Carbon-Halloysite Nanocomposites and Their Adsorption Characteristics for Pharmaceuticals—A Naproxen Case Study
Abstract
1. Introduction
2. Experimental
2.1. Materials and Reagents
- halloysite: 75.80%,
- iron oxides (mainly hematite and magnetite): 18.22%,
- iron and titanium oxides (mainly ilmenite): 2.4%.
2.2. Preparation of Carbon-Halloysite Nanocomposites
- (1)
- Raw halloysite: 15HS (15 wt.%—HS), 30HS (30 wt.%—HS), 45HS (45 wt.%—HS),
- (2)
- Calcined halloysite: 15HK1 (15 wt.%—HK1.00–0.500), 30HK1 (30 wt.%—HK1.00–0.500), 45HK1 (45 wt.%—HK1.00–0.500),
- (3)
- Calcined halloysite: 15HK05 (15 wt.%—HK0.500–0.250), 30HK05 (30 wt.%—HK0.500–0.250), 45HK05 (45 wt.%—HK0.500–0.250),
- (4)
- Calcined halloysite: 15HK025 (15 wt.%—HK0.250–0.125), 30HK025 (30 wt.%—HK0.250–0.125), 45HK025 (45 wt.%—HK0.250–0.125).
2.3. Characteristics of Carbon-Halloysite Nanocomposites
2.4. Adsorption Measurements
3. Results and Discussion
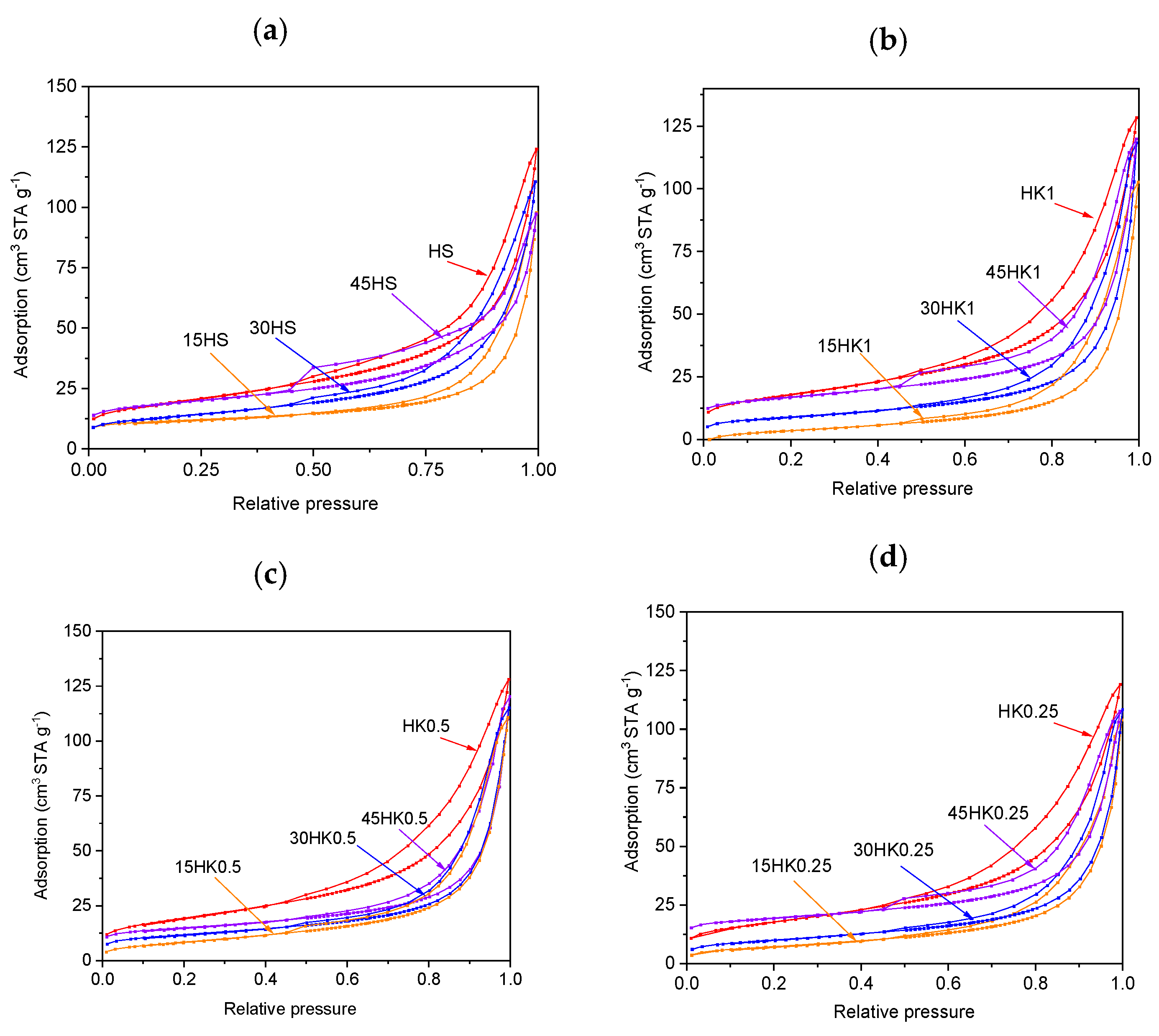
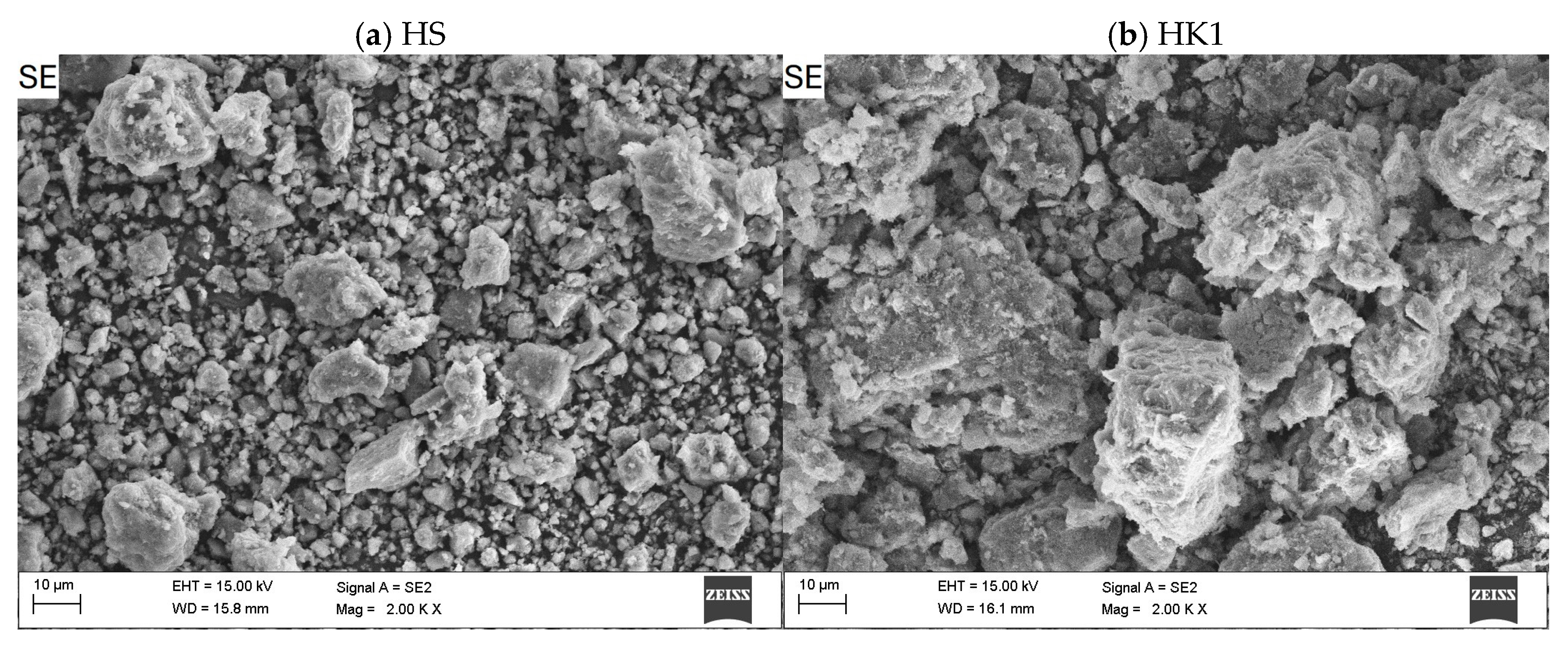
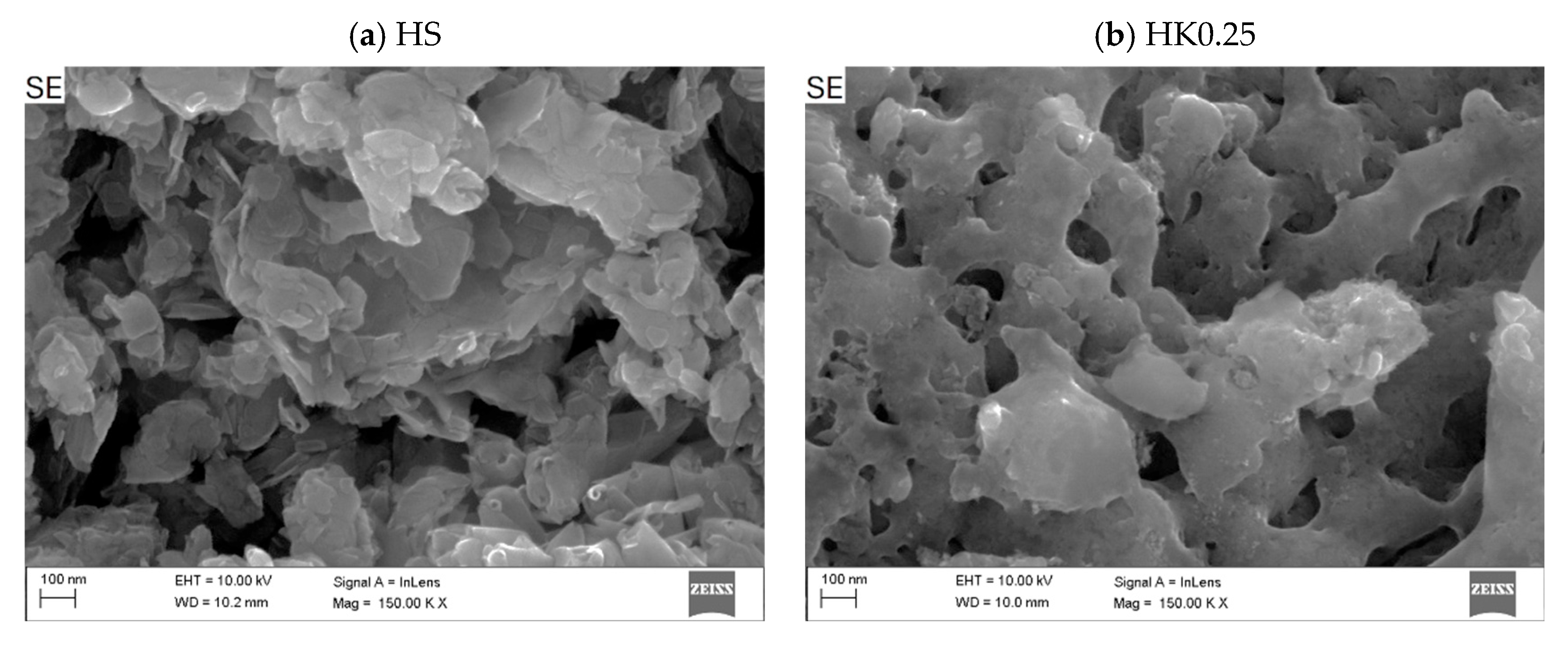
3.1. Characteristics of Adsorbents (Nanocomposites)
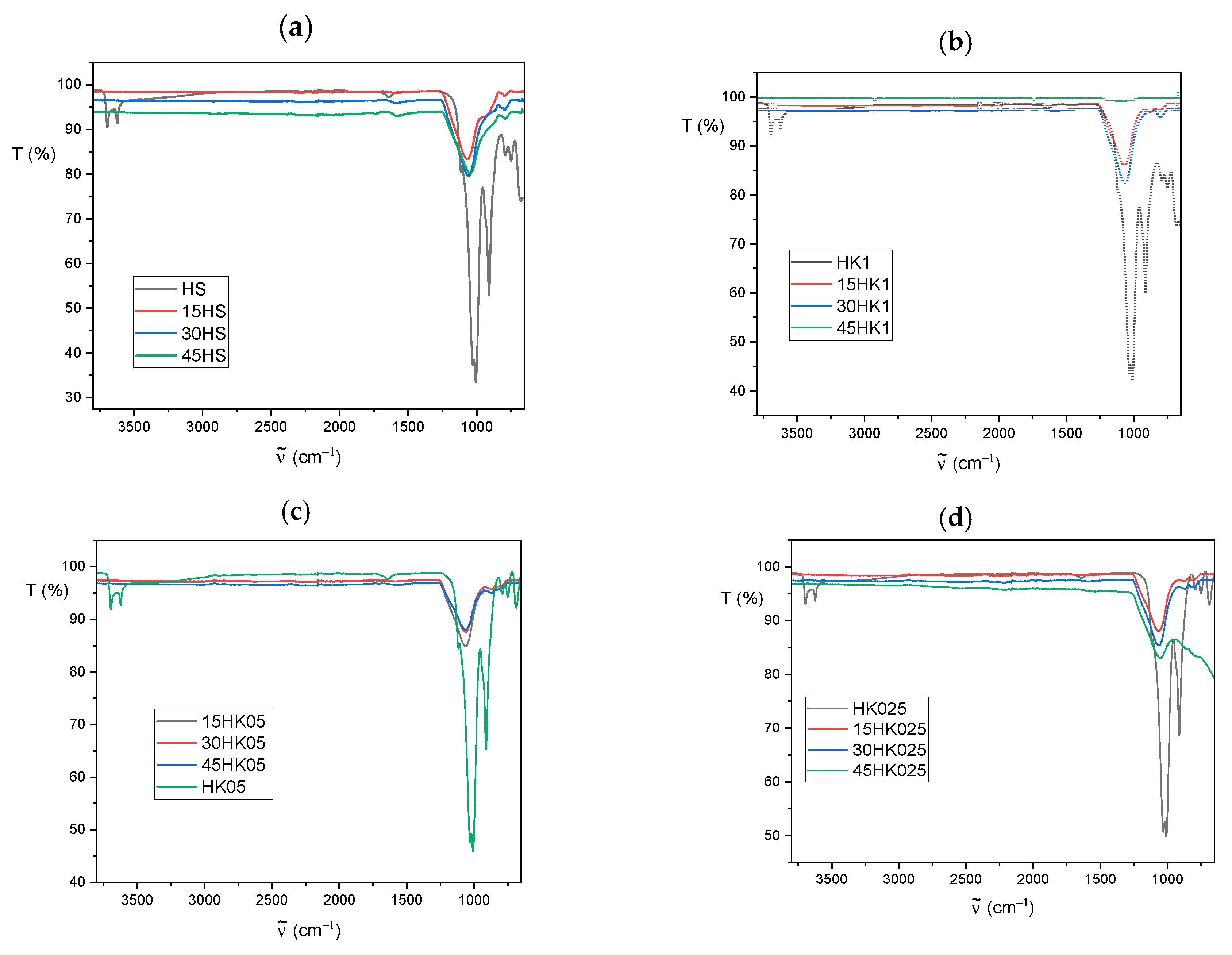
3.2. FT-IR Analysis
3.3. Adsorption Experiments
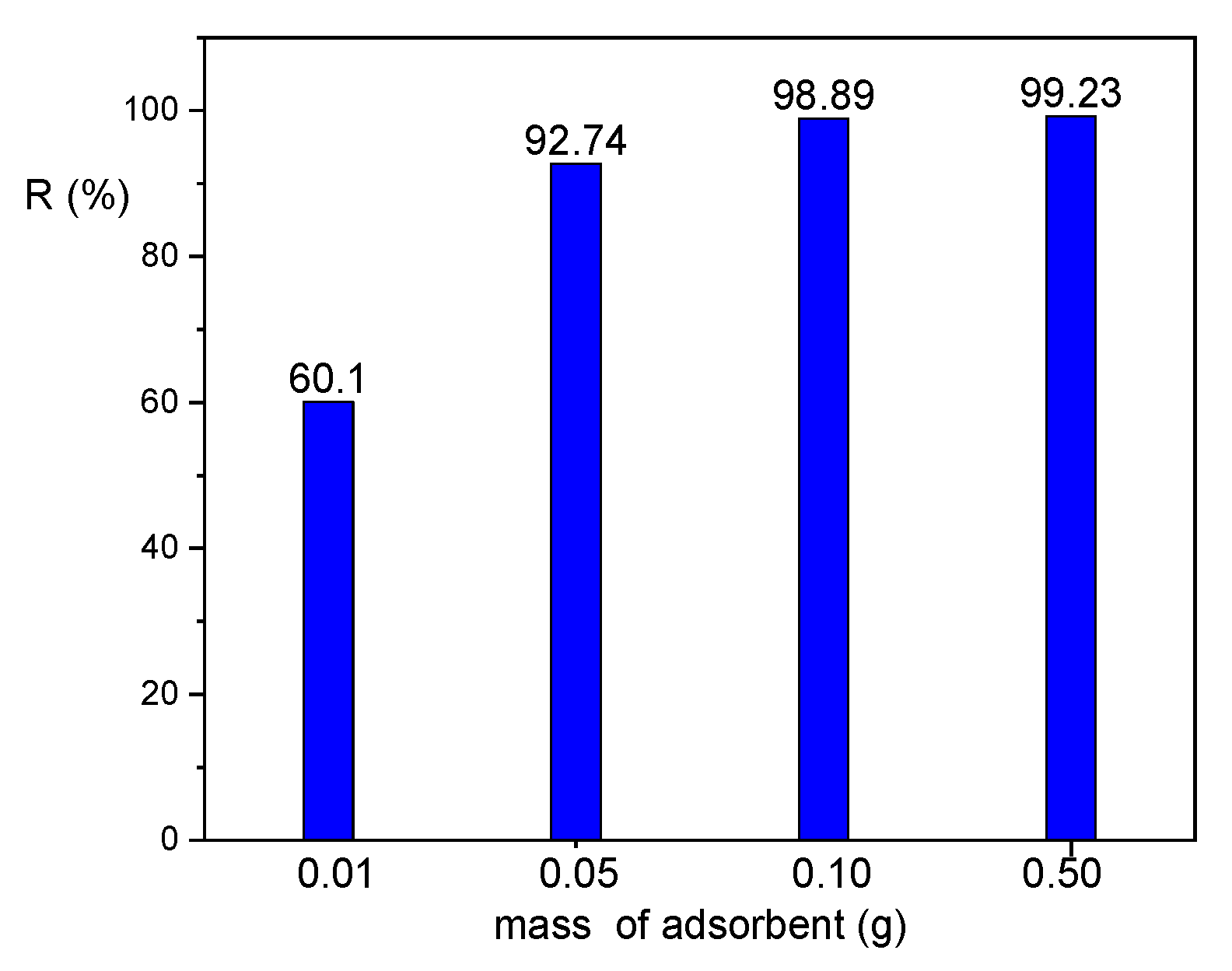
3.4. Effect of Adsorbent Dose
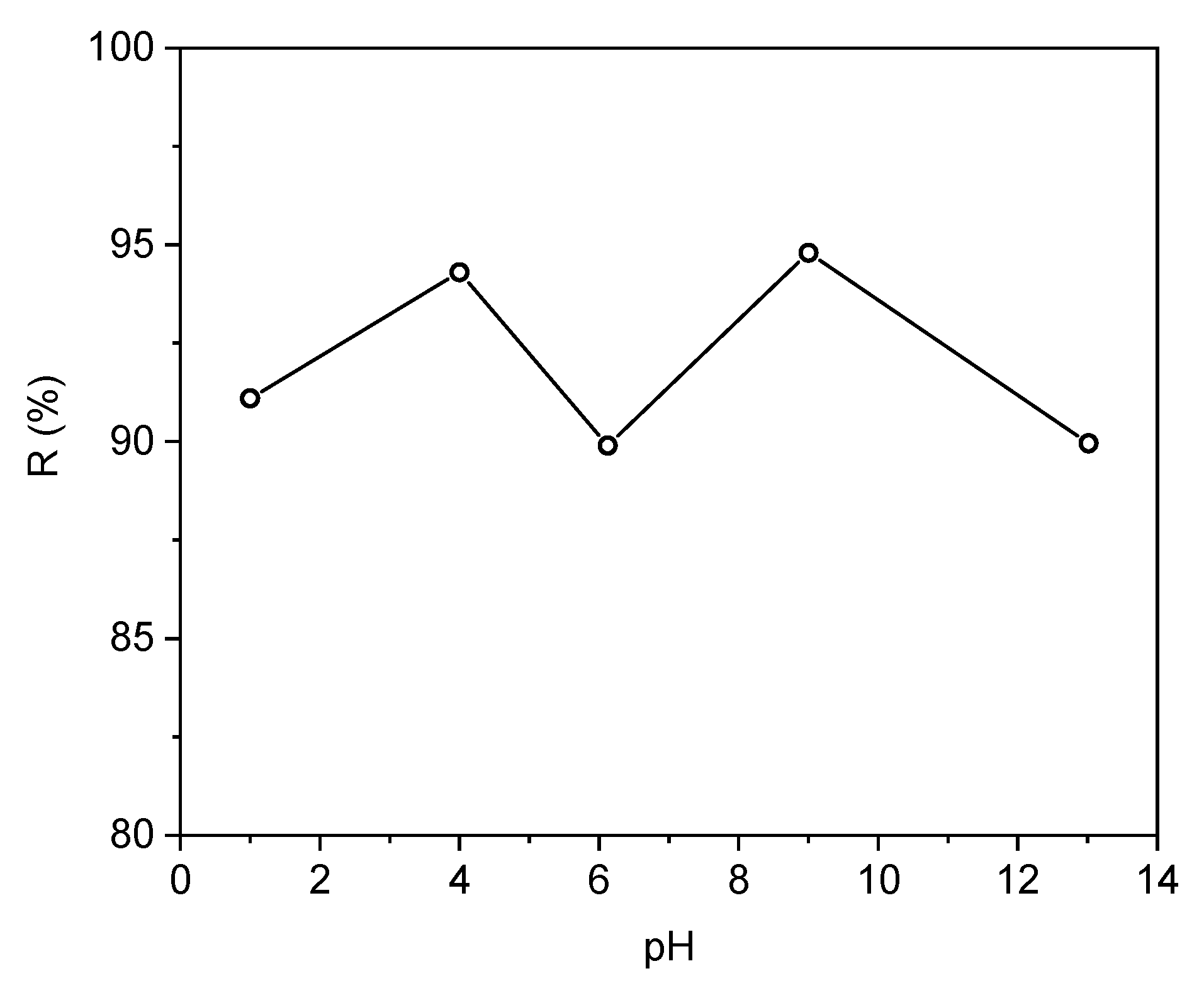
3.5. Effect of pH
3.6. Kinetic Models
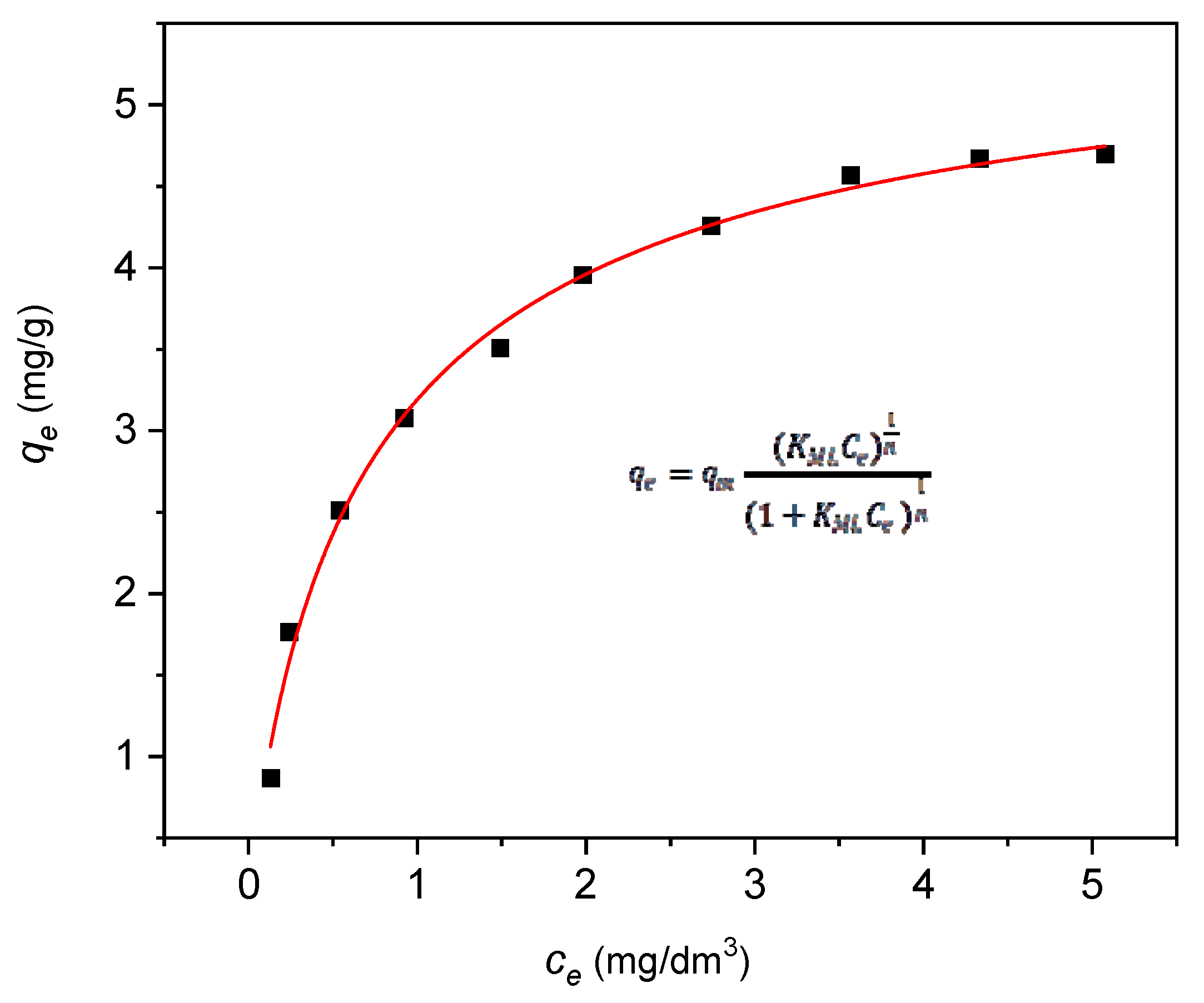
3.7. Adsorption Isotherms
4. Conclusions
- (1)
- The deposition of a carbon layer onto the halloysite carrier, represented by both raw halloysite (HS) and halloysite subjected to a controlled calcination process (HK), enabled the practical removal of the previous technological barrier related to the very low adsorptive separation efficiency of natural clays in relation to NPX with its characteristic chemical structure and charge distribution properties.
- (2)
- The reduction in the specific surface area of HS- and HK-based adsorbents, related to the surface deposition of carbon and the resulting changes in such obtained surface morphology of nanocomposites, was overcompensated by the much higher affinity of surface functional groups for NPX, which is directly related to the increased technological effect of NPX separation.
- (3)
- The SEM/EDS analysis indicates a combined effect of the adsorption process on and within the carbon layer and on the remaining unoccupied internal and external surface of the halloysite carrier (competitive adsorption on the actual surface structure).
- (4)
- The kinetic conditions of the process (pseudo-second-order kinetic model) indicate a favorable increase in the number of active sites formed after the deposition of a carbon layer on the surface of halloysite support. The presented kinetic data of the adsorption process and their theoretical interpretation refer to the aqueous model solution of naproxen.
- (5)
- The validation of the Langmuir multi-center adsorption isotherm model indicates a separation mechanism associated with the occurrence of multiple active centers on the nanocomposite adsorbent surface and the separation effect without dissociation of naproxen particles. The presented equilibrium data of the adsorption process and their theoretical interpretation refer to the aqueous model solution of naproxen.
