Modulating Heat Input to Optimize Corrosion Resistance of Nickel–Aluminum Bronze Manufactured by Cold Metal Transfer Additive Manufacturing
Abstract
:1. Introduction
2. Experiments and Methods
2.1. Materials and Specimen Fabrication
2.2. Material Characterization
2.3. Microhardness Tests
2.4. Electrochemical Experiment
3. Results and Discussion
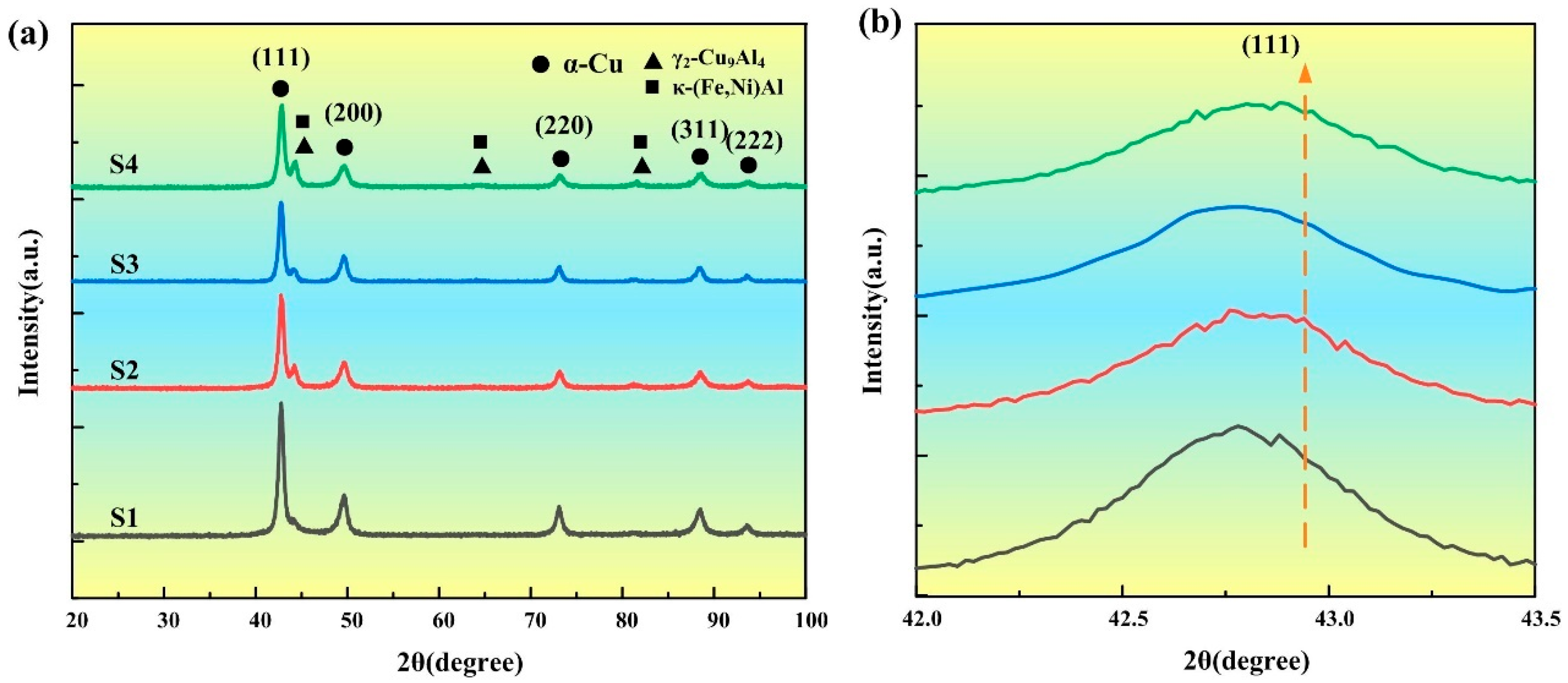
3.1. Phase Composition
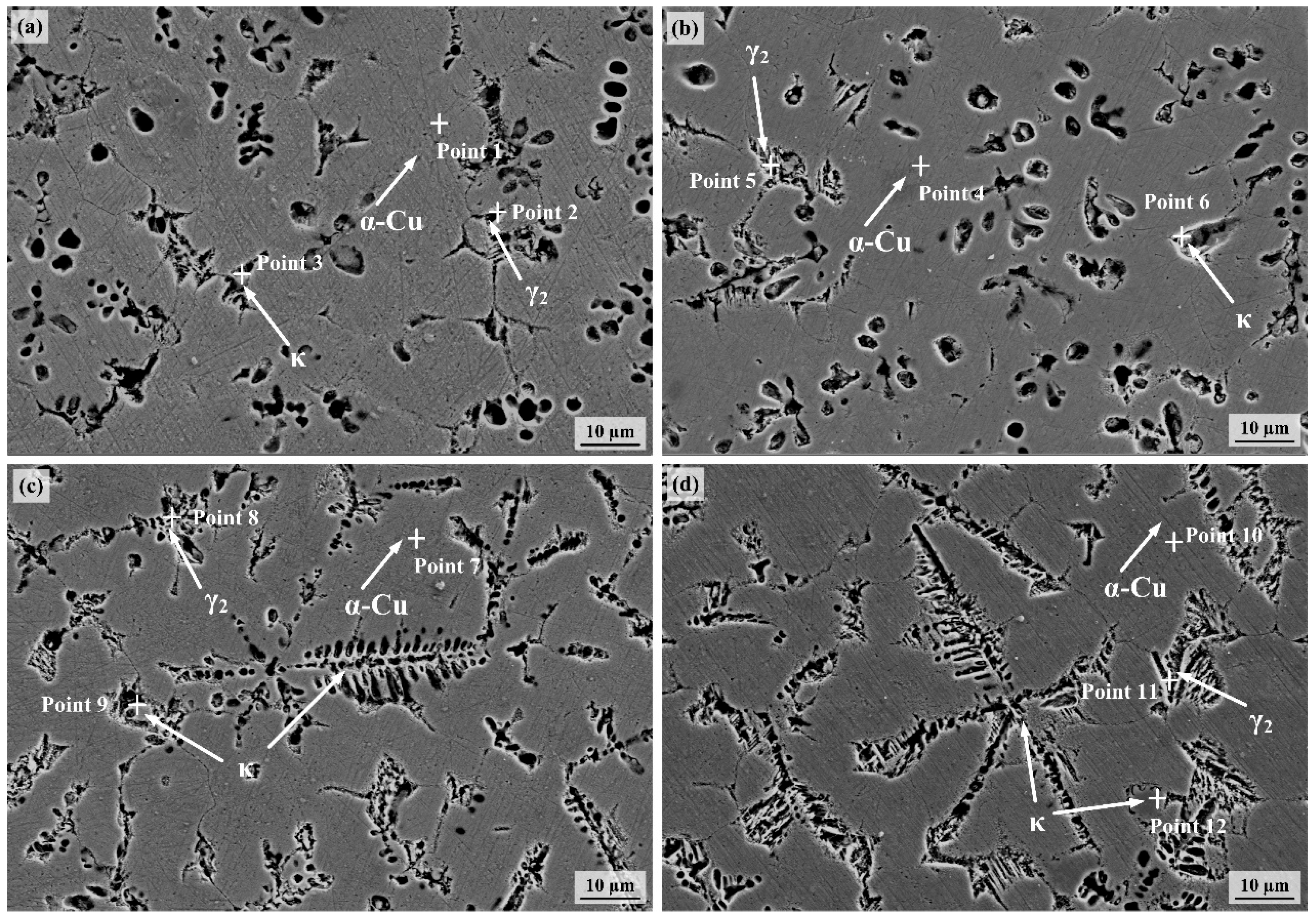
3.2. Microstructure Analysis
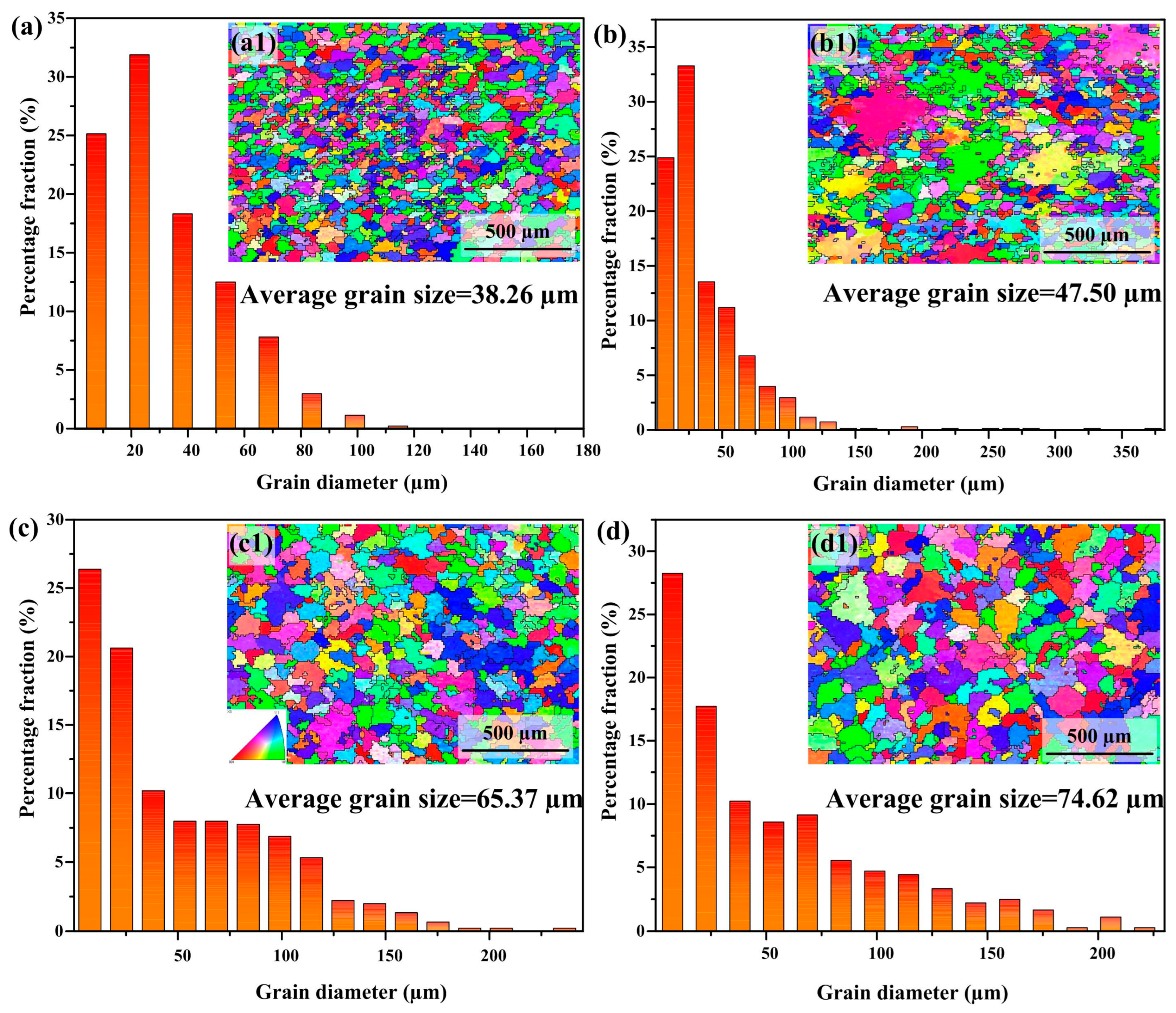
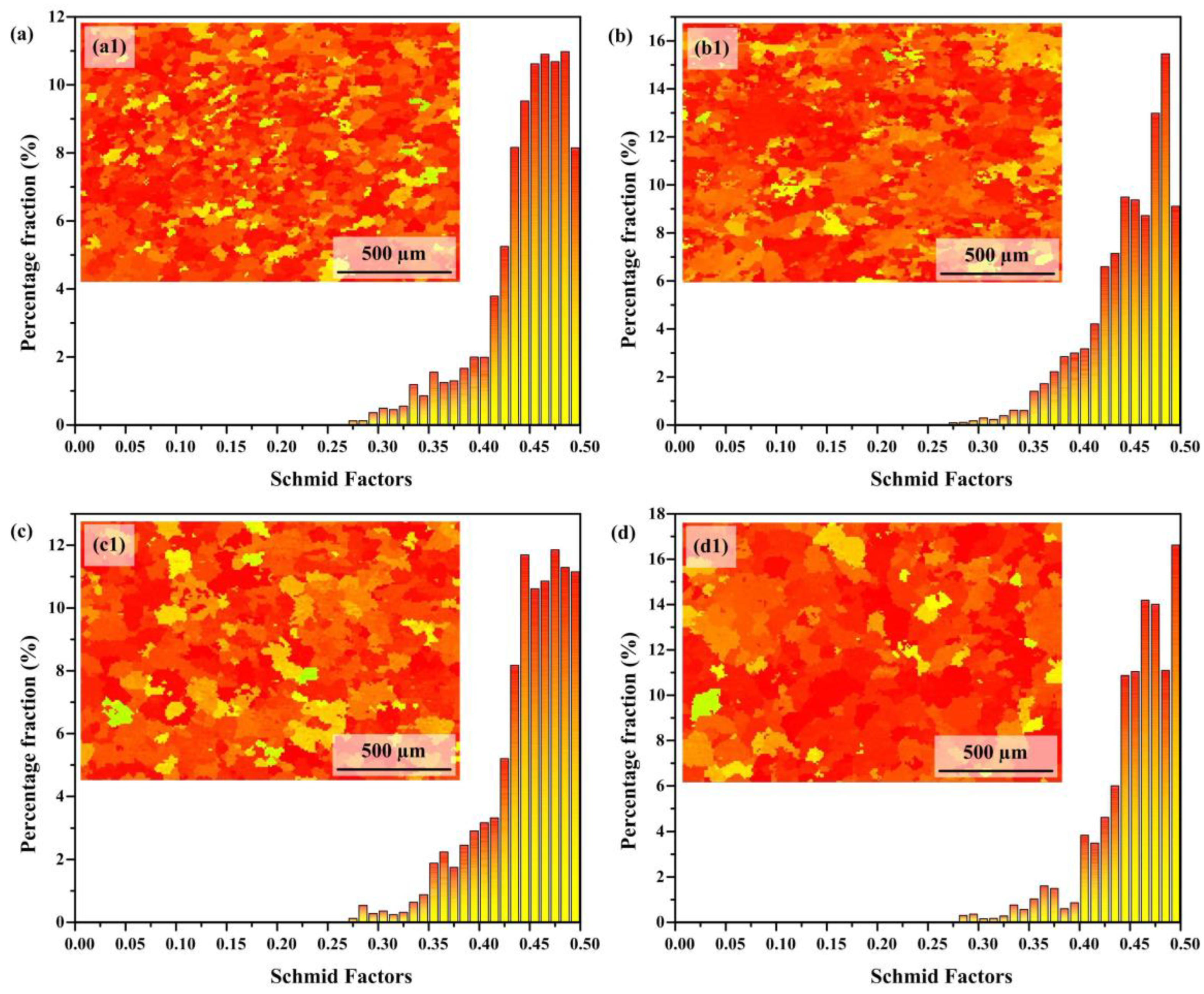
3.3. Electron Backscatter Diffraction Analysis
3.4. Microhardness
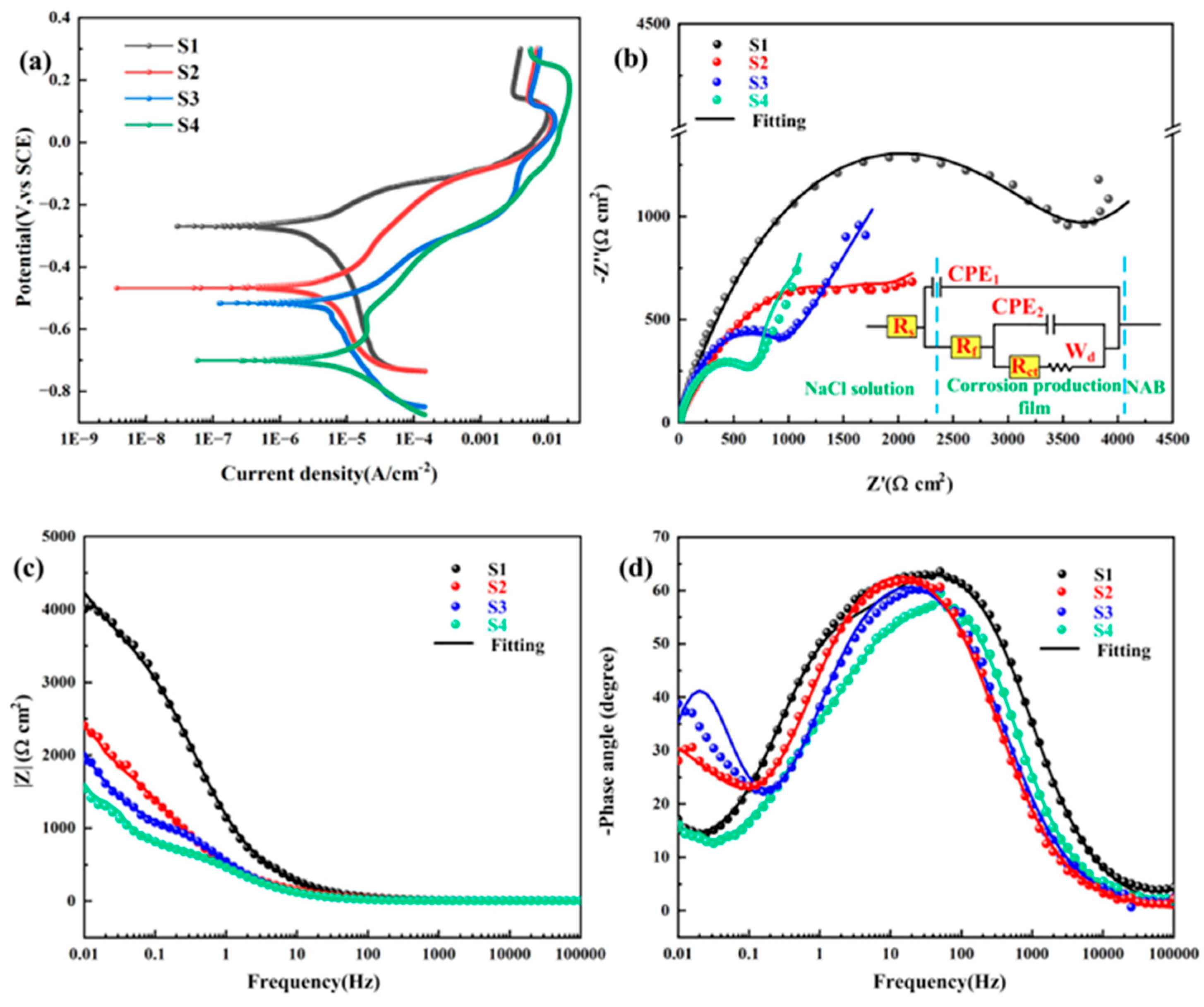
3.5. Corrosion Behavior
4. Conclusions
- (1)
- The NAB deposit layer is mainly composed of α-Cu, accompanied by a small amount of γ2-Cu9Al4 and κ phase, with varying degrees of lattice distortion. As the heat input gradually increases, the κ phase gradually coarsens.
- (2)
- As the heat input increases, both the crystalline domain dimensions and the crystallographic orientation parameter exhibit a proportional rise. The average grain size increases from 38.26 μm to 74.62 μm. The Schmidt factor increases from 0.41 to 0.47. The alloy phase gradually softens. When the heat input is 243.8 J/mm, the grain size is the smallest and the value of the Schmidt factor is the smallest.
- (3)
- The variation in grain size and the κ-phase precipitation strengthening mechanism exert a governing effect on the microhardness evolution within the as-deposited layers. The average microhardness decreased from 192.4 ± 6.9 HV to 166.9 ± 0.3 HV with the increase in input. The coarsening of grains and κ phase, as well as their uneven distribution, significantly reduced the microhardness.
- (4)
- With the increase in heat input, the corrosion potential decreased significantly from −269.61 ± 40 mV to −700.94 ± 15 mV, while the corrosion current density rose markedly from 2.56 ± 0.04 µA/cm2 to 7.52 ± 0.07 µA/cm2. The deterioration of corrosion-resistant properties is predominantly governed by two predominant mechanisms: (1) grain coarsening; and (2) the coarsening of precipitated phases, which induces Al segregation and consequently degrades the protective performance of the Al2O3 film.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, W.; Chen, Y.H.; Zhang, T.M.; Wen, T.T.; Feng, X.S.; Yin, L.M. Effects of Location on the Microstructure and Mechanical Properties of Cu-8Al-2Ni-2Fe-2Mn Alloy Produced Through Wire Arc Additive Manufacturing. J. Mater. Eng. Perform. 2020, 29, 4733–4744. [Google Scholar] [CrossRef]
