Fluid-Dynamic Crestal Sinus Floor Elevation in Atrophic Posterior Maxilla Implant Rehabilitation with Hyaluronic Acid: A Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
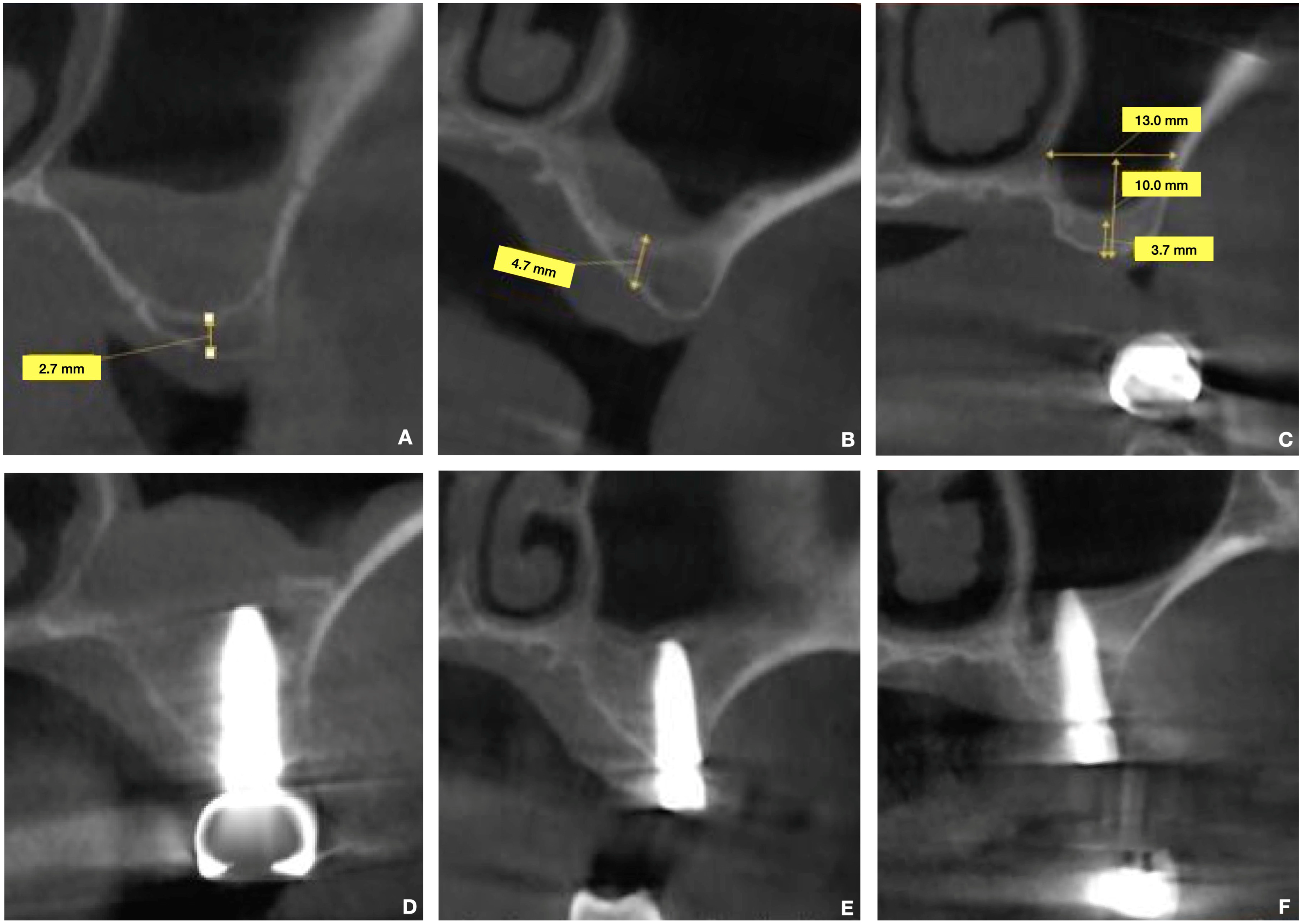
Radiological Examination (CBCT)
- Residual bone height (RBH): The vertical distance from the crest of the edentulous ridge to the floor of the maxillary sinus was measured at the intended implant sites.
