Comparative Assessment of Hygroscopic Properties and Thermal Performance of Activated Carbon-Based Physical Adsorbents and Advanced Composite Adsorbents
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
2.3. The Static Adsorption Test
2.4. The Adsorption Test in Fixed-Bed Reactor
3. Results and Discussion
3.1. Material Characterization
3.2. Heat Output Performance of CAC
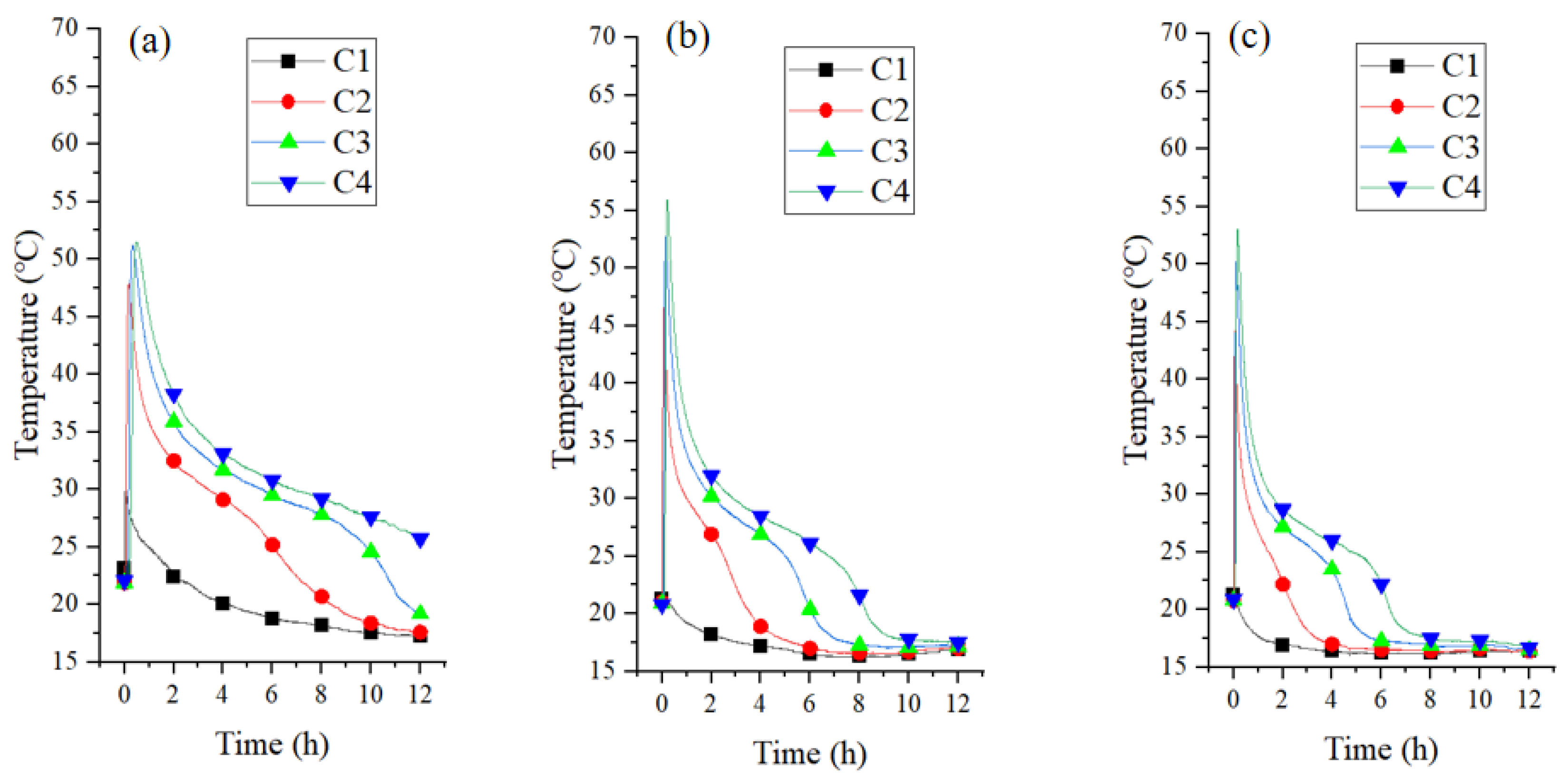
3.2.1. Bed Temperature at Different Air Velocities
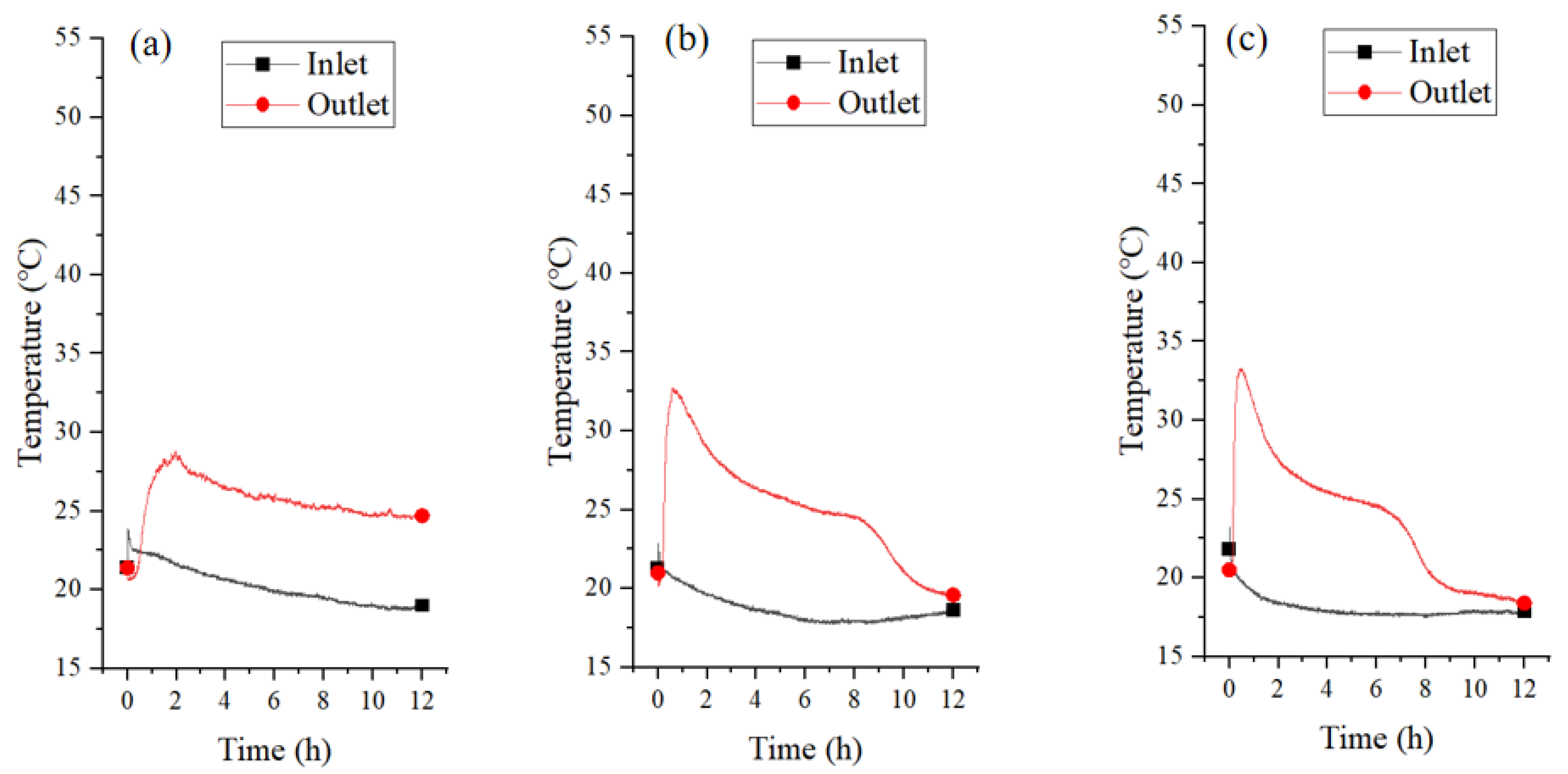
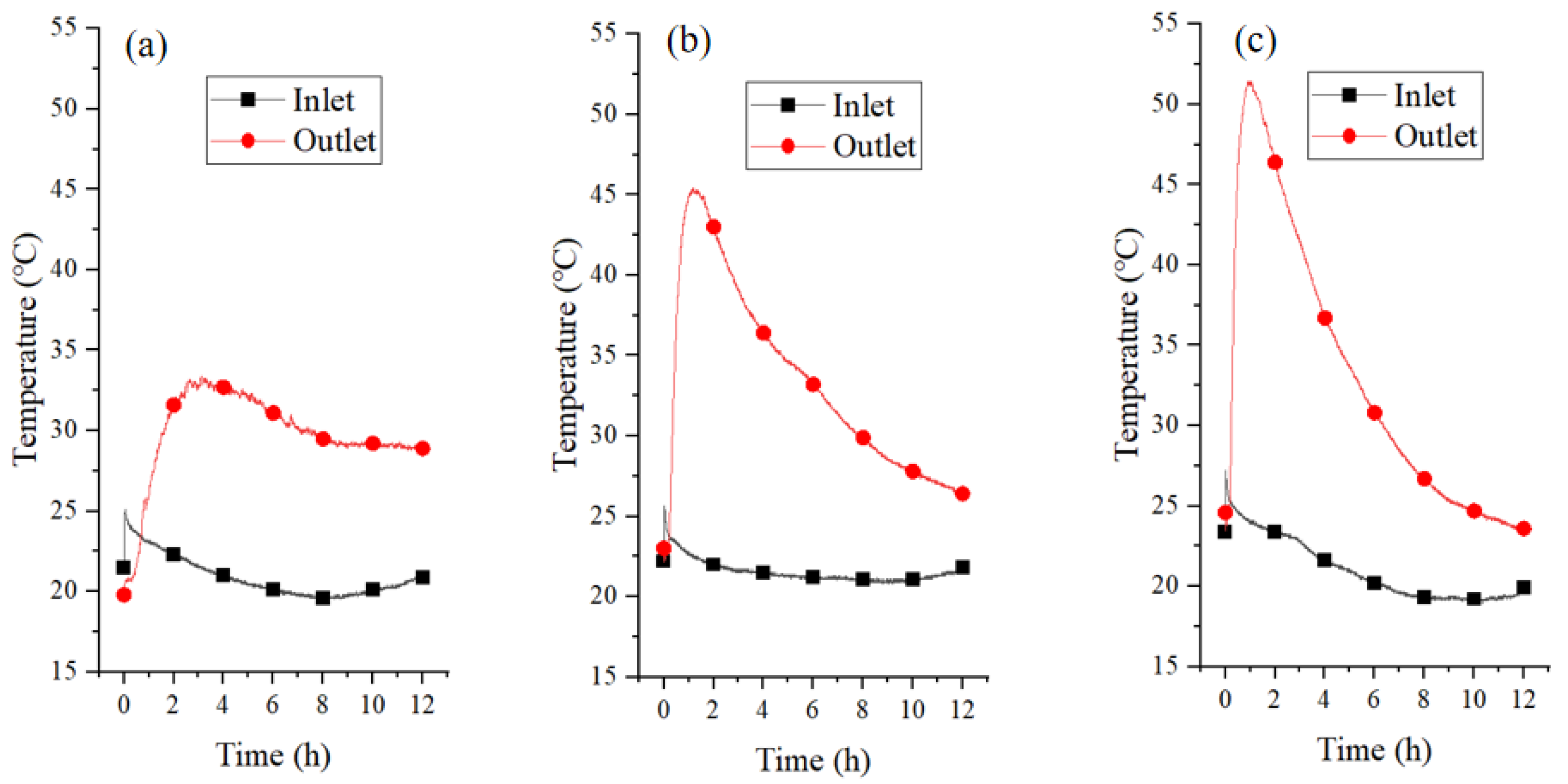
3.2.2. Temperature and Relative Humidity of Airflow
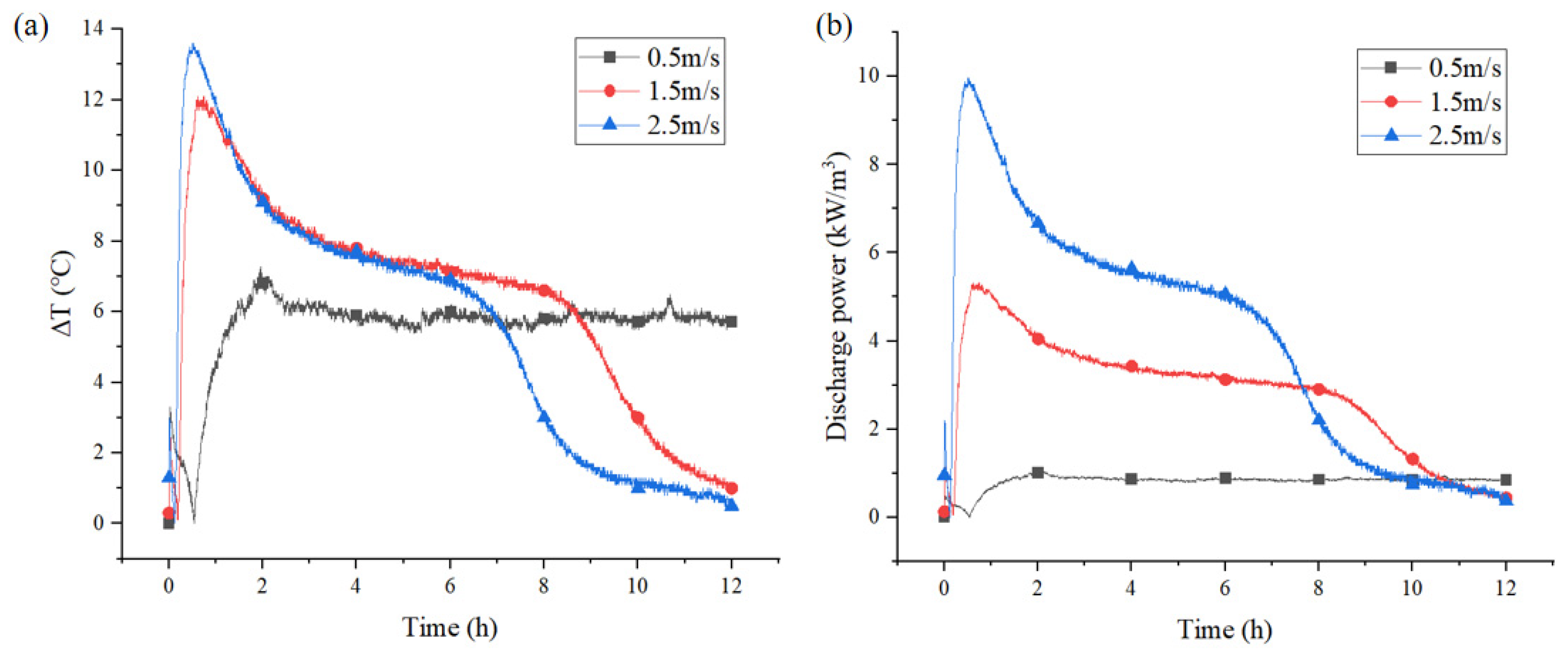
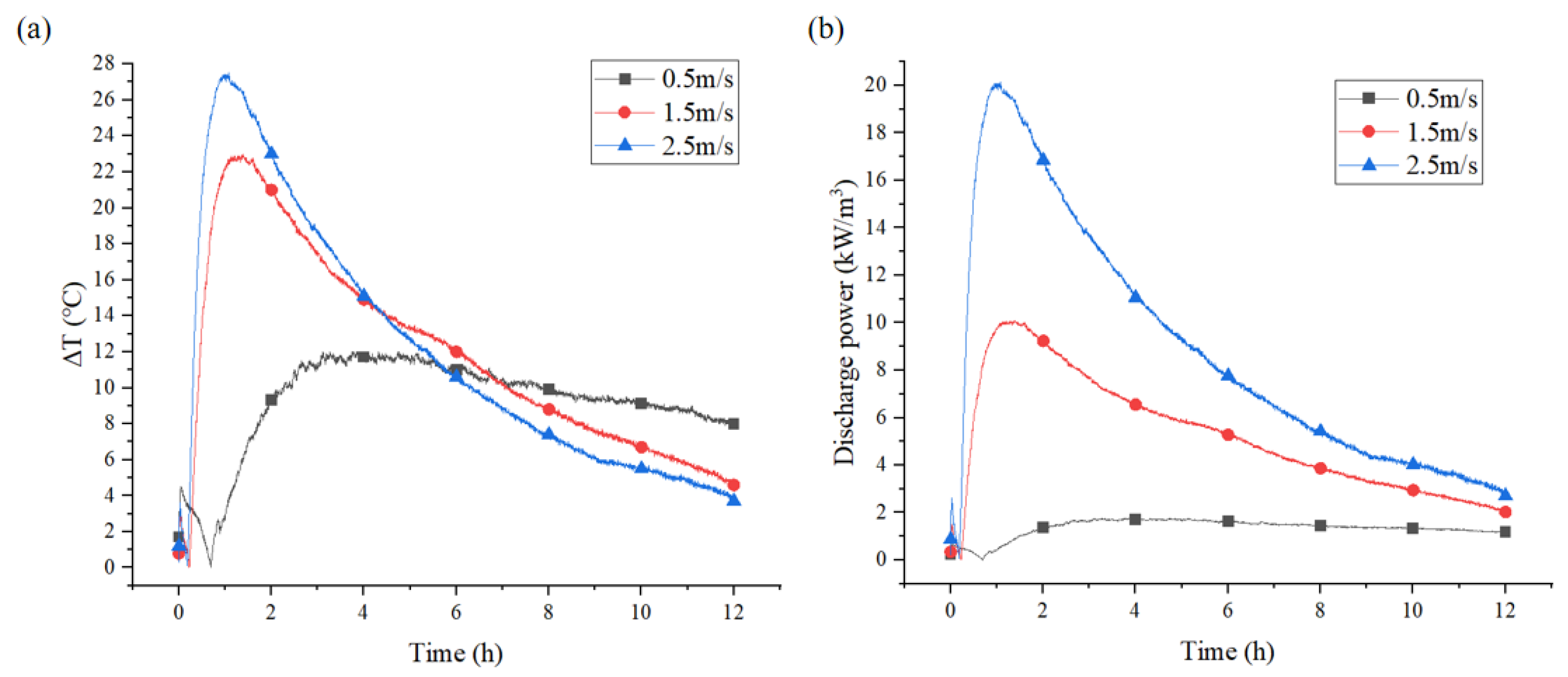
3.2.3. Effect of Different Flow Velocities on Discharge Power
3.3. Heat Output Performance of CAC/Ca
3.3.1. Bed Temperature at Different Air Velocities
3.3.2. Temperature and Relative Humidity of Airflow
3.3.3. Effect of Different Air Velocities on Discharge Power
3.4. The Comparison of CAC and CAC/Ca
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, M.; Liu, T.; Wang, X.; Liu, T.; Li, M.; Chen, G.; Jiang, D. Roles of thermal energy storage technology for carbon neutrality. Carbon Neutrality 2023, 2, 12. [Google Scholar] [CrossRef]
- Man, X.; Lu, H.; Xu, Q.; Wang, C.; Ling, Z. Review on the thermal property enhancement of inorganic salt hydrate phase change materials. J. Energy Storage 2023, 72, 108699. [Google Scholar] [CrossRef]
- Gbenou, T.R.S. Macroscopic and microscopic investigations of low-temperature thermochemical heat storage reactors: A review. Renew. Sustain. Energ. Rev. 2022, 161, 112152. [Google Scholar] [CrossRef]
- Ali, H.M.; Rehman, T.; Arıcı, M.; Said, Z.; Duraković, B.; Mohammed, H.I.; Kumar, R.; Rathod, M.K.; Buyukdagli, O.; Teggar, M. Advances in thermal energy storage: Fundamentals and applications. Prog. Energ. Combust. 2024, 100, 101109. [Google Scholar] [CrossRef]
- Zhang, X.; Ameli, H.; Dong, Z.; Vecchi, A.; Gallego-Schmid, A.; Strbac, G.; Sciacovelli, A. Values of latent heat and thermochemical energy storage technologies in low-carbon energy systems: Whole system approach. J. Energy Storage 2022, 50, 104126. [Google Scholar] [CrossRef]
