Synergistic Toughening Mechanisms in ZrO2/Multi-Walled Carbon Nanotubes-Reinforced CaZr4(PO4)6 Ceramics for Enhanced Mechanical Performance
Abstract
1. Introduction
2. Experimental
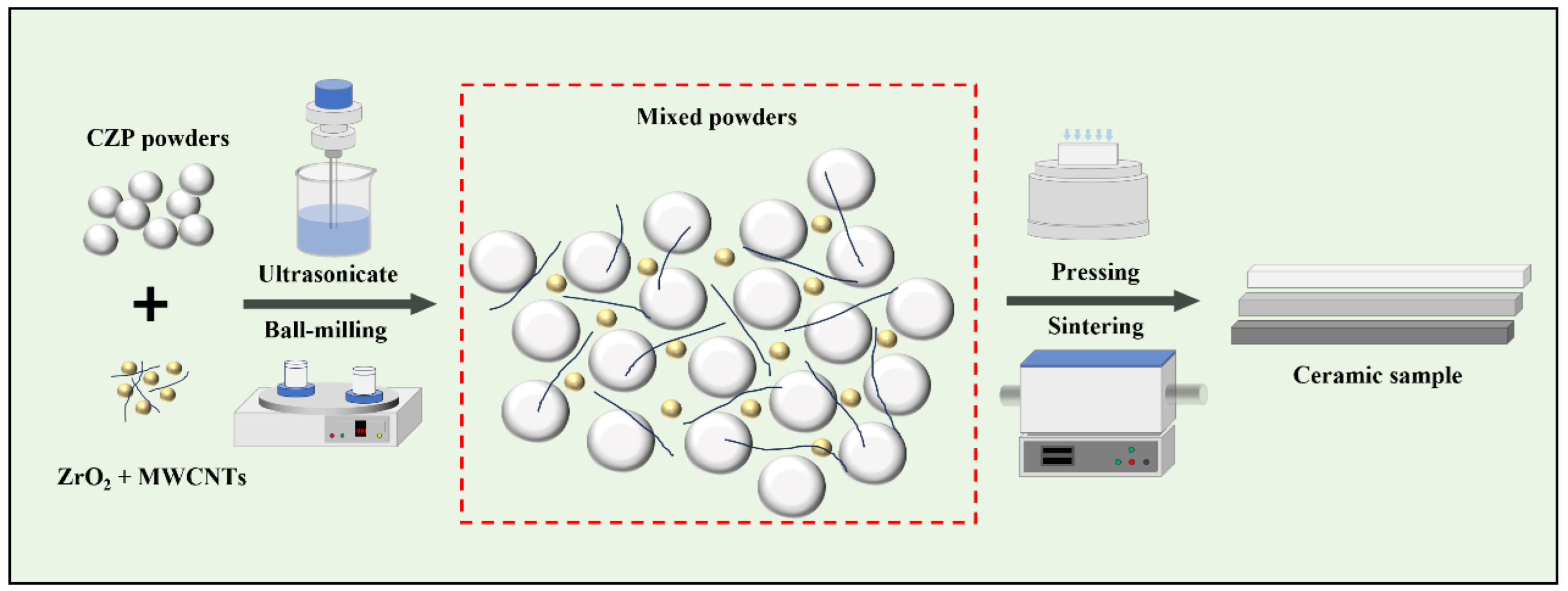
2.1. Materials Preparation
2.2. Characterization Methods
3. Results and Discussion