- Residual bone thickness: The buccolingual width of the alveolar ridge at the intended implant sites was assessed.
- Maxillary sinus width: On cross-sectional CBCT images, the width of the maxillary sinus was measured at a point 10 mm apical to the alveolar crest. This measurement encompassed the distance between the buccal and medial cortices of the maxillary sinus.
- Sinus membrane thickness: While noted, the primary focus for linear measurements was bone dimensions.
3. Surgical Protocol
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyne, P.J.; James, R.A. Grafting of the maxillary sinus floor with autogenous marrow and bone. J. Oral Surg. 1980, 38, 613–616. [Google Scholar] [PubMed]
- Danesh-Sani, S.A.; Wallace, S.S.; Movahed, A.; El Chaar, E.S.; Cho, S.C.; Khouly, I.; Testori, T. Maxillary Sinus Grafting with Biphasic Bone Ceramic or Autogenous Bone: Clinical, Histologic, and Histomorphometric Results from a Randomized Controlled Clinical Trial. Implant Dent. 2016, 25, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Stacchi, C.; Andolsek, F.; Berton, F.; Perinetti, G.; Navarra, C.O.; Di Lenarda, R. Intraoperative complications during sinus floor elevation with lateral approach: A systematic review. Int. J. Oral Maxillofac. Implant. 2017, 32, e107–e118. [Google Scholar] [CrossRef]
- Stacchi, C.; Coyac, B.R.; Helms, J.A. Biomechanical basis for bone healing and osseointegration of implants in sinus grafts. Clin. Implant Dent. Relat. Res. 2025, 27, e13424. [Google Scholar] [CrossRef] [PubMed]
- Valentini, P.; Stacchi, C. Prevention and management of intraoperative complications in maxillary sinus augmentation: A review. Clin. Implant Dent. Relat. Res. 2025, 27, e13397. [Google Scholar] [CrossRef]
- Wallace, S.S.; Froum, S.J. Effect of maxillary sinus augmentation on the survival of endosseous dental implants: A systematic review. Ann. Periodontol. 2003, 8, 328–343. [Google Scholar] [CrossRef]
- Scarano, A.; Cappucci, C.; Rapone, B.; Bugea, C.; Lorusso, F.; Serra, P.; Di Carmine, M.S. Volumetric evaluations of the maxillary sinus before and post regenerative surgery. Eur. Rev. Med. Pharmacol. Sci. 2023, 27 (Suppl. S3), 128–134. [Google Scholar] [PubMed]
- Tatum, H., Jr. Maxillary and sinus implant reconstructions. Dent. Clin. N. Am. 1986, 30, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Summers, R.B. A new concept in maxillary implant surgery: The osteotome technique. Compend. 1994, 15, 152, 154–156. [Google Scholar]
- Summers, R.B. The osteotome technique: Part 4—Future site development. Compend. Contin. Educ. Dent. 1995, 16, 1090, 1092 passim; 1094–1096, 1098, quiz 1099. [Google Scholar]
- Davarpanah, M.; Martinez, H.; Tecucianu, J.F.; Hage, G.; Lazzara, R. The modified osteotome technique. Int. J. Periodontics Restorative Dent. 2001, 21, 599–607. [Google Scholar]