- Salgado-Pizarro, R.; Calderón, A.; Svobodova-Sedlackova, A.; Fernández, A.I.; Barreneche, C. The relevance of thermochemical energy storage in the last two decades: The analysis of research evolution. J. Energy Storage 2022, 51, 104377. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, R. Sorption thermal energy storage: Concept, process, applications and perspectives. Energy Storage Mater. 2020, 27, 352–369. [Google Scholar] [CrossRef]
- Mehari, A.; Xu, Z.Y.; Wang, R.Z. Thermal energy storage using absorption cycle and system: A comprehensive review. Energy Convers. Manag. 2020, 206, 112482. [Google Scholar] [CrossRef]
- Ayisi, E.N.; Fraňa, K. The design and test for degradation of energy density of a silica gel-based energy storage system using low grade heat for desorption phase. Energies 2020, 13, 4513. [Google Scholar] [CrossRef]
- Qiu, Y.; Yang, Y.; Yang, N.; Tong, L.; Yin, S.; Wang, L.; Ding, Y. Thermochemical energy storage using silica gel: Thermal storage performance and nonisothermal kinetic analysis. Sol. Energy Mater. Sol. Cells 2023, 251, 112153. [Google Scholar] [CrossRef]
- Nonnen, T.; Preißler, H.; Kött, S.; Beckert, S.; Gläser, R. Salt inclusion and deliquescence in salt/zeolite X composites for thermochemical heat storage. Microporous Mesoporous Mater. 2020, 303, 110239. [Google Scholar] [CrossRef]
- D’Ans, P.; Courbon, E.; Permyakova, A.; Nouar, F.; Simonnet-Jégat, C.; Bourdreux, F.; Malet, L.; Serre, C.; Frère, M.; Steunou, N. A new strontium bromide MOF composite with improved performance for solar energy storage application. J. Energy Storage 2019, 25, 100881. [Google Scholar] [CrossRef]
- Aristov, Y.I.; Tokarev, M.M.; Freni, A.; Glaznev, I.S.; Restuccia, G. Kinetics of water adsorption on silica fuji davison RD. Microporous Mesoporous Mater. 2006, 96, 65–71. [Google Scholar] [CrossRef]
