Synergistic Removal of Diclofenac via Adsorption and Photocatalysis Using a Molecularly Imprinted Core–Shell Photocatalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microwave-Assisted Synthesis of Magnetic Core–Shell Fe3O4/SiO2/TiO2 Nanoparticles
2.3. Microwave-Assisted Polymerization of Diclofenac MIP (Fe3O4/SiO2/TiO2/MIP)
2.4. Materials Characterization
2.5. Adsorption, Photocatalysis and Photolysis Evaluation
2.6. Photocatalytic Mechanisms
3. Results and Discussion
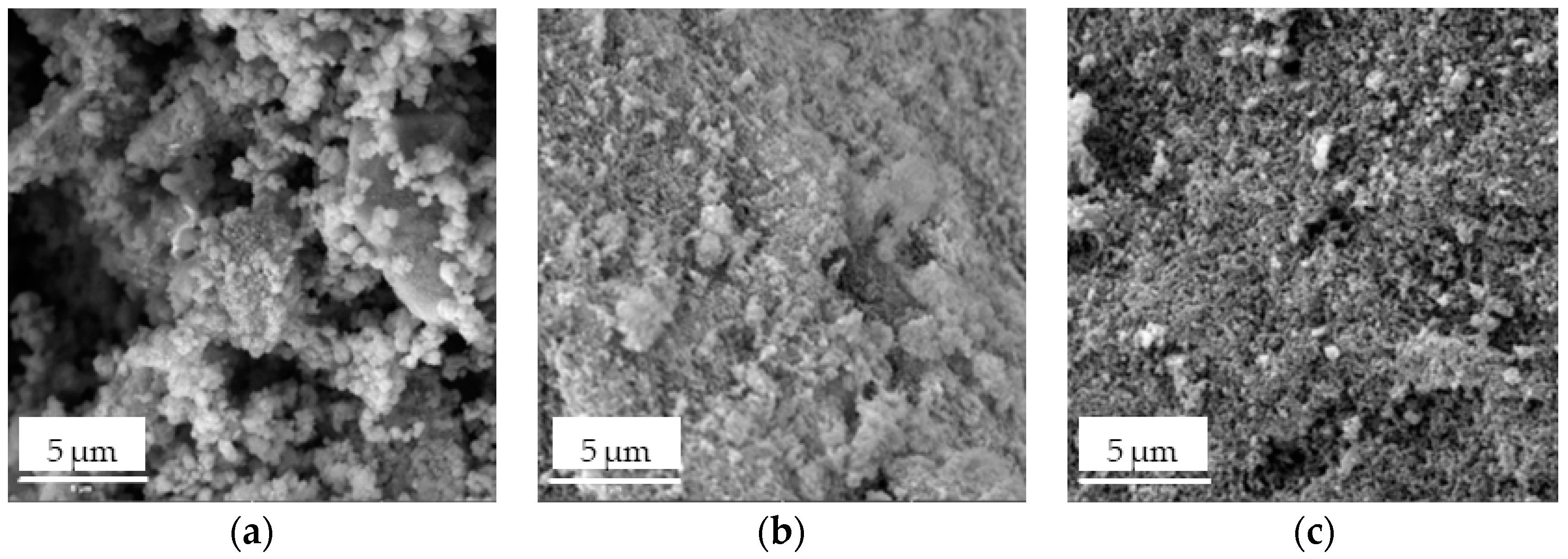
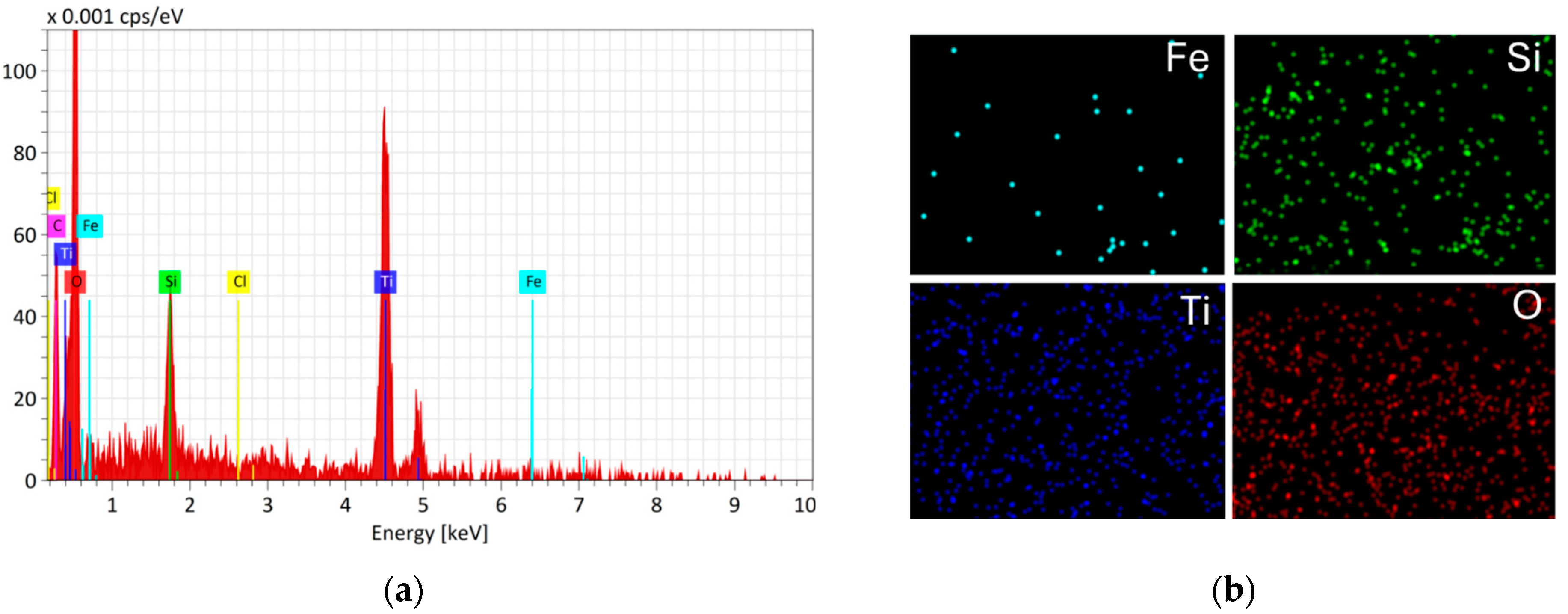
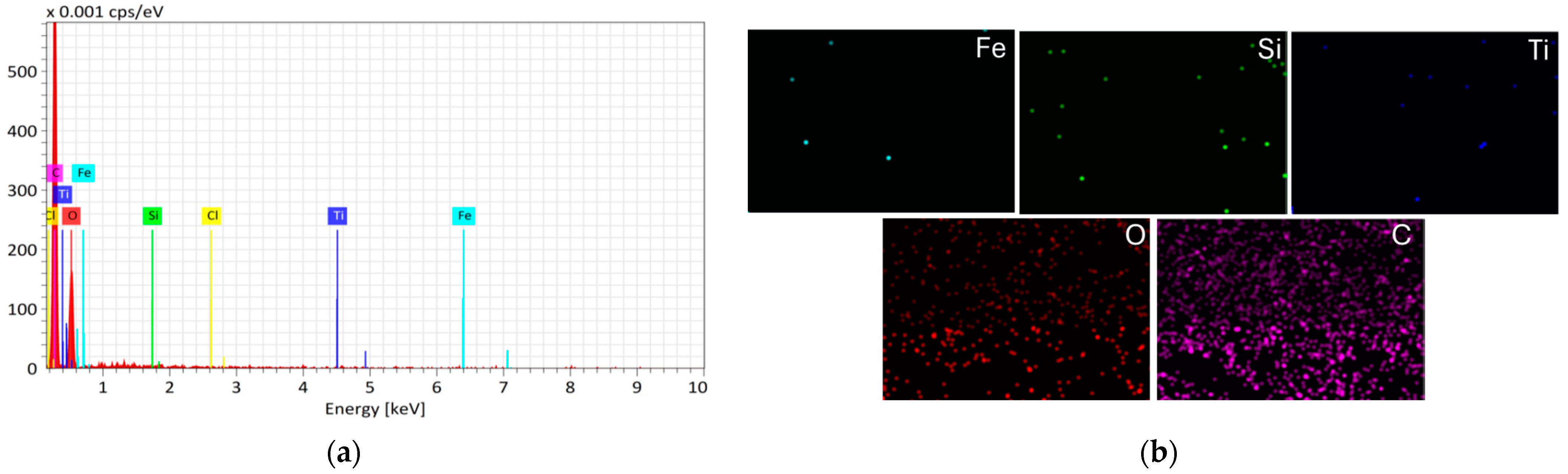
3.1. Characterisation of Core–Shell Fe3O4/SiO2/TiO2 and Fe3O4/SiO2/TiO2/MIP Nanoparticles
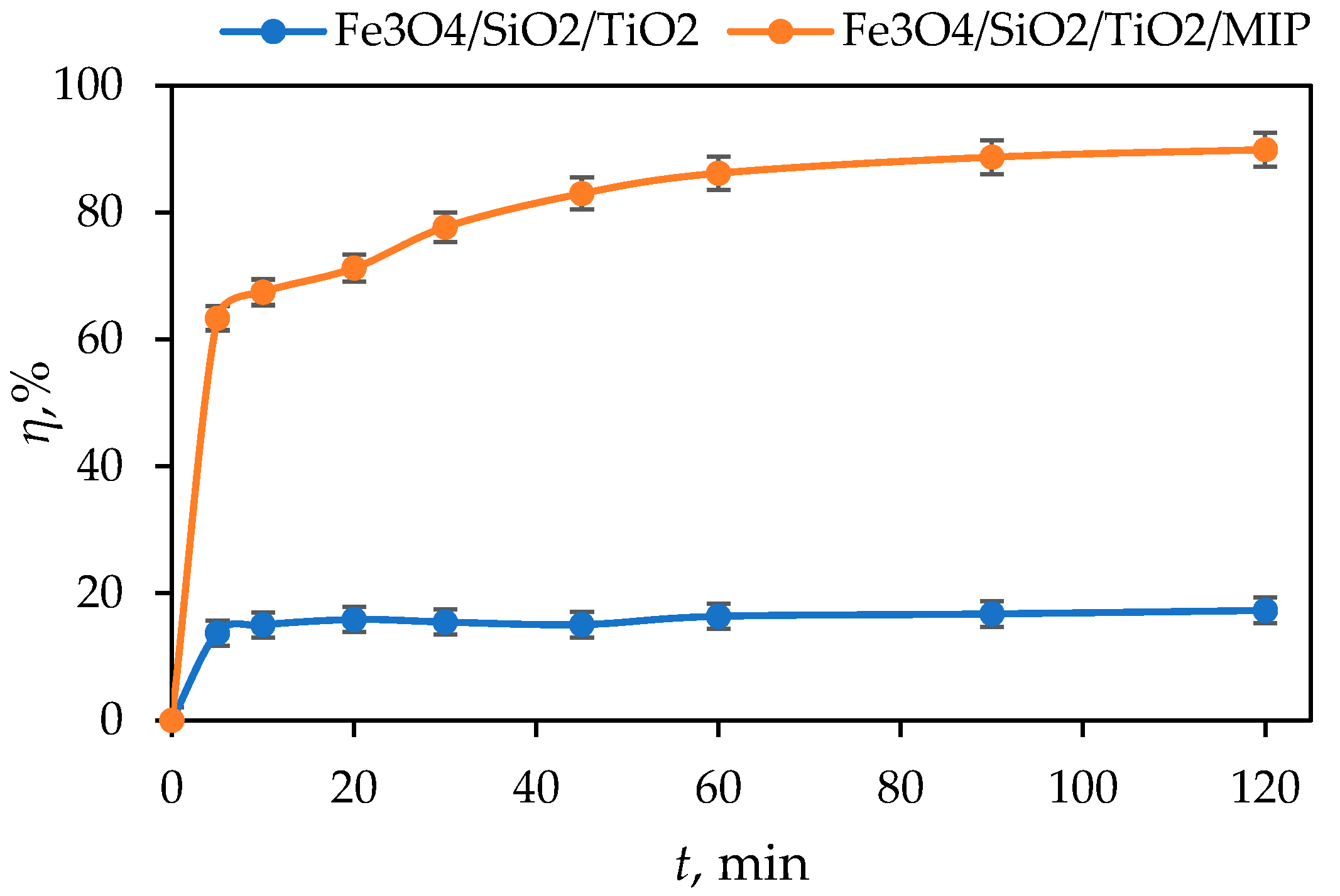
3.2. Adsorption Kinetic Studies
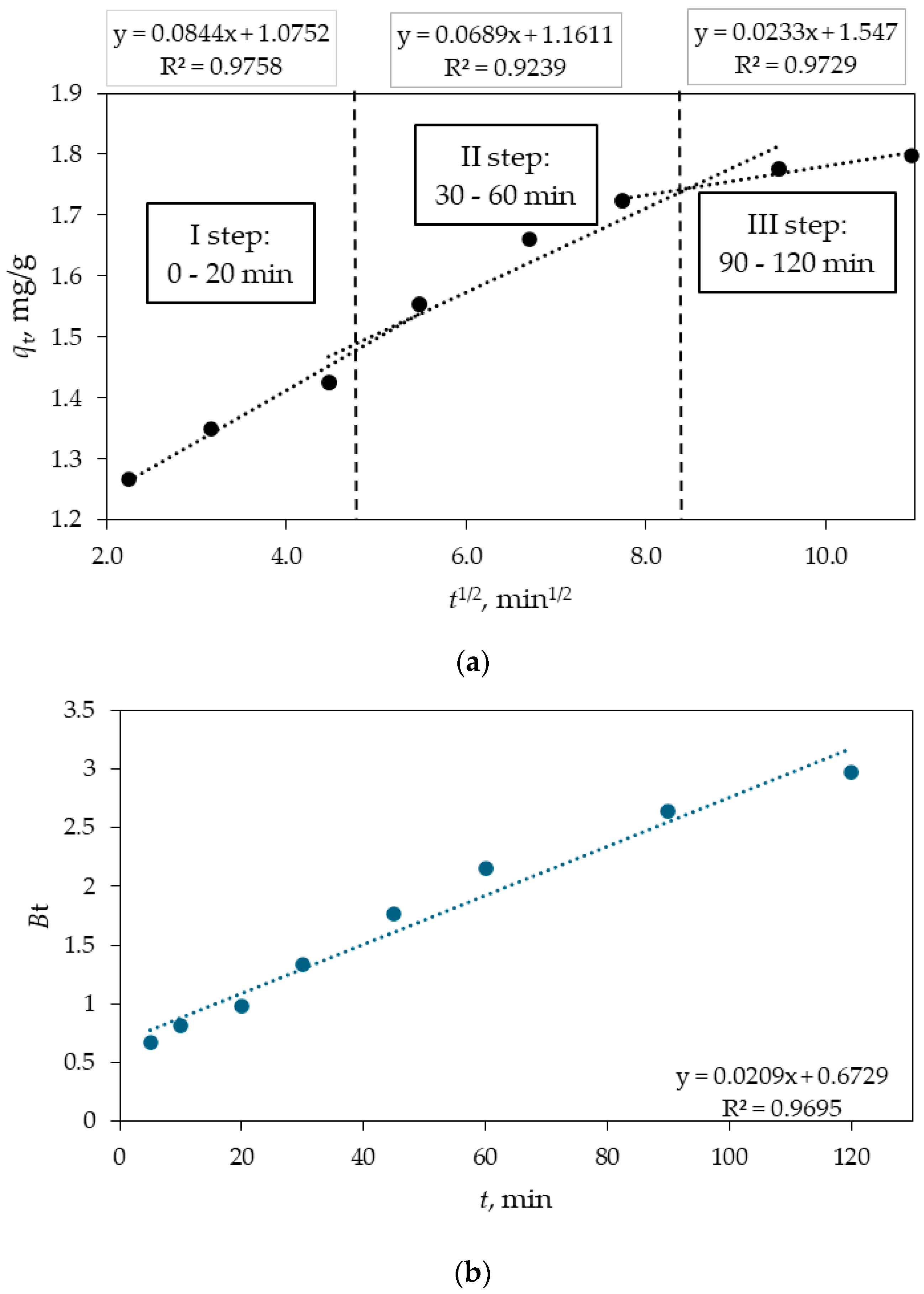
3.3. Intraparticle Diffusion Model
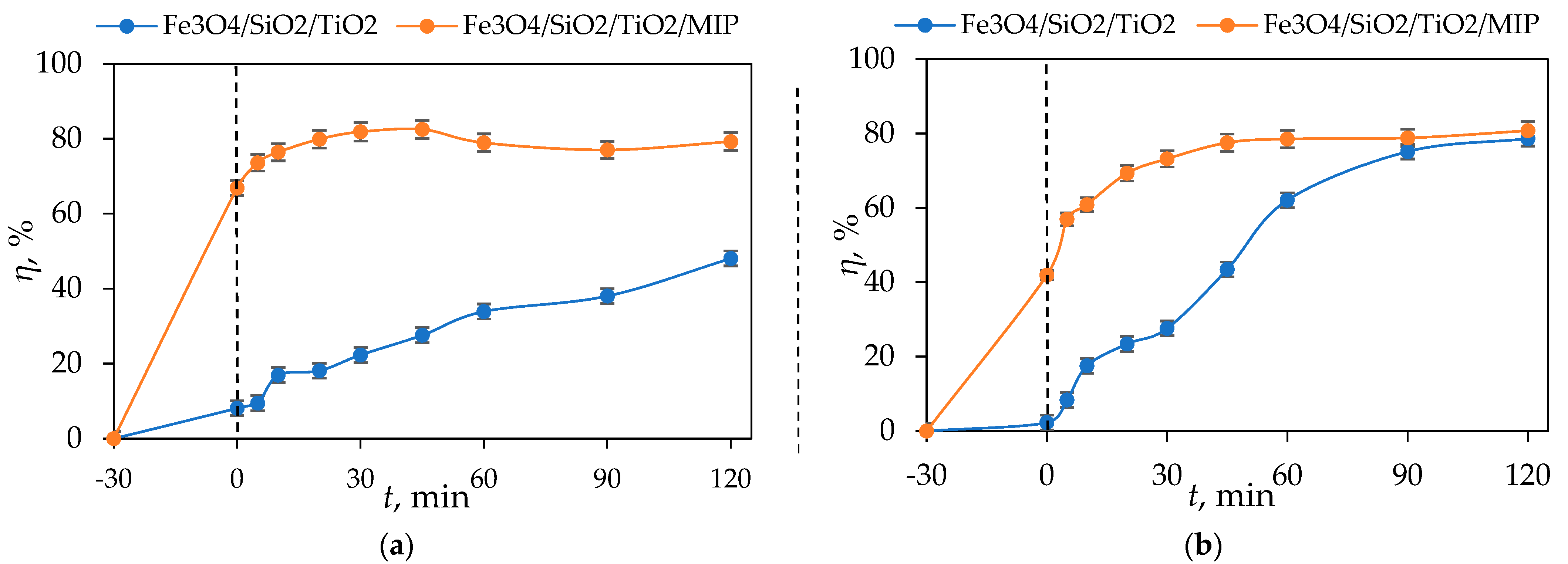
3.4. Photocatalytic Studies
