Effect of CeO2 Addition on the Microstructure and Properties of Induction Heating Ni-WC-CeO2 Composite Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
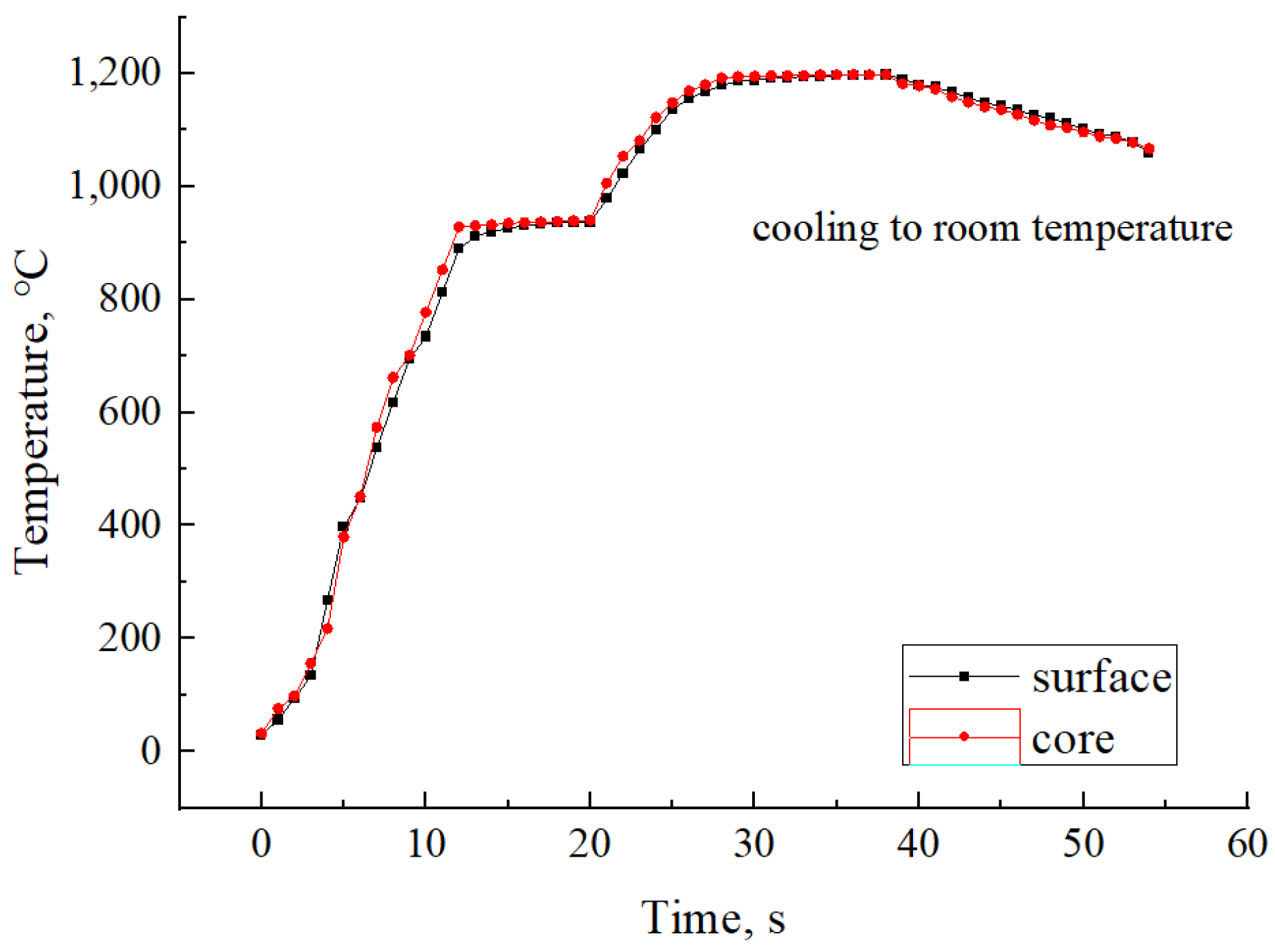
2.2. Production-Processes Control
3. Results and Discussion
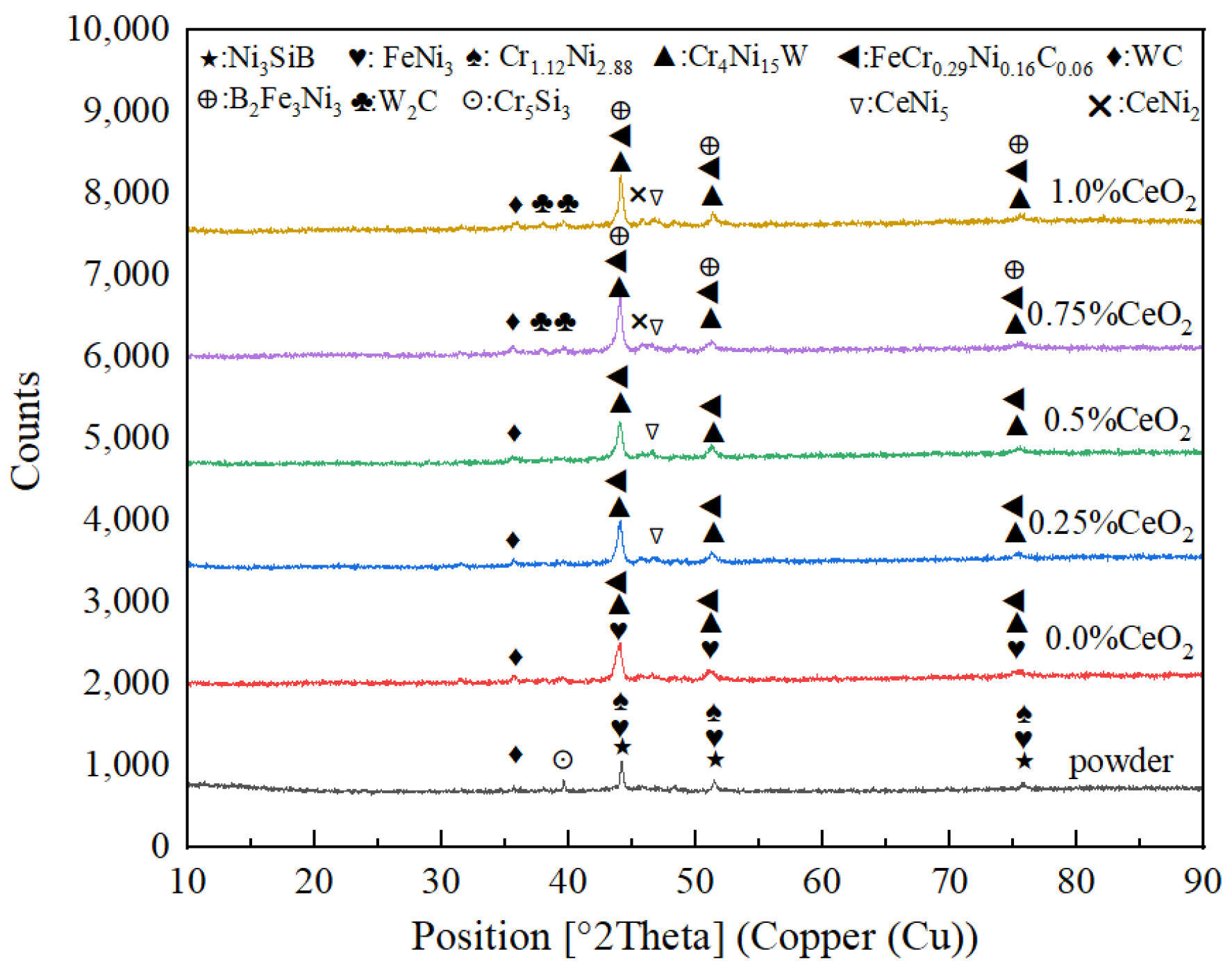
3.1. Influence of CeO2 Addition on Coating Phase Composition
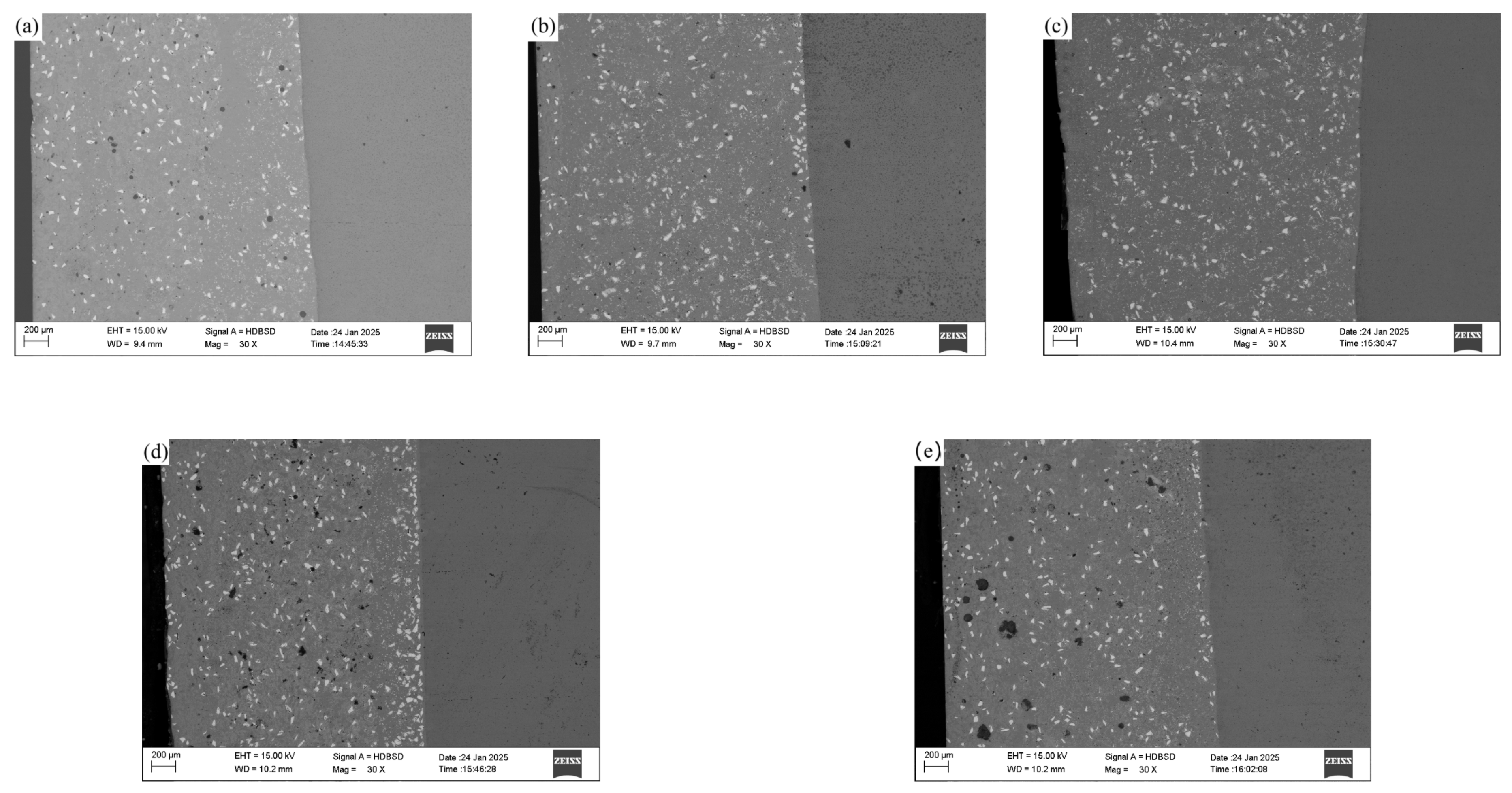
3.2. Influence of CeO2 Addition on Macroscopic Morphology of Coating
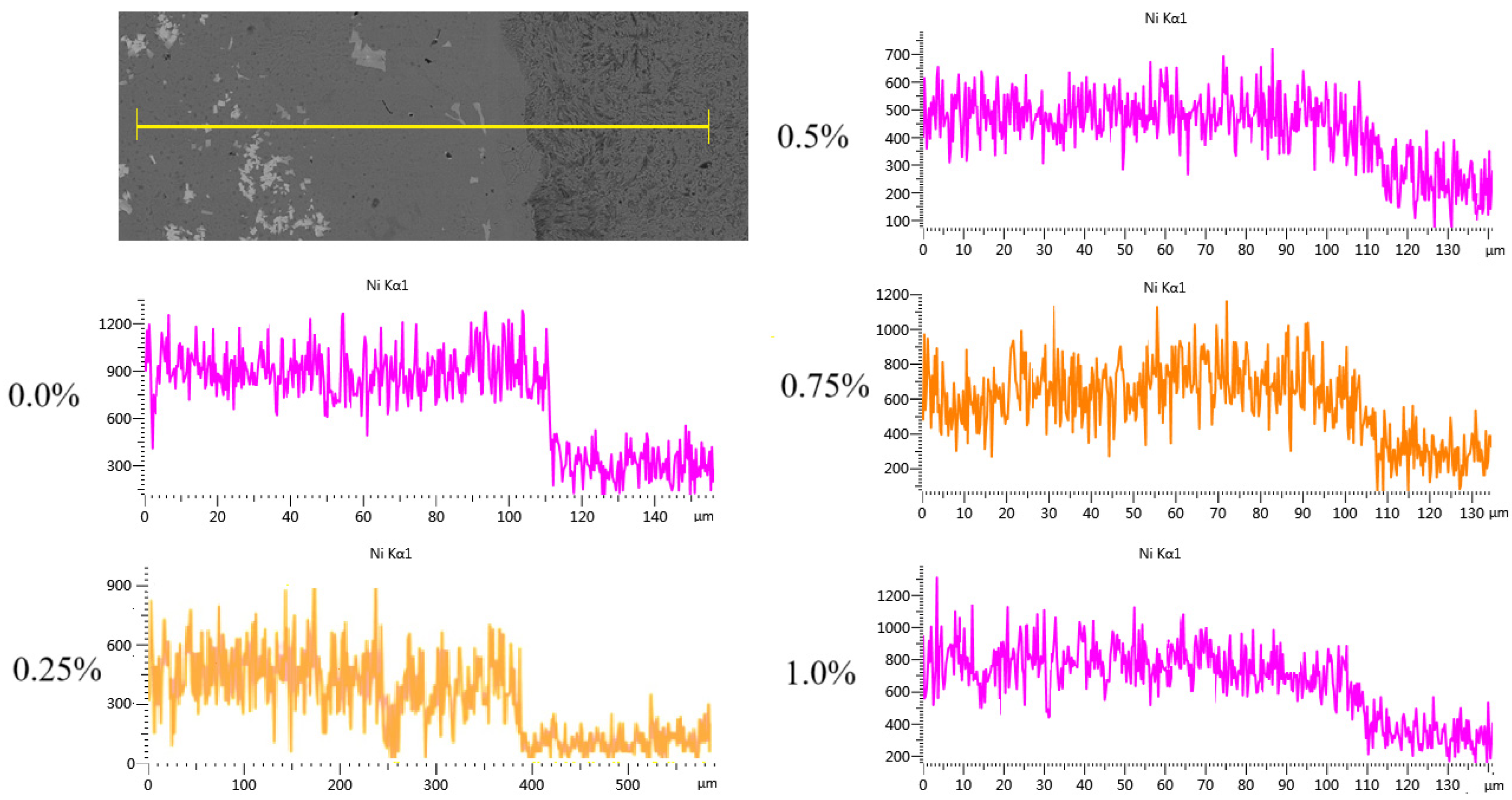
3.3. Effect of CeO2 Addition on Coating Bonding
3.4. Effect of CeO2 Addition on Coating Microstructure
3.5. Effect of CeO2 Addition on Cross-Section Microhardness of Coating
3.6. Effect of CeO2 Addition on Wear Resistance of Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ruktuev, A.A.; Lazurenko, D.V.; Kiseleva, N.A. Formation of the CoCrCuFeNi high entropy cladding layer by non-vacuum electron beam treatment. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1093, 012028. [Google Scholar] [CrossRef]
- China Composite Materials Society. Manufacturing Process and Equipment Technology of Advanced Composite Material Structure; China Railway Publishing House: Beijing, China, 2021; pp. 1–3. [Google Scholar]
- Wang, Q.; Li, Q.; Zhang, L.; Chen, D. Microstructure and properties of Ni-WC gradient composite coating prepared by laser cladding. Ceram. Int. 2022, 6, 7905–7917. [Google Scholar] [CrossRef]
- Meng, G.; Zhu, L.; Zhang, J. Statistical analysis and multi-objective process optimization of laser cladding TiC-Inconel718 composite coating. Opt. Int. J. Light Electron Opt. 2021, 240, 166828. [Google Scholar] [CrossRef]
