Synthesis of Porous Lithium Ion Sieve with High Purity for Li+ Adsorption
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Synthesis of Porous Cubic Mn2O3
2.2.2. Synthesis of LiMnO2 and Precursor Li1.6Mn1.6O4
2.2.3. Synthesis of Lithium Ion Sieve
2.2.4. Adsorption and Cycling Performance
2.2.5. Evaluation of Adsorption Selectivity
2.2.6. Evaluation of Adsorption Kinetics
2.2.7. Determination of Adsorption Isotherm
2.2.8. Analysis and Characterization
3. Results
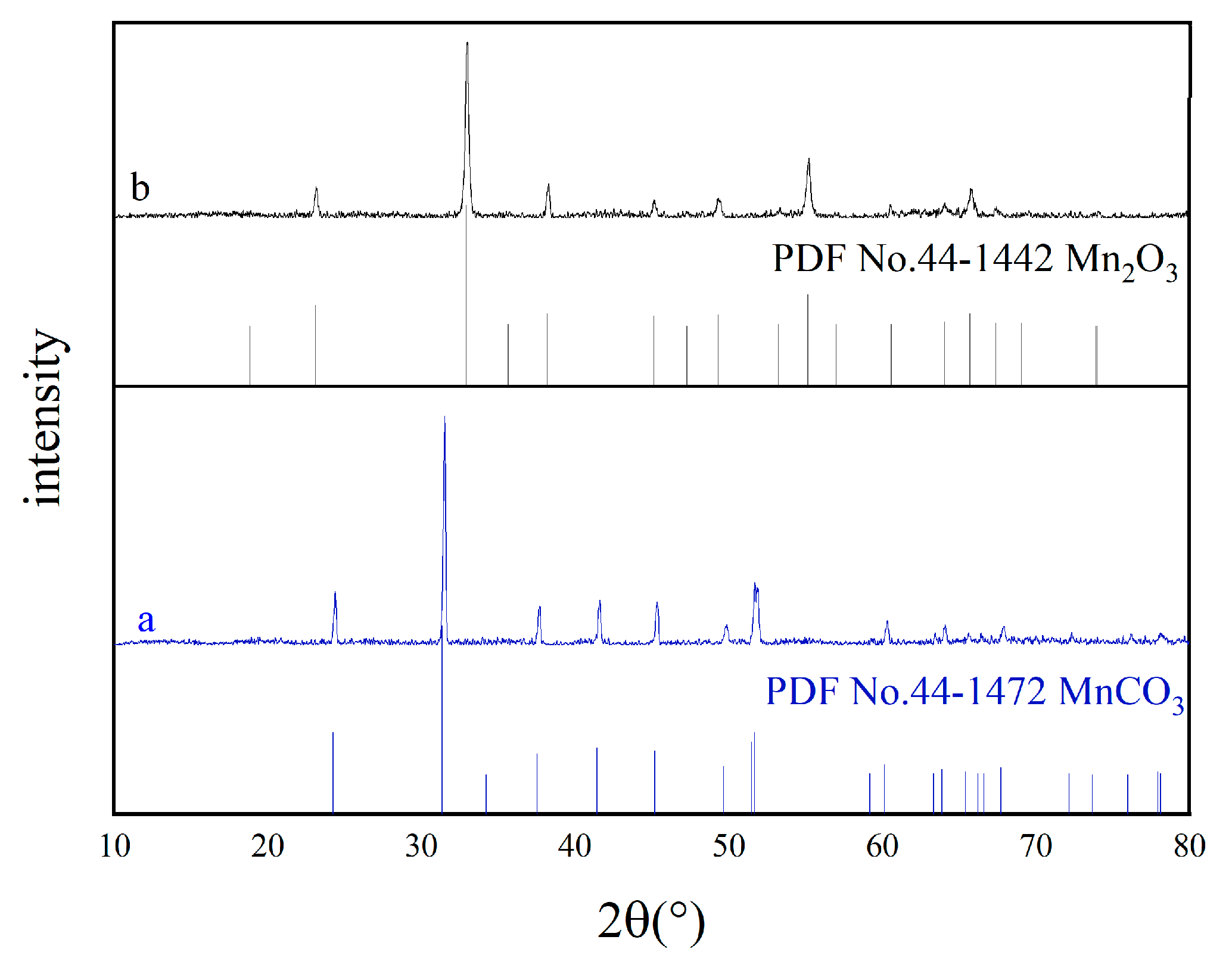
3.1. Properties of Porous Mn2O3
3.2. Synthesis of LiMnO2
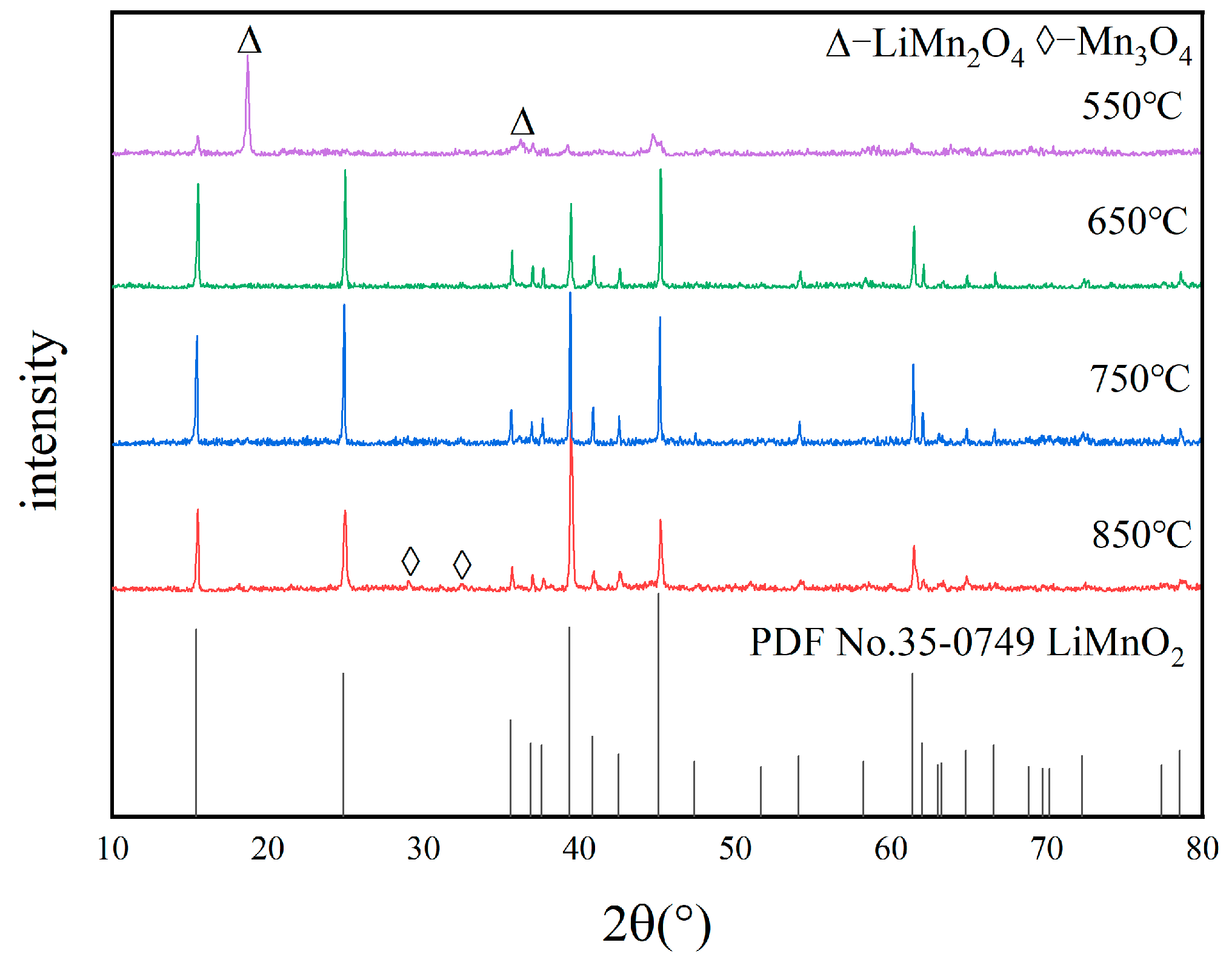
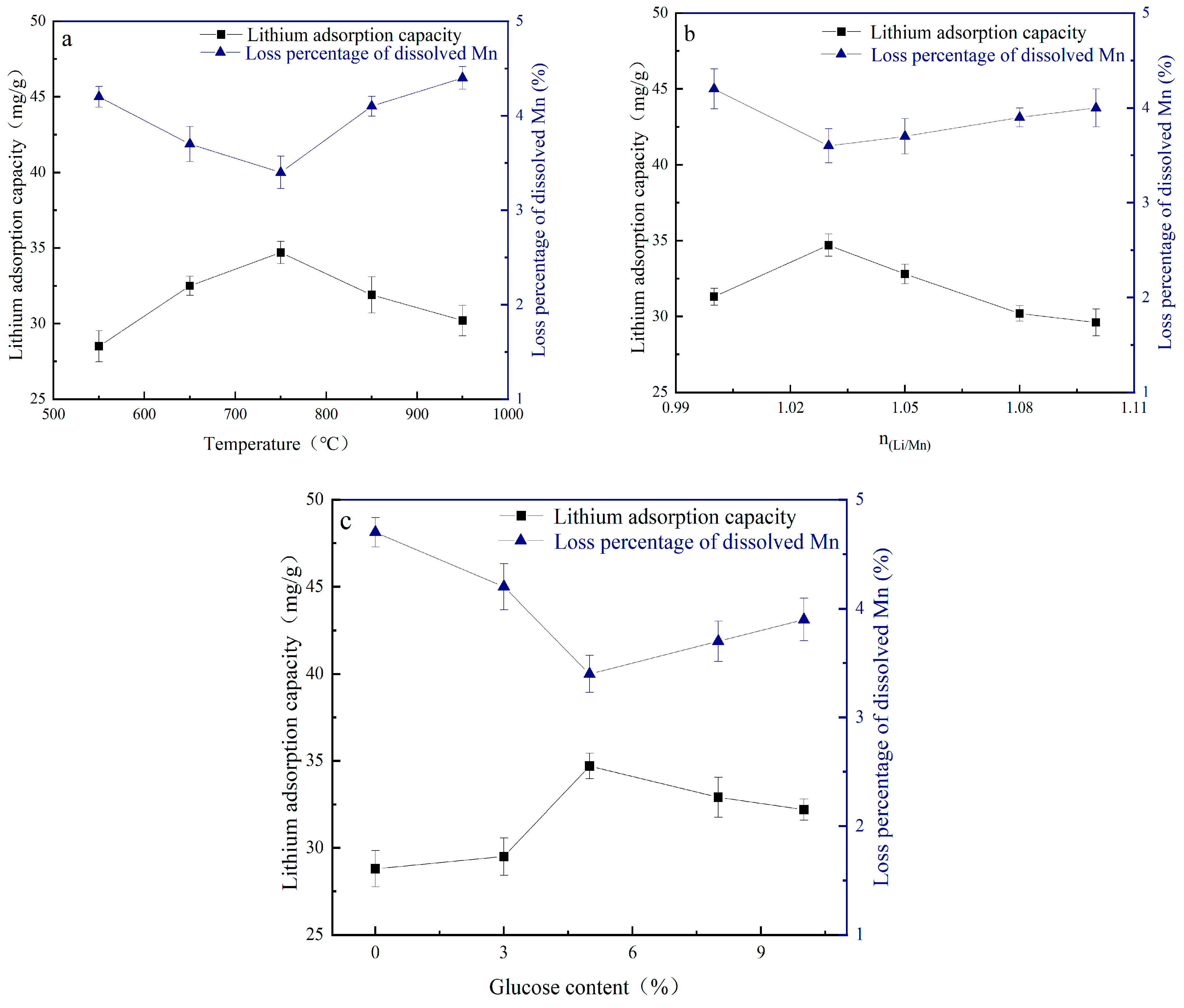
3.2.1. Optimization of Calcination Temperature
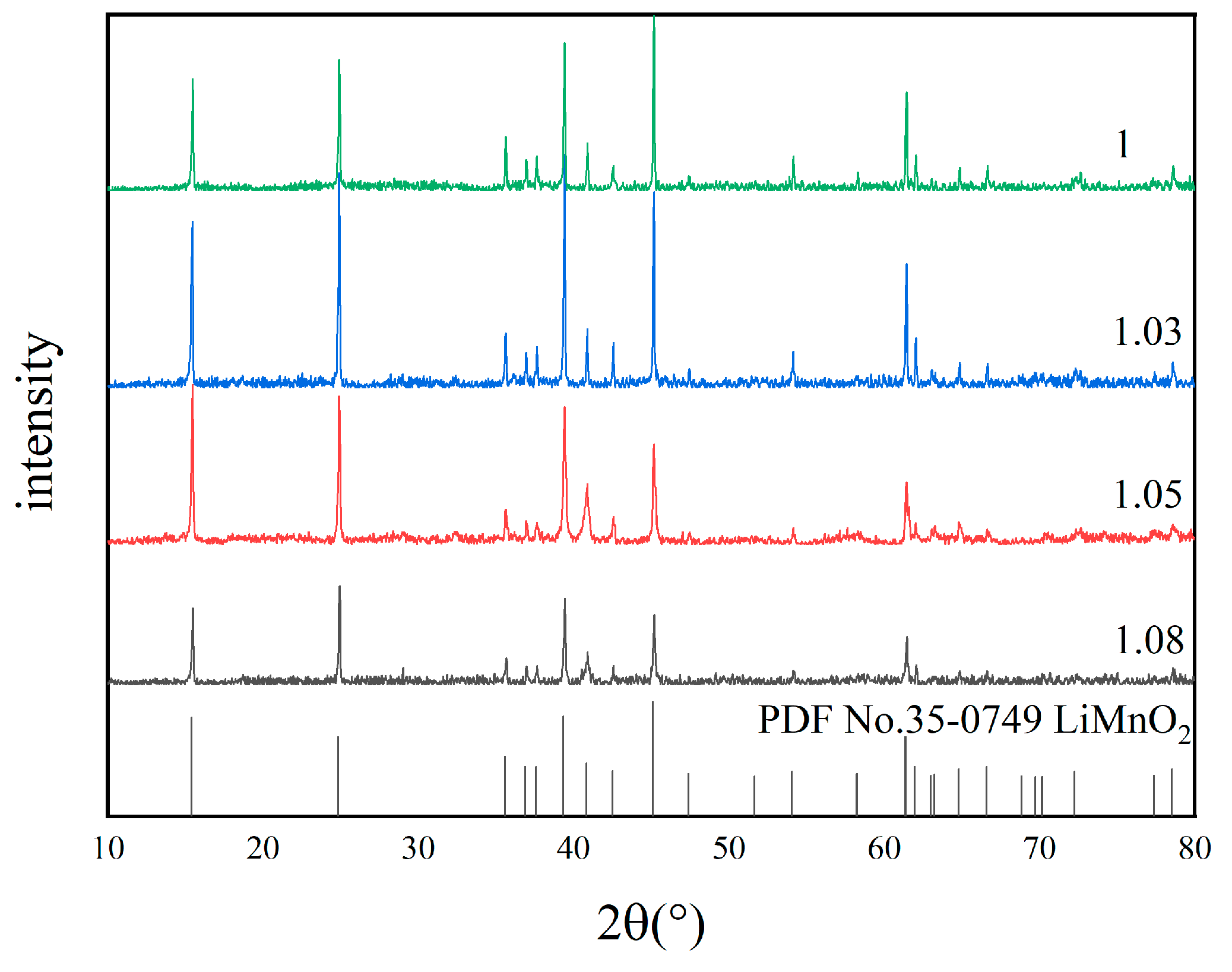
3.2.2. Optimization of Molar Ratio of Lithium to Manganese
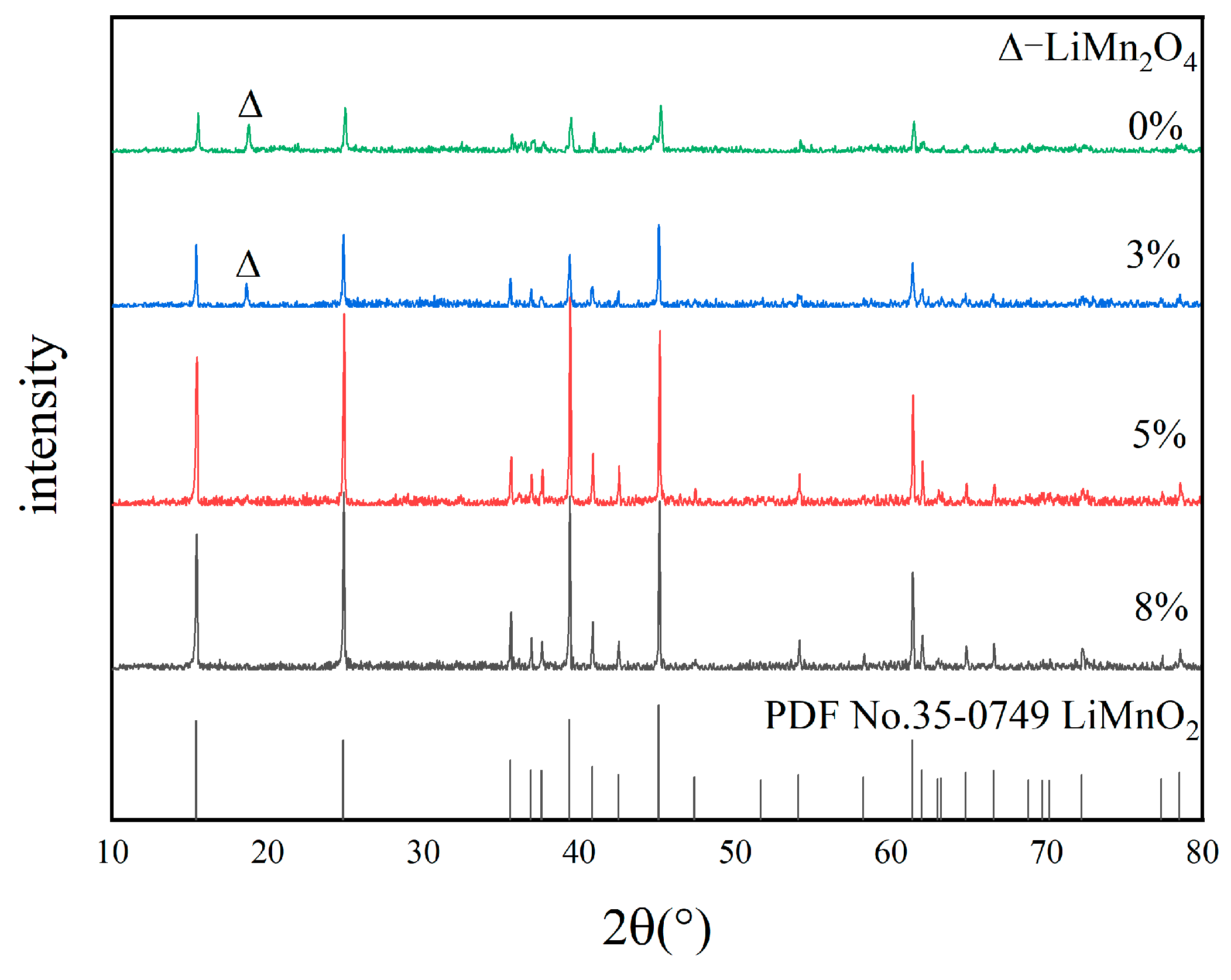
3.2.3. Optimization of Glucose Content
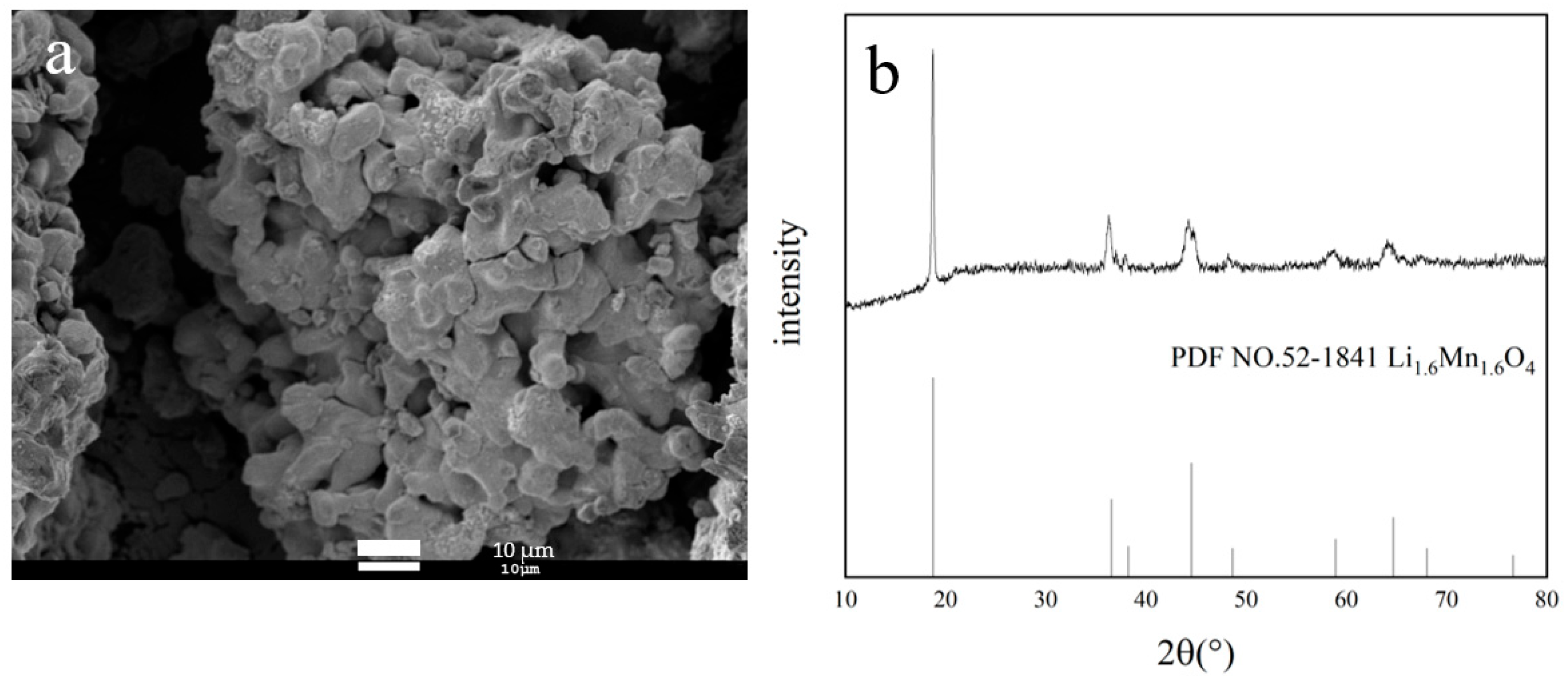
3.3. Characterization of Li1.6Mn1.6O4
4. Discussion
4.1. Adsorption Performance of H1.6Mn1.6O4
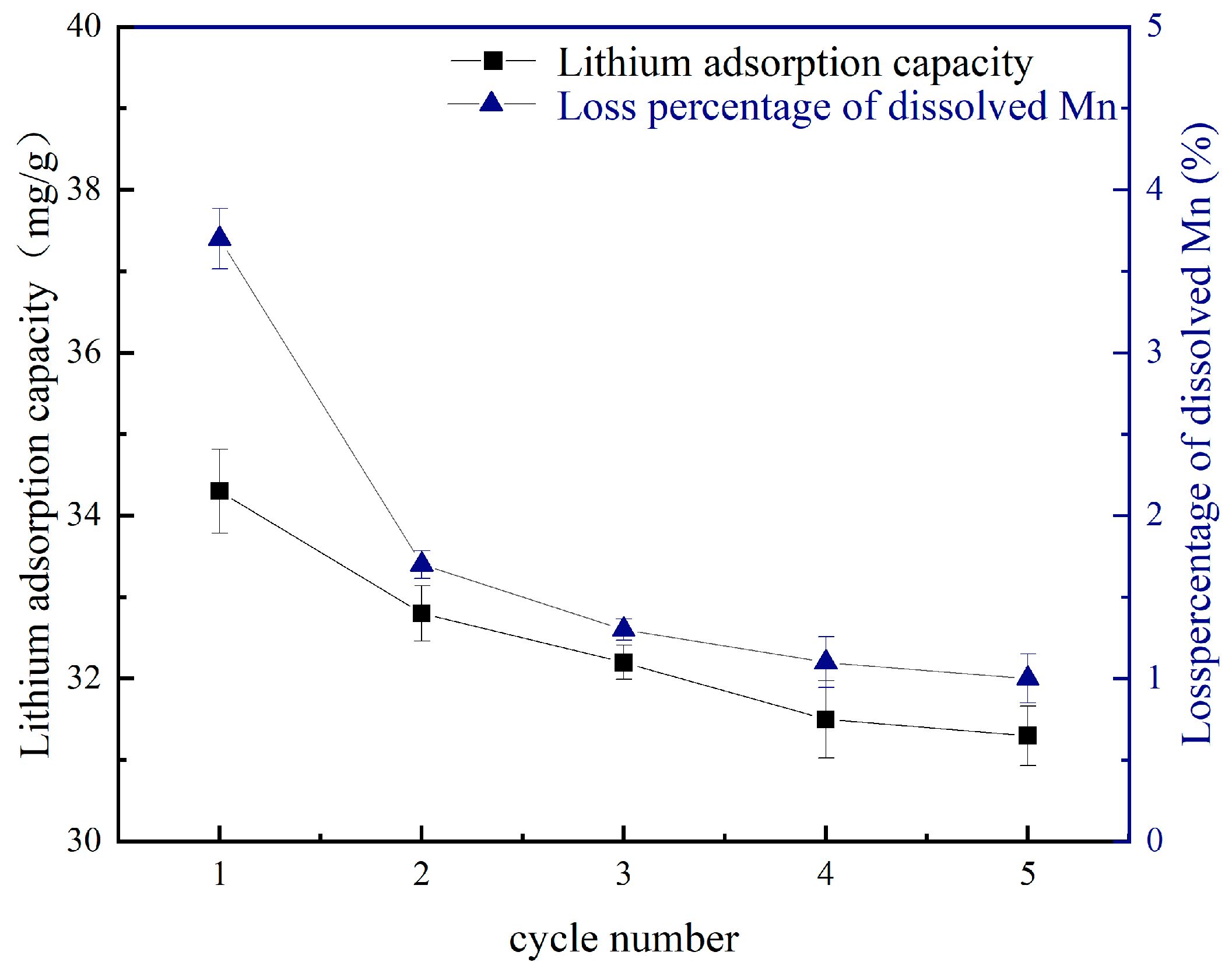
4.2. Cycling Performance
4.3. Adsorption Selectivity
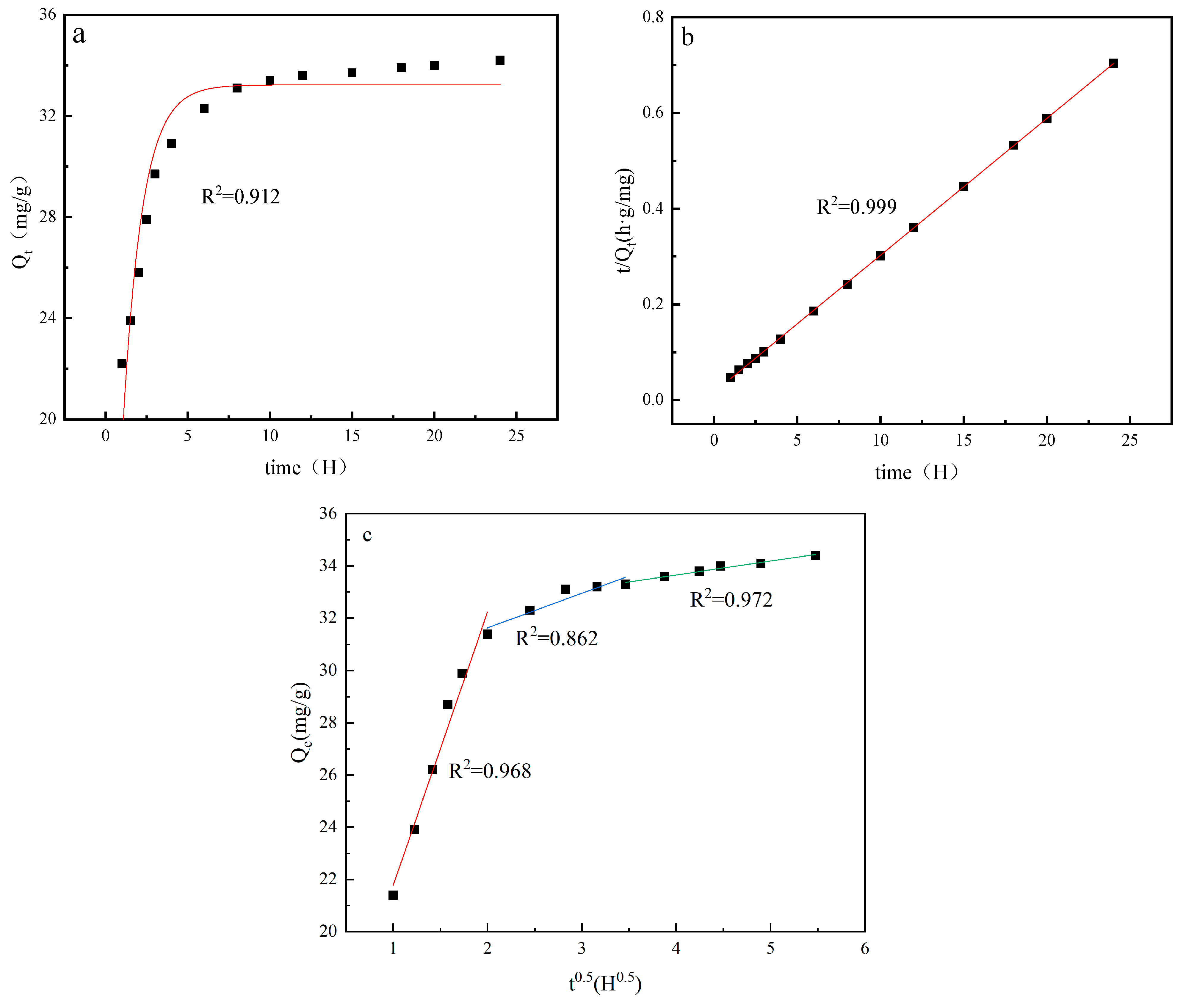
4.4. Adsorption Kinetics
4.5. Adsorption Isotherm
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Z.; Zhou, H.; Jia, S. Resource benefit evaluation of lithium recovery from new-energy vehicle batteries. Sustainability 2025, 17, 2922. [Google Scholar] [CrossRef]
- Gu, T.; Zhang, G.; Wang, Z.; Liu, L.; Zhang, L.; Wang, W.; Huang, Y.; Dan, Y.; Zhao, P.; He, Y.; et al. Review: The formation, characteristics, and resource utilization of lithium slag. Constr. Build. Mater. 2024, 432, 136648. [Google Scholar] [CrossRef]
