Sustainable Alginate–Hydrochar Composite Beads for 2-Nitrophenol Adsorption in Batch and Fixed-Bed Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Alginate–Hydrochar Composite Bead Preparation
2.3. Characterization
2.4. 2-Nitrophenol Batch Adsorption Experiments
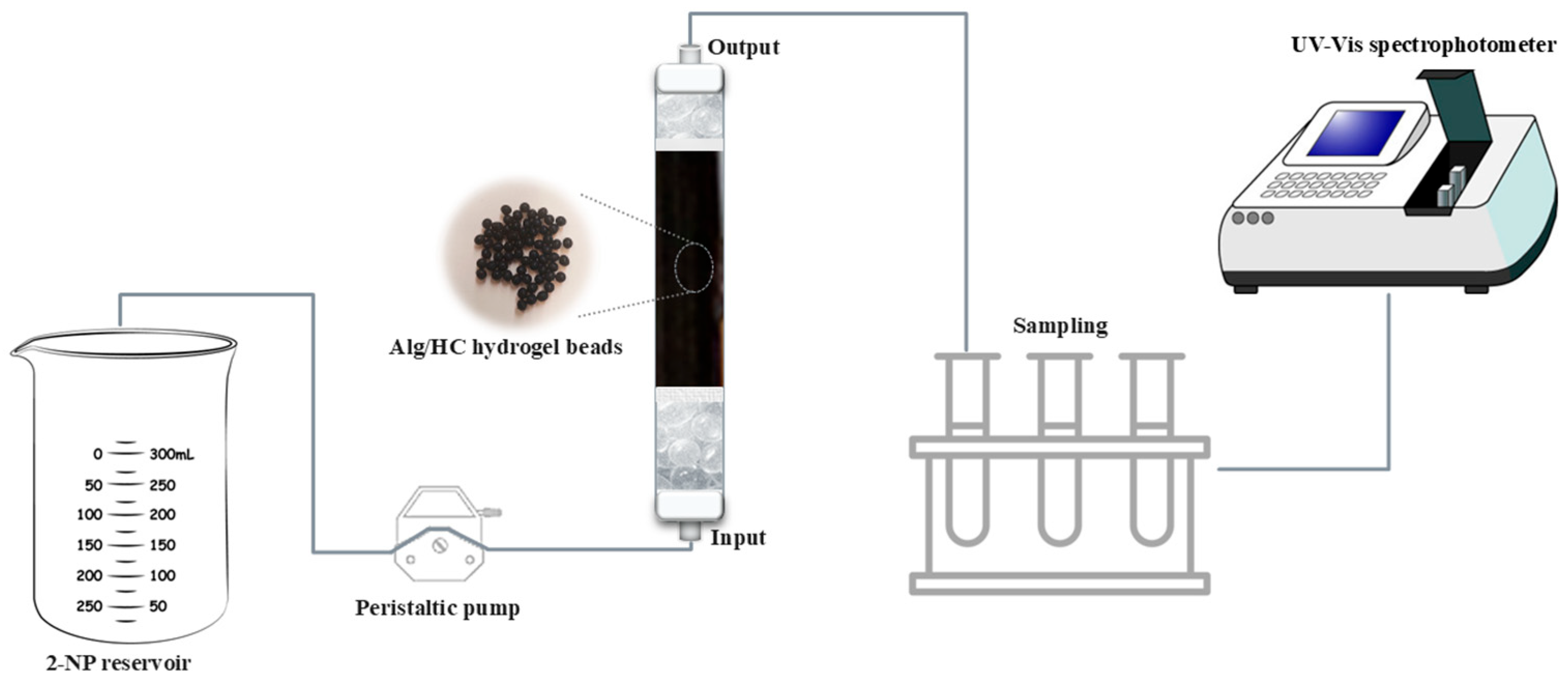
2.5. Continuous Column Adsorption Experiments
Determination of the Column Parameters
2.6. Regeneration Study in the Continuous System
2.7. Goodness-of-Fit Analysis
3. Results and Discussion
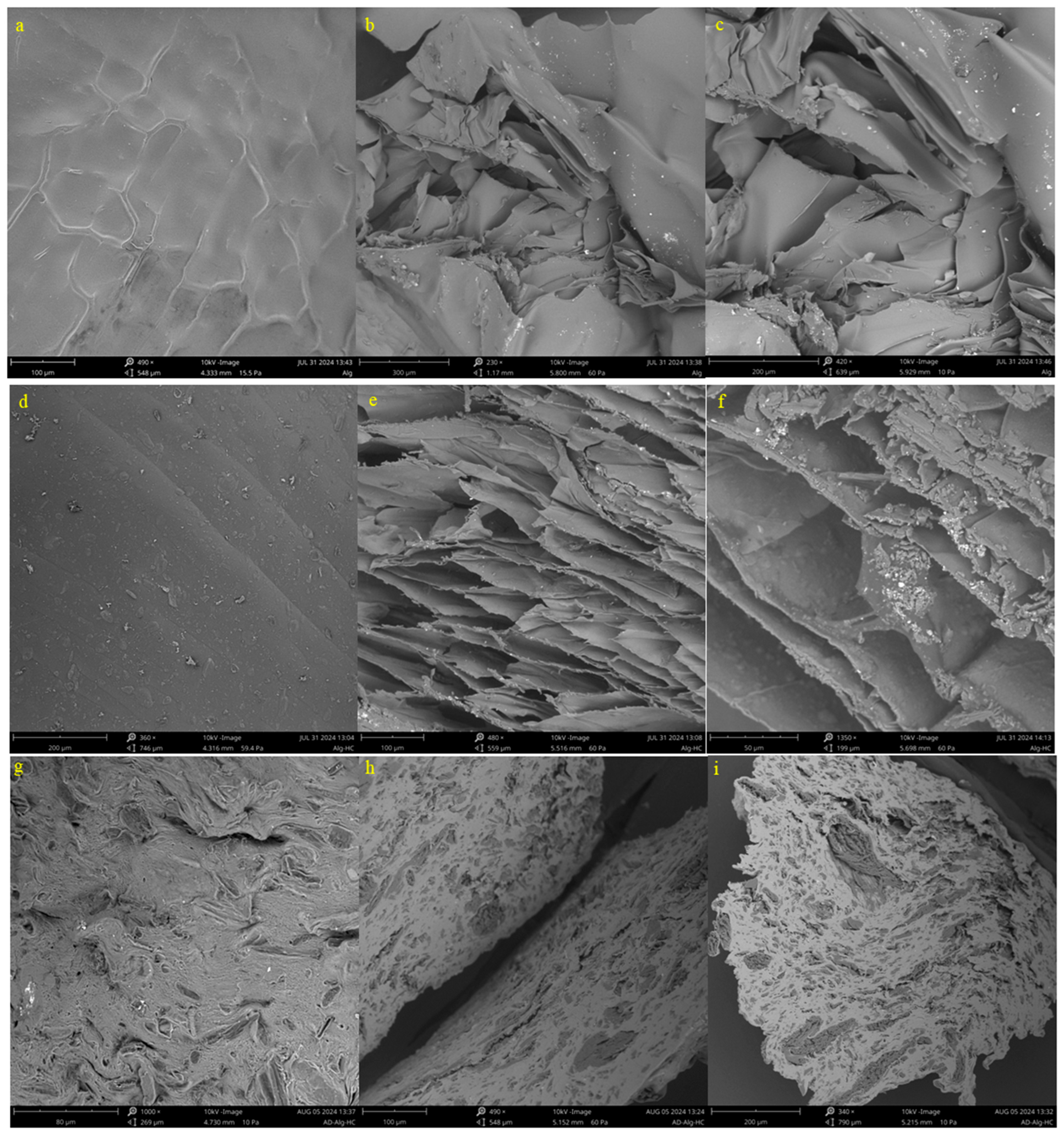
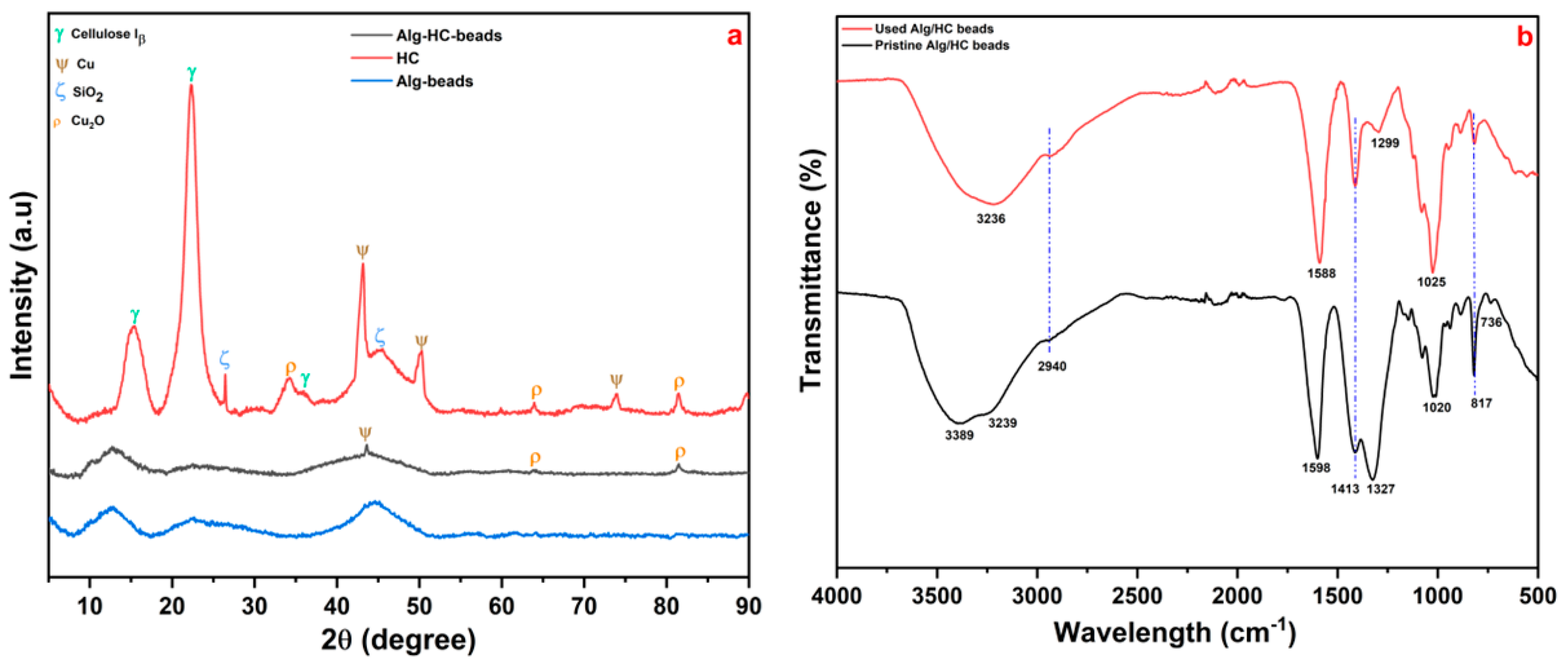
3.1. Alginate–Hydrochar Adsorbent Characterization
3.2. Batch Adsorption Performance
3.2.1. Batch Adsorption Kinetics of 2-Nitrophenol on Alginate–Hydrochar Beads
3.2.2. Batch Adsorption Isotherms and Thermodynamics of 2-Nitrophenol on Alginate–Hydrochar Beads
3.3. Fixed-Bed Adsorption Performance
3.4. Modeling of the Breakthrough Curves
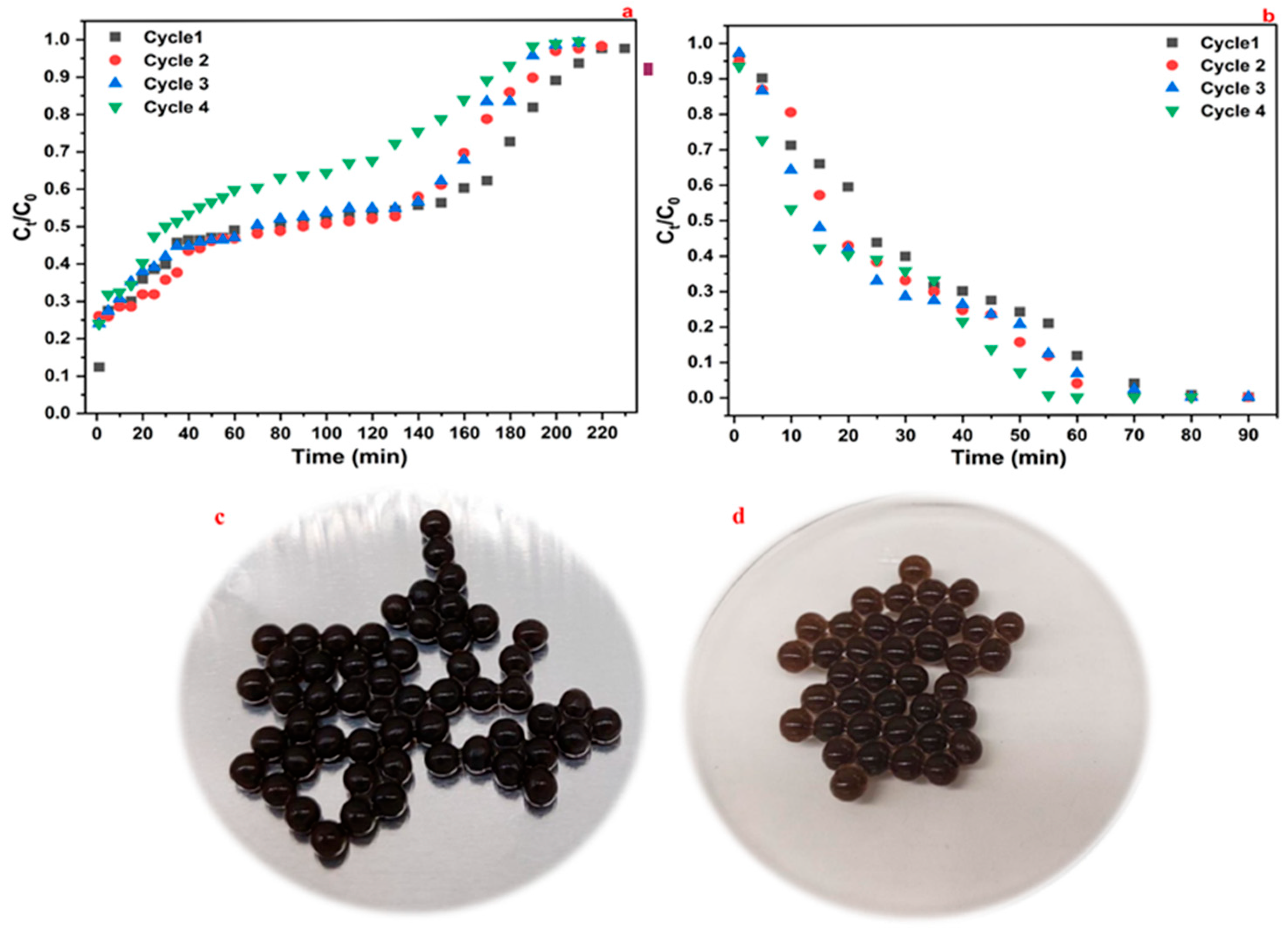
3.5. Packed-Bed Adsorbent Bead Regeneration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Etteieb, S.; Magdouli, S.; Komtchou, S.P.; Zolfaghari, M.; Tanabene, R.; Brar, K.K.; Calugaru Luliana, L.; Brar, S.K. Selenium Speciation and Bioavailability from Mine Discharge to the Environment: A Field Study in Northern Quebec, Canada. Environ. Sci. Pollut. Res. 2021, 28, 50799–50812. [Google Scholar] [CrossRef]
- Schwartz, H.; Marushka, L.; Chan, H.M.; Batal, M.; Sadik, T.; Ing, A.; Fediuk, K.; Tikhonov, C. Pharmaceuticals in Source Waters of 95 First Nations in Canada. Can. J. Public Health 2021, 112, 133–153. [Google Scholar] [CrossRef] [PubMed]
- Vaudreuil, M.A.; Vo Duy, S.; Munoz, G.; Sauvé, S. Pharmaceutical Pollution of Hospital Effluents and Municipal Wastewaters of Eastern Canada. Sci. Total Environ. 2022, 846, 157353. [Google Scholar] [CrossRef]