- Pan, L. Surface Modification Heat Treatment Technology and Application; Machinery Industry Press: Beijing, China, 2006; pp. 1–2. [Google Scholar]
- Zhang, N.; Xu, Y.; Han, M. Damage mechanism of Fe-based powder metallurgy friction materials in high energy braking. Powder Metall. Technol. 2023, 3, 275–285. [Google Scholar]
- Ou, Y.; Zhai, B.; Chen, W. Microstructure and mechanical properties of FeCrCoMn Ni matrix composites reinforced by Ti C particles. Powder Metall. Technol. 2024, 42, 338–345. [Google Scholar]
- Wang, Z.; Mu, J. Rapid Heat Treatment of Steel by Induction Heating; Chemical Industry Press: Beijing, China, 2012; pp. 23–24. [Google Scholar]
- Feng, W. Application of Induction Heating in Steel Wire Production. Met. Work. 2023, 8, 25–29. [Google Scholar]
- John, N. Welding Metallurgy & Weldability of Nickel-Base Alloys; Shanghai Scientific and Technological Literature Press: Shanghai, China, 2014; pp. 100–102. [Google Scholar]
- Chen, Z.; Chen, G.; Zhu, Q. Study on Argon Arc Surfacing Technology of Nickel Base Self-melting Alloy. Weld. Technol. 2019, 8, 58–61. [Google Scholar]
- Yang, S.; Qu, H.; Li, H. Properties of (Ti,W)C Particles Reinforced Ni-based Coating by Laser Cladding. J. Northeast. Univ. Nat. Sci. 2024, 7, 953–960. [Google Scholar]
- Ding, K.; Zhao, W.; Li, Z. Study on Influence of the Scanning Speed on Microstructure and Corrosion Resistance of Plasma Cladding WC Reinforced Nickel based Alloy Coating. Welded Pipe Tube 2023, 3, 20–25. [Google Scholar]
