Effect of Strengthening Mechanism of Alkali Curing on Mechanical Properties of Fly Ash Lightweight Aggregates and Its Concrete
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cement
2.1.2. Fly Ash
2.1.3. Alkaline Curing Solution
2.1.4. Others
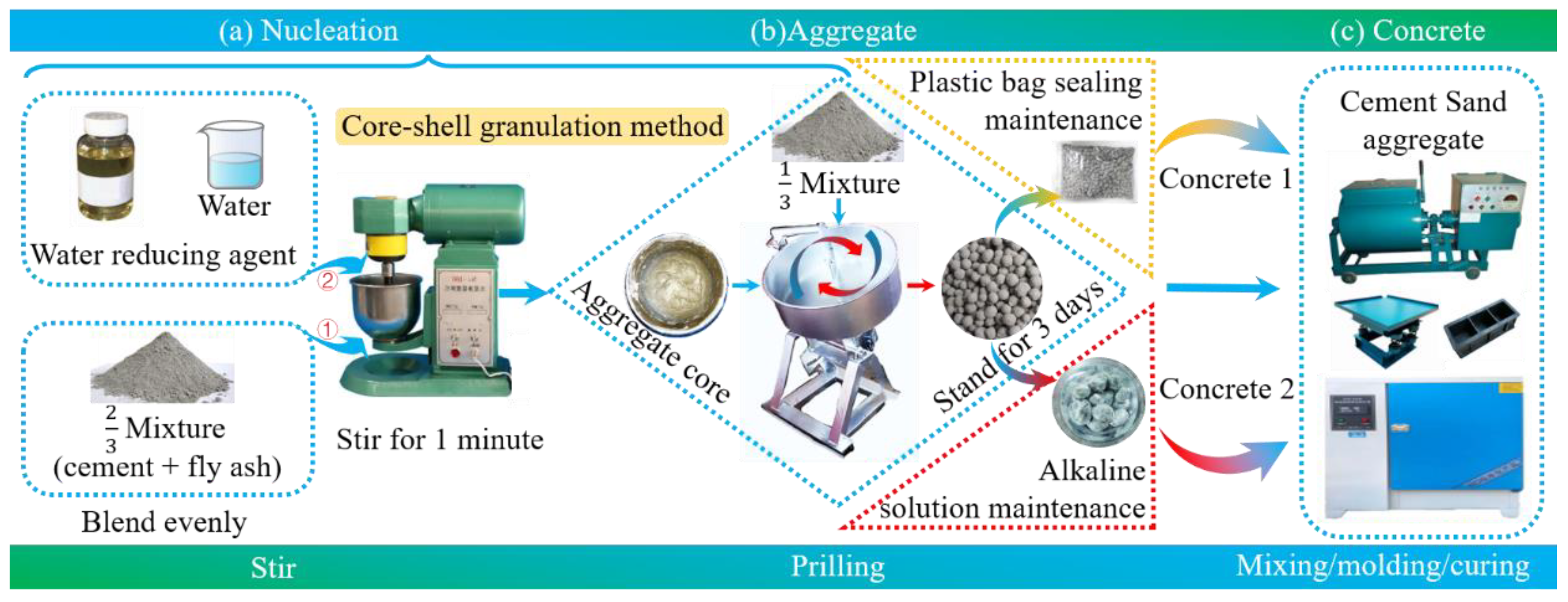
2.2. Preparation of Lightweight Aggregates and Its Concrete
2.2.1. Preparation of FULA
2.2.2. Preparation of FULA Concrete
2.3. Methods
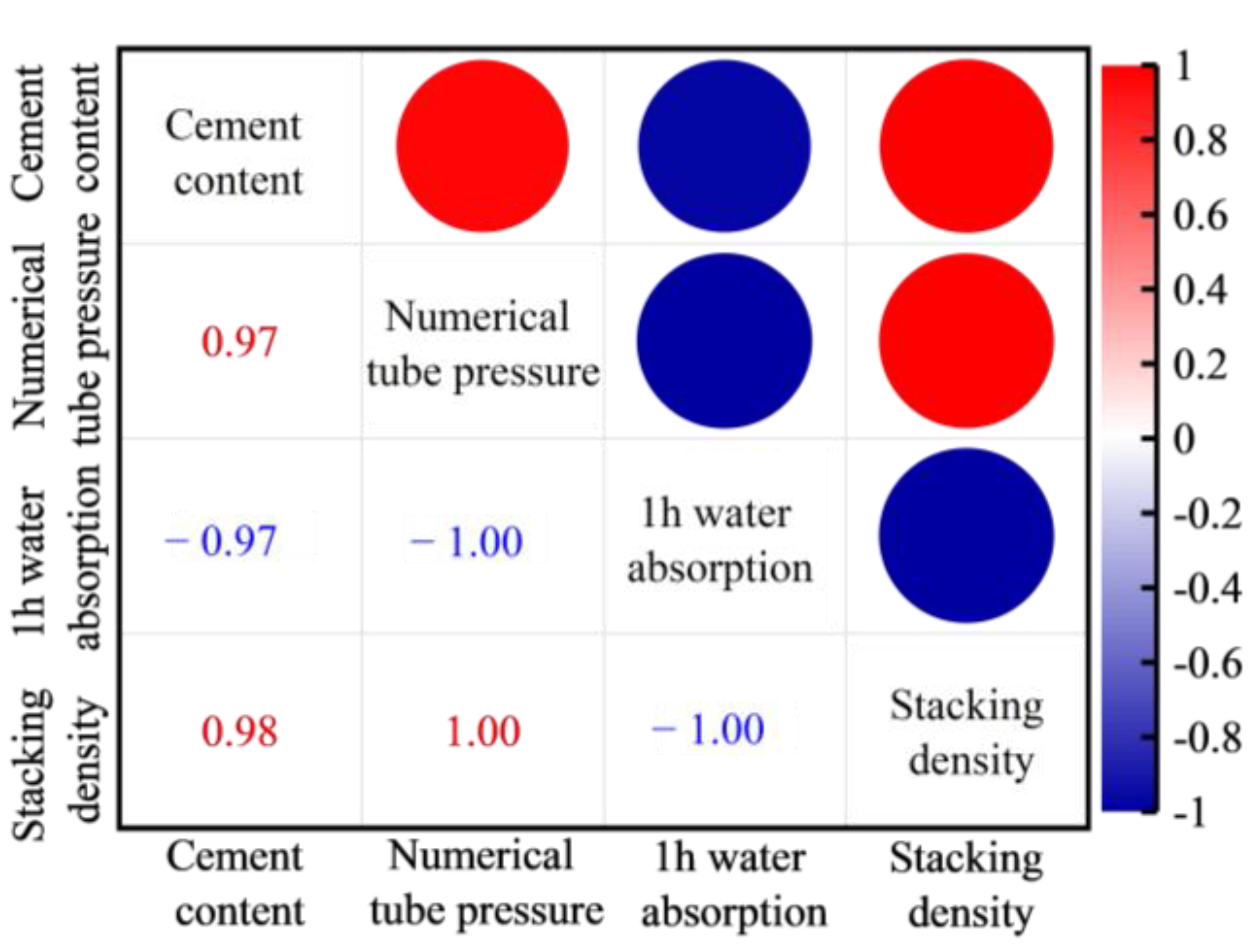
2.3.1. Effect of Cement Content on the Basic Properties of Ordinary FULA
2.3.2. Effect of Alkali Curing on the Basic Properties of FULA
- (1)
- Effect of Curing Solution Type on the Numerical Tube Pressure of FULA
- (2)
- Effect of Curing Solution Concentration on the Basic Properties of FULA
2.3.3. Effect of Alkali Curing on the Physical Phase Composition of FULA
2.3.4. Effect of Alkali Curing FULA on Mechanical Properties of Concrete
- (1)
- Effect of Aggregate Type on Mechanical Properties of Concrete
- (2)
- Effect of Water–Cement Ratio on Mechanical Properties of Concrete
2.3.5. Effect of Alkali Curing FULA on the Microstructure of the Interfacial Transition Zone
- (1)
- Effect of Alkali Curing FULA on Microhardness of Interfacial Transition Zone
- (2)
- Effect of Alkali Curing FULA on the Micro-Morphology of the Interfacial Transition Zone
3. Results and Discussion
3.1. Ordinary FULA Basic Performance Analysis
3.2. Alkali Curing FULA Basic Performance Analysis
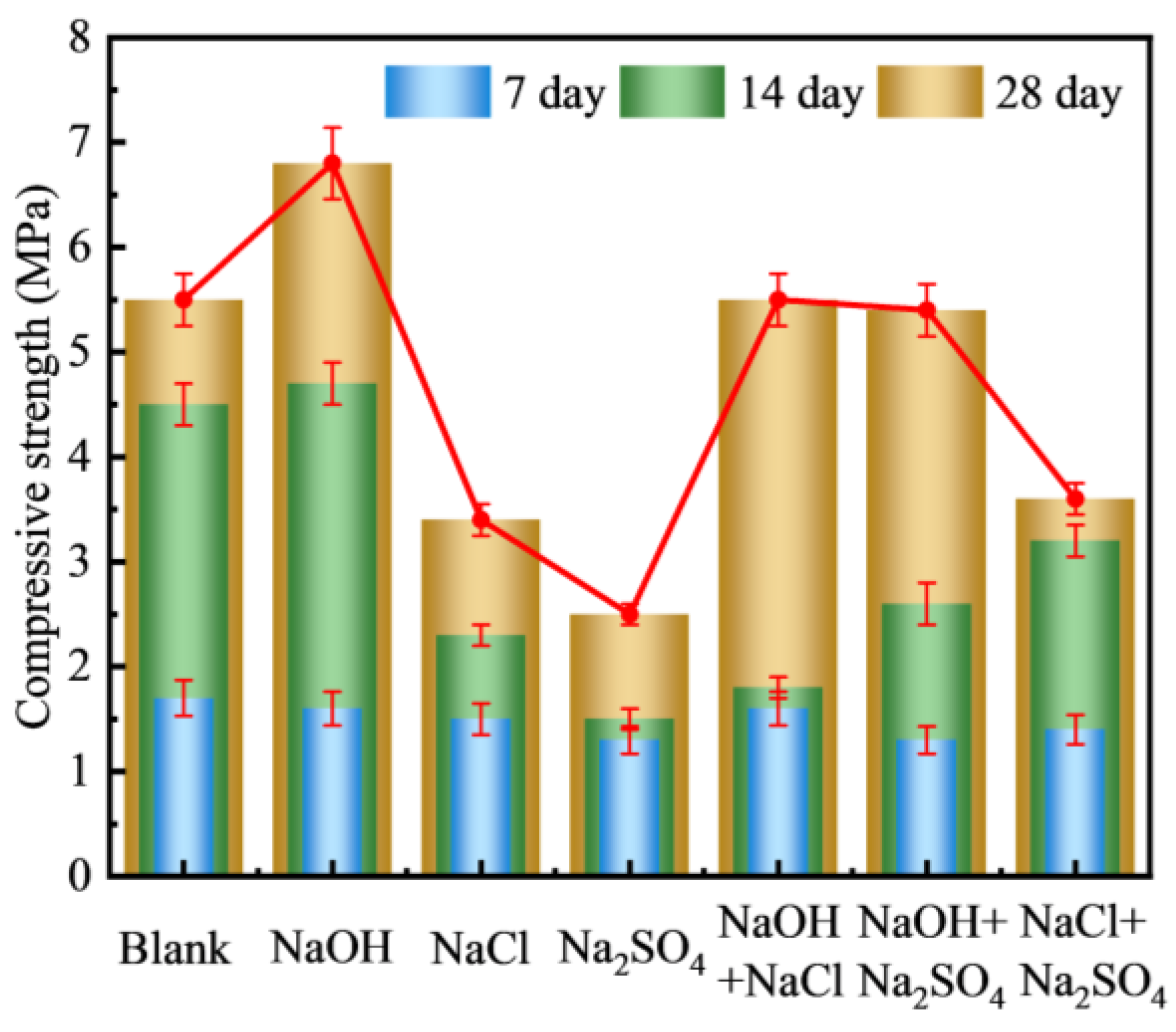
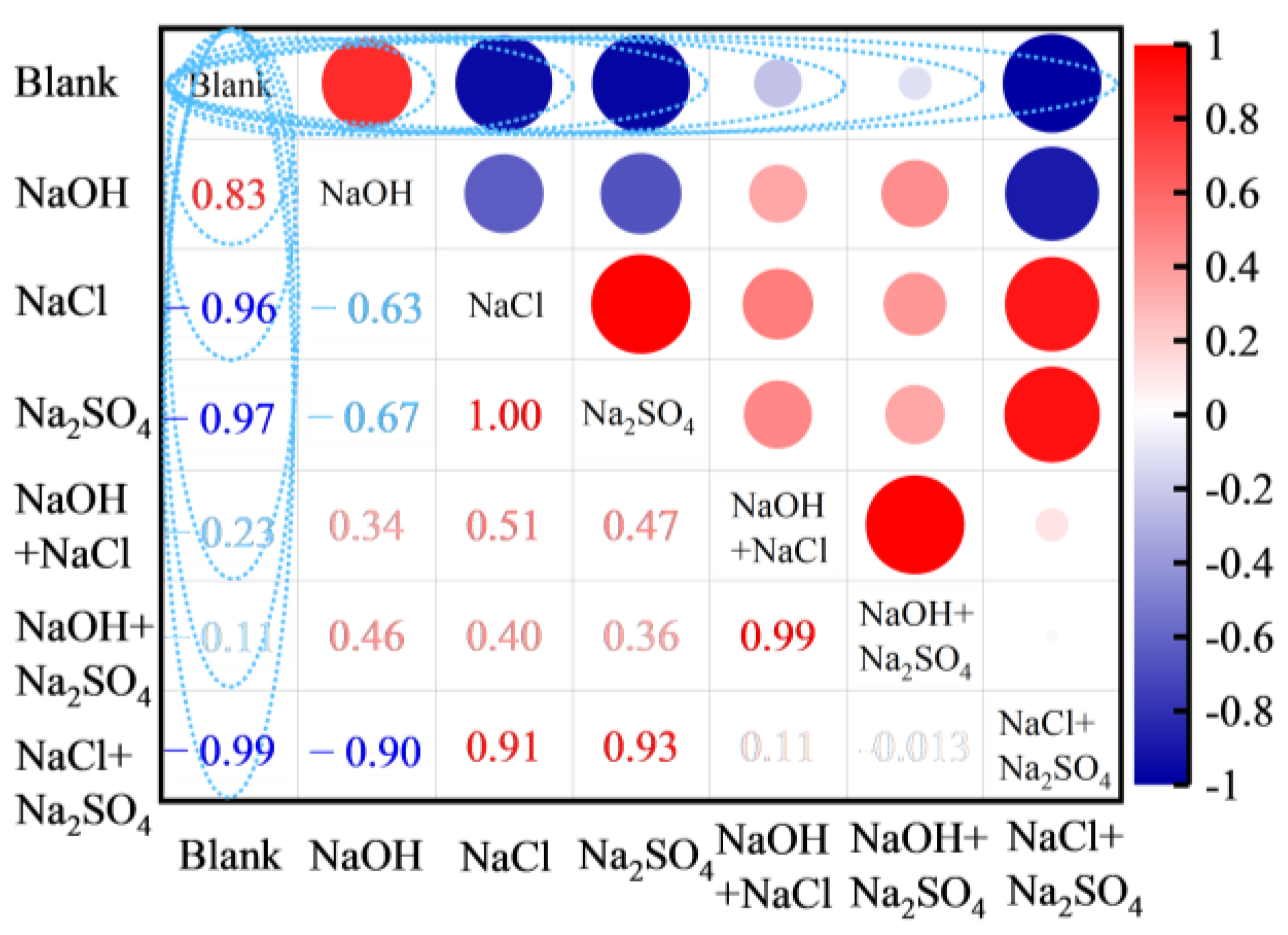
3.2.1. Types of Curing Solution
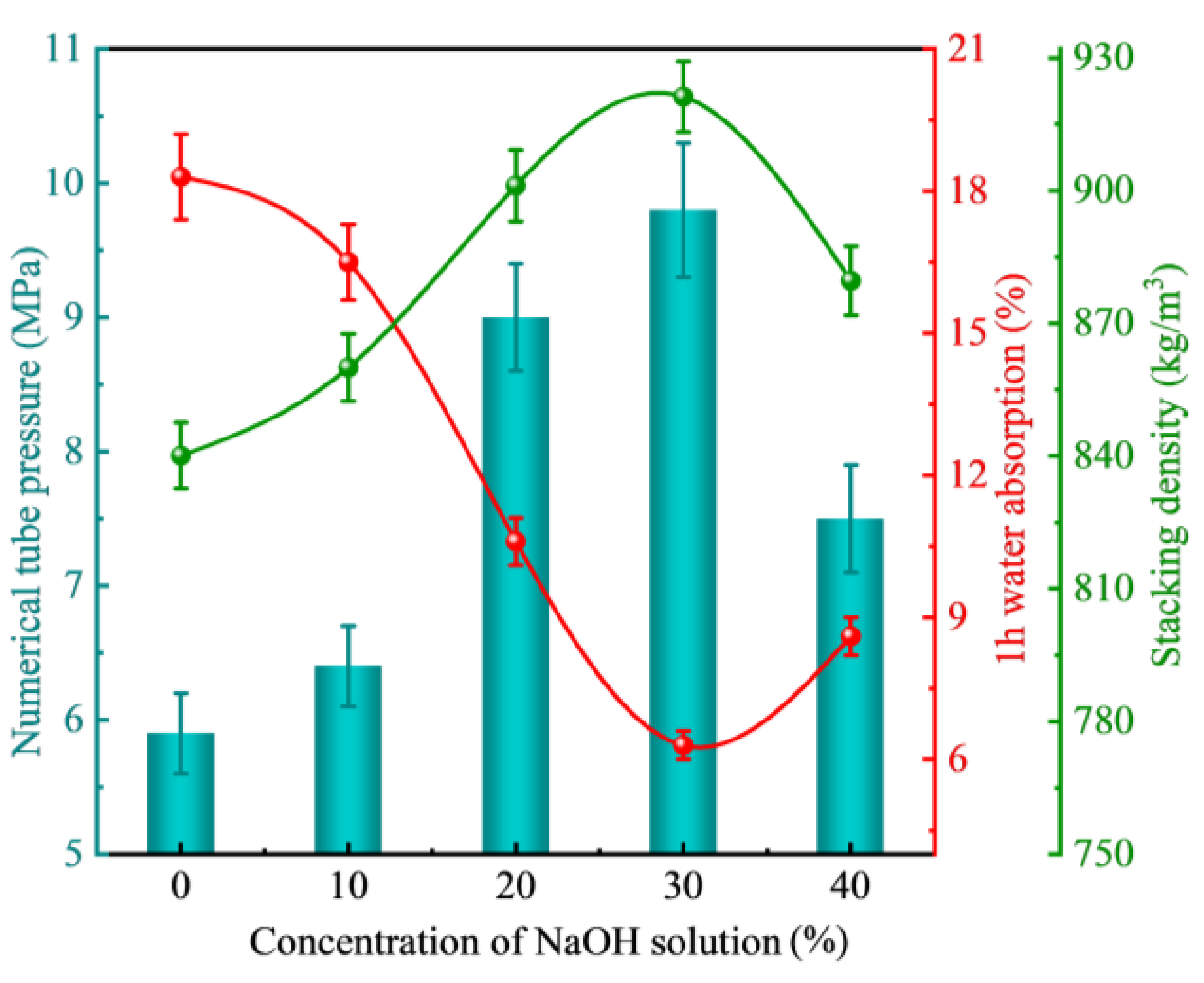
3.2.2. Concentration of Curing Solution
3.3. Physical Composition Analysis of Alkali Curing FULA
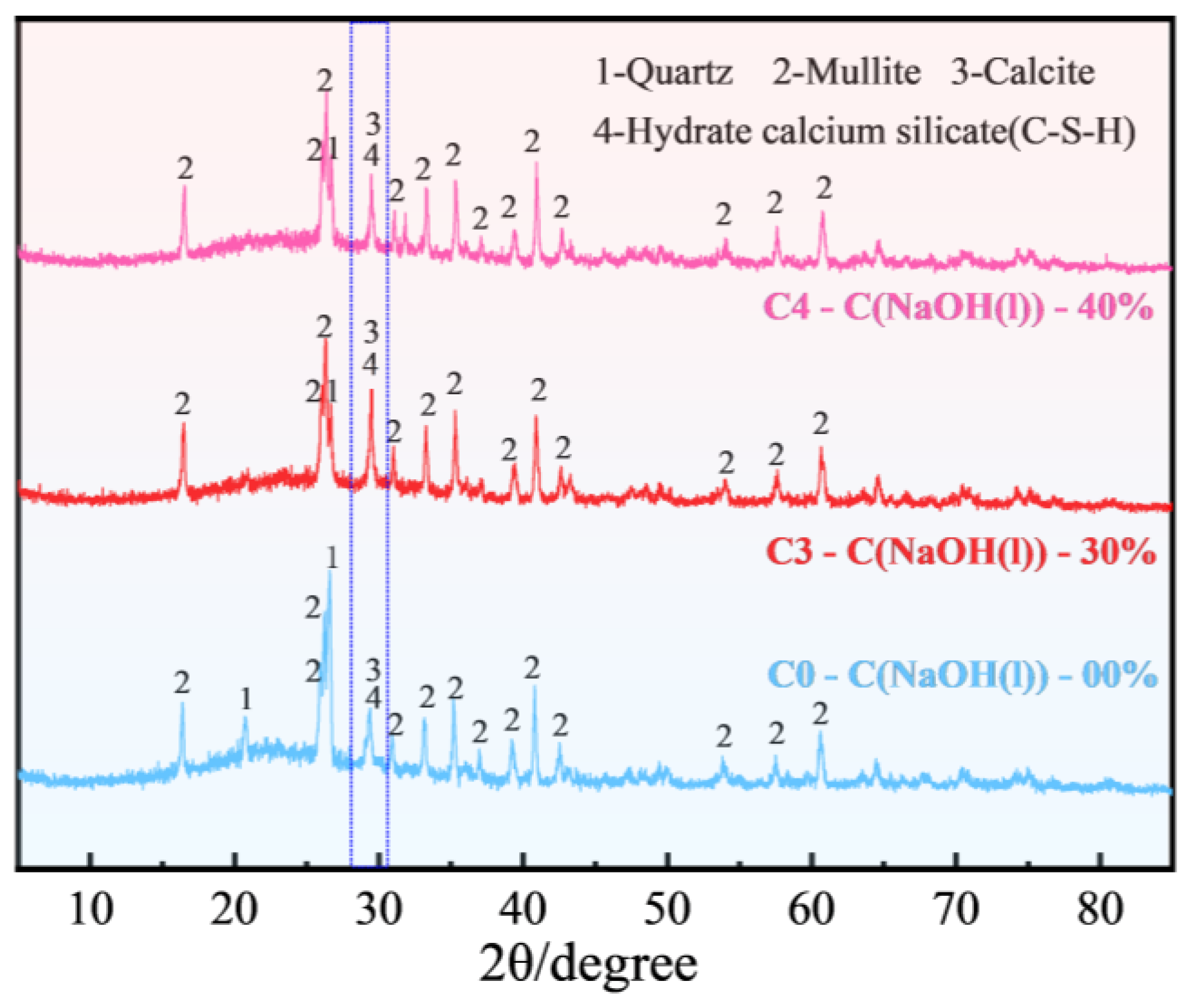
3.3.1. XRD
3.3.2. FT-IR