- (6)
- The proposed nanocomposite preparation method is cost-effective, technologically simple, and environmentally friendly, utilizing sucrose, water, and naturally occurring halloysite. Given the substantial NPX removal efficiency demonstrated in this study, the method holds promise for widespread application in the economical and effective removal of pharmaceutical contaminants from aqueous systems.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojcieszyńska, D.; Guzik, U. Naproxen in the environment: Its occurrence, toxicity to nontarget organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Tehrani, E.; Faraji, A.R.; Shojaei, N.; Shahinmehr, S.; Najafi, A.; Hekmatian, Z.; Tehrani, Z.; Bornas, B. An overview of the characteristics, toxicity, and treatment methods for the degradation of pharmaceutically active compounds: Naproxen as a case study. J. Environ. Chem. Eng. 2023, 11, 111575. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Weisman, S.M. Clinical pharmacology and cardiovascular safety of naproxen. Am. J. Cardiovasc. Drugs 2017, 17, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Barcella, C.A.; Lamberts, M.; McGettigan, P.; Fosbol, E.L.; Lindhardsen, J.; Torp-Pedersen, C.; Gislason, G.H.; Olsen, A.S. Differences in cardiovascular safety with non-steroidal anti-inflammatory drug therapy—A nationwide study in patients with osteoarthritis. Basic. Clin. Pharmacol. Toxicol. 2019, 124, 629–641. [Google Scholar] [CrossRef]
- CAguilar, M.; Chairez, I.; Rodriguez, J.L.; Tiznado, H.; Santillan, R.; Arrieta, D.; Poznyak, T. Inhibition effect of ethanol in naproxen degradation by catalytic ozonation with NiO. RSC Adv. 2019, 9, 14822–14833. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Mdluli, P.S.; Chimuka, L. An initial assessment of naproxen, ibuprofen and diclofenac in Ladysmith water resources in South Africa using molecularly imprinted solid-phase extraction followed by high performance liquid chromatography-photodiode array detection. S. Afr. J. Chem. 2017, 70, 145–153. [Google Scholar] [CrossRef]
- Garcia-Medina, A.L.; Galar-Martinez, M.; Garcia-Medina, S.; Gomez-Olivan, L.M.; Razo-Estrada, C. Naproxen-enriched artificial sediment induces oxidative stress and genotoxicity in Hyalella azteca. Water Air Soil. Pollut. 2015, 226, 195–210. [Google Scholar] [CrossRef]
- Grenni, P.; Patrolecco, L.; Ademollo, N.; Di Lenola, M.; Caracciolo, A.B. Capability of the natural microbial community in a river water ecosystem to degrade the drug naproxen. Environ. Sci. Pollut. Res. Int. 2014, 21, 13470–13479. [CrossRef]
- Lahti, M.; Oikari, A. Microbial transformation of pharmaceuticals naproxen, bisoprolol and diclofenac aerobic and anaerobic environments. Arch. Environ. Contam. Toxicol. 2011, 61, 202–210. [Google Scholar] [CrossRef]
- MBenotti, J.; Trenholm, R.A.; Vanderford, B.J.; Holady, J.C.; Stanford, B.D.; Snyder, S.A. Pharmaceuticals and endocrine disrupting compounds in U.S. drinking water. Environ. Sci. Technol. 2009, 43, 597–603. [Google Scholar] [CrossRef]