- Li, Y.; Lian, Y.; Sun, Y.J. Cavitation Erosion Behavior of Friction Stir Processed Nickel Aluminum Bronze. J. Alloys Compd. 2019, 795, 233–240. [Google Scholar] [CrossRef]
- Cai, X.; Chang, S.K.; Yang, M.M.; Li, S.J.; Wang, X.; Qiao, Y.X.; Zhou, J.; Xue, F. From cast to wire-arc DED: An investigation on NAB alloy MIC resistance. Surf. Coat. Technol. 2025, 251, 112910. [Google Scholar] [CrossRef]
- Rosalbino, F.; Carlini, R.; Soggia, F.; Zanicchi, G.; Scavino, G. Influence of rare earth metals addition on the corrosion behaviour of copper in alkaline environment. Corros. Sci. 2012, 58, 139–144. [Google Scholar] [CrossRef]
- Ding, Y.; Zhao, R.; Qin, Z.B.; Wu, Z.; Wang, L.Q.; Liu, L.; Lu, W.J. Evolution of the Corrosion Product Film on Nickel-Aluminum Bronze and Its Corrosion Behavior in 3.5 wt % NaCl Solution. Materials 2019, 12, 209. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, K.M.; Rayner, A.J.; Bishop, D.P.; Nasiri, A. Impact of fabrication method on the corrosion behavior of heat-treated nickel aluminum bronze (NAB) alloy: A comparison of laser powder bed fusion (L-PBF) and laser directed energy deposition (L-DED) techniques. Int. J. Electrochem. Sci. 2025, 708, 136069. [Google Scholar]
- Alam, S.; Marshall, R.I.; Sasaki, S. Metallurgical and tribological investigations of aluminum bronze bushes made by a novel centrifugal casting technique. Tribol. Int. 1996, 29, 487–492. [Google Scholar] [CrossRef]
- Feldshtein, E.E.; Devojno, O.; Kardapolava, M.; Lutsko, N.; Patalas-Maliszewska, J. On the Features of Composite Coating, Based on Nickel Alloy and Aluminum-Iron Bronze, Processed by Direct Metal Deposition. Materials 2021, 14, 957. [Google Scholar] [CrossRef]
- Cong, B.; Ouyang, R.; Qi, B.; Ding, J.L. Influence of cold metal transfer process and its heat input on weld bead geometry and porosity of aluminum-copper alloy welds. Rare Met. Mater. Eng. 2016, 45, 606–611. [Google Scholar] [CrossRef]
- Müller, V.; Fasselt, J.M.; Klötzer-Freese, C.; Kruse, T.; Kleba-Ehrhardt, R.; Biegler, M.; Rethmeier, M. Recycling Nickel Aluminium Bronze Grinding Chips to Feedstock for Directed Energy Deposition via Impact Whirl Milling: Investigation on Processability, Microstructure and Mechanical Properties. Addit. Manuf. 2025, 105, 104804. [Google Scholar] [CrossRef]
- Yang, J.; Wu, F.; Bai, B.; Wang, G.S.; Yang, L.; Zhou, S.F.; Lei, J.B. Effect of Cr additions on the microstructure and corrosion resistance of Diode laser clad CuAl10 coating. Surf. Coat. Technol. 2020, 381, 125215. [Google Scholar] [CrossRef]