- Kumari, P.; Tripathi, K.M.; Awasthi, K.; Gupta, R. Adsorptive Removal of Nitrophenols from Water by Biomass-Derived Carbon Nano-Onions. Ind. Eng. Chem. Res. 2023, 62, 19801–19812. [Google Scholar] [CrossRef]
- Adeola, A.O.; Paramo, L.; Fuoco, G.; Naccache, R. Emerging Hazardous Chemicals and Biological Pollutants in Canadian Aquatic Systems and Remediation Approaches: A Comprehensive Status Report. Sci. Total Environ. 2024, 954, 176267. [Google Scholar] [CrossRef]
- Hesaraki, S.A.H.; Prymak, O.; Heidelmann, M.; Ulbricht, M.; Fischer, L. Integrated In Situ Fabrication of CuO Nanorod-Decorated Polymer Membranes for the Catalytic Flow-Through Reduction of p-Nitrophenol. ACS Appl. Mater. Interfaces 2024, 16, 17517–17530. [Google Scholar] [CrossRef]
- Mateen, S.; Nawaz, R.; Qamar, M.T.; Ali, S.; Iqbal, S.; Aslam, M.; Raheel, M.; Awwad, N.S.; Ibrahium, H.A. Integration of WO3-Doped MoO3 with ZnO Photocatalyst for the Removal of 2-Nitrophenol in Natural Sunlight Illumination. Catalysts 2023, 13, 1262. [Google Scholar] [CrossRef]
- Dutt, S.; Singh, A.; Mahadeva, R.; Sundramoorthy, A.K.; Gupta, V.; Arya, S. A Reduced Graphene Oxide-Based Electrochemical Sensing and Eco-Friendly 4-Nitrophenol Degradation. Diam. Relat. Mater. 2024, 141, 110554. [Google Scholar] [CrossRef]
- Allouss, D.; Dupont, A.; Achouri, I.E.; Abatzoglou, N. Hydrothermal Conversion of Cu-Laden Biomass to One-Step Doped Hydrochar Used as a Potential Adsorbent for 2-Nitrophenol Removal. Sustain. Chem. Pharm. 2024, 39, 101505. [Google Scholar] [CrossRef]
- Bampi, J.; da Silva, T.C.; da Luz, C.; Pasquali, G.D.L.; Dervanoski, A.; Tochetto, G. Study of the Competitive Effect on the Adsorption of Phenol and 4-Nitrophenol in a Batch Reactor and Fixed-Bed Column Using Coconut Shell Activated Carbon. J. Water Process Eng. 2024, 65, 105825. [Google Scholar] [CrossRef]
- Patel, H. Fixed-Bed Column Adsorption Study: A Comprehensive Review. Appl. Water Sci. 2019, 9, 45. [Google Scholar] [CrossRef]