3.1. ZrO2 Single-Phase Toughening CZP Ceramics
3.2. ZrO2 and MWCNTs Composite Toughening CZP Ceramics
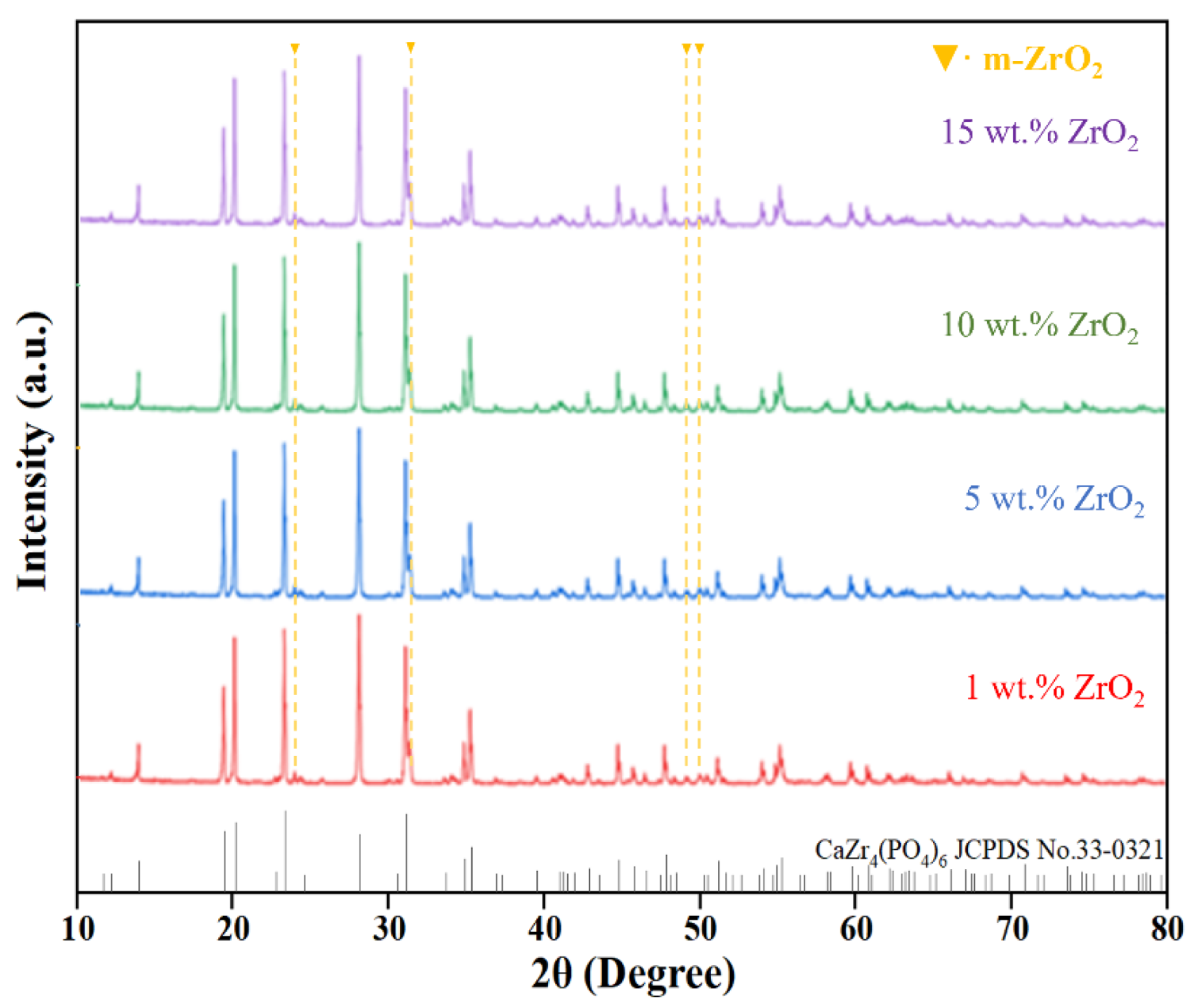
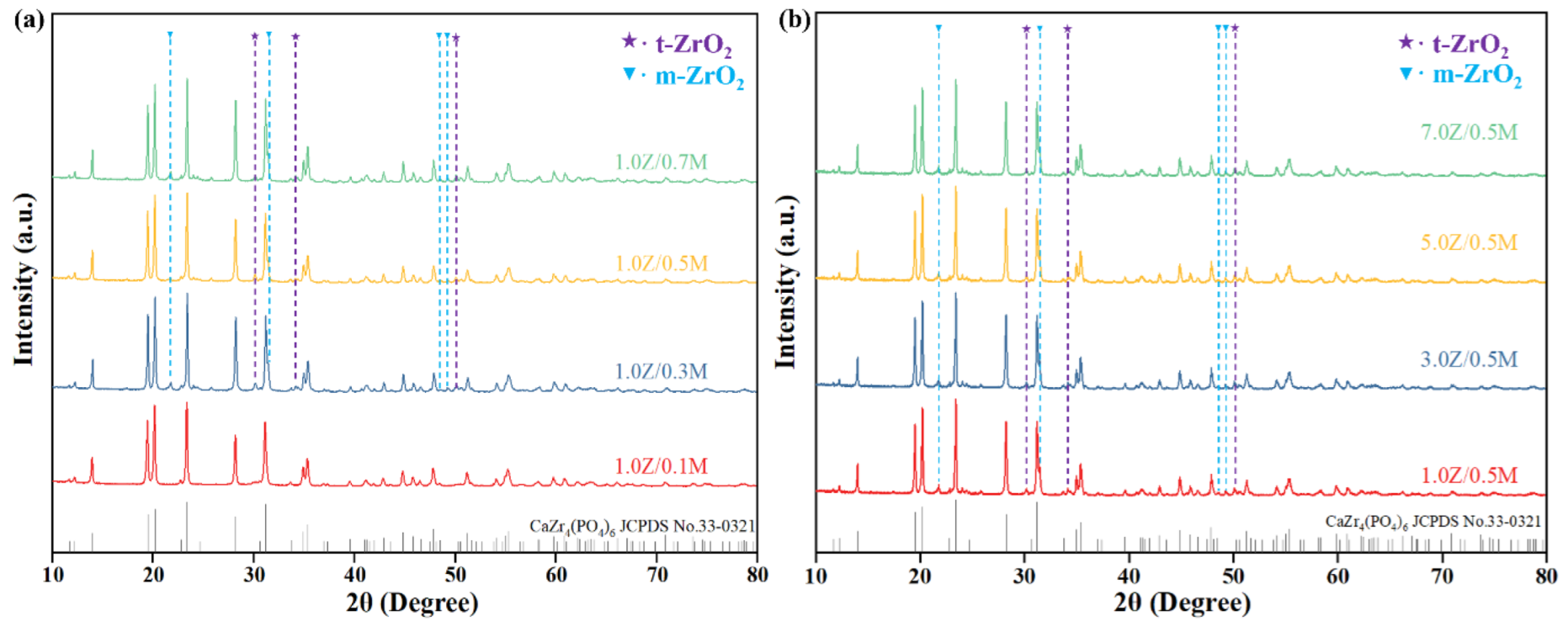