3.5. Scavenger Experiments
4. Conclusions
- The obtained particles were analysed via FTIR and XRD, which confirmed the successful synthesis of Fe3O4/SiO2/TiO2/MIP nanoparticles.
- Vibrating-sample magnetometry showed the superparamagnetic behaviour of the prepared samples.
- SEM images showed the increased surface roughness of the Fe3O4/SiO2/TiO2/MIP nanoparticles due to the synthesis of the imprinted polymer layer, while the washed Fe3O4/SiO2/TiO2/MIP nanoparticles exhibited higher porosity due to the removal of the template molecule. Elemental mapping confirmed the core–shell structure and successful molecular imprinting.
- The bandgap amounts to 1.7 eV, which can be identified as the optical bandgap of Fe3O4 and ~3 eV, which corresponds to titanium dioxides’ excitation.
- The Fe3O4/SiO2/TiO2 nanoparticles have the highest specific surface area (116.6 m2 g−1) but the lowest average pore diameter (8.5 nm), whereas adsorbed Fe3O4/SiO2/TiO2/MIP has the lowest specific surface area (49.7 m2 g−1) but highest average pore diameter (12.3 nm).
- The adsorption of DIC by Fe3O4/SiO2/TiO2 was only 16% after 120 min while the photocatalytic degradation by Fe3O4/SiO2/TiO2 nanoparticles under UV-A- and solar-simulated irradiation was around 40% and 80% after 120 min, respectively.
- The synergistic removal of DIC by Fe3O4/SiO2/TiO2/MIP achieved an adsorption of around 86% after 60 min, and a photocatalytic degradation of around 80% after 120 min of both UV-A and solar-simulated irradiation.
- The adsorption kinetic results indicate that the adsorption of DIC on Fe3O4/SiO2/TiO2/MIP nanoparticles could be controlled by the second-order model. The intraparticle diffusion model alongside the Boyd diagram suggest the rate controlling steps are both intraparticle diffusion and film diffusion.