- Wei, S.; Zhou, W.; Han, R.; Gao, J.; Zhao, G.; Qin, Y.; Wang, C. Influence of minerals with different porous structures on thermochemical heat storage performance of CaCl2-based composite sorbents. Sol. Energy Mater. Sol. Cells 2022, 243, 111769. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Y.; Li, Z. Adsorption of water on an MgSO4 (100) surface: A first-principles investigation. ChemPhysChem 2013, 14, 1969–1976. [Google Scholar] [CrossRef]
- Sögütoglu, L.-C.; Steiger, M.; Houben, J.; Biemans, D.; Fischer, H.R.; Donkers, P.; Huinink, H.; Adan, O.C.G. Understanding the hydration process of salts: The impact of a nucleation barrier. Cryst. Growth Des. 2019, 19, 2279–2288. [Google Scholar] [CrossRef]
- Huinink, H.; De Jong, S.; Houben, V. Hydration fronts in packed particle beds of salt hydrates: Implications for heat storage. J. Energy Storage 2023, 71, 108158. [Google Scholar] [CrossRef]
- Kiyabu, S.; Girard, P.; Siegel, D.J. Discovery of salt hydrates for thermal energy storage. J. Am. Chem. Soc. 2022, 144, 21617–21627. [Google Scholar] [CrossRef]
- Clark, R.-J.; Gholamibozanjani, G.; Woods, J.; Kaur, S.; Odukomaiya, A.; Al-Hallaj, S.; Farid, M. Experimental screening of salt hydrates for thermochemical energy storage for building heating application. J. Energy Storage 2022, 51, 104415. [Google Scholar] [CrossRef]
- Wijnhorst, R.; Demmenie, M.; Jambon-Puillet, E.; Ariese, F.; Bonn, D.; Shahidzadeh, N. Softness of hydrated salt crystals under deliquescence. Nat. Commun. 2023, 14, 1090. [Google Scholar] [CrossRef]
- Wei, S.; Han, R.; Su, Y.; Zhou, W.; Li, J.; Su, C.; Gao, J.; Zhao, G.; Qin, Y. Development of pomegranate-type CaCl2@C composites via a scalable one-pot pyrolysis strategy for solar-driven thermochemical heat storage. Energy Convers. Manag. 2020, 212, 112694. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Palomba, V.; Frazzica, A.; Cabeza, L.F. Innovative composite sorbent for thermal energy storage based on a SrBr2·6H2O filled silicone composite foam. J. Energy Storage 2019, 26, 100954. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Y.; Ding, B.; Yu, G.; Ye, F.; Xu, C. Structure and Hydration State Characterizations of MgSO4-zeolite 13x composite materials for long-term thermochemical heat storage. Sol. Energy Mater. Sol. Cells 2019, 200, 110047. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Zhao, J.; Wang, X.; Huang, H.; Wang, Y. Construction of biomass waste derived hierarchical porous biochar framework based lithium hydroxide composites for highly efficient and durable low temperature thermochemical heat storage. J. Clean. Prod. 2022, 359, 132047. [Google Scholar] [CrossRef]
- Li, W.; Klemeš, J.J.; Wang, Q.; Zeng, M. Characterisation and Sorption Behaviour of LiOH-LiCl@EG composite sorbents for thermochemical energy storage with controllable thermal upgradeability. Chem. Eng. J. 2021, 421, 129586. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, D. Effect of graphene oxide aerogel on dehydration temperature of graphene oxide aerogel stabilized MgCl2⋅6H2O composites. Sol. Energy 2019, 184, 202–208. [Google Scholar] [CrossRef]
- Garzón-Tovar, L.; Pérez-Carvajal, J.; Imaz, I.; Maspoch, D. Composite salt in porous metal-organic frameworks for adsorption heat transformation. Adv. Funct. Mater. 2017, 27, 1606424. [Google Scholar] [CrossRef]
- Permyakova, A.; Wang, S.; Courbon, E.; Nouar, F.; Heymans, N.; D’Ans, P.; Barrier, N.; Billemont, P.; De Weireld, G.; Steunou, N.; et al. Design of salt-metal organic framework composites for seasonal heat storage applications. J. Mater. Chem. A 2017, 5, 12889–12898. [Google Scholar] [CrossRef]
- Chen, B.; Johannes, K.; Horgnies, M.; Morin, V.; Kuznik, F. Characterization of an ettringite-based thermochemical energy storage material in an open-mode reactor. J. Energy Storage 2021, 33, 102159. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, Q.; Jia, X.; Jin, Y.; Li, Z.; Tan, L.; Ding, Y. Diatomite-based magnesium sulfate composites for thermochemical energy storage: Preparation and performance investigation. Sol. Energy 2021, 224, 907–915. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Y.; Zhang, Y.; Su, Y.; Riffat, S. A Study on Vermiculite-based salt mixture composite materials for low-grade thermochemical adsorption heat storage. Energy 2023, 278, 127986. [Google Scholar] [CrossRef]