3.2.1. Phase Analysis
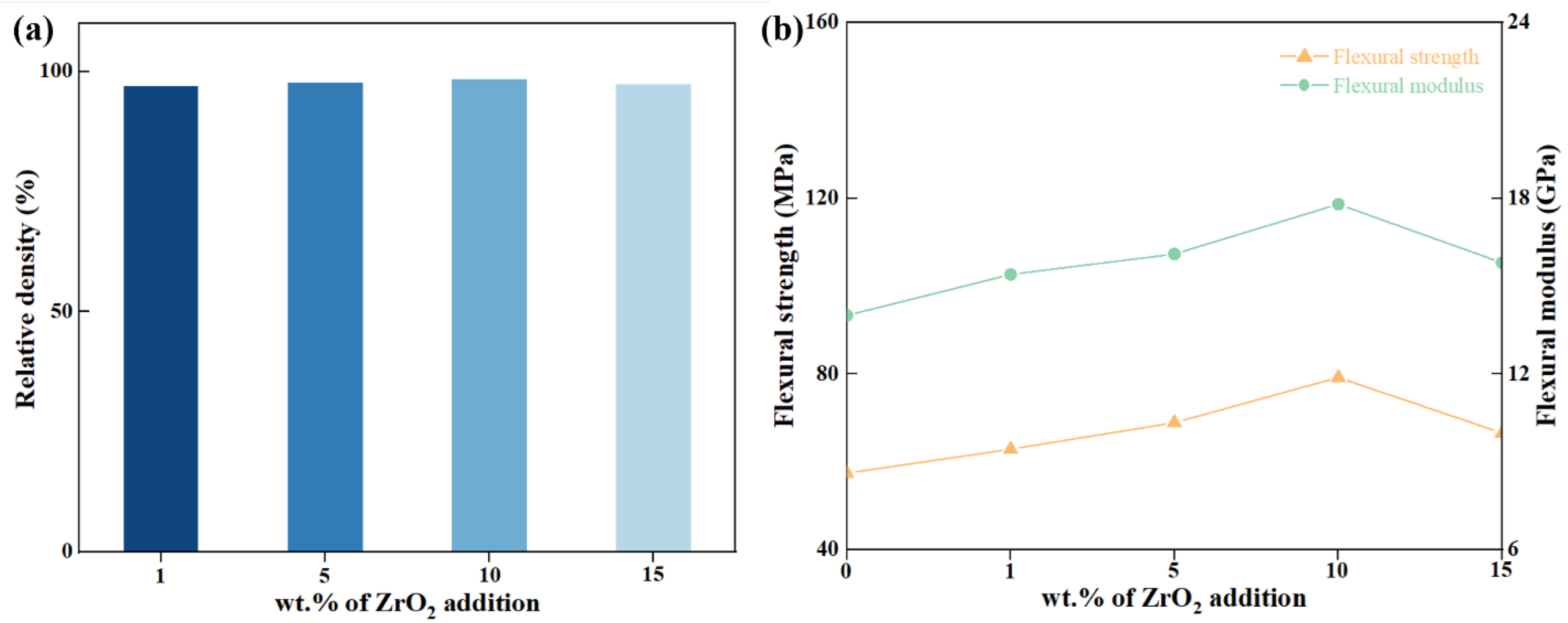
3.2.2. Mechanical Properties
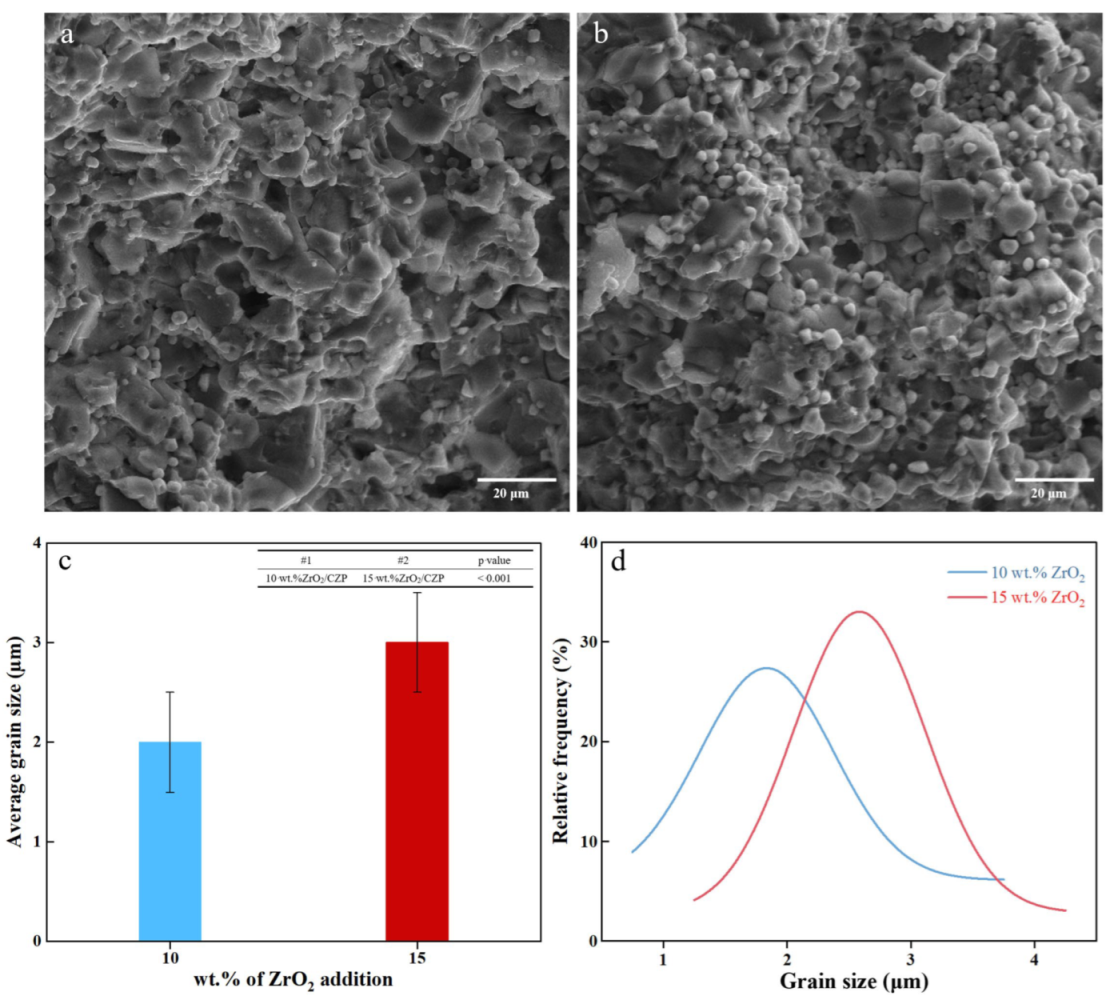