- Fe3O4/SiO2/TiO2/MIP demonstrates a degradation mechanism predominantly driven by singlet oxygen under SSL and a combination of singlet oxygen and hydroxyl radicals under UV light, while the Fe3O4/SiO2/TiO2 nanoparticles rely on superoxide radicals under UV light and a combination of superoxide radicals and photogenerated holes under SSL.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kyzas, G.Z.; Fu, J.; Lazaridis, N.K.; Bikiaris, D.N.; Matis, K.A. New Approaches on the Removal of Pharmaceuticals from Wastewaters with Adsorbent Materials. J. Mol. Liq. 2015, 209, 87–93. [Google Scholar] [CrossRef]
- Leyva-Díaz, J.C.; Batlles-Delafuente, A.; Molina-Moreno, V.; Molina, J.S.; Belmonte-Ureña, L.J. Removal of Pharmaceuticals from Wastewater: Analysis of the Past and Present Global Research Activities. Water 2021, 13, 2353. [Google Scholar] [CrossRef]
- Khasawneh, O.F.S.; Palaniandy, P. Occurrence and Removal of Pharmaceuticals in Wastewater Treatment Plants. Process. Saf. Environ. Prot. 2021, 150, 532–556. [Google Scholar] [CrossRef]
- Mousel, D.; Bastian, D.; Firk, J.; Palmowski, L.; Pinnekamp, J. Removal of Pharmaceuticals from Wastewater of Health Care Facilities. Sci. Total Environ. 2021, 751, 141310. [Google Scholar] [CrossRef]
- Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Removal of Pharmaceuticals during Wastewater Treatment and Environmental Risk Assessment Using Hazard Indexes. Environ. Int. 2010, 36, 15–26. [Google Scholar] [CrossRef]
- de Escobar, C.C.; Moreno Ruiz, Y.P.; dos Santos, J.H.Z.; Ye, L. Molecularly Imprinted TiO2 Photocatalysts for Degradation of Diclofenac in Water. Colloids Surf. A Physicochem. Eng. Asp. 2018, 538, 729–738. [Google Scholar] [CrossRef]
- Martínez, C.; Canle, L.M.; Fernández, M.I.; Santaballa, J.A.; Faria, J. Aqueous Degradation of Diclofenac by Heterogeneous Photocatalysis Using Nanostructured Materials. Appl. Catal. B 2011, 107, 110–118. [Google Scholar] [CrossRef]
- Sathishkumar, P.; Mohan, K.; Meena, R.A.A.; Balasubramanian, M.; Chitra, L.; Ganesan, A.R.; Palvannan, T.; Brar, S.K.; Gu, F.L. Hazardous Impact of Diclofenac on Mammalian System: Mitigation Strategy through Green Remediation Approach. J. Hazard. Mater. 2021, 419, 126135. [Google Scholar] [CrossRef]
- Angosto, J.M.; Roca, M.J.; Fernández-López, J.A. Removal of Diclofenac in Wastewater Using Biosorption and Advanced Oxidation Techniques: Comparative Results. Water 2020, 12, 3567. [Google Scholar] [CrossRef]
- Yu, H.; Nie, E.; Xu, J.; Yan, S.; Cooper, W.J.; Song, W. Degradation of Diclofenac by Advanced Oxidation and Reduction Processes: Kinetic Studies, Degradation Pathways and Toxicity Assessments. Water Res. 2013, 47, 1909–1918. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Titanium Dioxide Photocatalysis for Pharmaceutical Wastewater Treatment. Environ. Chem. Lett. 2014, 12, 27–47. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. TiO2 Photocatalyst for Water Treatment Applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Sharma, M.; Yadav, A.; Mandal, M.K.; Dubey, K.K. TiO2 Based Photocatalysis: A Valuable Approach for the Removal of Pharmaceuticals from Aquatic EnvironmentInt. J. Environ. Sci. Technol. 2023, 20, 4569–4584. [Google Scholar] [CrossRef]
- Bi, L.; Chen, Z.; Li, L.; Kang, J.; Zhao, S.; Wang, B.; Yan, P.; Li, Y.; Zhang, X.; Shen, J. Selective Adsorption and Enhanced Photodegradation of Diclofenac in Water by Molecularly Imprinted TiO2. J. Hazard. Mater. 2021, 407, 124759. [Google Scholar] [CrossRef] [PubMed]
- Sajini, T.; Mathew, B. A Brief Overview of Molecularly Imprinted Polymers: Highlighting Computational Design, Nano and Photo-Responsive Imprinting. Talanta Open 2021, 4, 100072. [Google Scholar] [CrossRef]
- Hasanah, A.N.; Susanti, I. Molecularly Imprinted Polymers for Pharmaceutical Impurities: Design and Synthesis Methods. Polymers 2023, 15, 3401. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Sun, L.; Guan, J.; Xu, Q.; Yang, X.; Wang, J.; Hu, X. Synthesis and Applications of Molecularly Imprinted Polymers Modified TiO2 Nanomaterials: A Review. Polymers 2018, 10, 1248. [Google Scholar] [CrossRef]
- Ariani, M.D.; Zuhrotun, A.; Manesiotis, P.; Hasanah, A.N. Magnetic Molecularly Imprinted Polymers: An Update on Their Use in the Separation of Active Compounds from Natural Products. Polymers 2022, 14, 1389. [Google Scholar] [CrossRef]
- Niu, M.; Pham-Huy, C.; He, H. Core-Shell Nanoparticles Coated with Molecularly Imprinted Polymers: A Review. Mikrochim. Acta 2016, 183, 2677–2695. [Google Scholar] [CrossRef]
- Zeb, S.; Wong, A.; Khan, S.; Hussain, S.; Sotomayor, M.D.P.T. Using Magnetic Nanoparticles/MIP-Based Electrochemical Sensor for Quantification of Tetracycline in Milk Samples. J. Electroanal. Chem. 2021, 900, 115713. [Google Scholar] [CrossRef]
