Preliminary Study on Electrochemical Deposition of Graphene on Steel Substrate via In Situ Oxidation Using Cyclic Voltammetry
Abstract
1. Introduction
2. Materials and Methods
2.1. Solutions
2.2. Specimens
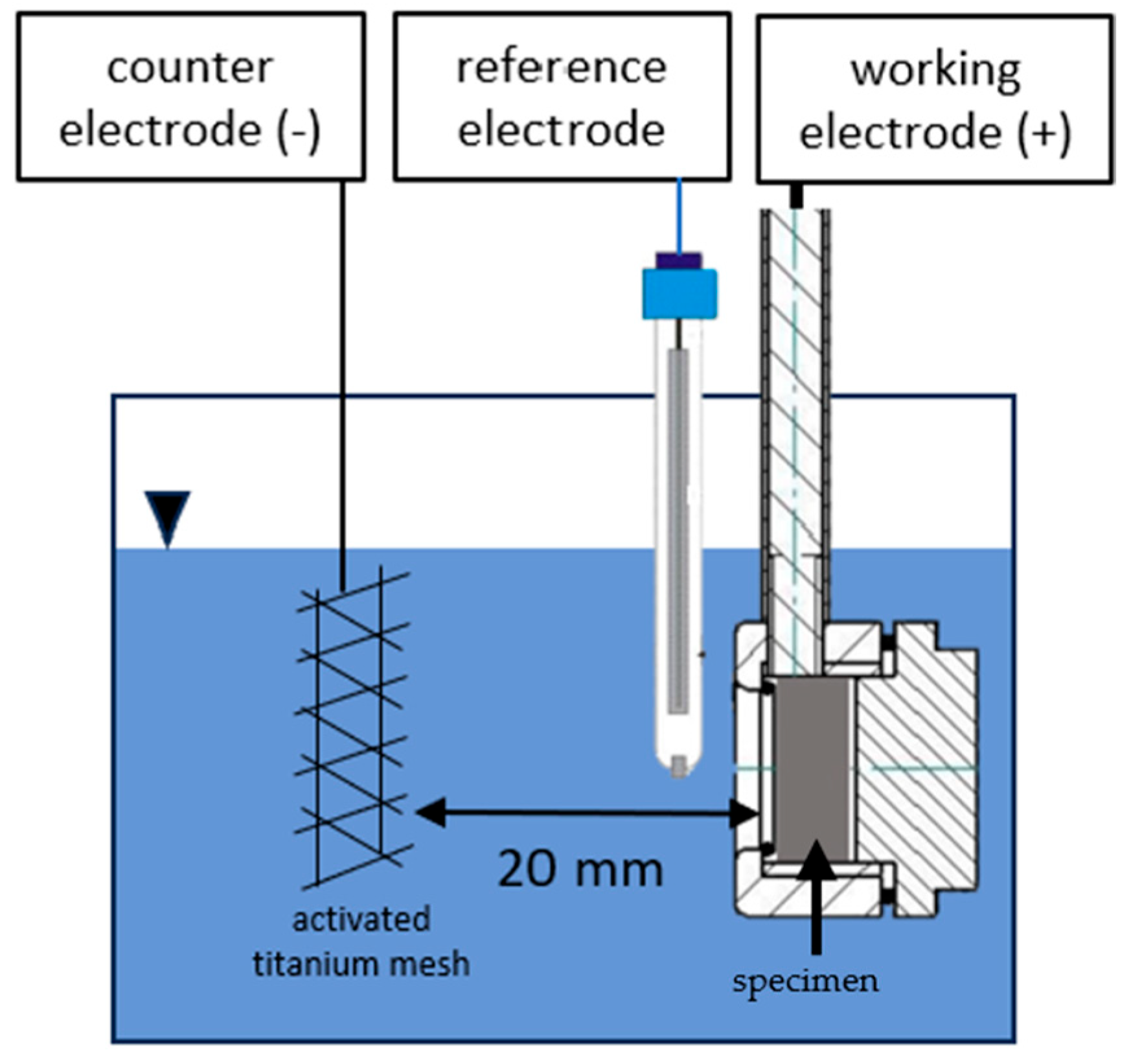
2.3. Potentiostatic Deposition
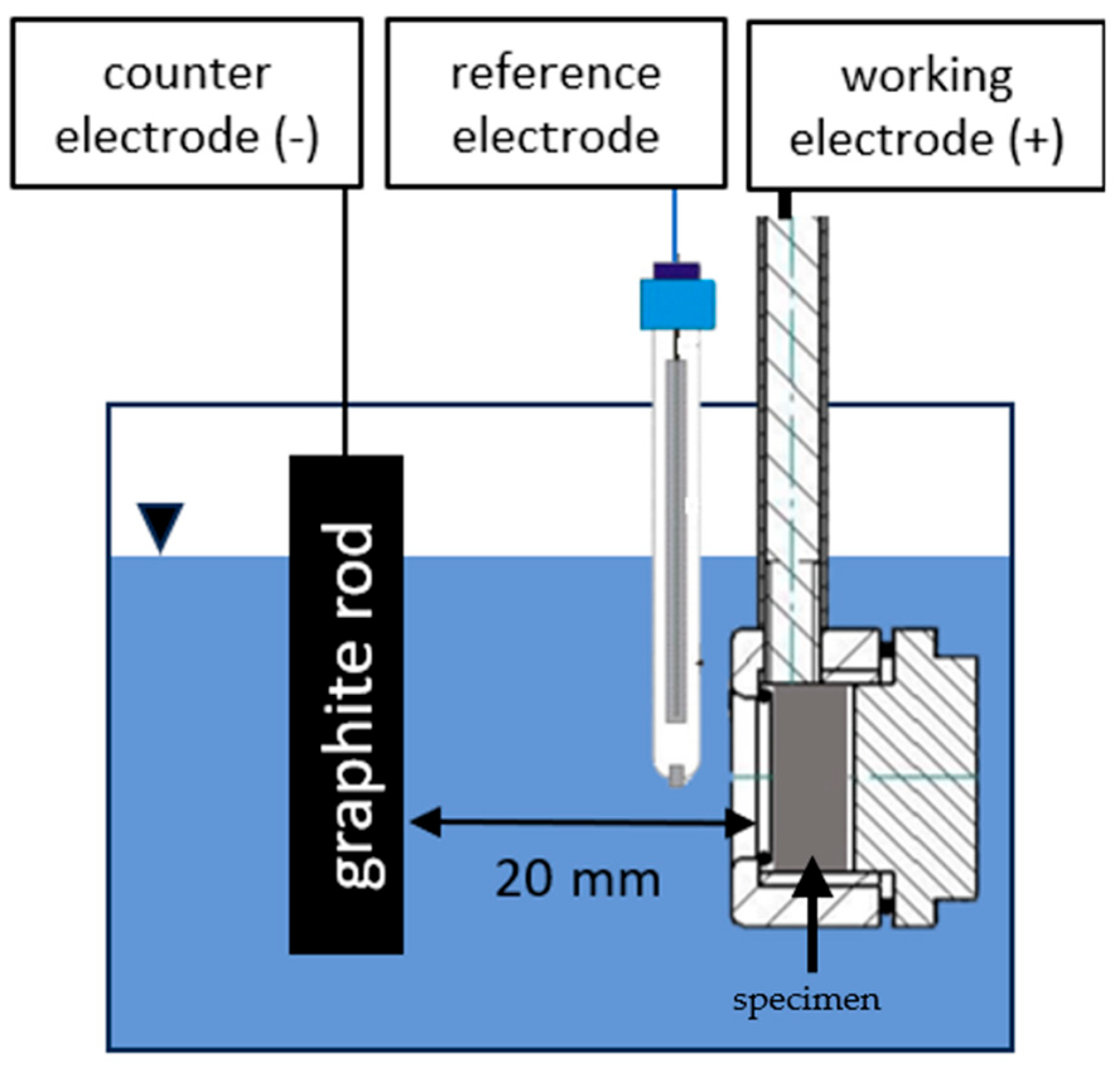
2.4. Cyclic Voltammetry Deposition
2.5. Coating Characterization Methods
2.6. Instruments and Software Used
3. Results and Discussion
