Effects of Grape Seed Extract-Modified Etchants on Collagenolytic Activity, Interface Formation, and Bonding Longevity of Adhesive–Dentin Interfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Etchant and Storage Solution Preparation
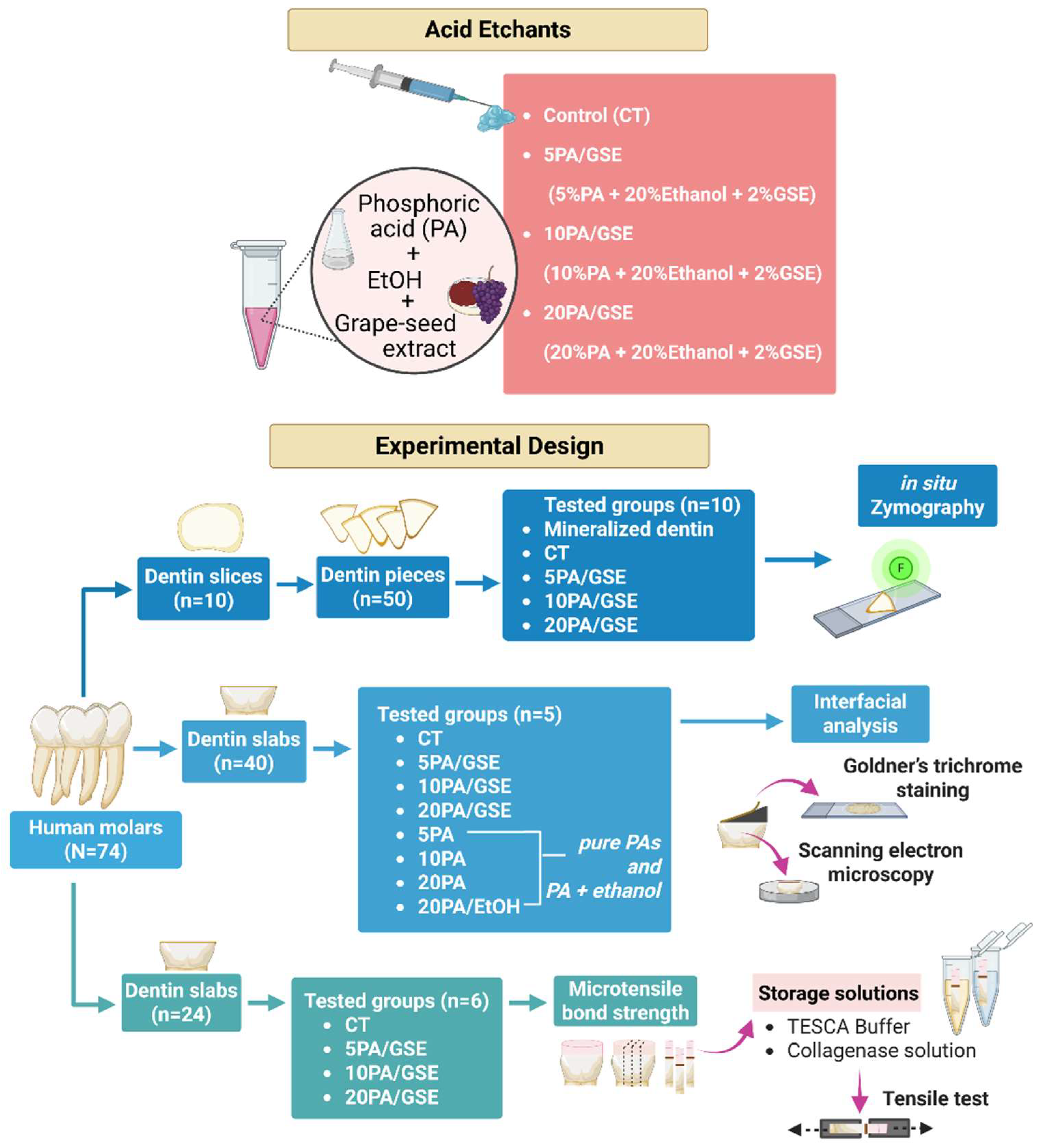
2.2. Tooth Preparation and Experimental Design
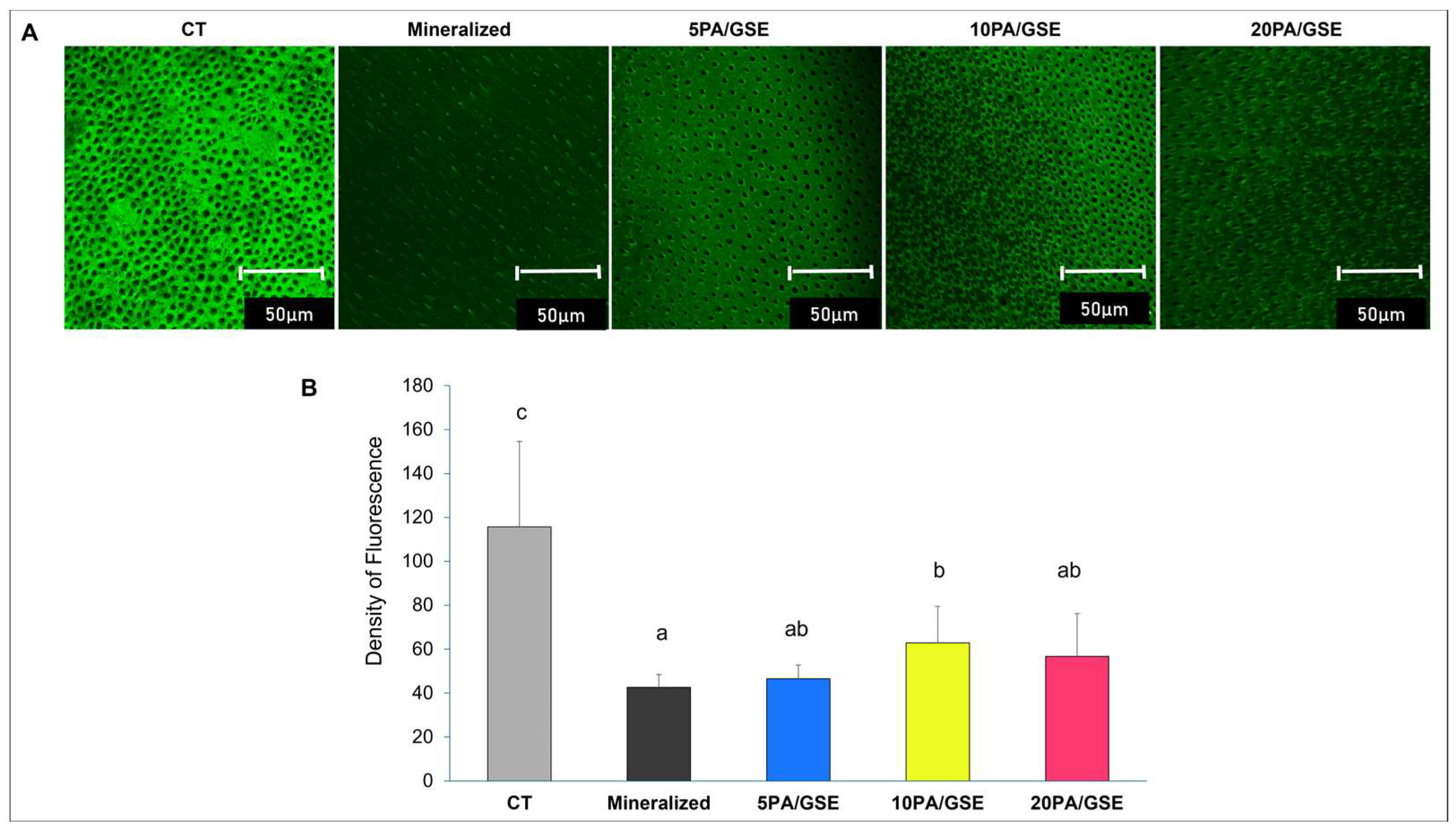
2.3. Collagenolytic Activity of Endogenous MMPs
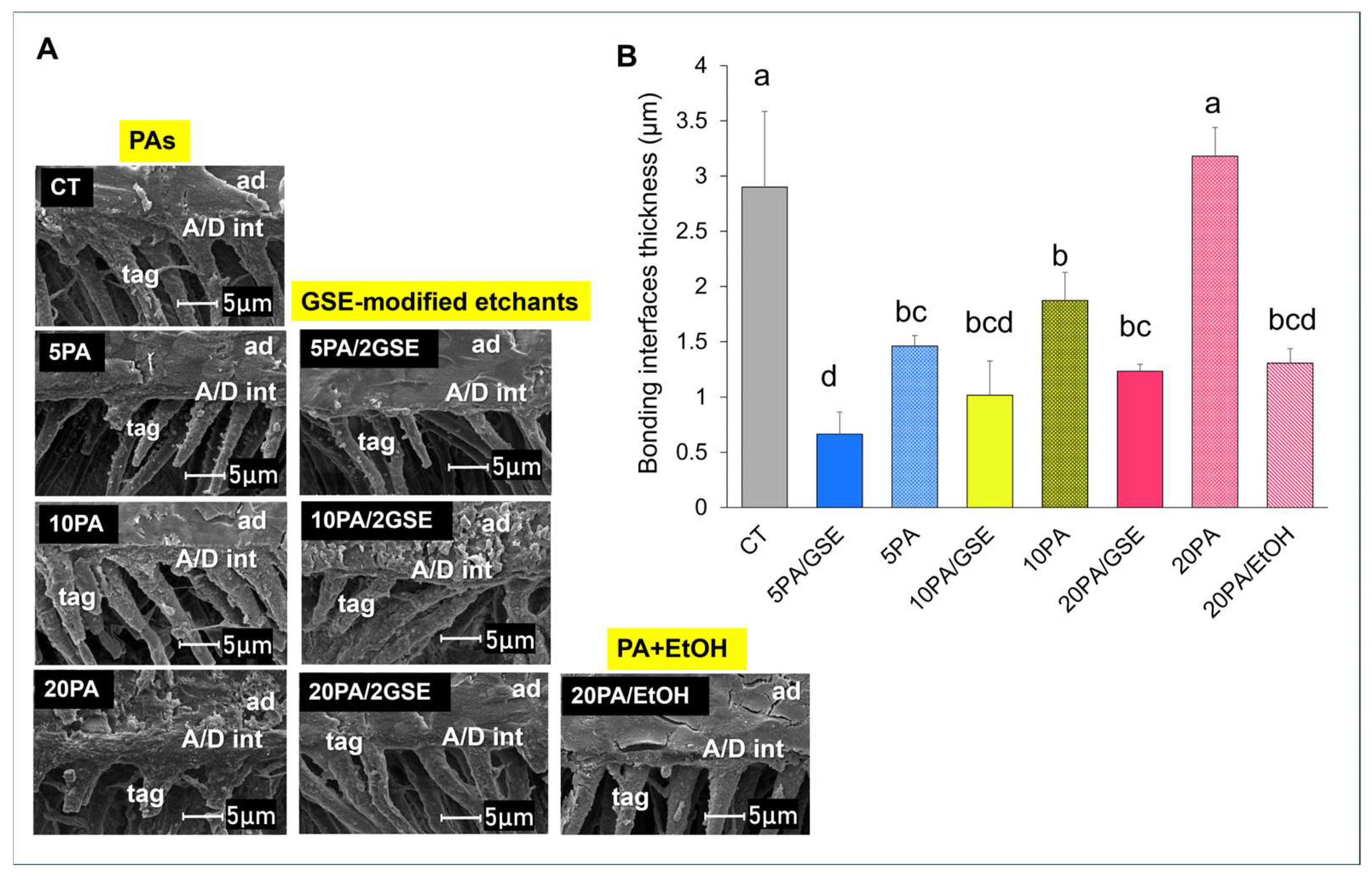
2.4. Adhesive/Dentin (A/D) Bonding Interfacial Analyses
2.4.1. Goldner’s Trichrome Differential Staining
2.4.2. Scanning Electron Microscopy (SEM)
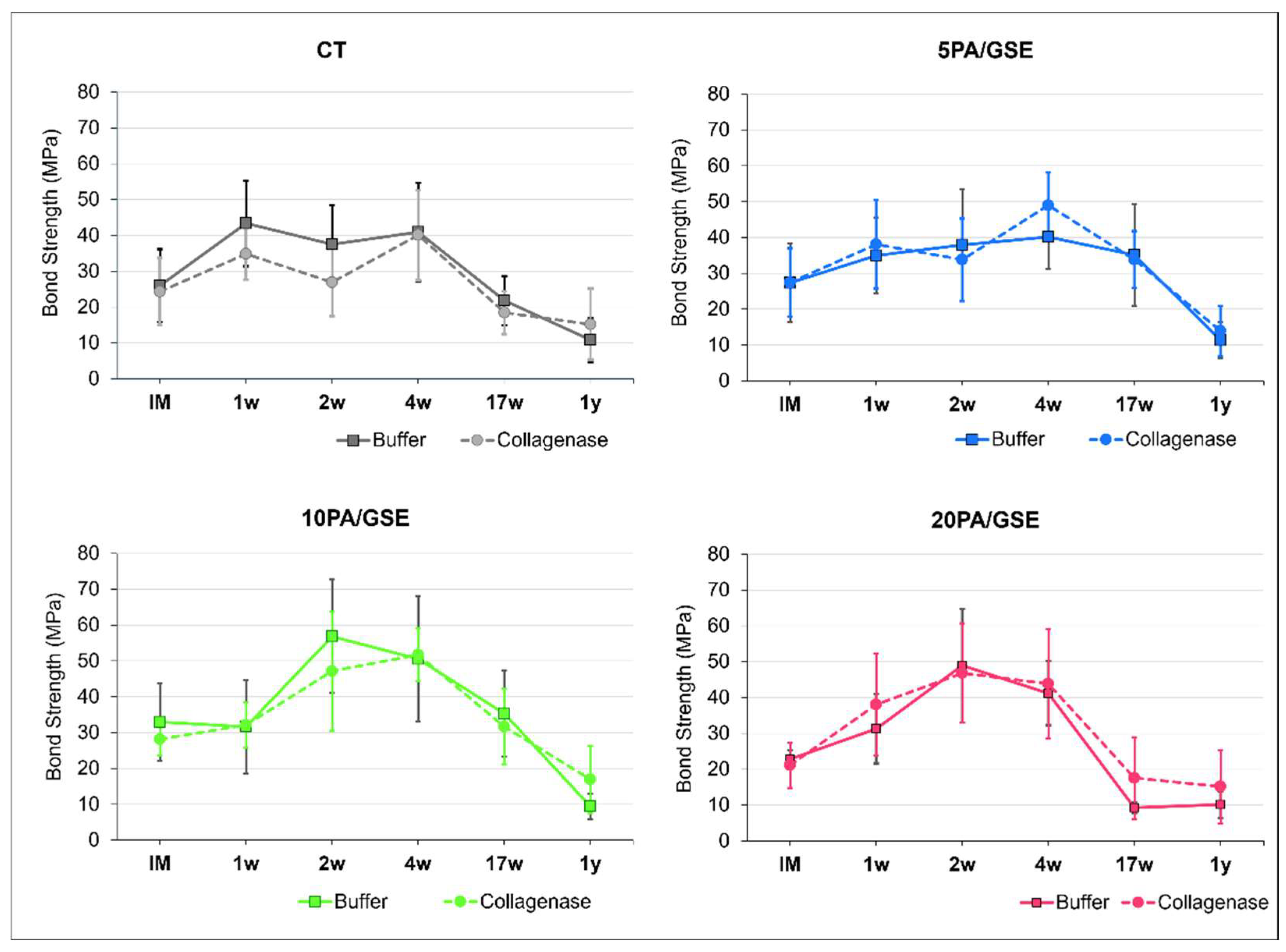
2.5. Microtensile Bond Strength Testing
2.6. Statistical Analysis
3. Results
3.1. Collagenolytic Activity of Endogenous MMPs
3.2. Differential Staining Technique
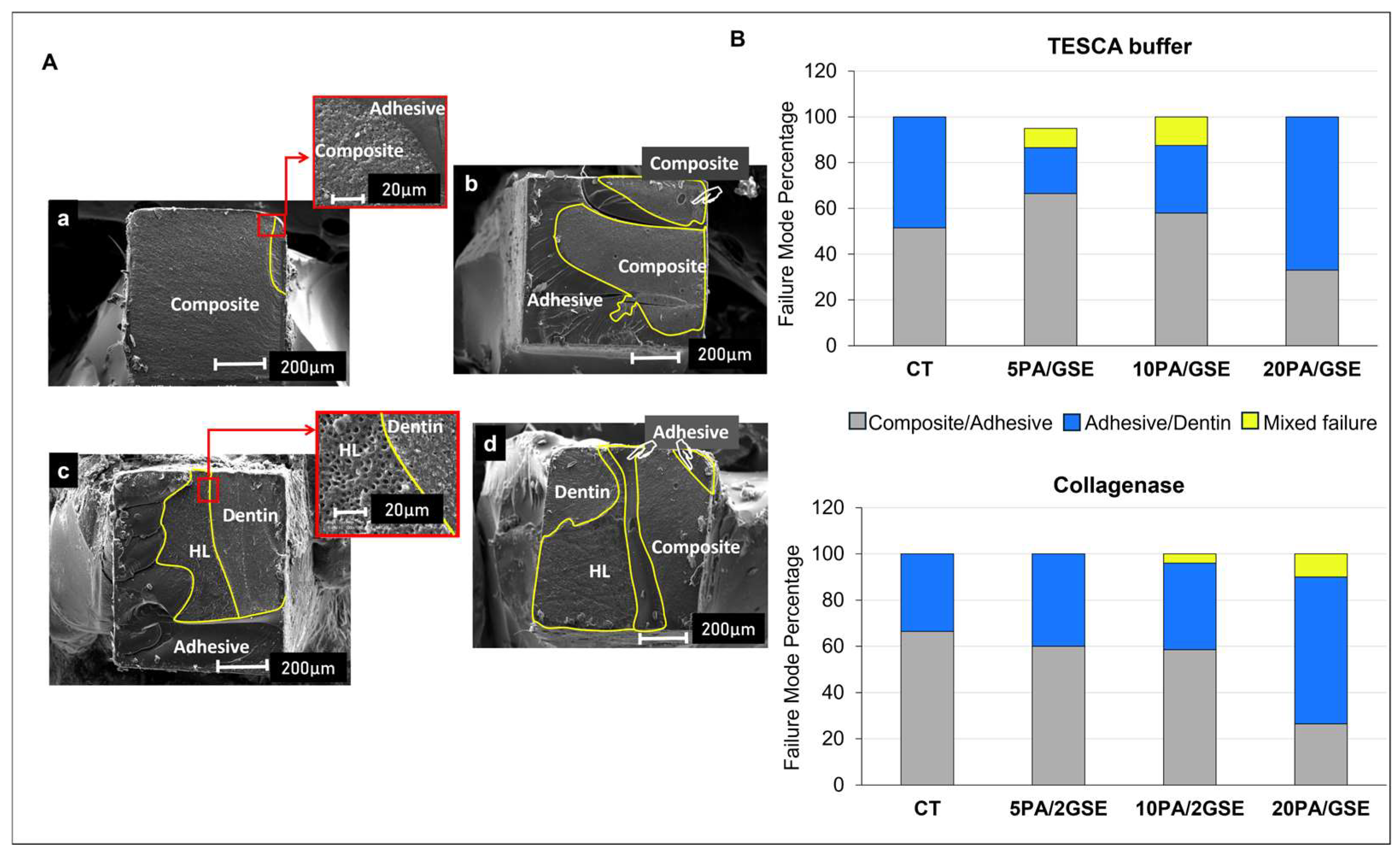
3.3. Scanning Electron Microscopy (SEM)
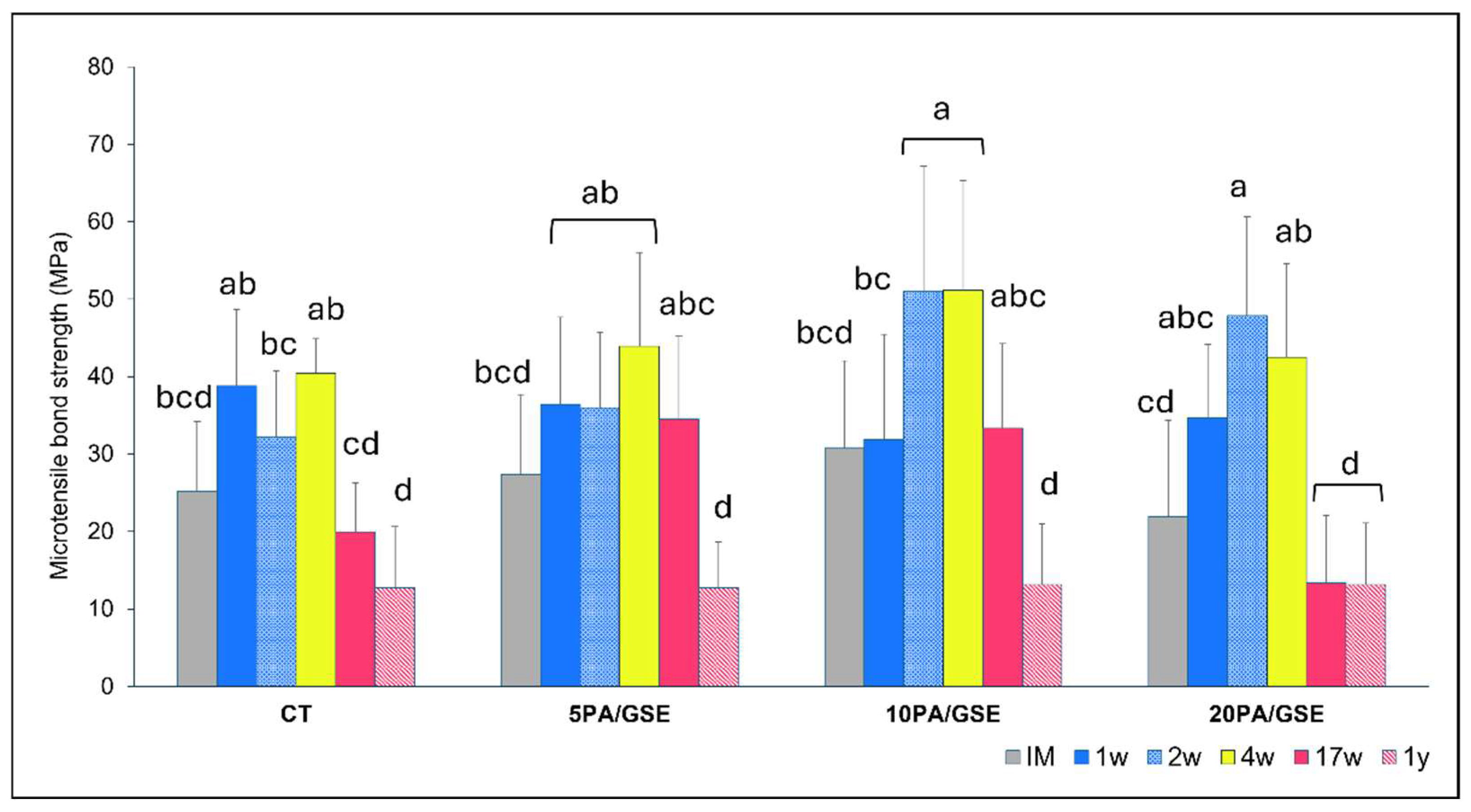
3.4. Microtensile Bond Strength
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eltahlah, D.; Lynch, C.D.; Chadwick, B.L.; Blum, I.R.; Wilson, N.H.F. An update on the reasons for placement and replacement of direct restorations. J. Dent. 2018, 72, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Nedeljkovic, I.; De Munck, J.; Vanloy, A.; Declerck, D.; Lambrechts, P.; Peumans, M.; Teughels, W.; Van Meerbeek, B.; Van Landuyt, K.L. Secondary caries: Prevalence, characteristics, and approach. Clin. Oral. Investig. 2020, 24, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Maravic, T.; Cunha, S.R.; Comba, A.; Cadenaro, M.; Tjäderhane, L.; Pashley, D.H.; Tay, F.R.; Mazzoni, A. Dentin bonding systems: From dentin collagen structure to bond preservation and clinical applications. Dent. Mater. 2018, 34, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Spencer, P.; Ye, Q.; Park, J.; Topp, E.M.; Misra, A.; Marangos, O.; Wang, Y.; Bohaty, B.S.; Singh, V.; Sene, F.; et al. Adhesive/Dentin interface: The weak link in the composite restoration. Ann. Biomed. Eng. 2010, 38, 1989–2003. [Google Scholar] [CrossRef]
- Wang, Y.; Spencer, P. Quantifying adhesive penetration in adhesive/dentin interface using confocal Raman microspectroscopy. J. Biomed. Mater. Res. 2002, 59, 46–55. [Google Scholar] [CrossRef]