- Turan, E.; Şahin, F. Molecularly Imprinted Biocompatible Magnetic Nanoparticles for Specific Recognition of Ochratoxin A. Sens. Actuators B Chem. 2016, 227, 668–676. [Google Scholar] [CrossRef]
- Santos, A.C.F.; de Araújo, O.R.P.; Moura, F.A.; Khan, S.; Tanaka, A.A.; Santana, A.E.G.; Pividori, M.I.; de Pilar Taboada-Sotomayor, M.; Goulart, M.O.F. Development of Magnetic Nanoparticles Modified with New Molecularly Imprinted Polymer (MIPs) for Selective Analysis of Glutathione. Sens. Actuators B Chem. 2021, 344, 130171. [Google Scholar] [CrossRef]
- Li, Y.; Ding, M.J.; Wang, S.; Wang, R.Y.; Wu, X.L.; Wen, T.T.; Yuan, L.H.; Dai, P.; Lin, Y.H.; Zhou, X.M. Preparation of Imprinted Polymers at Surface of Magnetic Nanoparticles for the Selective Extraction of Tadalafil from Medicines. ACS Appl. Mater. Interfaces 2011, 3, 3308–3315. [Google Scholar] [CrossRef]
- Lastovina, T.A.; Budnyk, A.P.; Kubrin, S.P.; Soldatov, A.V. Microwave-Assisted Synthesis of Ultra-Small Iron Oxide Nanoparticles for Biomedicine. Mendeleev Commun. 2018, 28, 167–169. [Google Scholar] [CrossRef]
- Li, C.; Wei, Y.; Liivat, A.; Zhu, Y.; Zhu, J. Microwave-Solvothermal Synthesis of Fe3O4 Magnetic Nanoparticles. Mater. Lett. 2013, 107, 23–26. [Google Scholar] [CrossRef]
- Lastovina, T.A.; Budnyk, A.P.; Soldatov, M.A.; Rusalev, Y.V.; Guda, A.A.; Bogdan, A.S.; Soldatov, A.V. Microwave-Assisted Synthesis of Magnetic Iron Oxide Nanoparticles in Oleylamine–Oleic Acid Solutions. Mendeleev Commun. 2017, 27, 487–489. [Google Scholar] [CrossRef]
- Kostyukhin, E.M.; Kustov, L.M. Microwave-Assisted Synthesis of Magnetite Nanoparticles Possessing Superior Magnetic Properties. Mendeleev Commun. 2018, 28, 559–561. [Google Scholar] [CrossRef]
- Gabelica, I.; Ćurković, L.; Mandić, V.; Panžić, I.; Ljubas, D.; Zadro, K. Rapid Microwave-Assisted Synthesis of Fe3O4/SiO2/TiO2 Core-2-Layer-Shell Nanocomposite for Photocatalytic Degradation of Ciprofloxacin. Catalysts 2021, 11, 1136. [Google Scholar] [CrossRef]
- Guedes, A.E.D.S.; Silva, A.R.V.; e Mello, V.S.; da Silva Pontes, F.; Alves, S.M.; Carvajal, T.L.R. Synthesis of Magnetic Nanoparticles by Microwave for Aplication in Lubricantion. J. Eng. Technol. Ind. Appl. 2016, 2, 41–46. [Google Scholar] [CrossRef]
- Pati, S.S.; Kalyani, S.; Mahendran, V.; Philip, J. Microwave Assisted Synthesis of Magnetite Nanoparticles. J. Nanosci. Nanotechnol. 2014, 14, 5790–5797. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.H.; Rather, M.A. Effect of Calcination Temperature on the Crystallite Size, Particle Size and Zeta Potential of TiO2 Nanoparticles Synthesized via Polyol-Mediated Method. Mater. Today Proc. 2020, 44, 482–488. [Google Scholar] [CrossRef]
- Bo, Z.; Dong, R.; Jin, C.; Chen, Z. High Photocatalytically Active Cocoons-like TiO2/SiO2 Synthesized by Hydrothermal Process and Subsequent Calcination at 900 °C. Mater. Sci. Semicond. Process. 2017, 72, 9–14. [Google Scholar] [CrossRef]
- Kebebe, D.; Belete, A.; Gebre-Mariam, T. Evaluation of Two Olibanum Resins as Rate Controlling Matrix Forming Excipients in Oral Sustained Release Tablets. Ethiop. Pharm. J. 2012, 28, 95–109. [Google Scholar] [CrossRef]
- Pal, T.; Paul, S.; Sa, B. Polymethylmethacrylate Coated Alginate Matrix Microcapsules for Controlled Release of Diclofenac Sodium. J. Pharm. Pharmacol. 2011, 2, 56–66. [Google Scholar] [CrossRef]
- Lu, W.; Shen, Y.; Xie, A.; Zhang, W. Green Synthesis and Characterization of Superparamagnetic Fe3O4 Nanoparticles. J. Magn. Magn. Mater. 2010, 322, 1828–1833. [Google Scholar] [CrossRef]
- Pajić, D.; Zadro, K.; Vanderberghe, R.E.; Nedkov, I. Superparamagnetic Relaxation in CuxFe3−xO4 (x = 0.5 and x = 1) Nanoparticles. J. Magn. Magn. Mater. 2004, 281, 353–363. [Google Scholar] [CrossRef][Green Version]
- Mazhari, M.P.; Khojasteh, H.; Sharifi, N.; Aspoukeh, P.; Mousavi, S.M. Development and Application of Multifunctional Fe3O4/SiO2/TiO2/Cu Nanocomposites for Sustainable Water Treatment. J. Solgel Sci. Technol. 2024, 110, 156–168. [Google Scholar] [CrossRef]
- Saragi, T.; Depi, B.L.; Butarbutar, S.; Permana, B. Risdiana The Impact of Synthesis Temperature on Magnetite Nanoparticles Size Synthesized by Co-Precipitation Method. J. Phys. Conf. Ser. 2018, 1013, 012190. [Google Scholar] [CrossRef]
- Ghorbanpour, M.; Lotfiman, S. Solid-State Immobilisation of Titanium Dioxide Nanoparticles onto Nanoclay. Micro. Nano. Lett. 2016, 11, 684–687. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.; Olivier, J.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Lowell, S.; Shields, J.; Thomas, M.; Thommes, M. Characterization of Porous Solids and Powders: Surface Area, Pore Size and Density; Springer Science + Business Media, LCC: Berlin, Germany, 2006; Volume 1, ISBN 9789048166336. [Google Scholar]