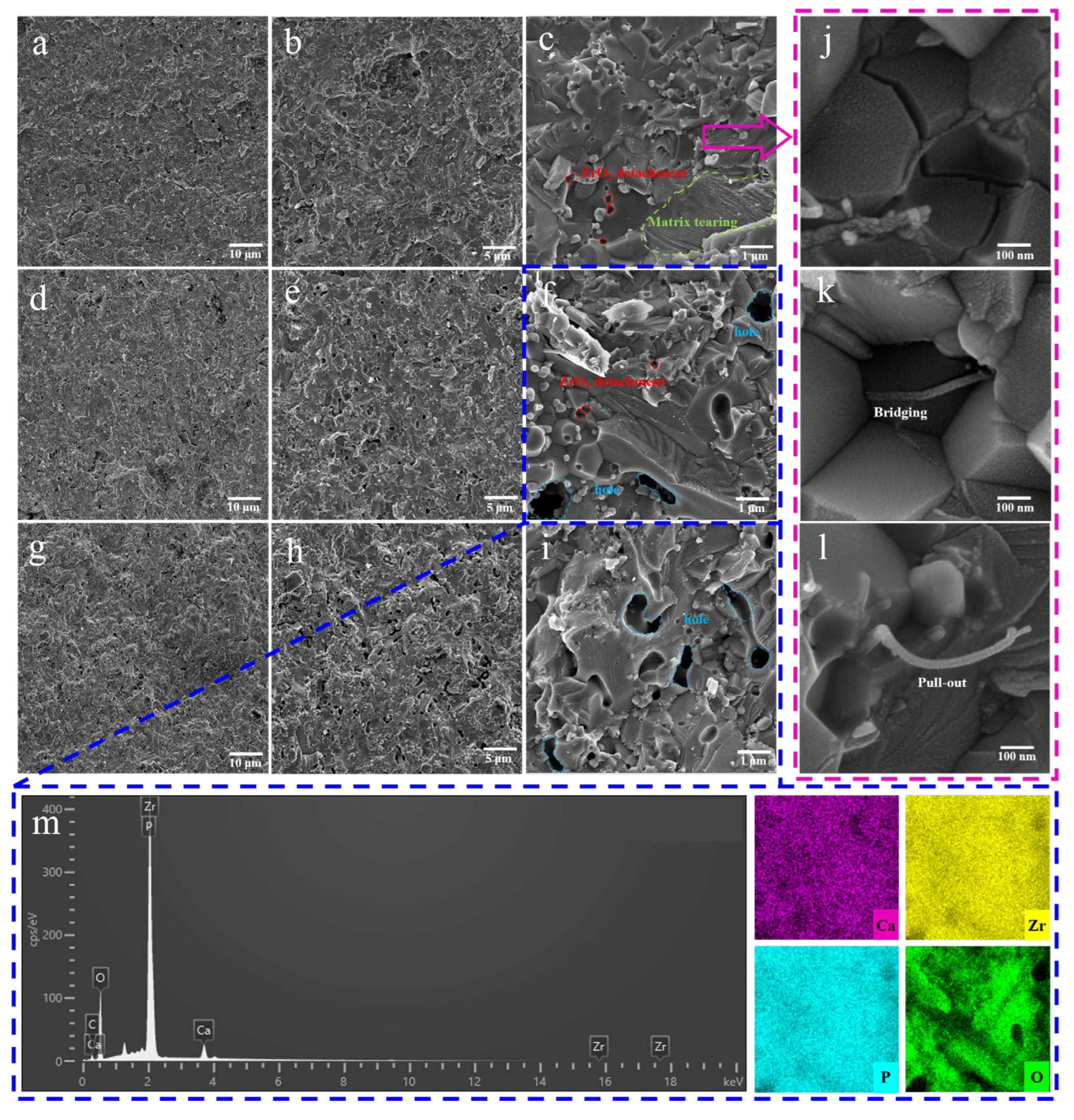
3.2.3. Morphological Analysis
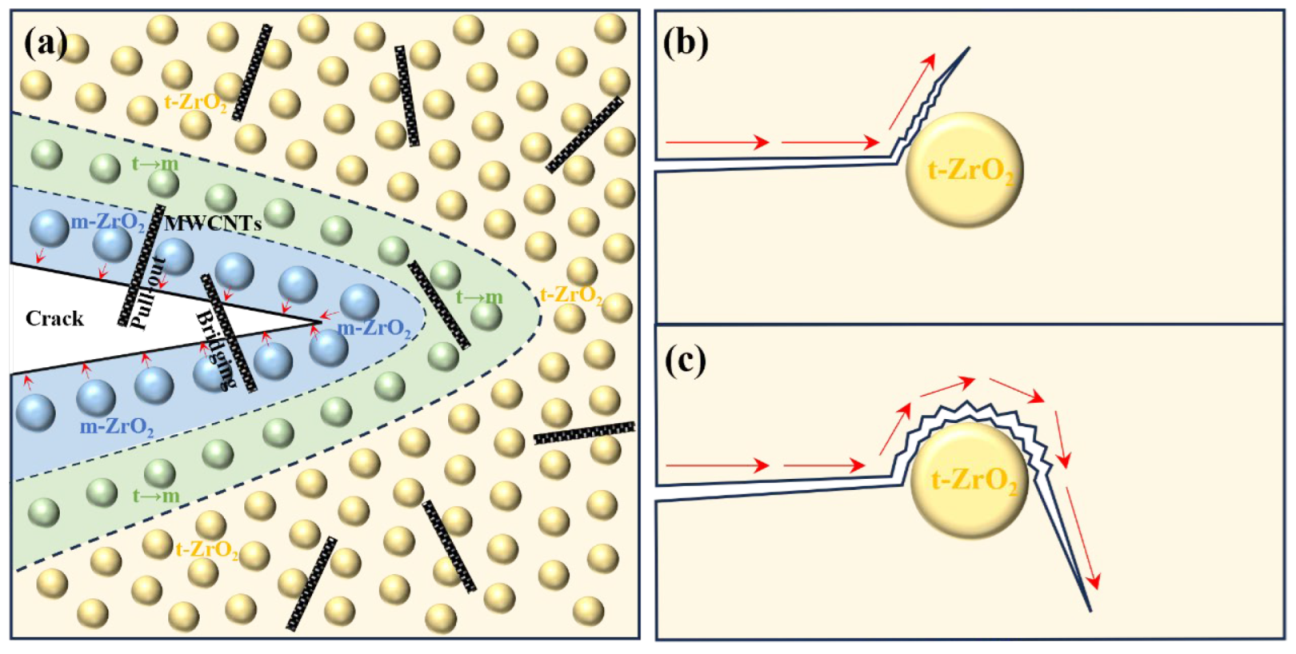
3.2.4. Toughening Mechanism
4. Conclusions
- 1.