- Dana, M.; Zetkov, I.; Hanzl, P. The influence of a ceramic recoater blade on 3D printing using direct metal laser sintering. Manuf. Tech. 2019, 19, 23–28. [Google Scholar]
- Utyaganova, V.; Vorontsov, A.; Gurianov, D.; Shamarin, N.; Chumaevskii, A.; Rubtsov, K.; Savchenko, N.; Tarasov, S. Effect of Mg admixing on strength and corrosion of electron beam additive manufactured AlSi12 on the AA5056 substrate. Mater. Charact. 2023, 204, 113172. [Google Scholar] [CrossRef]
- Lv, J.M.; Alexandrov, L.V.; Luo, K.Y.; Lu, H.F.; Lu, J.Z. Microstructural evolution and anisotropic regulation in tensile property of cold metal transfer additive manufactured Ti6Al4V alloys via ultrasonic impact treatment. Mater. Sci. Eng. A 2022, 859, 144177. [Google Scholar] [CrossRef]
- Gou, J.; Wang, Z.J.; Hu, S.S.; Shen, J.Q.; Tian, Y.B.; Zhao, G.C.; Chen, Y.Q. Effects of trace Nb addition on microstructure and properties of Ti–6Al–4V thin-wall structure prepared via cold metal transfer additive manufacturing. J. Alloys Compd. 2020, 829, 154481. [Google Scholar] [CrossRef]
- Chen, Z.W.; Liang, Y.N.; Xu, C.; Zhang, X.Y.; Fan, J.K.; Liu, J.; Kong, J.; Wang, K.H.; Peng, Y. Improving the product of strength and ductility and corrosion resistance by adding He shielding gas in the CMT additive manufacturing process of Ti6Al4V. J. Alloys Compd. 2024, 1005, 176078. [Google Scholar] [CrossRef]
- Dharmendra, C.; Hadadzadeh, A.; Amirkhiz, B.S.; Janaki Ram, G.D.; Mohammadi, M. Microstructural evolution and mechanical behavior of nickel aluminum bronze Cu-9Al-4Fe-4Ni-1Mn fabricated through wire-arc additive manufacturing. Addit. Manuf. 2019, 30, 100872. [Google Scholar] [CrossRef]
- Aliyu, A.; Bishop, D.P.; Nasiri, A. Effect of heat treatment on microstructural evolution and corrosion behavior of wirearc additive manufactured nickel aluminum bronze alloy. Corros. Sci. 2025, 249, 112812. [Google Scholar] [CrossRef]
- Shen, C.; Pan, Z.X.; Ding, D.H.; Yuan, L.; Nie, N.; Wang, Y.; Luo, D.Z.; Cuiuri, D.; Duin, S.V.; Li, H.J. The influence of post-production heat treatment on the multi-directional properties of nickel-aluminum bronze alloy fabricated using wire-arc additive manufacturing process. Addit. Manuf. 2018, 23, 411–421. [Google Scholar] [CrossRef]
- Peng, Y.H.; Zhao, L.J.; Cui, X.Y.; Xiong, T.Y.; Wang, J.Q. Effect of heat treatment on the gas-atomized nickel-aluminum bronze feedstock powders for cold spray. Surf. Coat. Technol. 2024, 478, 130458. [Google Scholar] [CrossRef]