- Pjetursson, B.E.; Lang, N.P. Sinus floor elevation utilizing the transalveolar approach. Periodontol. 2000 2014, 66, 59–71. [Google Scholar] [CrossRef]
- Vivek, G.K.; Ahmed, N.; Shetty, A.; Vaibhav, N.; Imran, M.; Umeshappa, H. Complications of conventional sinus augmentation techniques versus modified osteotome techniques in dental implant surgery: A 3-year retrospective clinical study. J. Maxillofac. Oral Surg. 2023, 22, 287–295. [Google Scholar]
- Farina, R.; Franceschetti, G.; Travaglini, D.; Consolo, U.; Minenna, L.; Schincaglia, G.P.; Riccardi, O.; Bandieri, A.; Maietti, E.; Trombelli, L. Morbidity following transcrestal and lateral sinus floor elevation: A randomized trial. J. Clin. Periodontol. 2018, 45, 1128–1139. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, D. Labyrinthine concussion and positional vertigo after osteotome site preparation. Implant Dent. 2004, 13, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Zadrożny, Ł.; Wagner, L.; Rosenbach, D. Minimally invasive transcrestal sinus floor elevation procedure in severely atrophic ridge: A case report. J. Oral Implantol. 2021, 47, 215–222. [Google Scholar] [CrossRef]
- Scarano, A.; Luongo, R.; Rampino, M.; Pedulla, E.; Bugea, C. Fluid dynamic transcrestal sinus floor elevation using a new surgical instrument, Flusilift, and hyaluronic acid as only biomaterial: A case report. J. Biomed. Res. Environ. Sci. 2021, 2, 1267–1273. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Andreasi Bassi, M.; Lopez, M.A.; Confalone, L.; Carinci, F. Hydraulic sinus lift technique in future site development: Clinical and histomorphometric analysis of human biopsies. Implant Dent. 2015, 24, 117–124. [Google Scholar] [CrossRef]
- Pommer, B.; Watzek, G. Gel-pressure technique for flapless transcrestal maxillary sinus floor elevation: A preliminary cadaveric study of a new surgical technique. Int. J. Oral Maxillofac. Surg. 2009, 24, 817–822. [Google Scholar]
- Pjetursson, B.E.; Rast, C.; Brägger, U.; Schmidlin, K.; Zwahlen, M.; Lang, N.P. Maxillary sinus floor elevation using the (transalveolar) osteotome technique with or without grafting material. Part I: Implant survival and patients’ perception. Clin. Oral Implant. Res. 2009, 20, 667–676. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Altairi, N.H.; Abotaleb, B.; Al-Iryani, G.; Halboub, E.; Alakhali, M.S. What is the most effective rehabilitation method for posterior maxillas with 4 to 8 mm of residual alveolar bone height below the maxillary sinus with implant-supported prostheses? A frequentist network meta-analysis. J. Oral Maxillofac. Surg. 2019, 77, 70.e1–70.e33. [Google Scholar] [CrossRef]