- Zheng, C.; Yong, Y.; Wang, Q.; Lin, Z.; Wang, Y.; Zhang, Y.; He, C. Removal of Pb(II) by Lignin-Sodium Alginate Composite in a Fixed-Bed Column. Environ. Technol. 2024, 45, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Sellaoui, L.; Luiz Dotto, G.; Bonilla-Petriciolet, A.; Ben Lamine, A. Understanding the Adsorption Mechanism of Phenol and 2-Nitrophenol on a Biopolymer-Based Biochar in Single and Binary Systems via Advanced Modeling Analysis. Chem. Eng. J. 2019, 371, 1–6. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Moreno-Pérez, J.; Hernández-Hernández, L.E.; Bonilla-Petriciolet, A.; Dotto, G.L.; Salau, N.P.G. Novel Biochar and Hydrochar for the Adsorption of 2-Nitrophenol from Aqueous Solutions: An Approach Using the PVSDM Model. Chemosphere 2021, 269, 128748. [Google Scholar] [CrossRef]
- Allouss, D.; Marrane, S.E.; Essamlali, Y.; Chakir, A.; Zahouily, M. Chemometrics for Optimization and Modeling of Cu (II) Continuous Adsorption onto Carboxymethylcellulose-Alginate Encapsulated Graphene Oxide Hydrogel Beads. Int. J. Environ. Sci. Technol. 2024, 21, 7061–7076. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-Based Nanomaterials: Fabrication Techniques, Properties, and Applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Allouss, D.; Essamlali, Y.; Amadine, O.; Chakir, A.; Zahouily, M. Response Surface Methodology for Optimization of Methylene Blue Adsorption onto Carboxymethyl Cellulose-Based Hydrogel Beads: Adsorption Kinetics, Isotherm, Thermodynamics and Reusability Studies. RSC Adv. 2019, 9, 37858–37869. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Z.; Shen, J.; Kang, J.; Zhao, S.; Wang, B.; Chen, Q.; Li, X. Dynamic Adsorption Models and Artificial Neural Network Prediction of Mercury Adsorption by a Dendrimer-Grafted Polyacrylonitrile Fiber in Fixed-Bed Column. J. Clean. Prod. 2021, 310, 127511. [Google Scholar] [CrossRef]