- Malacarne, J.; Carvalho, R.M.; de Goes, M.F.; Svizero, N.; Pashley, D.H.; Tay, F.R.; Yiu, C.K.; de Oliveira Carrilho, M.R. Water sorption/solubility of dental adhesive resins. Dent. Mater. 2006, 22, 973–980. [Google Scholar] [CrossRef]
- Mazzoni, A.; Pashley, D.H.; Nishitani, Y.; Breschi, L.; Mannello, F.; Tjäderhane, L.; Toledano, M.; Pashley, E.L.; Tay, F.R. Reactivation of inactivated endogenous proteolytic activities in phosphoric acid-etched dentine by etch-and-rinse adhesives. Biomaterials 2006, 27, 4470–4476. [Google Scholar] [CrossRef]
- Huang, B.; Stewart, C.A.; McCulloch, C.A.; Santerre, J.P.; Cvitkovitch, D.G.; Finer, Y. Streptococcus mutans Proteases Degrade Dentinal Collagen. Dent. J. 2022, 10, 223. [Google Scholar] [CrossRef]
- Gitalis, R.; Bae, J.H.; Preston, M.; Patel, M.; Liu, Z.; Sun, C.; Stewart, C.; Xiao, Y.; Siqueira, W.L.; Glogauer, M.; et al. Human neutrophils compromise the restoration-tooth interface. Acta Biomater. 2020, 117, 283–293. [Google Scholar] [CrossRef]
- Macedo, G.V.; Yamauchi, M.; Bedran-Russo, A.K. Effects of chemical cross-linkers on caries-affected dentin bonding. J. Dent. Res. 2009, 88, 1096–1100. [Google Scholar] [CrossRef]
- Hass, V.; Liu, H.; Cook, W.; Walker, M.P.; Wang, Y. Distinct effects of polyphenols and solvents on dentin collagen crosslinking interactions and biostability. Dent. Mater. 2021, 37, 1794–1805. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dusevich, V.; Wang, Y. Proanthocyanidins rapidly stabilize the demineralized dentin layer. J. Dent. Res. 2013, 92, 746–752. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Jaurequi, J.; Tang, B.W.; Nimni, M.E. Proanthocyanidin: A natural crosslinking reagent for stabilizing collagen matrices. J. Biomed. Mater. Res. A. 2003, 65, 118–124. [Google Scholar] [CrossRef]
- Hass, V.; Luque-Martinez, I.V.; Gutierrez, M.F.; Moreira, C.G.; Gotti, V.B.; Feitosa, V.P.; Koller, G.; Otuki, M.F.; Loguercio, A.D.; Reis, A. Collagen cross-linkers on dentin bonding: Stability of the adhesive interfaces, degree of conversion of the adhesive, cytotoxicity and in situ MMP inhibition. Dent. Mater. 2016, 32, 732–741. [Google Scholar] [CrossRef]