3.4. Mechanical Properties Analysis of Alkali Curing FULA Concrete
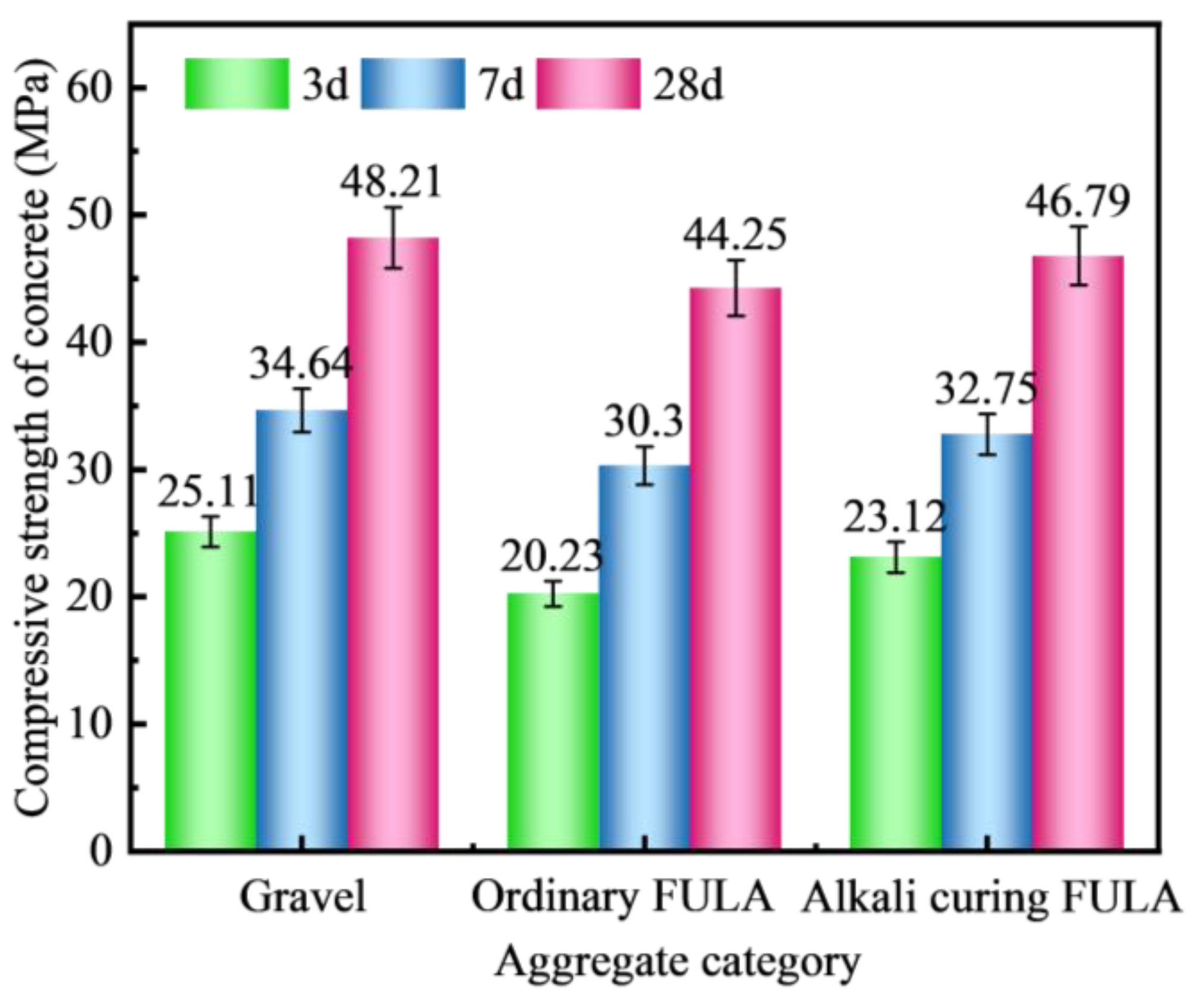
3.4.1. Types of Aggregates
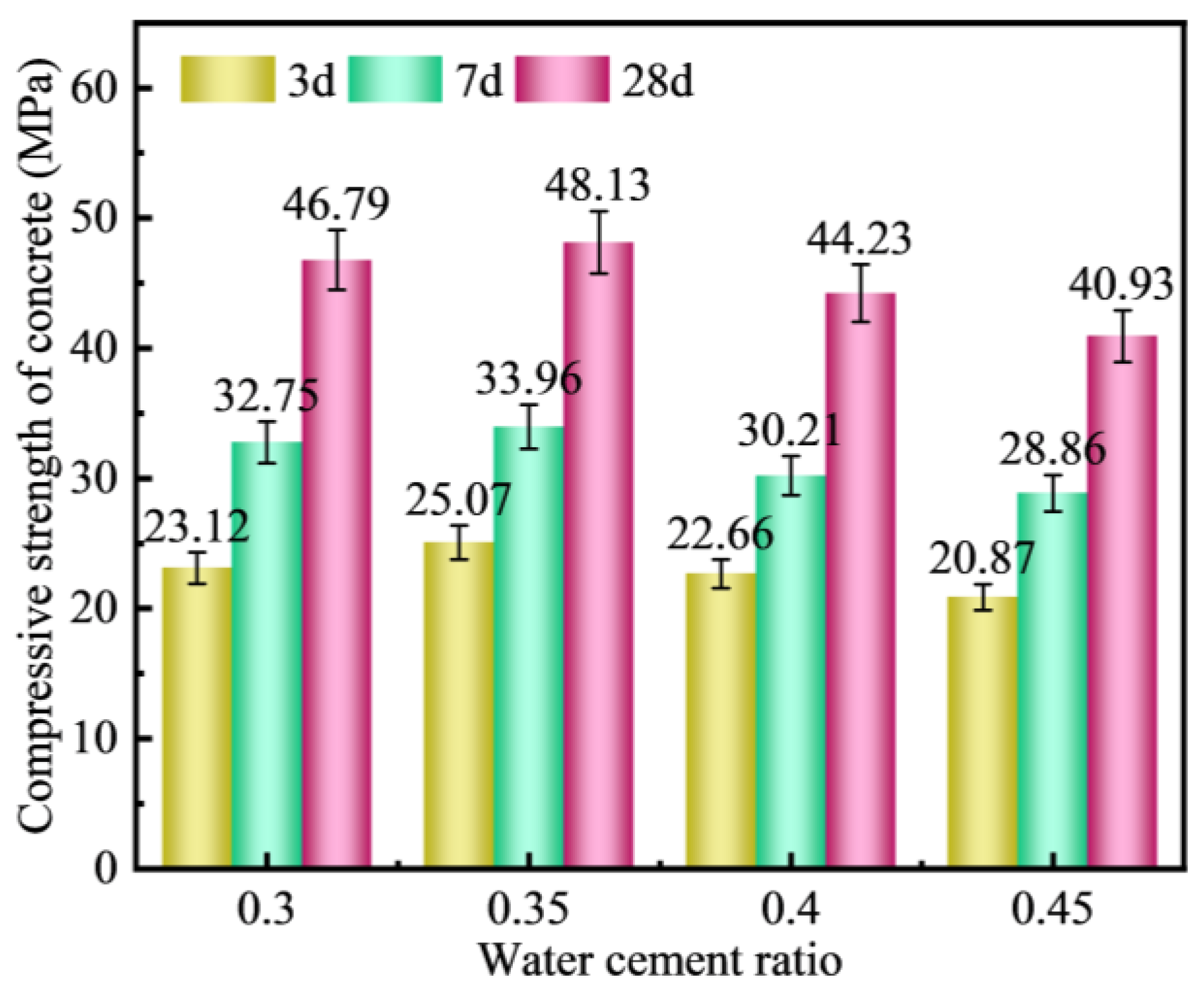
3.4.2. Water–Cement Ratio
3.5. Analysis of the Interfacial Transition Zone of Alkali Curing FULA Concrete
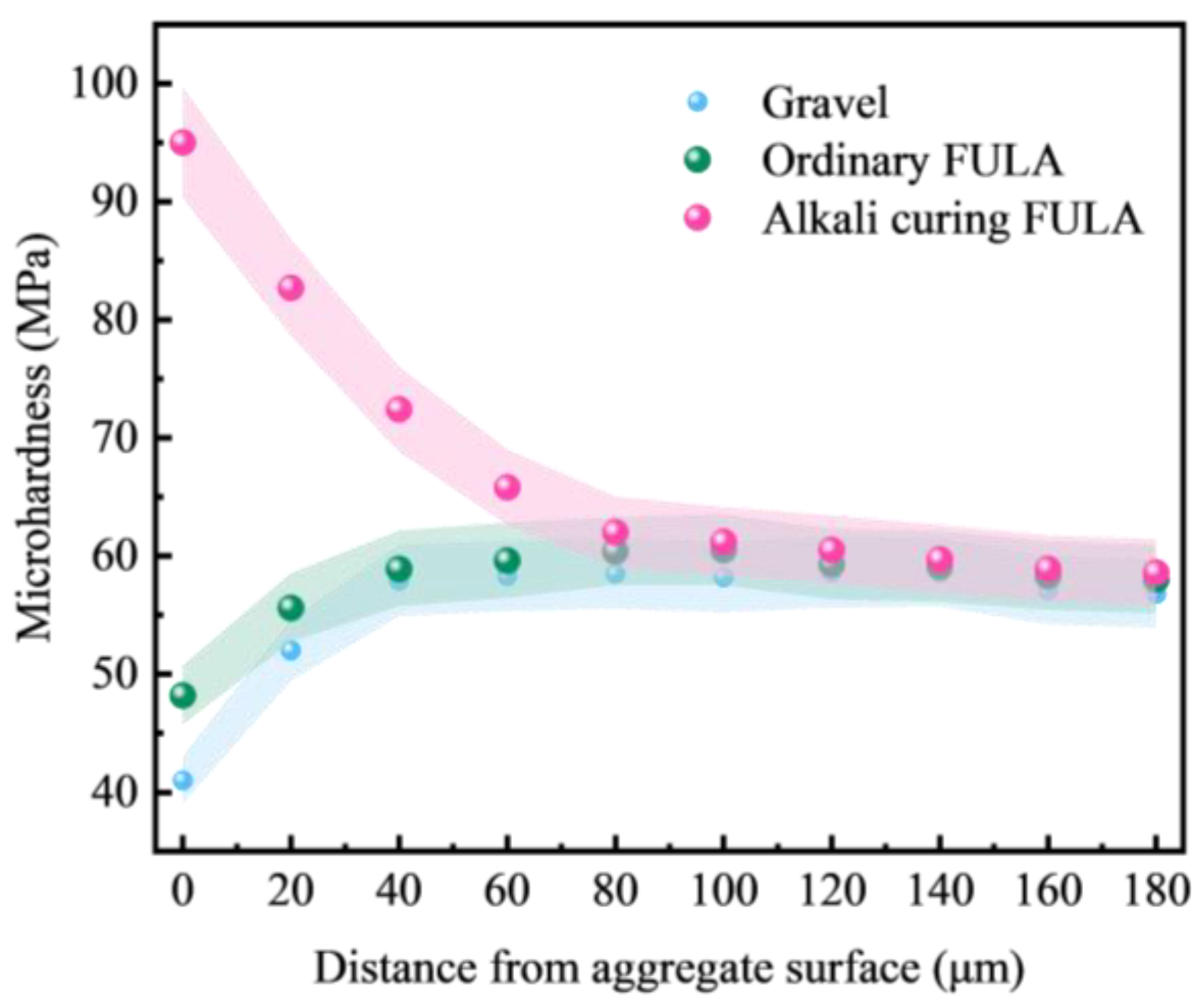
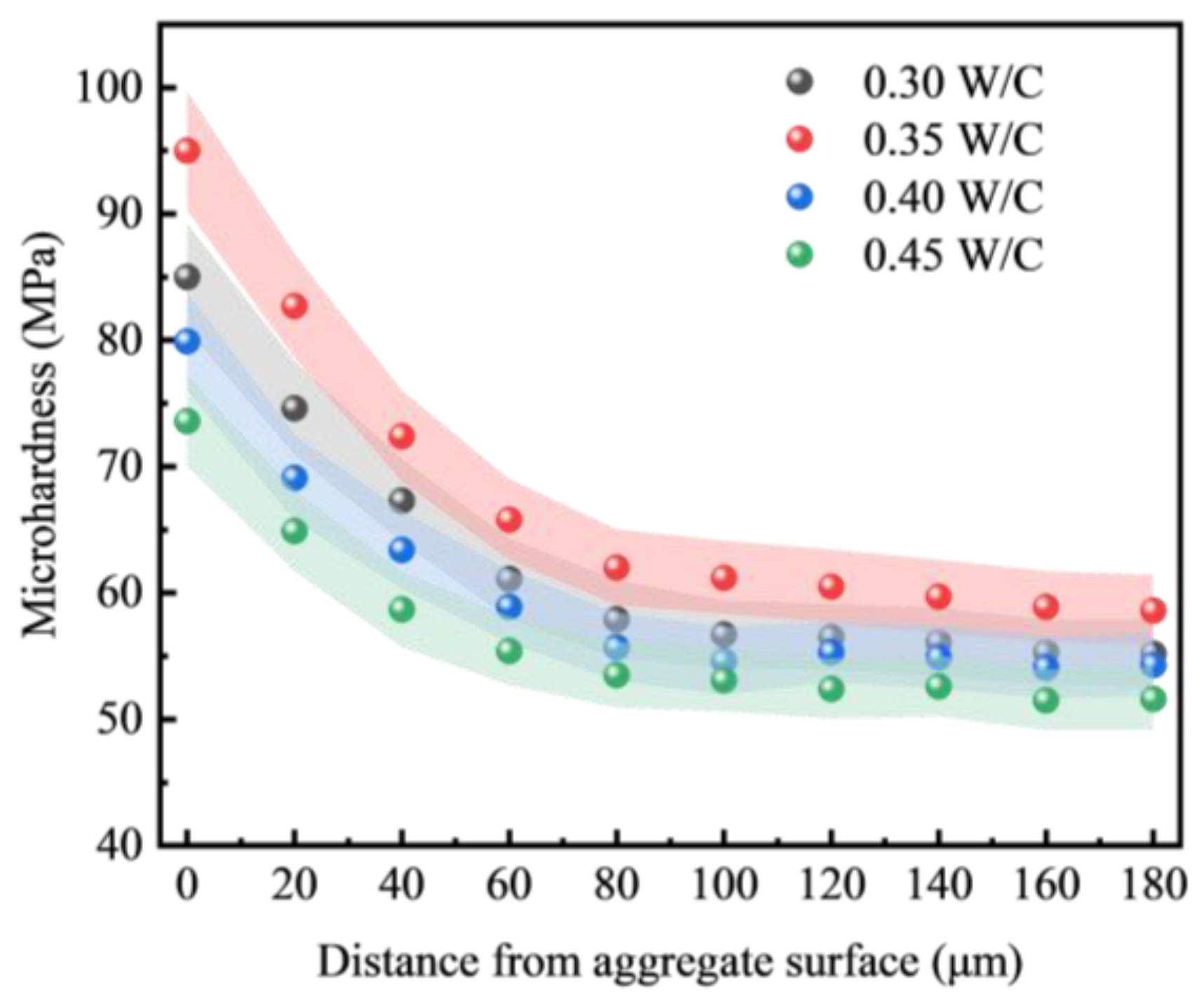
3.5.1. Microhardness
3.5.2. Micromorphology
4. Mechanistic Analysis
4.1. Mechanism of Reinforcement of FULA by Alkali Curing
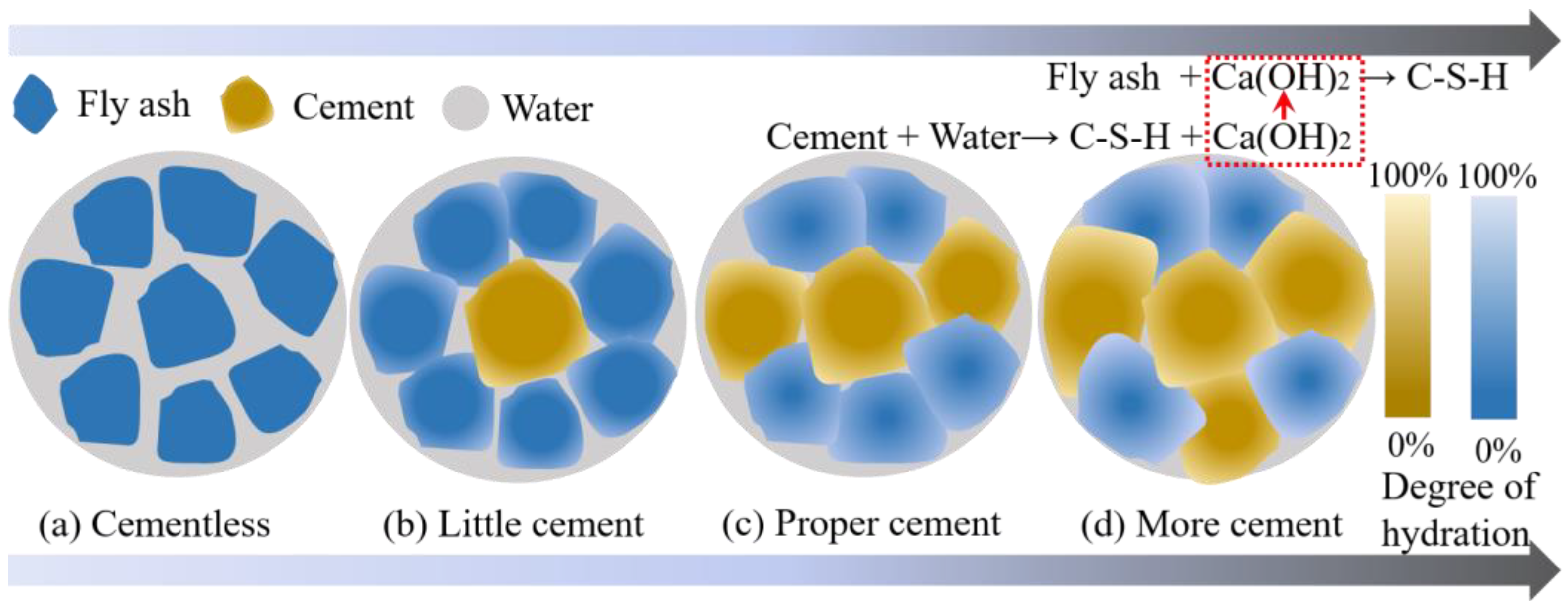
4.1.1. Cementation Mechanism of Cement on FULA
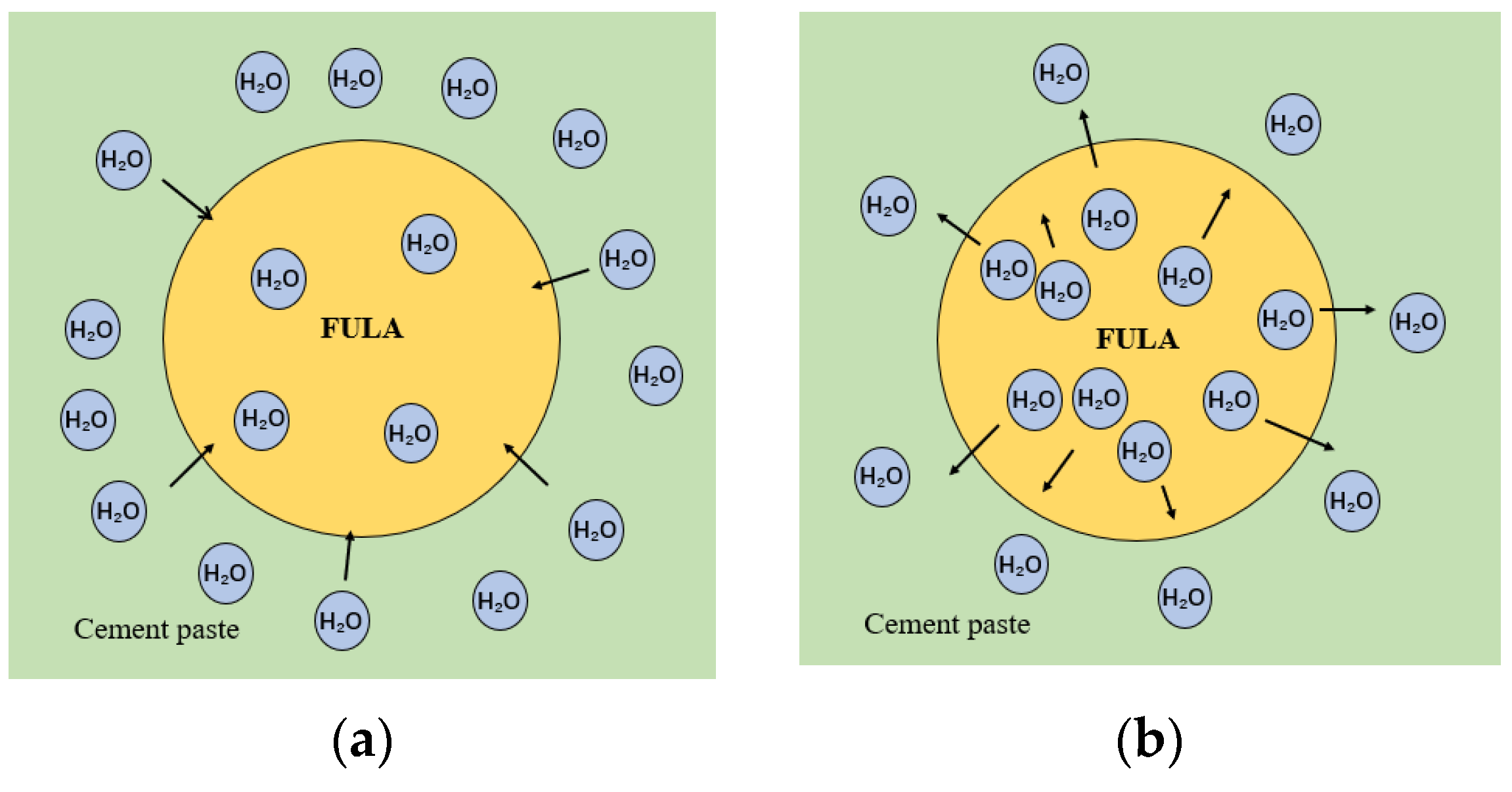
4.1.2. Mechanism of Reinforcement of Mechanical Properties of FULA by Alkali Curing
4.2. Mechanism of Reinforcement of Alkali Curing on FULA Concrete Interfacial Transition Zone
4.2.1. Mechanism of Different Aggregates on the Interfacial Transition Zone
4.2.2. Mechanism of the Effect of Water–Cement Ratio on the Interfacial Transition Zone
5. Conclusions
- (1)
- There is a positive correlation between the cement content and the numerical tube pressure and packing density of FULA. With the increase of cement content, the numerical tube pressure and packing density of FULA can be increased continuously. However, when the cement content is low, FULA is almost difficult to be molded. When the cement dosage is very high, FULA transforms to hardened cement stone, and the increase of numerical tube pressure and packing density slows down.
- (2)
- There are large differences in the effects of different types of alkaline solutions on the basic properties of FULA. Sodium hydroxide solution has the effect of increasing the basic properties of FULA (numerical tube pressure, bulk density, 1 h water absorption). In addition, sodium sulfate, sodium chloride, and their complex solutions are detrimental to the development of FULA basic properties at any concentration.