- Khalfa, L.; Sdiri, A.; Bagane, M.; Cervera, M.L. A Calcined Clay Fixed Bed Adsorption Studies for the Removal of Heavy Metals from Aqueous Solutions. J. Clean. Prod. 2021, 278, 123935. [Google Scholar] [CrossRef]
- Khadhri, N.; El Khames Saad, M.; Ben Mosbah, M.; Moussaoui, Y. Batch and Continuous Column Adsorption of Indigo Carmine onto Activated Carbon Derived from Date Palm Petiole. J. Environ. Chem. Eng. 2019, 7, 102775. [Google Scholar] [CrossRef]
- Cavas, L.; Karabay, Z.; Alyuruk, H.; Doĝan, H.; Demir, G.K. Thomas and Artificial Neural Network Models for the Fixed-Bed Adsorption of Methylene Blue by a Beach Waste Posidonia oceanica (L.) Dead Leaves. Chem. Eng. J. 2011, 171, 557–562. [Google Scholar] [CrossRef]
- Kletting, P.; Glatting, G. Model Selection for Time-Activity Curves: The Corrected Akaike Information Criterion and the F-Test. Z. Med. Phys. 2009, 19, 200–206. [Google Scholar] [CrossRef]
- Akpa, O.M.; Unuabonah, E.I. Small-Sample Corrected Akaike Information Criterion: An Appropriate Statistical Tool for Ranking of Adsorption Isotherm Models. Desalination 2011, 272, 20–26. [Google Scholar] [CrossRef]
- Allouss, D.; Makhado, E.; Zahouily, M. Recent Progress in Polysaccharide-Based Hydrogel Beads as Adsorbent for Water Pollution Remediation. Springer Ser. Mater. Sci. 2022, 323, 55–88. [Google Scholar] [CrossRef]
- Thakur, S.; Sharma, B.; Verma, A.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent Progress in Sodium Alginate Based Sustainable Hydrogels for Environmental Applications. J. Clean. Prod. 2018, 198, 143–159. [Google Scholar] [CrossRef]
- Ashrafi, S.D.; Safari, G.H.; Sharafi, K.; Kamani, H.; Jaafari, J. Adsorption of 4-Nitrophenol on Calcium Alginate-Multiwall Carbon Nanotube Beads: Modeling, Kinetics, Equilibriums and Reusability Studies. Int. J. Biol. Macromol. 2021, 185, 66–76. [Google Scholar] [CrossRef]
