Research Progress on Major Influencing Factors of Corrosion Behavior of Pipeline Steel in Supercritical CO2 Environment
Abstract
1. Introduction
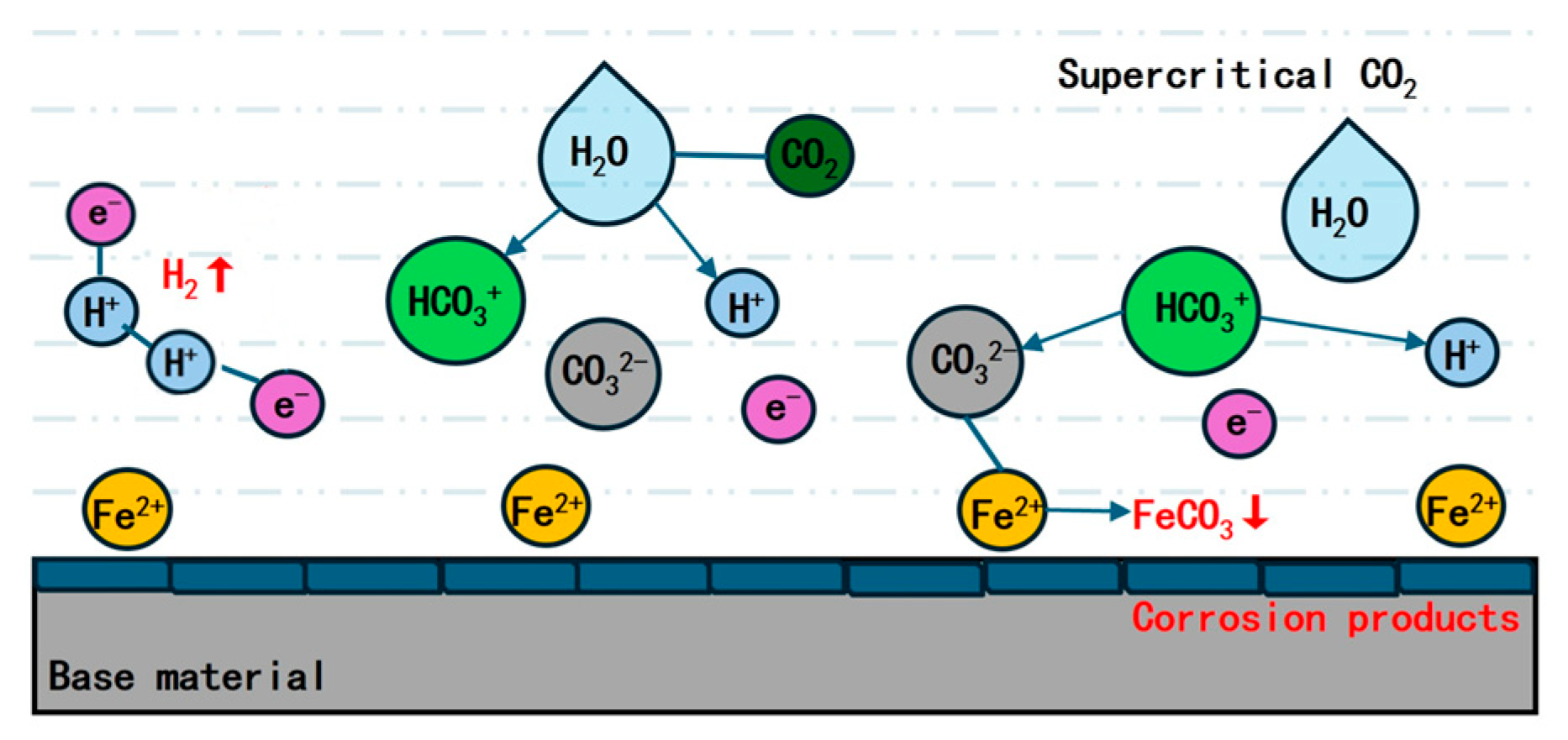
2. Corrosion Behavior of Carbon Steel in CO2
- (1)
- When CO2 dissolves in water, it reacts chemically with water molecules to form H2CO3. H2CO3 is categorized as a weak acid, and a fraction of it further dissociates into bicarbonate ions (HCO3−) and carbonate ions (CO32−).
- (2)
- Cathode reaction: The reduction of H+ ions occurs, leading to the acquisition of electrons and the formation of H2. Additionally, this reduction process involves H2CO3 and CO32−.
- (3)
- The anode reaction encompasses the dissolution of the Fe anode, the generation of Fe2+ ions, and the formation of carbonate compounds.
3. Factors Influencing Corrosion in Supercritical CO2 Transportation Pipelines
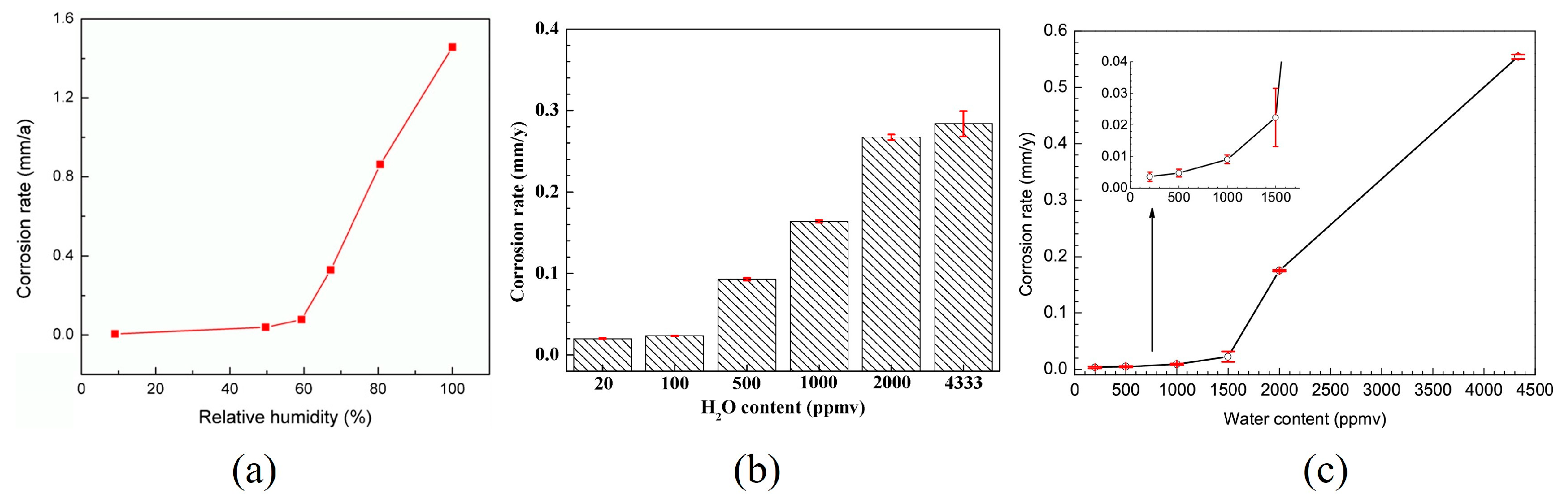
3.1. Water Content
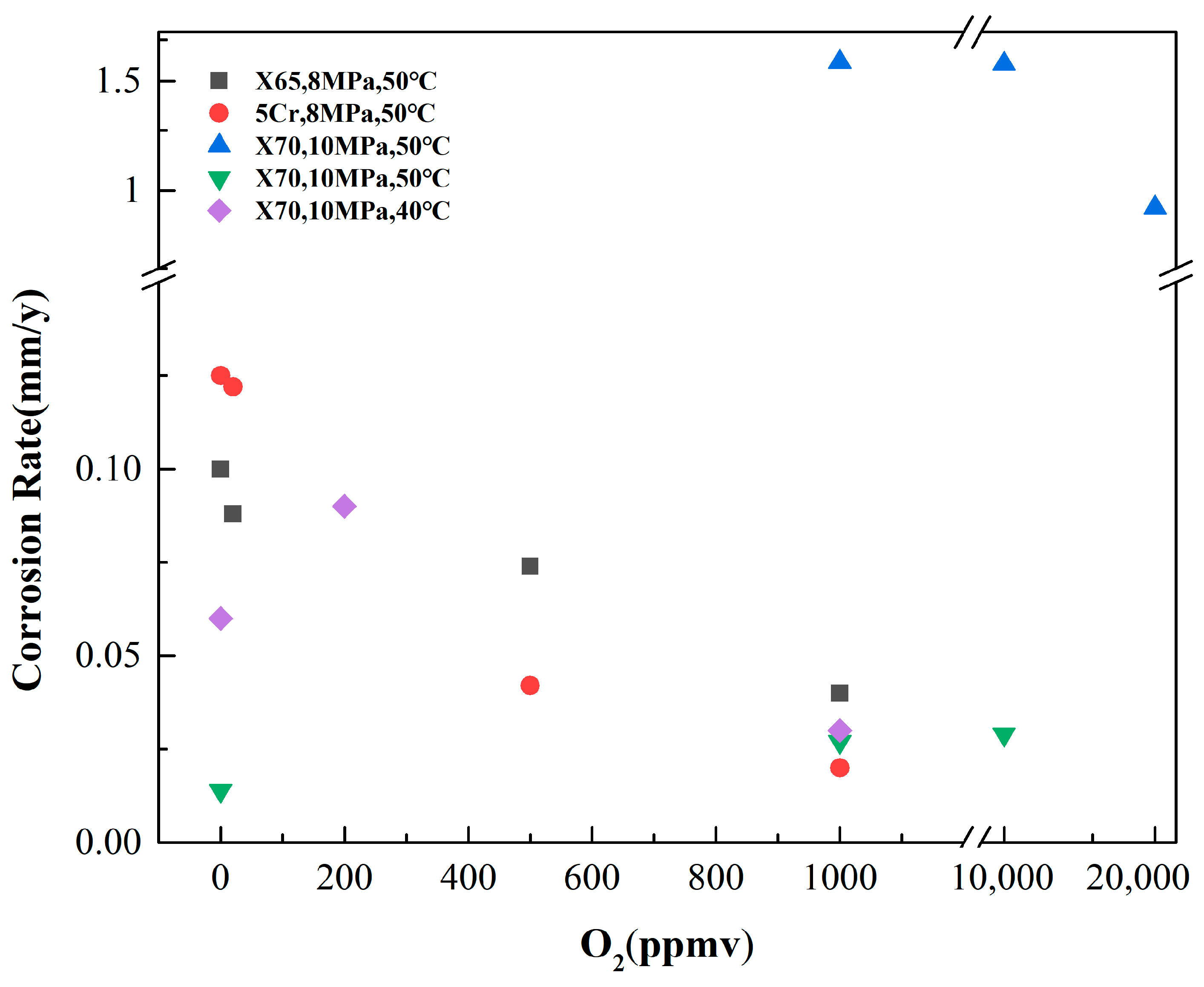
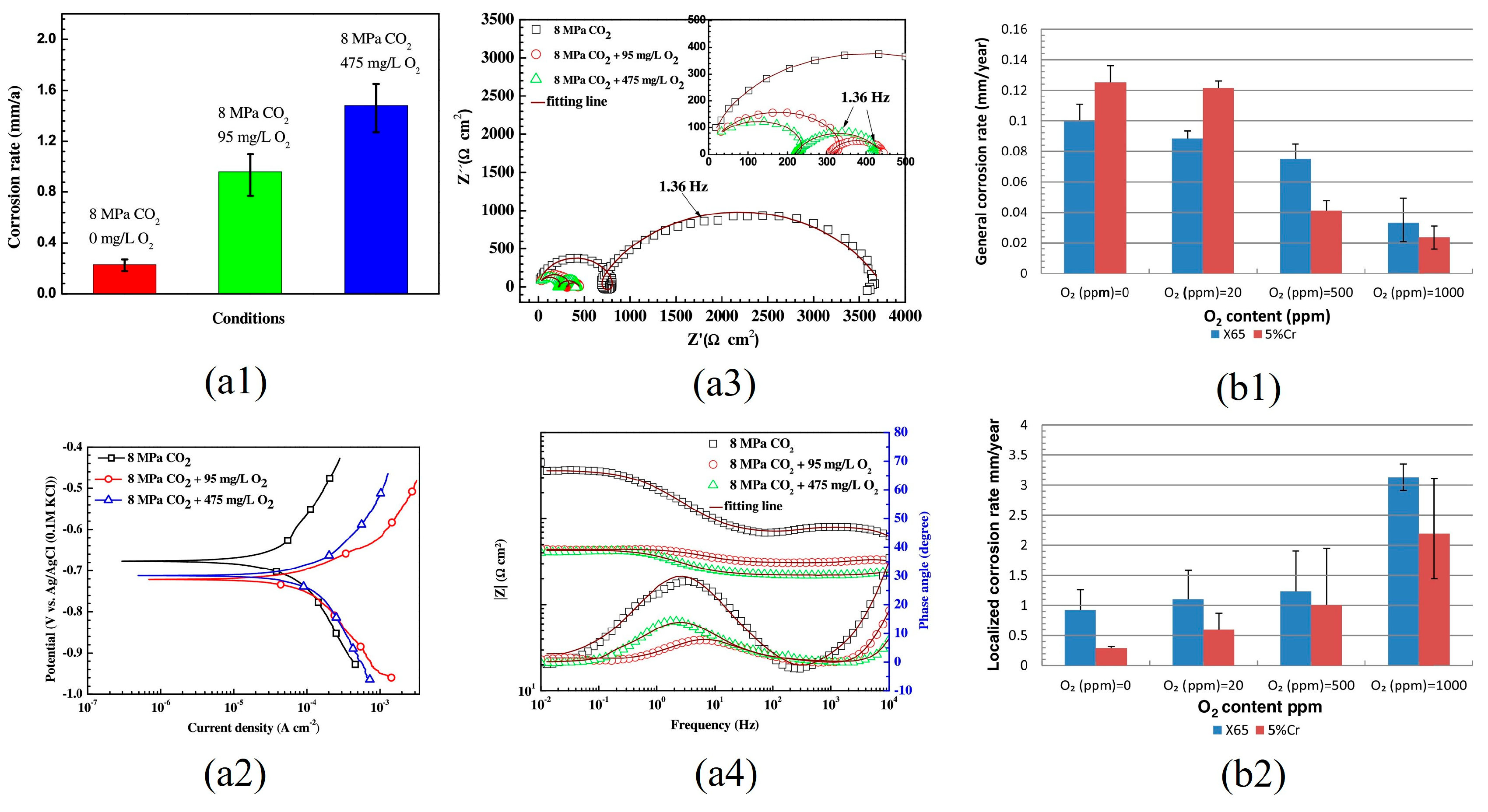
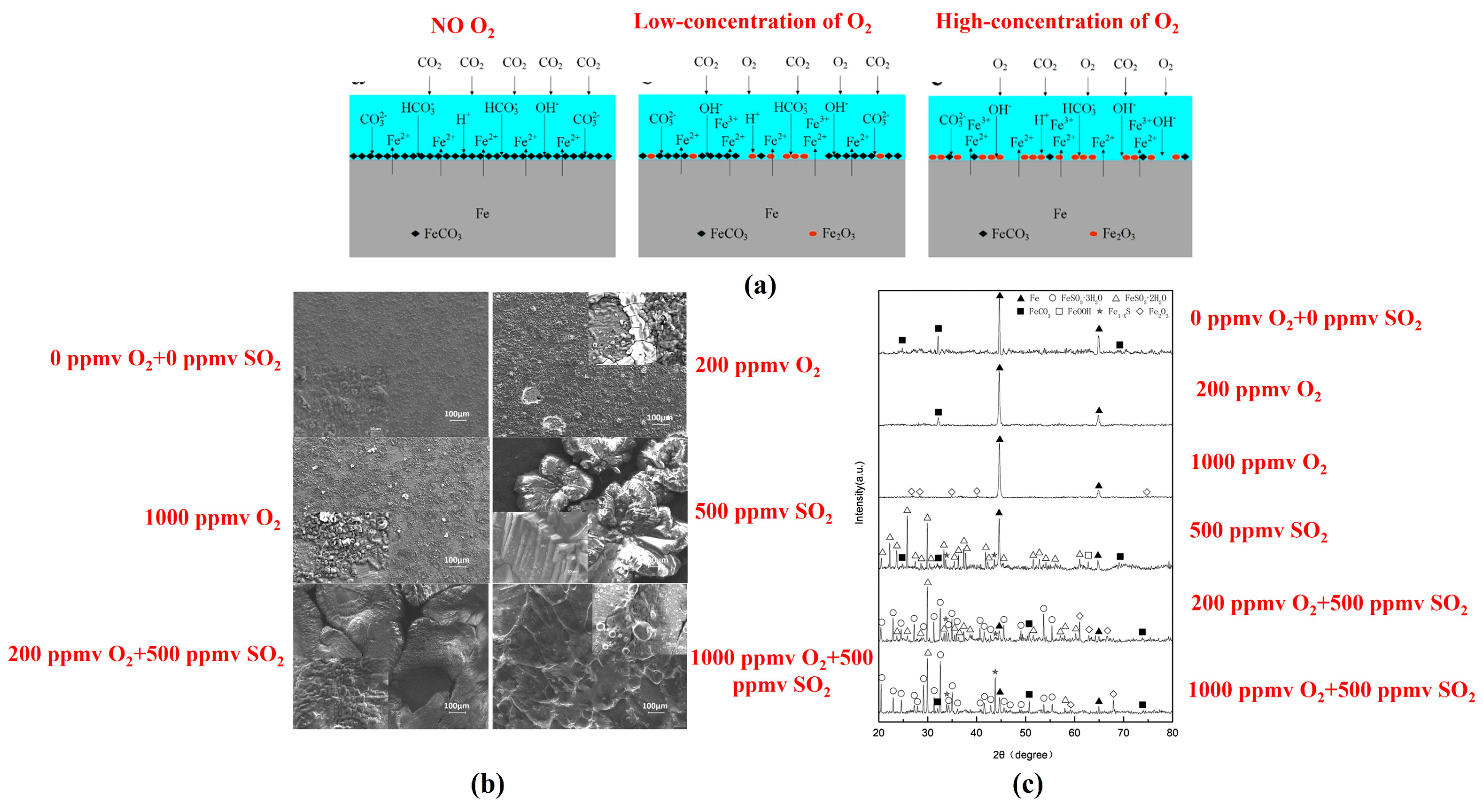
3.2. O2 Content
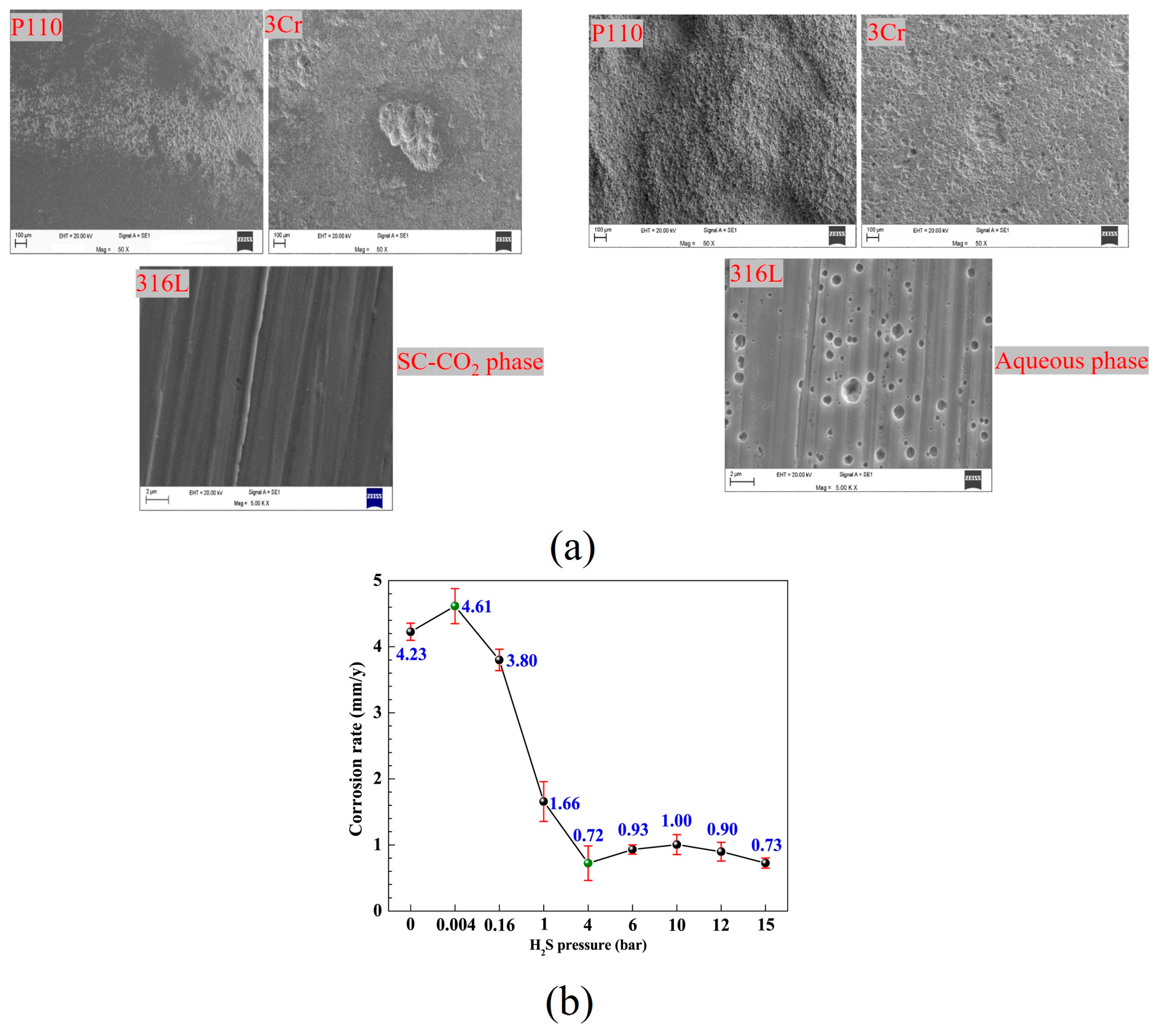
3.3. H2S Content
3.4. SO2 Content
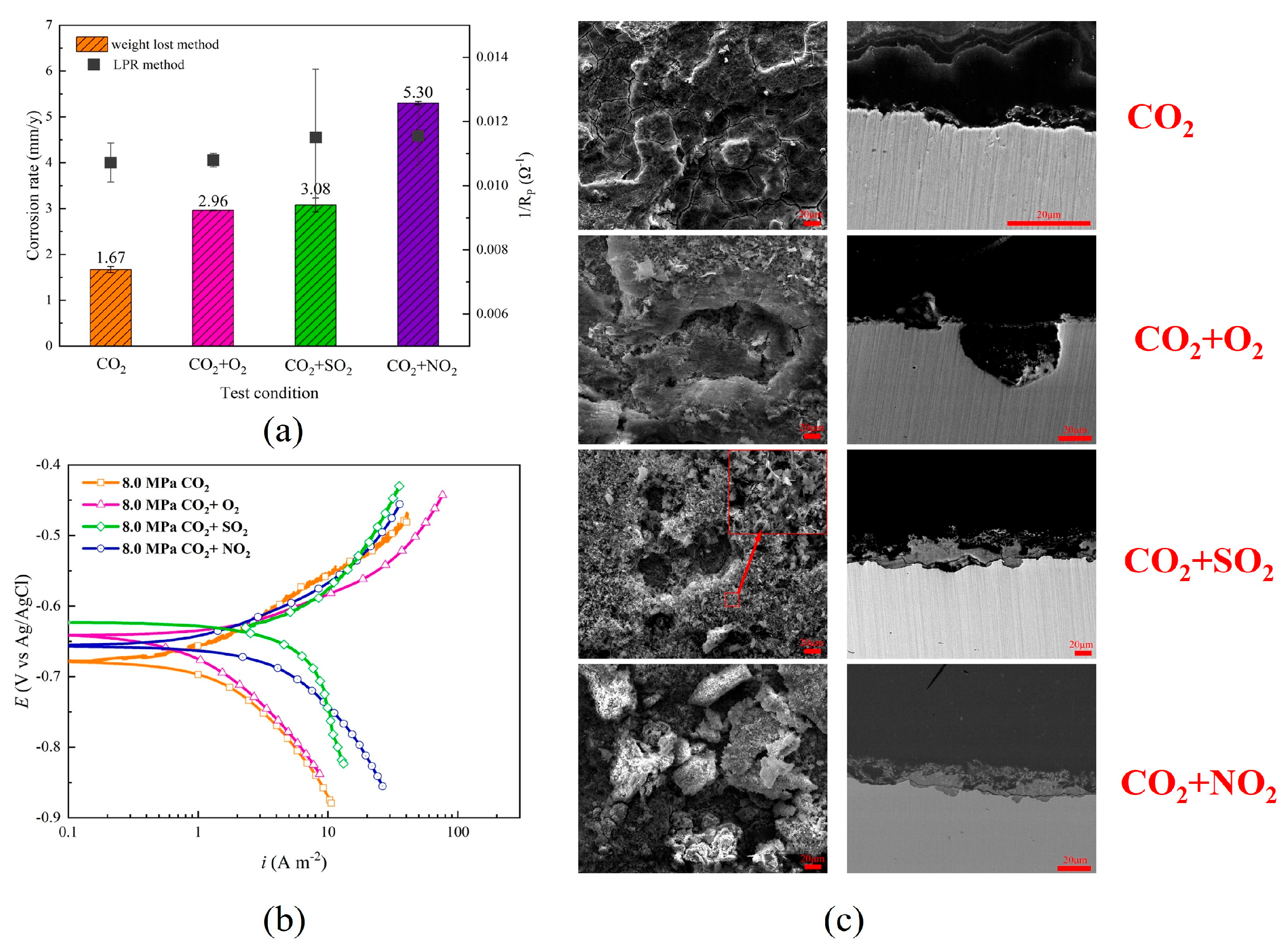
3.5. NO2 Content