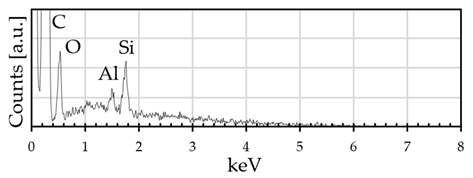
3.1. GE Powder
3.2. Potentiostatic
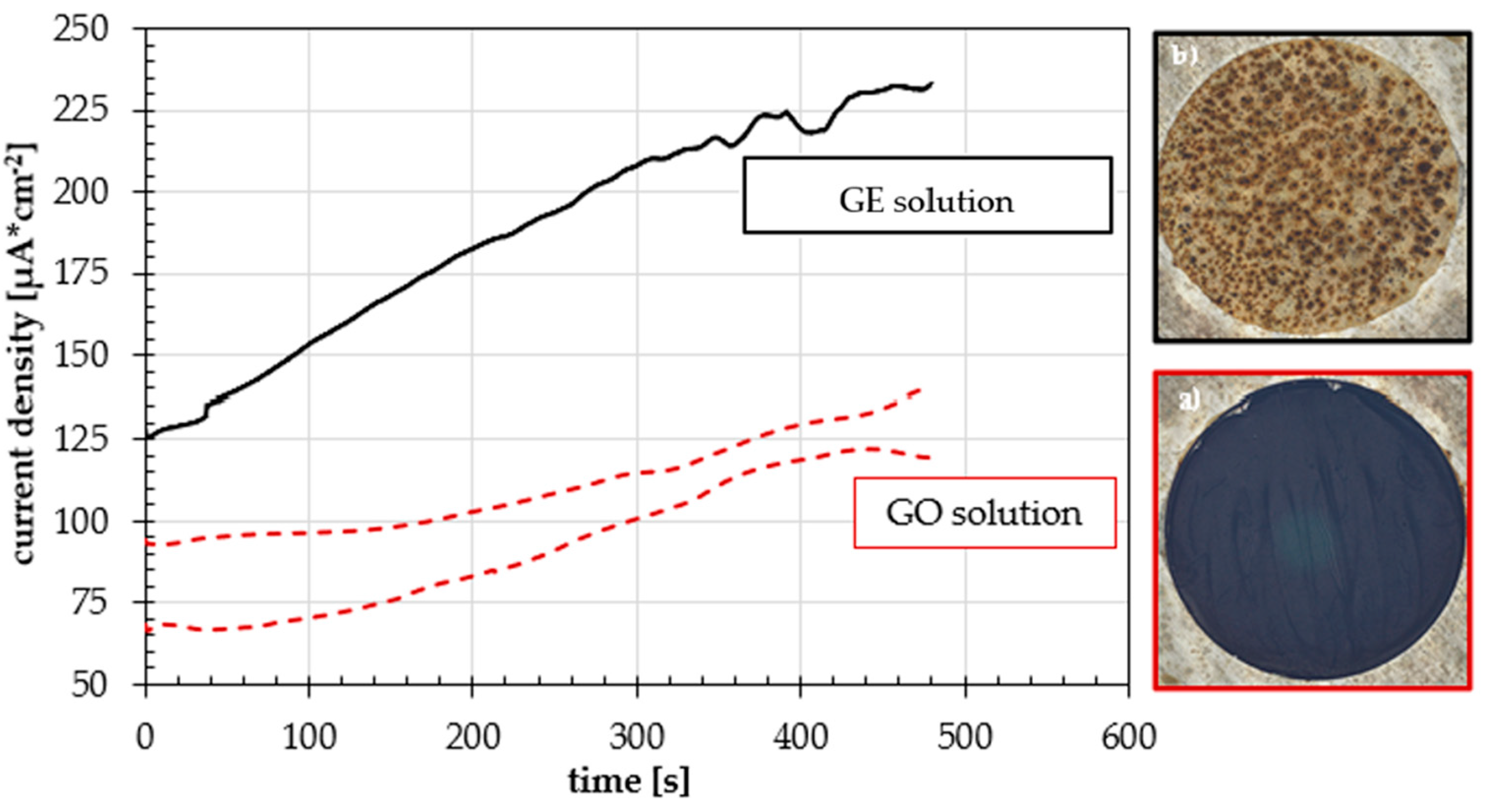
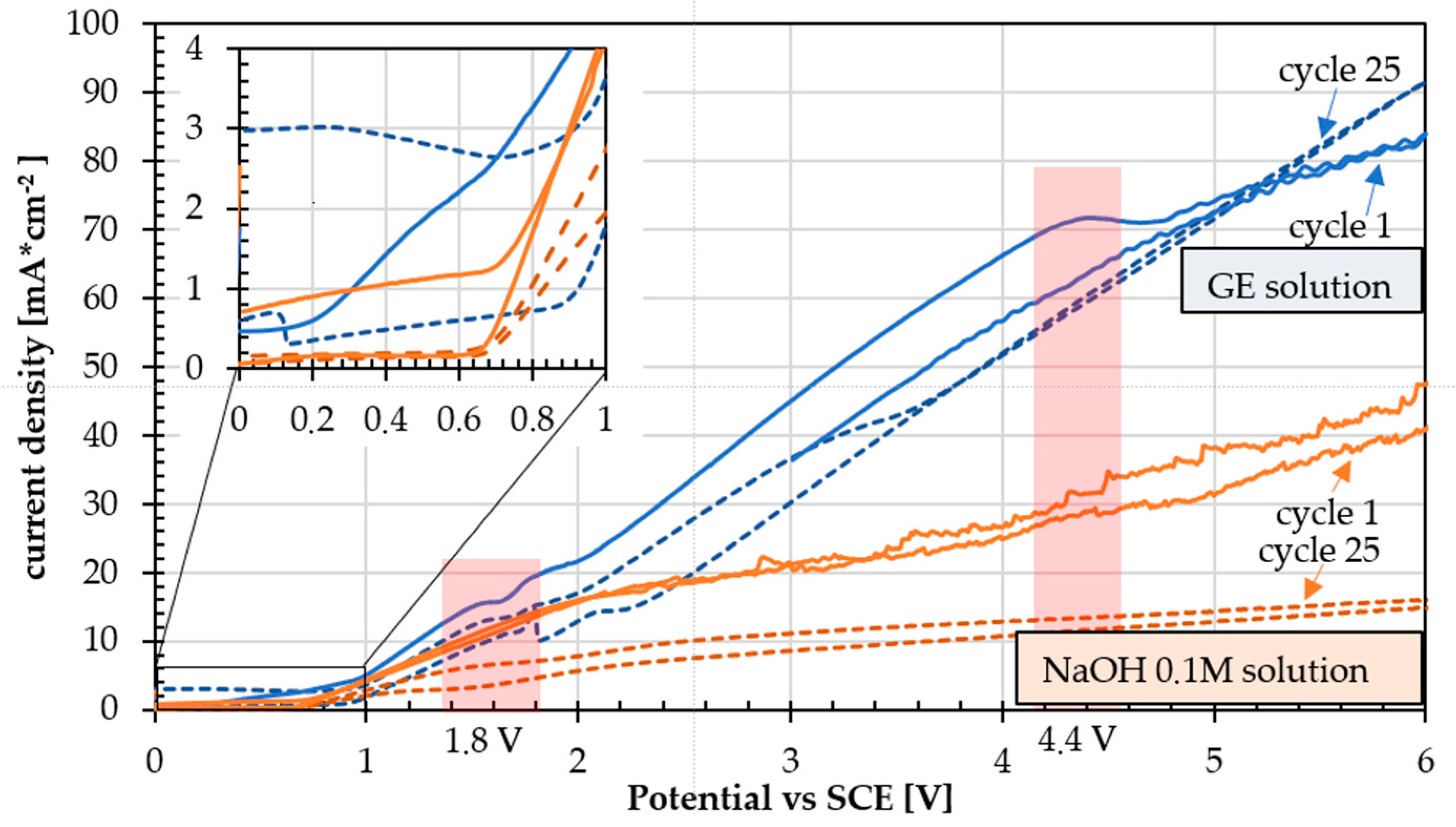
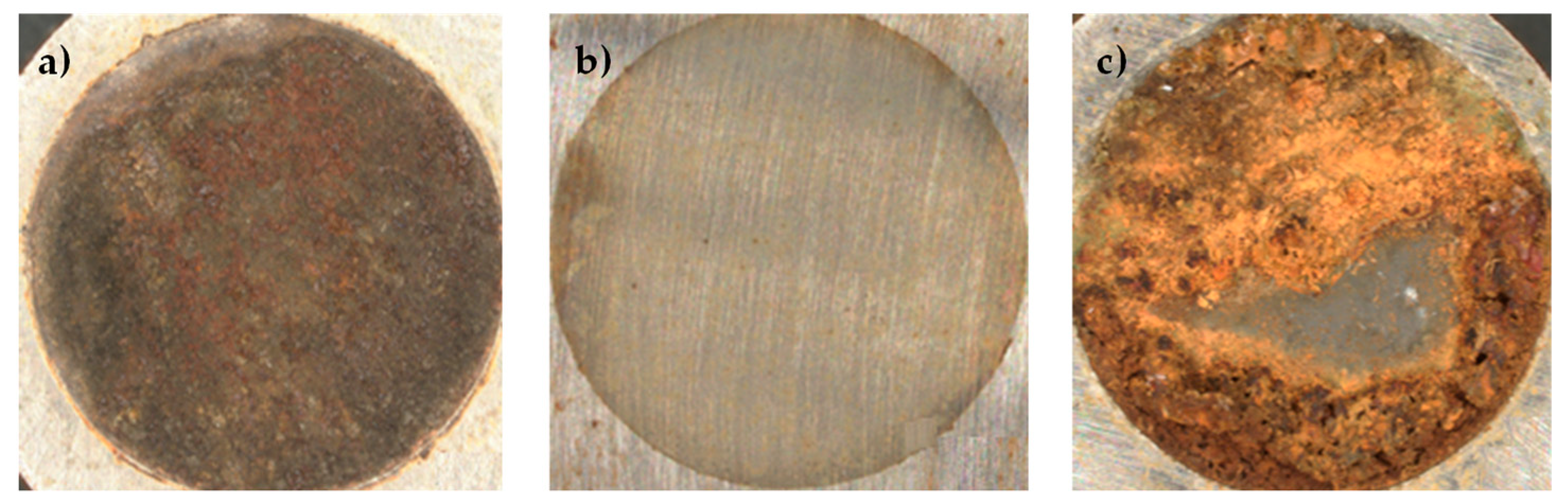
3.3. Cyclic Voltammetry
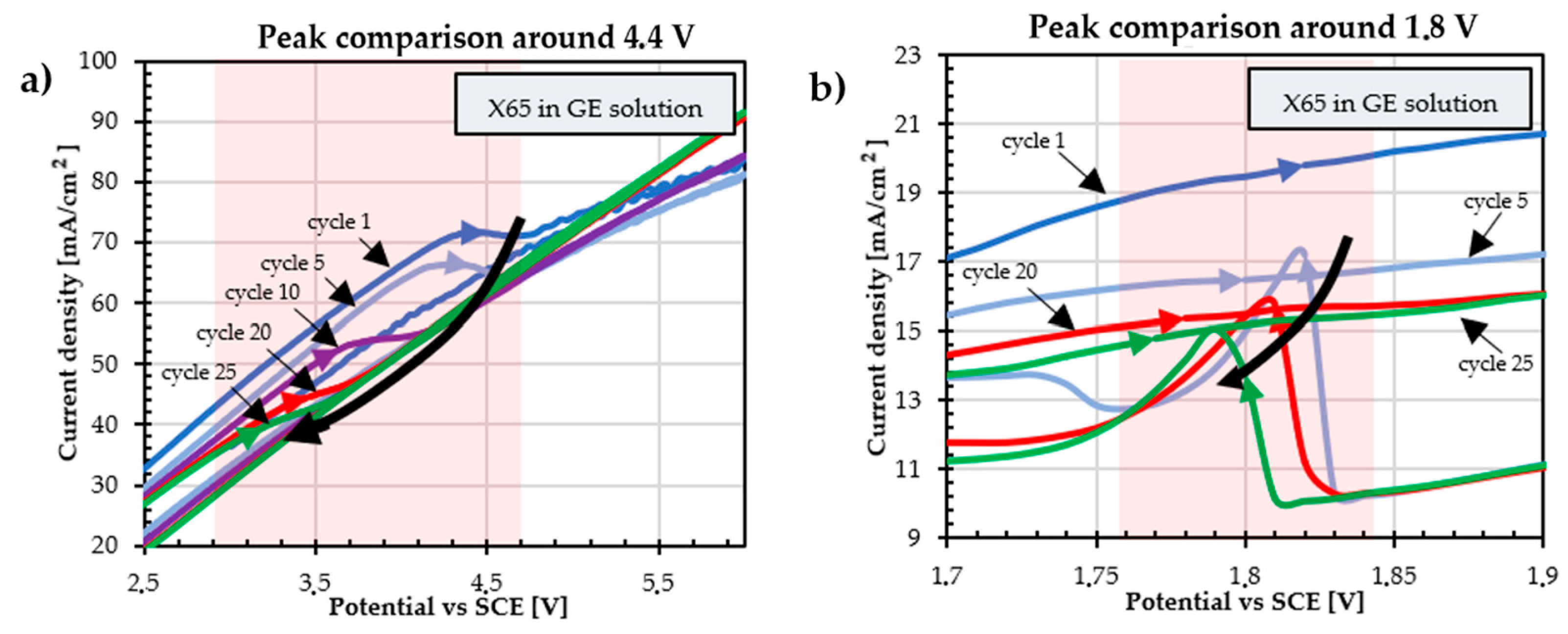
3.4. Effect of the Number of Cycles on the Coating
3.5. Effect of Potential Ranges
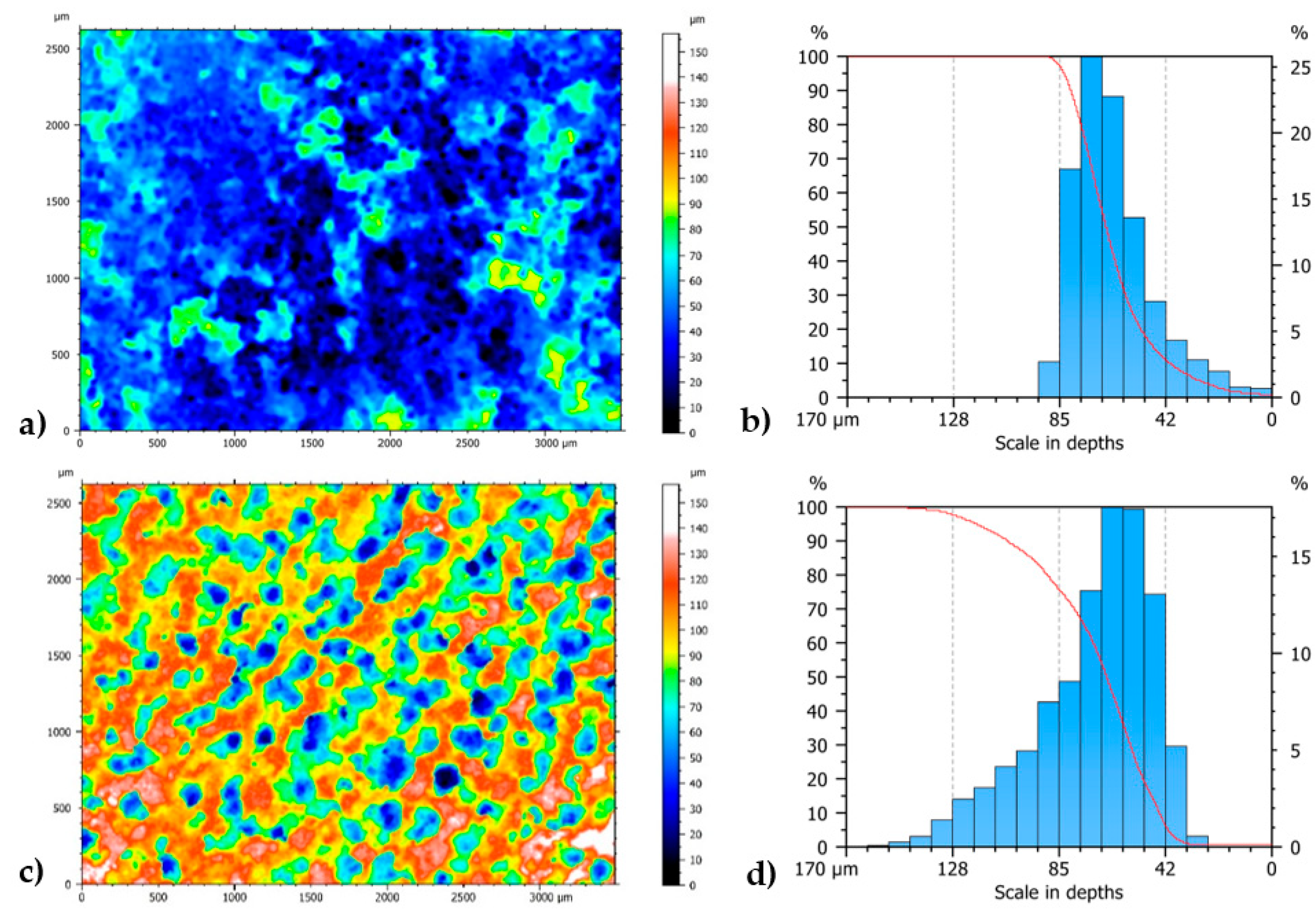
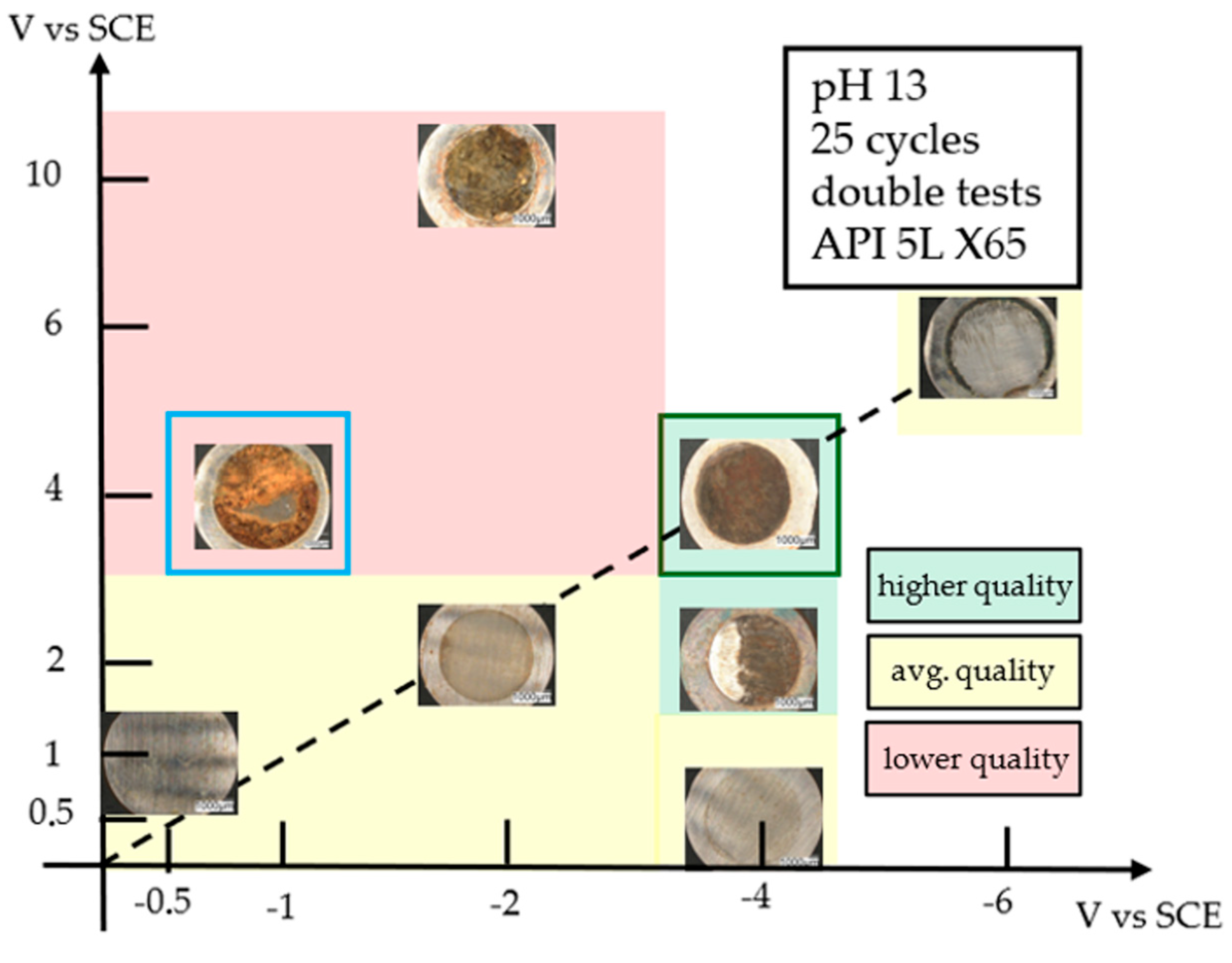
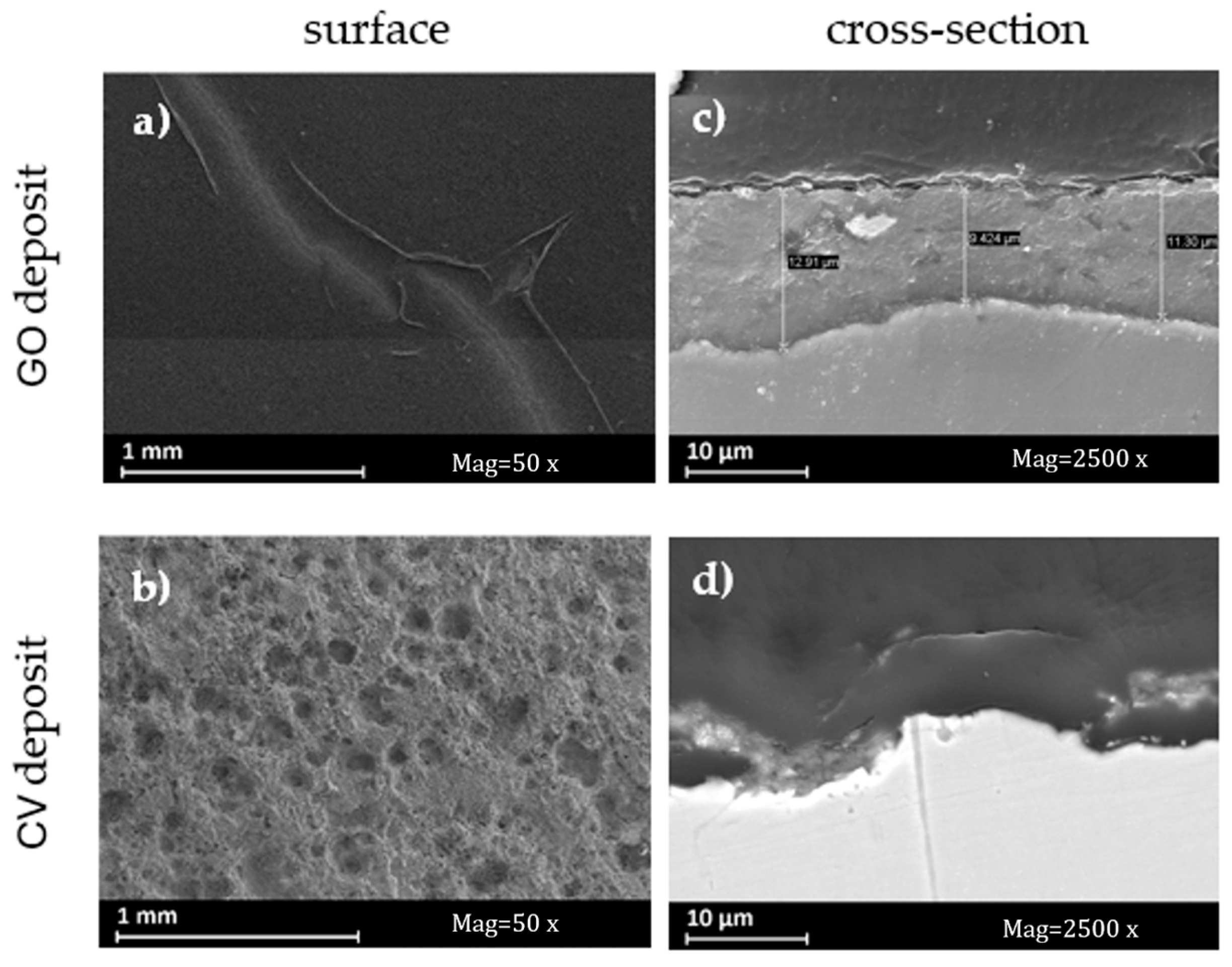