- Liu, M.; Li, H.; Chi, H.; Chen, S.; Wang, H.; Wang, C.; Ma, X.; Zhu, Z.; Li, F. A review on lithium extraction by electrochemical electrode deionization technology. J. Solid. State Electrochem. 2025, 29, 1577–1592. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, Z.; Liu, B.; Ma, T. Sustainable lithium supply for electric vehicle development in China towards carbon neutrality. Energy 2025, 320, 1135243. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Gao, L.; Wu, Y.; Chen, Y. Towards high adsorption performance and cycling stability for spinel lithium ion-sieve H1.6Mn1.6O4 by coating Li2ZrO3 and doping Zr. Colloids Surf. A 2022, 638, 128238. [Google Scholar] [CrossRef]
- Zheng, L.; Chen, G.; Wen, B.; Bao, W. Analysis of lithium demand for electric vehicles from supply and demand perspectives under China’s carbon peak and neutrality goals. Waste Manag. 2025, 202, 114822. [Google Scholar] [CrossRef]
- Sun, X.; Ouyang, M.; Hao, H. Surging lithium price will not impede the electric vehicle boom. Joule 2022, 6, 1738–1742. [Google Scholar] [CrossRef]
- Vega-Muratalla, V.O.; Ramírez-Márquez, C.; Lira-Barragán, L.F.; Ponce-Ortega, J.M. Review of lithium as a strategic resource for electric vehicle battery production: Availability, extraction, and future prospects. Resources 2024, 13, 148. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, T.; Lv, S.; Song, S.; Galván, H.; Quintana, M.; Zhao, Y. Adsorbents for lithium extraction from salt lake brine with high magnesium/lithium ratio: From structure-performance relationship to industrial applications. Desalination 2024, 597, 117480. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, B.; Lü, Y.; Wang, C.; Chen, Y. A review of lithium extraction from natural resources. Int. J. Miner. Metall. Mater. 2023, 30, 209–224. [Google Scholar] [CrossRef]