| Materials | Pressures (MPa) | Temperature (°C) | Environment | NO2 Content | Other Impurities | Corrosion Time (h) | Corrosion Rate (mm/y) | Corrosion Products | Reference |
|---|---|---|---|---|---|---|---|---|---|
| X80 | 8 | 35 | CO2-saturated 1wt% NaCl solution | 0 | 48 | 1.67 | FeCO3, Fe2O3 | [63] | |
| 100 ppmv | 5.30 | FeCO3, FeOOH | |||||||
| X65 | 10 | 50 | water-saturated CO2 phase | 0 | 24 | ~0.04 | FeCO3 | [69] | |
| 1000 ppmv | 1.72–1.76 | FeCO3, Fe(NO3)3∙9H2O, Fe2O3∙H2O | |||||||
| 0 | 120 | <0.04 | FeCO3 | ||||||
| 1000 ppmv | 0.44–0.48 | FeCO3, Fe(NO3)3∙9H2O, Fe2O3∙H2O |
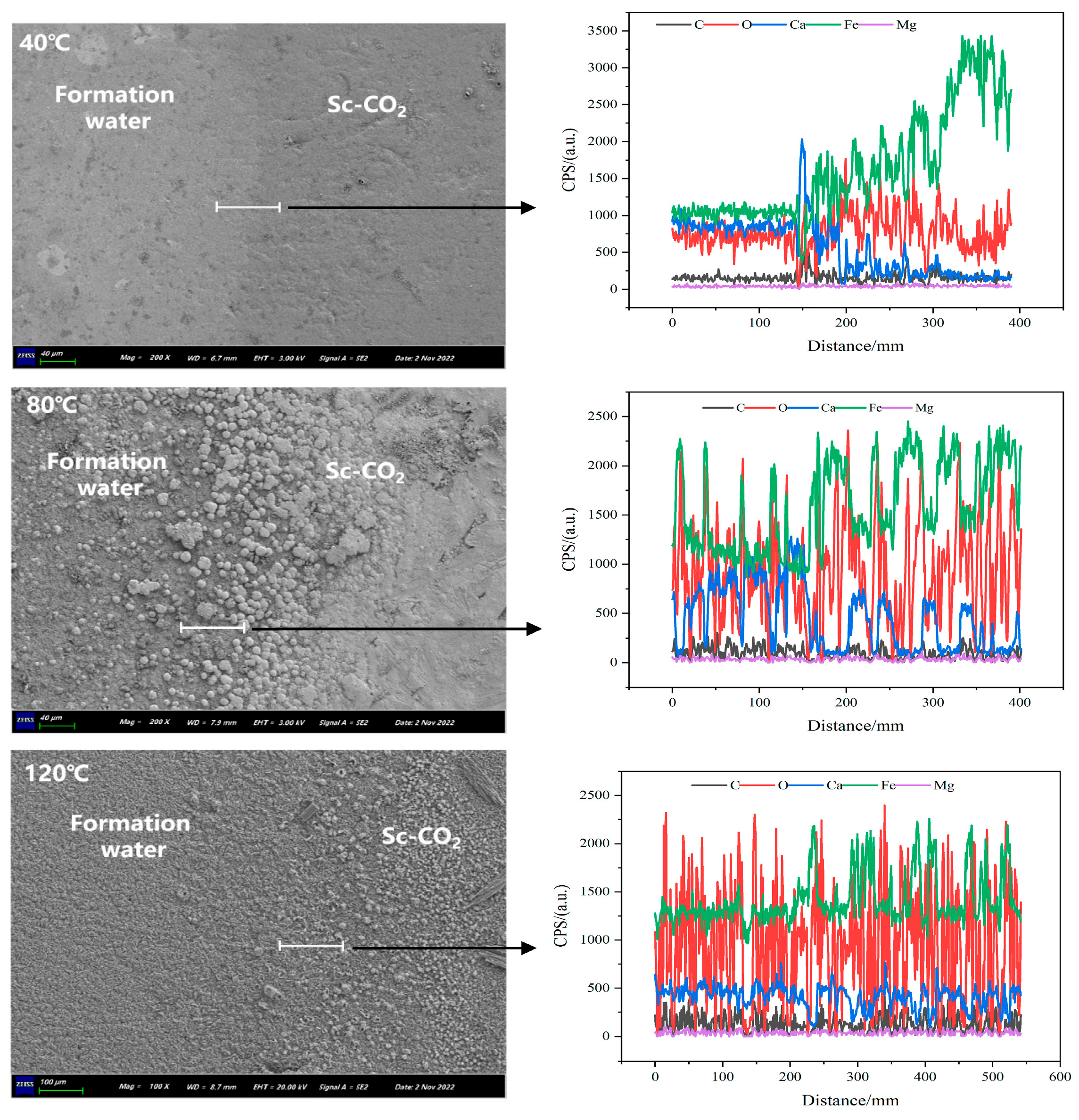
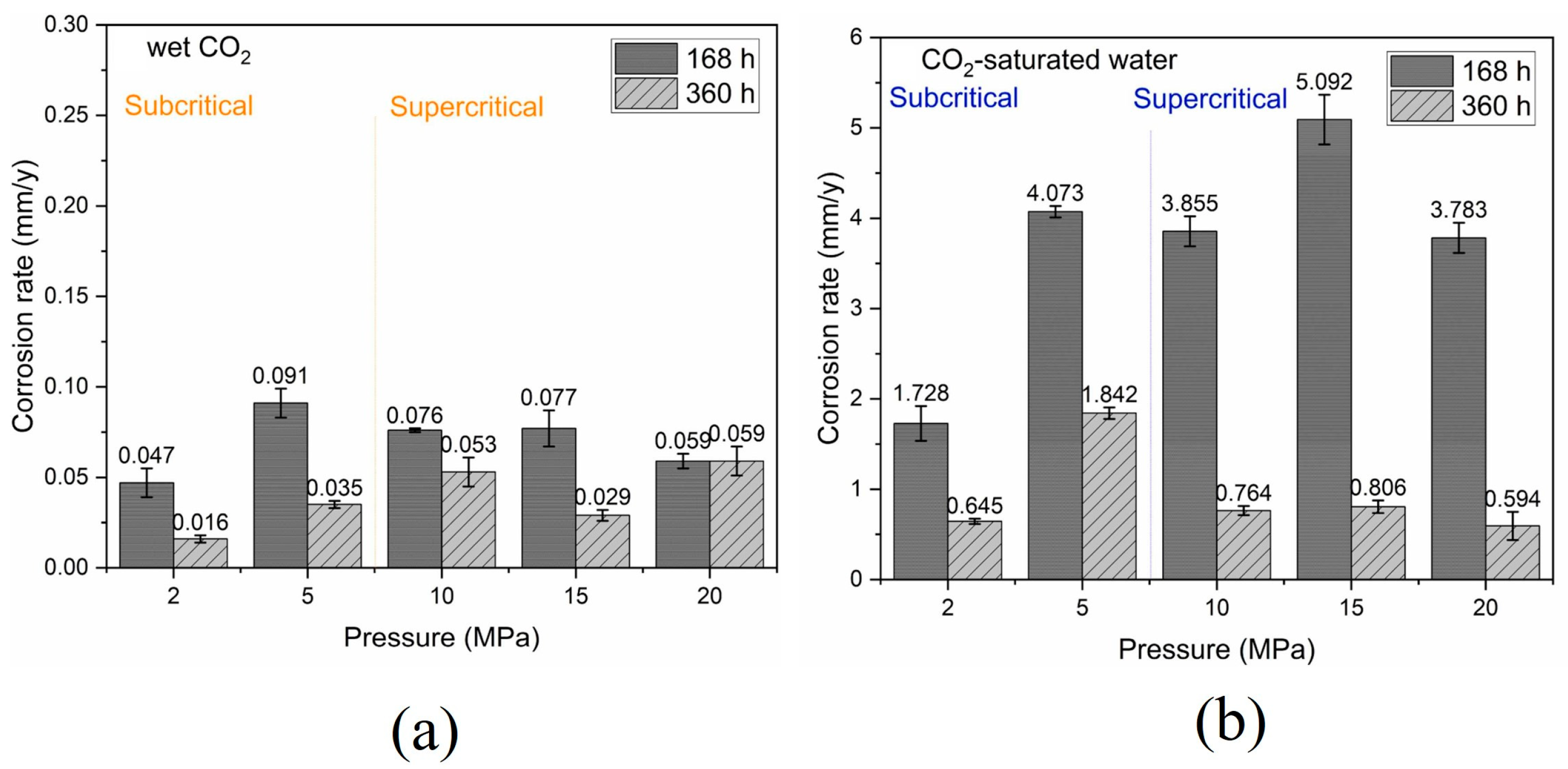
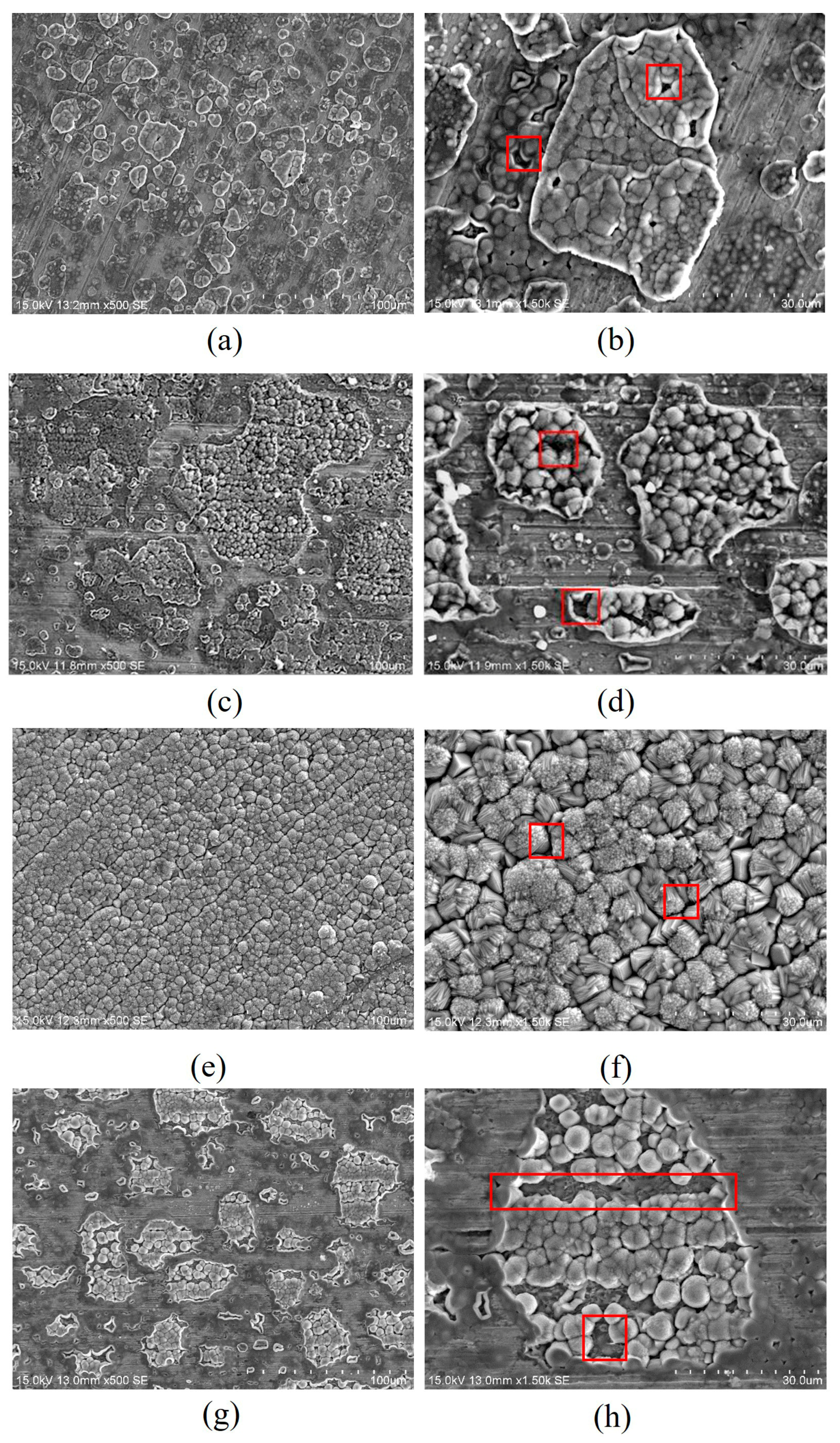
3.6. Influence of Working Conditions (Temperature, Pressure, Flow Rate, Time)
4. Corrosion of Welded Joints in Supercritical CO2 Environments
5. Conclusions
- When H2O is present in supercritical CO2 systems, gas impurities react with water to form corrosive products. Extensive studies have demonstrated that the corrosion rate increases significantly once the water content surpasses a specific critical threshold. However, the critical water content varies among different corrosion systems