1. Introduction
As a common environmental pollutant, fluoride is widely found in natural water bodies and industrial wastewater [
1]. Excessive concentrations of fluoride in water not only pose a threat to human health but may also lead to diseases such as skeletal fluoride deposition, abnormal bone development, and dental fluorosis and, in severe cases, even affect the structure and function of bones. In addition, an excessive accumulation of fluoride can also upset the balance of aquatic ecosystems and cause ecological hazards [
2,
3]. According to the standards of the World Health Organization (WHO), the concentration of fluorine in drinking water should be controlled at less than 1.5 mg L
−1 [
4]. However, in some areas, due to natural geological effects or industrial pollution, the concentration of fluorine in water bodies far exceeds this standard, resulting in fluorine pollution becoming a major environmental problem that needs to be solved urgently. Therefore, the development of efficient and economical water treatment technologies to remove fluoride ions from water has become an important research direction in the field of water treatment [
5].
Currently, common fluoride removal methods include adsorption [
6,
7] reverse osmosis [
8,
9], ion exchange [
10,
11], and precipitation [
12,
13]. Although effective, reverse osmosis is difficult to apply on a large scale due to its high cost and membrane clogging [
14]. Ion exchange requires expensive resin materials and is susceptible to interference from competing anions, and it requires frequent replacement or regeneration [
15]. The precipitation method is less costly, but its efficiency is limited by water quality conditions and may trigger secondary pollution [
16]. In contrast, the adsorption method has become a more ideal method for defluoridation because of its easy operation, high cost-effectiveness, good treatment effect, and low impact on the environment [
17,
18,
19]. However, the commonly used adsorbents, such as activated carbon, alumina and its base adsorbents, mixed metal oxides, and clay materials, generally have a low adsorption capacity, poor pH adaptability, and secondary pollution, which limits their wide application [
20,
21].
In recent years, layered double hydroxides (LDHs), also known as anionic clays, have shown excellent performance in various fields such as selective adsorption, catalysis, and separation, due to their unique interlayer structure and large specific surface area [
22]. The anions in the interlayers of LDHs are removed during a high-temperature calcination process to form layered oxides (LDOs). The general formula of LDHs can be expressed as
, where M
2+ and M
3+ are divalent and trivalent, respectively; 3+ are divalent and trivalent metal ions, respectively; and A is an interlayer anion [
23]. Due to their unique molecular structure, the charge density and interlayer spacing can be precisely adjusted by regulating the ratio of divalent metal (e.g., Ca
2+, Mg
2+) and trivalent metal (e.g., Al
3+, Fe
3+) ions, which endows LDO materials with a high specific surface area, a strong anion-exchange capacity, and selective adsorption properties for anionic pollutants [
24]. Therefore, LDOs are considered ideal adsorbents, especially for oxygen anions such as fluorine [
25]. However, the existing LDO materials still suffer from several limitations, such as slow adsorption kinetics, a limited pH tolerance (optimal pH range of 5–7), and irreversible collapse of the structure during regeneration. Thus, the development of LDO materials with a high adsorption capacity, wide pH adaptability, and good regeneration properties remains an important challenge in this field [
26,
27].
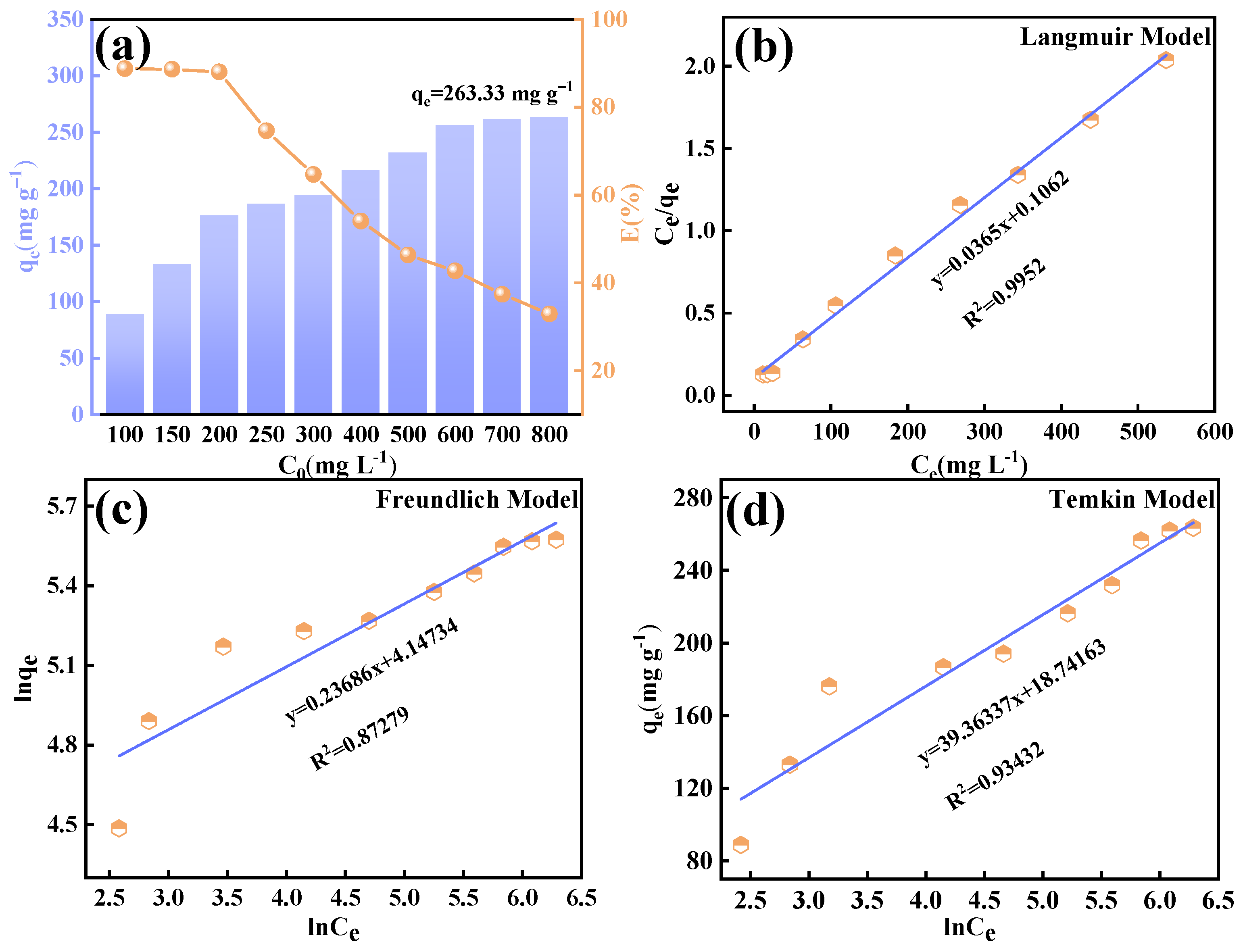
Based on the structural properties of layered bimetallic oxides (LDOs), this study innovatively developed a high-efficiency fluoride removal adsorbent, baked-state calcium aluminate (Ca12Al14O33). Using an optimized one-step co-precipitation method and a precisely controlled temperature calcination process, an active material with abundant surface hydroxyl groups and an ordered mesoporous structure was successfully synthesized. Its maximum fluoride adsorption capacity reached 263.33 mg g−1, which is significantly improved compared with most existing similar materials. Moreover, the synergistic fluoride removal mechanism, involving metal ion coordination and surface hydroxyl group ion exchange across a wide pH range (4~12), was systematically elucidated, overcoming the pH sensitivity limitations of traditional adsorbents. Through an integrated Na2CO3 elution–thermal regeneration cycling process, the material maintained 65.9% of its adsorption efficiency after five cycles, which is significantly better than the regeneration performance of current materials. The goal of this study is to develop a low-cost, high-capacity, and regenerable adsorbent material, offering a novel, efficient, and cost-effective solution for fluoride pollution control.
2. Experiment Section
2.1. Materials
Aluminum nitrate (Al(NO
3)
3·9H
2O), calcium nitrate (Ca(NO
3)
2·4H
2O), sodium hydroxide (NaOH), nitric acid (HNO
3), sodium fluoride (NaF), anhydrous ethanol (CH
3OH), acetic acid (CH
3COOH), sodium chloride (NaNO
3), trisodium citrate (C
6H
5Na
3O
7·2H
2O) and all reagents mentioned in the text were of analytically pure grade with purity greater than 99% without further purification, were purchased from Xilong Science Co. Ltd. (Shantou, Guangdong, China), deionised water was homemade by the laboratory. A standard stock solution of 1000 mg L
−1 fluoride was prepared by dissolving 2.21 g NaF in 1000 mL of deionized water at room temperature. Various experimental solutions were prepared by diluting the stock solution appropriately [
28].
The actual fluorine-containing wastewater used in the experiment came from Guangxi Southland Copper Co., Ltd. (Chongzuo, China). The concentration of F was about 218.29 mg L
−1, and the pH was 4.7. The composition of the actual F-containing smelting wastewater is shown in
Table 1.