- Larosa, C.; Salerno, M.; de Lima, J.S.; Merijs Meri, R.; da Silva, M.F.; de Carvalho, L.B.; Converti, A. Characterisation of Bare and Tannase-Loaded Calcium Alginate Beads by Microscopic, Thermogravimetric, FTIR and XRD Analyses. Int. J. Biol. Macromol. 2018, 115, 900–906. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. The Production of Carbon Materials by Hydrothermal Carbonization of Cellulose. Carbon. N. Y 2009, 47, 2281–2289. [Google Scholar] [CrossRef]
- de Araújo, T.P.; Quesada, H.B.; dos Santos, D.F.; da Silva Fonseca, B.C.; Barbieri, J.Z.; Bergamasco, R.; de Barros, M.A.S.D. Acetaminophen Removal by Calcium Alginate/Activated Hydrochar Composite Beads: Batch and Fixed-Bed Studies. Int. J. Biol. Macromol. 2022, 203, 553–562. [Google Scholar] [CrossRef]
- Benslima, A.; Sellimi, S.; Hamdi, M.; Nasri, R.; Jridi, M.; Cot, D.; Li, S.; Nasri, M.; Zouari, N. The Brown Seaweed Cystoseira Schiffneri as a Source of Sodium Alginate: Chemical and Structural Characterization, and Antioxidant Activities. Food Biosci. 2021, 40, 100873. [Google Scholar] [CrossRef]
- Arasteh, R.; Masoumi, M.; Rashidi, A.M.; Moradi, L.; Samimi, V.; Mostafavi, S.T. Adsorption of 2-Nitrophenol by Multi-Wall Carbon Nanotubes from Aqueous Solutions. Appl. Surf. Sci. 2010, 256, 4447–4455. [Google Scholar] [CrossRef]
- Kupeta, A.J.K.; Naidoo, E.B.; Ofomaja, A.E. Kinetics and Equilibrium Study of 2-Nitrophenol Adsorption onto Polyurethane Cross-Linked Pine Cone Biomass. J. Clean. Prod. 2018, 179, 191–209. [Google Scholar] [CrossRef]
- Putro, J.N.; Kurniawan, A.; Ismadji, S.; Ju, Y.H. Nanocellulose Based Biosorbents for Wastewater Treatment: Study of Isotherm, Kinetic, Thermodynamic and Reusability. Environ. Nanotechnol. Monit. Manag. 2017, 8, 134–149. [Google Scholar] [CrossRef]
- Wang, N.; Chen, J.; Wang, J.; Feng, J.; Yan, W. Removal of Methylene Blue by Polyaniline/TiO2 Hydrate: Adsorption Kinetic, Isotherm and Mechanism Studies. Powder Technol. 2019, 347, 93–102. [Google Scholar] [CrossRef]