- Ding, T.; Lin, K.; Yang, B.; Yang, M.; Li, J.; Li, W.; Gan, J. Biodegradation of naproxen by freshwater algae Cymbella sp. and Scenedesmus quadricauda and the comparative toxicity. Bioresour. Technol. 2017, 238, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Addison, R.S.; Parker-Scott, S.L.; Hooper, W.D.; Eadie, M.J.; Dickinson, R.G. Effect of naproxen co-administration on valproate disposition. Biopharm. Drug Dispos. 2000, 21, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Jallouli, N.; Elghniji, K.; Hentati, O.; Ribeiro, A.R.; Silva, A.M.T.; Ksibi, M. UV and solar photo-degradation of naproxen: TiO2 catalyst effect, reaction kinetics, products identification and toxicity assessment. J. Hazard. Mater. 2016, 304, 329–336. [Google Scholar] [CrossRef] [PubMed]
- DellaGreca, M.; Brigante, M.; Isidori, M.; Nardelli, A.; Previtera, L.; Rubino, M.; Temussi, F. Phototransformation and ecotoxicity of the drug naproxen-Na. Environ. Chem. Lett. 2004, 1, 237–241. [Google Scholar] [CrossRef]
- Xu, C.; Niu, L.; Guo, H.; Sun, X.; Chen, L.; Tu, W.; Dai, Q.; Ye, J.; Liu, W.; Liu, J. Long-term exposure to the non-steroidal anti-inflammatory drug (NSAID) naproxen causes thyroid disruption in zebrafish at environmentally relevant concentrations. Sci. Total Environ. 2019, 676, 387–395. [Google Scholar] [CrossRef]
- Li, Q.; Wang, P.; Chen, L.; Gao, H.; Wu, L. Acute toxicity and histopathological effects of naproxen in zebrafish (Danio rerio) early life stages. Environ. Sci. Pollut. Res. Int. 2016, 23, 18832–18841. [Google Scholar] [CrossRef]
- Górny, D.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Naproxen ecotoxicity and biodegradation by Bacillus thuringiensis B1. Ecotoxicol. Environ. Saf. 2019, 167, 505–512. [Google Scholar] [CrossRef]
- Zdarta, J.; Jankowska, K.; Wyszowska, M.; Kijeńska-Gawrońska, E.; Zgoła-Grześkowiak, A.; Pinelo, M.; Meyer, A.S.; Moszyński, D.; Jesionowski, T. Robust biodegradation of naproxen and diclofenac by laccase immobilized using electrospun nanofibers with enhanced stability and reusability. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109789. [Google Scholar] [CrossRef]
- Sriuttha, P.; Sirichanchuen, B.; Permsuwan, U. Hepatotoxicity of nonsteroidal anti-inflammatory drugs: A systematic review of randomized controlled trials. Int. J. Hepatol. 2018, 2018, 5253623. [Google Scholar] [CrossRef]
- Ríos, A.L.M.; Gutierrez-Suarez, K.; Carmona, Z.; Ramos, C.G.; Oliveira, L.F.S. Pharmaceuticals as emerging pollutants: Case naproxen an overview. Chemosphere 2022, 291, 132822. [Google Scholar] [CrossRef]
- Ebele, A.J.; Abdallah, M.A.E.; Harrad, S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017, 3, 1–16. [Google Scholar] [CrossRef]
- Vulava, V.M.; Cory, W.C.; Murphey, V.L.; Ulmer, C.Z. Sorption, photodegradation and chemical transformation of naproxen and ibuprofen in soils and water. Sci. Total Environ. 2016, 565, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Nielsen, A.H.; Vollertsen, J. Sorption and degradation potential of pharmaceuticals in sediments form a stormwater retention pond. Water 2019, 11, 526. [Google Scholar] [CrossRef]
- Packer, J.L.; Werner, J.J.; Latch, D.E.; McNeill, K.; Arnold, W.A. Photochemical fate of pharmaceuticals in the environment: Naproxen, diclofenac, clofibric acid and ibuprofen. Aquat. Sci. 2003, 65, 342–351. [Google Scholar] [CrossRef]