- (3)
- FULA after sodium hydroxide solution maintenance does not generate new substances, but the quartz minerals in the fly ash are significantly consumed, and the generation of hydration products C-S-H gel is significantly improved. However, the larger concentration of sodium hydroxide solution is not favorable to the generation of hydration products. The reason is that moderate amount of sodium hydroxide can promote the dissolution of fly ash, but excessive sodium hydroxide also has a dissolving effect on the hydration products.
- (4)
- Compared with gravel concrete and ordinary FULA concrete, the microhardness of the interfacial transition zone of alkali curing FULA concrete is significantly improved. The microhardness of the interfacial transition zone of gravel concrete and ordinary FULA concrete is lower than that of the cement stone matrix, and the closer to the aggregate, the more obvious the microhardness decrease. However, the microhardness of the interface transition zone of alkali curing FULA concrete is significantly higher than that of the cement stone matrix, and the closer to the aggregate, the more obvious the microhardness increase.
- (5)
- In this paper, FULA and its concrete with excellent performance were prepared by alkali curing method, but there are still more problems that need to be explored in depth. This paper focuses on the performance of short-term immersion FULA, and the change rule of mechanical properties of long-term immersion FULA is not yet understood. This paper focuses on the mechanical properties of FULA concrete, but the effect of FULA on the durability of concrete such as resistance to sulphate attack and carbonation is not involved.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature
| FULA | Fly Ash Unburned Lightweight Aggregate |
| ITZ | Interfacial Transition Zone |
References
- Bendixen, M.; Iversen, L.L.; Best, J.; Franks, D.M.; Hackney, C.R.; Latrubesse, E.M.; Tusting, L.S. Sand, gravel, and UN Sustainable Development Goals: Conflicts, synergies, and pathways forward. One Earth 2021, 4, 1095–1111. [Google Scholar] [CrossRef]
- Bisht, A. Sand futures: Post-growth alternatives for mineral aggregate consumption and distribution in the global south. Ecol. Econ. 2022, 191, 107233. [Google Scholar] [CrossRef]
- Marschke, M.; Rousseau, J. Sand ecologies, livelihoods and governance in Asia: A systematic scoping review. Resour. Policy 2022, 77, 102671. [Google Scholar] [CrossRef]
- Hou, H.; Su, L.; Guo, D.; Xu, H. Resource utilization of solid waste for the collaborative reduction of pollution and carbon emissions: Case study of fly ash. J. Clean. Prod. 2023, 383, 135449. [Google Scholar] [CrossRef]
- Lin, S.; Jiang, X.; Zhao, Y.; Yan, J. Zeolite greenly synthesized from fly ash and its resource utilization: A review. Sci. Total Environ. 2022, 851 Pt 1, 158182. [Google Scholar] [CrossRef]
- Wang, J.; Dong, H. Preparation and application of multi-source solid wastes as clean aggregates: A comprehensive review. Constr. Build. Mater. 2024, 418, 135414. [Google Scholar] [CrossRef]
- Jing, X.; Nan, Y.; Christian, W.; Cang, D.; Zhang, L. Strength reinforcement of coal fly ash based non-sintered lightweight aggregates by autoclave curing and H2O2-modified basalt fiber addition. J. Build. Eng. 2024, 94, 109803. [Google Scholar] [CrossRef]