- Isichei, T.O.; Okieimen, F.E. Adsorption of 2-Nitrophenol onto Water Hyacinth Activated Carbon-Kinetics and Equilibrium Studies. Environ. Pollut. 2014, 3, 99. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Mishra, T. Adsorption Isotherm Modeling for Methylene Blue Removal onto Magnetic Kaolinite Clay: A Comparison of Two-Parameter Isotherms. Appl. Water Sci. 2021, 11, 103. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, M.; Liu, N.; Zhang, F.; An, K.; Xiong, X.; Fan, S.; Sun, Q.; Le, T. Engineered Biochar Derived from Lemon Peel Waste for Highly Efficient Removal of Organic Pollutants from Water. Arab. J. Chem. 2023, 16, 105158. [Google Scholar] [CrossRef]
- González, J.A.; Bafico, J.G.; Villanueva, M.E.; Giorgieri, S.A.; Copello, G.J. Continuous Flow Adsorption of Ciprofloxacin by Using a Nanostructured Chitin/Graphene Oxide Hybrid Material. Carbohydr. Polym. 2018, 188, 213–220. [Google Scholar] [CrossRef]
- Allen, S.J.; Koumanova, B.; Kircheva, Z.; Nenkova, S. Adsorption of 2-Nitrophenol by Technical Hydrolysis Lignin: Kinetics, Mass Transfer, and Equilibrium Studies. Ind. Eng. Chem. Res. 2005, 44, 2281–2287. [Google Scholar] [CrossRef]
- Abaide, E.R.; Dotto, G.L.; Tres, M.V.; Zabot, G.L.; Mazutti, M.A. Adsorption of 2–Nitrophenol Using Rice Straw and Rice Husks Hydrolyzed by Subcritical Water. Bioresour. Technol. 2019, 284, 25–35. [Google Scholar] [CrossRef]
- Ai, L.; Li, M.; Li, L. Adsorption of Methylene Blue from Aqueous Solution with Activated Carbon/Cobalt Ferrite/Alginate Composite Beads: Kinetics, Isotherms, and Thermodynamics. J. Chem. Eng. Data 2011, 56, 3475–3483. [Google Scholar] [CrossRef]
- Mahmoud, A.E.D.; Franke, M.; Braeutigam, P. Experimental and Modeling of Fixed-Bed Column Study for Phenolic Compounds Removal by Graphite Oxide. J. Water Process Eng. 2022, 49, 103085. [Google Scholar] [CrossRef]