2.2. Preparation
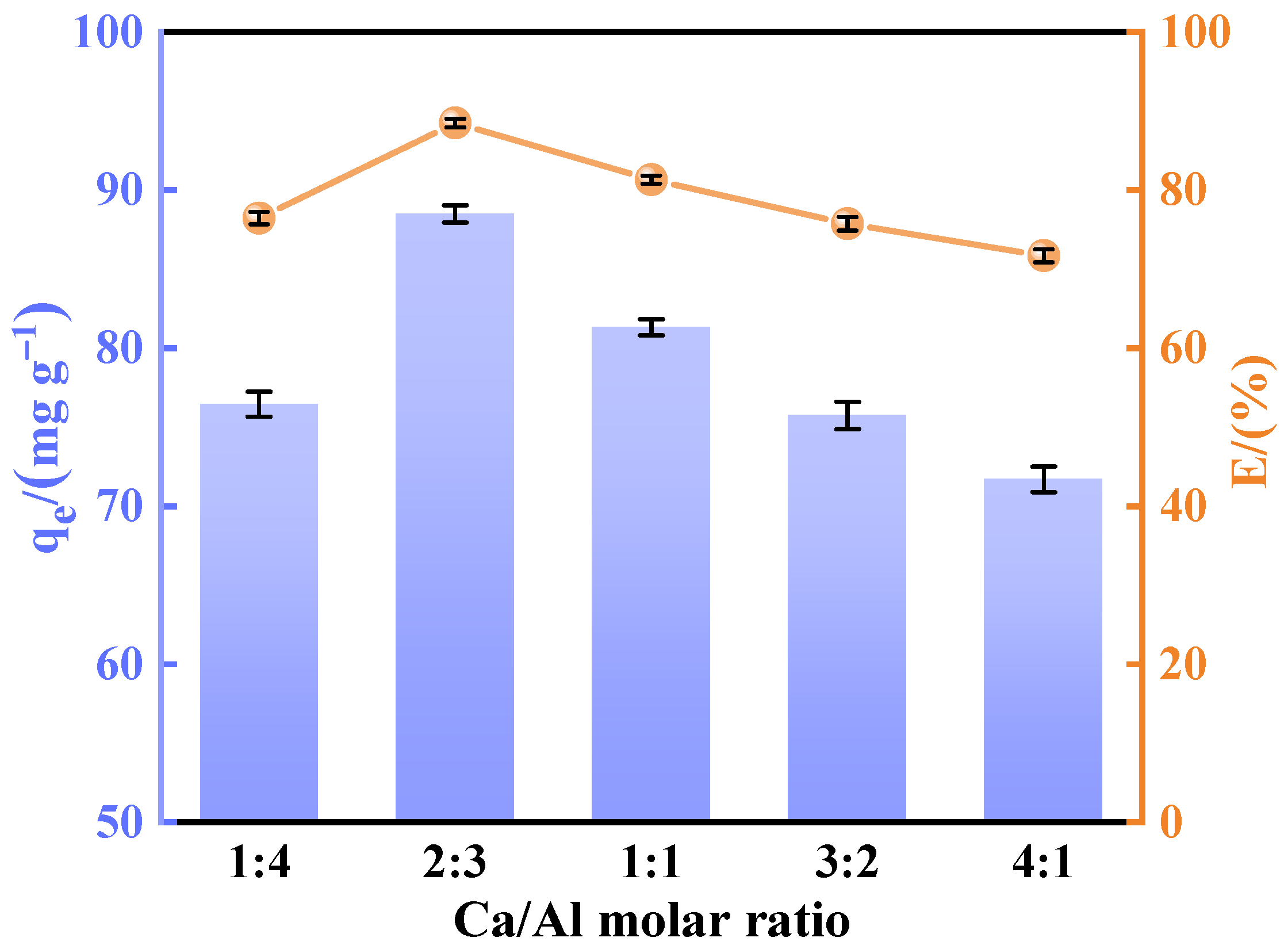
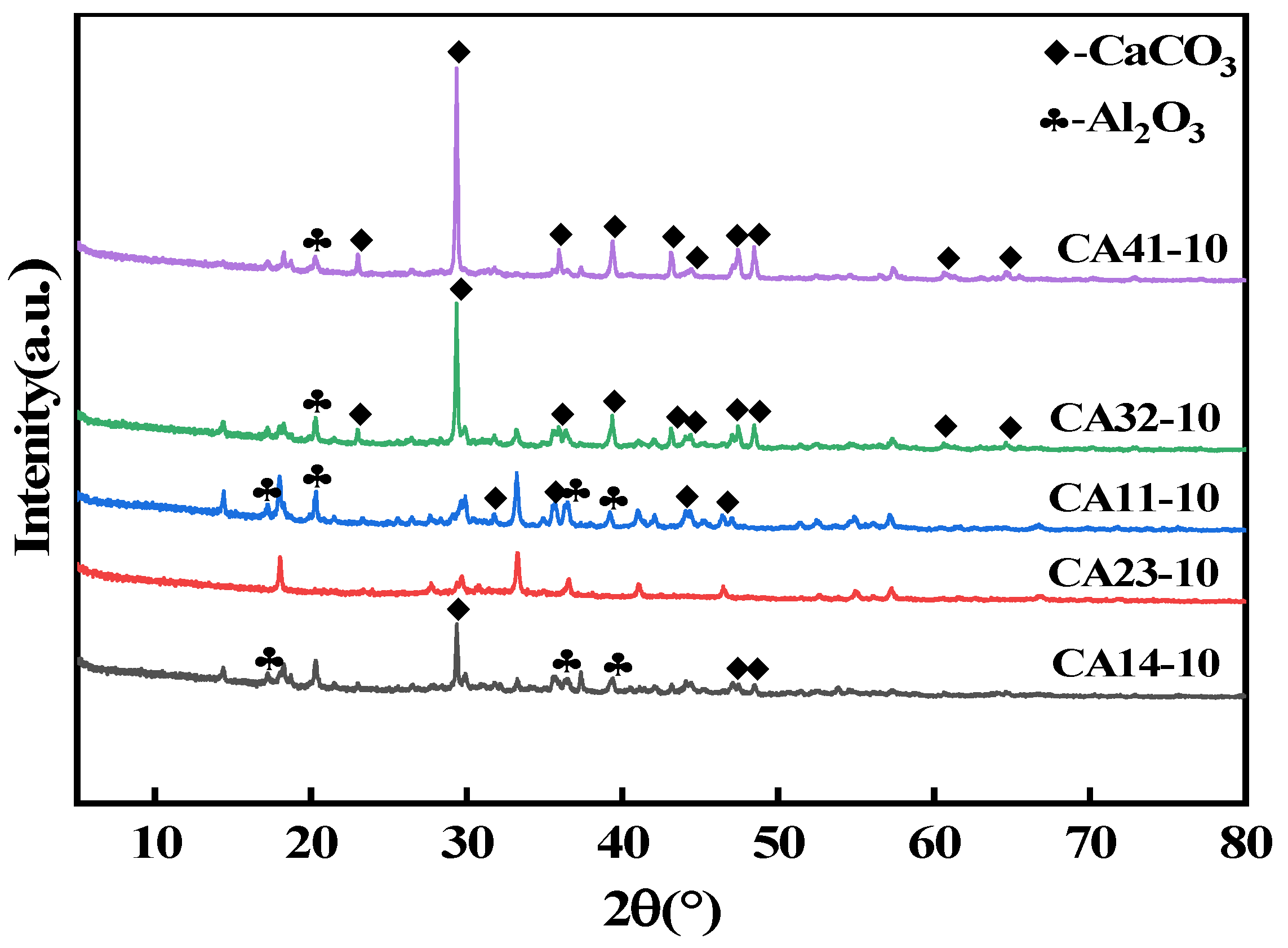
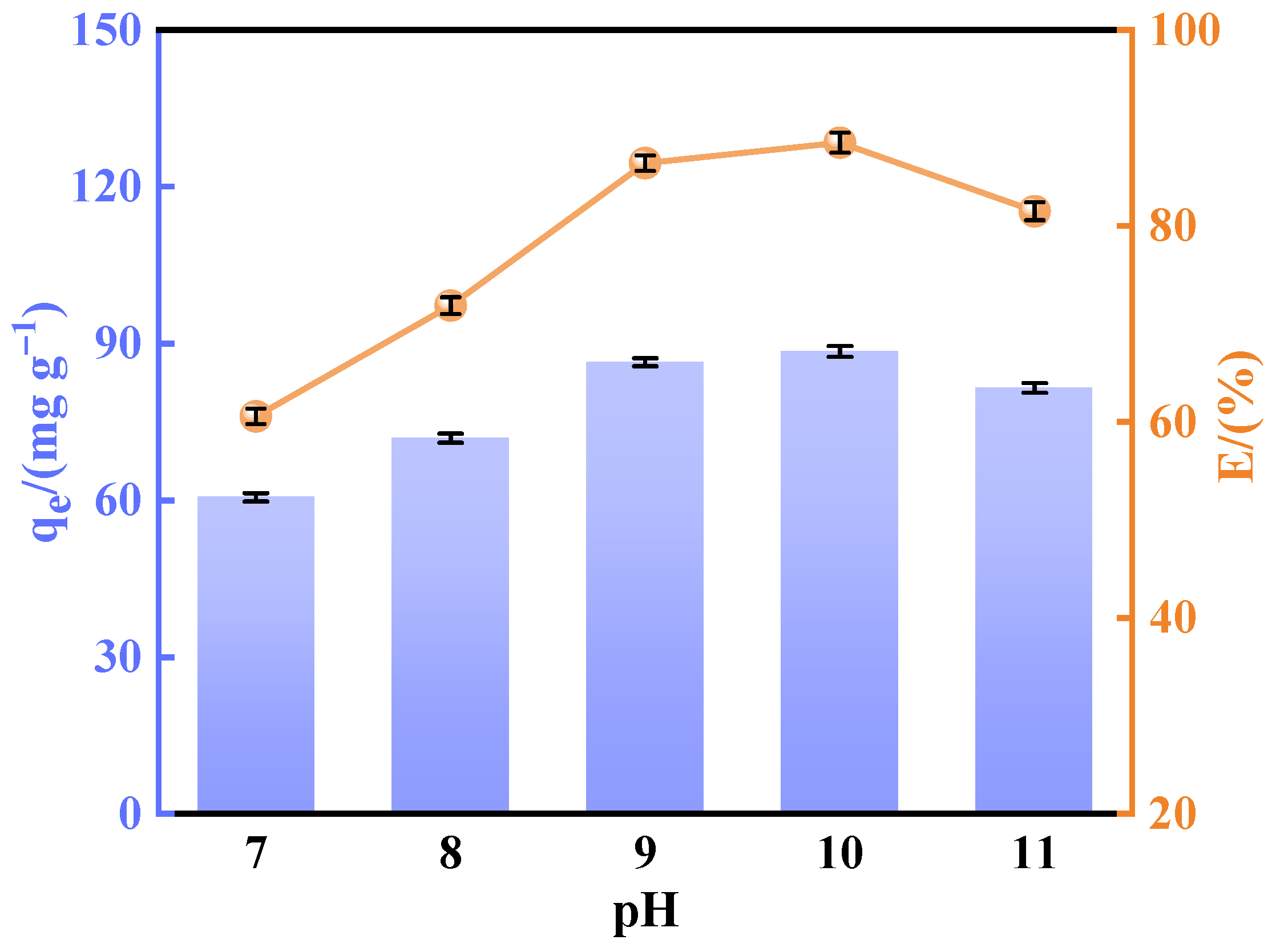
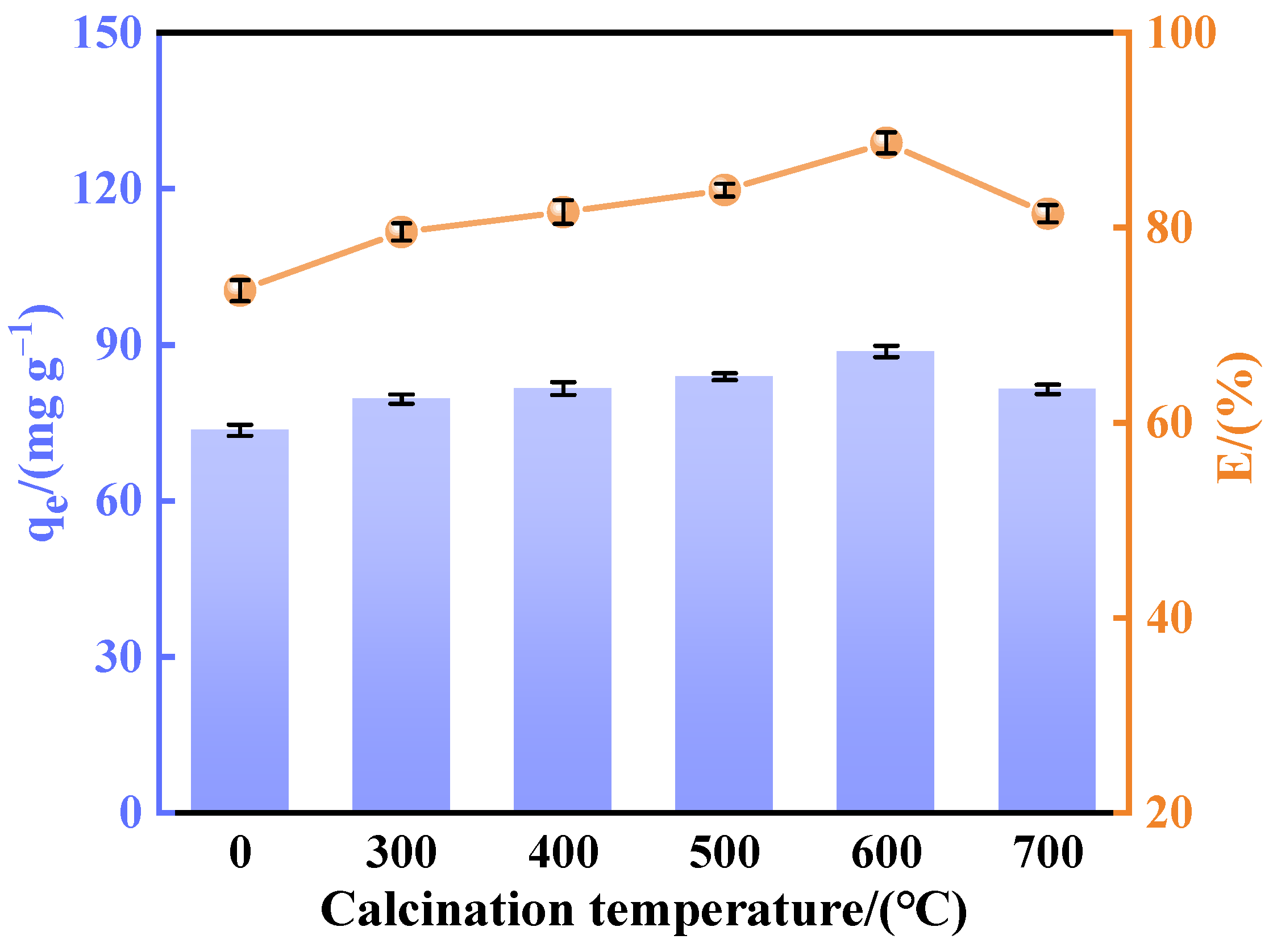
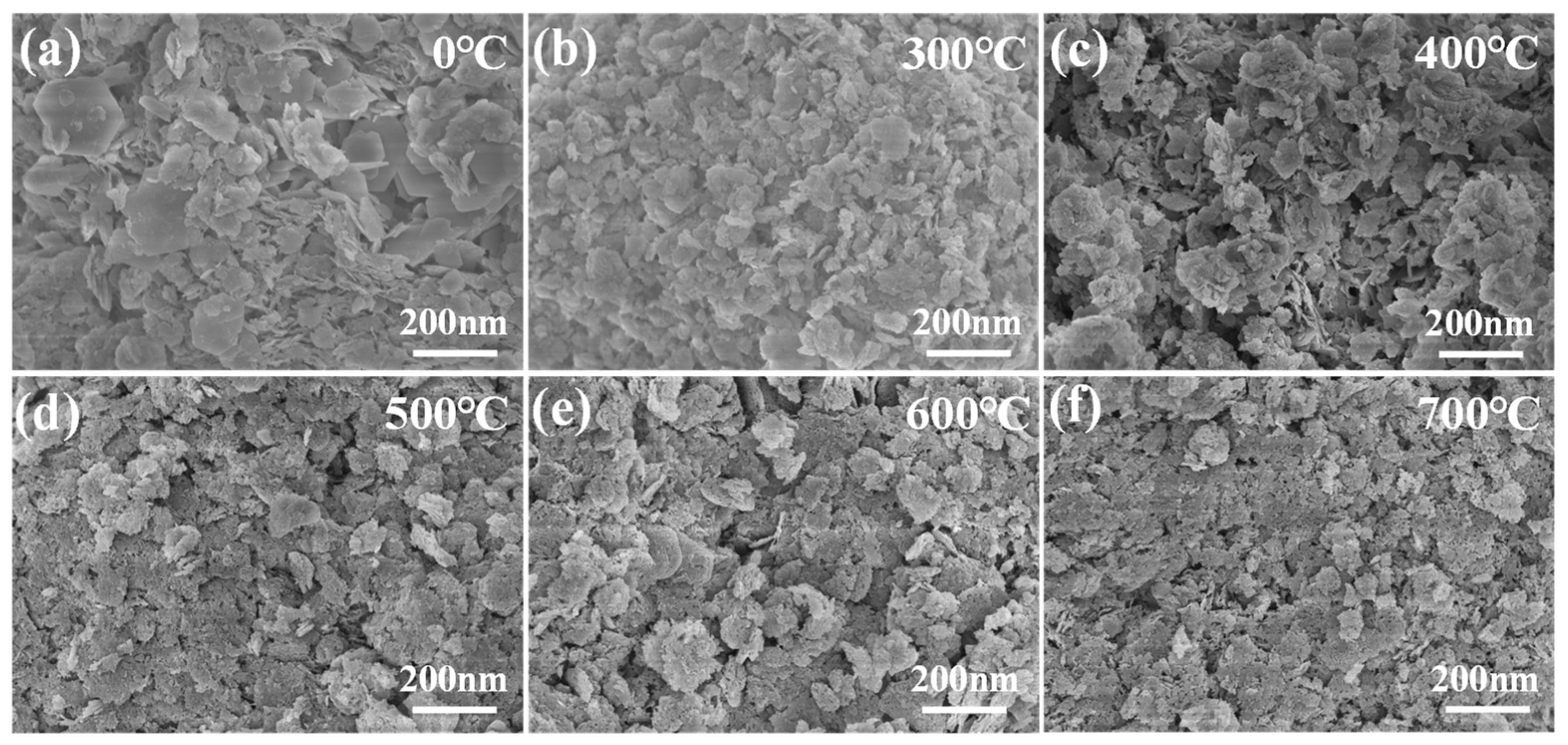
A batch of calcined-state mayenite (Ca12Al14O33) adsorbent materials were prepared by a simple co-precipitation method and calcination process. In a typical synthesis process, Ca(NO3)2·4H2O and Al(NO3)3·9H2O were dissolved in 120 mL of 40% ethanol in molar ratios of 1:4, 2:3, 1:1, 3:2, and 4:1, respectively, with ethanol acting as a dispersant. Then, under strong magnetic stirring, 5 mol L−1 of NaOH solution was slowly added dropwise, and the pH of the mixed solution was adjusted to 10 with continuous stirring for 12 h. At the end of the reaction, the solid precipitate was collected by centrifugation and washed several times with deionized water and ethanol and dried at 80 °C for 12 h. The samples were numbered CA14-10, CA23-10, CA11-10, CA32-10, and CA41-10. In order to optimize the best synthesis conditions, adsorbents with a molar ratio of Ca to Al of 2:3 were prepared at a solution pH 7, 8, 9, and 11, noted as CA23-7, CA23-8, CA23-9, and CA23-11, respectively. In addition, the CA23-10 samples were calcined at 300, 400, 500, 600, and 700 °C for 2 h with a temperature increase rate of 5 °C·min−1, denoted as CA23-10-300, CA23-10-400, CA23-10-500, CA23-10-600, and CA23-10-700, respectively. Ultimately, the calcination-obtained high-purity modified melilite adsorbent was pulverized into fine powder for the subsequent adsorption experiments.