- Calabrese, L.; Brancato, V.; Palomba, V.; Frazzica, A.; Cabeza, L.F. Magnesium sulphate-silicone foam composites for thermochemical energy storage: Assessment of dehydration behaviour and mechanical stability. Sol. Energy Mater. Sol. Cells 2019, 200, 109992. [Google Scholar] [CrossRef]
- Ur Rehman, A.; Zhao, T.; Shah, M.Z.; Khan, Y.; Hayat, A.; Dang, C.; Zheng, M.; Yun, S. Nanoengineering of MgSO4 nanohybrid on Mxene substrate for efficient thermochemical heat storage material. Appl. Energ. 2023, 332, 120549. [Google Scholar] [CrossRef]
- Aarts, J.; Van Ravensteijn, B.; Fischer, H.; Adan, O.; Huinink, H. Polymeric stabilization of salt hydrates for thermochemical energy storage. Appl. Energy 2023, 341, 121068. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Liu, X.; Sun, C.; Wu, Y. Fluidisable mesoporous silica composites for thermochemical energy storage. Energy 2023, 275, 127255. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Huang, Y.; Zhao, Y.; Li, X.; Xue, N.; Qu, Z.; Tang, Z.; Xie, L.; Li, Y.; et al. Rapid Preparation strategy of highly microporous activated carbons for gas adsorption, via tunable-energy-density microwave heating. Renew. Energy 2024, 225, 120260. [Google Scholar] [CrossRef]
- Li, J.; Zhou, W.; Huang, Y.; Zhao, Y.; Li, X.; Xue, N.; Qu, Z.; Tang, Z.; Xie, L.; Li, Y.; et al. Rapid, Simple and sustainable preparation of N-rich activated carbons with high performance for gas adsorption, via microwave heating. Sep. Purif. Technol. 2024, 330, 125464. [Google Scholar] [CrossRef]
- Wang, J.; Yan, L.; Ren, Q.; Fan, L.; Zhang, F.; Shi, Z. Facile hydrothermal treatment route of reed straw-derived hard carbon for high performance sodium ion battery. Electrochim. Acta 2018, 291, 188–196. [Google Scholar] [CrossRef]
