Transport Properties of Solutions in γ–FeOOH/CSH Pores of Steel Fiber-Reinforced Concrete (SFRC) Derived Using Molecular Dynamics
Abstract
1. Introduction
2. Experiment and Model
2.1. Experiment
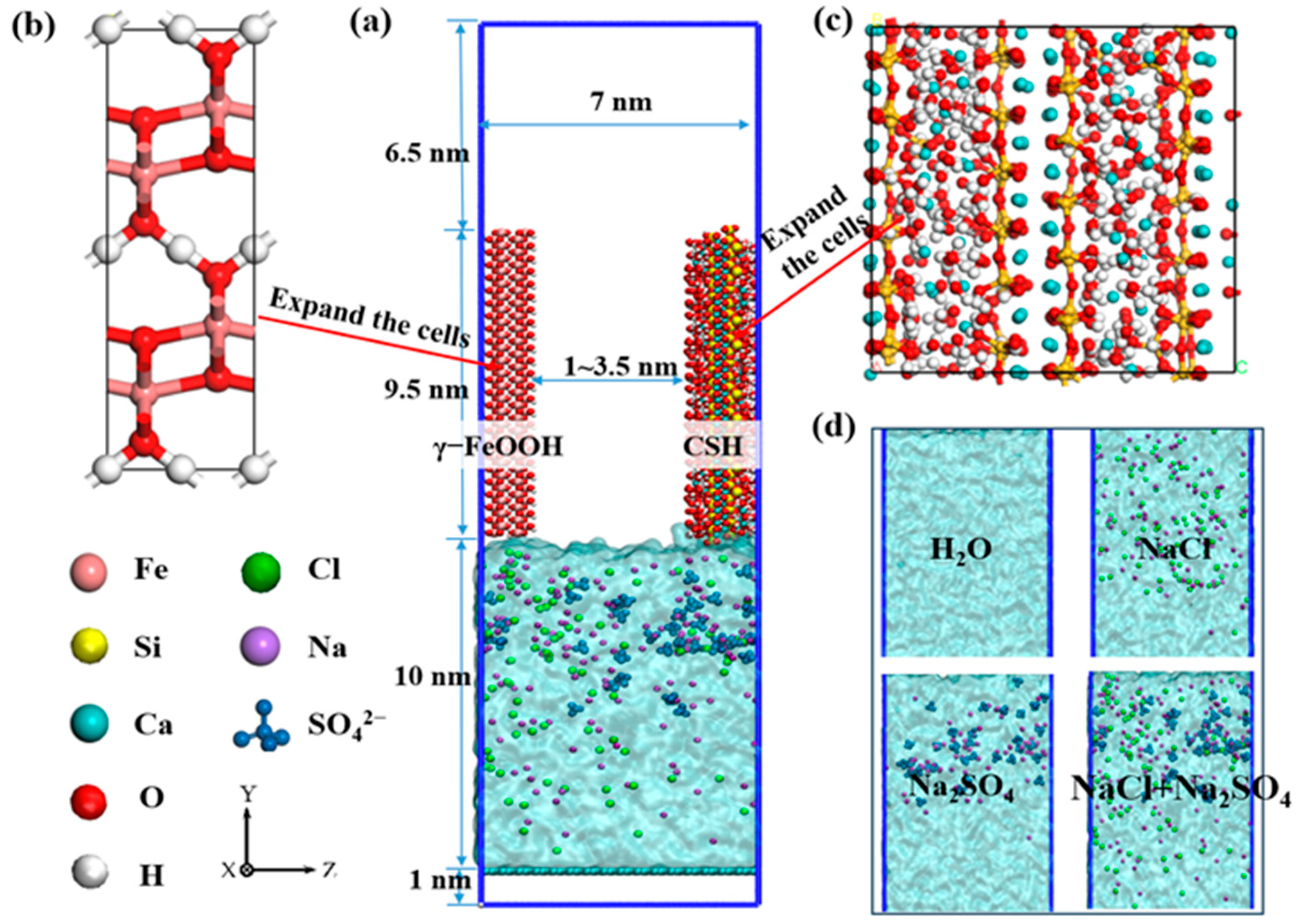
2.2. Model Construction
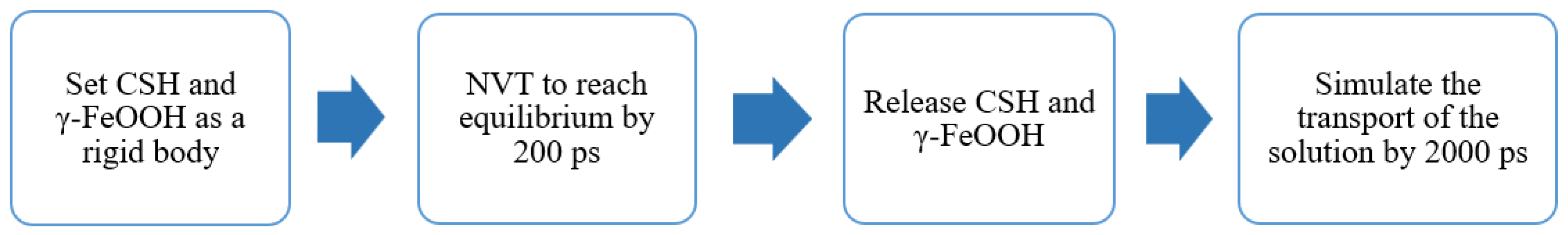
2.3. Force Field and Molecular Dynamics Procedure
3. Results and Discussion
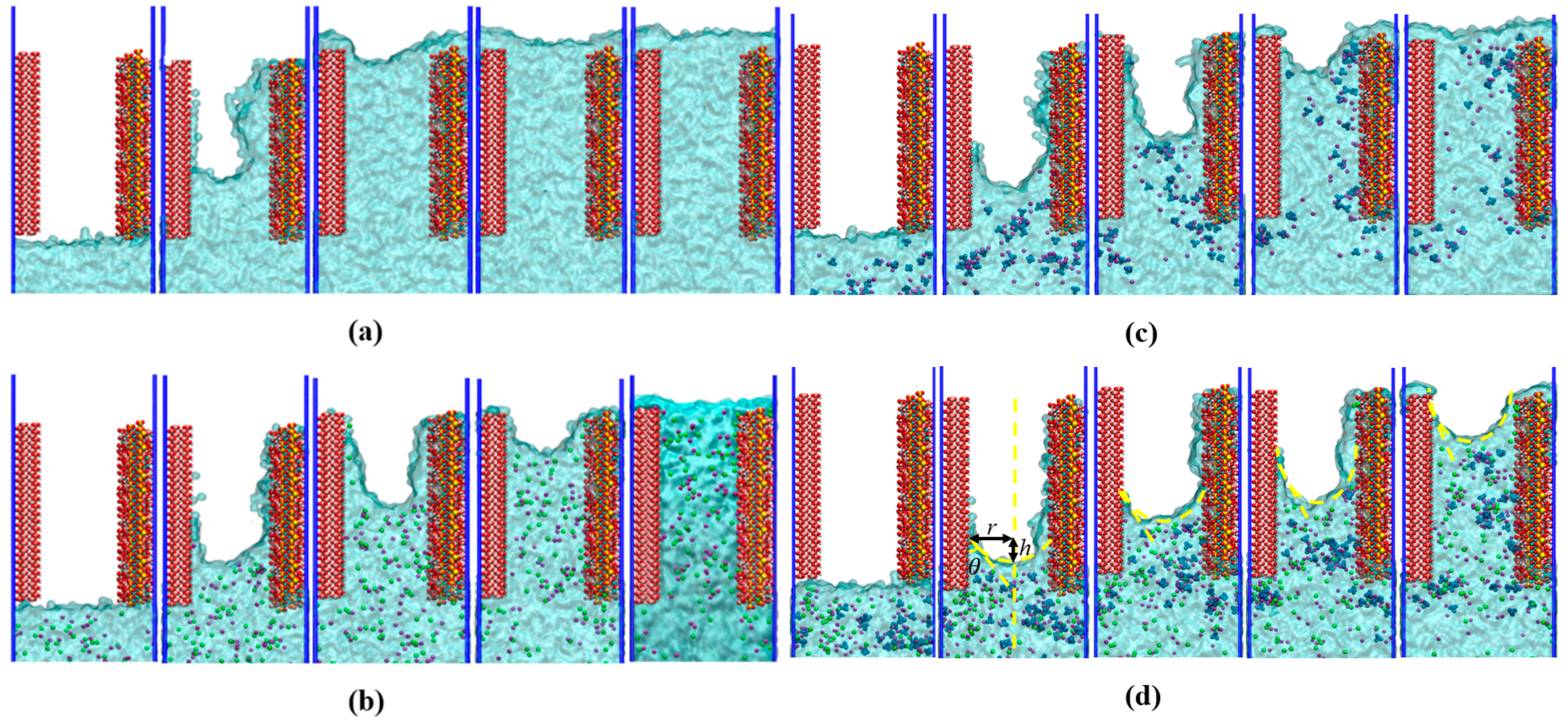