- Sotirakis, E.G.; Gonshor, A. Elevation of the maxillary sinus floor with hydraulic pressure. J. Oral Implantol. 2005, 31, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, S.; Andersson, S.; Gualini, F.; Sennerby, L. Bone reformation with sinus membrane elevation: A new surgical technique for maxillary sinus floor augmentation. Clin. Implant Dent. Relat. Res. 2004, 6, 165–173. [Google Scholar] [CrossRef]
- Yan, M.; Liu, R.; Bai, S.; Wang, M.; Xia, H.; Chen, J. Transalveolar sinus floor lift without bone grafting in atrophic maxilla: A meta-analysis. Sci. Rep. 2018, 8, 1451. [Google Scholar] [CrossRef]
- Borges, F.L.; Dias, R.O.; Piattelli, A.; Onuma, T.; Gouveia Cardoso, L.A.; Salomão, M.; Scarano, A.; Ayub, E.; Shibli, J.A. Simultaneous sinus membrane elevation and dental implant placement without bone graft: A 6-month follow-up study. J. Periodontol. 2011, 82, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Degidi, M.; Iezzi, G.; Pecora, G.; Piattelli, M.; Orsini, G.; Caputi, S.; Perrotti, V.; Mangano, C.; Piattelli, A. Maxillary sinus augmentation with different biomaterials: A comparative histologic and histomorphometric study in man. Implant Dent. 2006, 15, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Luongo, R.; Sgaramella, N.; Traini, T.; Bugea, C. Graftless maxillary sinus floor augmentation with simultaneous porcine bone layer insertion: A 1- to 5-year follow-up study. Int. J. Oral Maxillofac. Implant. 2020, 35, 808–815. [Google Scholar] [CrossRef] [PubMed]
- Ting, M.; Rice, J.G.; Braid, S.M.; Lee, C.Y.S.; Suzuki, J.B. Maxillary sinus augmentation for dental implant rehabilitation of the edentulous ridge: A comprehensive overview of systematic reviews. Implant Dent. 2017, 26, 438–446. [Google Scholar] [CrossRef]
- Cinar, I.C.; Zboun, M.; Gultekin, B.A.; Saglanmak, A.; Akay, A.S. Retrospective analysis of three different xenografts in maxillary sinus augmentation: Histologic and three-dimensional radiologic study. Quintessence Int. 2023, 54, 640–649. [Google Scholar]
- Scarano, A.; Piattelli, M.; Iezzi, G.; Mangano, F.G. Maxillary sinus augmentation with different biomaterials: A 5-year retrospective clinical and histological study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 29–37. [Google Scholar]
- Scarano, A.; Cholakis, A.K.; Piattelli, A. Histologic evaluation of sinus grafting materials after peri-implantitis-induced failure: A case series. Int. J. Oral Maxillofac. Implant. 2017, 32, e69–e75. [Google Scholar] [CrossRef] [PubMed]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic acid: Redefining its role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Zhang, L.T.; Liu, R.M.; Luo, Y.; Zhao, Y.J.; Chen, D.X.; Yu, C.Y.; Xiao, J.H. Hyaluronic acid promotes osteogenic differentiation of human amniotic mesenchymal stem cells via the TGF-beta/Smad signalling pathway. Life Sci. 2019, 232, 116669. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Chen, J.; Wang, R.; Hou, D.; Wu, G.; Liu, C.; Pathak, J.L. The paracrine effect of hyaluronic acid-treated endothelial cells promotes BMP-2-mediated osteogenesis. Bioengineering 2023, 10, 1227. [Google Scholar] [CrossRef]