- Wang, Y.; Green, A.; Yao, X.; Liu, H.; Nisar, S.; Gorski, J.P.; Hass, V. Cranberry Juice Extract Rapidly Protects Demineralized Dentin against Digestion and Inhibits Its Gelatinolytic Activity. Materials 2021, 14, 3637. [Google Scholar] [CrossRef]
- Epasinghe, D.J.; Yiu, C.K.; Burrow, M.F.; Tay, F.R.; King, N.M. Effect of proanthocyanidin incorporation into dental adhesive resin on resin-dentine bond strength. J. Dent. 2012, 40, 173–180. [Google Scholar] [CrossRef]
- Hechler, B.; Yao, X.; Wang, Y. Proanthocyanidins alter adhesive/dentin bonding strengths when included in a bonding system. Am. J. Dent. 2012, 25, 276–280. [Google Scholar]
- Hass, V.; da Maceno Oliveira, T.B.; Cardenas, A.F.M.; de Siqueira, F.S.F.; Bauer, J.R.; Abuna, G.; Sinhoreti, M.A.C.; de Souza, J.J. Is it possible for a simultaneous biomodification during acid etching on naturally caries-affected dentin bonding? Clin. Oral Investig. 2021, 25, 3543–3553. [Google Scholar] [CrossRef] [PubMed]
- Hass, V.; Luque-Martinez, I.; Muñoz, M.A.; Reyes, M.F.; Abuna, G.; Sinhoreti, M.A.; de Souza, J.J.; Loguercio, A.D. The effect of proanthocyanidin-containing 10% phosphoric acid on bonding properties and MMP inhibition. Dent. Mater. 2016, 32, 468–475. [Google Scholar] [CrossRef]
- Nisar, S.; Liu, H.; Hass, V.; Wang, Y. Dual-functional etchants that simultaneously demineralize and stabilize dentin render collagen resistant to degradation for resin bonding. Dent. Mater. 2023, 39, 1004–1012. [Google Scholar] [CrossRef]
- Liu, Y.; Dusevich, V.; Wang, Y. Addition of Grape Seed Extract Renders Phosphoric Acid a Collagen-stabilizing Etchant. J. Dent. Res. 2014, 93, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C.M. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Loguercio, A.D.; Malaquias, P.; Dos Santos, F.P.; Hass, V.; Stanislawczuk, R.; Lima, S.N.L.; Bandeca, M.C.; Reis, A. Acid Etching with Modified Phosphoric Acid to Increase the Longevity of the Bonded Interface. J. Adhes. Dent. 2017, 19, 195–201. [Google Scholar]
- De-Paula, D.M.; Lomonaco, D.; Ponte, A.M.P.; Cordeiro, K.E.; Moreira, M.M.; Mazzetto, S.E.; Feitosa, V.P. Influence of collagen cross-linkers addition in phosphoric acid on dentin biomodification and bonding of an etch-and-rinse adhesive. Dent. Mater. 2020, 36, e1–e8. [Google Scholar] [CrossRef]