- Thommes, M.; Cychosz, K. Physical Adsorption Characterization of Nanoporous Materials: Progress and Challenges. Adsorption 2014, 20, 233–250. [Google Scholar] [CrossRef]
- John, L.; Yu, G.G.; Alexander, N. Density Functional Theory Methods for Characterization of Porous Materials. Colloids Surf. A Physicochem. Eng. Asp. 2013, 437, 3–32. [Google Scholar]
- Zhang, H.; Xiao, Y.; Peng, Y.; Tian, L.; Wang, Y.; Tang, Y.; Cao, Y.; Wei, Z.; Wu, Z.; Zhu, Y.; et al. Selective Degradation of Ceftriaxone Sodium by Surface Molecularly Imprinted BiOCl/Bi3NbO7 Heterojunction Photocatalyst. Sep. Purif. Technol. 2023, 315, 123716. [Google Scholar] [CrossRef]
- Gryshchenko, A.O.; Bottaro, C.S. Development of Molecularly Imprinted Polymer in Porous Film Format for Binding of Phenol and Alkylphenols from Water. Int. J. Mol. Sci. 2014, 15, 1338–1357. [Google Scholar] [CrossRef]
- Bakhtiar, S.; Bhawani, S.A.; Shafqat, S.R. Synthesis and Characterization of Molecular Imprinting Polymer for the Removal of 2-Phenylphenol from Spiked Blood Serum and River Water. Chem. Biol. Technol. 2019, 6, 15. [Google Scholar] [CrossRef]
- Zheng, T.; Wang, Q.; Shi, Z.; Zhang, Z.; Ma, Y. Microwave Regeneration of Spent Activated Carbon for the Treatment of Ester-Containing Wastewater. RSC Adv. 2016, 6, 60815–60825. [Google Scholar] [CrossRef]
- Simonin, J.P. On the Comparison of Pseudo-First Order and Pseudo-Second Order Rate Laws in the Modeling of Adsorption Kinetics. J. Chem. Eng. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Chu, J.; Dong, C.; Qi, J.; Yuan, Y. Synthesis of Core-Shell Magnetic Molecular Imprinted Polymer by the Surface RAFT Polymerization for the Fast and Selective Removal of Endocrine Disrupting Chemicals from Aqueous Solutions. Environ. Pollut. 2010, 158, 2317–2323. [Google Scholar] [CrossRef] [PubMed]
- Zbair, M.; Ainassaari, K.; Drif, A.; Ojala, S.; Bottlinger, M.; Pirilä, M.; Keiski, R.L.; Bensitel, M.; Brahmi, R. Toward New Benchmark Adsorbents: Preparation and Characterization of Activated Carbon from Argan Nut Shell for Bisphenol A Removal. Environ. Sci. Pollut. Res. 2018, 25, 1869–1882. [Google Scholar] [CrossRef] [PubMed]
- Prasetya, N.; Wöll, C. Removal of Diclofenac by Adsorption Process Studied in Free-Base Porphyrins Zr-Metal Organic Frameworks (Zr-MOFs). RSC Adv. 2023, 13, 22998–23009. [Google Scholar] [CrossRef]
- Al-Khateeb, L.A.; Hakami, W.; Salam, M.A. Removal of Non-Steroidal Anti-Inflammatory Drugs from Water Using High Surface Area Nanographene: Kinetic and Thermodynamic Studies. J. Mol. Liq. 2017, 241, 733–741. [Google Scholar] [CrossRef]
- Thilagan, J.; Gopalakrishnan, S.; Kannadasan, T. Chitosan Immobilised on Red Soil: Isotherms, Kinetics and Mechanism. Int. J. Pharm. Chem. S. 2013, 2, 1055–1066. [Google Scholar]
- Zhang, Y.; Yin, Z.; Dai, C.; Zhou, X.; Chen, W. Interfacial Thermodynamics and Kinetics of Sorption of Diclofenac on Prepared High Performance Flower-like MoS2. J. Colloid. Interface Sci. 2016, 481, 210–219. [Google Scholar] [CrossRef]
- Maia, G.S.; de Andrade, J.R.; da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Diclofenac Sodium onto Commercial Organoclay: Kinetic, Equilibrium and Thermodynamic Study. Powder Technol. 2019, 345, 140–150. [Google Scholar] [CrossRef]
- Kalam, S.; Abu-Khamsin, S.A.; Kamal, M.S.; Patil, S. Surfactant Adsorption Isotherms: A Review. ACS Omega 2021, 6, 32342–32348. [Google Scholar] [CrossRef]
- Tonk, S.; Rápó, E. Linear and Nonlinear Regression Analysis for the Adsorption of Remazol Dye by Romanian Brewery Waste By-Product, Saccharomyces Cerevisiae. Int. J. Mol. Sci. 2022, 23, 11827. [Google Scholar] [CrossRef]
- Hussain, D.; Khan, S.A.; Khan, T.A.; Alharthi, S.S. Efficient Liquid Phase Confiscation of Nile Blue Using a Novel Hybrid Nanocomposite Synthesized from Guar Gum-Polyacrylamide and Erbium Oxide. Sci. Rep. 2022, 12, 14656. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, J.; Gai, Q.-Y.; Fu, Y.-J.; Wang, Y. Selective Adsorption and Photodegradation of Potentially Hazardous Salicylic Acid by a Novel Magnetic Surface Molecularly Imprinted Photocatalyst (SMIP-Fe0@b-TiO2/CQDs). J. Environ. Chem. Eng. 2025, 13, 115065. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Jiao, J.; Gai, Q.Y.; Fu, Y.J.; Cao, R.Z. Selective Adsorption and Photodegradation of Salicylic Acid by a Novel Magnetic Molecularly Imprinted Mesoporous TiO2 Co-Doped with Silver Nanoparticles and Carbon Nanotubes (Fe3O4@mTiO2-Ag-CNTs-MIPs) under Visible Light. J. Photochem. Photobiol. A Chem. 2025, 459, 116071. [Google Scholar] [CrossRef]
- Anirudhan, T.S.; Christa, J.; Shainy, F. Magnetic Titanium Dioxide Embedded Molecularly Imprinted Polymer Nanocomposite for the Degradation of Diuron under Visible Light. React. Funct. Polym. 2020, 152, 104597. [Google Scholar] [CrossRef]