- Bennici, S.; Dutournié, P.; Cathalan, J.; Zbair, M.; Nguyen, M.H.; Scuiller, E.; Vaulot, C. Heat storage: Hydration investigation of MgSO4/active carbon composites, from material development to domestic applications scenarios. Renew. Sustain. Energ. Rev. 2022, 158, 112197. [Google Scholar] [CrossRef]
- Yang, X.; Li, S.; Zhao, J.; Huang, H.; Deng, L. Development of covalent-organic frameworks derived hierarchical porous hollow carbon spheres based lioh composites for thermochemical heat storage. J. Energy Chem. 2022, 73, 301–310. [Google Scholar] [CrossRef]
- Sundaram, P.; Sathishkumar, A.; Liu, J.; Prabakaran, R.; Kumar, P.G.; Pragathi, P.; Kim, S.C. Coconut shell-derived activated carbon-enhanced water phase change material for cold thermal energy storage. Environ. Sci. Pollut. Res. 2024. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Ma, F.; Fu, Z.; Li, C.; An, Q.; Zhu, C.; Dai, J. Encapsulation of stearic-palmitic acid in alkali-activated coconut shell and corn cob biochar to optimize energy storage. J. Energy Storage 2023, 66, 107418. [Google Scholar] [CrossRef]
- Hekimoğlu, G. Utilizing biomass-derived activated carbon hybrids for enhanced thermal conductivity and latent heat storage in form-stabilized composite PCMs. Energy Sources Part A 2024, 46, 11395–11412. [Google Scholar] [CrossRef]
- Sun, N.; Liu, H.; Xu, B. Facile synthesis of high performance hard carbon anode materials for sodium ion batteries. J. Mater. Chem. A 2015, 3, 20560–20566. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Wang, R.; Feng, P. Air humidity assisted sorption thermal battery governed by reaction wave model. Energy Storage Mater. 2020, 27, 9–16. [Google Scholar] [CrossRef]
- Lin, Y.C.; Fan, Y.B.; Chen, S.; Zhang, X.J.; Frazzica, A.; Jiang, L. Wave Analysis method for air humidity assisted sorption thermal battery: A new perspective. Energy Convers. Manag. 2023, 277, 116638. [Google Scholar] [CrossRef]
- Esaki, T.; Yasuda, M.; Kobayashi, N. Experimental evaluation of the heat output/input and coefficient of performance characteristics of a chemical heat pump in the heat upgrading cycle of CaCl2 hydration. Energy Convers. Manag. 2017, 150, 365–374. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, T.; Wang, R. A new strategy of dual-material reactors for stable thermal output of sorption thermal battery. Energy 2024, 293, 130692. [Google Scholar] [CrossRef]