- Satya, A.; Harimawan, A.; Sri Haryani, G.; Johir, M.A.H.; Nguyen, L.N.; Nghiem, L.D.; Vigneswaran, S.; Ngo, H.H.; Setiadi, T. Fixed-Bed Adsorption Performance and Empirical Modeling of Cadmium Removal Using Adsorbent Prepared from the Cyanobacterium Aphanothece Sp Cultivar. Environ. Technol. Innov. 2021, 21, 101194. [Google Scholar] [CrossRef]
- Taka, A.L.; Klink, M.J.; Mbianda, X.Y.; Naidoo, E.B. Chitosan Nanocomposites for Water Treatment by Fixed-Bed Continuous Flow Column Adsorption: A Review. Carbohydr. Polym. 2021, 255, 117398. [Google Scholar] [CrossRef]
- Abdallah, M.M.; Ahmad, M.N.; Walker, G.; Leahy, J.J.; Kwapinski, W. Batch and Continuous Systems for Zn, Cu, and Pb Metal Ions Adsorption on Spent Mushroom Compost Biochar. Ind. Eng. Chem. Res. 2019, 58, 7296–7307. [Google Scholar] [CrossRef]
- Chu, K.H. Breakthrough Curve Analysis by Simplistic Models of Fixed Bed Adsorption: In Defense of the Century-Old Bohart-Adams Model. Chem. Eng. J. 2020, 380, 122513. [Google Scholar] [CrossRef]





| 2-NP Concentration (mg/L) | Temperature (K) | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol K) |
|---|---|---|---|---|
| 10 | 298.15 | 1.42 | 100.69 | 332.94 |
| 308.15 | −1.90 | |||
| 318.15 | 0.46 | −41.50 | −131.88 | |
| 328.15 | 1.78 | |||
| 30 | 298.15 | 0.52 | −11.30 | −39.64 |
| 308.15 | 0.92 | |||
| 318.15 | −0.36 | −47.14 | −147.03 | |
| 328.15 | 1.10 |
| Column adsorption experiments | Q (mL/min) | 3.8 | 3.8 | 3.8 | 5.2 | 3.8 | 3.8 |
| Z (cm) | 35 | 55 | 15 | 35 | 35 | 35 | |
| C0 (mg/L) | 10 | 10 | 10 | 10 | 20 | 5 | |
| Thomas model | KTh (mL/(mg min)) | 0.0004 | 0.0003 | 0.002 | 0.003 | 0.0005 | 0.003 |
| q0 (mg/g) | 235 | 337 | 152 | 97 | 235 | 40 | |
| R2 | 0.867 | 0.803 | 0.946 | 0.961 | 0.838 | 0.817 | |
| RMSE | 0.07 | 0.008 | 0.05 | 0.06 | 0.10 | 0.11 | |
| AICC | 264 | 484 | 174 | 155 | 184 | 158 | |