- Wang, D.C.; Tao, X.P.; Wang, X.G.; Zhang, R.; Zhou, Z.J.; Zhang, C.H.; Zhang, S.; Wu, C.L.; Sun, X.F.; Zhou, Y.Z.; et al. Normalizing temperature dependence on microstructure, mechanical and wear properties of a novel high-vanadium high-speed steel. Mater. Charact. 2025, 224, 115047. [Google Scholar] [CrossRef]
- Li, T.C.; Zhang, S.P.; Xie, M.; Song, X.L.; Lei, J.B. Directed energy deposition of aluminium bronze/GNPs composites: Microstructural evolution for enhanced wear resistance. Tribol. Int. 2024, 197, 109761. [Google Scholar] [CrossRef]
- Wu, H.; Wang, Z.Y.; Wang, M.S.; Wang, R.; Zhang, S.; Zhang, C.H.; Wu, C.L.; Chen, H.T.; Chen, J. Microstructure evolution, corrosion and corrosive wear properties of NbC-reinforced FeNiCoCr-based high entropy alloys coatings fabricated by laser cladding. Eng. Fail. Anal. 2025, 171, 109352. [Google Scholar] [CrossRef]
- Marginean, G.; Utu, D. Microstructure refinement and alloying of WC–CoCr coatings by electron beam treatment. Surf. Coat. Technol. 2010, 205, 1985–1989. [Google Scholar] [CrossRef]
- Zhang, R.; Tao, X.P.; Wang, X.G.; Zhang, R.; Zhang, C.H.; Zhang, S.; Wu, C.L.; Sun, X.F.; Zhou, Y.Z.; Cui, C.Y. Effect of tempering temperature on the microstructure, mechanical and wear failure behaviours of a novel high vanadium high-speed steel. Steel Res. Int. 2025; early view. [Google Scholar] [CrossRef]
- Yang, Q.; Zhao, L.R. Characterization of nano-layered multilayer coatings using modified Bragg law. Mater. Charact. 2008, 59, 1285–1291. [Google Scholar] [CrossRef]
- Huang, S.W.; Zhou, P.F.; Luo, F.X.; Chen, H.M.; Xie, W.B.; Zhang, W.J.; Wang, H.; Yang, B.; Peng, B.F. Effects of Ni and Mn contents on precipitation and strengthening behavior in Cu-Ni-Mn ternary alloys. Mater. Charact. 2023, 199, 112775. [Google Scholar] [CrossRef]
- Svirid, A.E.; Pushin, V.G.; Kuranova, N.N.; Luk’yanov, A.V.; Pushin, A.; Uksusnikov, A.N.; Ustyugov, Y.M. The structure–phase transformations and mechanical properties of the shape memory effect alloys based on the system Cu-Al-Ni. Mater. Today Proc. 2017, 4, 4758–4762. [Google Scholar] [CrossRef]
- Zhuang, L.Z.; Buekenhout, L.; Duszczyk, J. Reaction phase-forming and mechanical properties of Fe3Al produced from elemental powders. Scr. Met. Mater. 1994, 7, 909–914. [Google Scholar] [CrossRef]
- Wen, J.; Cui, H.; Wei, N.; Song, X.; Zhang, G.; Wang, C.; Song, Q. Effect of phase composition and microstructure on the corrosion resistance of Ni-Al intermetallic compounds. J. Alloys Compd. 2017, 695, 2424–2433. [Google Scholar] [CrossRef]