- Yuan, H.; Li, M.; Cui, L.; Wang, L.; Cheng, F. Electrochemical extraction technologies of lithium: Development and challenges. Desalination 2025, 598, 118416. [Google Scholar] [CrossRef]
- He, M.-Y.; Luo, C.G.; Yang, H.J.; Kong, F.C.; Li, Y.L.; Deng, L.; Zhang, X.Y.; Yang, K.Y. Sources and a proposal for comprehensive exploitation of lithium brine deposits in the Qaidam Basin on the northern Tibetan Plateau, China: Evidence from Li isotopes. Ore Geol. Rev. 2020, 117, 103277. [Google Scholar] [CrossRef]
- Yu, H.; Naidu, G.; Zhang, C.; Wang, C.; Razmjou, A.; Han, D.; He, T.; Shon, H. Metal-based adsorbents for lithium recovery from aqueous resources. Desalination 2022, 539, 115951. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, S.; Srinivasakannan, C.; Li, S.; Yin, S.; Zhang, L.; Jiang, X.; Zhou, G.; Zhang, N. Lithium extraction from salt lake brines with high magnesium/lithium ratio: A review. Environ. Chem. Lett. 2023, 21, 1611–1626. [Google Scholar] [CrossRef]
- Yang, Y.; Bi, S.; Wang, F.; Pan, X.; Yang, T.; Hu, S.; Tang, S.; Jia, Q. Solid membrane-based aqueous lithium extraction and adsorption: Advances, challenges, and prospects. Chem. Eng. J. 2025, 510, 161749. [Google Scholar] [CrossRef]
- Sun, S.-Y.; Nie, X.Y.; Huang, J.; Yu, J.G. Molecular simulation of diffusion behavior of counterions within polyelectrolyte membranes used in electrodialysis. J. Membr. Sci. 2020, 595, 117528. [Google Scholar] [CrossRef]
- Hu, Y.; Su, H.; Zhu, Z.; Pang, Q. Environmentally benign techniques of lithium extraction from salt lakes: A review. Environ. Chem. Let. 2024, 22, 105–120. [Google Scholar] [CrossRef]
- Su, H.; Li, Z.; Zhang, J.; Liu, W.; Zhu, Z.; Wang, L.; Qi, T. Combining selective extraction and easy stripping of lithium using a ternary synergistic solvent extraction system through regulation of Fe3+ Coordination. ACS Sustain. Chem. Eng. 2020, 8, 1971–1979. [Google Scholar] [CrossRef]