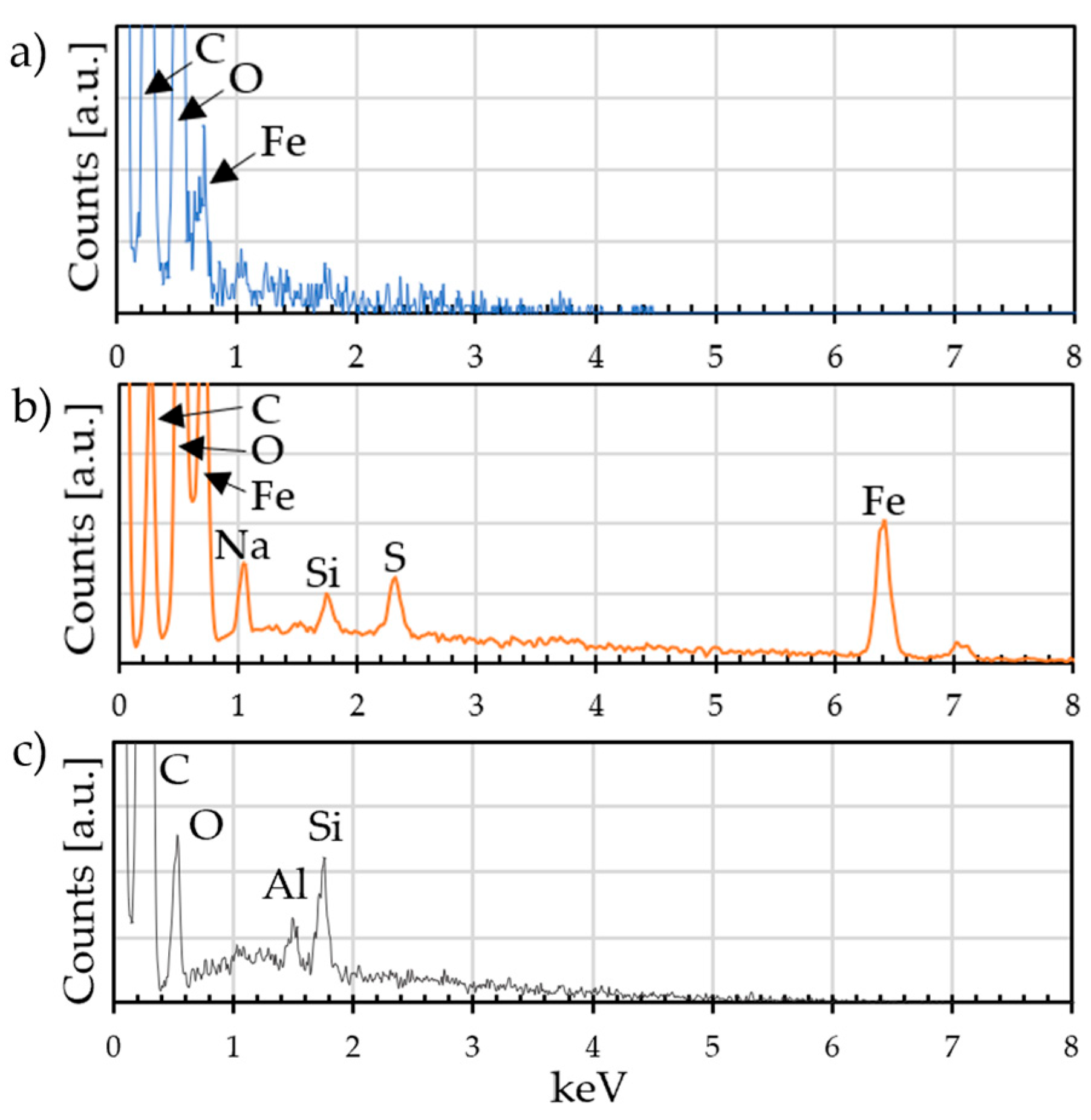
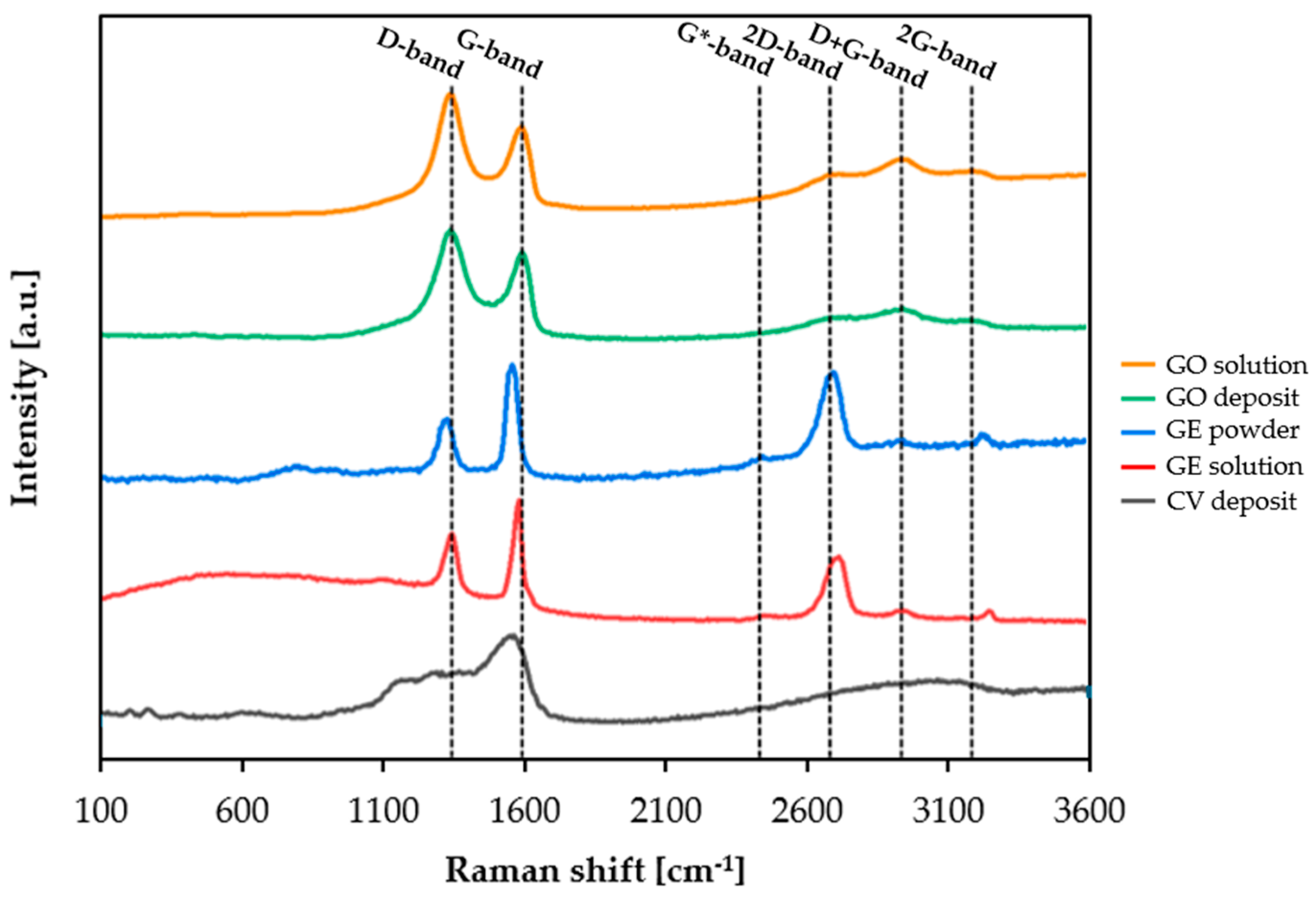
3.6. Coating Characterization
4. Conclusions
- The potentiostatic deposition technique allows for the formation of uniform, smooth graphene oxide (GO) coatings with poor adhesion. However, this method is not suitable for the deposition of non-oxidized graphene.
- The deposition of graphene on a metal surface by direct electrochemical in situ oxidation