- Qu, Z.; Zhang, B.; Wang, L. Analysis on Microstructure and Electrochemical Corrosion of Nickel-based Alloy Coating Prepared by Thermal Spraying-Induction Fusion Welding. Hot Work. Technol. 2023, 8, 92–96. [Google Scholar]
- Hua, S.; Pang, M. Effect of Ti3SiC2 on properties of Ni based layers prepared by laser cladding on Q235 steel surface. Trans. Mater. Heat Treat. 2021, 10, 133–140. [Google Scholar]
- Zhao, N.; Tao, L.; Guo, H. Microstructure and Wear Resistance of Laser Cladded Ni-based Coatings with Nanometer La2O3 Addition. Rare Met. Mater. Eng. 2017, 8, 2092–2096. [Google Scholar]
- Rohini, B.S. Synthesis of CeO2 Nanopowders for Advanced Forensic Applications; LAP Lambert Academic Publishing: Salebr, Germany, 2024; pp. 1–10. [Google Scholar]
- Jiao, M.; Hu, K.; Sun, Q. First principles study on lattice dynamics and thermal transport properties of CeO2. Chin. J. Comput. Phys. 2025, 1, 1–10. [Google Scholar]
- Wu, J.; Su, Y.; Guo, Y. Effect of CeO2 Adding Content on the Microstructure and Tribological Properties of lron-Based Alloy Coatings. Mater. Prot. 2023, 9, 83–90. [Google Scholar]
- Xu, H.; Lin, C.; Liu, J. Effect of CeO2 Adding Content on Microstructure and Properties of Laser Cladding WC Reinforced Nickel-Based Alloy Coating. Mater. Mech. Eng. 2021, 7, 2021–2028. [Google Scholar]
- Qin, N.; Wu, X.; Zhang, S.X. Effeet of CeO2 Doping on Corrosion Resistance of CoCrFeNiMo0.2 High-Entropy Alloy Coating. Appl. Laser 2024, 10, 103683. [Google Scholar]
- Wu, Z.; Miao, L.; Feng, D. The Influence of CeO2 Content on the Microstructure and Properties of 45 steel Surface Laser Cladding Ni-WC Coating. Chin. Rare Earths 2022, 3, 100–110. [Google Scholar]