- When ZrO2 was used as a single-phase toughening agent, the optimal additive amount was found to be 10 wt.%, which resulted in the maximum relative density of Z/C ceramics. The flexural strength of the ceramics reached 71.60 MPa, representing a 137% increase compared to pure-phase CZP. Analysis indicated that the toughening mechanism is primarily attributable to the particle toughening effect of ZrO2.
- 2.
- The incorporation of ZrO2/MWCNTs as a composite toughening agent resulted in the ceramic sample 1.0Z/0.3M/C exhibiting remarkable mechanical properties. Specifically, the sample achieved a flexural strength of 138.43 MPa and a flexural modulus of 21.25 GPa.
- 3.
- A comprehensive examination of the toughening mechanism of the composite toughening agent revealed that the phase-transformation toughening mechanism of ZrO2—including the transformation between m-ZrO2 and t-ZrO2, the counteraction of compressive stress through phase transformation to prevent crack propagation, and the generation of microcracks that consume energy and cause crack deflection—interacted with the bridging and pull-out toughening mechanisms of MWCNTs. This interaction significantly enhanced the mechanical properties of CZP. However, excessive addition of agents can lead to agglomeration of the toughening materials, greatly reducing the sintering activity and the mechanical strength of CZP ceramics.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chenu, S.; Lebullenger, R.; Bénard-Rocherullé, P.; Calvez, G.; Guillou, O.; Rocherullé, J.; Kidari, A.; Pomeroy, M.J.; Hampshire, S. Glass reactive sintering as an alternative route for the synthesis of NZP glass-ceramics. J. Mater. Sci. 2012, 47, 486–492. [Google Scholar] [CrossRef]
- Wei, Y.F.; Luo, P.; Wang, J.X.; Wen, J.W.; Zhan, L.; Zhang, X.; Yang, S.Y.; Wang, J. Microwave-sintering preparation, phase evolution and chemical stability of Na1−2xSrxZr2(PO4)3 ceramics for immobilizing simulated radionuclides. J. Nucl. Mater. 2020, 540, 152366. [Google Scholar] [CrossRef]
- Wu, B.; Ning, H.X.; Zhu, H.Z.; Chen, J.J.; Wang, K.; Zhang, D.Y.; Wang, F.; Liao, Q.L. Synthesis and characterization of sodium-aluminophosphate based glass-ceramics containing NZP phase for HLW immobilization. J. Nucl. Mater. 2024, 588, 154832. [Google Scholar] [CrossRef]
- Savinykh, D.O.; Khainakov, S.A.; Boldin, M.S.; Orlova, A.I.; Aleksandrov, A.A.; Popov, A.A.; Murashov, A.A.; Garcia-Granda, S.; Nokhrin, A.V.; Chuvil, V.N. Synthesis, Thermal Expansion Behavior and Sintering of Sodium Zirconium Nickel and Calcium Zirconium Nickel Phosphates. Inorg. Mater. 2021, 57, 529–540. [Google Scholar] [CrossRef]
- Pet, V.I.; Asabina, E.A. Thermophysical properties of NZP ceramics (A review). Glass Ceram. 2004, 61, 233–239. [Google Scholar] [CrossRef]
- Pet, V.I.; Asabina, E.A.; Markin, A.V.; Smirnova, N.N.; Kitaev, D.B. Thermodynamic data of the NZP compounds family. J. Therm. Anal. Calorim. 2005, 80, 695–700. [Google Scholar] [CrossRef]
- Liu, Y.X.; Zhang, X.L.; Liu, R.X.; Jiang, K.; Zhou, C.L.; Liu, F.T.; Li, K. Excellent mechanical property of fast hot pressure sintering CaZr4(PO4)6 low thermal expansion ceramics. Ceram. Int. 2022, 48, 36758–36763. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, Y.Y.; Song, Y.Y.; Yang, L.P.; Liu, F.T. Mechanical and thermal expansion studies on Ca0.5Sr0.5Zr4−xTixP6O24 ceramics. Ceram. Int. 2018, 44, 16698–16702. [Google Scholar] [CrossRef]
- Kumar, M.; Vasanthavel, S.; Khan, M.I.K.; Dhayalan, A.; Kannan, S. Investigation on the structural, mechanical and in vitro biocompatibility features of CaZr4(PO4)6 influenced by Zn2+ substitutions. J. Biomed. Mater. Res. B 2020, 108, 1546–1558. [Google Scholar] [CrossRef]