- Zheng, K.X.; Yu, D.T.; Liu, J.L.; Wu, C.L.; Zhang, S.; Zhang, C.H.; Wang, Q.; Zhang, D. Laser cladding of FeCoCrNiTi high-entropy alloy coatings to modulate the microstructure and enhance the tribo-corrosion behavior on 304 stainless steel. Surf. Coat. Technol. 2025, 505, 132114. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Wang, M.S.; Wu, H.; Xu, T.Z.; Wu, C.L.; Zhang, C.H.; Zhang, S.; Chen, H.T.; Chen, J. Effect of aging treatment on cavitation erosion resistance and slurry erosion resistance of laser directed energy deposition martensitic aging steel. Mater. Today Commun. 2025, 46, 112588. [Google Scholar] [CrossRef]
- Jeng, Y.R.; Tsai, P.C.; Chiang, S.H. Effects of grain size and orientation on mechanical and tribological characterizations of nanocrystalline nickel films. Wear 2013, 303, 262–268. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, S.; Hu, Y.; Meng, Q.H.; Fu, B.; Zhao, Y.W.; Wu, C.L.; Li, G.M.; Qin, J.; Wang, J.P. Effects of welding current on microstructure evolution and mechanical behavior of WC-Co/X32 steel joints by resistance spot welding. Int. J. Refract. Met. Hard Mater. 2025, 128, 107048. [Google Scholar] [CrossRef]
- Varghese, P.; Vetrivendan, E.; Dash, M.K.; Ningshen, S.; Kamaraj, M.; Mudali, U.K. Weld overlay coating of Inconel 617 M on type 316 L stainless steel by cold metal transfer process. Surf. Coat. Technol. 2019, 357, 1004–1013. [Google Scholar] [CrossRef]
- Aliyu, A.; Bishop, D.P.; Nasiri, A. Effect of heat input on bead geometry and mechanical properties in wire arc additive manufacturing of a nickel aluminum bronze alloy. J. Mater. Res. Technol. 2024, 30, 8043–8053. [Google Scholar] [CrossRef]
- Cai, X.; Yang, M.M.; Qiao, Y.X.; Wang, Z.; Zhou, J.; Xue, F.X. Experimental investigation on wear resistance and corrosion behavior of nickel-aluminum bronze alloy fabricated by wire-arc additive manufacturing. J. Mater. Res. Technol. 2023, 26, 5801–5815. [Google Scholar] [CrossRef]
- Hasan, F.; Jahanafrooz, A.; Lorimer, G.W.; Ridley, N. The Morphology, Crystallography, and Chemistry of Phases in As-Cast Nickel-Aluminum Bronze. Metall. Trans. A 1982, 13, 1337. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, Y.F.; Liu, L.; Lv, W.; Hu, W. Effect of heat treatment on microstructure evolution and erosion–corrosion behavior of a nickel–aluminum bronze alloy in chloride solution. Corros. Sci. 2015, 98, 260–270. [Google Scholar] [CrossRef]
- Aung, N.N.; Zhou, W. Effect of grain size and twins on corrosion behaviour of AZ31B magnesium alloy. Corros. Sci. 2010, 52, 589–594. [Google Scholar] [CrossRef]