- Zhang, J.; Hui, S.; Liu, W.; Wang, L.; Zhu, Z.; Qi, T. A new process to produce battery grade lithium carbonate from salt lake brines by purification, synergistic solvent extraction and carbon dioxide stripping. Hydrometallurgy 2023, 215, 105991. [Google Scholar] [CrossRef]
- Ye, S.; Yang, C.; Sun, Y.; Guo, C.; Wang, J.; Chen, Y.; Zhong, C.; Qiu, T. Application and mechanism of lithium-ion sieves in the recovery of lithium-containing wastewater: A review. Water Air Soil. Pollut. 2024, 235, 272. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, Y.; Sun, S.; Yu, J. Adsorption of lithium ions on lithium-aluminum hydroxides: Equilibrium and kinetics. Can. J. Chem. Eng. 2020, 98, 544–555. [Google Scholar] [CrossRef]
- Fu, Y.Y.; Yang, X.Y.; Huang, H.Q.; Wang, Y.Y.; Li, J.S. Role of process parameters on phase purity of o-LiMnO2 and synthesis of L1.6Mn1.6O4 as lithium ion-sieve. Chin. J. Inorg. Chem. 2020, 36, 536–546. [Google Scholar] [CrossRef]
- Wang, Q.; Yao, M.; Zhu, A.; Wang, Q.; Wu, H.; Zhang, Y. Semi-metallic superionic layers suppressing voltage fading of Li-rich layered oxide towards superior-stable Li-ion batteries. Angew. Chem. Int. Ed. 2023, 62, 202309049. [Google Scholar] [CrossRef]
- Qiu, Z.; Wang, M.; Chen, Y.; Zhang, T.; Yang, D.; Qiu, F. Li4Mn5O12 doped cellulose acetate membrane with low Mn loss and high stability for enhancing lithium extraction from seawater. Desalination 2021, 506, 115003. [Google Scholar] [CrossRef]
- Bao, L.; Tang, W.; Sun, S. Semi-wet synthesis of high-performance Li1.6Mn1.6O4 for selective lithium extraction from Salt Lakes Brine. Desalination 2024, 584, 117730. [Google Scholar] [CrossRef]