- Sasaky, T.; Watanabe, C. Stimulation of osteoinduction in bound wound healing by high-molecular hyaluronic acid. Bone 1995, 16, 9–15. [Google Scholar] [CrossRef]
- Huang, L.; Cheng, Y.Y.; Koo, P.L.; Lee, K.M.; Qin, L.; Cheng, J.C.; Kumta, S.M. The effect of hyaluronan on osteoblast proliferation and differentiation in rat calvarial-derived cell cultures. J. Biomed. Mater. Res. A 2003, 66, 880–884. [Google Scholar] [CrossRef]
- Kablik, J.; Monheit, G.D.; Yu, L.; Chang, G.; Gershkovich, J. Comparative physical properties of hyaluronic acid dermal fillers. Dermatol. Surg. 2009, 35 (Suppl. S1), 302–312. [Google Scholar] [CrossRef]
- Kauffmann, F.; Fickl, S.; Sculean, A.; Fischer, K.R.; Friedmann, A. Alveolar ridge alterations after lateral guided bone regeneration with and without hyaluronic acid: A prospective randomized trial with morphometric and histomorphometric evaluation. Quintessence Int. 2023, 54, 712–722. [Google Scholar]
- D’Albis, G.; D’Albis, V.; Palma, M.; Plantamura, M.; Nizar, A.K. Use of hyaluronic acid for regeneration of maxillofacial bones. Genesis 2022, 60, e23497. [Google Scholar] [CrossRef]
- Zou, X.; Li, H.; Chen, L.; Baatrup, A.; Bünger, C.; Lind, M. Stimulation of porcine bone marrow stromal cells by hyaluronan, dexamethasone and rhBMP-2. Biomaterials 2004, 25, 5375–5385. [Google Scholar] [CrossRef]
- Zhai, P.; Peng, X. The application of hyaluronic acid in bone regeneration. Int. J. Biol. Macromol. 2020, 148, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lee, J. The optimal dosage of hyaluronic acid for bone regeneration in rat calvarial defects. J. Periodontal Implant Sci. 2023, 53, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, A.; Jung, R.; Bilhan, H.; Ghawi-Begovic, H.A.; Kauffmann, F.; Diehl, D. Reconstructive surgical therapy of peri-implant defects with ribose cross-linked collagen matrix and crosslinked hyaluronic acid—A prospective case series. Clin. Oral Investig. 2024, 28, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Alcântara, C.; Castro, M. Hyaluronic acid accelerates bone repair in human dental sockets: A randomized triple-blind clinical trial. Braz. Oral Res. 2018, 32, e84. [Google Scholar] [CrossRef]
- Abaza, G.; Abdel Gaber, H.K. Injectable platelet rich fibrin versus hyaluronic acid with bovine derived xenograft for alveolar ridge preservation. A randomized controlled clinical trial with histomorphometric analysis. Clin. Implant Dent. Relat. Res. 2024, 26, 88–102. [Google Scholar] [CrossRef]