and subsequent reduction via cyclic voltammetry represents a novel technique that has yet to be documented in the existing literature.
- Raman analyses have confirmed the presence of graphene, which was oxidized prior to reduction by cyclic voltammetry and deposited at the surface.
- SEM, EDX, and interferometric observations of the deposits show discontinuities, both in terms of morphology and composition, that can be attributed to the deterioration of the substrate, which has resulted in a reduction in the quality of the coating.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capros, P.; Paroussos, L.; Fragkos, P.; Tsani, S.; Boitier, B.; Wagner, F.; Busch, S.; Resch, G.; Blesl, M.; Bollen, J. Description of models and scenarios used to assess European decarbonisation pathways. Energy Strat. Rev. 2014, 2, 220–230. [Google Scholar] [CrossRef]
- Bennoua, S.; Le Duigou, A.; Quéméré, M.-M.; Dautremont, S. Role of hydrogen in resolving electricity grid issues. Int. J. Hydrogen Energy 2015, 40, 7231–7245. [Google Scholar] [CrossRef]
- Chamandoust, H.; Hashemi, A.; Bahramara, S. Energy management of a smart autonomous electrical grid with a hydrogen storage system. Int. J. Hydrogen Energy 2021, 46, 17608–17626. [Google Scholar] [CrossRef]
- Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC126763 (accessed on 10 September 2024).