- El Ouaragli, J.; Xiao, Z.; Tao, M.; Granados-Focil, S.; Van Dessel, S. A novel passive polymer-sorbent thermal battery for low-temperature energy applications: A numerical feasibility study. J. Energy Storage 2022, 56, 105971. [Google Scholar] [CrossRef]
- Xu, C.; Yu, Z.; Xie, Y.; Ren, Y.; Ye, F.; Ju, X. Study of the hydration behavior of zeolite-MgSO4 composites for long-term heat storage. Appl. Therm. Eng. 2018, 129, 250–259. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, X.K.; Yan, T.; Zhang, H.; Wang, L.W.; Pan, W.G. Preparation and thermal properties of zeolite 13X/MgSO4-LiCl binary-salt composite material for sorption heat storage. Appl. Therm. Eng. 2024, 245, 122905. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, Z.C.; Wang, Y.H.; Li, W.Y.; Liu, S.L. Comprehensive study of a volcanic-based hydrated salt thermochemical energy storage composites for buildings heating in China’s low-latitude plateau region: Development, characterisation, and analysis. J. Energy Storage 2024, 103, 114396. [Google Scholar] [CrossRef]
- Shervani, S.; Strong, C.; Tezel, F.H. Simultaneous impregnation and microencapsulation of CaCl2 using silica gel and methyl cellulose for thermal energy storage applications. Sci. Rep. 2024, 14, 7183. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.M.; Luo, J.X.; Jin, Y.H.; Ke, F.L.; Wang, W.; Yin, Q. Experimental study on thermochemical heat storage performance of expanded perlite-based SrCl2/CaCl2 binary hydrated salt composites. J. Energy Storage 2025, 117, 116199. [Google Scholar] [CrossRef]
- Chen, J.B.; Zhang, Y.; Chen, Z.W.; Gan, G.H.; Su, Y.H. Impact of porous host materials on the compromise of thermochemical energy storage performance. Renew. Energy 2025, 245, 122784. [Google Scholar] [CrossRef]
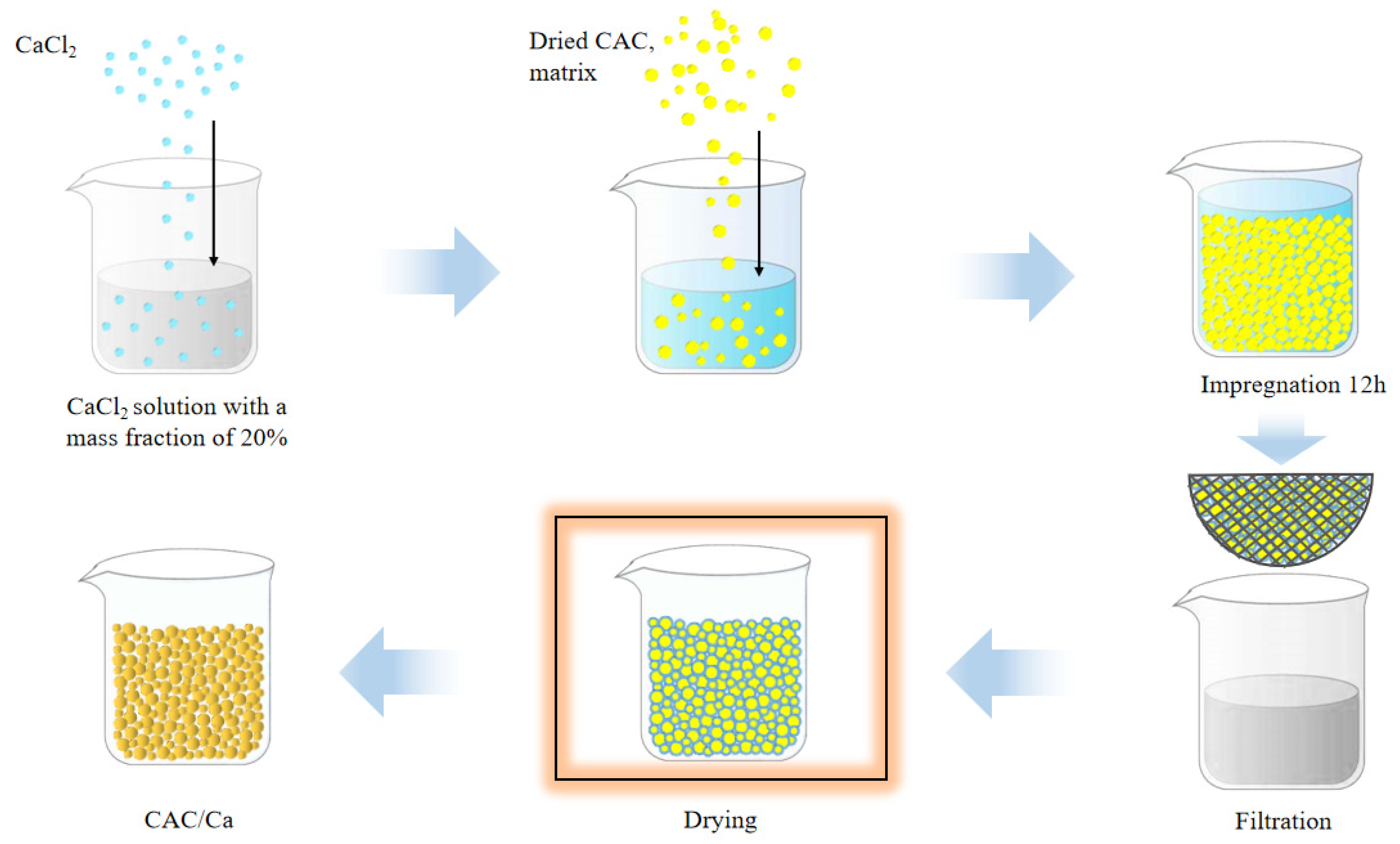