- Sokół, A.; Borowska, K.; Karpińska, J. Investigating the influence of some environmental factors on the stability of paracetamol, naproxen and diclofenac in simulated natural conditions. Pol. J. Environ. Stud. 2017, 26, 293–302. [Google Scholar] [CrossRef]
- Topp, E.; Hendel, J.G.; Lapen, D.R.; Chapman, R. Fate of the nonsteroidal anti-inflammatory drug naproxen in agricultural soil receiving liquid municipal biosolids. Environ. Toxicol. Chem. 2008, 27, 2005–2010. [Google Scholar] [CrossRef] [PubMed]
- Cleuvers, M. Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen and acetylsalicylic acid. Ecotoxicol. Environ. Saf. 2004, 59, 309–315. [Google Scholar] [CrossRef]
- Melvin, S.D.; Cameron, M.C.; Lanctot, C.M. Individual and mixture toxicity of pharmaceuticals naproxen, carbamazepine and sulfamethoxazole to Australian striped marsh frog tadpoles (Limnodynastes peronii). J. Toxicol. Environ. Health A 2014, 77, 337–345. [Google Scholar] [CrossRef]
- Feng, L.; Song, W.; Oturan, N.; Karbasi, M.; van Hullebusch, E.D.; Esposito, G.; Giannakis, S.; Oturan, M.A. Electrochemical oxidation of Naproxen in aqueous matrices: Elucidating the intermediates’ eco-toxicity, by assessing its degradation pathways via experimental and density functional theory (DFT) approaches. Chem. Eng. J. 2023, 451, 138483. [Google Scholar] [CrossRef]
- Eslami, A.; Amini, M.M.; Asadi, A.; Safari, A.A.; Daglioglu, N. Photocatalytic degradation of ibuprofen and naproxen in water over NS-TiO2 coating on polycarbonate: Process modeling and intermediates identification. Inorg. Chem. Commun. 2020, 115, 107888. [Google Scholar] [CrossRef]
- Jureczko, M.; Przystaś, W. Pleurotus ostreatus and Trametes versicolor, fungal strains as remedy for recalcitrant pharmaceuticals removal current knowledge and future perspectives. Miomed J. Sci. Tech. Res. 2018, 3, BJSTR.MS.ID.000903. [Google Scholar] [CrossRef]
- Lloret, L.; Eibes, G.; Lu-Chau, T.A.; Moreira, M.T.; Feijoo, G.; Lema, J.M. Laccase-catalyzed degradation of anti-inflammatories and estrogens. Biochem. Eng. J. 2010, 51, 124–131. [Google Scholar] [CrossRef]
- Marco-Urrea, E.; Perez-Trujillo, M.; Blanquez, P.; Vicent, T.; Caminal, G. Biodegradation of analgesic naproxen by Trametes versicolor and identification of intermediates using HPLC-DADMS and NMR. Bioresour. Technol. 2010, 101, 2159–2166. [Google Scholar] [CrossRef] [PubMed]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Degradation of selected pharmaceutical and personal care products (PPCPs) by white-rot fungi. World J. Microbiol. Biotechnol. 2011, 27, 1839–1846. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Biotransformation of three pharmaceutical active compounds by the fungus Phanerochaete chrysosporium in a fed batch stirred reactor under air and oxygen supply. Biodegradation 2012, 23, 145–156. [Google Scholar] [CrossRef]
- NTran, H.; Urase, T.; Kusakabe, O. Biodegradation characteristics of pharmaceutical substances by whole fungal culture Trametes versicolor and its laccase. J. Water Environ. Technol. 2010, 8, 125–139. [Google Scholar] [CrossRef]