and materials. Under identical water content conditions, the localized corrosion rate can be several dozen times higher than the average corrosion rate. Controlling water content is crucial for mitigating pipeline corrosion. Compared to the average corrosion rate, taking the local corrosion rate into account is more reasonable for controlling water content in supercritical CO2 fluids. It is crucial to emphasize the impact of water content on the localized corrosion behavior of pipeline steel in future research.
- A small amount of O2 accelerates the corrosion of steel; however, at high concentrations, O2 can induce passivation of the substrate, thereby significantly reducing the corrosion rate. O2 can facilitate local corrosion of steel in supercritical CO2 environments and significantly influence the rapid progression of localized corrosion. Acidic gases, such as H2S, SO2 and NO2, can accelerate the corrosion rate of steel, and their influence on the corrosion behavior of steel in the water phase is significantly greater than that in the CO2 phase. Among these gases, NO2 exhibits the most significant impact, often leading to localized corrosion. H2S and SO2 do not accelerate the localized corrosion rate of steel. When various types of gas impurities are present, the composition of corrosion products becomes more complex, and the corrosion products form a multi-layer structure. The synergistic effects of multiple impurities are more detrimental than those of individual impurities, and water content plays a crucial role in multi-impurity systems. The influence of the synergistic effect of multiple impurities on the corrosion behavior of pipeline steel requires further investigation and merits considerable attention.
- Temperature and pressure significantly influence the formation and properties of corrosion product films. Numerous studies have demonstrated that, within a specific temperature range, elevated temperatures can promote the densification of corrosion product films, thereby enhancing the protective effect on the substrate surface. Moreover, the effects of pressure exhibit variability across different systems. The corrosion rate of steel increases with the flow velocity of supercritical CO2 fluid, and the dynamic flow of the fluid can lead to localized corrosion of steel. The morphology and structure of the corrosion products evolve as the corrosion time increases.
