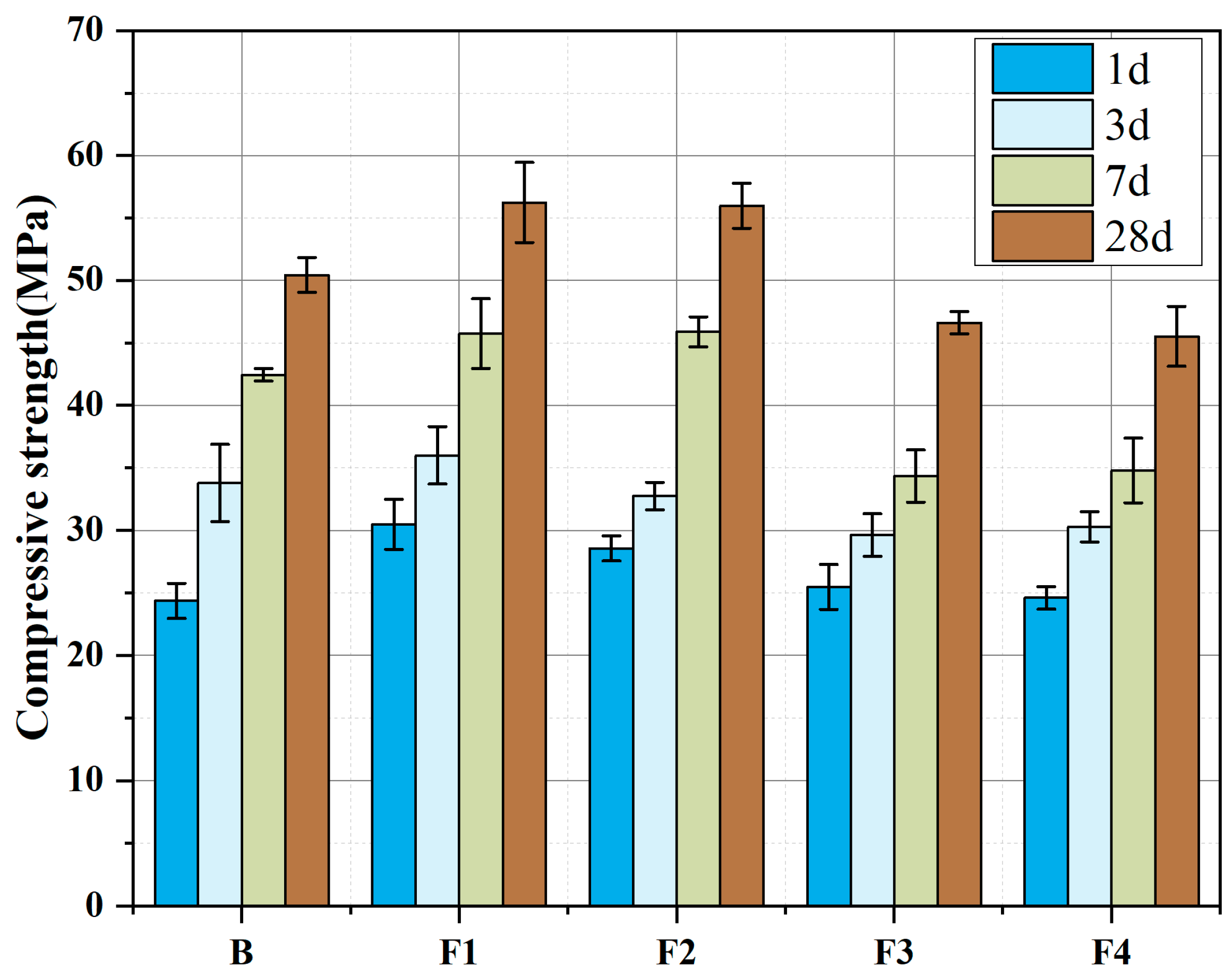
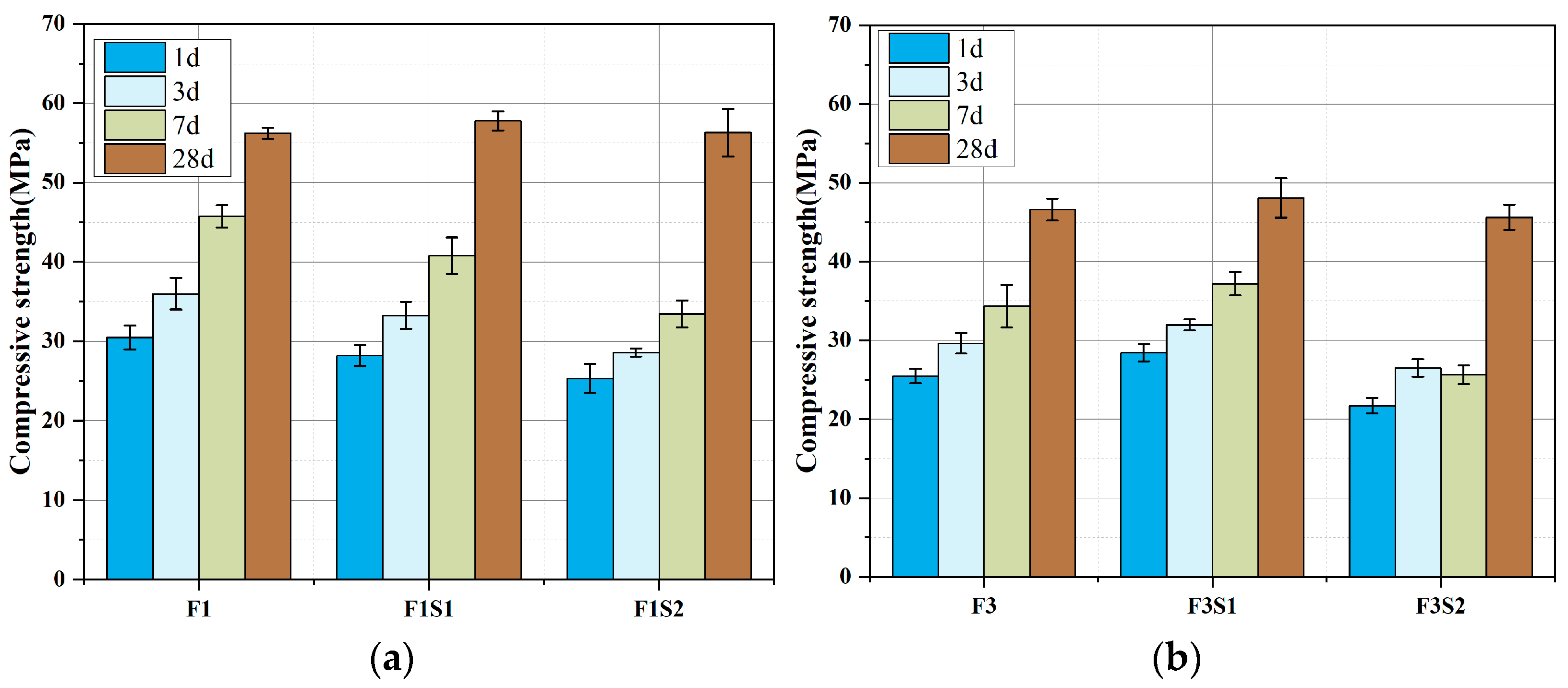
3.3. Cement Strength Development
3.4. Microstructural Analysis
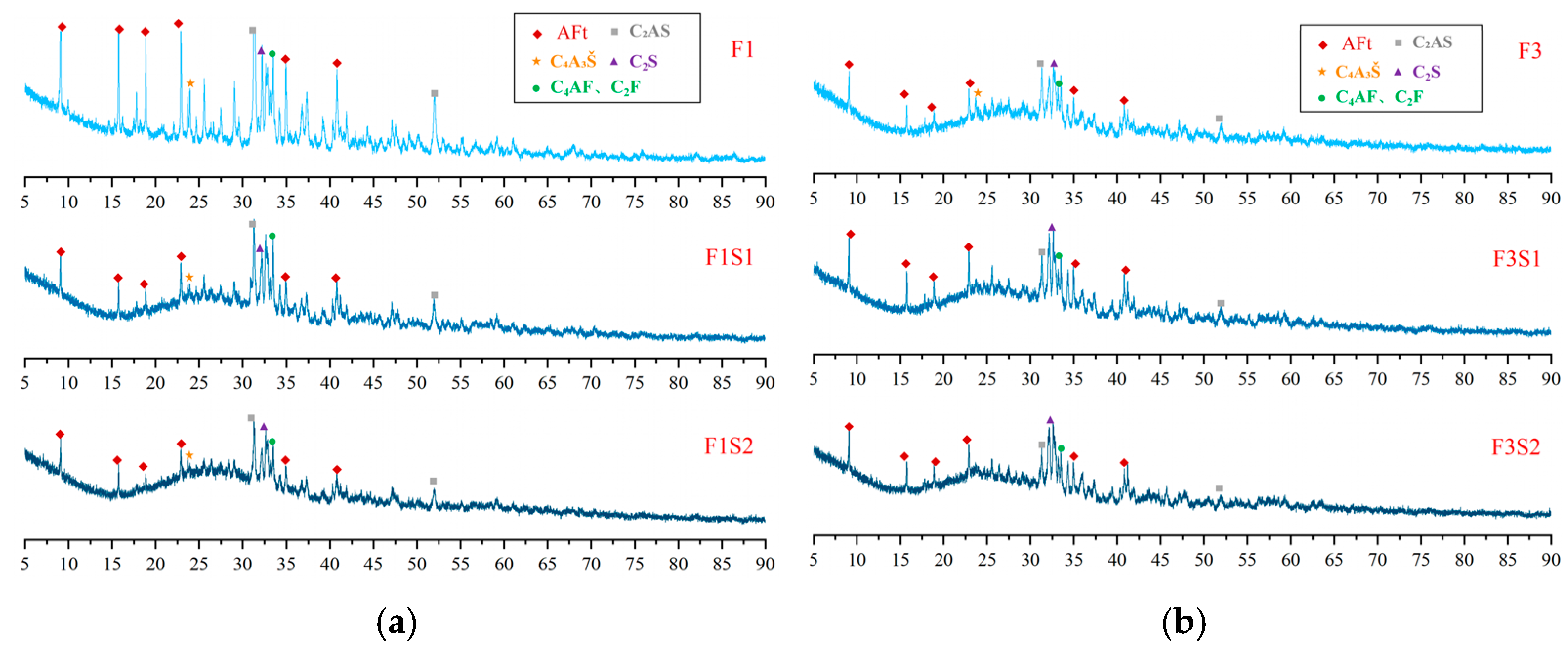