- Tra, V.T.; Pham, V.T.; Tran, T.D.; Tran, T.H.; Tran, T.K.; Nguyen, T.P.T.; Nguyen, V.T.; Dao, T.V.H.; Tran, P.Y.N.; Le, V.G.; et al. Enhance Diclofenac Removal in Wastewater by Photocatalyst Process Combination with Hydrogen Peroxide. Case Stud. Chem. Environ. Eng. 2023, 8, 100506. [Google Scholar] [CrossRef]
- Salaeh, S.; Juretic Perisic, D.; Biosic, M.; Kusic, H.; Babic, S.; Lavrencic Stangar, U.; Dionysiou, D.D.; Loncaric Bozic, A. Diclofenac Removal by Simulated Solar Assisted Photocatalysis Using TiO2-Based Zeolite Catalyst; Mechanisms, Pathways and Environmental Aspects. J. Chem. Eng. 2016, 304, 289–302. [Google Scholar] [CrossRef]
- Apopei, P.; Orha, C.; Popescu, M.I.; Lazau, C.; Manea, F.; Catrinescu, C.; Teodosiu, C. Diclofenac Removal from Water by Photocatalysis- Assisted Filtration Using Activated Carbon Modified with N-Doped TiO2. Process. Saf. Environ. Prot. 2020, 138, 324–336. [Google Scholar] [CrossRef]
- Bagal, M.V.; Gogate, P.R. Degradation of Diclofenac Sodium Using Combined Processes Based on Hydrodynamic Cavitation and Heterogeneous Photocatalysis. Ultrason. Sonochem. 2014, 21, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Jauris, I.M.; Matos, C.F.; Saucier, C.; Lima, E.C.; Zarbin, A.J.G.; Fagan, S.B.; Machado, F.M.; Zanella, I. Adsorption of Sodium Diclofenac on Graphene: A Combined Experimental and Theoretical Study. Phys. Chem. Chem. Phys. 2016, 18, 1526–1536. [Google Scholar] [CrossRef]
- Salvestrini, S.; Fenti, A.; Chianese, S.; Iovino, P.; Musmarra, D. Diclofenac Sorption from Synthetic Water: Kinetic and Thermodynamic Analysis. J. Environ. Chem. Eng. 2020, 8, 104105. [Google Scholar] [CrossRef]
- Martinez-Costa, J.I.; Leyva-Ramos, R.; Padilla-Ortega, E. Sorption of Diclofenac from Aqueous Solution on an Organobentonite and Adsorption of Cadmium on Organobentonite Saturated with Diclofenac. Clays Clay. Miner. 2018, 66, 515–528. [Google Scholar] [CrossRef]
- Suriyanon, N.; Punyapalakul, P.; Ngamcharussrivichai, C. Mechanistic Study of Diclofenac and Carbamazepine Adsorption on Functionalized Silica-Based Porous Materials. J. Chem. Eng. 2013, 214, 208–218. [Google Scholar] [CrossRef]
- Varga, M.; ELAbadsa, M.; Tatár, E.; Mihucz, V.G. Removal of Selected Pharmaceuticals from Aqueous Matrices with Activated Carbon under Batch Conditions. Microchem. J. 2019, 148, 661–672. [Google Scholar] [CrossRef]
- Cuccarese, M.; Brutti, S.; De Bonis, A.; Teghil, R.; Mancini, I.M.; Masi, S.; Caniani, D. Removal of Diclofenac from Aqueous Solutions by Adsorption on Thermo-Plasma Expanded Graphite. Sci. Rep. 2021, 11, 3427. [Google Scholar] [CrossRef] [PubMed]
- Kocabıyık, B.; Üner, O.; Geçgel, Ü. Diclofenac Sodium Adsorption in Aqueous Media by Activated Carbon Obtained from Einkorn (Triticum monococcum L.) Husk. Adsorption 2024, 30, 1033–1046. [Google Scholar] [CrossRef]
- Orimolade, B.; Arotiba, O. Enhanced Photoelectrocatalytic Degradation of Diclofenac Sodium Using a System of Ag-BiVO4/BiOI Anode and Ag-BiOI Cathode. Sci. Rep. 2022, 12, 4214. [Google Scholar] [CrossRef] [PubMed]
- Pelaez, M.; Falaras, P.; Likodimos, V.; O’Shea, K.; de la Cruz, A.A.; Dunlop, P.S.M.; Byrne, J.A.; Dionysiou, D.D. Use of Selected Scavengers for the Determination of NF-TiO2 Reactive Oxygen Species during the Degradation of Microcystin-LR under Visible Light Irradiation. J. Mol. Catal. A Chem. 2016, 425, 183–189. [Google Scholar] [CrossRef]
- Lalhriatpuia, C.; Tiwari, D.; Tiwari, A.; Lee, S.M. Immobilized Nanopillars-TiO2 in the Efficient Removal of Micro-Pollutants from Aqueous Solutions: Physico-Chemical Studies. J. Chem. Eng. 2015, 281, 782–792. [Google Scholar] [CrossRef]
- Thanh Doan Nguyen, T.; Nguyen, D.; Phong Vo, P.; Ngoc Doan, H.; Thinh, H.; Pham, N.; Ha Hoang, V.; Tien Le, K.; Kinashi, K.; Tan Huynh, V.; et al. The Roles of Ethanol and Isopropanol as Hole Scavengers in the Photoreduction Reaction of Graphene Oxide by TiO2: A Competition of 2 Oxygenated Groups Removal and Carbon Defects Invasion. J. Mol. Liq. 2023, 381, 121831. [Google Scholar] [CrossRef]
- Schneider, J.T.; Firak, D.S.; Ribeiro, R.R.; Peralta-Zamora, P. Use of Scavenger Agents in Heterogeneous Photocatalysis: Truths, Half-Truths, and Misinterpretations. Phys. Chem. Chem. Phys. 2020, 22, 15723–15733. [Google Scholar] [CrossRef]
- Trenczek-Zajac, A.; Synowiec, M.; Zakrzewska, K.; Zazakowny, K.; Kowalski, K.; Dziedzic, A.; Radecka, M. Scavenger-Supported Photocatalytic Evidence of an Extended Type I Electronic Structure of the TiO2@Fe2O3 Interface. ACS Appl. Mater. Interfaces 2022, 14, 38255–38269. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. The Photocatalytic Degradation of Sodium Diclofenac in Different Water Matrices Using g-C3N4 Nanosheets: A Study of the Intermediate By-Products and Mechanism. J. Environ. Chem. Eng. 2021, 9, 105827. [Google Scholar] [CrossRef]