- Yin, Y.; Zhao, K.; Li, H. Effect of CeO2 Addition on Quality of Laser Cladding Ni60A/TC4 Composite Layers under high pressure. Hot Work. Technol. 2025, 8, 119–124. [Google Scholar]
- Wu, Y. Performance Comparison of Structural Ste e ls in Chine se and American Standards. J. Zhejiang Univ.-Sci. A Appl. Phys. Eng. 2020, 9, 26–43. [Google Scholar]
- Jiang, S.; Zhao, C. Principle & Application of Elements of induction Heating; Tianjin Science and Technology Translation Publishing House: Tianjing, China, 1993; pp. 1–3. [Google Scholar]
- Wang, Y.; Yang, S.; Cui, L. Influence of Induction Quenching Heating Power on Wear Resistance of 42CrMoA Steel for Crankshaft. Heat Treat. Technol. Equip. 2025, 1, 30–36. [Google Scholar]
- Xiao, L.; Yin, C.; Yang, L. Effect of Cr3C2 addition amount on microstructure and mechanical properties of ultrafine grained WC-6%Co cemented carbide. Cem. Carbides 2024, 1, 28–36. [Google Scholar]
- Chen, S.; Tian, F.; Li, G. Phase Diagram Analysis & Application; Metallurgical Industry Press: Beijing, China, 2007; pp. 100–110. [Google Scholar]
- Chen, F.; Li, Y. Phase Diagram Principle and Metallurgical Phase Diagram; Metallurgical Industry Press: Beijing, China, 2002; pp. 203–210. [Google Scholar]
- Dong, J. Squeezing and Microstructure Control of Nickel Base Alloy Pipes; Metallurgical Industry Press: Beijing, China, 2007; pp. 1–4. [Google Scholar]
- Technical Qualification Appraisal Committee for Nondestructive Testing Personnel of Ordnance Industries. In Manual for Quick Inquiry of Magnetic Characteristic Curve of Common Steel Materials; Machinery Industry Press: Beijing, China, 2004; pp. 10–20.