- Domaradzka, D.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Cometabolic degradation of naproxen by Planococcus sp. strain S5. Water Air Soil Pollut. 2015, 226, 297. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Domaradzka, D.; Guzik, U.; Wojcieszyńska, D. Bacillus thuringiensis B1 is a gram-positive bacteria able to degrade naproxen and ibuprofen. Water Air Soil. Pollut. 2016, 227, 197. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Domaradzka, D.; Hupert-Kocurek, K.; Guzik, U. Bacterial degradation of naproxen—Undisclosed pollutant in the environment. J. Environ. Manag. 2014, 145, 157–161. [Google Scholar] [CrossRef]
- Wojcieszyńska, D.; Domaradzka, D.; Hupert-Kocurek, K.; Guzik, U. Enzymes involved in naproxen degradation by Planococcus sp. S5. Pol. J. Microbiol. 2016, 65, 177–182. [Google Scholar] [CrossRef]
- Hasan, Z.; Choi, E.J.; Jhung, S.H. Adsorption of naproxen and clofibric acid over a metal–organic framework MIL-101 functionalized with acidic and basic groups. Chem. Eng. J. 2013, 219, 537–544. [Google Scholar] [CrossRef]
- Suriyanon, N.; Permrungruang, J.; Kaosaiphun, J.; Wongrueng, A.; Ngamcharussrivichai, C.; Punyapalakul, P. Selective adsorption mechanisms of antilipidemic and non-steroidal anti-inflammatory drug residues on functionalized silica-based porous materials in a mixed solute. Chemosphere 2015, 136, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Peldszus, S.; Huck, P.M. Adsorption characteristics of selected pharmaceuticals and an endocrine disrupting compound—Naproxen, carbamazepine and nonylphenol on activated carbon. Water Res. 2008, 42, 2873–2882. [Google Scholar] [CrossRef]
- Baccar, R.; Sarr, M.; Bouzid, J.; Feki, M.; Blanquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211, 310–317. [Google Scholar] [CrossRef]
- Hasan, Z.; Jeon, J.; Jhung, S.H. Adsorptive removal of naproxen and clofibric acid from water using metal-organic frameworks. J. Hazard. Mater. 2012, 209, 151–157. [Google Scholar] [CrossRef]
- Phasuphan, W.; Praphairaksit, N.; Imyim, A. Removal of ibuprofen, diclofenac, and naproxen from water using chitosan-modified waste tire crumb rubber. J. Mol. Liq. 2019, 294, 111554. [Google Scholar] [CrossRef]
- Husein, D.Z.; Hassanien, R.; Al-Hakkani, M.F. Green-synthesized copper nano-adsorbent for the removal of pharmaceutical pollutants from real wastewater samples. Heliyon 2019, 5, e02339. [Google Scholar] [CrossRef]
- Jung, C.; Boateng, L.K.; Flora, J.R.; Oh, J.; Braswell, M.C.; Son, A.; Yoon, Y. Competitive adsorption of selected non-steroidal anti-inflammatory drugs on activated biochars: Experimental and molecular modeling study. Chem. Eng. J. 2015, 264, 1–9. [Google Scholar] [CrossRef]
- Ilbay, Z.; Sahin, S.; Kerkez, O.Z.G.E.; Bayazit, S.S. Isolation of naproxen from wastewater using carbon-based magnetic adsorbents. Int. J. Environ. Sci. Technol. 2015, 12, 3541–3550. [Google Scholar] [CrossRef]
- Mlunguza, N.Y.; Ncube, S.; Mahlambi, P.N.; Chimuka, L.; Madikizela, L.M. Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- Soares, S.F.; Trindade, T.; Daniel-da-Silva, A.L. Enhanced removal of non-steroidal inflammatory drugs from water by quaternary chitosan-based magnetic nanosorbents. Coatings 2021, 11, 964. [Google Scholar] [CrossRef]