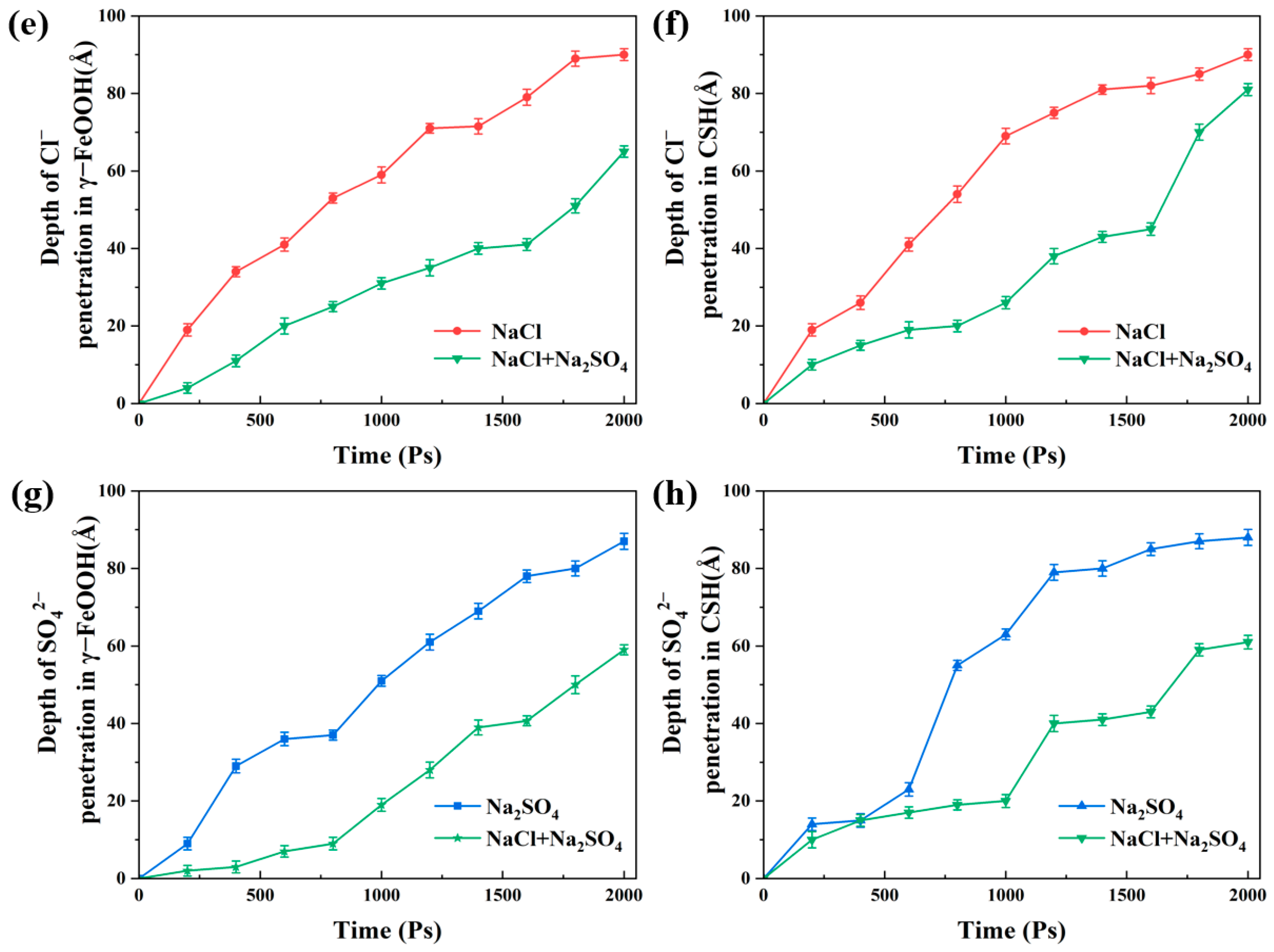
3.1. Water and Ions Transport in the γ–FeOOH/CSH Pore
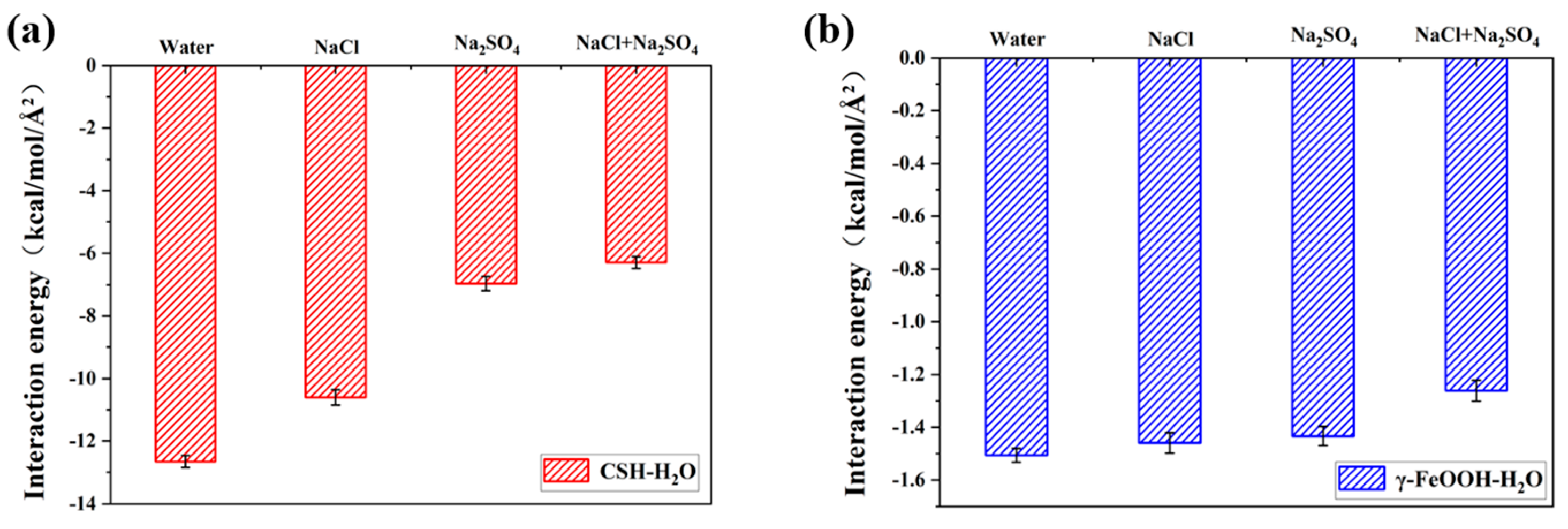
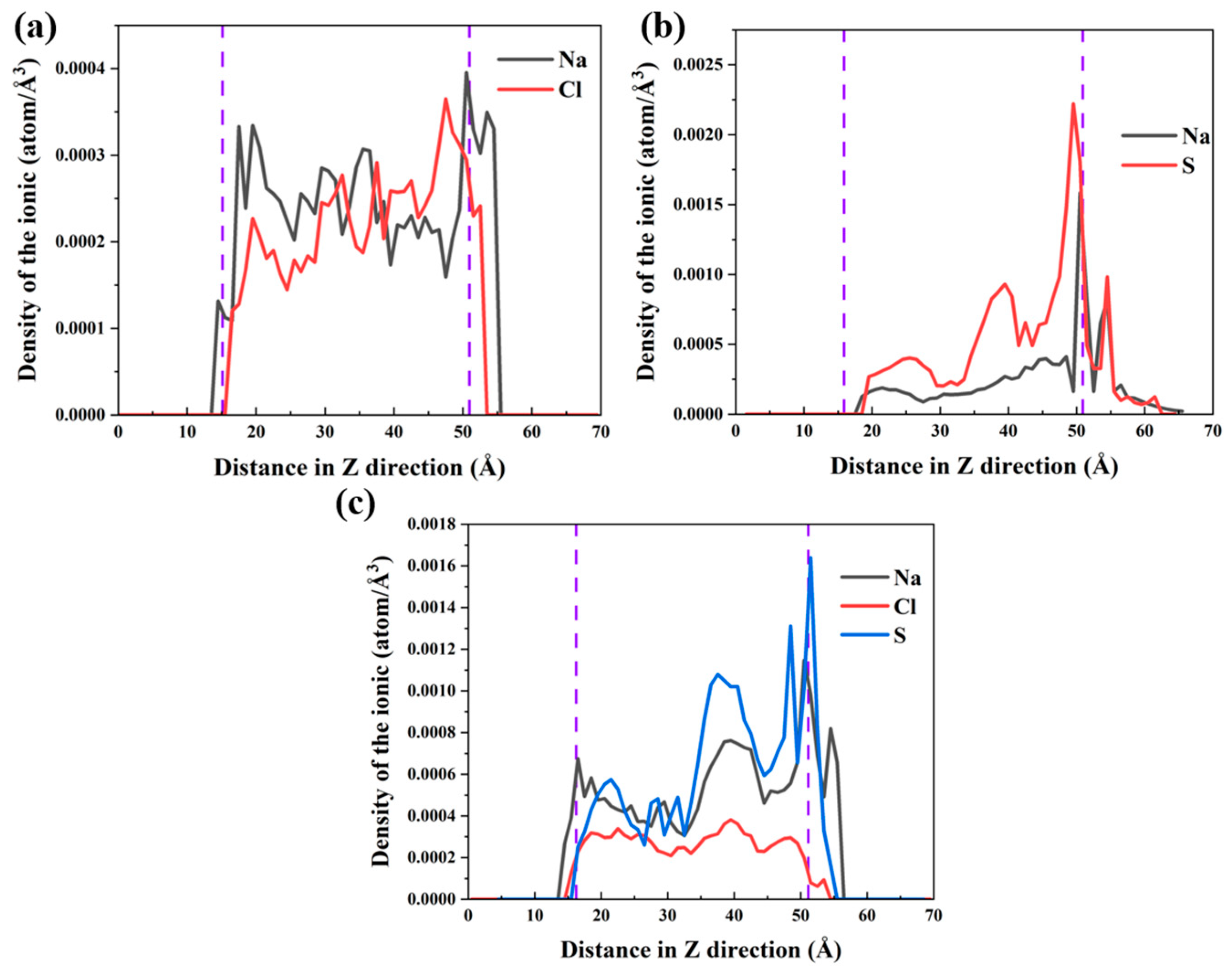
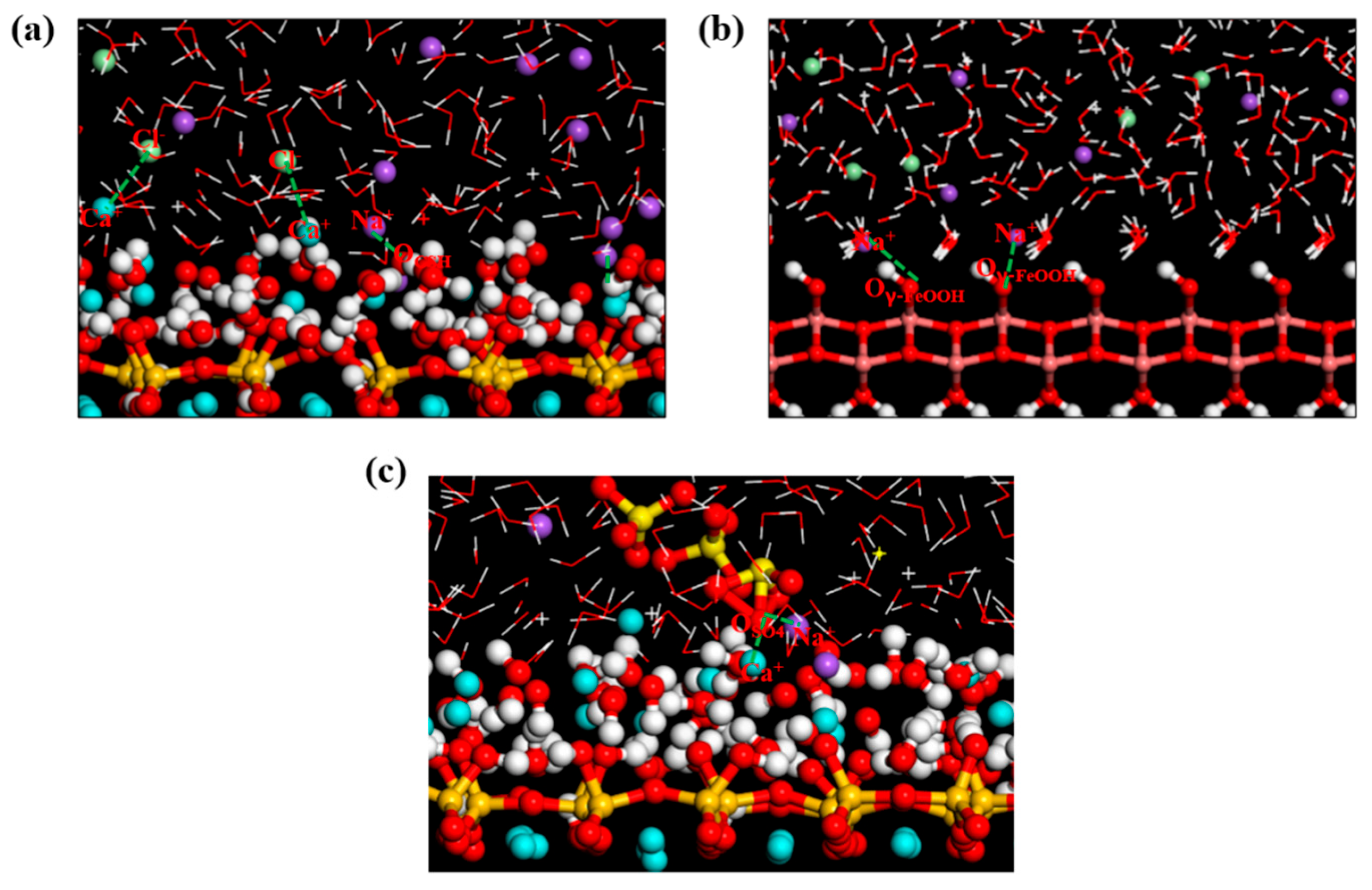
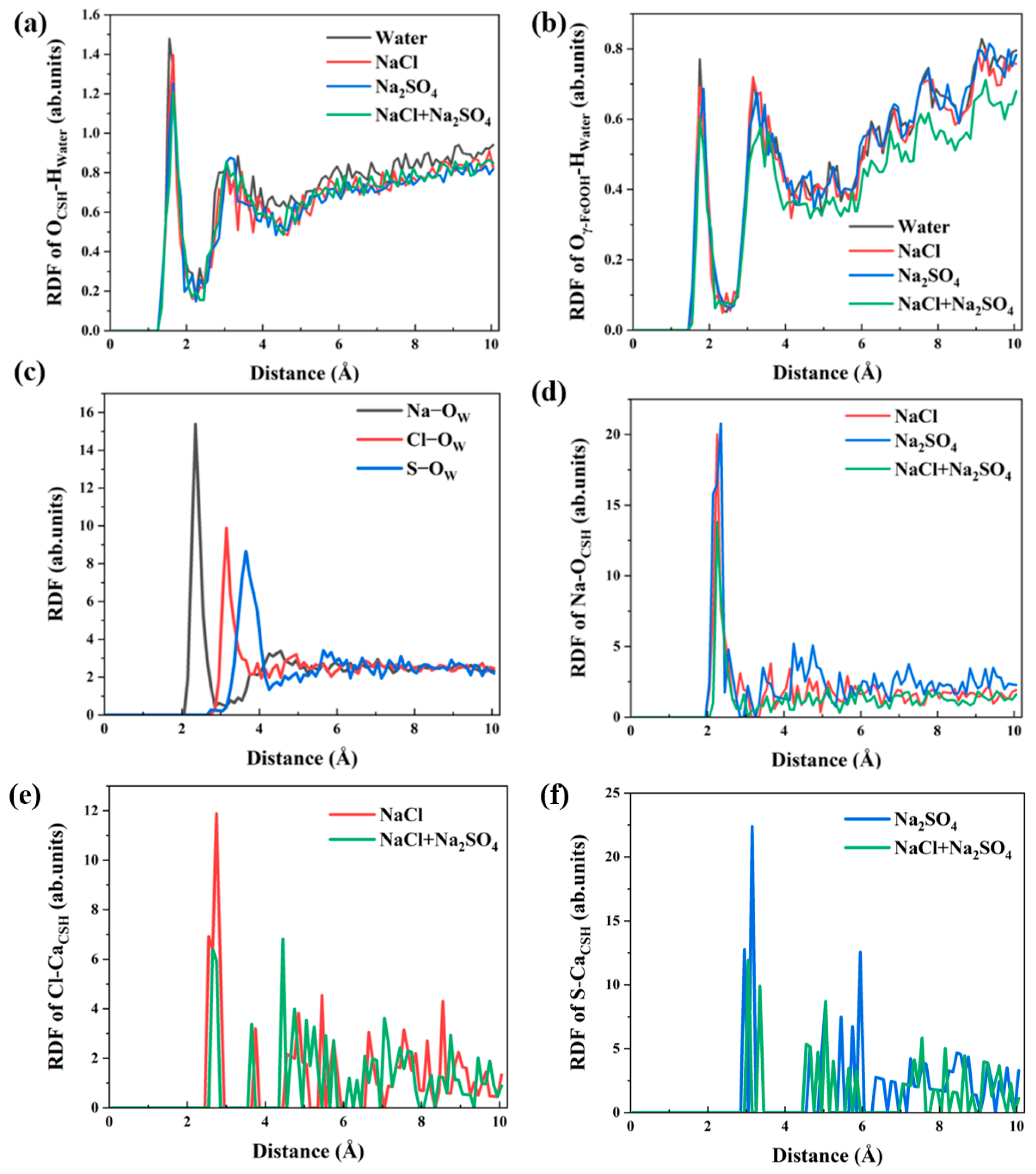
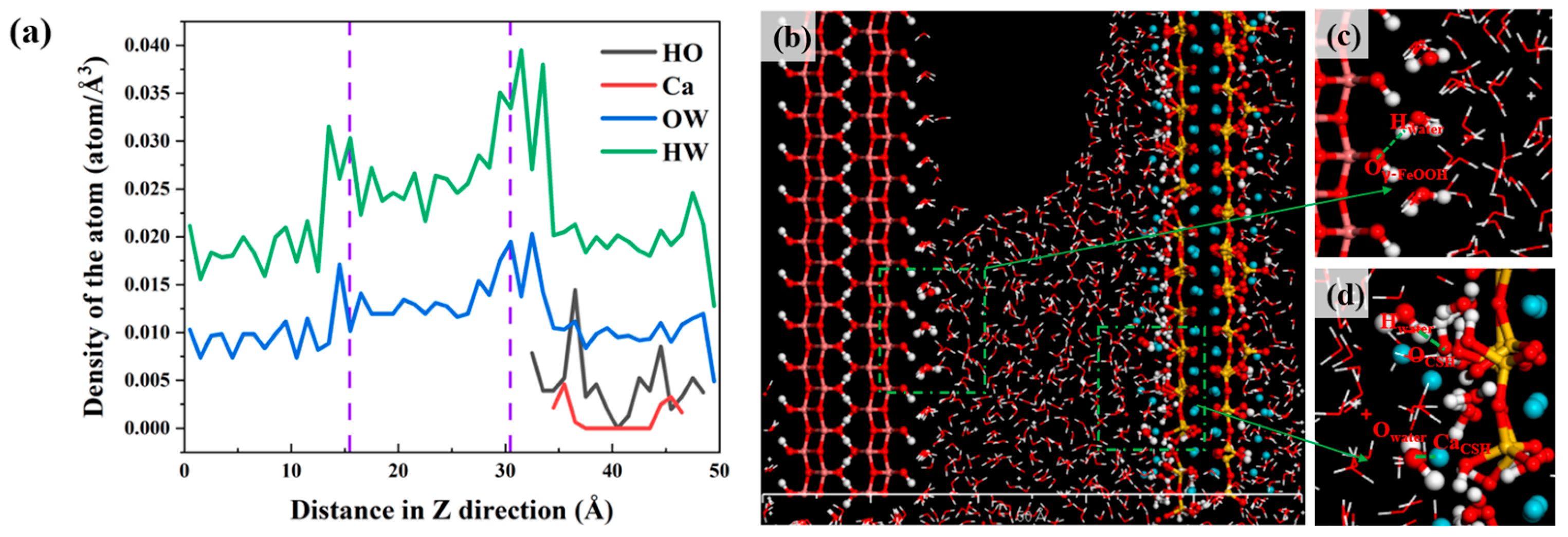
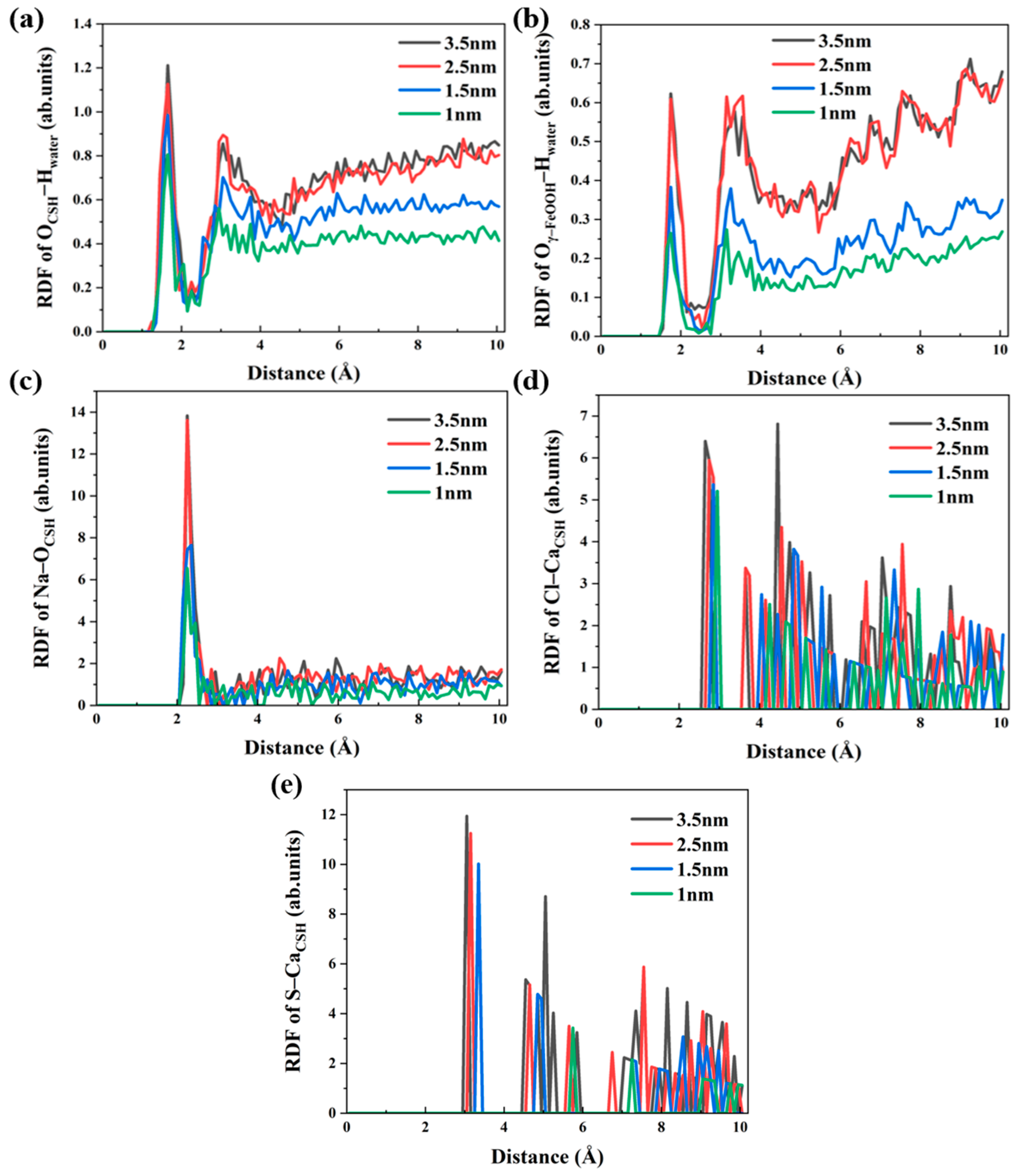
3.2. Local Structure of Different Solutions in the γ–FeOOH/CSH Pore