- Lou, J.; Yu, J.; Li, C. Mechanism of N2O Formation over Supported Pt/CeO2 Catalysts. J. Mater. Sci. Eng. 2021, 1, 1–9. [Google Scholar]
- Sistema, P. Periodic Table of Elements; People’s Literature Publishing House: Beijing, China, 2017; pp. 35–59. [Google Scholar]
- Yang, C.; Ding, X.; Tian, Y. Microstructure and cavitation corrosion properties of CeO2 modified multiscale WC-CoCr coatings. Funct. Mater. 2023, 4, 0427–0433. [Google Scholar]
- Yang, D.; Zhang, G.; Wang, Q. Effect and Influence of Rare Earth Oxides on Laser Cladding Nickel-Based Alloy Coatings. Appl. Laser 2023, 3, 9–18. [Google Scholar]
- He, L.; Tan, Y.; Tan, H. Microstructure and Tribological Properties of WC-CeO2/Ni-base Alloy Composite Coatings. Rare Met. Mater. Eng. 2014, 4, 0823–0829. [Google Scholar] [CrossRef]
- Tang, R.; Tian, R. Binary Alloy Phase Diagram and Intermediate Phase Crystal Structure; Central South University Press: Changsha, China, 2009; pp. 100–102. [Google Scholar]