- Nodeh, M.K.M.; Radfard, M.; Zardari, L.A.; Nodeh, H.R. Enhanced removal of naproxen from wastewater using silica magnetic nanoparticles decorated onto graphene oxide; parametric and equilibrium study. Sep. Sci. Technol. 2018, 53, 2476–2485. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; He, L.; Meng, L.; Lu, H.; Li, X. Preparation of poly (4-vinylpyridine)-functionalized magnetic Al-MOF for the removal of naproxen from aqueous solution. J. Hazard. Mater. 2020, 383, 121144. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P. Chapter 7—Thermal-Treatment-Induced Deformations and Modifications of Halloysite. In Developments in Clay Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 137–166. [Google Scholar]
- Sołtys, J.; Schomburg, J.; Sakiewicz, P.; Pytliński, A.; Jóźwiak, K.; Sołtys, B. Haloizyt ze złoża Dunino jako surowiec do wytwarzania sorbentów mineralnych. In Sorbenty Mineralne: Surowce, Energetyka, Ochrona Środowiska, Nowoczesne Technologie; Ratajczak, T., Rzepa, G., Bajda, T., Eds.; AGH: Kraków, Poland, 2013; pp. 457–469. ISBN 978-83-7464-629-1. [Google Scholar]
- Piotrowski, K.; Sakiewicz, P.; Gołombek, K. Halloysite as main nanostructural filler in multifunctional mixed matrix membranes—Review of applications and new possibilities. Desalination Water Treat. 2021, 243, 91–106. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption. In Surface Area and Porosity, 2nd ed.; Academic Press: London, UK, 1982. [Google Scholar]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Annabi-Bergaya, F.; Yan, W.; Fan, M.; Liu, D.; He, H. Changes in structure, morphology, porosity, and surface activity of mesoporous halloysite nanotubes under heating. Clays Clay Miner. 2012, 60, 561–573. [Google Scholar] [CrossRef]
- Joussein, E.; Petit, S.; Delvaux, B. Behavior of halloysite clay under formamide treatment. Appl. Clay Sci. 2007, 35, 17–24. [Google Scholar] [CrossRef]
- Cheng, H.; Frost, R.L.; Yang, J.; Liu, Q.; He, J. Infrared and infrared emission spectroscopic study of typical Chinese kaolinite and halloysite. Spectrochim. Acta Part A 2010, 77, 1014–1020. [Google Scholar] [CrossRef]
- Szczepanik, B.; Frydel, L.; Słomkiewicz, P.M.; Banaś, D.; Stabrawa, I.; Kubala-Kukuś, A. Adsorptive removal of chloroxylenol and chlorophene from aqueous solutions using carbon-halloysite nanocomposites obtained from corrugated cardboard as a carbon precursor. Desalination Water Treat. 2023, 288, 93–103. [Google Scholar] [CrossRef]
- Szczepanik, B.; Rędzia, N.; Frydel, L.; Słomkiewicz, P.; Kołbus, A.; Styszko, K.; Dziok, T.; Samojeden, B. Synthesis and Characterization of Halloysite/Carbon Nanocomposites for Enhanced NSAIDs Adsorption from Water. Materials 2019, 12, 3754. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, B.; Banaś, D.; Kubala-Kukuś, A.; Szary, K.; Słomkiewicz, P.; Rędzia, N.; Frydel, L. Surface Properties of Halloysite-Carbon Nanocomposites and Their Application for Adsorption of Paracetamol. Materials 2020, 13, 5647. [Google Scholar] [CrossRef] [PubMed]