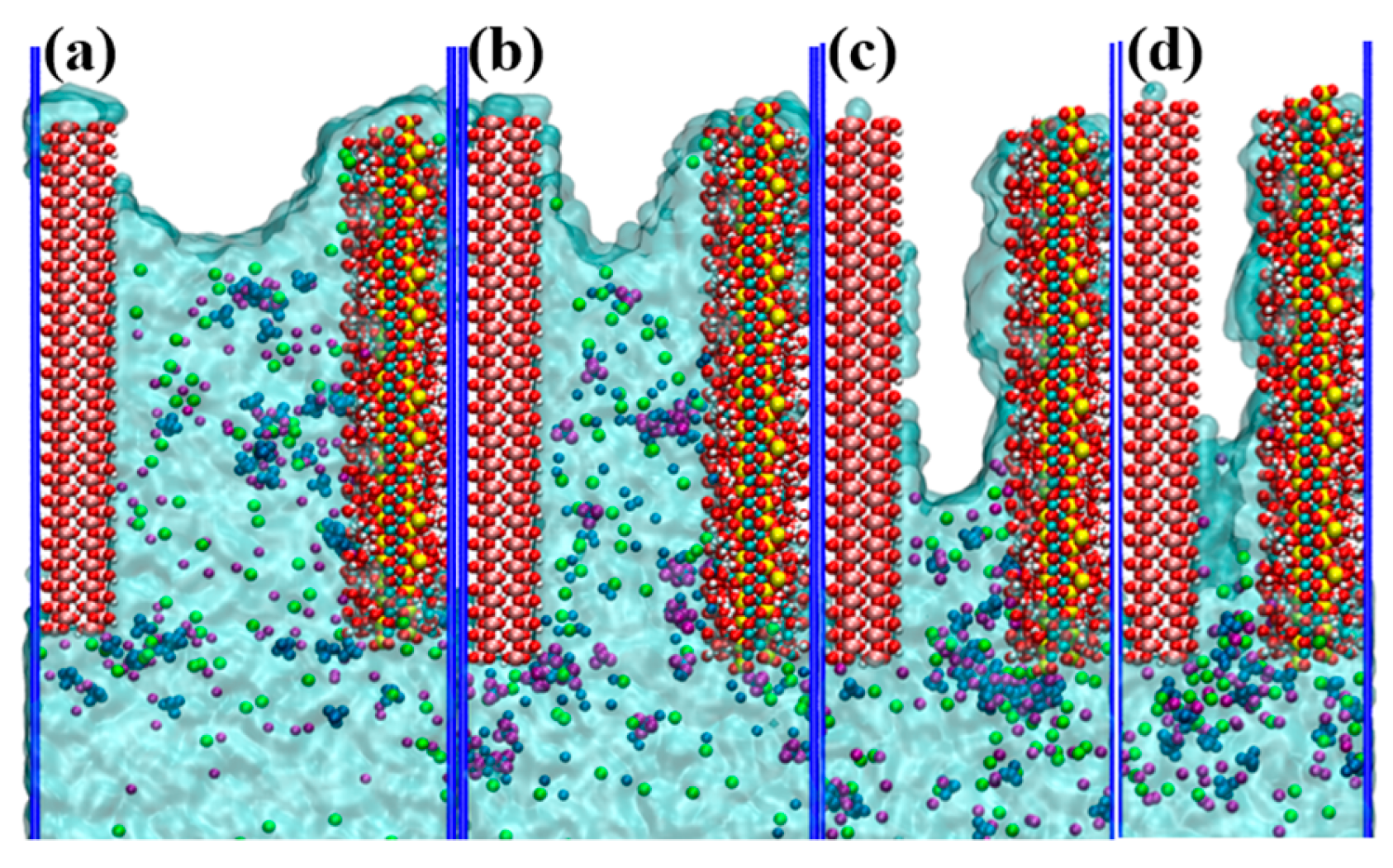
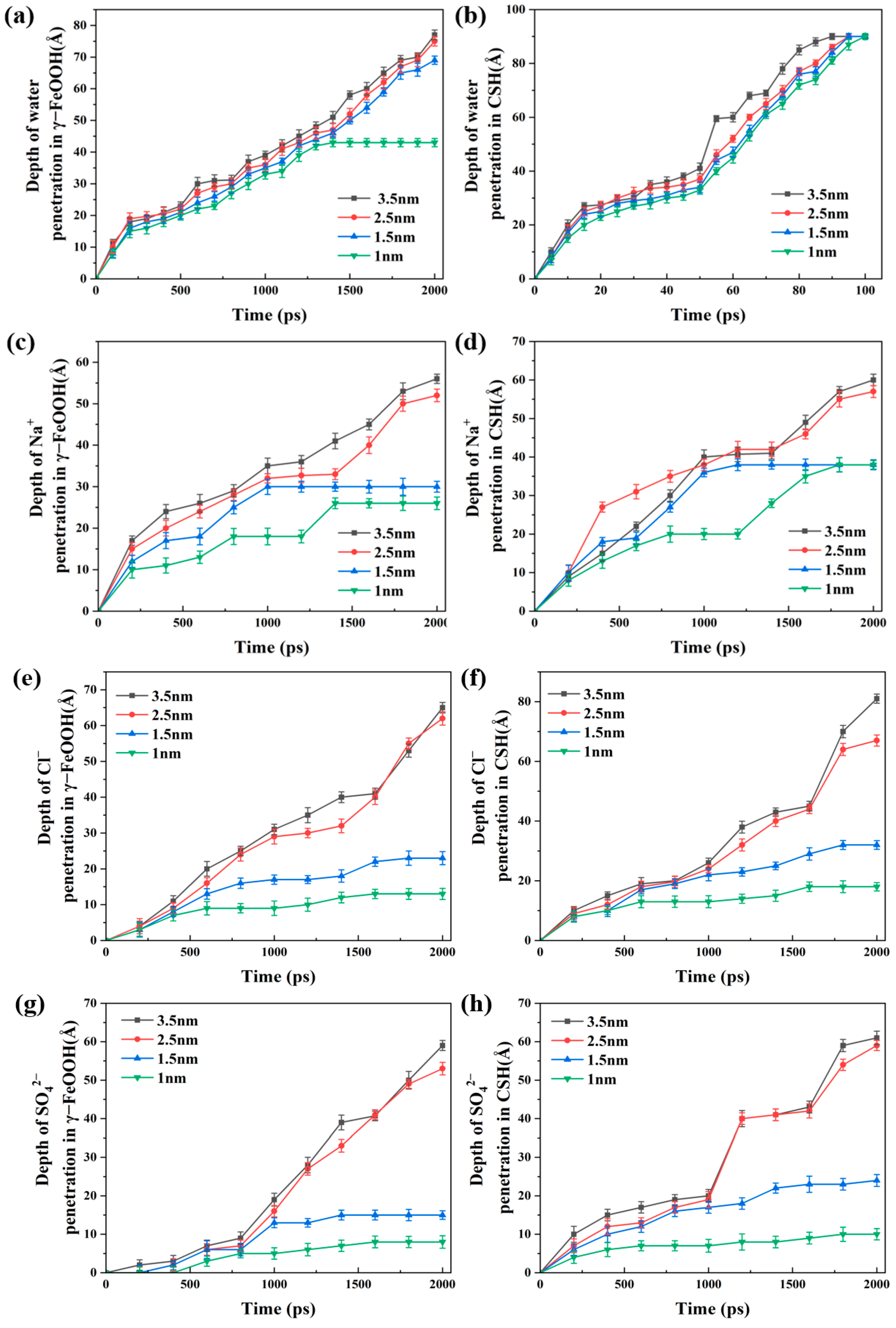
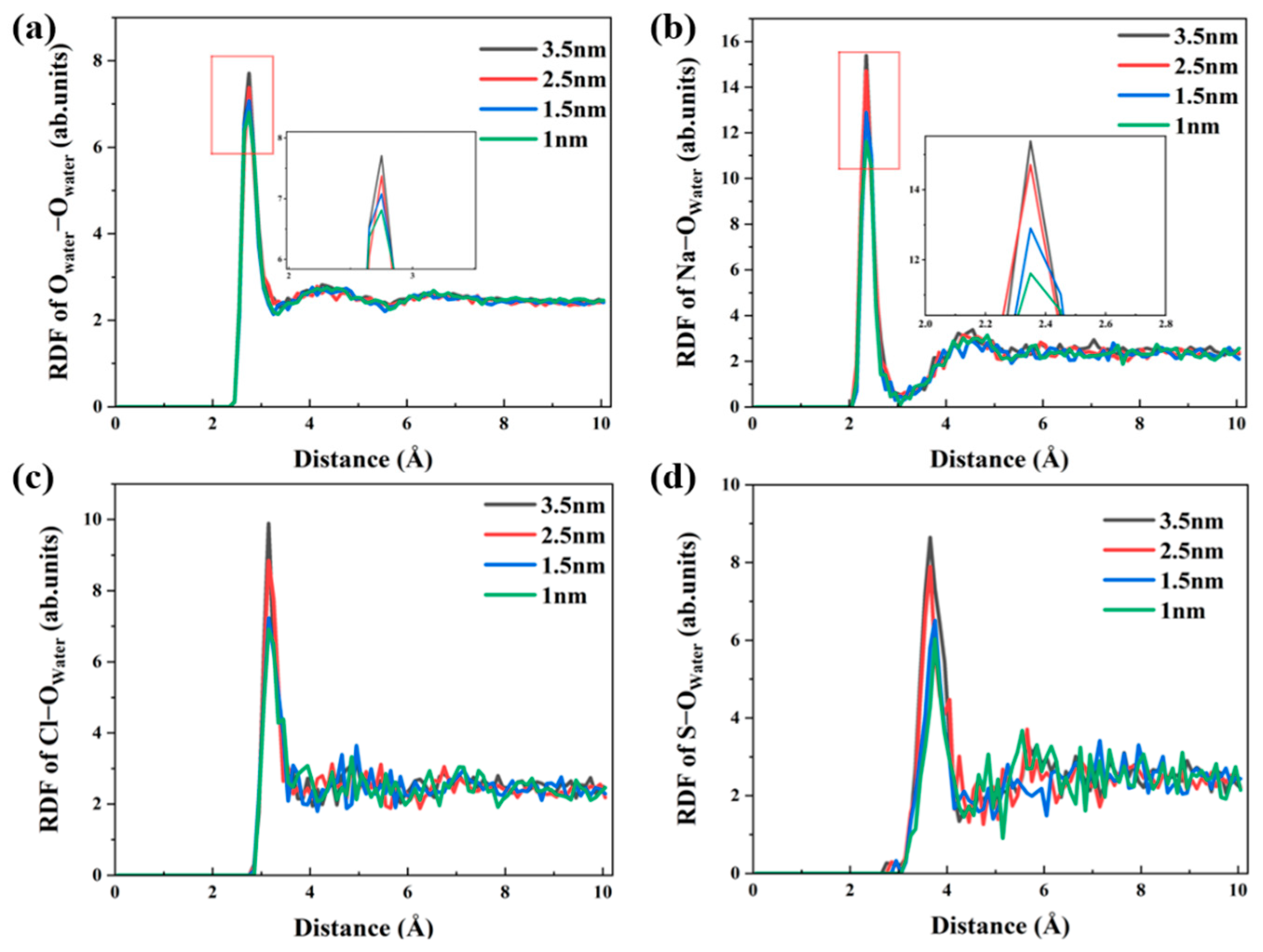
3.3. Water and Ions Transport in Different Pore Widths
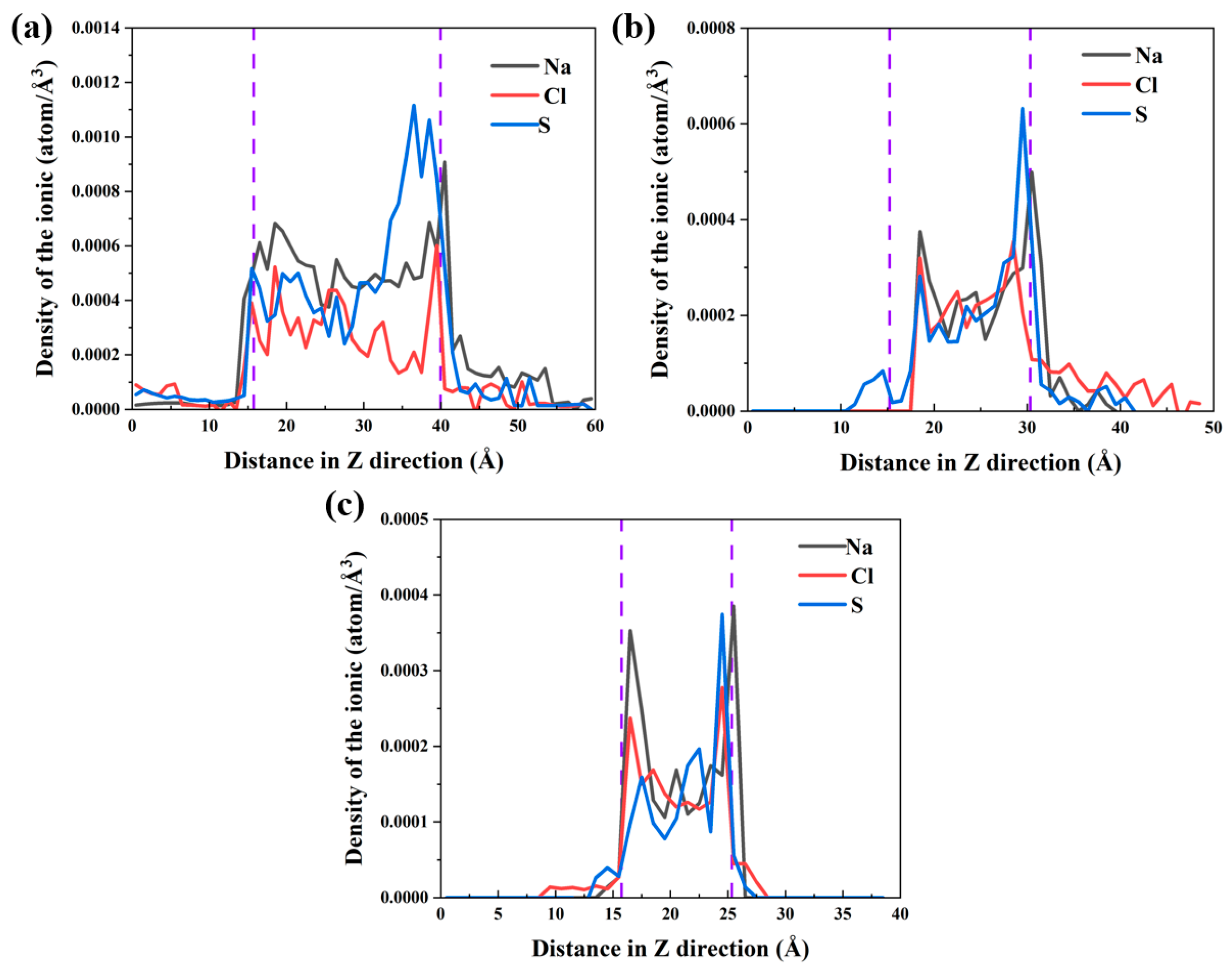
3.4. Local Structures of Water and Ions in Nano–Pores with Different Widths
4. Conclusions
- (1)
- The transport of solutions in nanopores was meniscus-shaped with contact angles less than 90° on both substrates, which was consistent with capillary transport and confirms the hydrophilic nature of both CSH and γ–FeOOH.
- (2)
- The penetration depths of different solutions were ordered as follows: D(water) > D(NaCl) > D(Na2SO4) > D(NaCl + Na2SO4). In all the solutions, water molecules penetrated along the CSH side faster than along the γ–FeOOH side. Ions penetrated more slowly than water molecules on both sides.
- (3)
- Water molecules in the solution formed hydrogen bonds with γ–FeOOH and CSH, facilitating transport and driving ion movement. The nanopores immobilized different ions via distinct adsorption mechanisms: the Na+, Cl−, and SO42− ions formed Na–OCSH, Cl–CaCSH, and S–CaCSH ion pairs with CSH, while Na–Oγ–FeOOH ion paired with γ–FeOOH. In the mixed solution of NaCl + Na2SO4, water and ion transport were inhibited by the formation of large clusters of ion pairs and their adsorption onto the CSH side.
- (4)
- The transport rate of water and ions reduced with decreasing pore width, and the difference between the penetration depths of the water molecules and ions increased due to capillary action. For pores with less than 2.5 nm width, the Na+, Cl−, and SO42− ions tended to remain in the entry region of the nanopore.