- Wang, Y.; Li, M. Controlled Rolling and Controlled Cooling of Steel; Metallurgical Industry Press: Beijing, China, 2009; pp. 10–12. [Google Scholar]
- Wen, S. Principles of Tribology; Tsinghua University Press: Beijing, China, 2018; pp. 10–12. [Google Scholar]
- Wang, J.; Dong, G. Basic Tribology; Xidian University Press: Xi’an, China, 2018; pp. 4–6. [Google Scholar]
- Tian, X.; Chen, B.; Yang, X. Effect of WC Content on Microstructure and Properties of WC/Ni60 Laser Cladding Layer. China Laser 2025, 2, 0402205. [Google Scholar]


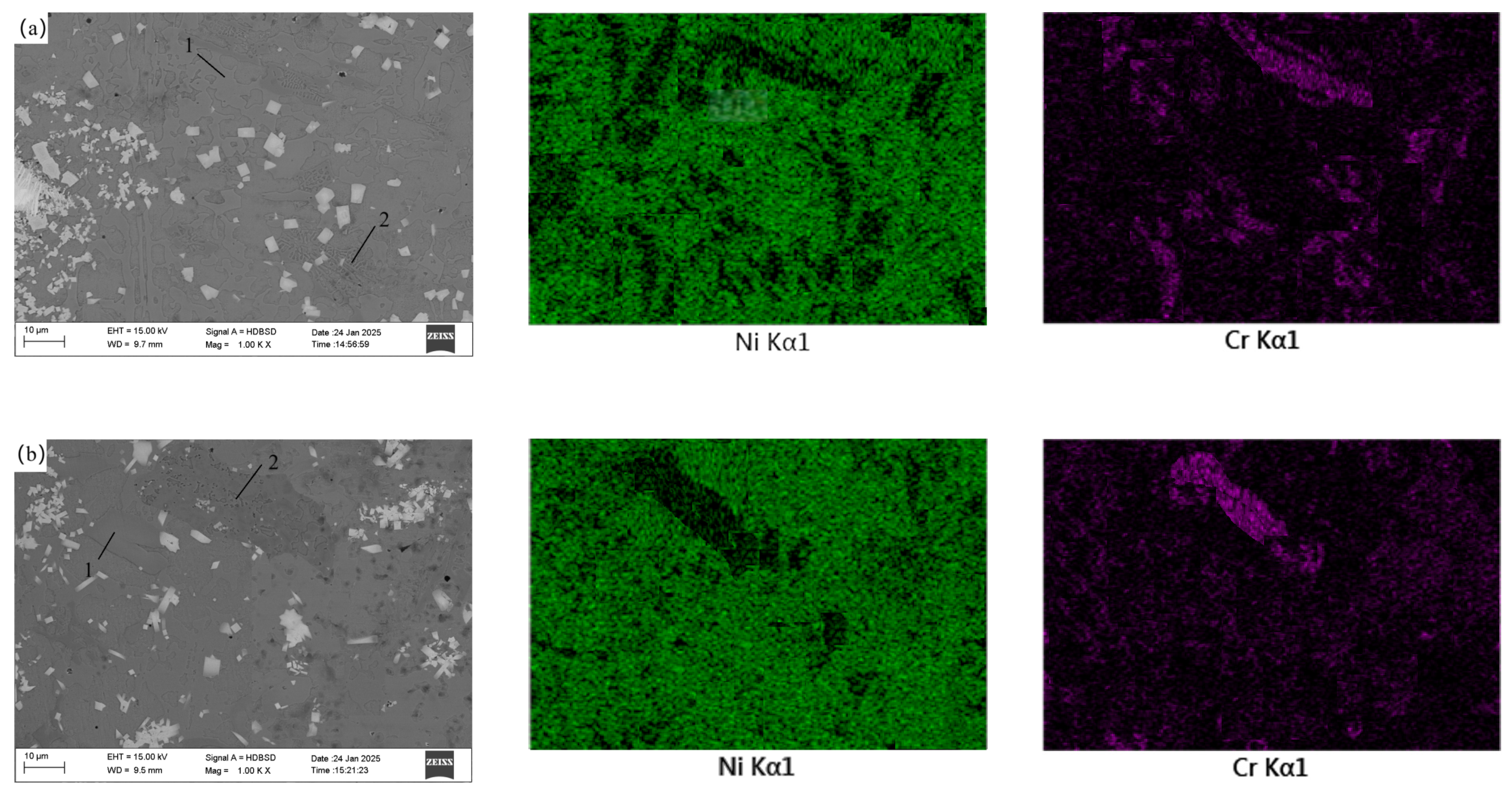



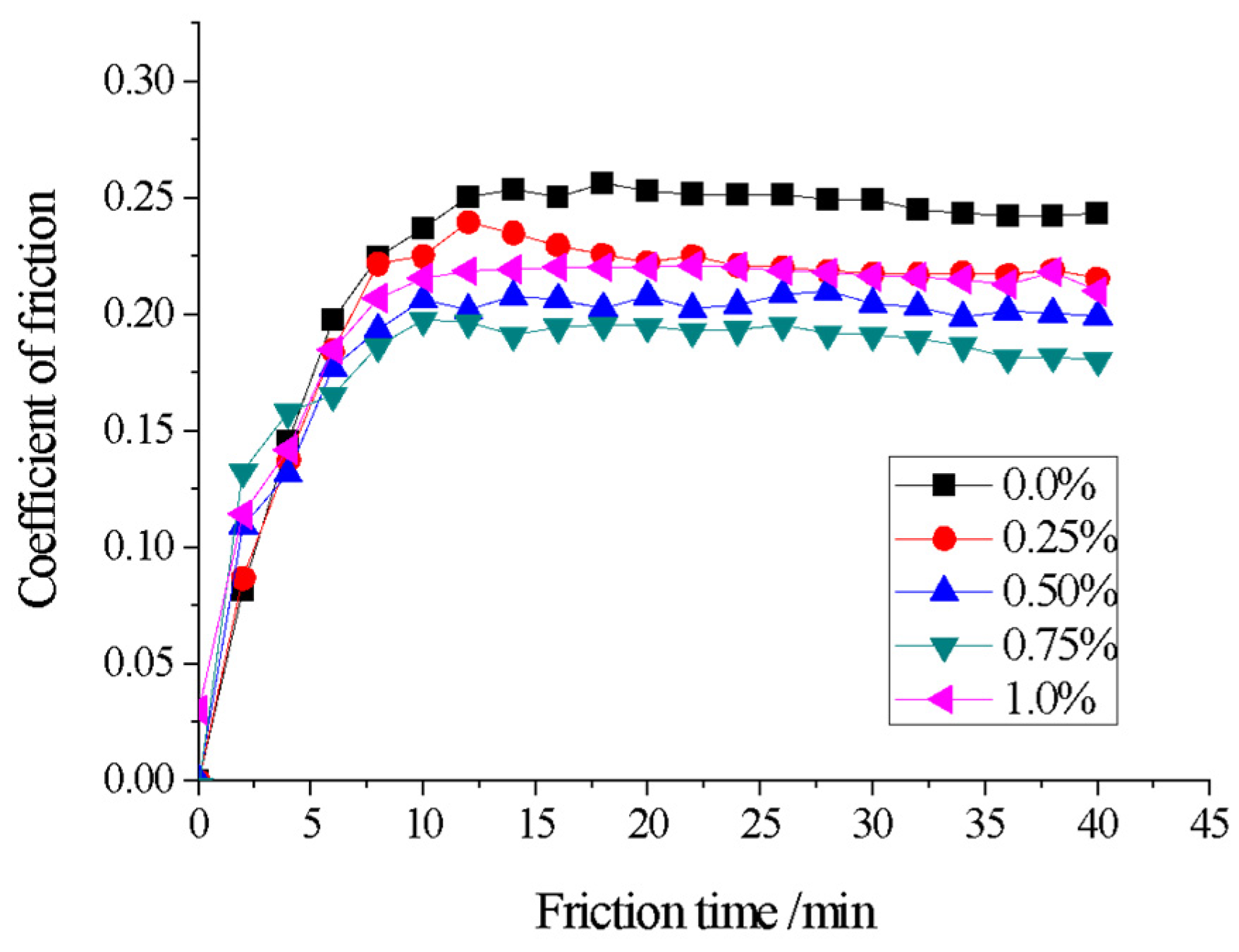
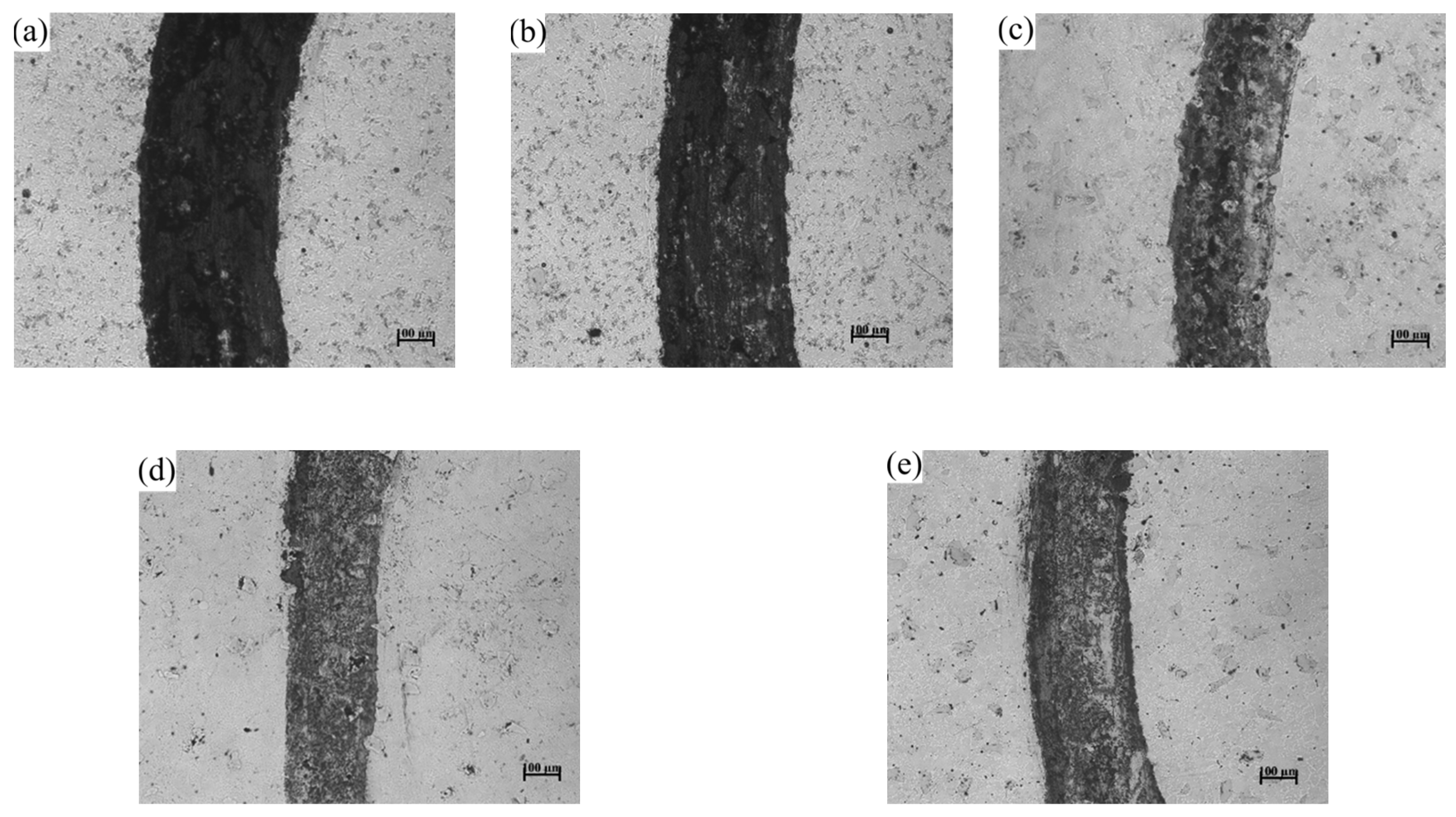
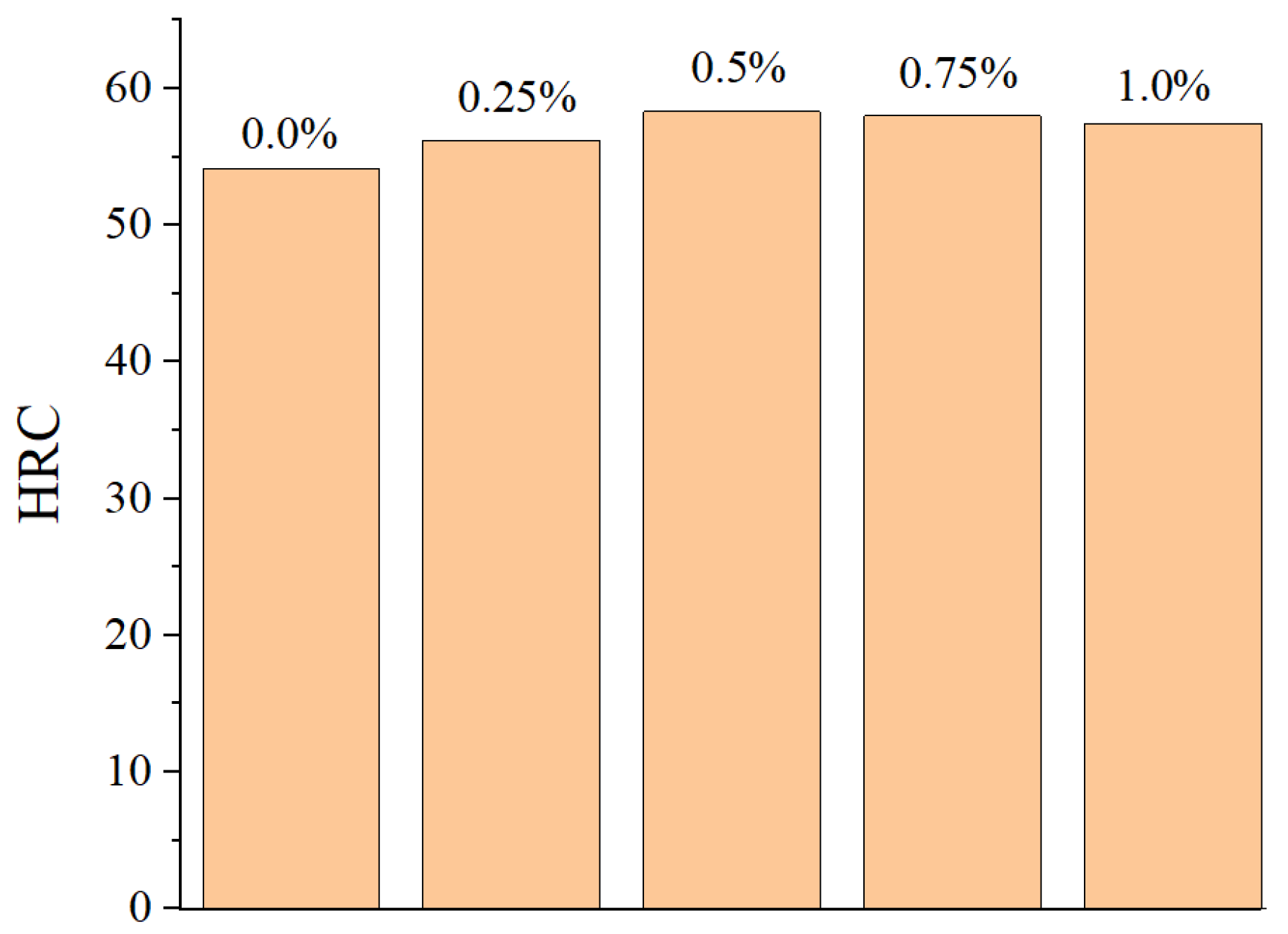
| Element | C | Mn | Si | P | S | Cu | Fe |
|---|---|---|---|---|---|---|---|