- Qian, F.; Zhao, B.; Guo, M.; Li, J.; Liu, Z.; Wu, Z. K-gradient doping to stabilize the spinel structure of Li1.6Mn1.6O4 for Li+ recovery. Dalton Trans. 2020, 49, 10939–10948. [Google Scholar] [CrossRef]
- Chitrakar, R.; Kanoh, H.; Miyai, Y.; Ooi, K. A new type of manganese oxide (MnO2·0.5H2O) derivedfrom Li1.6Mn1.6O4 and its lithium ion-sieve properties. Chem. Mater. 2000, 12, 3151–3157. [Google Scholar] [CrossRef]
- Hayashi, F.; Kurokawa, S.; Shiiba, H.; Wagata, H.; Wagata, H.; Yubbuta, K.; Oishi, S.; Nishikiori, H.; Teshima, K. Exceptional flux growth and chemical transformation of metastable orthorhombic LiMnO2 cuboids into hierarchically-structured porous H1.6Mn1.6O4 rods as Li ion sieves. Crys. Growth Des. 2016, 16, 6178–6185. [Google Scholar] [CrossRef]
- Ariza, M.; Jones, D.; Rozière, J.; Chitrakar, R.; Ooi, K. Probing the local structure and the role of protons in lithium sorption processes of a new lithium-rich manganese oxide. Chem. Mater. 2006, 18, 1885–1890. [Google Scholar] [CrossRef]
- Tang, W.; Kanoh, H.; Ooi, K. Lithium ion extraction from orthorhombic LiMnO2 in ammonium peroxodisulfate solutions. J. Solid. State Chem. 1999, 142, 19–28. [Google Scholar] [CrossRef]
- Feng, Q.; Kanoh, H.; Ooi, K. Manganese oxide porous crystals. J. Mater. Chem. 1999, 9, 319–333. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, L.; Liu, X.; Zhong, Z.; Deng, W.; Yang, P. The preparation path, adsorption characteristics and manganese dissolution loss mechanism of manganese-based ion-sieve MnO2·0.5H2O for liquid phase lithium resource recovery. J. Environ. Chem. Eng. 2025, 13, 115154. [Google Scholar] [CrossRef]
- Li, X.; Tao, B.; Jia, Q.; Guo, R. Preparation and adsorption performance of multi-morphology H1.6Mn1.6O4 for lithium extraction. Chin. J. Chem. Eng. 2021, 34, 68–76. [Google Scholar] [CrossRef]