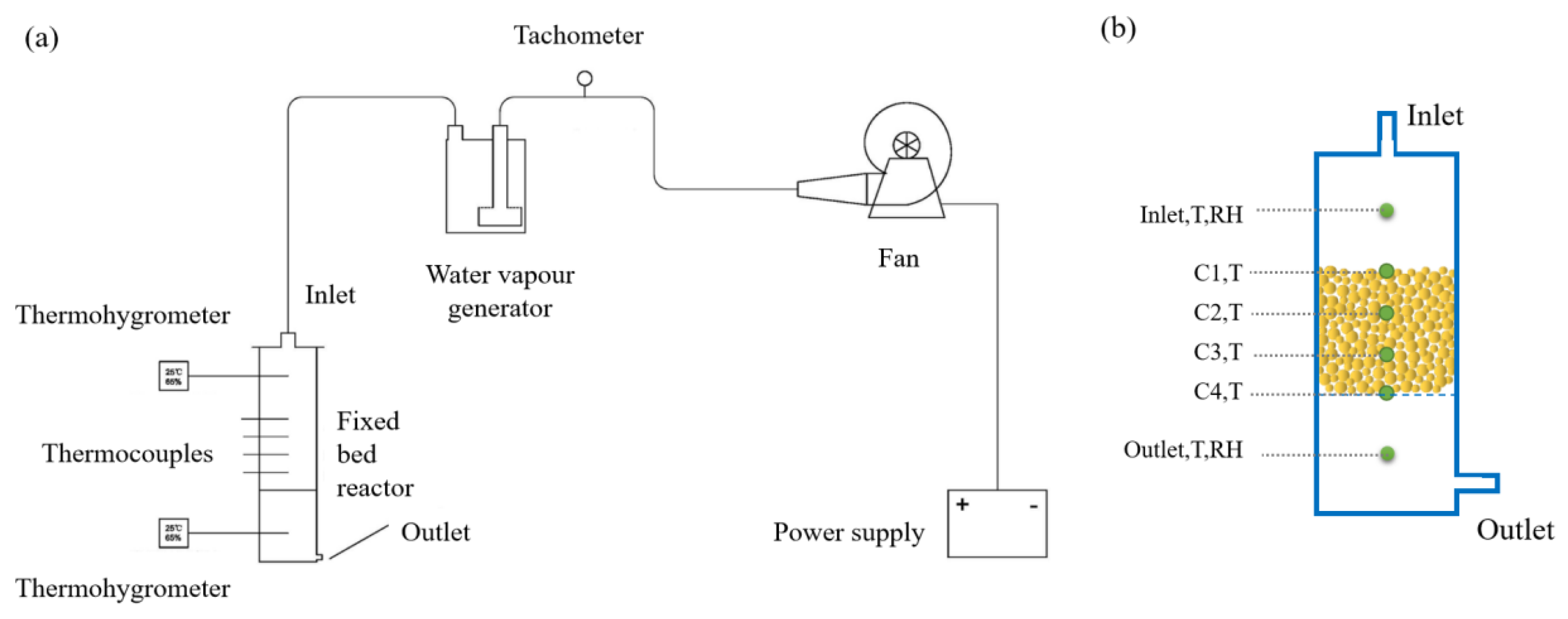
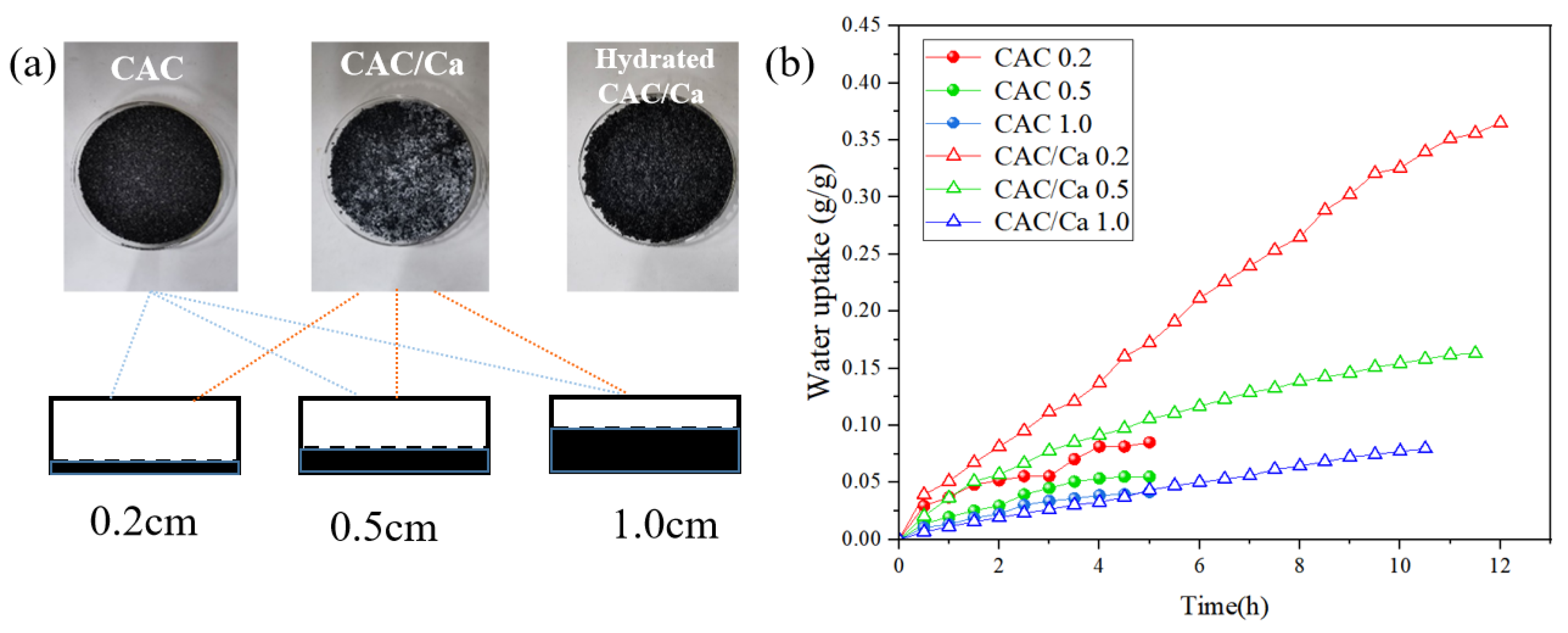

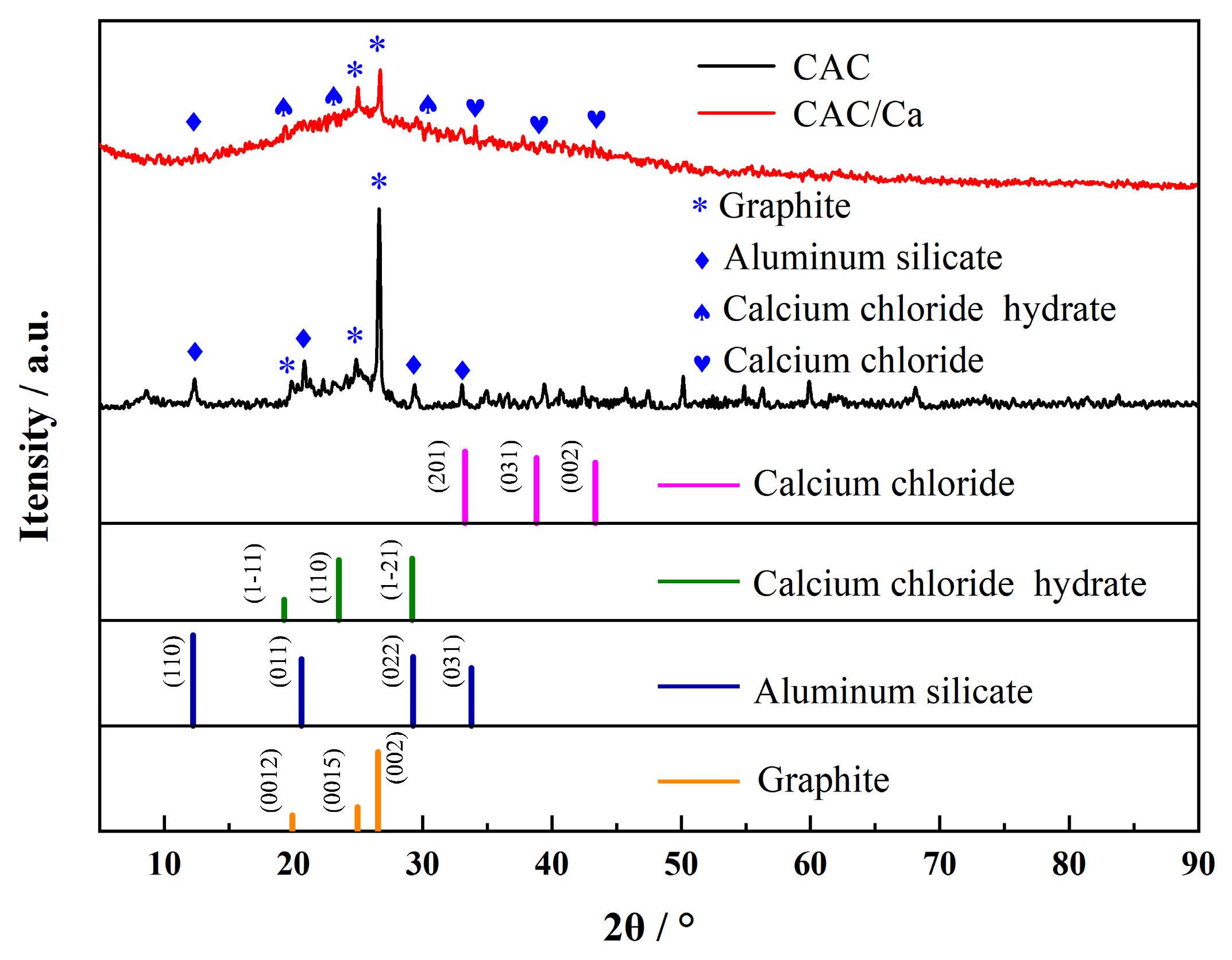
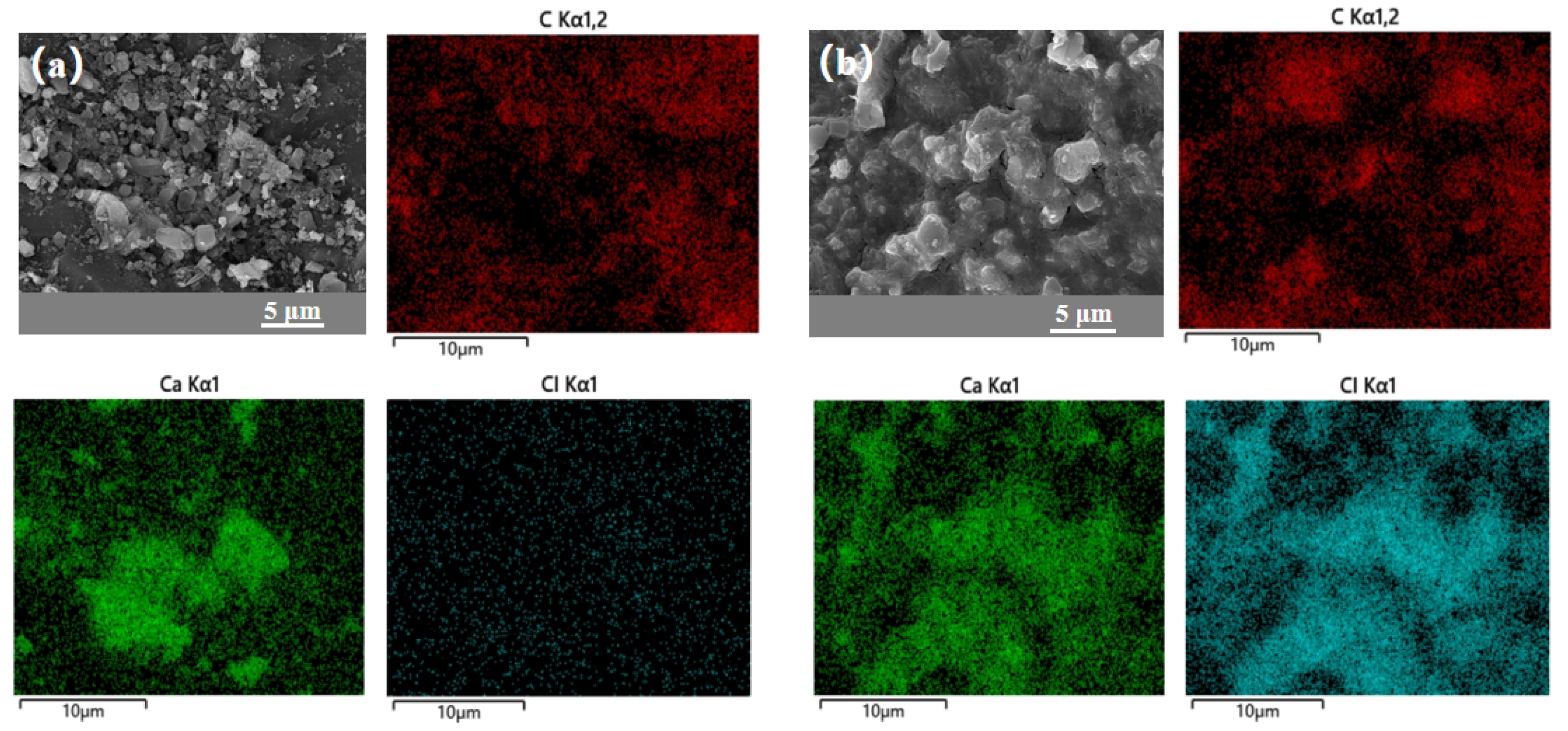
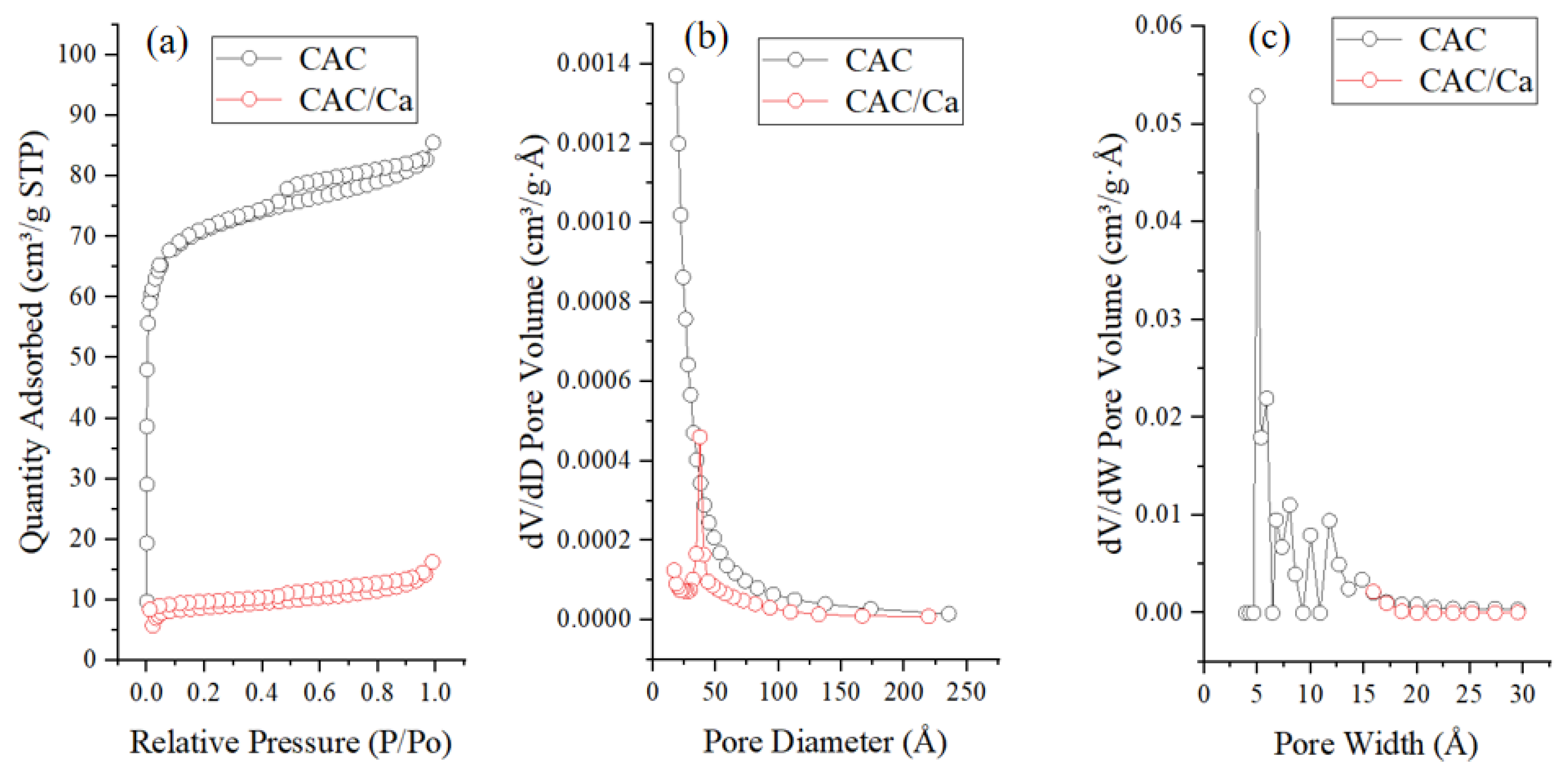
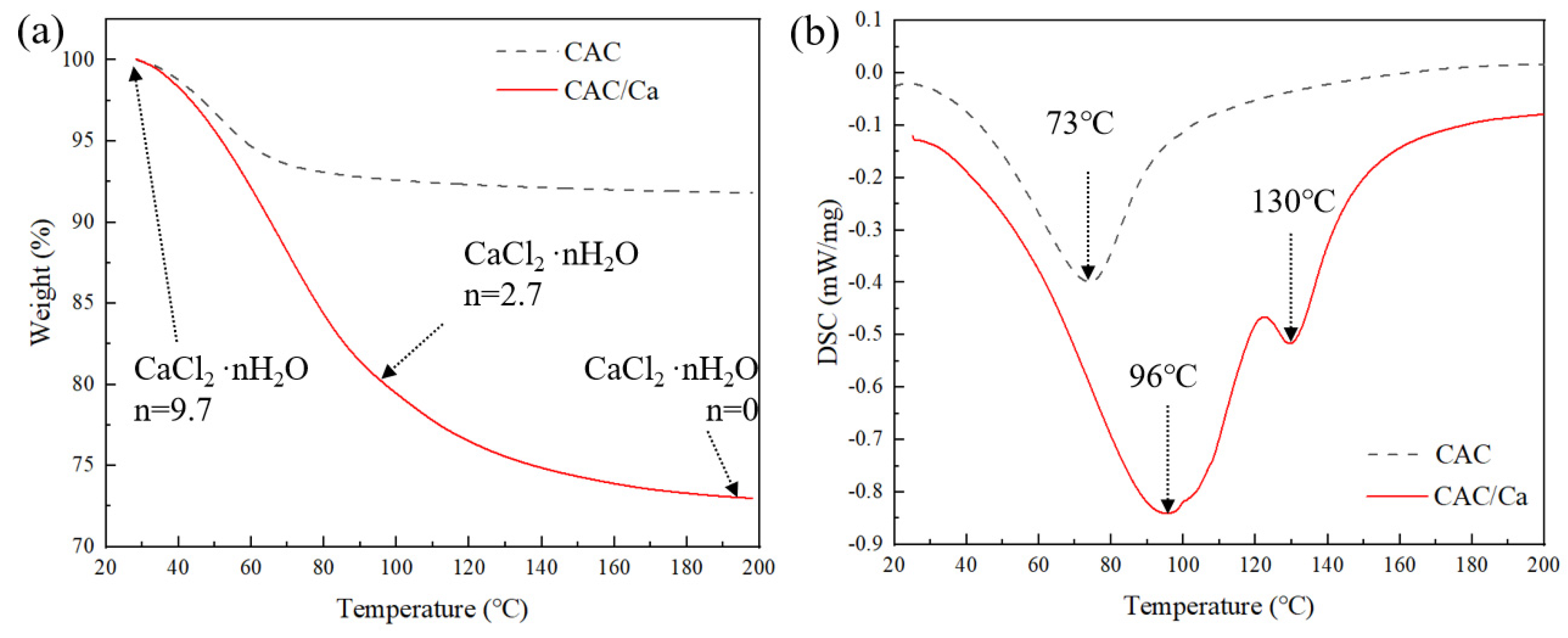


| Sample | SBET (m2/g) | Vmicro (cm3/g) | Vtotal (m3/g) | Dave (nm) | CaCl2 Content (%) | Water Uptake 0.2 cm, 5 h (g/g) |
|---|---|---|---|---|---|---|
| CAC | 231.26 | 0.08 | 0.13 | 3.8 | 0 | 0.09 |
| CAC/Ca | 28.74 | 0.01 | 0.03 | 7.0 | 24 | 0.17 |
| Sample | 0.5 m/s | 1.5 m/s | 2.5 m/s |
|---|---|---|---|
| CAC | 18 | 56 | 78 |
| CAC/Ca | 29 | 113 | 183 |
| Sample | Heat Release Density from Fixed-Bed Test/(kJ/kg) | Heat Storage Density from DSC/(kJ/kg) | Heat Storage Efficiency/% |
|---|---|---|---|
| CAC | 156 | 205 | 76 |
| CAC/Ca | 547 | 783 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, S.; Fan, Z.; Zhang, S.; Xiao, Y.; Wang, C.; Peng, S.; Zhang, X. Comparative Assessment of Hygroscopic Properties and Thermal Performance of Activated Carbon-Based Physical Adsorbents and Advanced Composite Adsorbents. Materials 2025, 18, 2280. https://doi.org/10.3390/ma18102280
Wei S, Fan Z, Zhang S, Xiao Y, Wang C, Peng S, Zhang X. Comparative Assessment of Hygroscopic Properties and Thermal Performance of Activated Carbon-Based Physical Adsorbents and Advanced Composite Adsorbents. Materials. 2025; 18(10):2280. https://doi.org/10.3390/ma18102280
Chicago/Turabian StyleWei, Siyu, Zhengpeng Fan, Songyu Zhang, Yutong Xiao, Chunhao Wang, Shanbi Peng, and Xueying Zhang. 2025. "Comparative Assessment of Hygroscopic Properties and Thermal Performance of Activated Carbon-Based Physical Adsorbents and Advanced Composite Adsorbents" Materials 18, no. 10: 2280. https://doi.org/10.3390/ma18102280
APA StyleWei, S., Fan, Z., Zhang, S., Xiao, Y., Wang, C., Peng, S., & Zhang, X. (2025). Comparative Assessment of Hygroscopic Properties and Thermal Performance of Activated Carbon-Based Physical Adsorbents and Advanced Composite Adsorbents. Materials, 18(10), 2280. https://doi.org/10.3390/ma18102280