- Research on welded joints of pipeline steel in the supercritical CO2 environment remains relatively limited. Existing studies indicate that distinct morphologies of corrosion products form in the HAZ, WM, and BM of X80 welded joints under supercritical CO2 conditions. However, a comprehensive and in-depth understanding of the corrosion mechanism remains insufficient. Specifically, there is a lack of comprehensive investigation into the influence of water content and impurity gases on the corrosion behavior of different pipeline steel welded joints.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, S.; Jiao, X.; Zheng, D.; Zhang, Y.; Xie, H.; Guo, Z. Demand and Fluctuation Range of China’s Coal Production under the Dual Carbon Target. Energy Rep. 2024, 11, 3267–3282. [Google Scholar] [CrossRef]
- Wen, W.; Su, Y.; Tang, Y.; Zhang, X.; Hu, Y.; Ben, Y.; Qu, S. Evaluating Carbon Emissions Reduction Compliance Based on “dual Control” Policies of Energy Consumption and Carbon Emissions in China. J. Environ. Manag. 2024, 367, 121990. [Google Scholar] [CrossRef]
- Zhu, H.; Cao, S.; Su, Z.; Zhuang, Y. China’s Future Energy Vision: Multi-Scenario Simulation Based on Energy Consumption Structure under Dual Carbon Targets. Energy 2024, 301, 131751. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Tian, J. The Evolutionary Analysis of Investment in CCS-EOR under Dual Carbon Target—From the Perspective of Multi-Agent Involvement. Int. J. Greenh. Gas Control 2024, 135, 104107. [Google Scholar] [CrossRef]
- Dou, L.; Sun, L.; Lyu, W.; Wang, M.; Gao, F.; Gao, M.; Jiang, H. Trend of Global Carbon Dioxide Capture, Utilization and Storage Industry and Challenges and Countermeasures in China. Pet. Explor. Dev. 2023, 50, 1246–1260. [Google Scholar] [CrossRef]
- Wang, W.; Guang, Y.; Liu, W.; Shen, K.; Huffman, M.; Wang, Q. Experimental Investigation of Stress Corrosion on Supercritical CO2 Transportation Pipelines against Leakage for CCUS Applications. Energy Rep. 2023, 9, 266–276. [Google Scholar] [CrossRef]
- Global Status of CCS 2024. Available online: https://www.globalccsinstitute.com/resources/global-status-report/ (accessed on 23 January 2025).
- Kim, T.W.; Yoon, H.C.; Lee, J.Y. Review on Carbon Capture and Storage (CCS) from Source to Sink; Part 1: Essential Aspects for CO2 Pipeline Transportation. Int. J. Greenh. Gas Control 2024, 137, 104208. [Google Scholar] [CrossRef]
- Peletiri, S.P.; Rahmanian, N.; Mujtaba, I.M. CO2 Pipeline Design: A Review. Energies 2018, 11, 2184. [Google Scholar] [CrossRef]
- Miao, L.; Feng, L.; Ma, Y. Comprehensive Evaluation of CCUS Technology: A Case Study of China’s First Million-Tonne CCUS-EOR Project. Environ. Impact Assess. Rev. 2025, 110, 107684. [Google Scholar] [CrossRef]
- Yang, L.; Rui, W.; Zhao, Q.M.; Zhou, Y.L.; Xin, F.; Xue, Z.J. CO2-Enhanced Oil Recovery with CO2 Utilization and Storage: Progress and Practical Applications in China. Unconv. Resour. 2024, 4, 100096. [Google Scholar] [CrossRef]
- Sarrade, S.; Feron, D.; Rouillard, F.; Perrin, S.; Robin, R.; Ruiz, J.-C.; Turc, H.-A. Overview on Corrosion in Supercritical Fluids. J. Supercrit. Fluids 2017, 120, 335–344. [Google Scholar] [CrossRef]
- Bacca, K.R.G.; Lopes, N.F.; Batista, G.d.S.; dos Santos, C.A.; Costa, E.M.d. Electrochemical, Mechanical, and Tribological Properties of Corrosion Product Scales Formed on X65 Steel under CO2 Supercritical Pressure Environments. Surf. Coat. Technol. 2022, 446, 128789. [Google Scholar] [CrossRef]
- Eldevik, F.; Graver, B.; Torbergsen, L.E.; Saugerud, O.T. Development of a Guideline for Safe, Reliable and Cost Efficient Transmission of CO2 in Pipelines. Energy Procedia 2009, 1, 1579–1585. [Google Scholar] [CrossRef]
- Wang, Z.M.; Song, G.L.; Zhang, J. Corrosion Control in CO2 Enhanced Oil Recovery From a Perspective of Multiphase Fluids. Front. Mater. 2019, 6, 272. [Google Scholar] [CrossRef]
- Luo, X.; Wang, M.; Oko, E.; Okezue, C. Simulation-Based Techno-Economic Evaluation for Optimal Design of CO2 Transport Pipeline Network. Appl. Energy 2014, 132, 610–620. [Google Scholar] [CrossRef]
- El-Kady, A.H.; Amin, M.T.; Khan, F.; El-Halwagi, M.M. Analysis of CO2 Pipeline Regulations from a Safety Perspective for Offshore Carbon Capture, Utilization, and Storage (CCUS). J. Clean. Prod. 2024, 439, 140734. [Google Scholar] [CrossRef]
- Nath, F.; Mahmood, M.N.; Yousuf, N. Recent Advances in CCUS: A Critical Review on Technologies, Regulatory Aspects and Economics. Geoenergy Sci. Eng. 2024, 238, 212726. [Google Scholar] [CrossRef]
- Li, C.; Xiang, Y.; Song, C.; Ji, Z. Assessing the Corrosion Product Scale Formation Characteristics of X80 Steel in Supercritical CO2-H2O Binary Systems with Flue Gas and NaCl Impurities Relevant to CCUS Technology. J. Supercrit. Fluids 2019, 146, 107–119. [Google Scholar] [CrossRef]
- Li, Q.K.; Kutana, A.; Penev, E.S.; Yakobson, B.I. Iron Corrosion in the “Inert” Supercritical CO2, Ab Initio Dynamics Insights: How Impurities Matter. Matter 2022, 5, 751–762. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Charpentier, T.; Ward, M.; Neville, A. Relating Iron Carbonate Morphology to Corrosion Characteristics for Water-Saturated Supercritical CO2 Systems. J. Supercrit. Fluids 2015, 98, 183–193. [Google Scholar] [CrossRef]
- Zhang, G.A.; Zeng, Y.; Guo, X.P.; Jiang, F.; Shi, D.Y.; Chen, Z.Y. Electrochemical Corrosion Behavior of Carbon Steel under Dynamic High Pressure H2S/CO2 Environment. Corros. Sci. 2012, 65, 37–47. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, M.; Choi, Y.S. State-of-the-Art Overview of Pipeline Steel Corrosion in Impure Dense CO2 for CCS Transportation: Mechanisms and Models. Corros. Eng. Sci. Technol. 2017, 52, 485–509. [Google Scholar] [CrossRef]
- NACE Standard RP0775-2005; Standard Recommended Practice: Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Oilfield Operations. NACE International: Houston, TX, USA, 2005.
- Xiang, Y.; Song, C.; Li, C.; Yao, E.; Yan, W. Characterization of 13Cr Steel Corrosion in Simulated EOR-CCUS Environment with Flue Gas Impurities. Process Saf. Environ. Prot. 2020, 140, 124–136. [Google Scholar] [CrossRef]
- Ma, G. Corrosion Behavior of N80 Steel in Underground Supercritical CO2 Environments. Chem. Tech. Fuels Oils 2024, 60, 491–500. [Google Scholar] [CrossRef]
- Simonsen, K.R.; Hansen, D.S.; Pedersen, S. Challenges in CO2 Transportation: Trends and Perspectives. Renew. Sustain. Energy Rev. 2024, 191, 114149. [Google Scholar] [CrossRef]
- Slavchov, R.I.; Iqbal, M.H.; Faraji, S.; Madden, D.; Sonke, J.; Clarke, S.M. Corrosion Maps: Stability and Composition Diagrams for Corrosion Problems in CO2 Transport. Corros. Sci. 2024, 236, 112204. [Google Scholar] [CrossRef]
- Li, Y.; Wang, W.; Chen, Z.F.; Li, Y.X. Integrity Assessment of Supercritical CO2 Transport Pipelines. Geoenergy Sci. Eng. 2023, 221, 211355. [Google Scholar] [CrossRef]
- Fu, L.; Ren, Z.; Si, W.; Ma, Q.; Huang, W.; Liao, K.; Huang, Z.; Wang, Y.; Li, J.; Xu, P. Research Progress on CO2 Capture and Utilization Technology. J. CO2 Util. 2022, 66, 102260. [Google Scholar] [CrossRef]
- Jayakumar, A.; Gomez, A.; Mahinpey, N. Post-Combustion CO2 Capture Using Solid K2CO3: Discovering the Carbonation Reaction Mechanism. Appl. Energy 2016, 179, 531–543. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Y. Advancing the Mechanistic Understanding of Corrosion in Supercritical CO2 with H2O and O2 Impurities. Corros. Sci. 2023, 213, 110981. [Google Scholar] [CrossRef]
- Yan, T.; Xu, L.-C.; Zeng, Z.-X.; Pan, W.-G. Mechanism and Anti-Corrosion Measures of Carbon Dioxide Corrosion in CCUS: A Review. iScience 2024, 27, 108594. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Yang, Z.; Liu, J.; Li, Z. A Comprehensive Review of Metal Corrosion in a Supercritical CO2 Environment. Int. J. Greenh. Gas Control 2019, 90, 102814. [Google Scholar] [CrossRef]
- Jiang, X.; Qu, D.; Song, X.; Liu, X.; Zhang, Y. Critical Water Content for Corrosion of X65 Mild Steel in Gaseous, Liquid and Supercritical CO2 Stream. Int. J. Greenh. Gas Control 2019, 85, 11–22. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. Comparison of Corrosion Behaviour for X-65 Carbon Steel in Supercritical CO2-Saturated Water and Water-Saturated/Unsaturated Supercritical CO2. J. Supercrit. Fluids 2015, 97, 224–237. [Google Scholar] [CrossRef]
- Liu, G.Y.; Fan, X.X.; Wang, C.L.; Zhao, X.F.; Meng, L.; Hu, Q.H.; Li, Y.X. Study on the pitting corrosion behavior of X65 steel in supercritical and dense-phase CO2 based on in-situ electrochemical noise measurement. Process Saf. Environ. Prot. 2025, 197, 107060. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Yang, X.; Li, Z.; Ni, W. The Upper Limit of Moisture Content for Supercritical CO2 Pipeline Transport. J. Supercrit. Fluids 2012, 67, 14–21. [Google Scholar] [CrossRef]
- Liu, J.; Yao, D.; Chen, K.; Wang, C.; Sun, C.; Pan, H.; Meng, F.; Chen, B.; Wang, L. Effect of H2O Content on the Corrosion Behavior of X52 Steel in Supercritical CO2 Streams Containing O2, H2S, SO2 and NO2 Impurities. Energies 2023, 16, 6119. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.; Liu, S.; Wang, Y. Effect of Water Content on the Corrosion Behavior of X65 Pipeline Steel in Supercritical CO2-H2O-O2-H2S-SO2 Environment as Relevant to CCS Application. Corros. Sci. 2018, 137, 151–162. [Google Scholar] [CrossRef]
- Xu, M.; Li, W.; Zhou, Y.; Yang, X.; Wang, Z.; Li, Z. Effect of Pressure on Corrosion Behavior of X60, X65, X70, and X80 Carbon Steels in Water-Unsaturated Supercritical CO2 Environments. Int. J. Greenh. Gas Control 2016, 51, 357–368. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. The Effect of O2 Content on the Corrosion Behaviour of X65 and 5Cr in Water-Containing Supercritical CO2 Environments. Appl. Surf. Sci. 2015, 356, 499–511. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, Q.; Wang, Z.; Liu, J.; Li, Z. Effect of High-Concentration O2 on Corrosion Behavior of X70 Steel in Water-Containing Supercritical CO2 with SO2. Corrosion 2016, 73, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Barker, R.; Hua, Y.; Neville, A. Internal Corrosion of Carbon Steel Pipelines for Dense-Phase CO2 Transport in Carbon Capture and Storage (CCS)—A Review. Int. Mater. Rev. 2017, 62, 1–31. [Google Scholar] [CrossRef]
- NACE Standard RP0775-2023; Standard Recommended Practice: Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Hydrocarbon Operations. NACE International: Houston, TX, USA, 2023.