- Xu, N.; Liu, J.; Han, L.; Feng, B.; Li, Y.; Yang, Y.; Bian, S. Preparation, modification and adsorption properties of spinel-type H1.6Mn1.6O4 lithium-ion sieves with spiny nanotube morphology. J. Mater. Sci. 2023, 58, 4707–4725. [Google Scholar] [CrossRef]
- Li, H.; Qin, J.; Zhao, K.; Guo, Y.; Tong, B.; Samadiy, M.; Alimov, U.; Deng, T. Novel lithium ion-sieve spinning fiber composite of PVDF-HMO for lithium recovery from geothermal water. J. Clean. Prod. 2024, 434, 139997. [Google Scholar] [CrossRef]
- Xu, J.; Chen, P. Preparation and characterization of lithium-ion sieve with attapulgite. Desalination 2024, 571, 117111. [Google Scholar] [CrossRef]
- Li, X. Preparation and Properties of Bifunctional Layered Lithium Manganate Pre-Lithiation Agent. Master’s Thesis, Central South University, Changsha, China, 1 June 2023. [Google Scholar]
- Sun, X.M.; Li, Y.D. Colloidal carbon spheres and their core/shell structures with noble-metal nanoparticles. Angew. Chem. Int. Ed. 2004, 43, 597–601. [Google Scholar] [CrossRef]
- Shao, Y.; Ren, B.; Jiang, H.; Zhou, B.; Lv, L.; Ren, J.; Dong, L.; Li, J.; Liu, Z. Dual-porosity Mn2O3 cubes for highly efficient dye adsorption. J. Hazard. Mater. 2017, 333, 222–231. [Google Scholar] [CrossRef]
- Karshyga, Z.; Yersaiynova, A.; Yessengaziyev, A.; Orynbayev, B.; Kvyatkovskaya, M.; Silachyov, I. Synthesis of manganese oxide sorbent for the extraction of lithium from hydromineral raw materials. Materials 2023, 16, 7548. [Google Scholar] [CrossRef]
- Liu, R.; Zhao, S.; Zhang, M.; Feng, F.; Shen, Q. High interfacial lithium storage capability of hollow porous Mn2O3 nanostructures obtained from carbonate precursors. Chem. Commun. 2015, 51, 5728–5731. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Zhang, H.; Zhang, W.; Jiang, J.; Guo, R. An ion-sieve-tailored biomimetic porous nanofiber as an efficient adsorbent for extraction of lithium from brine. New J. Chem. 2023, 47, 4187–4191. [Google Scholar] [CrossRef]