- Rey, Y.C.D.; Palma-Dibb, R.G.; França, R.; Paula-Silva, F.W.G.; Guedes, D.F.C.; Fiuza, C.; Fernandes, A.C.B.C.J.; Faraoni, J.J.; Roselino, L.M.R. Phosphoric acid containing proanthocyanidin enhances bond stability of resin/dentin interface. Braz. Dent. J. 2022, 33, 62–70. [Google Scholar] [CrossRef] [PubMed]
- DeVito-Moraes, A.G.; Francci, C.; Vidal, C.M.; Scaffa, P.M.; Nesadal, D.; Yamasaki, L.C.; Nicolau, J.; Nascimento, F.D.; Pashley, D.H.; Carrilho, M.R. Phosphoric acid concentration affects dentinal MMPs activity. J. Dent. 2016, 53, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Scaffa, P.; Carrilho, M.; Tjäderhane, L.; Di Lenarda, R.; Polimeni, A. Effects of etch-and-rinse and self-etch adhesives on dentin MMP-2 and MMP-9. J. Dent. Res. 2013, 92, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Nascimento, F.D.; Carrilho, M.; Tersariol, I.; Papa, V.; Tjäderhane, L.; Di Lenarda, R.; Tay, F.R.; Pashley, D.H.; Breschi, L. MMP activity in the hybrid layer detected with in situ zymography. J. Dent. Res. 2012, 91, 467–472. [Google Scholar] [CrossRef]
- Spencer, P.; Wang, Y.; Walker, M.P.; Wieliczka, D.M.; Swafford, J.R. Interfacial chemistry of the dentin/adhesive bond. J. Dent. Res. 2000, 79, 1458–1463. [Google Scholar] [CrossRef]
- Wang, Y.; Spencer, P. Evaluation of the interface between one-bottle adhesive systems and dentin by Goldner’s trichrome. Am. J. Dent. 2005, 18, 66–72. [Google Scholar]
- Martin-De Las Heras, S.; Valenzuela, A.; Overall, C.M. The matrix metalloproteinase gelatinase A in human dentine. Arch. Oral. Biol. 2000, 45, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Tjäderhane, L.; Larjava, H.; Sorsa, T.; Uitto, V.J.; Larmas, M.; Salo, T. The activation and function of host matrix metalloproteinases in dentin matrix breakdown in caries lesions. J. Dent. Res. 1998, 77, 1622–1629. [Google Scholar] [CrossRef]
- Nascimento, F.D.; Minciotti, C.L.; Geraldeli, S.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Pashley, D.H.; Breschi, L. Cysteine cathepsins in human carious dentin. J. Dent. Res. 2011, 90, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Tersariol, I.L.; Geraldeli, S.; Minciotti, C.L.; Nascimento, F.D.; Pääkkönen, V.; Martins, M.T.; Carrilho, M.R.; Pashley, D.H.; Tay, F.R.; Salo, T.; et al. Cysteine cathepsins in human dentin-pulp complex. J. Endod. 2010, 36, 475–481. [Google Scholar] [CrossRef]