- Peng, B.; Wang, J.; Dong, X.; Yang, F.; Sheng, C.; Liu, Y. Enhancement of mechanical and durability properties of preplaced lightweight aggregate concrete. Adv. Concr. Constr. 2023, 15, 419–430. [Google Scholar]
- Rodacka, M.; Domagala, L.; Szydlowski, R. Assessment of Properties of Structural Lightweight Concrete with Sintered Fly Ash Aggregate in Terms of Its Suitability for Use in Prestressed Members. Materials 2023, 16, 5429. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Niu, S.; Han, K.; Wang, Y.; Zhu, J.; Yang, Z.; Liu, J.; Zhang, H.; Sun, X.; Liang, B.; et al. Properties of artificial lightweight aggregates prepared from coal and biomass co-fired fly ashes and sewage sludge fly ash. Ceram. Int. 2024, 50, 28609–28618. [Google Scholar] [CrossRef]
- Ślosarczyk, A.; Garbalińska, H.; Strzałkowski, J. Lightweight alkali-activated composites containing sintered fly ash aggregate and various amounts of silica aerogel. J. Build. Eng. 2023, 74, 106879. [Google Scholar] [CrossRef]
- Kim, D.; Song, H.; Yu, J.; Sim, S.; Jeon, D.; Oh, J.E. Development of lightweight and low-crystalline artificial aggregate using cementless fly ash binder for thermal neutron shielding concrete manufacture. Cem. Concr. Compos. 2024, 152, 105674. [Google Scholar] [CrossRef]
- Narattha, C.; Chaipanich, A. Phase characterizations, physical properties and strength of environment-friendly cold-bonded fly ash lightweight aggregates. J. Clean. Prod. 2018, 171, 1094–1100. [Google Scholar] [CrossRef]
- Xue, K.; Qi, J.; Yang, X.; Liu, M.; Su, M.; Peng, X.; Ju, C.; Zhang, Y.; Wu, Y. Study on preparation and activation enhancement effect of cold bonded multi-solid waste wrap-shell lightweight aggregates (SWSLAs) with low cement content. Case Stud. Constr. Mater. 2024, 20, e2897. [Google Scholar] [CrossRef]