- Božić, D.; Ćatović, I. Treatment of intrabony defects with a combination of hyaluronic acid and deproteinized porcine bone mineral. Materials 2021, 14, 6795. [Google Scholar] [CrossRef]
- Gurbuz, E.; Dursun, E. Microcomputed tomographic analysis of bone microarchitecture after sinus augmentation with hyaluronic matrix: A case-control study. Oral Maxillofac. Surg. 2022, 26, 431–437. [Google Scholar] [CrossRef]
- Kim, J.; Ben Amara, H.; Park, J.; Kim, S.; Kim, T.; Seol, Y.; Lee, Y.; Ku, Y.; Rhyu, I.; Koo, K. Biomodification of compromised extraction sockets using hyaluronic acid and rhBMP-2: An experimental study in dogs. J. Periodontol. 2019, 90, 416–424. [Google Scholar] [CrossRef]
- Song, D.S.; Kim, C.H.; Kim, B.J.; Kim, J.H. Tenting effect of dental implant on maxillary sinus lift without grafting. J. Dent. Sci. 2020, 15, 278–285. [Google Scholar] [CrossRef]
- Stacchi, C.; Spinato, S.; Lombardi, T.; Bernardello, F.; Bertoldi, C.; Zaffe, D.; Nevins, M. Minimally invasive management of implant-supported rehabilitation in the posterior maxilla, Part I. Sinus floor elevation: Biologic principles and materials. Int. J. Periodontics Restor. Dent. 2020, 40, e85–e93. [Google Scholar] [CrossRef]
- Dogan, E.; Dursun, E.; Tosun, E.; Bilgic, E.; Akman, A.C.; Orhan, K.; Celik, H.H.; Korkusuz, P.; Caglayan, F. Evaluation of hyaluronic matrix efficacy in sinus augmentation: A randomized-controlled histomorphometric and micro-computed tomography analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Rapone, B.; Amuso, D.; Inchingolo, F.; Lorusso, F. Hyaluronic acid fillers enriched with glycine and proline in eyebrow augmentation procedure. Aesthetic Plast. Surg. 2022, 46, 419–428. [Google Scholar] [CrossRef]
- Scarano, A.; Qorri, E.; Sbarbati, A.; Gehrke, S.A.; Frisone, A.; Amuso, D.; Tari, S.R. The efficacy of hyaluronic acid fragments with amino acid in combating facial skin aging: An ultrasound and histological study. J. Ultrasound 2024, 27, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Chavda, S.; Rabbani, S.A.; Wadhwa, T. Role and effectiveness of intra-articular injection of hyaluronic acid in the treatment of knee osteoarthritis: A systematic review. Cureus 2022, 14, e24503. [Google Scholar] [CrossRef] [PubMed]
- Brunel, G.; Piantoni, P.; Piotrowski, B.; Baysse, E. Action of hyaluronic acid on the wound healing following extraction. Dent. Inform. 2004, 7, 385–391. [Google Scholar]
- Scarano, P.; Tari, S.R.; Di Nardo Di Maio, F.; Di Carmine, M.; Scarano, A. Hyaluronic acid enriched with amino acid used to fill bone defect after cyst nasopalatine enucleation: A case report. Ann. Stomatol. 2023, 3, 19–23. [Google Scholar]