3.4.1. Hydration Product Composition
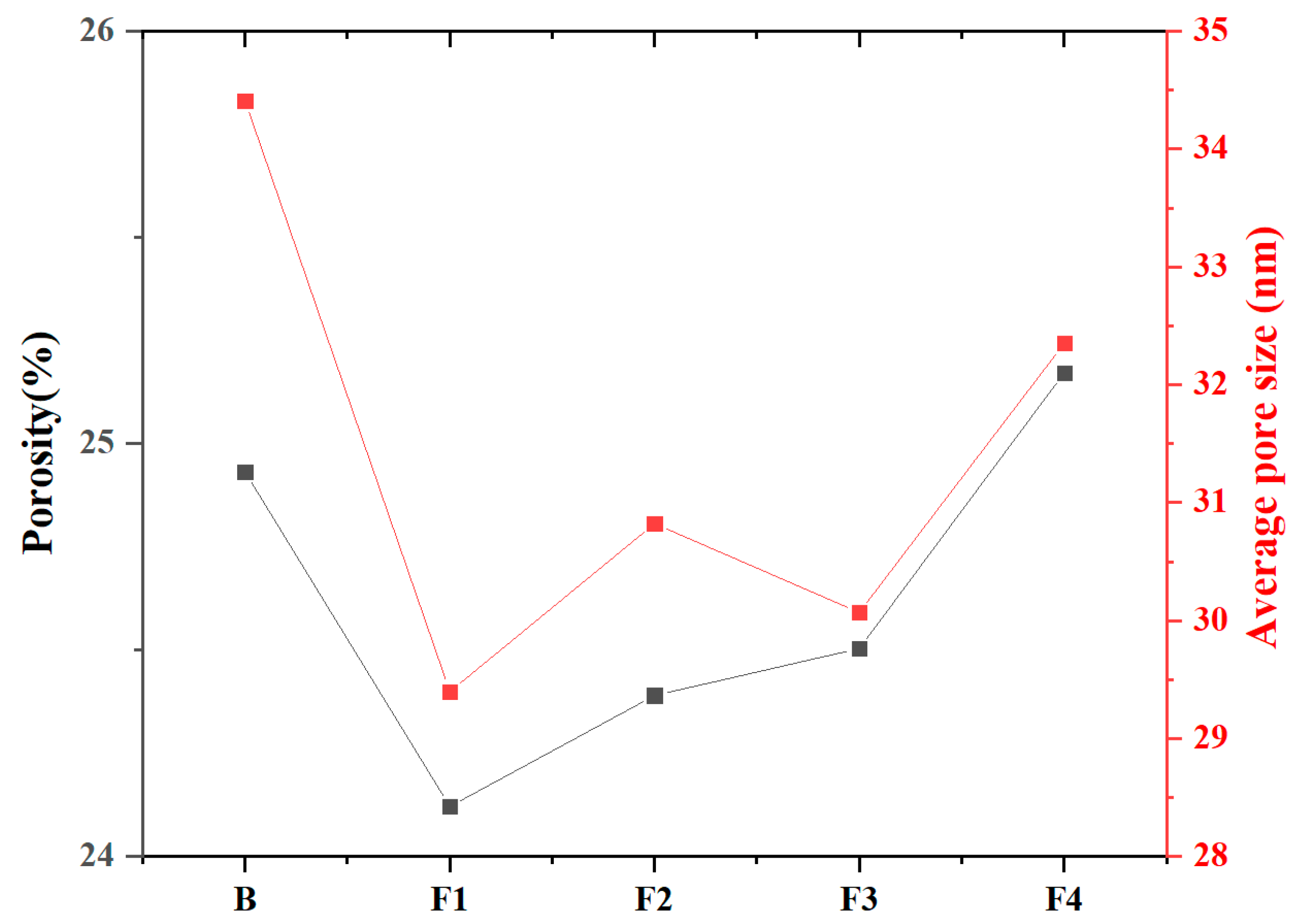
3.4.2. Microporous Structure
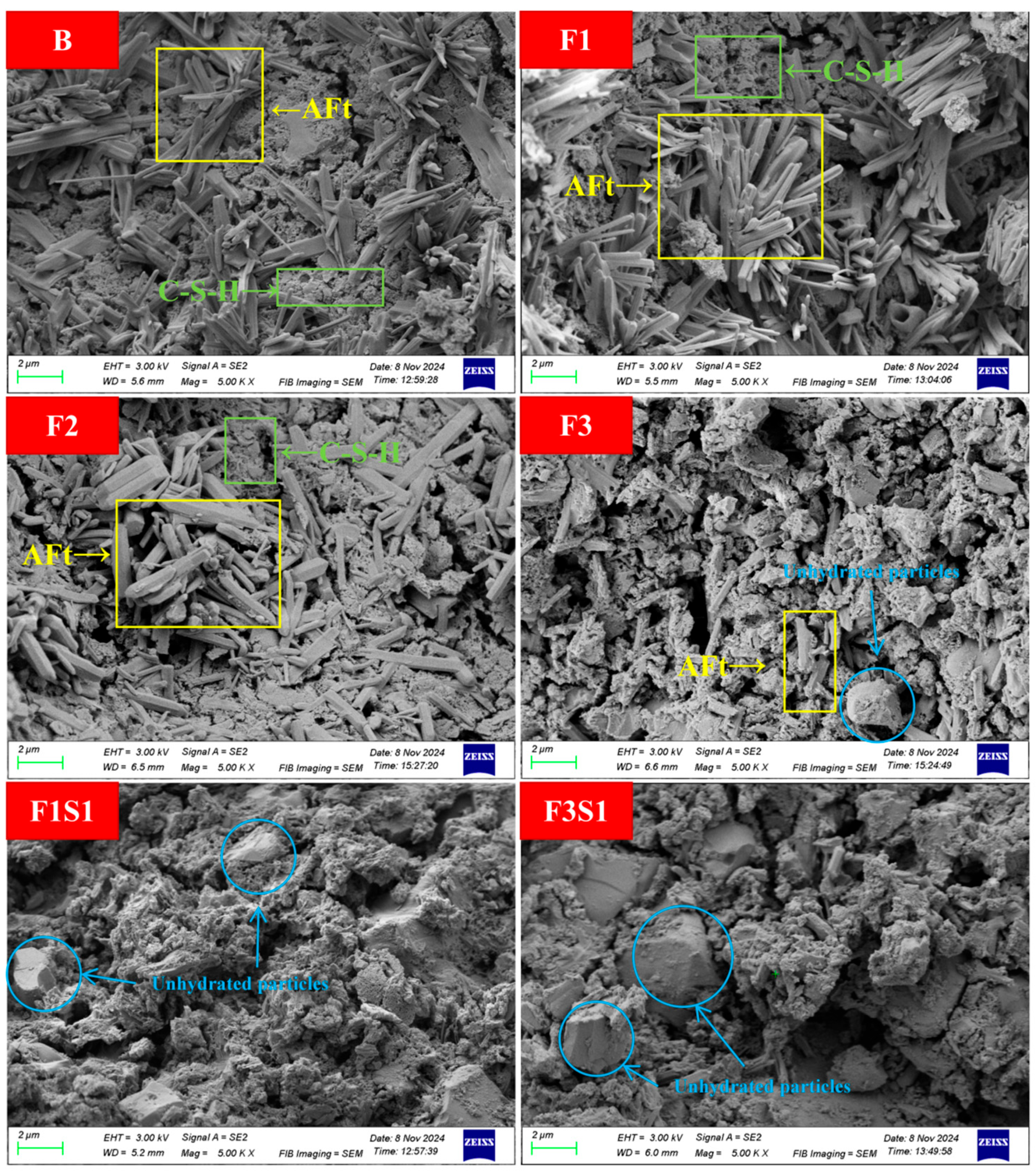
3.4.3. Microstructural Morphology
4. Conclusions
- The ferrite phase plays a critical role in regulating the mineralogical evolution of CSA clinker. Higher ferrite-phase content helps C4A3Š and C2S align more closely with their corresponding target design values, demonstrating that the ferrite phase promotes their stabilization during clinkering while reducing low-activity transitional products like C2AS.