- Rashad, A.M.; Mosleh, Y.A.; Mokhtar, M.M. Thermal insulation and durability of alkali-activated lightweight slag mortar modified with silica fume and fly ash. Constr. Build. Mater. 2024, 411, 134255. [Google Scholar] [CrossRef]
- Kong, L.; Fan, Z.; Lu, J.; Zhang, L. Microstructure evolution of interfacial transition zone between alkali-activated fly ash/slag matrix and aggregate. Mater. Struct. 2022, 55, 203. [Google Scholar] [CrossRef]
- Risdanareni, P.; Wang, J.; Boon, N.; De Belie, N. Alkali activated lightweight aggregate as bacterial carrier in manufacturing self-healing mortar. Constr. Build. Mater. 2023, 368, 130375. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, G.; Han, X.; Gao, Q.; Ren, J. Research on the design method of mix proportion of ceramsite lightweight aggregate concrete. Constr. Build. Mater. 2024, 433, 136665. [Google Scholar] [CrossRef]
- Zou, S.; Lu, J.-X.; Xiao, J.; Duan, Z.; Chau, C.K.; Sham, M.L.; Poon, C.S. Development and characteristics of novel high-strength lightweight core-shell aggregate. Constr. Build. Mater. 2023, 393, 132080. [Google Scholar] [CrossRef]
- Leong, G.W.; Mo, K.H.; Ibrahim, Z.; Radwan, M.K.; Ling, T.-C.; Sinoh, S.S. Lightweight cementitious composites incorporating fly ash cenospheres and perlite microspheres. Constr. Build. Mater. 2023, 404, 133226. [Google Scholar] [CrossRef]
- Kockal, N.U.; Ozturan, T. Durability of lightweight concretes with lightweight fly ash aggregates. Constr. Build. Mater. 2011, 25, 1430–1438. [Google Scholar] [CrossRef]
- Kourti, I.; Cheeseman, C.R. Properties and microstructure of lightweight aggregate produced from lignite coal fly ash and recycled glass. Resour. Conserv. Recycl. 2010, 54, 769–775. [Google Scholar] [CrossRef]