| Wt./% | ≤0.25 | 0.80~1.20 | ≤0.40 | ≤0.04 | ≤0.05 | ≥0.20 | Bal. |
| Substances and Properties | C | Cr | Si | B | Fe | Ni | Mesh |
|---|---|---|---|---|---|---|---|
| Wt./% | 0.5~1.1 | 15~20 | 3.5~5.5 | 3.0~4.5 | ≤5.0 | Bal. | −150~+320 |
| Substances and Properties | Cu | Fe | Nitric Acid Insoluble | Other Rare Earths | Burn Weightless | Purity |
|---|---|---|---|---|---|---|
| Wt./% | <0.002 | <0.002 | <0.03 | <0.5 | <0.2 | ≥99 |
| Powder Material | 0.0% CeO2 | 0.25% CeO2 | 0.5% CeO2 | 0.75% CeO2 | 1.0% CeO2 |
|---|---|---|---|---|---|
| Ni60A + 15% WC, Wt./g | 4.9800 | 4.9675 | 4.9551 | 4.9426 | 4.9302 |
| CeO2, Wt./g | 0 | 0.0125 | 0.0249 | 0.0374 | 0.0498 |
| (a) | (b) | (c) | (d) | (e) | |
|---|---|---|---|---|---|
| Rate of wear/mm3·N−1·m−1 | 2.2 | 1.9 | 1.6 | 1.5 | 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miao, L.; Miao, H.; Xie, S.; Liu, P.; Li, Y.; Liu, J. Effect of CeO2 Addition on the Microstructure and Properties of Induction Heating Ni-WC-CeO2 Composite Coatings. Materials 2025, 18, 2175. https://doi.org/10.3390/ma18102175
Miao L, Miao H, Xie S, Liu P, Li Y, Liu J. Effect of CeO2 Addition on the Microstructure and Properties of Induction Heating Ni-WC-CeO2 Composite Coatings. Materials. 2025; 18(10):2175. https://doi.org/10.3390/ma18102175
Chicago/Turabian StyleMiao, Lu, Heqi Miao, Shangpeng Xie, Peibin Liu, Yanhui Li, and Jihui Liu. 2025. "Effect of CeO2 Addition on the Microstructure and Properties of Induction Heating Ni-WC-CeO2 Composite Coatings" Materials 18, no. 10: 2175. https://doi.org/10.3390/ma18102175
APA StyleMiao, L., Miao, H., Xie, S., Liu, P., Li, Y., & Liu, J. (2025). Effect of CeO2 Addition on the Microstructure and Properties of Induction Heating Ni-WC-CeO2 Composite Coatings. Materials, 18(10), 2175. https://doi.org/10.3390/ma18102175