- Tabuchi, M.; Nabeshima, Y.; Takeuchi, T.; Kageyama, H.; Tatsumi, T.; Akimoto, J.; Shibuya, H.; Imaizumi, J. Synthesis and electrochemical characterization of Fe and Ni substituted Li2MnO3-an effective means to use Fe for constructing “Co-free” Li2MnO3 based positive electrode material. J. Power Sources 2011, 196, 3611–3622. [Google Scholar] [CrossRef]
- Yu, Q.; Morioka, E.; Sasaki, K. Characterization of lithium ion sieve derived from biogenic Mn oxide. Microporous Mesoporous Mat. 2013, 179, 122–127. [Google Scholar] [CrossRef]
- Chen, L.; Xu, X.; Song, J.; Zhu, X.; Qi, Z. Microwave assisted hydrothermal synthesis of MnO2·0.5H2O ion-sieve for lithium ion selective adsorption. Sep. Sci. Technol. 2016, 51, 874–882. [Google Scholar] [CrossRef]
- Yin, R.; Zhao, Z.; Xu, W.; He, L. LiMn2O4 submicronization: Shorten Li diffusion pathway for enhancing electrochemical lithium extraction and cycle performance. Sep. Purif. Technol. 2025, 359, 130394. [Google Scholar] [CrossRef]
- Li, N.; Gan, K.; Lu, D.; Zhang, J.; Wang, L. Preparation of three-dimensional macroporous-mesoporous lithium ion sieve with high Li+ adsorption capacity. Res. Chem. Intermed. 2018, 44, 1105–1117. [Google Scholar] [CrossRef]
- Wang, C.; Zhai, Y.; Wang, X.; Zeng, M. Preparation and characterization of lithium λ-MnO2 ion-sieves. Front. Chem. Sci. Eng. 2014, 8, 471–477. [Google Scholar] [CrossRef]