- Tezvergil-Mutluay, A.; Agee, K.A.; Hoshika, T.; Carrilho, M.; Breschi, L.; Tjäderhane, L.; Nishitani, Y.; Carvalho, R.M.; Looney, S.; Tay, F.R. The requirement of zinc and calcium ions for functional MMP activity in demineralized dentin matrices. Dent. Mater. 2010, 26, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Massova, I.; Kotra, L.P.; Fridman, R.; Mobashery, S. Matrix metalloproteinases: Structures, evolution, and diversification. FASEB J. 1998, 12, 1075–1095. [Google Scholar] [CrossRef]
- Sulkala, M.; Tervahartiala, T.; Sorsa, T.; Larmas, M.; Salo, T.; Tjäderhane, L. Matrix metalloproteinase-8 (MMP-8) is the major collagenase in human dentin. Arch. Oral. Biol. 2007, 52, 121–127. [Google Scholar] [CrossRef]
- Iwasa, M.; Tsubota, K.; Shimamura, Y.; Ando, S.; Miyazaki, M.; Platt, J.A. pH changes upon mixing of single-step self-etching adhesives with powdered dentin. J. Adhes. Dent. 2011, 13, 207–212. [Google Scholar]
- Tezvergil-Mutluay, A.; Mutluay, M.; Seseogullari-Dirihan, R.; Agee, K.A.; Key, W.O.; Scheffel, D.L.; Breschi, L.; Mazzoni, A.; Tjäderhane, L.; Nishitani, Y.; et al. Effect of phosphoric acid on the degradation of human dentin matrix. J. Dent. Res. 2013, 92, 87–91. [Google Scholar] [CrossRef]
- Tjäderhane, L.; Nascimento, F.D.; Breschi, L.; Mazzoni, A.; Tersariol, I.L.; Geraldeli, S.; Tezvergil-Mutluay, A.; Carrilho, M.; Carvalho, R.M.; Tay, F.R.; et al. Strategies to prevent hydrolytic degradation of the hybrid layer-A review. Dent. Mater. 2013, 29, 999–1011. [Google Scholar] [CrossRef]
- Parise Gré, C.; Pedrollo Lise, D.; Ayres, A.P.; De Munck, J.; Tezvergil-Mutluay, A.; Seseogullari-Dirihan, R.; Lopes, G.C.; Van Landuyt, K.; Van Meerbeek, B. Do collagen cross-linkers improve dentin’s bonding receptiveness? Dent. Mater. 2018, 34, 1679–1689. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Grandi, V.; Carlotto, L.; Bortoli, G.; Patzlaff, R.; Rodrigues Accorinte Mde, L.; Loguercio, A.D. Effect of smear layer thickness and acidity of self-etching solutions on early and long-term bond strength to dentin. J. Dent. 2005, 33, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, E. Photopolymerization kinetics of multi-functional monomers Progress in Polymer. Science 2001, 26, 605–665. [Google Scholar]