- Jo, B.; Park, S.; Park, J. Properties of concrete made with alkali-activated fly ash lightweight aggregate (AFLA). Cem. Concr. Compos. 2007, 29, 128–135. [Google Scholar] [CrossRef]
- Shi, Y.; Guo, W.; Jia, Y.; Xue, C.; Qiu, Y.; Zhao, Q.; Wang, D. Preparation of non-sintered lightweight aggregate ceramsite based on red mud-carbide slag-fly ash: Strength and curing method optimization. J. Clean. Prod. 2022, 372, 133788. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Chowdhury, S.; Mohapatra, S.; Gaur, A.; Dwivedi, G.; Soni, A. Study of various properties of geopolymer concrete—A review. Mater. Today Proc. 2021, 46, 5687–5695. [Google Scholar] [CrossRef]
- GB/T 17431.2-2010; Lightweight Aggregates and Its Test Methods-Part 2: Test Methods for Lightweight Aggregates. China National Standardization Administration Committee. Standards Press of China: Beijing, China, 2010.
- GB/T 50081-2019; Standard for Test Methods of Concrete Physical and Mechanical Properties. China National Standardization Administration Committee. Standards Press of China: Beijing, China, 2019.
- Edelmann, D.; Móri, T.F.; Székely, G.J. On relationships between the Pearson and the distance correlation coefficients. Stat. Probab. Lett. 2021, 169, 108960. [Google Scholar] [CrossRef]
- Zhang, M.; Li, W.; Zhang, L.; Jin, H.; Mu, Y.; Wang, L. A Pearson correlation-based adaptive variable grouping method for large-scale multi-objective optimization. Inf. Sci. 2023, 639, 118737. [Google Scholar] [CrossRef]
- Tu, Y.; Yu, H.; Ma, H.; Han, W.; Diao, Y. Experimental study of the relationship between bond strength of aggregates interface and microhardness of ITZ in concrete. Constr. Build. Mater. 2022, 352, 128990. [Google Scholar] [CrossRef]