- (5)
- The Na–OCSH, Cl–CaCSH, and S–CaCSH ion pairs formed by CSH with the Na+, Cl−, and SO42− ions were significantly stronger than the hydrogen bonds between CSH and water molecules. As a result, water transport continued while the ions were retained on the CSH surface for extended periods. This led to the separation of ions from water. For pores with less than 2.5 nm width, the proximity of the substrate surfaces caused the ion pairs adsorbed on the two surfaces to interact, forming a large cluster of ion pairs such as Oγ–FeOOH–Na–SO4–Ca and Oγ–FeOOH–Na–Cl–Ca. This partially blocked the pore entrance and hindered the transport of water molecules and ions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faella, C.; Lima, C.; Martinelli, E.; Pepe, M.; Realfonzo, R. Mechanical and durability performance of sustainable structural concretes: An experimental study. Cem. Concr. Comp. 2016, 71, 85–96. [Google Scholar] [CrossRef]
- Peng, Y.; Yu, L.; Qian, J.; Li, W.; Zhang, T.; Zhou, L. Dynamic tensile behavior and crack propagation in coral aggregate seawater shotcrete: Experimental investigation and numerical simulation. Cem. Concr. Comp. 2025, 159, 06010. [Google Scholar] [CrossRef]
- Li, Z.Q.; Nie, L.; Xue, Y.; Li, W.; Fan, K. Model Testing on the Processes, Characteristics, and Mechanism of Water Inrush Induced by Karst Caves Ahead and Alongside a Tunnel. Rock. Mech. Rock. Eng. 2025, 58, 1–18. [Google Scholar] [CrossRef]
- Liu, H.; Li, K.; Li, Y.; Liu, S.; Dong, Z.; Cui, H.; Liu, W. Corrosion behavior of steel fiber reinforced concrete under ambipolar stray current interference. Constr. Build. Mater. 2024, 411, 134302. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Q.; Wang, X.; Yu, L.; Li, Z. Synergistic effect of particle size, carboxymethyl starch and Na2CO3 on rheological and filtration property of bentonite-based material. Case. Stud. Constr. Mat. 2024, 21, e03537. [Google Scholar] [CrossRef]
- Xu, L.; Zhu, X. Detection model for key nodes of manufacturing industry chain stability under complex networks: A naval architecture and marine engineering equipment industry chain example. Kybernetes 2025, 54, 2892–2915. [Google Scholar] [CrossRef]
- Gholamhoseini, A.; Khanlou, A.; MacRae, G.; Scott, A.; Hicks, S.; Leon, R. An experimental study on strength and serviceability of reinforced and steel fibre reinforced concrete (SFRC) continuous composite slabs. Eng. Struct. 2016, 114, 171–180. [Google Scholar] [CrossRef]
- Li, W.; Yu, L.; Zhang, T. Quantitative analysis of grain size effect on tensile mechanical behavior of granite based on multi-level force chain networks. Comp. Part. Mech. 2024, 11, 2245–2266. [Google Scholar] [CrossRef]
- Xiang, D.; Liu, S.; Li, Y.; Liu, Y. Improvement of flexural and cyclic performance of bridge deck slabs by utilizing steel fiber reinforced concrete (SFRC). Constr. Build. Mater. 2022, 329, 127184. [Google Scholar] [CrossRef]
- Song, W.; Yin, J. Hybrid effect evaluation of steel fiber and carbon fiber on the performance of the fiber reinforced concrete. Materials 2016, 9, 704. [Google Scholar] [CrossRef]
- Li, W.; Yu, L.Y.; Tan, Y.Z.; Wu, L.R.; Qian, J.Y. Mechanical properties and impact behavior of frozen clay: Insights from static mechanical tests, fly-plate tests, and split-Hopkinson pressure bar analysis. Phys. Fluids 2024, 36, 057138. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, C.; Gao, Z.; Wang, F. A review on fracture properties of steel fiber reinforced concrete. J. Build. Eng. 2023, 67, 105975. [Google Scholar] [CrossRef]
- Abbass, W.; Khan, M.I.; Mourad, S. Evaluation of mechanical properties of steel fiber reinforced concrete with different strengths of concrete. Constr. Build. Mater. 2018, 168, 556–569. [Google Scholar] [CrossRef]
- Sharma, S.; Arora, V.V.; Kumar, S.; Daniel, Y.N.; Sharma, A. Durability study of high-strength steel fiber-reinforced concrete. ACI. Mater. J. 2018, 115, 219–225. [Google Scholar] [CrossRef]
- Tian, J.; Wu, X.; Zuo, Y.; Yuan, J.; Wang, W.W. Interface behaviors between smart-functional ECC and steel rebar under coupling effect of sustained load and chloride ion erosion: Corrosion resistance, mechanical and self-sensing properties. Constr. Build. Mater. 2024, 438, 137205. [Google Scholar] [CrossRef]
- Li, H.; Wu, Y.; Zhou, A.; Lu, F.; Lei, Z.; Zeng, B.; Zhu, K. Cracking pattern and bearing capacity of steel fiber-reinforced concrete single-layer tunnel lining. Sustainability 2023, 13, 10665. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of soft matter nanomechanics by atomic force microscopy and optical tweezers: A comprehensive review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef]
- Hwang, J.P.; Jung, M.S.; Kim, M.; Ann, K.Y. Corrosion risk of steel fibre in concrete. Constr. Build. Mater. 2015, 101, 239–245. [Google Scholar] [CrossRef]
- Yoo, D.Y.; Shin, W.; Chun, B. Corrosion effect on tensile behavior of ultra–high–performance concrete reinforced with straight steel fibers. Cem. Concr. Comp. 2020, 109, 103566. [Google Scholar] [CrossRef]
- Tai, Y.; Yang, L.; Gao, D.; Kang, K.; Cao, Z.; Zhao, P. Properties evolution and deterioration mechanism of steel fiber reinforced concrete (SFRC) under the coupling effect of carbonation and chloride attack. J. Build. Eng. 2024, 95, 110275. [Google Scholar] [CrossRef]
- Marcos–Meson, V.; Fischer, G.; Edvardsen, C.; Skovhus, T.L.; Michel, A. Durability of Steel Fibre Reinforced Concrete (SFRC) exposed to acid attack—A literature review. Constr. Build. Mater. 2019, 200, 490–501. [Google Scholar] [CrossRef]
- Feng, G.; Zhu, D.; Guo, S.; Rahman, M.Z.; Jin, Z.; Shi, C. A review on mechanical properties and deterioration mechanisms of FRP bars under severe environmental and loading conditions. Cem. Concr. Comp. 2022, 134, 104758. [Google Scholar] [CrossRef]
- Arnittali, M.; Rissanou, A.N.; Harmandaris, V. Structure of biomolecules through molecular dynamics simulations. Procedia Comput. Sci. 2019, 156, 69–78. [Google Scholar] [CrossRef]
- Hou, D.; Lu, C.; Zhao, T.; Zhang, P.; Ding, Q. Structural, dynamic and mechanical evolution of water confined in the nanopores of disordered calcium silicate sheets. Microfluid. Nanofluid. 2015, 19, 1309–1323. [Google Scholar] [CrossRef]
- Hou, D.; Jia, Y.; Yu, J.; Wang, P.; Liu, Q.F. Transport properties of sulfate and chloride ions confined between calcium silicate hydrate surfaces: A molecular dynamics study. J. Phys. Chem. C 2018, 122, 28021–28032. [Google Scholar] [CrossRef]
- Yang, J.; Jia, Y.; Hou, D.; Wang, P.; Jin, Z.; Shang, H.; Li, S.; Zhao, T. Na and Cl immobilization by size controlled calcium silicate hydrate nanometer pores. Constr. Build. Mater. 2019, 202, 622–635. [Google Scholar] [CrossRef]
- Maddalena, R.; Li, K.; Chater, P.A.; Michalik, S.; Hamilton, A. Direct synthesis of a solid calcium-silicate-hydrate (CSH). Constr. Build. Mater. 2019, 223, 554–565. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Kirkpatrick, R.J. Innovation in use and research on cementitious material. Cem. Concr. Res. 2008, 38, 128–136. [Google Scholar] [CrossRef]