- Ferracane, J.L.; Mitchem, J.C.; Condon, J.R.; Todd, R. Wear and marginal breakdown of composites with various degrees of cure. J. Dent. Res. 1997, 76, 1508–1516. [Google Scholar] [CrossRef]
- Schneider, L.F.; Consani, S.; Ogliari, F.; Correr, A.B.; Sobrinho, L.C.; Sinhoreti, M.A. Effect of time and polymerization cycle on the degree of conversion of a resin composite. Oper. Dent. 2006, 31, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Haslam, E. Natural polyphenols (vegetable tannins) as drugs: Possible modes of action. J. Nat. Prod. 1996, 59, 205–215. [Google Scholar] [CrossRef]
- Cadenaro, M.; Antoniolli, F.; Sauro, S.; Tay, F.R.; Di Lenarda, R.; Prati, C.; Biasotto, M.; Contardo, L.; Breschi, L. Degree of conversion and permeability of dental adhesives. Eur. J. Oral. Sci. 2005, 113, 525–530. [Google Scholar] [CrossRef]
- Spencer, P.; Wang, Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J. Biomed. Mater. Res. 2002, 62, 447–456. [Google Scholar] [CrossRef]
- Perdigão, J.; Reis, A.; Loguercio, A.D. Dentin adhesion and MMPs: A comprehensive review. J. Esthet. Restor. Dent. 2013, 25, 219–241. [Google Scholar] [CrossRef]
- Stewart, C.A.; Finer, Y. Biostable, antidegradative and antimicrobial restorative systems based on host-biomaterials and microbial interactions. Dent. Mater. 2019, 35, 36–52. [Google Scholar] [CrossRef]
- Xie, Q.; Bedran-Russo, A.K.; Wu, C.D. In vitro remineralization effects of grape seed extract on artificial root caries. J. Dent. 2008, 36, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Pashley, D.H.; Yoshiyama, M. Two modes of nanoleakage expression in single-step adhesives. J. Dent. Res. 2002, 81, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Pianet, I.; Andre, Y.; Ducasse, M.A.; Tarascou, I.; Lartigue, J.C.; Pinaud, N.; Fouquet, E.; Dufourc, E.J.; Laguerre, M. Modeling procyanidin self-association processes and understanding their micellar organization: A study by diffusion NMR and molecular mechanics. Langmuir ACS J. Surf. Colloids 2008, 24, 11027–11035. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Cartalade, D.; Pautaux, J.L.; Cheynier, V.; Verhet, A. Flavan-3-ol aggregation in model ethanolic solutions: Incidence of polyphenol structure, concentration, ethanol content, and ionic strength. Langmuir ACS J. Surf. Colloids 2003, 19, 10563–10572. [Google Scholar] [CrossRef]
- Nassar, M.; Hiraishi, N.; Islam, M.S.; Otsuki, M.; Tagami, J. Age-related changes in salivary biomarkers. J. Dent. Sci. 2014, 9, 85–90. [Google Scholar] [CrossRef]
- Uitto, V.J.; Suomalainen, K.; Sorsa, T. Salivary collagenase. Origin, characteristics and relationship to periodontal health. J. Periodontal Res. 1990, 25, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Vidal, C.M.; Aguiar, T.R.; Phansalkar, R.; McAlpine, J.B.; Napolitano, J.G.; Chen, S.N.; Araújo, L.S.N.; Pauli, G.F.; Bedran-Russo, A. Galloyl moieties enhance the dentin biomodification potential of plant-derived catechins. Acta Biomater. 2014, 10, 3288–3294. [Google Scholar] [CrossRef]
- Madhan, B.; Krishnamoorthy, G.; Rao, J.R.; Nair, B.U. Role of green tea polyphenols in the inhibition of collagenolytic activity by collagenase. Int. J. Biol. Macromol. 2007, 41, 16–22. [Google Scholar] [CrossRef]
- Yoshida, Y.; Van Meerbeek, B.; Nakayama, Y.; Snauwaert, J.; Hellemans, L.; Lambrechts, P.; Vanherle, G.; Wakasa, K. Evidence of chemical bonding at biomaterial-hard tissue interfaces. J. Dent. Res. 2000, 79, 709–714. [Google Scholar] [CrossRef]
- Coutinho, E.; Yoshida, Y.; Inoue, S.; Fukuda, R.; Snauwaert, J.; Nakayama, Y.; De Munck, J.; Lambrechts, P.; Suzuki, K.; Van Meerbeek, B. Gel phase formation at resin-modified glass-ionomer/tooth interfaces. J. Dent. Res. 2007, 86, 656–661. [Google Scholar] [CrossRef]
- Jin, X.; Han, F.; Wang, Q.; Yuan, X.; Zhou, Q.; Xie, H.; Niu, L.; Chen, C. The roles of 10-methacryloyloxydecyl dihydrogen phosphate and its calcium salt in preserving the adhesive-dentin hybrid layer. Dent. Mater. 2022, 38, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Van Meerbeek, B.; Peumans, M.; Poitevin, A.; Mine, A.; Van Ende, A.; Neves, A.; De Munck, J. Relationship between bond-strength tests and clinical outcomes. Dent. Mater. 2010, 26, e100–e121. [Google Scholar] [CrossRef] [PubMed]