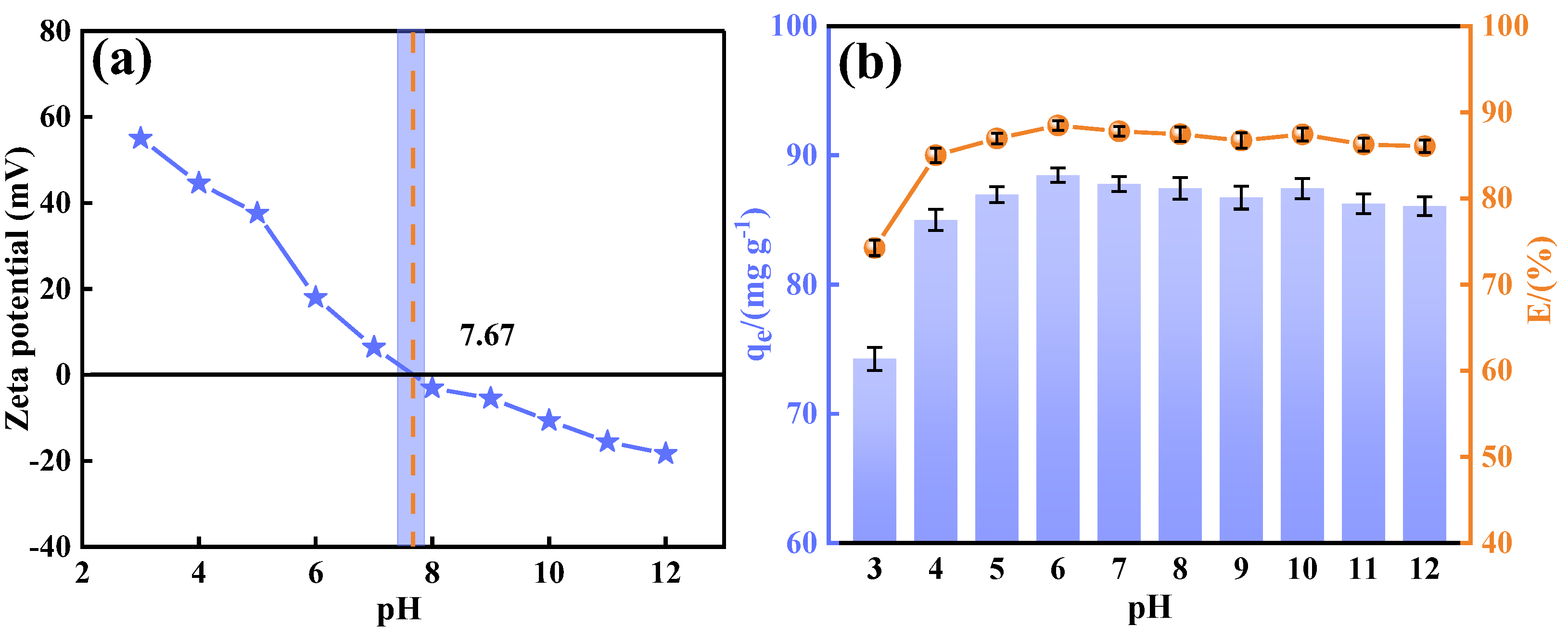
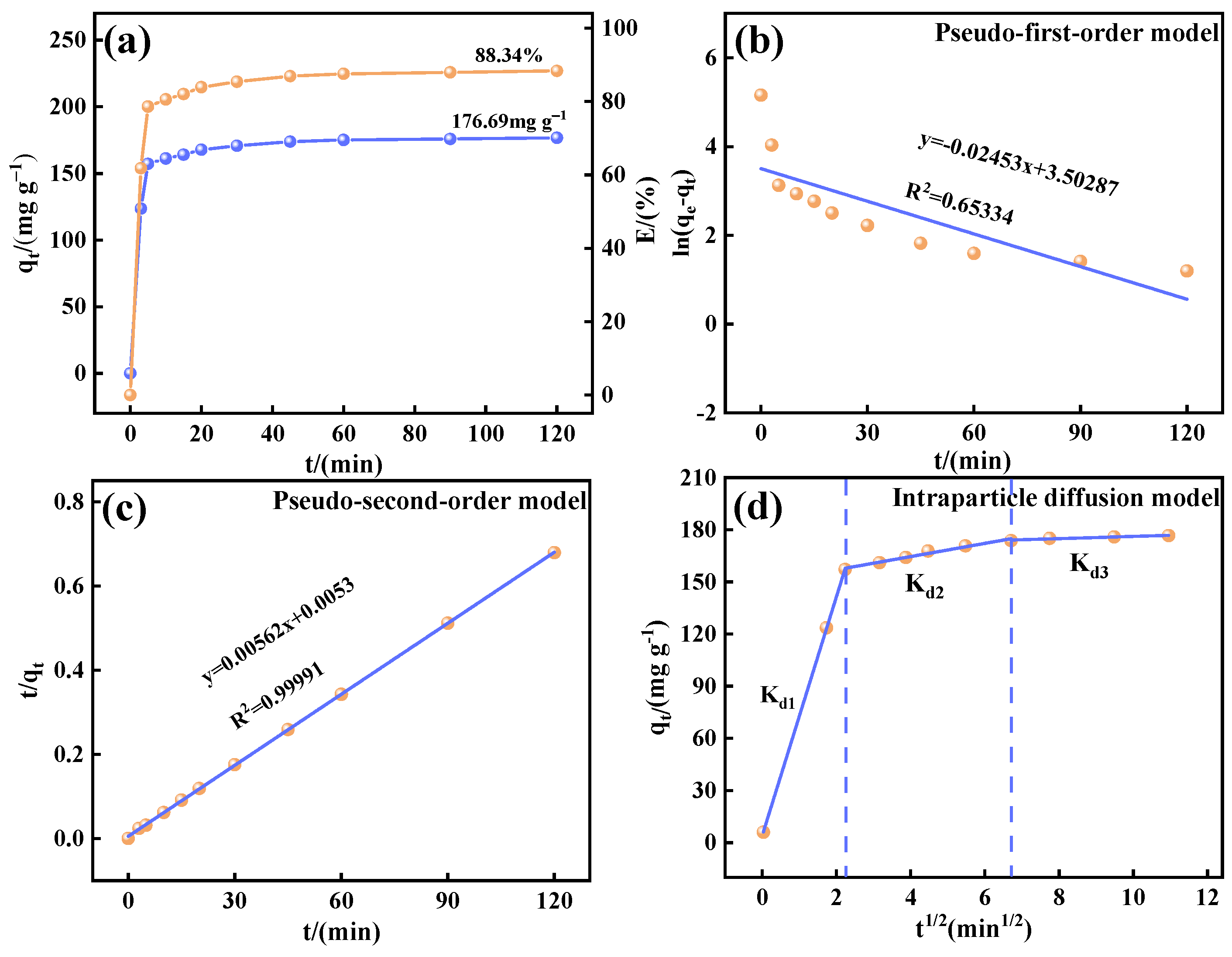
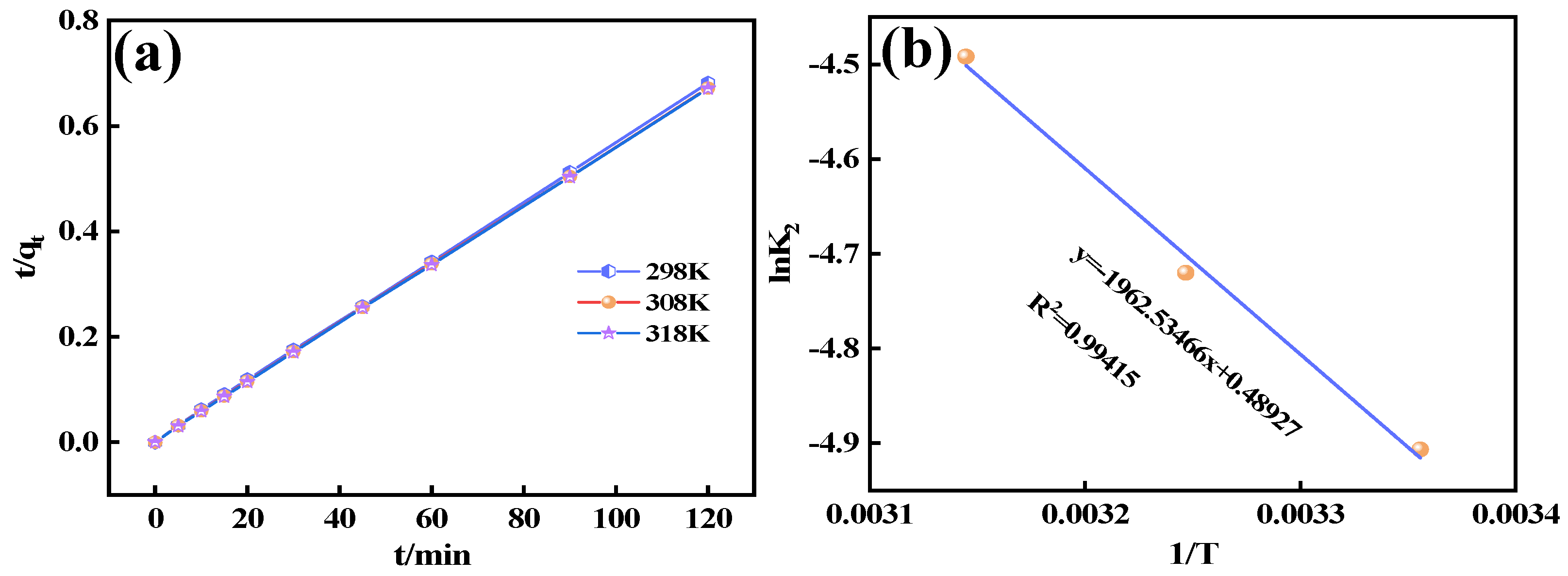
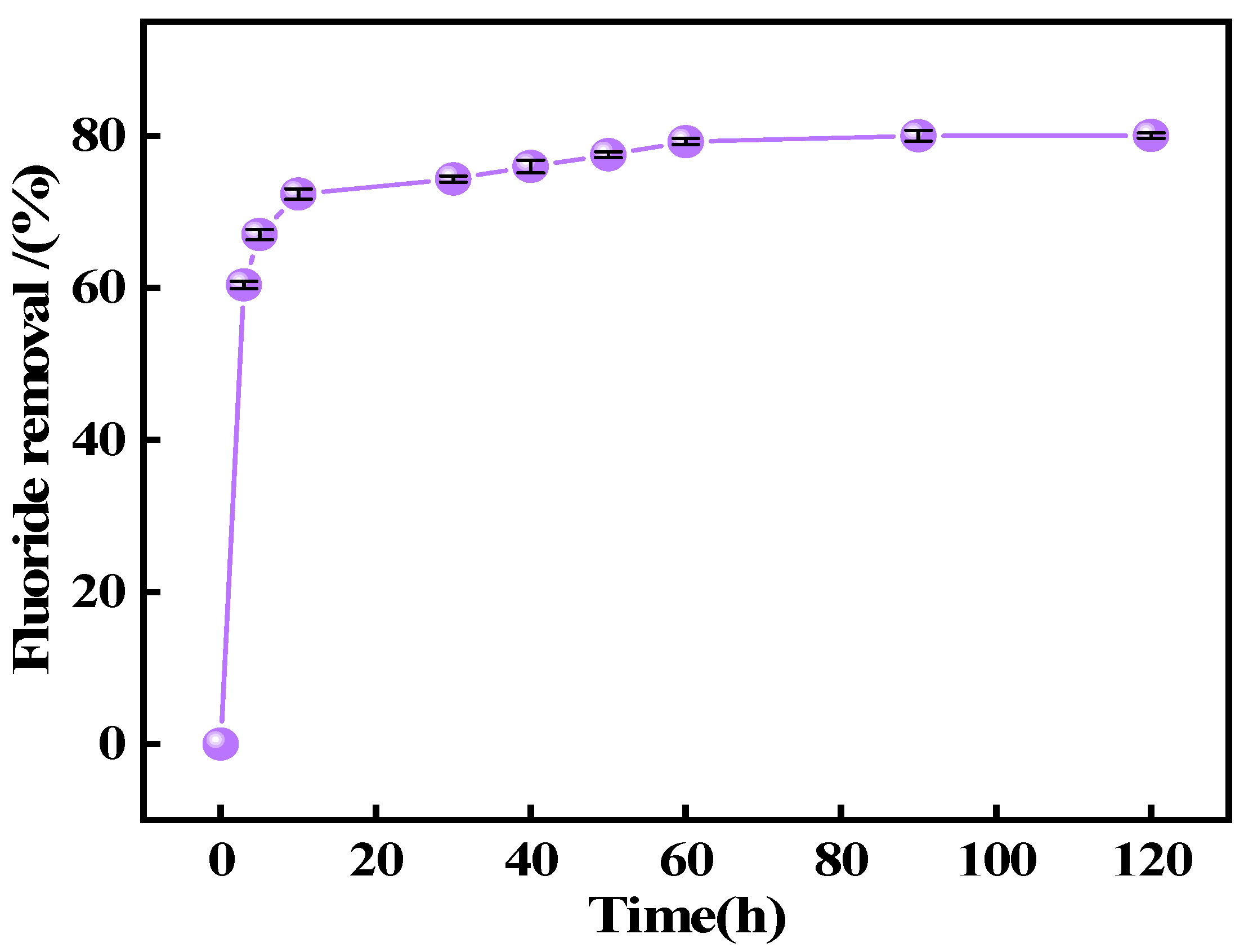
2.3. Adsorption Experiments
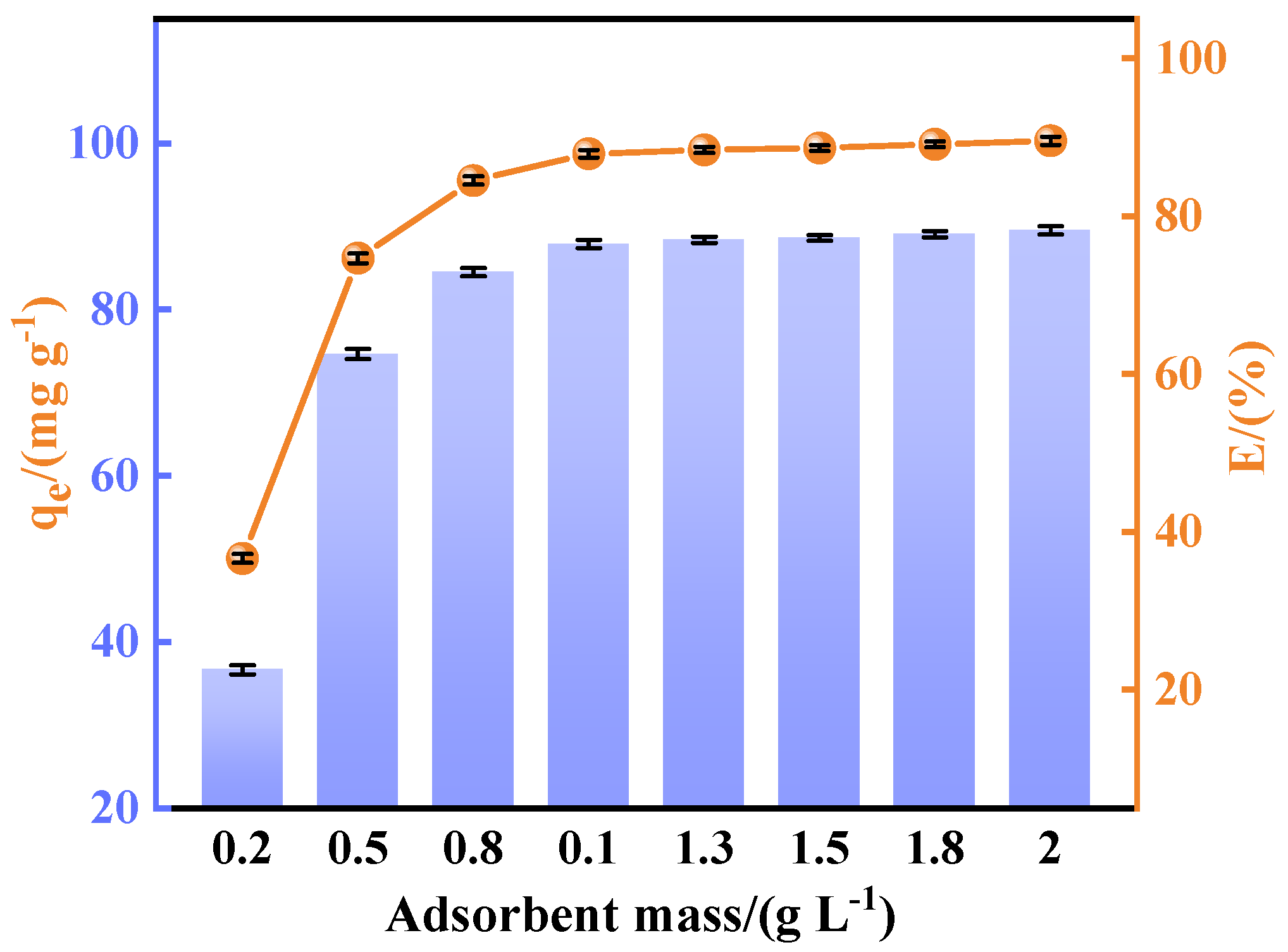
This study systematically investigated the fluoride adsorption performance of calcined-state mayenite (Ca
12Al
14O
33) in water through batch adsorption experiments. All batch experiments were conducted in a thermostatic orbital shaker (200 rpm) using 100 mL of fluoride solution (100 mg L
−1). The systematic parameter screening included adsorbent mass (0.2~2 g L
−1) and solution pH (3~12). The adsorption kinetics, thermodynamics, and isotherms were investigated at different times (5~120 min), fluoride concentrations (100~800 ppm), and temperatures (25~45 °C) under an adsorbent dosing of 1 g L
−1 and pH 7 ± 0.1. Post-adsorption suspensions were filtered through 0.45 μm membranes, with residual F
− concentrations determined by an ion meter (PXSJ-6F, Nanjing Everich Medicare Import & Export Co., Nanjing, China) equipped with a fluoride ion-selective electrode (PF-2-01, Shanghai Yidian Scientific Instrument Co., Shanghai, China), TISAB buffer was introduced prior to the measurements to eliminate ionic interference. Each trial included duplicate runs, with adsorption capacity (
qₑ) and removal efficiency (
Rₑ) calculated via Equations (1) and (2), respectively.