- Bernal, V.; Erto, A.; Giraldo, L.; Moreno-Piraján, J.C. Effect of solution pH on the adsorption of paracetamol on chemically modified activated carbons. Molecules 2017, 22, 1032. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances, Kungliga Svenska Vetenskapsakademiens. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second-order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Gamoudi, S.; Srasra, E. Adsorption of organic dyes by HDPy+-modified clay: Effect of molecular structure on the adsorption. J. Mol. Struct. 2019, 1193, 522–531. [Google Scholar] [CrossRef]
- Lou, Z.; Xing, S.; Xiao, X.; Shan, W.; Xiong, Y.; Fan, Y. Selective adsorption of Re (VII) by chitosan modified with imidazolium-based ionic liquid. Hydrometallurgy 2018, 179, 141–148. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, T.; Zhu, N.; Li, D.; Chen, Z.; Lang, Q.; Liu, Z.; Jiao, W. Enhanced adsorption of Pb (II) onto modified hydrochar: Modeling and mechanism analysis. Bioresour. Technol. 2019, 288, 121593. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y. Modified bentonite adsorption of organic pollutants of dye wastewater. Mater. Chem. Phys. 2017, 202, 266–276. [Google Scholar] [CrossRef]
- Zhuang, Y.; Yu, F.; Ma, J.; Chen, J.H. Enhanced adsorption removal of antibiotics from aqueous solutions by modified alginate/graphene double network porous hydrogel. J. Colloid. Interface Sci. 2017, 507, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Komers, R.; Tomanova, D.; Beranek, L. Adsorption of Weak Bases from the Gas Phase on Organic Ion-Exchangers. J. Catal. 1973, 30, 343–349. [Google Scholar] [CrossRef]
- Kurczewska, J.; Cegłowski, M.; Schroeder, G. PAMAM-halloysite Dunino hybrid as an effective adsorbent of ibuprofen and naproxen from aqueous solutions. Appl. Clay Sci. 2020, 190, 105603. [Google Scholar] [CrossRef]
- Madikizela, L.M.; Chimuka, L. Synthesis, adsorption and selectivity studies of a polymer imprinted with naproxen, ibuprofen and diclofenac. J. Environ. Chem. Eng. 2016, 4, 4029–4037. [Google Scholar] [CrossRef]
- Thiebault, T.; Boussafir, M.; Le Forestier, L.; Le Milbeau, C.; Monnin, L.; Guégan, R. Competitive adsorption of a pool of pharmaceuticals onto a raw clay mineral. RSC Adv. 2016, 6, 65257–65265. [Google Scholar] [CrossRef]
- Kurtulbaş, E.; Bilgin, M.; Şahin, S.; Bayazit, Ş.S. Comparison of different polymeric resins for naproxen removal from wastewater. J. Mol. Liq. 2017, 241, 633–637. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Hakami, W.; Salam, M.A. Removal of non-steroidal anti-inflammatory drugs from water using high surface area nanographene: Kinetic and thermodynamic studies. J. Mol. Liq. 2017, 241, 733–741. [Google Scholar] [CrossRef]
- Rafati, L.; Ehrampoush, M.H.; Rafati, A.A.; Mokhtari, M.; Mahvi, A.H. Modeling of adsorption kinetic and equilibrium isotherms of naproxen onto functionalized nanoclay composite adsorbent. J. Mol. Liq. 2016, 224, 832–841. [Google Scholar] [CrossRef]
- Sun, W.; Li, H.; Li, H.; Li, S.; Cao, X. Adsorption mechanisms of ibuprofen and naproxen to UiO-66 and UiO-66-NH2: Batch and DFT calculation. Chem. Eng. J. 2019, 360, 645–653. [Google Scholar] [CrossRef]






| Compound | Naproxen |
|---|---|