- Gangloff, R.P.; Somerday, B.P. (Eds.) The problem, its characterisation and effects on particular alloy classes. In Gaseous Hydrogen Embrittlement of Materials in Energy Technologies; Woodhead Publishing Limited: Cambridge, UK, 2012; Volume 1. [Google Scholar]
- Lei, Y.; Hosseini, E.; Liu, L.; Scholes, C.A.; Kentish, S.E. Internal polymeric coating materials for preventing pipeline hydrogen embrittlement and a theoretical model of hydrogen diffusion through coated stee. Int. J. Hydrogen Energy 2022, 47, 31409–31419. [Google Scholar] [CrossRef]
- Shen, S.; Song, X.; Li, Q.; Li, X.; Zhu, R.; Yang, G. Effect of CrxCy–NiCr coating on the hydrogen embrittlement of 17-4 PH stainless steel using the smooth bar tensile test. J. Mater. Sci. 2019, 54, 7356–7368. [Google Scholar] [CrossRef]
- Morimoto, T.; Kumai, T. Prevention of Hydrogen Embrittlement Using Ultra Rapid Cooling Thermal Spraying Gun. ISIJ Int. 2017, 57, 1461–1467. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Prevention of Hydrogen Embrittlement in Steels. ISIJ Int. 2016, 56, 24–36. [Google Scholar] [CrossRef]
- Khare, A.; Vishwakarma, M.; Ahmed, S. Combating Hydrogen Embrittlement With Graphene Based Coatings. Int. J. Adv. Res. Eng. Technol. 2019, 10, 234–251. [Google Scholar] [CrossRef]
- Coatings and Liners for Hydrogen Service Pipelines. Available online: https://www.csagroup.org/ (accessed on 10 September 2024).
- Wan, H.; Cheng, Z.l.; Song, D.; Chen, C. Preparation and performance study of waterborne epoxy resin/non-covalent modified graphene oxide hydrogen barrier coatings. Int. J. Hydrogen Energy 2024, 53, 218–228. [Google Scholar] [CrossRef]
- Cai, K.; Jiang, B. Preparation and Characterization of Composite Hydrogen Barrier Coatings with (Graphene–Epoxy Resin)/(Silicon Carbide–Epoxy Resin)/(Graphene–Epoxy Resin) Sandwich Structures. Coatings 2025, 15, 518. [Google Scholar] [CrossRef]
- Soldano, C.; Mahmood, A.; Dujardin, E. Production, properties and potential of graphene. Carbon 2010, 48, 2127–2150. [Google Scholar] [CrossRef]
- Prasai, D.; Tuberquia, J.C.; Harl, R.R.; Jennings, G.K.; Bolotin, K.I. Graphene: Corrosion-Inhibiting Coating. ACS Nano 2012, 6, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Berry, V. Impermeability of graphene and its applications. Carbon 2013, 62, 1–10. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Van Der Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef]
- Sun, P.Z.; Yang, Q.; Kuang, W.J.; Stebunov, Y.V.; Xiong, W.Q.; Yu, J.; Nair, R.R.; Katsnelson, M.I.; Yuan, S.J.; Grigorieva, I.V.; et al. Limits on gas impermeability of graphene. Nature 2020, 579, 229–232. [Google Scholar] [CrossRef]
- Nam, T.H.; Lee, J.H.; Choi, S.R.; Yoo, J.B.; Kim, J.G. Graphene coating as a protective barrier against hydrogen embrittlement. Int. J. Hydrogen Energy 2014, 39, 11810–11817. [Google Scholar] [CrossRef]
- Shi, K.; Xiao, S.; Ruan, Q.; Wu, H.; Chen, G.; Zhou, C.; Jiang, S.; Xi, K.; He, M.; Chu, P.K. Hydrogen permeation behavior and mechanism of multi-layered graphene coatings and mitigation of hydrogen embrittlement of pipe steel. Appl. Surf. Sci. 2022, 573, 151529. [Google Scholar] [CrossRef]
- Miglietta, M.L.; Polichetti, T.; Massera, E.; Mauro, A.D.G.D.; Alfano, B. Direct Growth Of Graphene On Stainless Steel As Protective Layer Against Hydrogen Corrosion. In Proceedings of the 2023 IEEE Nanotechnology Materials and Devices Conference (NMDC), Paestum, Italy, 22–25 October 2023; pp. 257–258. [Google Scholar] [CrossRef]
- Saeed, M.; Alshammari, Y.; Majeed, S.A.; Al-Nasrallah, E. Chemical Vapour Deposition of Graphene—Synthesis, Characterisation, and Applications: A Review. Molecules 2020, 25, 3856. [Google Scholar] [CrossRef]
- Li, X.; Cai, W.; An, J.; Kim, S.; Nah, J.; Yang, D.; Piner, R.; Velamakanni, A.; Jung, I.; Tutuc, E.; et al. Large-Area Synthesis of High-Quality and Uniform Graphene Films on Copper Foils. Science 2009, 324, 1312–1314. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, B.; Bulin, C.; Li, R.; Xing, R. High-efficient Synthesis of Graphene Oxide Based on Improved Hummers Method. Sci. Rep. 2016, 6, 36143. [Google Scholar] [CrossRef]
- Jabbar, A.; Yasin, G.; Khan, W.Q.; Anwar, M.Y.; Korai, R.M.; Nizam, M.N.; Muhyodin, G. Electrochemical deposition of nickel graphene composite coatings: Effect of deposition temperature on its surface morphology and corrosion resistance. RSC Adv. 2017, 7, 31100–31109. [Google Scholar] [CrossRef]
- Liu, C.; Wei, D.; Huang, X.; Mai, Y.; Zhang, L.; Jie, X. Electrodeposition of Co–Ni–P/graphene oxide composite coating with enhanced wear and corrosion resistance. J. Mater. Res. 2019, 34, 1726–1733. [Google Scholar] [CrossRef]
- Tseluikin, V.; Dzhumieva, A.; Yakovlev, A.; Mostovoy, A.; Lopukhova, M. Electrodeposition of Graphene Oxide Modified Composite Coatings Based on Nickel-Chromium Alloy. Crystals 2021, 11, 415. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, J.-G. Electroplating of reduced-graphene oxide on austenitic stainless steel to prevent hydrogen embrittlement. Int. J. Hydrogen Energy 2017, 42, 27428–27437. [Google Scholar] [CrossRef]
- Behunová, D.M.; Gallios, G.; Girman, V.; Kolev, H.; Kaňuchová, M.; Dolinská, S.; Václavíková, M. Electrophoretic Deposition of Graphene Oxide on Stainless Steel Substrate. Nanomaterials 2021, 11, 1779. [Google Scholar] [CrossRef]
- Yu, Q.; Wei, L.; Yang, X.; Wang, C.; Chen, J.; Du, H.; Shen, W.; Kang, F.; Huang, Z.-H. Electrochemical synthesis of graphene oxide from graphite flakes exfoliated at room temperature. Appl. Surf. Sci. 2022, 598, 153788. [Google Scholar] [CrossRef]