where
C0 and
Ct are the initial and post-adsorption fluorine concentrations (mg L
−1), respectively;
V is the solution volume (L); and m is the mass of adsorbent (g).
2.4. Characterization Tools
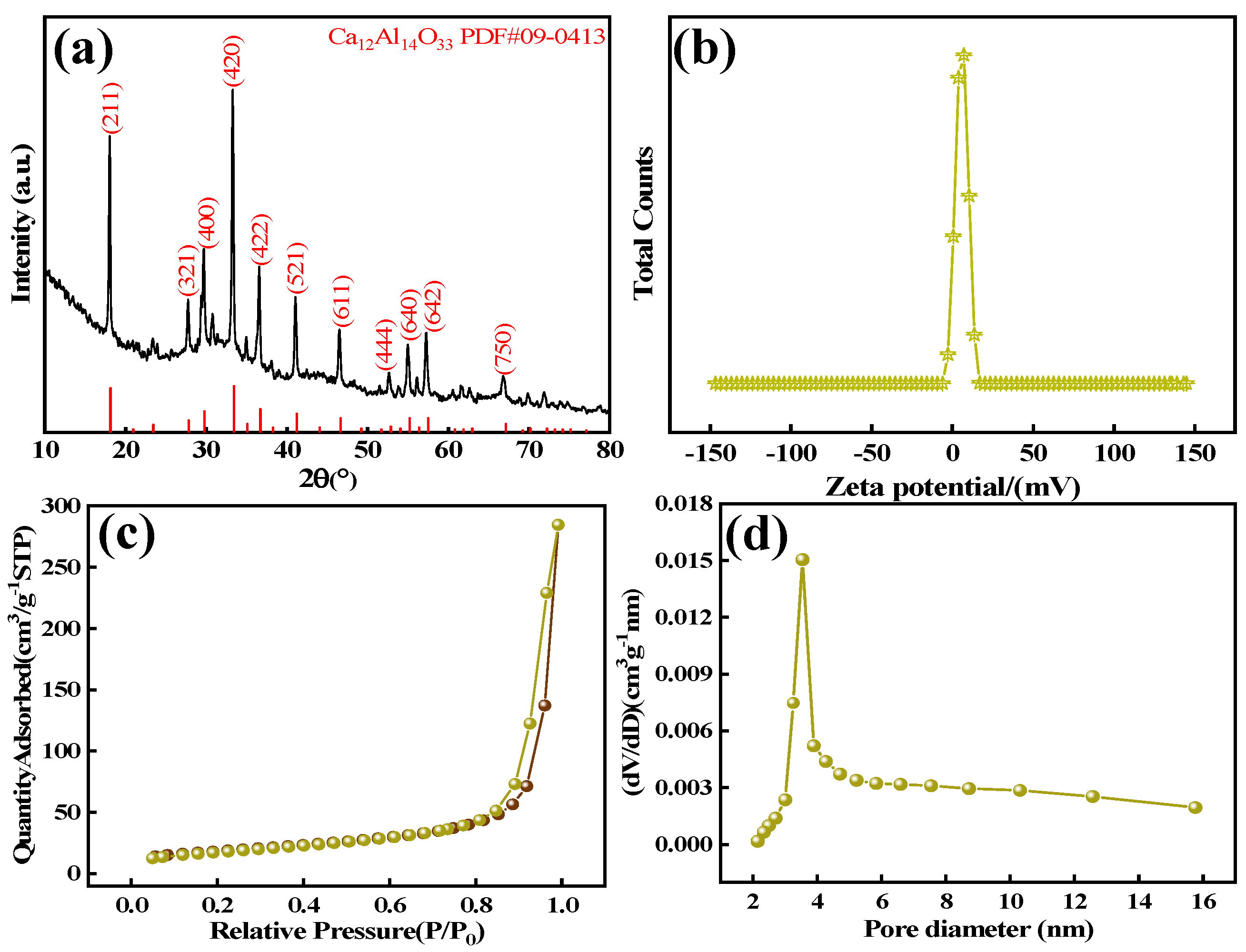
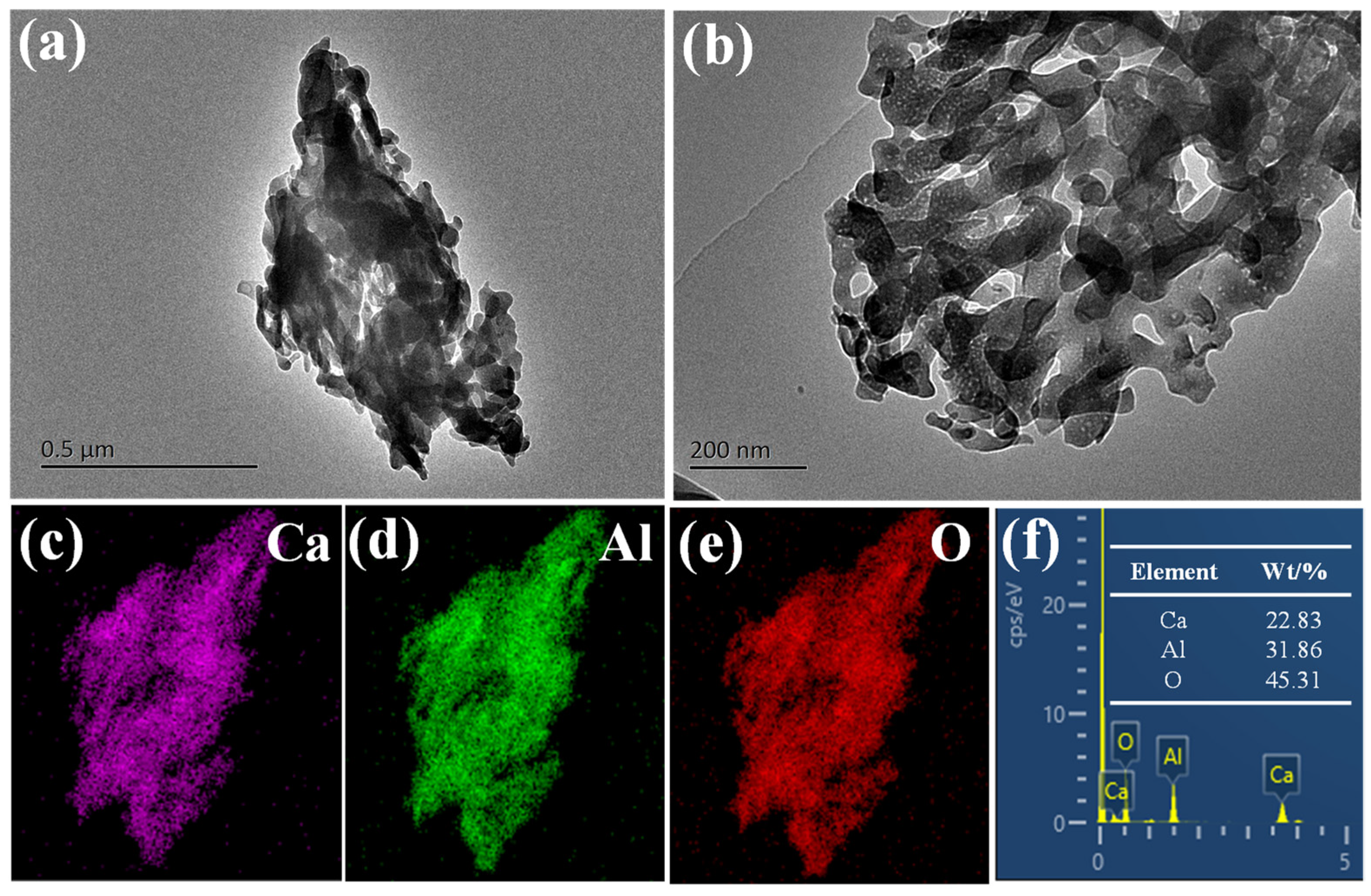
The pH value of the solution was measured by a PHS-3C pH meter (Shanghai Puchun Measure Instrument Co., Ltd., Shanghai, China), and the morphological characteristics and elemental distribution of the materials were observed by field-emission scanning electron microscope (SEM) model S-4800 (Hitachi, Tokyo, Japan) and transmission electron microscope (TEM) model JEM-2100F (JEOL Ltd., Tokyo, Japan) and X’Pert PRO X-ray diffractometer (XRD) (Panalytical, Almelo, The Netherlands). Structural–physical phase analyses of the materials were carried out by the BET and BJH methods, NoVA 1200e (Quantachrome Instruments, Inc., Boynton Beach, FL, USA) surface area, and porosity analyzer to determine the specific surface area, pore volume, and pore size distribution of the adsorbent and the Nicolet 6700-NXR Fourier-transfer infrared spectrometer (FT-IR) (Thermo Fisher Scientific Inc., Waltham, MA, USA) and EscaLab 250Xi X-ray photoelectron spectroscopy (XPS) (Thermo Fisher Scientific Inc., Waltham, MA, USA) to study the changes in the functional groups and the surface chemical properties of the materials.