- The hydration characteristics of CSA cements are modulated by ferrite-phase content as evidenced by three distinct heat release peaks. Increasing ferrite phase suppresses the initial dissolution peak and prolongs the secondary reaction stage (4–6 h) by reducing AFt formation rates as ferrite phases partially replace sulfoaluminate components.
- Moderate ferrite-phase content (15–20%) optimizes the strength development of calcium sulfoaluminate (CSA) cement: Early-stage strength (1–3 days) improves due to the ferrite phase (C4AF) promoting rapid nucleation of ettringite (AFt), while later-stage strength (28 days) benefits from pore structure refinement through secondary reaction products of the ferrite phase, achieving a balance between early hydration activity and long-term microstructural optimization.
- Excessive ferrite content (>25%) causes adverse effects in the strength development of CSA: During clinkering, an elevated Fe2O3/Al2O3 ratio reduces C4A3Š formation and limits early AFt production, while increased proportions of low-reactivity C2F further degrade performance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Habert, G.; Miller, S.A.; John, V.M.; Provis, J.L.; Favier, A.; Horvath, A.; Scrivener, K.L. Environmental impacts and decarbonization strategies in the cement and concrete industries. Nat. Rev. Earth Environ. 2020, 1, 559–573. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in alternative cementitious binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M. Gartner Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–Belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Péra, J.; Ambroise, J. New applications of calcium sulfoaluminate cement. Cem. Concr. Res. 2004, 34, 671–676. [Google Scholar] [CrossRef]
- Hargis, C.W.; Telesca, A.; Monteiro, P.J. Calcium sulfoaluminate (Ye’elimite) hydration in the presence of gypsum, calcite, and vaterite. Cem. Concr. Res. 2014, 65, 15–20. [Google Scholar] [CrossRef]
- Canbek, O.; Erdoğan, S.T. Influence of production parameters on calcium sulfoaluminate cements. Constr. Build. Mater. 2020, 239, 117866. [Google Scholar] [CrossRef]
- Tang, X.; Zhan, S.; Xu, Q.; He, K. Mechanical performance and chloride penetration of calcium sulfoaluminate concrete in marine tidal zone. Materials 2023, 16, 2905. [Google Scholar] [CrossRef]
- Wang, L.; Zhou, H.; Lian, S.; Tang, X. Drying–Wetting Correlation Analysis of Chloride Transport Behavior and Mechanism in Calcium Sulphoaluminate Cement Concrete. Materials 2024, 17, 4600. [Google Scholar] [CrossRef]
- Tan, B.; Okoronkwo, M.U.; Kumar, A.; Ma, H. Durability of calcium sulfoaluminate cement concrete. J. Zhejiang Univ. Sci. A 2020, 21, 118–128. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, D.; Ma, G.; Ji, X.; Zhou, W. Early Hydration of Calcium Sulfoaluminate Cement at Elevated Temperatures. ACS Sustain. Chem. 2024, 12, 13654–13668. [Google Scholar] [CrossRef]
- Andac, Ö.; Glasser, F.P. Microstructure and microchemistry of calcium sulfoaluminate cement. MRS Online Proc. Libr. (OPL) 1994, 370, 143. [Google Scholar] [CrossRef]
- Park, S.; Jeong, Y.; Moon, J.; Lee, N. Hydration characteristics of calcium sulfoaluminate (CSA) cement/portland cement blended pastes. J. Build. Eng. 2021, 34, 101880. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Sánchez-Herrero, M.J.; Fernández-Jiménez, A.; Palomo, A. C4A3Š hydration in different alkaline media. Cem. Concr. Res. 2013, 46, 41–49. [Google Scholar] [CrossRef]