| Yoon–Nelson model | KYN (min−1) | 0.004 | 0.003 | 0.016 | 0.03 | 0.01 | 0.012 |
| τ (min) | 212 | 488 | 60 | 65 | 108 | 73 | |
| R2 | 0.872 | 0.803 | 0.946 | 0.956 | 0.838 | 0.817 | |
| RMSE | 0.01 | 0.08 | 0.05 | 0.06 | 0.10 | 0.11 | |
| AICC | 158 | 482 | 172 | 155 | 183 | 158 | |
| Bohart–Adams model | KAB (mL/(mg min)) | 0.030 | 0.003 | 0.002 | 0.010 | 0.006 | 0.024 |
| N0 (mg/L) | 21 | 72 | 176 | 46 | 61 | 19 | |
| R2 | 0.963 | 0.886 | 0.915 | 0.924 | 0.815 | 0.934 | |
| RMSE | 0.020 | 0.030 | 0.000 | 0.030 | 0.060 | 0.000 | |
| AICC | 15 | 92 | 21 | 25 | 7 | 23 | |
| Clark model | A | 0.019 | 0.012 | 1.662 | 0.018 | 1.0 | 0.007 |
| n | 1.0 | 1.0 | 1.7 | 1.0 | 0.9 | 1.0 | |
| r (min−1) | 0.003 | 0.002 | 0.015 | 0.020 | 0.004 | 0.010 | |
| R2 | 0.868 | 0.814 | 0.941 | 0.969 | 0.873 | 0.840 | |
| RMSE | 0.075 | 0.085 | 0.057 | 0.049 | 0.089 | 0.101 | |
| AICC | 262 | 486 | 169 | 162 | 193 | 164 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allouss, D.; Abatzoglou, N.; Achouri, I.E. Sustainable Alginate–Hydrochar Composite Beads for 2-Nitrophenol Adsorption in Batch and Fixed-Bed Systems. Materials 2025, 18, 2412. https://doi.org/10.3390/ma18102412
Allouss D, Abatzoglou N, Achouri IE. Sustainable Alginate–Hydrochar Composite Beads for 2-Nitrophenol Adsorption in Batch and Fixed-Bed Systems. Materials. 2025; 18(10):2412. https://doi.org/10.3390/ma18102412
Chicago/Turabian StyleAllouss, Dalia, Nicolas Abatzoglou, and Inès Esma Achouri. 2025. "Sustainable Alginate–Hydrochar Composite Beads for 2-Nitrophenol Adsorption in Batch and Fixed-Bed Systems" Materials 18, no. 10: 2412. https://doi.org/10.3390/ma18102412
APA StyleAllouss, D., Abatzoglou, N., & Achouri, I. E. (2025). Sustainable Alginate–Hydrochar Composite Beads for 2-Nitrophenol Adsorption in Batch and Fixed-Bed Systems. Materials, 18(10), 2412. https://doi.org/10.3390/ma18102412