- Lama, G.C.; Santillo, C.; Recupido, F.; Liu, J.; Verdolotti, L.; Marzella, R.; Polichetti, T.; Kaciulis, S.; Lavorgna, M. Autoclave-mediated reduction of graphene oxide for enhanced conductive films. Appl. Surf. Sci. 2024, 657, 159741. [Google Scholar] [CrossRef]
- Alfano, B.; Polichetti, T.; Miglietta, M.L.; Massera, E.; Schiattarella, C.; Ricciardella, F.; Di Francia, G. Fully eco-friendly H2 sensing device based on Pd-decorated graphene. Sens. Actuators B Chem. 2017, 239, 1144–1152. [Google Scholar] [CrossRef]
- ASTM G5-14; Standard Reference Test Method for Making Potentiodynamic Anodic Polarization Measurements. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Aoki, K.; Kato, N. Analysis of the cyclic voltammograms associated with deposition or precipitation of the electrochemical product. J. Electroanal. Chem. Interfacial Electrochem. 1988, 245, 51–60. [Google Scholar] [CrossRef]
- Mbayachi, V.B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E.R.; Khan, A.U. Graphene synthesis, characterization and its applications: A review. Results Chem. 2021, 3, 100163. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T. Cyclic voltammetry evaluation of inhibitors for localised corrosion in alkaline solutions. Electrochim. Acta 2014, 124, 156–164. [Google Scholar] [CrossRef]
- de Rességuier, T.; Kurakevych, O.O.; Chabot, A.; Petitet, J.P.; Solozhenko, V.L. Structural changes and phase stability of graphitelike BC3 under explosive shock-wave loading. J. Appl. Phys. 2010, 108, 083522. [Google Scholar] [CrossRef]
- Wall, M. Raman Spectroscopy Optimizes Graphene Characterization. AM&P Tech. Artic. 2012, 170, 35–38. [Google Scholar]
- Ferralis, N. Probing mechanical properties of graphene with Raman spectroscopy. J. Mater. Sci. 2010, 45, 5135–5149. [Google Scholar] [CrossRef]
- Saito, R.; Hofmann, M.; Dresselhaus, G.; Jorio, A.; Dresselhaus, M.S. Raman spectroscopy of graphene and carbon nanotubes. Adv. Phys. 2011, 60, 413–550. [Google Scholar] [CrossRef]
- Vejpravová, J. Mixed sp2–sp3 nanocarbon materials: A status quo review. Nanomaterials 2021, 11, 2469. [Google Scholar] [CrossRef] [PubMed]
- Brzhezinskaya, M.; Irzhak, A.; Irzhak, D.; Kang, T.W.; Kononenko, O.; Matveev, V.; Panin, G.; Roshchupkin, D. Direct growth of graphene film on piezoelectric La3Ga5.5Ta0.5O14 crystal. Rapid Res. Lett. 2016, 10, 639–644. [Google Scholar] [CrossRef]
- Brzhezinskaya, M.; Kapitanova, O.O.; Kononenko, O.V.; Koveshnikov, S.; Korepanov, V.; Roshchupkin, D. Large-scalable graphene oxide films with resistive switching for non-volatile memory applications. J. Alloys Compd. 2020, 849, 156699. [Google Scholar] [CrossRef]
- Kononenko, O.; Brzhezinskaya, M.; Zotov, A.; Korepanov, V.; Levashov, V.; Matveev, V.; Roshchupkin, D. Influence of numerous Moiré superlattices on transport properties of twisted multilayer graphene. Carbon 2022, 194, 52–61. [Google Scholar] [CrossRef]
- Elias, D.C.; Nair, R.R.; Mohiuddin, T.M.G.; Morozov, S.V.; Blake, P.; Halsall, M.P.; Ferrari, A.C.; Boukhvalov, D.W.; Katsnelson, M.I.; Geim, A.K.; et al. Control of Graphene’s Properties by Reversible Hydrogenation: Evidence for Graphane. Science 2009, 323, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Delfino, F.; Ros, C.; Palardonio, S.M.; Carretero, N.M.; Murcia-López, S.; Morante, J.R.; Martorell, J.; Fthenakis, Z.G.; Sgroi, M.F.; Tozzini, V.; et al. Multi-methodological analysis of hydrogen desorption from graphene. Carbon 2024, 227, 119211. [Google Scholar] [CrossRef]
- Openov, L.A.; Podlivaev, A.I. Thermal desorption of hydrogen from graphane. Tech. Phys. Lett. 2010, 36, 31–33. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Q.; Sun, Q.; Chen, X.S.; Kawazoe, Y.; Jena, P. Ferromagnetism in Semihydrogenated Graphene Sheet. Nano Lett. 2009, 9, 3867–3870. [Google Scholar] [CrossRef] [PubMed]



| Weight % | C max | Mn max | P max | S max |
|---|---|---|---|---|
| API 5L X65 | 1.45 | 0.03 | 0.03 | 0.03 |
| Atomic % | C | O | Al | Si |
|---|---|---|---|---|
| Graphene powder | 95 | 3 | traces | traces |
| Atomic % | C | O | Fe | S | Na | Al | Si |
|---|---|---|---|---|---|---|---|
| GO deposit | 50 | 50 | traces | / | / | / | / |
| CV deposit | 30 | 41 | 26 | 1 | 2 | / | / |
| Graphene powder | 95 | 3 | / | / | / | traces | traces |
| Sample | D-Position | G-Position | ID/IG |
|---|---|---|---|
| GO solution | 1349.61 | 1601.83 | 1.30 |
| GO deposit | 1349.14 | 1601.43 | 1.22 |
| GE powder | 1336.83 | 1565.71 | 0.65 |
| GE solution | 1355.71 | 1589.99 | 0.79 |
| CV deposit | 1319.75 | 1571.78 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelucchi, M.; Alfano, B.; Lama, G.C.; Rosa, R.P.; Cabrini, M. Preliminary Study on Electrochemical Deposition of Graphene on Steel Substrate via In Situ Oxidation Using Cyclic Voltammetry. Materials 2025, 18, 2440. https://doi.org/10.3390/ma18112440
Pelucchi M, Alfano B, Lama GC, Rosa RP, Cabrini M. Preliminary Study on Electrochemical Deposition of Graphene on Steel Substrate via In Situ Oxidation Using Cyclic Voltammetry. Materials. 2025; 18(11):2440. https://doi.org/10.3390/ma18112440
Chicago/Turabian StylePelucchi, Mattia, Brigida Alfano, Giuseppe Cesare Lama, Raphael Palucci Rosa, and Marina Cabrini. 2025. "Preliminary Study on Electrochemical Deposition of Graphene on Steel Substrate via In Situ Oxidation Using Cyclic Voltammetry" Materials 18, no. 11: 2440. https://doi.org/10.3390/ma18112440
APA StylePelucchi, M., Alfano, B., Lama, G. C., Rosa, R. P., & Cabrini, M. (2025). Preliminary Study on Electrochemical Deposition of Graphene on Steel Substrate via In Situ Oxidation Using Cyclic Voltammetry. Materials, 18(11), 2440. https://doi.org/10.3390/ma18112440