- Telesca, A.; Marroccoli, M.; Pace, M.L.; Tomasulo, M.; Valenti, G.L.; Monteiro, P.J.M. A hydration study of various calcium sulfoaluminate cements. Cem. Concr. Compos. 2014, 53, 224–232. [Google Scholar] [CrossRef]
- Ye, S.; Feng, P.; Liu, J. Dissolution and early hydration of tetracalcium aluminoferrite (C4AF) in water and in aqueous sulfate solutions. Cem. Concr. Res. 2024, 186, 107676. [Google Scholar] [CrossRef]
- Mrak, M.; Winnefeld, F.; Lothenbach, B.; Dolenec, S. The influence of calcium sulfate content on the hydration of belite-calcium sulfoaluminate cements with different clinker phase compositions. Mater. Struct. 2021, 54, 1–17. [Google Scholar] [CrossRef]
- Seo, J.; Nawaz, A.; Jang, J.G.; Lee, H.K. Modifications in hydration kinetics and characteristics of calcium aluminate cement upon blending with calcium sulfoaluminate cement. Constr. Build. Mater. 2022, 342, 127958. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhao, J.; Cui, L. Exploration of hydration and durability properties of ferroaluminate cement with compare to Portland cement. Constr. Build. Mater. 2022, 319, 126138. [Google Scholar] [CrossRef]
- Shao, Y.; Lu, X.; Li, Q.; Dong, Y.; Zhang, L.; Jiang, C.; Cheng, X. Study on the preparation and sulfate resistance of Portland cement clinker with the high Fe/Al ratio of ferrite phase. Cem. Concr. Compos. 2022, 134, 104699. [Google Scholar] [CrossRef]
- Zhang, P.; Qi, D.; Hao, L.; Wang, Z.; Liu, H.; Zhang, D.; Xie, Y.; Zhao, E. Effect of w/b ratio and supplemental cementitious material on the chloride penetration and corrosion resistance of ferroaluminate cement concrete. Constr. Build. Mater. 2024, 431, 136481. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, J.; Li, Z. Investigations on the hydration characteristics of blended ferroaluminate cement containing granulated blast furnace slag. Constr. Build. Mater. 2024, 417, 135243. [Google Scholar] [CrossRef]
- Xue, J.; Li, S.; Zhang, Z.; Liu, S.; Li, G.; Guan, X. Hydration, mechanical properties, and corrosion resistance of ferroaluminate cement in the presence of FA and GGBS. J. Build. Eng. 2025, 102, 111974. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.L.; Feng, J.R.; Zheng, G.F.; Yang, Q.C.; Du, P.; Zhu, J.; Cheng, X. Calcium ferroaluminate cements made with red mud: Synthetic issues and mechanical properties. Ceram. Silikáty 2024, 68, 195–204. [Google Scholar] [CrossRef]
- Shi, N.; Ma, Y.; Zhang, X.; Li, J.; Lu, X.; Zhang, L.; Cheng, X. The Effect of Calcium Sulfate on the Hydration and Properties of Red Mud-Based Calcium Ferroaluminate Cement Clinker. Materials 2024, 17, 5064. [Google Scholar] [CrossRef]
- Iacobescu, R.I.; Pontikes, Y.; Koumpouri, D.; Angelopoulos, G.N. Synthesis, characterization and properties of calcium ferroaluminate belite cements produced with electric arc furnace steel slag as raw material. Cem. Concr. Compos. 2013, 44, 1–8. [Google Scholar] [CrossRef]
- Wu, S.; Yao, X.; Ren, C.; Yao, Y.; Zhang, C.; Wu, C.; Wang, W. Effect of iron on the preparation of iron-rich calcium sulfoatablluminate cement using gypsum as the sole calcium oxide source and its incorporation into mineral phases. Constr. Build. Mater. 2021, 290, 123214. [Google Scholar] [CrossRef]