- He, L.; Zhang, Q.; Chen, W.; Wang, Y.; Wang, M.; Huang, Y.; Xu, Y. Unraveling Short-Term O2 Contamination on under Deposit Corrosion of X65 Pipeline Steel in CO2 Saturated Solution. Corros. Sci. 2024, 233, 112113. [Google Scholar] [CrossRef]
- Sun, J.; Sun, C.; Wang, Y. Effects of O2 and SO2 on Water Chemistry Characteristics and Corrosion Behavior of X70 Pipeline Steel in Supercritical CO2 Transport System. Ind. Eng. Chem. Res. 2018, 57, 2365–2375. [Google Scholar] [CrossRef]
- Basilico, E.; Marcelin, S.; Mingant, R.; Kittel, J.; Fregonese, M.; Barker, R.; Owen, J.; Neville, A.; Ropital, F. Effect of O2 Contamination on Carbon Steel Pseudo-Passive Scales in CO2 Aqueous Solutions. Corros. Sci. 2022, 205, 110388. [Google Scholar] [CrossRef]
- Li, Y.Y.; Jiang, Z.N.; Zhang, Q.H.; Lei, Y.; Wang, X.; Zhang, G.A. Unveiling the Influential Mechanism of O2 on the Corrosion of N80 Carbon Steel under Dynamic Supercritical CO2 Conditions. Corros. Sci. 2022, 205, 110436. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, X.P.; Zhang, G.A. Corrosion Behaviour of X65 Carbon Steel in Supercritical-CO2 Containing H2O and O2 in Carbon Capture and Storage (CCS) Technology. Corros. Sci. 2017, 118, 118–128. [Google Scholar] [CrossRef]
- Wang, W.; Shen, K.; Tang, S.; Shen, R.; Parker, T.; Wang, Q. Synergistic Effect of O2 and SO2 Gas Impurities on X70 Steel Corrosion in Water-Saturated Supercritical CO2. Process Saf. Environ. Prot. 2019, 130, 57–66. [Google Scholar] [CrossRef]
- Visser, E.; Hendriks, C.; Barrio, M.; Mølnvik, M.J.; Koeijer, G.; Liljemark, S.; Le Gallo, Y. Dynamis CO2 Quality Recommendations. Int. J. Greenh. Gas Control 2008, 2, 478–484. [Google Scholar] [CrossRef]
- Li, K.; Zeng, Y.; Luo, J.L. Influence of H2S on the General Corrosion and Sulfide Stress Cracking of Pipelines Steels for Supercritical CO2 Transportation. Corros. Sci. 2021, 190, 109639. [Google Scholar] [CrossRef]
- Sun, C.; Ding, T.; Sun, J.; Lin, X.; Zhao, W.; Chen, H. Insights into the Effect of H2S on the Corrosion Behavior of N80 Steel in Supercritical CO2 Environment. J. Mater. Res. Technol. 2023, 26, 5462–5477. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.; Luo, J.L. Unlocking the Impurity-Induced Pipeline Corrosion Based on Phase Behavior of Impure CO2 Streams. Corros. Sci. 2020, 165, 108367. [Google Scholar] [CrossRef]
- Jou, F.; Carroll, J.; Mather, A.; Otto, F. Solubility of Mixtures of Hydrogen-Sulfide and Carbon-Dioxide in Aqueous N-Methyldiethanolamine Solutions. J. Chem. Eng. Data 1993, 38, 75–77. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Corrosion of Low Alloy Steel and Stainless Steel in Supercritical CO2/H2O/H2S Systems. Corros. Sci. 2016, 111, 637–648. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Effect of Small Amount of H2S on the Corrosion Behavior of Carbon Steel in the Dynamic Supercritical CO2 Environments. Corros. Sci. 2016, 103, 132–144. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; He, S.; Zhang, L.; Xing, X.; Li, H.; Lu, M. Unraveling the Effect of H2S on the Corrosion Behavior of High Strength Sulfur-Resistant Steel in CO2/H2S/Cl− Environments at Ultra High Temperature and High Pressure. J. Nat. Gas Sci. Eng. 2022, 100, 104477. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Sun, J.; Lin, X.; Wang, Y. Probing the Initial Corrosion Behavior of X65 Steel in CCUS-EOR Environments with Impure Supercritical CO2 Fluids. Corros. Sci. 2021, 189, 109585. [Google Scholar] [CrossRef]
- Xiang, Y.; Wang, Z.; Xu, C.; Zhou, C.; Li, Z.; Ni, W. Impact of SO2 Concentration on the Corrosion Rate of X70 Steel and Iron in Water-Saturated Supercritical CO2 Mixed with SO2. J. Supercrit. Fluids 2011, 58, 286–294. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. The Influence of SO2 on the Tolerable Water Content to Avoid Pipeline Corrosion during the Transportation of Supercritical CO2. Int. J. Greenh. Gas Control 2015, 37, 412–423. [Google Scholar] [CrossRef]
- Li, C.; Xiang, Y.; Wang, R.T.; Yuan, J.; Xu, Y.H.; Li, W.G.; Zheng, Z.G. Exploring the inffuence of ffue gas impurities on the electrochemical corrosion mechanism of X80 steel in a supercritical CO2-saturated aqueous environment. Corros. Sci. 2023, 211, 110899. [Google Scholar] [CrossRef]
- Sun, C.; Sun, J.; Wang, Y.; Lin, X.; Li, X.; Cheng, X.; Liu, H. Synergistic Effect of O2, H2S and SO2 Impurities on the Corrosion Behavior of X65 Steel in Water-Saturated Supercritical CO2 System. Corros. Sci. 2016, 107, 193–203. [Google Scholar] [CrossRef]
- Jiang, X.; Song, X.L.; Yu, C.; Xie, H.; Qu, D.R.; Hua, J. Investigation of SO2-induced corrosion of X65 steel in gaseous, liquid, and supercritical CO2 environments through experimental and thermodynamic approaches. J. Supercrit. Fluids 2025, 221, 106574. [Google Scholar] [CrossRef]
- Mahlobo, M.G.R.; Premlall, K.; Olubambi, P.A. Effect of Exposure Time with SO2 as an Impurity on the Corrosion Behaviour of Pipeline Steel in CCS Transportation. Corros. Eng. Sci. Technol. 2022, 57, 44–54. [Google Scholar] [CrossRef]
- Morland, B.; Truls, N.; Tjelta, M.; Svenningsen, G. Effect of SO2, O2, NO2, and H2O Concentrations on Chemical Reactions and Corrosion of Carbon Steel in Dense Phase CO2. Corrosion 2019, 75, 1327–1338. [Google Scholar] [CrossRef]
- Dugstad, A.; Halseid, M.; Morland, B. Effect of SO2 and NO2 on Corrosion and Solid Formation in Dense Phase CO2 Pipelines. Energy Procedia 2013, 37, 2877–2887. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Y.; Sun, J.; Lin, X.; Li, X.; Liu, H.; Cheng, X. Effect of Impurity on the Corrosion Behavior of X65 Steel in Water-Saturated Supercritical CO2 System. J. Supercrit. Fluids 2016, 116, 70–82. [Google Scholar] [CrossRef]
- Sun, C.; Yan, X.; Sun, J.; Pang, J.; Zhao, W.; Lin, X. Unraveling the Effect of O2, NO2 and SO2 Impurities on the Stress Corrosion Behavior of X65 Steel in Water-Saturated Supercritical CO2 Streams. Corros. Sci. 2022, 209, 110729. [Google Scholar] [CrossRef]
- Kairy, S.K.; Zhou, S.; Turnbull, A.; Hinds, G. Corrosion of Pipeline Steel in Dense Phase CO2 Containing Impurities: A Critical Review of Test Methodologies. Corros. Sci. 2023, 214, 110986. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Allahkaram, S.R.; Kermani, M.B. The Effects of Temperature and pH on the Characteristics of Corrosion Product in CO2 Corrosion of Grade X70 Steel. Mater. Des. 2010, 31, 3559–3563. [Google Scholar] [CrossRef]