- Gao, M.; Jia, C.; Ni, D.; Luan, Y.; Ma, Z.J. Influence of microstructure modification on corrosion resistance of friction stir processing biodegradable Mg-Zn-Nd alloy. Mater. Technol. 2022, 37, 57–62. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.H.; Tu, X.H.; Li, W. The effects of subsurface microstructure evolution on fretting wear resistance of nickel-based alloy. Wear 2018, 416, 81–88. [Google Scholar] [CrossRef]
- Culpan, E.A.; Rose, G. Microstructural characterization of cast Nickel Aluminum bronze. J. Mater. Sci. 1978, 13, 1647–1657. [Google Scholar] [CrossRef]
- Wang, X.Y.; Li, D.Y. Mechanical and electrochemical behavior of nanocrystalline surface of 304 stainless steel. Electrochim. Acta 2002, 47, 3939–3947. [Google Scholar] [CrossRef]
- Tao, S.; Li, D.Y. Tribological, mechanical and electrochemical properties of nanocrystalline copper deposits produced by pulse electrodeposition. Nanotechnology 2006, 17, 65–78. [Google Scholar] [CrossRef]
- Elkedim, O.; Cao, H.S.; Meunier, C.; Gaffet, E. Preparation of nanocrystalline copper by hot and cold compaction: Characterization of mechanical and electrochemical properties. Mater. Sci. Forum. 1998, 269, 843–848. [Google Scholar] [CrossRef]
- Zhai, W.Z.; Zhao, Y.J.; Zhou, R.H.; Lu, W.L.; Zhai, W.C.; Liu, X.J.; Zhou, L.P.; Chang, S.P. Additively manufactured (Fe, Ni)Al-reinforced nickel aluminum bronze with nearly-isotropic mechanical properties in build and transverse directions. Mater. Charact. 2022, 184, 111706. [Google Scholar] [CrossRef]
- Jorcin, J.B.; Orazem, M.E.; Pébère, N.; Tribollet, B. CPE analysis by local electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 1473–1479. [Google Scholar] [CrossRef]
- El-Sherif, R.M.; Ismail, K.M.; Badawy, W.A. Effect of Zn and Pb as alloying elements on the electrochemical behavior of brass in NaCl solutions. Electrochim. Acta 2004, 49, 5139–5150. [Google Scholar] [CrossRef]
- Qin, Z.B.; Luo, Q.; Zhang, Q.; Wu, Z.; Liu, L.; Shen, B.; Hu, W.B. Improving corrosion resistance of nickel-aluminum bronzes by surface modification with chromium ion implantation. Surf. Coat. Technol. 2018, 334, 402–409. [Google Scholar] [CrossRef]
- Qin, Z.B.; Xia, D.H.; Zhang, Y.W.; Wu, Z.; Liu, L.; Lv, Y.T.; Liu, Y.C.; Hu, W.B. Microstructure modification and improving corrosion resistance of laser surface quenched nickel–aluminum bronze alloy. Corros. Sci. 2020, 174, 108744. [Google Scholar] [CrossRef]
- Wang, T.; Wang, Z.Y.; Wang, R.; Wang, M.S.; Wu, C.L.; Zhang, C.H.; Zhang, S.; Chen, H.T.; Chen, J. Effect of solution aging treatment on corrosion resistance and erosion resistance of laser metal deposition 17-4PHss. Eng. Fail. Anal. 2025, 169, 109167. [Google Scholar] [CrossRef]