| Group | Final Composition (Percentage by Total Weight) | Application Protocol | pH |
|---|---|---|---|
| Control (CT): Scotchbond Universal Etchant; 3M ESPE, St. Paul, MN, USA, Lot. 542642 | 32% phosphoric acid (approximately), water, synthetic amorphous silica, polyethylene glycol, aluminum oxide. | - Apply the acid etchant gel to the dentin; - Allow to react for 15 s; - Rinse thoroughly with deionized water for 30 s. | ~0.1 |
| 5PA/GSE | 5% phosphoric acid, 73% deionized water, 20% ethanol and 2% GSE. | - Apply the acid etchant to the dentin; - Allow to react for 30 s; - Rinse thoroughly with deionized water for 30 s. | ~1.15 |
| 10PA/GSE | 10% phosphoric acid, 68% deionized water, 20% ethanol and 2% GSE. | - Apply the acid etchant to the dentin; - Allow to react for 30 s; - Rinse thoroughly with deionized water for 30 s. | ~0.85 |
| 20PA/GSE | 20% phosphoric acid, 58% deionized water, 20% ethanol and 2% GSE. | - Apply the acid etchant to the dentin; - Allow to react for 30 s; - Rinse thoroughly with deionized water for 30 s. | ~0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hass, V.; Yao, X.; Wang, Y. Effects of Grape Seed Extract-Modified Etchants on Collagenolytic Activity, Interface Formation, and Bonding Longevity of Adhesive–Dentin Interfaces. Materials 2025, 18, 2416. https://doi.org/10.3390/ma18112416
Hass V, Yao X, Wang Y. Effects of Grape Seed Extract-Modified Etchants on Collagenolytic Activity, Interface Formation, and Bonding Longevity of Adhesive–Dentin Interfaces. Materials. 2025; 18(11):2416. https://doi.org/10.3390/ma18112416
Chicago/Turabian StyleHass, Viviane, Xiaomei Yao, and Yong Wang. 2025. "Effects of Grape Seed Extract-Modified Etchants on Collagenolytic Activity, Interface Formation, and Bonding Longevity of Adhesive–Dentin Interfaces" Materials 18, no. 11: 2416. https://doi.org/10.3390/ma18112416
APA StyleHass, V., Yao, X., & Wang, Y. (2025). Effects of Grape Seed Extract-Modified Etchants on Collagenolytic Activity, Interface Formation, and Bonding Longevity of Adhesive–Dentin Interfaces. Materials, 18(11), 2416. https://doi.org/10.3390/ma18112416