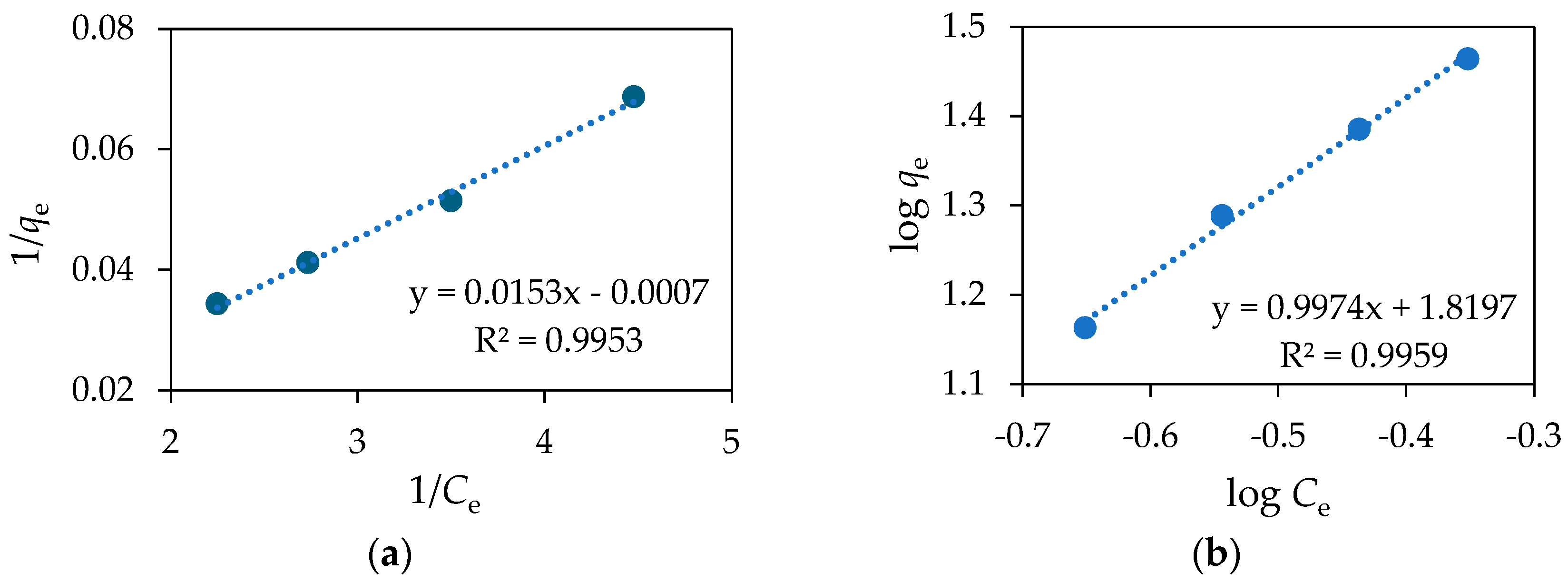

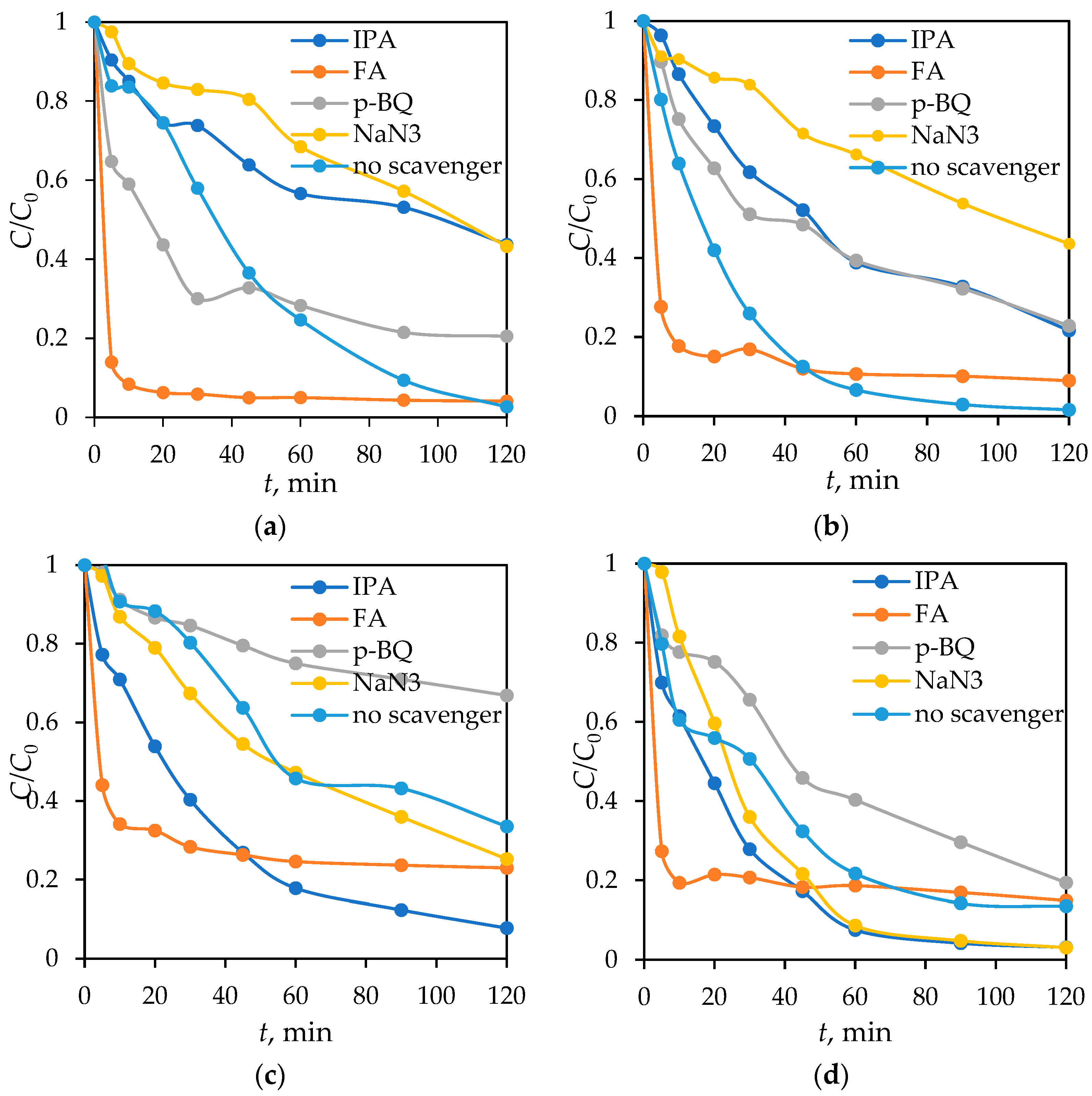
| Scavengers | Reactive Oxygen Species (ROSs) |
|---|---|
| isopropanol (IPA) | hydroxyl radicals (●OH) |
| formic acid (FA) | holes (h+) |
| p-benzoquinone (p-BQ) | superoxide radicals (O2●−) |
| sodium azide (NaN3) | singlet oxygen (1O2) |
| Material | Specific Surface Area | Average Pore Diameter | Pore Volume |
|---|---|---|---|
| SBET, m2 g−1 | davg, nm | Vpore, cm3 g−1 | |
| Fe3O4/SiO2/TiO2 | 116.6 | 8.5 | 0.2591 |
| Fe3O4/SiO2/TiO2/MIP adsorbed | 49.7 | 12.3 | 0.1820 |
| Fe3O4/SiO2/TiO2/MIP washed | 55.7 | 11.4 | 0.1858 |
| Kinetic Model | ||||||
|---|---|---|---|---|---|---|
| Pseudo-First Order | Pseudo-Second Order | |||||
| qe,exp, mg g−1 | k1, min−1 | qe,cal, mg g−1 | R2 | k2, g mg−1 min−1 | qe,cal, mg g−1 | R2 |
| 1.80 | 0.04 | 0.70 | 0.9952 | 0.12 | 1.86 | 0.9991 |
| Intraparticle Diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|
| First Stage of Sorption | Second Stage of Sorption | Third Stage of Sorption | ||||||
| kp1, mg g−1 min−1/2 | C1 | R2 | kp2, mg g−1 min−1/2 | C1 | R2 | kp3, mg g−1 min−1/2 | C3 | R2 |
| 0.084 | 1.07 | 0.9758 | 0.069 | 1.16 | 0.9239 | 0.023 | 1.55 | 0.9729 |
| Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| Qm, mg g−1 | KL, dm−3 mg−1 | R2 | 1/n | KF, mg g−1 | R2 | RL |
| 65.36 | 21.86 | 0.9953 | 1.00 | 66.02 | 0.9959 | 0.005 |
| Photocatalyst | Light Source | Degradation Efficiency, % | Ref. |
|---|---|---|---|
| TiO2 (P25) MIP | UV | 60 | [6] |
| TiO2 (P25) MIP | UV | 70 | [15] |
| TiO2 (P25) | UV (4 × 2.78 W m−2) UV/H2O2 | 20 80 | [65] |
| TiO2-based zeolite | SSL (1247.8 W m−2) | 90 | [66] |
| AC-TiO2-N | UV-immersed lamp | 80 | [67] |
| TiO2 | UV-immersed lamp | 50 | [68] |
| Fe3O4/SiO2/TiO2/MIP | UV (98.5 W m−2) SSL (1028.6 W m−2) | 80 | This work |
| Sorbent | Diclofenac Concentration, mg dm−3 | Sorption Capacity, mg g−1 | Ref. |
|---|---|---|---|
| Reduced graphene oxide (rGO) | 40 | 22.64 | [69] |
| Granular activated carbon | 5 100 | 35 150 | [70] |
| Organobentonite with surfactant hexadecyltrimethylammonium | 600 | 125–250 | [71] |
| Hexagonal mesoporous silicate | 0.1 | 0.032 | [72] |
| Powdered activated carbon | 5 | 4.47 | [73] |
| Thermo-plasma expanded graphite | 100 | 400 | [74] |
| Einkorn husk activated carbon | 50 | 147.06 | [75] |
| Fe3O4/SiO2/TiO2/MIP | 10 | 1.86 | This work |
| Material | Pseudo-First Order | Pseudo-Second Order | |||
|---|---|---|---|---|---|
| Lamp | k1, min−1 | R2 | k2, dm3 mg−1 min−1 | R2 | |
| Fe3O4/SiO2/TiO2 | UV | 0.0044 | 0.9799 | 0.0204 | 0.9797 |
| SSL | 0.0137 | 0.9695 | 0.1546 | 0.9646 | |
| Fe3O4/SiO2/TiO2/MIP | UV | 0.0027 | 0.9515 | 0.0982 | 0.9245 |
| SSL | 0.013 | 0.9273 | 0.126 | 0.8258 | |
| Material | UV | SSL | |||||
|---|---|---|---|---|---|---|---|
| k1, min−1 | t1/2, min | R2 | k1, min−1 | t1/2, min | R2 | ||
| Fe3O4/SiO2/TiO2 | IPA | 0.0213 | 32.54 | 0.9718 | 0.0302 | 22.95 | 0.9533 |
| FA | 0.0065 | 106.64 | 0.8452 | 0.0051 | 135.91 | 0.8445 | |
| p-BQ | 0.0033 | 210.04 | 0.9349 | 0.0131 | 52.91 | 0.9861 | |
| NaN3 | 0.0115 | 60.27 | 0.9933 | 0.0319 | 21.73 | 0.9656 | |
| No scavenger | 0.0098 | 70.73 | 0.9560 | 0.0173 | 40.07 | 0.9785 | |
| Fe3O4/SiO2/TiO2/MIP | IPA | 0.0064 | 108.30 | 0.9490 | 0.0127 | 54.58 | 0.9829 |
| FA | 0.0100 | 69.31 | 0.8129 | 0.0115 | 60.27 | 0.8906 | |
| p-BQ | 0.0115 | 60.27 | 0.7831 | 0.0115 | 60.27 | 0.9530 | |
| NaN3 | 0.0066 | 105.02 | 0.9776 | 0.0067 | 103.45 | 0.9925 | |
| No scavenger | 0.0562 | 12.33 | 0.9773 | 0.0456 | 15.20 | 0.9995 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabelica, I.; Radovanović-Perić, F.; Matijašić, G.; Tolić Čop, K.; Ćurković, L.; Mutavdžić Pavlović, D. Synergistic Removal of Diclofenac via Adsorption and Photocatalysis Using a Molecularly Imprinted Core–Shell Photocatalyst. Materials 2025, 18, 2300. https://doi.org/10.3390/ma18102300
Gabelica I, Radovanović-Perić F, Matijašić G, Tolić Čop K, Ćurković L, Mutavdžić Pavlović D. Synergistic Removal of Diclofenac via Adsorption and Photocatalysis Using a Molecularly Imprinted Core–Shell Photocatalyst. Materials. 2025; 18(10):2300. https://doi.org/10.3390/ma18102300
Chicago/Turabian StyleGabelica, Ivana, Floren Radovanović-Perić, Gordana Matijašić, Kristina Tolić Čop, Lidija Ćurković, and Dragana Mutavdžić Pavlović. 2025. "Synergistic Removal of Diclofenac via Adsorption and Photocatalysis Using a Molecularly Imprinted Core–Shell Photocatalyst" Materials 18, no. 10: 2300. https://doi.org/10.3390/ma18102300
APA StyleGabelica, I., Radovanović-Perić, F., Matijašić, G., Tolić Čop, K., Ćurković, L., & Mutavdžić Pavlović, D. (2025). Synergistic Removal of Diclofenac via Adsorption and Photocatalysis Using a Molecularly Imprinted Core–Shell Photocatalyst. Materials, 18(10), 2300. https://doi.org/10.3390/ma18102300