- Yao, X.; Yang, S.; Huang, Y.; Wu, S.; Yao, Y.; Wang, W. Effect of CaSO4 batching in raw material on the iron-bearing mineral transition of ferric-rich sulfoaluminate cement. Constr. Build. Mater. 2020, 250, 118783. [Google Scholar] [CrossRef]
- Zheng, M.; Huang, J.; Yang, M.; Song, B.; Yang, L.; Wang, F. A composite low-carbon cement strategy: High ferrite cement with addition of ferroaluminate cement. Constr. Build. Mater. 2024, 456, 139250. [Google Scholar] [CrossRef]
- Liao, Y.; Cai, Z.; Deng, F.; Ye, J.; Wang, K.; Tang, S. Hydration behavior and thermodynamic modelling of ferroaluminate cement blended with steel slag. J. Build. Eng. 2024, 97, 110833. [Google Scholar] [CrossRef]
- Wu, S.; Cui, Y.; Yao, X.; Ren, C.; Yao, Y.; Wang, W. Preparation of iron-rich sulfoaluminate cement by regulating Fe-bearing minerals. Ceram. Int. 2025, in press. [CrossRef]
- Meng, F.; Lu, D.; Wang, G.; Wang, S.; Zhou, X.; Du, X. Experimental study on pore structures and mechanical properties of ferroaluminate cement under sulfate attack. J. Build. Eng. 2024, 97, 110905. [Google Scholar] [CrossRef]
- Bogue, R.H. Calculation of the compounds in Portland cement. Ind. Chem. Anal. Ed. 1929, 1, 192–197. [Google Scholar] [CrossRef]
- Chen, I.A.; Juenger, M.C. Incorporation of coal combustion residuals into calcium sulfoaluminate-belite cement clinkers. Cem. Concr. Compos. 2012, 34, 893–902. [Google Scholar] [CrossRef]
- ASTM C1702; Standard Test Method for Measurement of Heat of Hydration of Hydraulic Cementitious Materials Using Isothermal Conduction Calorimetry. ASTM: West Conshohocken, PA, USA, 2017.
- Abir, F.Z.; Mesnaoui, M.; Abouliatim, Y.; Nibou, L.; Labbilta, T.; El Hafiane, Y.; Smith, A. Effect of the addition of iron oxide on the microstructure of ye’elimite. Cem. Concr. Res. 2022, 151, 106625. [Google Scholar] [CrossRef]
- Ndzila, J.S.; Liu, S.; Jing, G.; Wang, S.; Ye, Z. The effect of Fe3+ ion substitution on the crystal structure of ye’elimite. Ceram. Silik 2020, 64, 18–28. [Google Scholar] [CrossRef]
- Irico, S.; Gastaldi, D.; Canonico, F.; Magnacca, G. Investigation of the microstructural evolution of calcium sulfoaluminate cements by thermoporometry. Cem. Concr. Res. 2013, 53, 239–247. [Google Scholar] [CrossRef]
- Tao, Y.; Wan, D.; Zhang, W.; Wang, F.; Hu, S. Intrinsic reactivity and dissolution characteristics of tetracalcium aluminoferrite. Cem. Concr. Res. 2021, 146, 106485. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, P.; Yang, L.; Rao, M.; Wang, F. Improvement of the hydration kinetics of high ferrite cement: Synergic effect of gypsum and C3S–C4AF systems. ACS Sustain. Chem. 2021, 9, 15127–15137. [Google Scholar] [CrossRef]
- Lu, X.; Ye, Z.; Wang, S.; Du, P.; Li, C.; Cheng, X. Study on the preparation and properties of belite-ye’elimite-alite cement. Constr. Build. Mater. 2018, 182, 399–405. [Google Scholar] [CrossRef]
- Bullerjahn, F.; Zajac, M.; Haha, M.B.; Scrivener, K.L. Factors influencing the hydration kinetics of ye’elimite; effect of mayenite. Cem. Concr. Res. 2019, 116, 113–119. [Google Scholar] [CrossRef]
- Winnefeld, F.; Martin, L.H.; Müller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Hargis, C.W.; Kirchheim, A.P.; Monteiro, P.J.; Gartner, E.M. Early age hydration of calcium sulfoaluminate (synthetic ye’elimite, C4A3S) in the presence of gypsum and varying amounts of calcium hydroxide. Cem. Concr. Res. 2013, 48, 105–115. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye’elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Cuesta, A.; Ichikawa, R.U.; Londono-Zuluaga, D.; De la Torre, A.G.; Santacruz, I.; Turrillas, X.; Aranda, M.A. Aluminum hydroxide gel characterization within a calcium aluminate cement paste by combined Pair Distribution Function and Rietveld analyses. Cem. Concr. Res. 2017, 96, 1–12. [Google Scholar] [CrossRef]