- Ozgur, C. Preparation and characterization of LiMn2O4 ion-sieve with high Li+ adsorption rate by ultrasonic spray pyrolysis. Solid. State Ionics 2010, 181, 1425–1428. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhou, Y.; Qi, G.; Zeng, J.; Sun, Y.; Shen, Y.; Li, X.; Ren, X.; Dong, S.; et al. Practical synthesis of manganese oxide MnO2·0.5H2O for an advanced and applicable lithium ion-sieve. J. Solid. State Chem. 2021, 293, 121768. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, T.; Ma, L.; Hai, C.; Sun, Y.; Dong, S.; He, X.; Xu, Q.; Chen, J.; Su, H.; et al. Factors affecting the efficiency of electrochemical lithium extraction: A systematic review from materials to processes technology. Desalination 2025, 602, 118570. [Google Scholar] [CrossRef]
- Liu, Q.; Dong, J.; Yu, Y. Preparation of layered lithium ion-sieve by solid-phase method and their lithium extraction performance. Desalin Water Treat. 2024, 319, 100547. [Google Scholar] [CrossRef]
- Song, L.; Liu, M.; Nian, M.; Yang, G. Preparation of HMn2O4 lithium-ion sieves with low manganese dissolution loss for improved cycling stability. RSC Adv. 2024, 14, 19795–19805. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Yang, X.; Huang, Y.; Yao, R. Synthesis of Porous Lithium Ion Sieve with High Purity for Li+ Adsorption. Materials 2025, 18, 2373. https://doi.org/10.3390/ma18102373
Zhu J, Yang X, Huang Y, Yao R. Synthesis of Porous Lithium Ion Sieve with High Purity for Li+ Adsorption. Materials. 2025; 18(10):2373. https://doi.org/10.3390/ma18102373
Chicago/Turabian StyleZhu, Jing, Xiyun Yang, Yongqiang Huang, and Rongzheng Yao. 2025. "Synthesis of Porous Lithium Ion Sieve with High Purity for Li+ Adsorption" Materials 18, no. 10: 2373. https://doi.org/10.3390/ma18102373
APA StyleZhu, J., Yang, X., Huang, Y., & Yao, R. (2025). Synthesis of Porous Lithium Ion Sieve with High Purity for Li+ Adsorption. Materials, 18(10), 2373. https://doi.org/10.3390/ma18102373