- Qin, Z.B.; Zhang, Q.; Luo, Q.; Wu, Z.; Shen, B.; Liu, L.; Hu, W.B. Microstructure design to improve the corrosion and cavitation corrosion resistance of a nickel-aluminum bronze. Corros. Sci. 2018, 139, 255–266. [Google Scholar] [CrossRef]
- Siemek, K.; Eseev, M.K.; Horodek, P.; Kobets, A.G.; Kuziv, I.V. Defects studies of nickel aluminum bronze subjected to cavitation. Appl. Surf. Sci. 2021, 546, 149107. [Google Scholar] [CrossRef]
- Zhang, H.F.; Wu, H.; Wang, Z.Y.; Wang, R.; Zhang, S.; Zhang, C.H.; Wu, C.L.; Chen, J.; Chen, H.T. In-situ synthesized (Ti,Nb)C reinforced austenitic stainless steel by laser cladding: New insights into microstructure evolution and corrosion behavior. Mater. Today Commun. 2025, 42, 111604. [Google Scholar] [CrossRef]
- Yang, F.F.; Kang, H.J.; Guo, E.Y.; Li, R.G.; Chen, Z.N.; Zeng, Y.H.; Wang, T.M. The role of nickel in mechanical performance and corrosion behaviour of nickel-aluminium bronze in 3.5 wt.% NaCl solution. Corros. Sci. 2018, 139, 333–345. [Google Scholar] [CrossRef]
- Wu, H.; Wang, M.S.; Zhang, S.; Wang, R.; Zhang, C.H.; Wu, C.L.; Chen, H.T.; Chen, J. Cavitation erosion characteristics and dominant factors of a novel FeNiCoCrMo0.3Nb0.5 hypoeutectic high entropy alloys coating. Wear 2025, 572–573, 206043. [Google Scholar] [CrossRef]
- Song, S.L.; Li, D.G.; Chen, D.R.; Liang, P. The role of Ti in cavitation erosion and corrosion behaviours of NAB alloy in 3.5% NaCl solution. J. Alloys Compd. 2022, 919, 165728. [Google Scholar] [CrossRef]
- Whartan, J.A.; Barik, R.C.; Kear, G.; Wood, R.J.K.; Stokes, K.R.; Walsh, F.C. The corrosion of nickel–aluminium bronze in seawater. Corros. Sci. 2005, 47, 3336–3367. [Google Scholar] [CrossRef]
- Kayvandarian, F.; Zanganeh, D.; Khodabakhshi, F.; Malekan, M. Corrosion properties of submerged friction stir modified as-cast nickel-aluminum bronze (NAB) compositions. Electrochim. Acta 2024, 501, 144775. [Google Scholar] [CrossRef]



| Cu | Al | Fe | Ni | Mn | Pb | Si | Zn | |
|---|---|---|---|---|---|---|---|---|
| ERCuNiAl | Bal. | 8.73 | 5.9 | 4.52 | 1.39 | 0.005 | 0.058 | 0.002 |
| Substrate | Bal. | 8.62 | 2.32 | 0.005 | 0.258 | 0.027 | 0.149 | 0.021 |
| Samples | Current (A) | Voltage (V) | WFS (m/min) | TS (mm/min) | Stick Out (mm) | HI (J/mm) |
|---|---|---|---|---|---|---|
| S1 | 137 | 12.7 | 6 | 480 | 14 | 243.8 |
| S2 | 158 | 15.0 | 7 | 378.8 | ||
| S3 | 184 | 17.1 | 8 | 502.8 | ||
| S4 | 209 | 19.3 | 9 | 644.7 |
| Sample | Point | Cu | Al | Ni | Fe | Mn | Phases Identified |
|---|---|---|---|---|---|---|---|
| S1 | 1 | 85.2 | 5.7 | 3.9 | 3.8 | 1.4 | α-Cu |