- Liu, Y.Z.; Jiang, X.S.; Shi, J.L.; Luo, Y.; Tang, Y.J.; Wu, Q.; Luo, Z.P. Research on the interface properties and strengthening-toughening mechanism of nanocarbon-toughened ceramic matrix composites. Nanotechnol. Rev. 2020, 9, 190–208. [Google Scholar] [CrossRef]
- Trunec, M.; Stastny, P.; Kastyl, J.; Roupcova, P.; Chlup, Z. 2Y-TZP ceramics with high strength and toughness by optimizing the microstructure. J. Eur. Ceram. Soc. 2024, 44, 3258–3266. [Google Scholar] [CrossRef]
- Zhao, C.; Lu, H.; Wang, H.B.; Liu, X.M.; Fang, Z.Z.; Hou, C.; Song, X.Y. Seeding ductile nanophase in ceramic grains. Mater. Horiz. 2024, 11, 1908–1922. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Liang, S.S.; Li, Y.J.; Zhou, C.C.; Yu, H.B.; Li, J. ZrO2 Matrix Toughened Ceramic Material-Strength and Toughness. Adv. Eng. Mater. 2022, 24, 2101278. [Google Scholar] [CrossRef]
- Basu, B. Toughening of yttria-stabilised tetragonal zirconia ceramics. Int. Mater. Rev. 2005, 50, 239–256. [Google Scholar] [CrossRef]
- Jiang, W.T.; Lu, H.; Chen, J.H.; Luo, L.; Liu, X.M.; Wang, H.B.; Song, X.Y. Toughening Ceramic-Based Composites by Homogenizing the Lattice Strain at Phase Boundaries. Acs Appl. Mater. Interfaces 2023, 15, 19604–19615. [Google Scholar] [CrossRef] [PubMed]
- Elsaka, S.E.; Elnaghy, A.M. Mechanical properties of zirconia reinforced lithium silicate glass-ceramic. Dent. Mater. 2016, 32, 908–914. [Google Scholar] [CrossRef]
- Zheng, Y.T.; Yu, W.J.; Yu, Y.D.; Su, X.Y.; Yang, P. Fracture morphology and toughening mechanism of Al2O3/ZrO2 eutectic ceramic prepared by the combustion synthesis under ultra-high temperature. Int. J. Refract. Met. Hard Mater. 2019, 81, 21–26. [Google Scholar] [CrossRef]
- Li, J.Q.; Huang, C.Z.; Wang, Z.; Shi, Z.Y.; Xu, L.H.; Huang, S.Q.; Qu, M.N.; Xu, Z.K.; Zhang, D.J.; Guo, B.S.; et al. A new type of high flexural strength micro-nano-composite ceramic tool material Ti(C,N)/WC/ZrO2 for integrated micro milling cutter. Int. J. Refract. Met. Hard Mater. 2024, 120, 106628. [Google Scholar] [CrossRef]
- Mittal, G.; Dhand, V.; Rhee, K.Y.; Park, S.J.; Lee, W.R. A review on carbon nanotubes and graphene as fillers in reinforced polymer nanocomposites. J. Ind. Eng. Chem. 2015, 21, 11–25. [Google Scholar] [CrossRef]
- Nurazzi, N.M.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication; Functionalization, and Application of Carbon Nanotube-Reinforced Polymer Composite: An Overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef]
- Vasudevan, S.; Kothari, A.; Sheldon, B.W. Direct observation of toughening and R-curve behavior in carbon nanotube reinforced silicon nitride. Scripta Mater. 2016, 124, 112–116. [Google Scholar] [CrossRef]
- Bocanegra-Bernal, M.H.; Echeberria, J.; Ollo, J.; Garcia-Reyes, A.; Domínguez-Rios, C.; Reyes-Rojas, A.; Aguilar-Elguezabal, A. A comparison of the effects of multi-wall and single-wall carbon nanotube additions on the properties of zirconia toughened alumina composites. Carbon 2011, 49, 1599–1607. [Google Scholar] [CrossRef]
- Liao, N.; Jia, D.C.; Yang, Z.H.; Zhou, Y.; Li, Y.W.; Jia, X.Y. Strengthening and toughening effects of MWCNTs on Si2BC3N ceramics sintered by SPS technique. Mat. Sci. Eng. A-Struct. 2018, 710, 142–150. [Google Scholar] [CrossRef]
- Akinribide, O.J.; Akinwamide, S.O.; Olubambi, P.A. Morphological analysis and high-temperature phase stability of spark plasma sintered TiN-MWCNTs ceramic composite. J. Aust. Ceram. Soc. 2023, 59, 521–532. [Google Scholar] [CrossRef]
- Gao, H.; Tang, X.N.; Zhang, H.; He, Y.P.; Zhou, T.; Shen, J.Y.; Zhu, L.H.; Si, T. Mechanical strength enhancement of CaZr4P6O24 ceramics with multi-walled carbon nanotubes additions. J. Alloys Compd. 2024, 976, 173310. [Google Scholar] [CrossRef]