- Sui, P.; Sun, J.; Hua, Y.; Liu, H.; Zhou, M.; Zhang, Y.; Liu, J.; Wang, Y. Effect of Temperature and Pressure on Corrosion Behavior of X65 Carbon Steel in Water-Saturated CO2 Transport Environments Mixed with H2S. Int. J. Greenh. Gas Control 2018, 73, 60–69. [Google Scholar] [CrossRef]
- Hua, Y.; Barker, R.; Neville, A. Effect of Temperature on the Critical Water Content for General and Localised Corrosion of X65 Carbon Steel in the Transport of Supercritical CO2. Int. J. Greenh. Gas Control 2014, 31, 48–60. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, L.; Gan, M.; Su, X.; Wang, Y.; Xue, Q.; Mei, K.; Fu, X. Temperature and Reaction Time’s Effects on N80 Steel Corrosion Behavior in Supercritical CO2 and Formation Water Environments. Appl. Sci. 2024, 14, 728. [Google Scholar] [CrossRef]
- Azevedo Rodrigues, T.R.S.; Marcolino, J.B.; Moraes, M.K.; Lopes, N.F.; Costa, E.M. Influence of CO2 Subcritical and Supercritical Pressures on the Protective Properties of Corrosion Product Scales Formed on X65 Steel. J. Supercrit. Fluids 2024, 206, 106184. [Google Scholar] [CrossRef]
- Li, W.; Pots, B.F.M.; Brown, B.; Kee, K.E.; Nesic, S. A Direct Measurement of Wall Shear Stress in Multiphase Flow—Is It an Important Parameter in CO2 Corrosion of Carbon Steel Pipelines? Corros. Sci. 2016, 110, 35–45. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Effect of Flow Rate on Localized Corrosion of X70 Steel in Supercritical CO2 Environments. Corros. Sci. 2018, 136, 339–351. [Google Scholar] [CrossRef]
- Zeng, L.; Lv, T.; Chen, H.; Ma, T.; Fang, Z.; Shi, J. Flow Accelerated Corrosion of X65 Steel Gradual Contraction Pipe in High CO2 Partial Pressure Environments. Arab. J. Chem. 2023, 16, 104935. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Zhu, G.; Zhang, G. Effects of temperature and flow rate on the corrosion behavior of N80 carbon steel in supercritical CO2 environment (China). Surf. Technol. 2020, 49, 35–41. (In Chinese) [Google Scholar] [CrossRef]
- Wang, Y.; Wang, B.; Xing, X.; He, S.; Zhang, L.; Lu, M. Effects of Flow Velocity on the Corrosion Behaviour of Super 13Cr Stainless Steel in Ultra-HTHP CO2-H2S Coexistence Environment. Corros. Sci. 2022, 200, 110235. [Google Scholar] [CrossRef]
- Wang, X.; Yang, P.; Li, R.; Tong, G.; Chen, J.; Wang, Y. Investigation of Supercritical CO2 Corrosion Behavior of X80 Carbon Steel in Pipelines: An in Situ Experimental and DFT Study. J. Supercrit. Fluids 2024, 213, 106371. [Google Scholar] [CrossRef]
- Hua, Y.; Jonnalagadda, R.; Zhang, L.; Neville, A.; Barker, R. Assessment of General and Localized Corrosion Behavior of X65 and 13Cr Steels in Water-Saturated Supercritical CO2 Environments with SO2/O2. Int. J. Greenh. Gas Control 2017, 64, 126–136. [Google Scholar] [CrossRef]
- Wang, L.W.; Liu, Z.Y.; Cui, Z.Y.; Du, C.W.; Wang, X.H.; Li, X.G. In Situ Corrosion Characterization of Simulated Weld Heat Affected Zone on API X80 Pipeline Steel. Corros. Sci. 2014, 85, 401–410. [Google Scholar] [CrossRef]
- Sharma, S.K.; Maheshwari, S. A Review on Welding of High Strength Oil and Gas Pipeline Steels. J. Nat. Gas Sci. Eng. 2017, 38, 203–217. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, F.; Jia, H.; Chen, C.; Feng, Q.; Dai, L.; Zhang, Z. Study on the Effect of Microstructure on Toughness Dispersion of X70 Steel Girth Weld. Eng. Fail. Anal. 2024, 164, 108671. [Google Scholar] [CrossRef]
- Li, J.; Wang, D.; Xie, F. Failure Analysis of CO2 Corrosion of Natural Gas Pipeline under Flowing Conditions. Eng. Fail. Anal. 2022, 137, 106265. [Google Scholar] [CrossRef]
- Ding, H.X.; Xiang, Y.; Lu, W.P.; Yan, K.; Ren, J.R.; Yan, W.; Yao, E.D.; Zhao, X.H. Selective adsorption and corrosion mechanism of SO2 and its hydrates on X65 welded joints steel in CO2-saturated aqueous solution. Corros. Sci. 2024, 238, 112373. [Google Scholar] [CrossRef]
- Bai, F.; Ding, H.; Tong, L.; Pan, L. Microstructure and Properties of the Interlayer Heat-Affected Zone in X80 Pipeline Girth Welds. Prog. Nat. Sci. Mater. Int. 2020, 30, 110–117. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, L. Early Corrosion Stage of Welded Carbon Steel Joints in CO2-Saturated Oilfield Water. Mater. Test. 2020, 62, 129–137. [Google Scholar] [CrossRef]
- Yan, Y.; Deng, H.; Xiao, W.; Ou, T.; Cao, X. Corrosion Behaviour of X80 Pipeline Steel Welded Joint in H2O-Saturated Supercritical-CO2 Environment. Int. J. Electrochem. Sci. 2020, 15, 1713–1726. [Google Scholar] [CrossRef]
- Yao, B.; Gao, Q.; Zhang, Z.; Yan, Y.; Deng, H.; Lan, W. Effect of Microstructure of Welded Joint of X80 pipeline Steel on Its Corrosion Behaviors in Supercritical CO2 (China). Corros. Prot. 2022, 43, 36–41. (In Chinese) [Google Scholar]

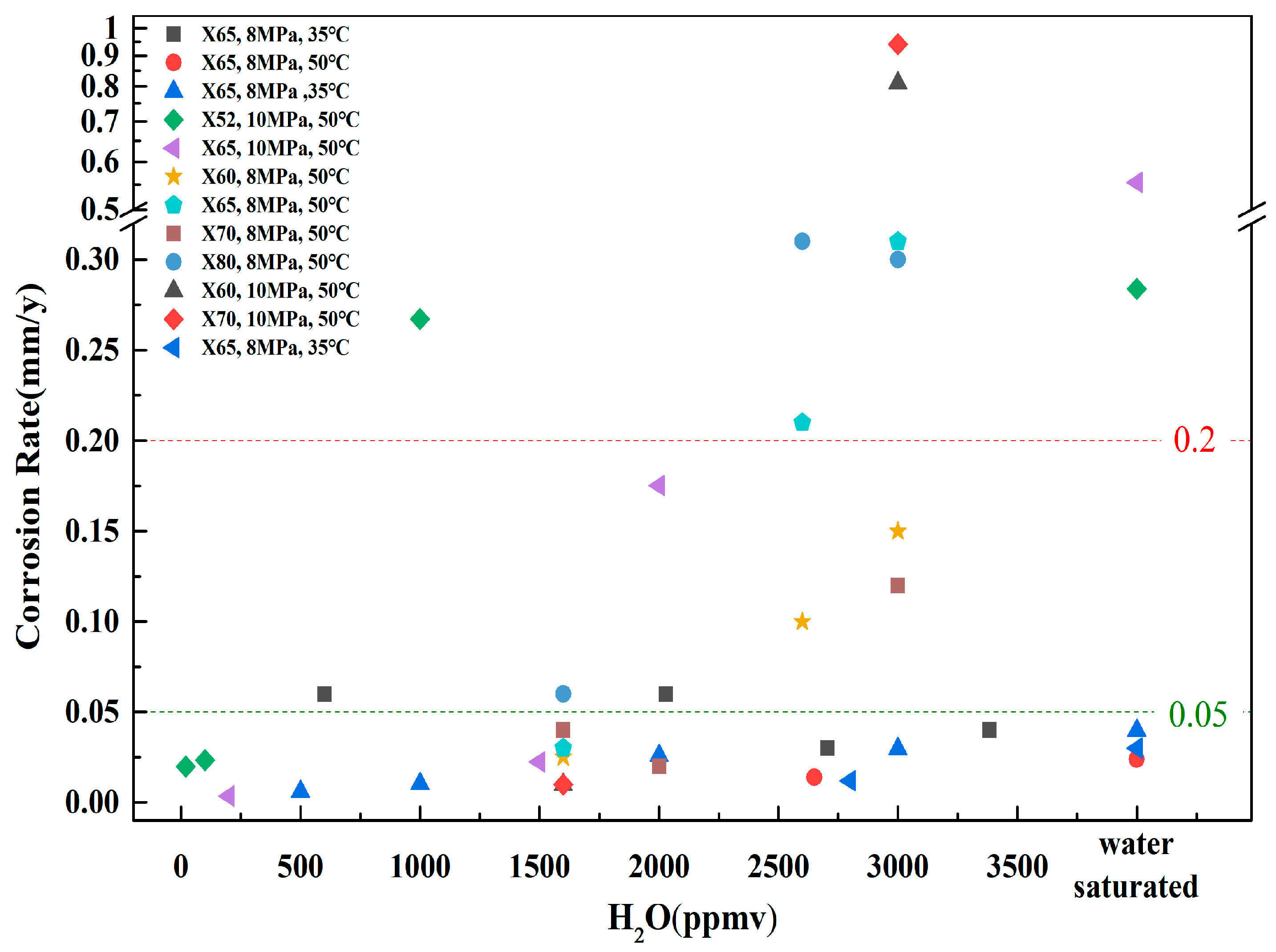
| Materials | Pressures (MPa) | Temperature (°C) | Water Content | Other Impurities | Test Period (h) | Corrosion Rate (mm/y) | Local Corrosion Rate (mm/y) | Reference |
|---|---|---|---|---|---|---|---|---|
| X65 | 8 | 35 | 2029 ppm (60% RH) | 336 | <0.02 | [35] | ||
| 72 | <0.1 | |||||||
| 2705 ppm (80% RH) | ||||||||
| 3382 ppm (water-saturated CO2 phase) | ||||||||
| CO2-saturated water phase | 1 | |||||||
| X65 | 8 | 50 | 700 ppm, 1600 ppm | 48 | No measurable corrosion | No measurable corrosion | [36] | |
| 2650 ppm | 0.014 | 0.2 | ||||||
| 3400 ppm (water-saturated CO2 phase) | 0.024 | 1.4 | ||||||
| 300 mL (CO2-saturated water phase) | 96 | 4.1 | ||||||
| X65 | 8 | 35 | 500 ppmv | 72 | 0.0061 | 0.137 | [37] | |
| 1000 ppmv | 0.0103 | 0.322 | ||||||
| 2000 ppmv | 0.0259 | 0.697 | ||||||
| 3000 ppmv | 0.0298 | 0.825 | ||||||
| 5000 ppmv | 0.0398 | 1.127 | ||||||
| X70 | 10 | 50 | 0.95 g (50% RH) | SO2 2.0 mol%, O2 1000 ppm | 120 | 0.0387 | [38] | |
| 1.32 g (70% RH) | 0.3–0.4 | |||||||
| 1.67 g (88% RH) | 0.8–0.9 | |||||||
| 3 g (100% RH) | 1.4–1.5 | |||||||
| X52 | 10 | 50 | 20 ppmv | O2 200 ppmv, H2S 200 ppmv, SO2 200 ppmv | 72 | 0.0199 | [39] | |
| 100 ppmv | 0.0234 | |||||||
| 1000 ppmv | 0.2671 | |||||||
| 4333 ppmv (water-saturated CO2 phase) | 0.2838 | |||||||
| X65 | 10 | 50 | 200 ppmv | O2 200 ppmv, H2S 200 ppmv, SO2 200 ppmv | 72 | 0.0036 | [40] | |
| 1500 ppmv | 0.0224 | |||||||
| 2000 ppmv | 0.1752 | |||||||
| 4333 ppmv (water-saturated CO2 phase) | 0.5546 | |||||||
| X60, X65, X70, X80 | 8 | 50 | 1600 ppm | SO2 3000 ppm, O2 1000 ppm | 72 | 0.025–0.06 | 0.2–3.25 | [41] |
| 2600 ppm, 3000 ppm | 0.1–0.31 | |||||||