| 2 | 78.1 | 8.5 | 4.9 | 6.8 | 1.7 | γ2 | |
| 3 | 10.2 | 18.8 | 28.7 | 41.1 | 1.2 | κ | |
| S2 | 4 | 84.5 | 5.2 | 3.7 | 5.3 | 1.3 | α-Cu |
| 5 | 78.8 | 8.8 | 4.4 | 6.9 | 1.1 | γ2 | |
| 6 | 8.5 | 17.9 | 31.1 | 40.7 | 1.8 | κ | |
| S3 | 7 | 83.1 | 6.1 | 3.9 | 5.6 | 1.3 | α-Cu |
| 8 | 77.8 | 11.3 | 4.7 | 6.2 | 1.9 | γ2 | |
| 9 | 20.6 | 18.2 | 26.8 | 33.4 | 1.0 | κ | |
| S4 | 10 | 81.8 | 5.2 | 4.9 | 6.6 | 1.5 | α-Cu |
| 11 | 73.3 | 9.3 | 8.1 | 7.3 | 2.0 | γ2 | |
| 12 | 6.8 | 16.8 | 27.5 | 48.2 | 0.7 | κ |
| Specimens | E (mV) | icorr (µA/cm2) |
|---|---|---|
| S1 | −269.61 ± 40 | 2.56 ± 0.04 |
| S2 | −466.62 ± 10 | 3.85 ± 0.11 |
| S3 | −517.77 ± 20 | 5.03 ± 0.08 |
| S4 | −700.94 ± 15 | 7.52 ± 0.07 |
| Gradient | Rs (Ω·cm2) | Qf (µF·cm−2·sn−1) | n1 | Rf (Ω·cm2) | Qct (µF·cm−2·sn−1) | n2 | Rct (Ω·cm2) | Wd (S·cm−2·s1/2) | Chi-Squared |
|---|---|---|---|---|---|---|---|---|---|
| S1 | 8.149 | 11.700 × 10−5 | 0.80 | 1152 | 1.067 × 10−4 | 0.80 | 2485 | 34.60 × 10−4 | 8.21 × 10−4 |
| S2 | 7.702 | 1. 651 × 10−4 | 0.82 | 1047 | 4.893 × 10−4 | 0.63 | 1966 | 99.55 × 10−4 | 5.36 × 10−4 |
| S2 | 7.714 | 3. 141 × 10−4 | 0.79 | 947 | 5.076 × 10−3 | 0.85 | 1284 | 27.82 × 10−4 | 5.41 × 10−4 |
| S4 | 7.102 | 3.254 × 10−4 | 0.78 | 185.8 | 5.941 × 10−4 | 0.80 | 707 | 10.10 × 10−4 | 4.83 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, R.; Wang, Z.; Wang, M.; Wang, R.; Zhang, S.; Zhang, C.; Wu, C.; Chen, H.; Chen, J. Modulating Heat Input to Optimize Corrosion Resistance of Nickel–Aluminum Bronze Manufactured by Cold Metal Transfer Additive Manufacturing. Materials 2025, 18, 2205. https://doi.org/10.3390/ma18102205
Huo R, Wang Z, Wang M, Wang R, Zhang S, Zhang C, Wu C, Chen H, Chen J. Modulating Heat Input to Optimize Corrosion Resistance of Nickel–Aluminum Bronze Manufactured by Cold Metal Transfer Additive Manufacturing. Materials. 2025; 18(10):2205. https://doi.org/10.3390/ma18102205
Chicago/Turabian StyleHuo, Renjie, Zheying Wang, Mingsheng Wang, Rui Wang, Song Zhang, Chunhua Zhang, Chenliang Wu, Haitao Chen, and Jiang Chen. 2025. "Modulating Heat Input to Optimize Corrosion Resistance of Nickel–Aluminum Bronze Manufactured by Cold Metal Transfer Additive Manufacturing" Materials 18, no. 10: 2205. https://doi.org/10.3390/ma18102205
APA StyleHuo, R., Wang, Z., Wang, M., Wang, R., Zhang, S., Zhang, C., Wu, C., Chen, H., & Chen, J. (2025). Modulating Heat Input to Optimize Corrosion Resistance of Nickel–Aluminum Bronze Manufactured by Cold Metal Transfer Additive Manufacturing. Materials, 18(10), 2205. https://doi.org/10.3390/ma18102205