| Molecular structure | 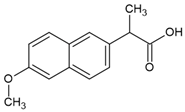 |
| IUPAC name | (2S)-2-(6-methoxynaphthalen-2-yl)propanoic acid |
| Chemical formula | C14H14O3 |
| Molecular weight | 230.26 g mol−1 |
| Water solubility | 15.9 mg·dm−3 (25 °C) |
| pKa * | 4.15 |
| logKOW ** | 3.18 |
| Adsorbent | SBET (m2 g−1) | Vt (cm3 g−1) | Vmi (cm3 g−1) | Vme (cm3 g−1) | Mesoporosity (%) |
|---|---|---|---|---|---|
| HS | 45.64 | 0.1925 | 0.0036 | 0.1906 | 99 |
| 15HS | 27.23 | 0.1348 | 0.0027 | 0.1321 | 98 |
| 30HS | 43.37 | 0.1559 | 0.0015 | 0.1544 | 99 |
| 45HS | 54.75 | 0.1340 | 0.0057 | 0.1283 | 96 |
| HK1 | 62.58 | 0.1896 | 0.0020 | 0.1876 | 99 |
| 15HK1 | 29.67 | 0.1423 | 0.0016 | 0.1407 | 99 |
| 30HK1 | 31.20 | 0.1369 | 0.0037 | 0.1332 | 97 |
| 45HK1 | 41.87 | 0.1508 | 0.0039 | 0.1469 | 97 |
| HK05 | 69.74 | 0.1891 | 0.0020 | 0.1871 | 99 |
| 15HK05 | 33.73 | 0.1611 | 0.0009 | 0.1602 | 99 |
| 30HK05 | 39.67 | 0.1695 | 0.0031 | 0.1664 | 98 |
| 45HK05 | 42.15 | 0.1671 | 0.0022 | 0.1649 | 99 |
| HK025 | 64.01 | 0.1757 | 0.0018 | 0.1739 | 97 |
| 15HK025 | 30.35 | 0.1410 | 0.0007 | 0.1403 | 99 |
| 30HK025 | 34.61 | 0.1518 | 0.0013 | 0.1505 | 99 |
| 45HK025 | 42.15 | 0.1671 | 0.0022 | 0.1649 | 99 |
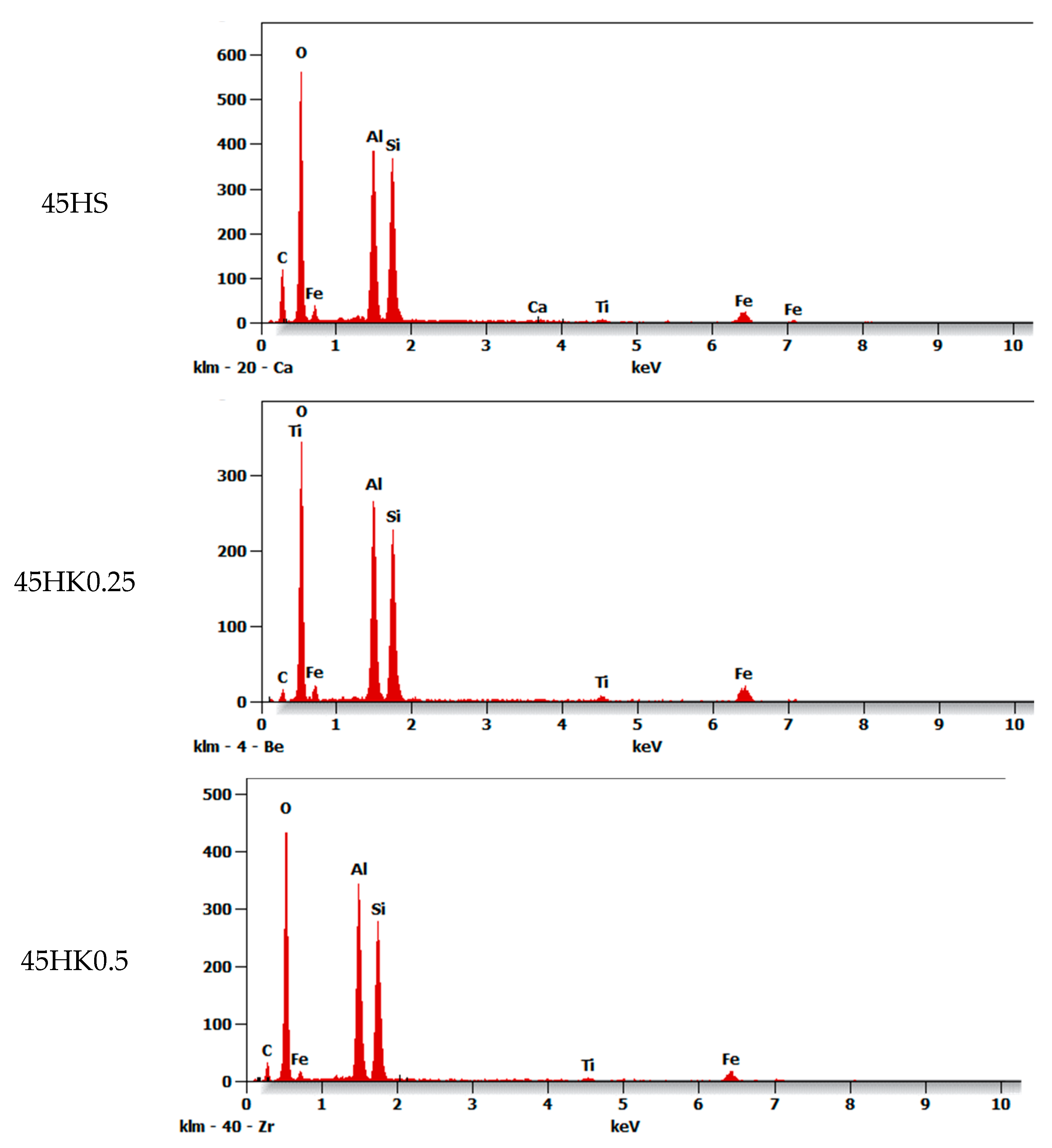
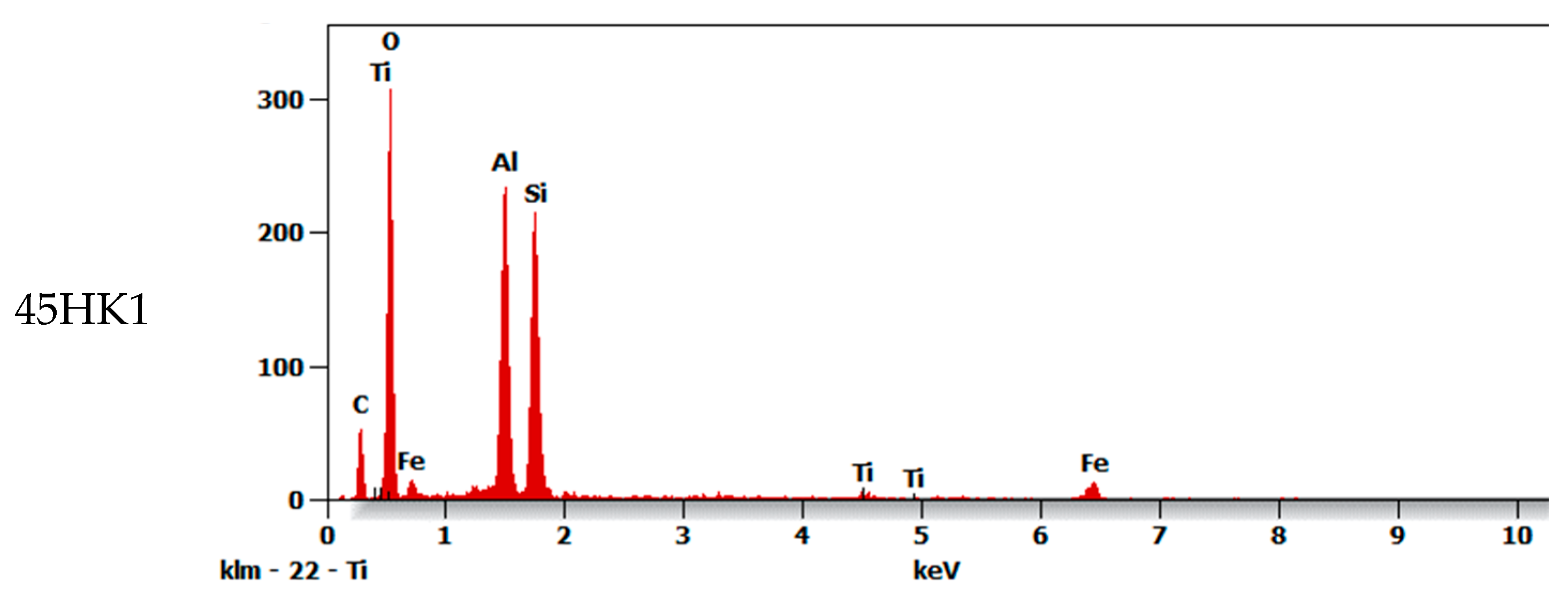
| Composite | C | O | Al | Si | Ti | Fe |
|---|---|---|---|---|---|---|
| 45HS | 12.2 | 42.3 | 15.0 | 17.1 | 1.3 | 11.7 |
| 45HK0.25 | 2.4 | 42.4 | 18.7 | 19.6 | 2.2 | 14.7 |
| 45HK0.5 | 11.3 | 41.4 | 16.0 | 16.1 | 1.3 | 13.2 |
| 45HK1 | 10.3 | 42.6 | 16.5 | 18.6 | 1.2 | 11.0 |
| Adsorbate | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||
|---|---|---|---|---|
| Naproxen | k1 (min−1) | R2 | k2 (g mg−1 min−1) | R2 |
| 0.00923 | 0.85353 | 0.03166 | 0.9991 | |
| Adsorbate | kd1 (mg g−1 min−1/2) | c1 (mg g−1) | R12 | kd2 (mg g−1 min−1/2) | c2 (mg g−1) | R22 |
|---|---|---|---|---|---|---|
| Naproxen | 0.4101 | 0.938 | 0.6523 | 0.0096 | 2.372 | 0.7456 |
| Langmuir Multi-Center Model | |||
|---|---|---|---|
| KMF (dm3∙mg−1)1/n | qm (mg∙g−1) | n | R2 |
| 1.34 | 5.69 | 1.19 | 0.9934 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słomkiewicz, P.; Szczepanik, B.; Sakiewicz, P.; Gołombek, K.; Piotrowski, K. Carbon-Halloysite Nanocomposites and Their Adsorption Characteristics for Pharmaceuticals—A Naproxen Case Study. Materials 2025, 18, 2433. https://doi.org/10.3390/ma18112433
Słomkiewicz P, Szczepanik B, Sakiewicz P, Gołombek K, Piotrowski K. Carbon-Halloysite Nanocomposites and Their Adsorption Characteristics for Pharmaceuticals—A Naproxen Case Study. Materials. 2025; 18(11):2433. https://doi.org/10.3390/ma18112433
Chicago/Turabian StyleSłomkiewicz, Piotr, Beata Szczepanik, Piotr Sakiewicz, Klaudiusz Gołombek, and Krzysztof Piotrowski. 2025. "Carbon-Halloysite Nanocomposites and Their Adsorption Characteristics for Pharmaceuticals—A Naproxen Case Study" Materials 18, no. 11: 2433. https://doi.org/10.3390/ma18112433
APA StyleSłomkiewicz, P., Szczepanik, B., Sakiewicz, P., Gołombek, K., & Piotrowski, K. (2025). Carbon-Halloysite Nanocomposites and Their Adsorption Characteristics for Pharmaceuticals—A Naproxen Case Study. Materials, 18(11), 2433. https://doi.org/10.3390/ma18112433