| 10 | 1600 ppm, 2000 ppm, 2600 ppm | <0.01 | 0.04–6.02 | |||||
| 3000 ppm | 0.81–0.94 | |||||||
| X65 | 8 | 35 | 300 ppm, 650 ppm, 1200 ppm | O2 1000 ppm | 48 | No measurable corrosion | No measurable corrosion | [42] |
| 2800 ppm | 0.010–0.014 | 0.8 | ||||||
| 34,000 ppm (water-saturated CO2 phase) | 0.03 | 3.1 | ||||||
| 34,000 ppm (water-saturated CO2 phase) | 0.10 | 0.9 | ||||||
| 5Cr | 8 | 35 | 300 ppm, 650 ppm, 1200 ppm | O2 1000 ppm | 48 | No measurable corrosion | No measurable corrosion | [42] |
| 2800 ppm | <0.01 | 0.7 | ||||||
| 34,000 ppm (water-saturated CO2 phase) | 0.02 | 2.2 | ||||||
| 34,000 ppm (water-saturated CO2 phase) | 0.125 | 0.3 | ||||||
| X70 | 10 | 50 | 45% RH | O2 0.1 mol% | 72 | 0.03 | 0.03 | [43] |
| 60% RH | 0.1 | |||||||
| 75% RH | 0.90 | ~1.5 | ||||||
| 88% RH | ~1.1 | ~3 | ||||||
| 100% RH | 1.61 | 7.03 | ||||||
| 45% RH | O2 1.0 mol% | 0.03 | 0.03 | |||||
| 50% RH | 0.50 | |||||||
| 60% RH | 0.63 | |||||||
| 75% RH | 0.90 | |||||||
| 88% RH | 0.90–1.0 | 9 | ||||||
| 100% RH | 1.6 | 5.23 |
| Materials | Pressures (MPa) | Temperature (°C) | Environment | O2 Content | Other Impurities | Test Period (h) | Corrosion Rate (mm/y) | Local Corrosion Rate (mm/y) | Corrosion Products | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| X65 | 8 | 35 | water-saturated CO2 phase | 0 ppm | 48 | 0.10 | 0.9 | FeCO3, Fe2O3, FeOOH, Fe3O4 | [42] | |
| 20 ppm | 0.088 | 1.0–1.2 | ||||||||
| 500 ppm | 0.072–0.076 | 1.2–1.3 | ||||||||
| 1000 ppm | 0.04 | 3 | ||||||||
| 5Cr | 8 | 35 | water-saturated CO2 phase | 0 ppm | 48 | 0.125 | 0.3 | FeCO3, Fe2O3, Cr2O3, FeOOH, Cr(OH)3, Fe3O4 | [42] | |
| 20 ppm | 0.120–0.124 | 0.5–0.6 | ||||||||
| 500 ppm | 0.040–0.044 | 1.0 | ||||||||
| 1000 ppm | 0.02 | 2.2 | ||||||||
| X70 | 10 | 50 | water-saturated CO2 phase | 0.1 mol% | SO2 2 mol% | 72 | 1.61 | 7.03 | FeO FeSO4∙xH2O | [43] |
| 1.0 mol% | 1.6 | 5.23 | ||||||||
| 2.0 mol% | 0.94 | 3.3 | ||||||||
| X70 | 10 | 50 | water-saturated CO2 phase | 0 ppm | 120 | 0.014 | FeCO3, Fe2O3 | [47] | ||
| 1000 ppm | 0.027 | |||||||||
| 10,000 ppm | 0.029 | |||||||||
| N80 | 8 | 65 | simulated formation water phase | 0 bar | 48 | 27.86 | FeCO3, Fe2O3, FeOOH | [49] | ||
| 5 bar | 13.15 | |||||||||
| X65 | 8 | 50 | water-saturated CO2 phase | 0 mg/L | 96 | 0.25 | FeCO3, Fe2O3 | [50] | ||
| 95 mg/L | 0.91 | |||||||||
| 475 mg/L | 1.52 | |||||||||
| X70 | 10 | 40 | water-saturated CO2 phase | 0 | 48 | 0.06 | FeCO3 | [51] | ||
| 200 ppmv | 0.09 | |||||||||
| 1000 ppmv | 0.03 | Fe2O3 |
| Materials | Pressures (MPa) | Temperature (°C) | Environment | H2S Content | Other Impurities | Test Period (h) | Corrosion Rate (mm/y) | Local Corrosion Rate (mm/y) | Corrosion Products | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| P110 | 10 | 80 | water-saturated CO2 phase | 50 ppmv | 240 | <0.2 | 0.52 | FeCO3, mackinawite, Cr(OH)3 | [57] | |
| 3Cr | >0.4 | 0.84 | ||||||||
| 316L | 0 | 0 | FeCO3, mackinawite | |||||||
| P110 | CO2-saturated water phase | 10.37 | <6 | FeCO3, mackinawite, pyrrhotie, Cr(OH)3 | ||||||
| 3Cr | 2.71 | <2 | ||||||||
| 316L | <0.02 | >0.024 | FeCO3, Cr(OH)3, nickel sulfide, mackinawite | |||||||
| X65 | 10 | 80 | water-saturated CO2 phase | 0 | 240 | 0.17 | 0.29 | FeCO3 | [58] | |
| 50 ppmv | 0.24 | 0.48 | FeCO3, Fe1−xS, Fe1+xS, | |||||||
| CO2-saturated NaCl solution | 0 ppmv | 8.46 | 9.19 | FeCO3 | ||||||
| 50 ppmv | 15.48 | 2.45 | FeCO3, FeS, Fe1−xS | |||||||
| Q125 | 14.2 | 140 | water-saturated CO2 phase | 1.33 KPa | 168 | 0.015 | FeCO3, Fe1−xS, Cr(OH)3 | [59] | ||
| 7.24 KPa | 0.041 | |||||||||
| CO2-saturated formation water phase | 1.33 KPa | 0.081 | ||||||||
| 7.24 KPa | 0.051 | |||||||||
| X65 | 8 | 50 | water-saturated CO2 phase | 0.08 bar | SO2 0.08 bar, O2 0.08 bar | 1.5 | >20 | FeS, FeSO4∙xH2O, FeCO3 | [60] | |
| 72 | 2.57 | FeS, FeSO4∙xH2O, FeCO3, FeOOH, S |
| Materials | Pressures (MPa) | Temperature (°C) | Environment | SO2 Content | Other Impurities | Test Period (h) | Corrosion Rate (mm/y) | Corrosion Products | Reference |
|---|---|---|---|---|---|---|---|---|---|
| X70 | 10 | 50 | water-saturated CO2 phase | 0 ppm | 120 | 0.014 | FeSO3, FeCO3, | [47] | |
| 200 ppm | 0.269 | ||||||||
| 600 ppm | 0.345 | ||||||||
| 1000 ppm | 0.423 | ||||||||
| 1000 ppm | O2 1000 ppm | 0.842 | FeSO3, FeSO4, FeCO3, FeOOH | ||||||
| X70 | 10 | 40 | water-saturated CO2 phase | 500 ppmv | 48 | 1.1 | FeSO3∙2H2O, FeCO3, FeOOH | [51] | |
| O2 200 ppmv | 1.24 | FeSO3∙2H2O, FeSO3∙3H2O, FeCO3, Fe2O3 | |||||||
| O2 1000 ppmv | 0.6 | ||||||||
| X70 | 10 | 50 | water-saturated CO2 phase | 0.2 mol% | O2 1000 ppm | 288 | 0.2 | FeSO4∙7H2O, | [61] |
| 0.7 mol% | 0.6–0.7 | FeSO4∙4H2O, FeSO3∙3H2O | |||||||
| 1.4 mol% | 0.75–0.9 | FeSO4∙4H2O, α-FeOOH | |||||||
| 2.0 mol% | 0.9 | FeSO4∙4H2O | |||||||
| X80 | 8 | 35 | CO2-saturated water phase | 0 | 48 | 1.67 | FeCO3, Fe2O3 | [63] | |
| 5% | 3.08 | FeCO3, FeSO3∙xH2O, FeS | |||||||
| X65 | 10 | 50 | water-saturated CO2 phase | 0 | 120 | 0.015 | FeCO3, | [64] | |
| 1000 ppmv | 0.469 | FeSO3 FeSO3∙xH2O, FeSO4∙4H2O, FeCO3, Fe2O3∙H2O | |||||||
| X65 | 8 | 35 | water-saturated CO2 phase | 0 | 72 | 0.1 | FeCO3 | [65] | |
| 50 ppm | 0.37 | FeCO3 FeSO3∙3H2O | |||||||
| 100 ppm | 0.72 | ||||||||
| carbon steel | 9.5 | 60 | CO2 phase | 0 | H2O 1000 ppm | 1512 | 0.00352 | FeCO3, α-FeOOH, Fe3O4 | [66] |
| 0.50% | 0.3375 | γ-FeOOH | |||||||
| 1.50% | 0.5–0.6 | ||||||||
| 5% | 1.396 | FeCO3, FeSO3∙3H2O |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Gao, Q.; Zhou, Y.; Pan, R. Research Progress on Major Influencing Factors of Corrosion Behavior of Pipeline Steel in Supercritical CO2 Environment. Materials 2025, 18, 2424. https://doi.org/10.3390/ma18112424
Liu Z, Gao Q, Zhou Y, Pan R. Research Progress on Major Influencing Factors of Corrosion Behavior of Pipeline Steel in Supercritical CO2 Environment. Materials. 2025; 18(11):2424. https://doi.org/10.3390/ma18112424
Chicago/Turabian StyleLiu, Zhe, Qian Gao, Yong Zhou, and Ruijuan Pan. 2025. "Research Progress on Major Influencing Factors of Corrosion Behavior of Pipeline Steel in Supercritical CO2 Environment" Materials 18, no. 11: 2424. https://doi.org/10.3390/ma18112424
APA StyleLiu, Z., Gao, Q., Zhou, Y., & Pan, R. (2025). Research Progress on Major Influencing Factors of Corrosion Behavior of Pipeline Steel in Supercritical CO2 Environment. Materials, 18(11), 2424. https://doi.org/10.3390/ma18112424