- Tang, X.; Xu, Q.; Qian, K.; Ruan, S.; Lian, S.; Zhan, S. Effects of cyclic seawater exposure on the mechanical performance and chloride penetration of calcium sulfoaluminate concrete. Constr. Build. Mater. 2021, 303, 124139. [Google Scholar] [CrossRef]
- Londono-Zuluaga, D.; Tobón, J.I.; Aranda, M.A.G.; Santacruz, I.; De la Torre, A.G. Clinkering and hydration of belite-alite-yeīelimite cement. Cem. Concr. Compos. 2017, 80, 333–341. [Google Scholar] [CrossRef]





| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | TiO2 | SO3 | Loss | |
|---|---|---|---|---|---|---|---|---|
| Limestone | 51.16 | 2.26 | 0.74 | 0.41 | 4.16 | - | 0.11 | 40.55 |
| Bauxite | 0.81 | 30.89 | 46.51 | 6.12 | 0.17 | 2.23 | 0.11 | 12.13 |
| Gypsum | 28.71 | 1.93 | 0.90 | 0.29 | 0.73 | 0.03 | 43.98 | 22.30 |
| P2O5 | Cl | Cr2O3 | MnO | CuO | SrO | ZrO2 | |
|---|---|---|---|---|---|---|---|
| Limestone | 0.0553 | 0.0499 | - | 0.0196 | - | 0.0125 | - |
| Bauxite | 0.1441 | 0.0202 | 0.0290 | 0.0114 | 0.0053 | 0.0202 | 0.0457 |
| Gypsum | 0.0202 | 0.0855 | - | - | - | 0.0171 | - |
| Samples | C4A3Š | C2S | C4AF | CŠ |
|---|---|---|---|---|
| B | 44 | 45 | 10 | 1 |
| F1 | 39 | 45 | 15 | 1 |
| F2 | 34 | 45 | 20 | 1 |
| F3 | 29 | 45 | 25 | 1 |
| F4 | 24 | 45 | 30 | 1 |
| F1S1 | 34 | 50 | 15 | 1 |
| F1S2 | 29 | 55 | 15 | 1 |
| F3S1 | 24 | 50 | 25 | 1 |
| F3S2 | 19 | 55 | 25 | 1 |
| Samples | Limestone | Bauxite | SiO2 | Fe2O3 | Gypsum |
|---|---|---|---|---|---|
| B | 57.48 | 33.27 | 0 | 0 | 9.25 |
| F1 | 58.71 | 31.32 | 0.39 | 1.26 | 8.32 |
| F2 | 59.86 | 29.32 | 1.02 | 2.44 | 7.36 |
| F3 | 61.01 | 27.32 | 1.64 | 3.62 | 6.41 |
| F4 | 62.17 | 25.31 | 2.26 | 4.81 | 5.45 |
| F1S1 | 60.85 | 27.78 | 2.57 | 1.47 | 7.34 |
| F1S2 | 62.97 | 24.26 | 4.74 | 1.67 | 6.36 |
| F3S1 | 63.14 | 23.78 | 3.82 | 3.82 | 5.43 |
| F3S2 | 65.26 | 20.27 | 5.98 | 4.02 | 4.47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lian, S.; Shao, Y.; Wang, C.; Bi, Y.; Ma, J.; Han, K.; Zhu, A.; Ying, G. Unveiling the Influence and Mechanisms of Enhancing Ferrite-Phase Composition on the Properties of Calcium Sulfoaluminate Cement. Materials 2025, 18, 2457. https://doi.org/10.3390/ma18112457
Lian S, Shao Y, Wang C, Bi Y, Ma J, Han K, Zhu A, Ying G. Unveiling the Influence and Mechanisms of Enhancing Ferrite-Phase Composition on the Properties of Calcium Sulfoaluminate Cement. Materials. 2025; 18(11):2457. https://doi.org/10.3390/ma18112457
Chicago/Turabian StyleLian, Songsong, Yu Shao, Chenyu Wang, Yutian Bi, Jiaxing Ma, Kangzhan Han, Anzhe Zhu, and Guogang Ying. 2025. "Unveiling the Influence and Mechanisms of Enhancing Ferrite-Phase Composition on the Properties of Calcium Sulfoaluminate Cement" Materials 18, no. 11: 2457. https://doi.org/10.3390/ma18112457
APA StyleLian, S., Shao, Y., Wang, C., Bi, Y., Ma, J., Han, K., Zhu, A., & Ying, G. (2025). Unveiling the Influence and Mechanisms of Enhancing Ferrite-Phase Composition on the Properties of Calcium Sulfoaluminate Cement. Materials, 18(11), 2457. https://doi.org/10.3390/ma18112457