- Pilloni, A.; Marini, L.; Gagliano, N.; Canciani, E.; Dellavia, C.; Cornaghi, L.B.; Costa, E.; Rojas, M.A. Clinical, histological, immunohistochemical, and biomolecular analysis of hyaluronic acid in early wound healing of human gingival tissues: A randomized, split-mouth trial. J. Periodontol. 2023, 94, 868–888. [Google Scholar] [CrossRef]
- Elnawam, H.; Thabet, A.; Mobarak, A.; Khalil, N.M.; Abdallah, A.; Nouh, S.; Elbackly, R. Bovine pulp extracellular matrix hydrogel for regenerative endodontic applications: In vitro characterization and in vivo analysis in a necrotic tooth model. Head Face Med. 2024, 20, 61. [Google Scholar] [CrossRef]
- Miglani, A.; Vishnani, R.; Reche, A.; Buldeo, J.; Wadher, B. Hyaluronic acid: Exploring its versatile applications in dentistry. Cureus 2023, 15, e46349. [Google Scholar] [CrossRef]


| Category | Value |
|---|---|
| Total Implants | 58 |
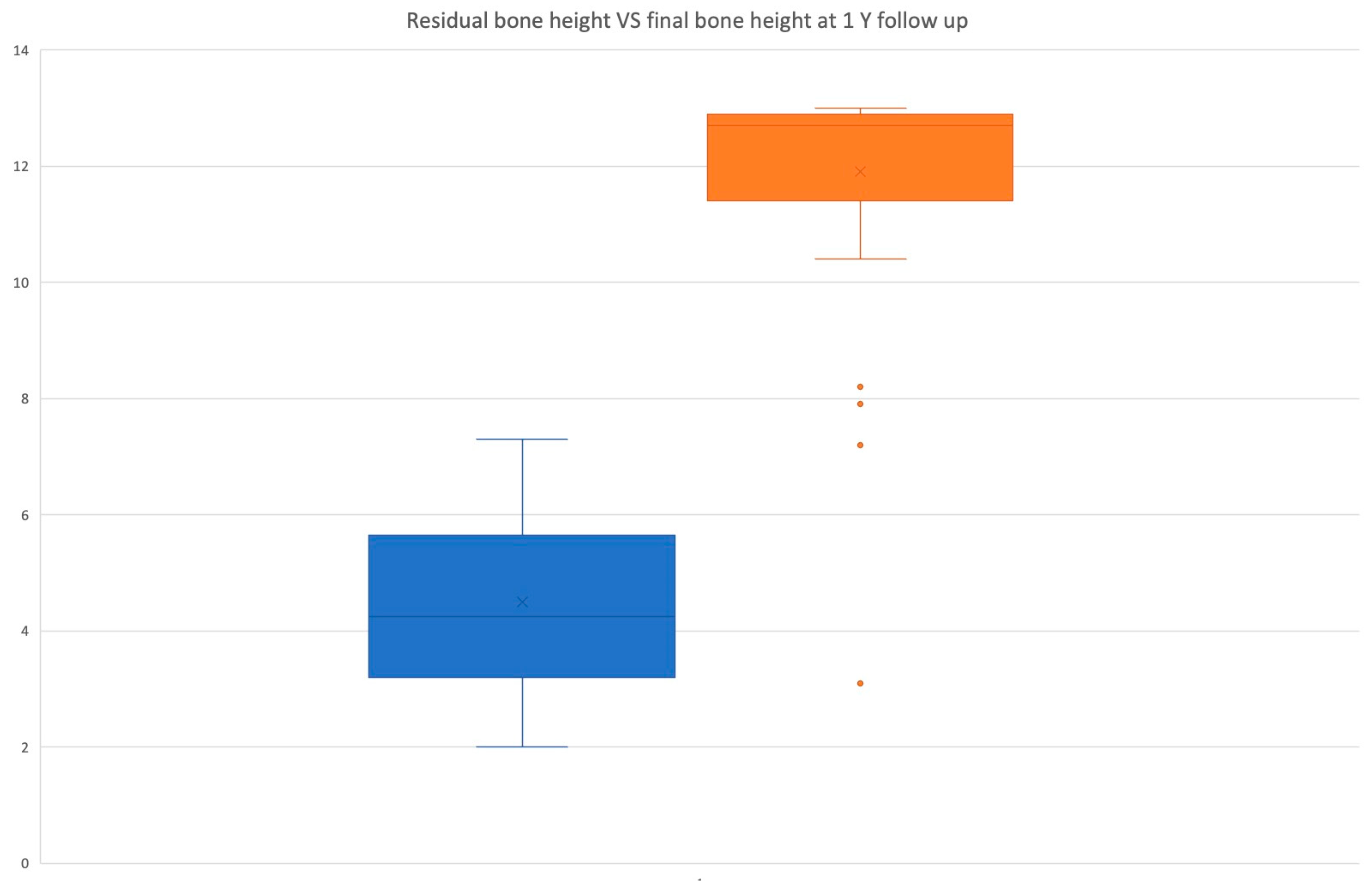
| Residual Bone Height (RBH) | 4.5 ± 1.4 mm |
| Medial-Lateral Wall Distance | 12.46 ± 2.6 mm |
| Residual Bone Thickness | 7.8 ± 1.2 mm |
| Sinus Membrane Thickness | 3.5 ± 3.3 mm |
| Torque (Primary Stability) | 35–50 Ncm |
| Osstell Value (ISQ) | 71.4 ± 2.79 |
| Final Bone Height | 12.05 ± 1.2 mm |
| Bone Gain | 7.5 ± 1.77 mm |
| Implant Protrusion Length (IPL) | 8.5 ± 1.4 mm |
| Regeneration Difference | 0.9 ± 1.2 mm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scarano, A.; Luongo, R.; De Filippis, I.; Scarano, A.; Qorri, E.; Sforza, F.; Rampino, M.; Bugea, C. Fluid-Dynamic Crestal Sinus Floor Elevation in Atrophic Posterior Maxilla Implant Rehabilitation with Hyaluronic Acid: A Prospective Study. Materials 2025, 18, 2230. https://doi.org/10.3390/ma18102230
Scarano A, Luongo R, De Filippis I, Scarano A, Qorri E, Sforza F, Rampino M, Bugea C. Fluid-Dynamic Crestal Sinus Floor Elevation in Atrophic Posterior Maxilla Implant Rehabilitation with Hyaluronic Acid: A Prospective Study. Materials. 2025; 18(10):2230. https://doi.org/10.3390/ma18102230
Chicago/Turabian StyleScarano, Alessandro, Roberto Luongo, Ilaria De Filippis, Antonio Scarano, Erda Qorri, Francesco Sforza, Mario Rampino, and Calogero Bugea. 2025. "Fluid-Dynamic Crestal Sinus Floor Elevation in Atrophic Posterior Maxilla Implant Rehabilitation with Hyaluronic Acid: A Prospective Study" Materials 18, no. 10: 2230. https://doi.org/10.3390/ma18102230
APA StyleScarano, A., Luongo, R., De Filippis, I., Scarano, A., Qorri, E., Sforza, F., Rampino, M., & Bugea, C. (2025). Fluid-Dynamic Crestal Sinus Floor Elevation in Atrophic Posterior Maxilla Implant Rehabilitation with Hyaluronic Acid: A Prospective Study. Materials, 18(10), 2230. https://doi.org/10.3390/ma18102230