- Jebli, M.; Jamin, F.; Malachanne, E.; Garcia-Diaz, E.; El Youssoufi, M. Experimental characterization of mechanical properties of the cement-aggregate interface in concrete. Constr. Build. Mater. 2018, 161, 16–25. [Google Scholar] [CrossRef]




| Component | C3S | C2S | C3A | C4AF | Others |
|---|---|---|---|---|---|
| Content | 54.04 | 22.84 | 8.39 | 10.42 | 4.31 |
| Category | SiO2 | A12O3 | Fe2O3 | CaO | MgO | f-CaO | Na2O | Loss |
|---|---|---|---|---|---|---|---|---|
| Cement | 23.48 | 5.36 | 3.43 | 65.10 | 2.11 | 0.39 | 0.47 | 0.13 |
| Fly ash | 58.13 | 32.75 | 1.14 | 1.09 | 0.93 | 0.94 | 5.02 |
| Categories | D1 | D2 | D3 |
|---|---|---|---|
| Cement | 400 | 400 | 400 |
| Coarse aggregate | 1000 | 770 | 770 |
| Fine Aggregate | 550 | 550 | 550 |
| Water | 120 | 120 | 120 |
| water reducer | 0.5% | 0.5% | 0.5% |
| Coarse aggregate type | Gravel | Ordinary FULA | Alkali curing FULA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xu, Z.; Ji, Y. Effect of Strengthening Mechanism of Alkali Curing on Mechanical Properties of Fly Ash Lightweight Aggregates and Its Concrete. Materials 2025, 18, 89. https://doi.org/10.3390/ma18010089
Liu J, Xu Z, Ji Y. Effect of Strengthening Mechanism of Alkali Curing on Mechanical Properties of Fly Ash Lightweight Aggregates and Its Concrete. Materials. 2025; 18(1):89. https://doi.org/10.3390/ma18010089
Chicago/Turabian StyleLiu, Jun, Zhishan Xu, and Yongsheng Ji. 2025. "Effect of Strengthening Mechanism of Alkali Curing on Mechanical Properties of Fly Ash Lightweight Aggregates and Its Concrete" Materials 18, no. 1: 89. https://doi.org/10.3390/ma18010089
APA StyleLiu, J., Xu, Z., & Ji, Y. (2025). Effect of Strengthening Mechanism of Alkali Curing on Mechanical Properties of Fly Ash Lightweight Aggregates and Its Concrete. Materials, 18(1), 89. https://doi.org/10.3390/ma18010089