- Chen, F.; Yan, K.; Zhou, J.P.; Zhu, Y.S.; Hong, J. High toughness Si3N4 ceramic composites synergistically toughened by multilayer graphene/β-Si3N4 whisker: Preparation and toughening mechanism investigation. J. Alloys Compd. 2022, 921, 166183. [Google Scholar] [CrossRef]
- Guan, C.; Chen, G.; Kai, X.Z.; Huang, L.Y.; Zhao, P.F.; Chen, W.H.; Zhang, M.; Zhao, Y.T. Strengthening-toughening of graphene nanoplates and in situ ZrB2 nanoparticles reinforced AA6111 matrix composites with discontinuous layered structures. Mat. Sci. Eng. A-Struct. 2022, 853, 143750. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Yin, Z.B.; Hong, D.B.; Yuan, J.T. Preparation of complex-shaped Al2O3/SiCp/SiCw ceramic tool by two-step microwave sintering. Ceram. Int. 2020, 46, 27362–27372. [Google Scholar] [CrossRef]
- Shi, Y.J.; Li, W.X.; Zhang, X.R.; Jin, J.C.; Wang, J.L.; Dong, Y.; Mu, J.B.; Wang, G.S.; Zhang, X.L.; Zhang, Z.X. Preparation and toughening mechanism of Al2O3 composite ceramics toughened by B4C@TiB2 core-shell units. J. Adv. Ceram. 2023, 12, 2371–2381. [Google Scholar] [CrossRef]
- Cui, E.Z.; Zhao, J.; Wang, X.C. Determination of microstructure and mechanical properties of graphene reinforced Al2O3-Ti(C, N) ceramic composites. Ceram. Int. 2019, 45, 20593–20599. [Google Scholar] [CrossRef]

| Reagents | Purity | Manufacturer |
|---|---|---|
| CaCO3 | 99.00% | Tianjin Damao Chemicals Reagent Factory, Tianjin, China |
| ZrOCl2·8H2O | 99.00% | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| (NH4)2HPO4 | 99.00% | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| ZrO2 (diameter ≤ 100 nm) | 99.99% | Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China |
| MWCNTs (length 3–12 µm diameter 8–15 nm) | 99.00% | Suzhou Tanfeng Technology Inc., Suzhou, China |
| Sample | Relative Density (%) | Flexural Strength (MPa) | Flexural Modulus (GPa) |
|---|---|---|---|
| CZP | - | 57.47 | 14.00 |
| 1 wt.% Z/C | 96.94 *** | 62.91 *** | 15.46 *** |
| 5 wt.% Z/C | 97.61 *** | 67.97 *** | 16.15 *** |
| 10 wt.% Z/C | 98.43 *** | 71.60 *** | 17.86 *** |
| 15 wt.% Z/C | 97.24 *** | 66.59 *** | 15.81 *** |
| Sample | Relative Density (%) | Flexural Strength (MPa) | Flexural Modulus (GPa) |
|---|---|---|---|
| CZP | - | 57.47 | 14.00 |
| 1.0Z/0.1M/C | 84.21 *** | 107.45 *** | 20.30 *** |
| 1.0Z/0.3M/C | 88.34 *** | 138.43 *** | 21.25 *** |
| 1.0Z/0.5M/C | 91.51 *** | 123.03 *** | 21.01 *** |
| 1.0Z/0.7M/C | 61.27 *** | 56.21 *** | 8.53 *** |
| 3.0Z/0.5M/C | 92.26 *** | 111.95 *** | 20.50 *** |
| 5.0Z/0.5M/C | 94.64 *** | 108.53 *** | 20.30 *** |
| 7.0Z/0.5M/C | 96.52 *** | 109.91 *** | 20.25 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, J.; Si, T.; Gao, H.; Zhu, L.; Zhang, H.; Gao, X.; Tang, X. Synergistic Toughening Mechanisms in ZrO2/Multi-Walled Carbon Nanotubes-Reinforced CaZr4(PO4)6 Ceramics for Enhanced Mechanical Performance. Materials 2025, 18, 2289. https://doi.org/10.3390/ma18102289
Shen J, Si T, Gao H, Zhu L, Zhang H, Gao X, Tang X. Synergistic Toughening Mechanisms in ZrO2/Multi-Walled Carbon Nanotubes-Reinforced CaZr4(PO4)6 Ceramics for Enhanced Mechanical Performance. Materials. 2025; 18(10):2289. https://doi.org/10.3390/ma18102289
Chicago/Turabian StyleShen, Junyao, Tian Si, Huan Gao, Linhua Zhu, Heng Zhang, Xin Gao, and Xiaoning Tang. 2025. "Synergistic Toughening Mechanisms in ZrO2/Multi-Walled Carbon Nanotubes-Reinforced CaZr4(PO4)6 Ceramics for Enhanced Mechanical Performance" Materials 18, no. 10: 2289. https://doi.org/10.3390/ma18102289
APA StyleShen, J., Si, T., Gao, H., Zhu, L., Zhang, H., Gao, X., & Tang, X. (2025). Synergistic Toughening Mechanisms in ZrO2/Multi-Walled Carbon Nanotubes-Reinforced CaZr4(PO4)6 Ceramics for Enhanced Mechanical Performance. Materials, 18(10), 2289. https://doi.org/10.3390/ma18102289