4. Conclusions
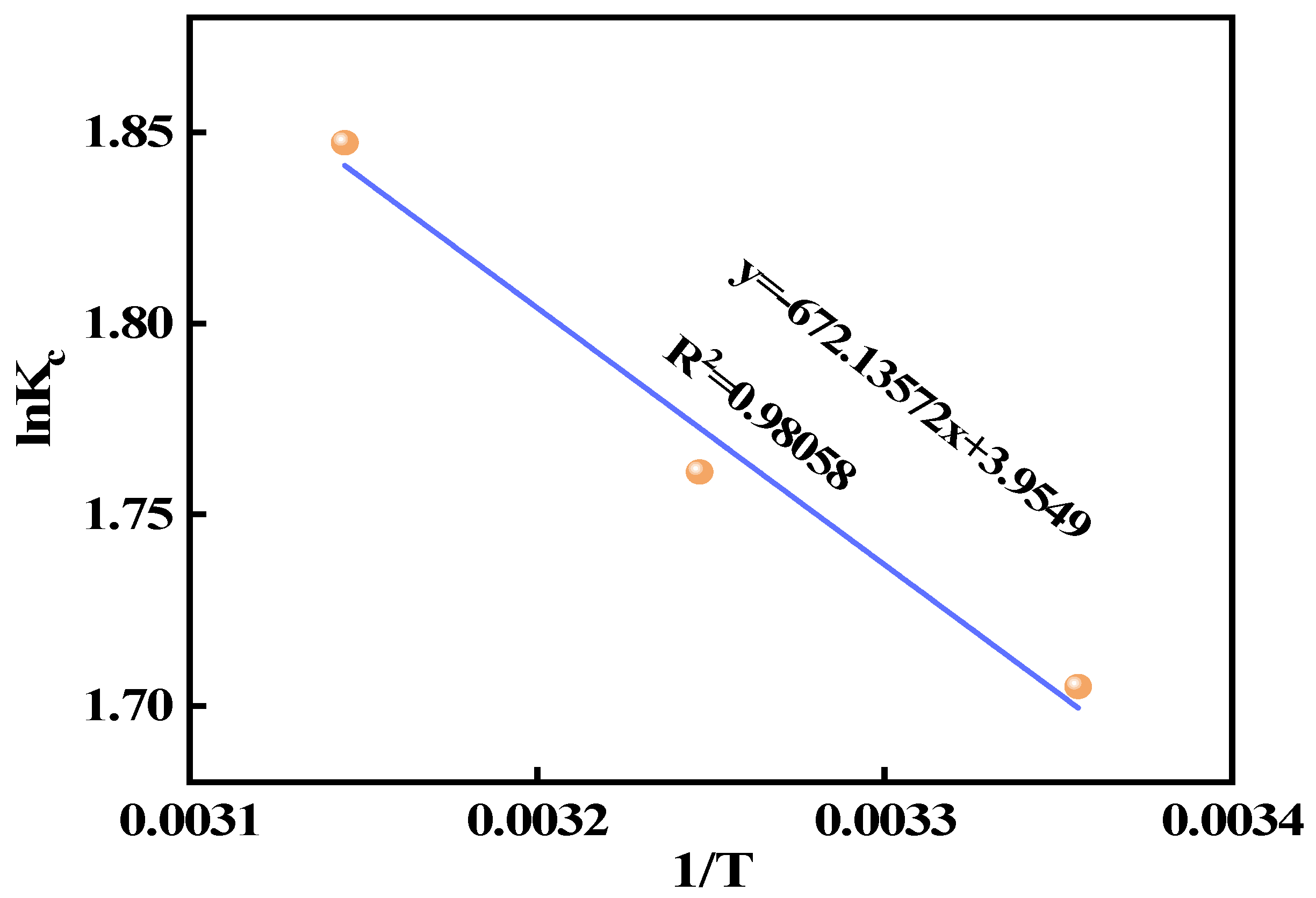
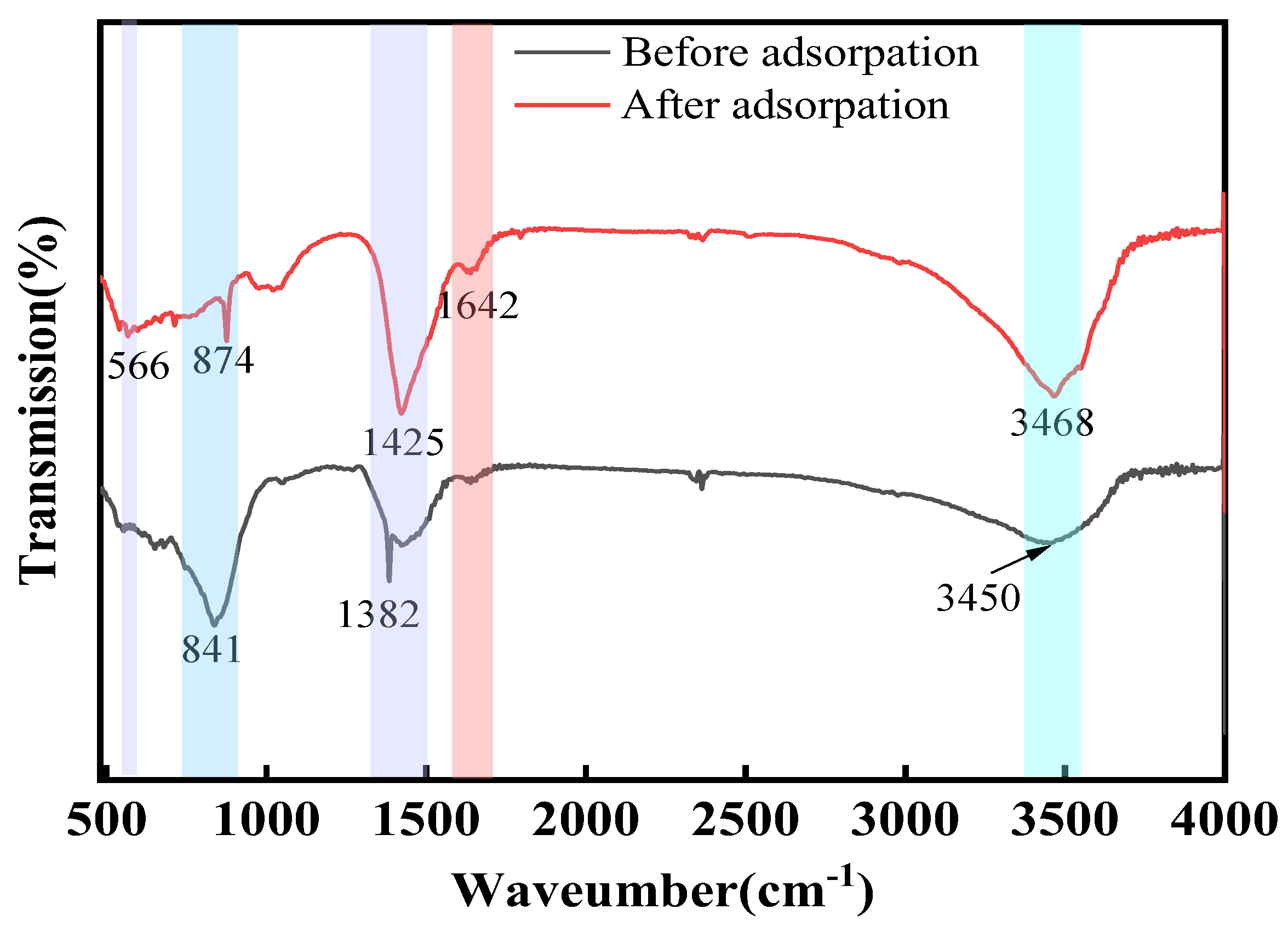
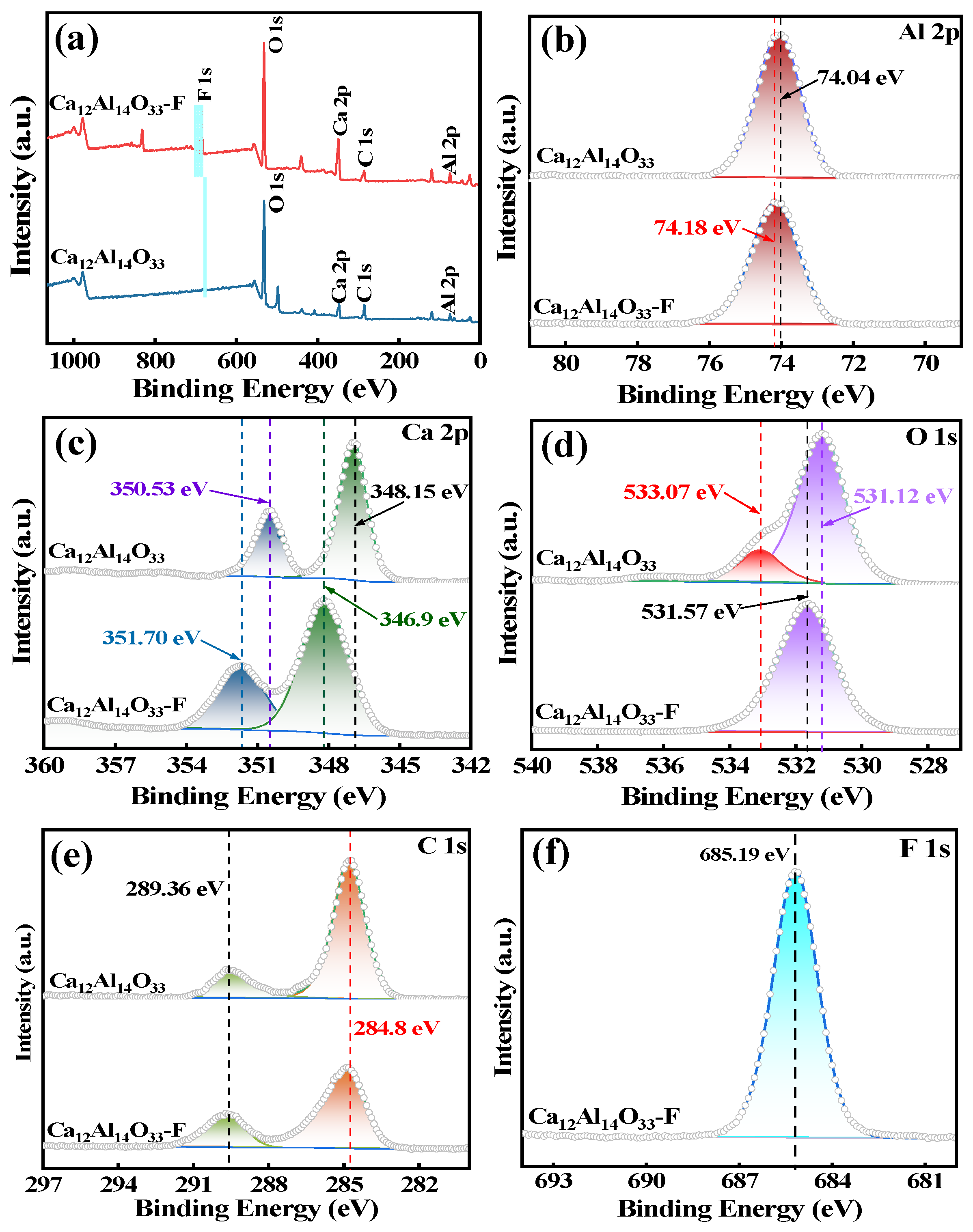
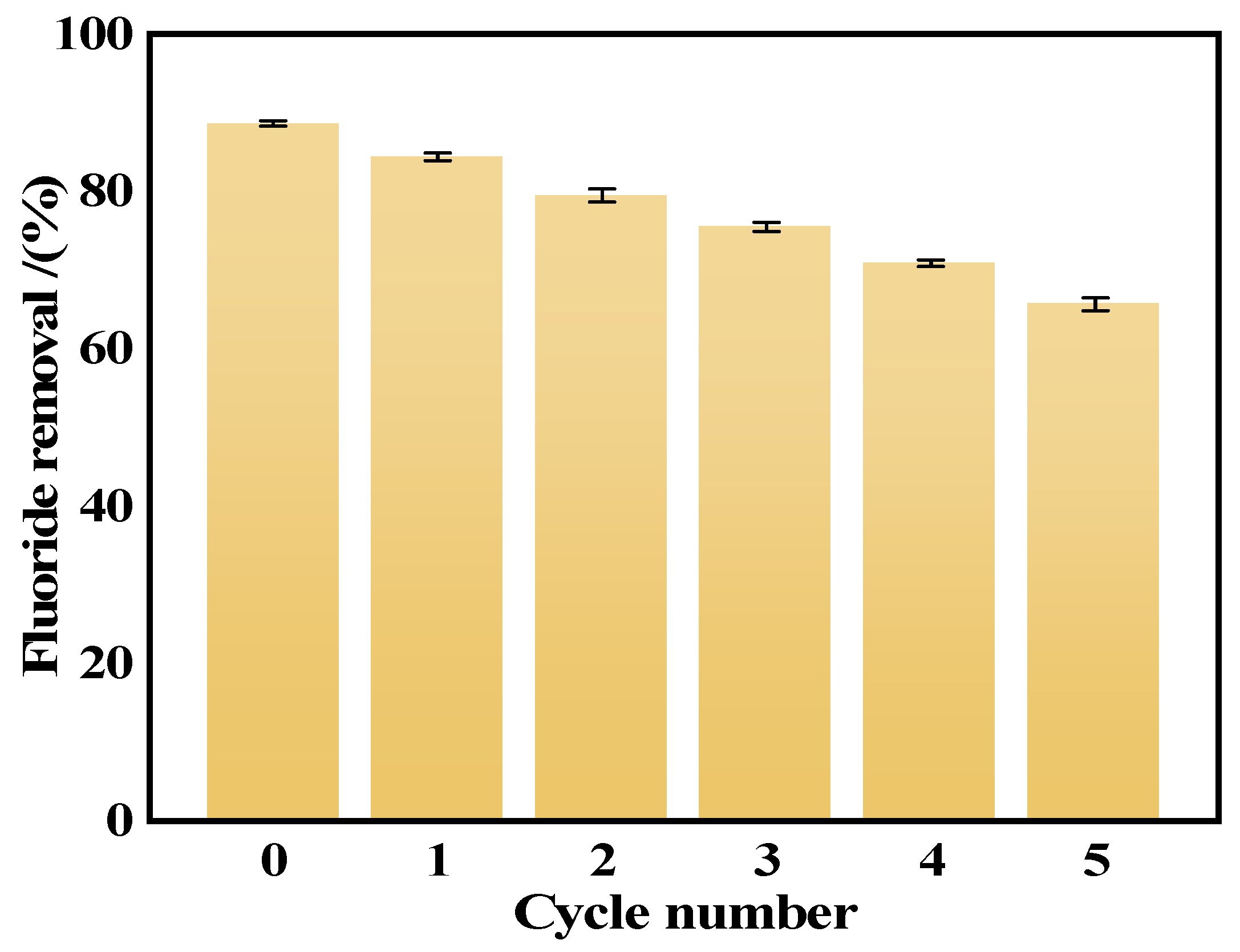
In this study, we successfully synthesized a highly efficient fluoride ion adsorbent, calcined-state mayenite (Ca12Al14O33), by optimizing the co-precipitation–calcination process. Under optimal synthesis conditions (calcium/aluminum molar ratio of 2:3, pH 10, calcination temperature of 600 °C), the material exhibited a high specific surface area (69.42 m2 g−1), an ordered mesoporous structure (pore size of 5.138 nm), and abundant surface hydroxyl groups. The saturated adsorption capacity of the Ca12Al14O33 for fluoride ions was 263.33 mg g−1, significantly surpassing that of most comparable adsorbents. The adsorption kinetics followed a quasi-second-order model, and the Langmuir adsorption isotherm best described the process. Fluoride removal occurred mainly via ion exchange between the metal ions and surface hydroxyl groups. Thermodynamic analysis indicated that the adsorption process is spontaneous, endothermic, and entropy-increasing, making it suitable for practical applications. After five adsorption–desorption cycles, the material retained 65.92% of its initial adsorption capacity, demonstrating excellent structural stability and regeneration potential. Notably, in real fluorine-containing smelting wastewater, the material effectively removed fluoride ions. Compared to traditional adsorbents, Ca12Al14O33 offers significant advantages in adsorption capacity, pH adaptability, and regeneration performance, making it an efficient and cost-effective solution for wastewater treatment.