- Hashim, A.A.; Al-Mosawi, A.I.; Abdulsada, S.A. Investigating the mechanical properties, durability, microstructure, and embodied CO2 emissions of silica fume-infused sustainable concrete. Int. J. Appl. Ceram. Technol. 2025, e15136. [Google Scholar] [CrossRef]
- Li, S.; Jensen, O.M.; Yu, Q. Influence of steel fiber content on the rate-dependent flexural performance of ultra-high performance concrete with coarse aggregates. Constr. Build. Mater. 2022, 318, 125935. [Google Scholar] [CrossRef]
- Yang, K.H.; Cho, A.R.; Song, J.K.; Nam, S.H. Hydration products and strength development of calcium hydroxide–based alkali–activated slag mortars. Constr. Build. Mater. 2012, 29, 410–419. [Google Scholar] [CrossRef]
- Farges, F.; Lefrère, Y.; Rossano, S.; Berthereau, A.; Calas, G.; Brown, G.E., Jr. The effect of redox state on the local structural environment of iron in silicate glasses: A combined XAFS spectroscopy, molecular dynamics, and bond valence study. J. Non. Cryst. Solids 2004, 344, 176–188. [Google Scholar] [CrossRef]
- Leung, C.K.Y.; Wan, K.T.; Chen, L. A novel optical fiber sensor for steel corrosion in concrete structures. Sensors 2008, 8, 1960–1976. [Google Scholar] [CrossRef] [PubMed]
- Vayer, M.; Reynaud, I.; Erre, R. XPS characterisations of passive films formed on martensitic stainless steel: Qualitative and quantitative investigations. J. Mater. Sci. 2000, 35, 2581–2587. [Google Scholar] [CrossRef]
- Hou, D.; Xu, X.; Ge, Y.; Wang, P.; Chen, J.; Zhang, J. Molecular structure, dynamics and adsorption behavior of water molecules and ions on [0 1 0] surface of γ-FeOOH: A molecular dynamics approach. Constr. Build. Mater. 2019, 224, 785–795. [Google Scholar] [CrossRef]
- Hamid, S.A. The crystal structure of the 11Å natural tobermorite Ca2.25[Si3O7.5(OH)1.5]1H2O. Z. Kristallogr. Cryst. Mater. 1981, 154, 189–198. [Google Scholar] [CrossRef]
- Manzano, H.; Moeini, S.; Marinelli, F.; Van Duin, A.C.; Ulm, F.J.; Pellenq, R.J. Confined water dissociation in microporous defective silicates: Mechanism, dipole distribution, and impact on substrate properties. J. Am. Chem. Soc. 2012, 134, 2208–2215. [Google Scholar]
- Zhang, W.; Zhang, M.; Hou, D. Nanoscale insights into the anti-erosion performance of concrete: A molecular dynamics study. Appl. Surf. Sci. 2022, 593, 153403. [Google Scholar] [CrossRef]
- Hou, D.; Yang, Q.; Wang, P.; Jin, Z.; Wang, M.; Zhang, Y.; Wang, X. Unraveling disadhesion mechanism of epoxy/CSH interface under aggressive conditions. Cem. Concr. Res. 2021, 146, 106489. [Google Scholar] [CrossRef]
- Thompson, A.P.; Aktulga, H.M.; Berger, R.; Bolintineanu, D.S.; Brown, W.M.; Crozier, P.S.; Plimpton, S.J. LAMMPS—A flexible simulation tool for particle–based materials modeling at the atomic, meso, and continuum scales. Comput. Phys. Commun. 2022, 271, 108171. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. Model. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Pouvreau, M.; Greathouse, J.A.; Cygan, R.T.; Kalinichev, A.G. Structure of hydrated kaolinite edge surfaces: DFT results and further development of the ClayFF classical force field with metal–O–H angle bending terms. J. Phys. Chem. C 2019, 123, 11628–11638. [Google Scholar] [CrossRef]
- Luty, B.A.; van Gunsteren, W.F. Calculating electrostatic interactions using the particle–particle particle–mesh method with nonperiodic long–range interactions. J. Phys. Chem. 1996, 100, 2581–2587. [Google Scholar] [CrossRef]
- Hou, D.; Li, T.; Wang, P. Molecular dynamics study on the structure and dynamics of NaCl solution transport in the nanometer channel of CASH gel. ACS Sustain. Chem. Eng. 2018, 6, 9498–9509. [Google Scholar] [CrossRef]
- Zehtab, B.; Tarighat, A. Molecular dynamics simulation to assess the effect of temperature on diffusion coefficients of different ions and water molecules in CSH. Mech. Time-Depend. Mat. 2018, 22, 483–497. [Google Scholar] [CrossRef]
- Wang, P.; Jia, Y.; Li, T.; Hou, D.; Zheng, Q. Molecular dynamics study on ions and water confined in the nanometer channel of Friedel’s salt: Structure, dynamics and interfacial interaction. Phys. Chem. Chem. Phys. 2018, 20, 27049–27058. [Google Scholar] [CrossRef]
- Hou, D.; Gao, L.; Chen, D.; Wang, P.; Wang, J.; Zhou, Y.; Zhang, J. Molecular-scale insights on structure-efficiency relationship of silane-based waterproofing agents. Constr. Build. Mater. 2022, 327, 126985. [Google Scholar] [CrossRef]
- Hou, D.; Li, T. Influence of aluminates on the structure and dynamics of water and ions in the nanometer channel of calcium silicate hydrate (C–S–H) gel. Phys. Chem. Chem. Phys. 2018, 20, 2373–2387. [Google Scholar] [CrossRef]
- Suwito, A.; Cai, X.C.; Xi, Y. Parallel finite element methods for coupled chloride penetration and moisture diffusion in concrete. Int. J. Numer. Anal. Mod. 2006, 3, 481–503. [Google Scholar]
- Hou, D.; Li, Z. Molecular dynamics study of water and ions transport in nano–pore of layered structure: A case study of tobermorite. Micropor. Mesopor. Mater. 2014, 195, 9–20. [Google Scholar]
- Li, D.; Zhao, W.; Hou, D.; Zhao, T. Molecular dynamics study on the chemical bound, physical adsorbed and ultra–confined water molecules in the nano–pore of calcium silicate hydrate. Constr. Build. Mater. 2017, 151, 563–574. [Google Scholar] [CrossRef]
- Khaleel Ibrahim, S.; Abbas Hadi, N.; Movahedi Rad, M. Experimental and numerical analysis of steel-polypropylene hybrid fibre reinforced concrete deep beams. Polymers 2023, 15, 2340. [Google Scholar] [CrossRef]



| Cement | Silica Fume | Fly Ash | Water | Superplasticizer | Steel Fiber | Quartz Sand | Coarse Aggregate |
|---|---|---|---|---|---|---|---|
| 559.68 | 79.95 | 159.908 | 127.9264 | 15.9908 | 157 | 799.54 | 600 |
| H2O | Na+ | Cl− | SO42− | |
|---|---|---|---|---|
| Water | 5290 | 0 | 0 | 0 |
| NaCl | 5223 | 84 | 84 | 0 |
| Na2SO4 | 5223 | 68 | 0 | 34 |
| NaCl + Na2SO4 | 4948 | 152 | 84 | 34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luan, Y.; Wang, R.; Huang, C.; Jivkov, A.; Zhang, L. Transport Properties of Solutions in γ–FeOOH/CSH Pores of Steel Fiber-Reinforced Concrete (SFRC) Derived Using Molecular Dynamics. Materials 2025, 18, 2176. https://doi.org/10.3390/ma18102176
Luan Y, Wang R, Huang C, Jivkov A, Zhang L. Transport Properties of Solutions in γ–FeOOH/CSH Pores of Steel Fiber-Reinforced Concrete (SFRC) Derived Using Molecular Dynamics. Materials. 2025; 18(10):2176. https://doi.org/10.3390/ma18102176
Chicago/Turabian StyleLuan, Yalin, Runan Wang, Changxin Huang, Andrey Jivkov, and Lianzhen Zhang. 2025. "Transport Properties of Solutions in γ–FeOOH/CSH Pores of Steel Fiber-Reinforced Concrete (SFRC) Derived Using Molecular Dynamics" Materials 18, no. 10: 2176. https://doi.org/10.3390/ma18102176
APA StyleLuan, Y., Wang, R., Huang, C., Jivkov, A., & Zhang, L. (2025). Transport Properties of Solutions in γ–FeOOH/CSH Pores of Steel Fiber-Reinforced Concrete (SFRC) Derived Using Molecular Dynamics. Materials, 18(10), 2176. https://doi.org/10.3390/ma18102176






