Chromium Substitution Extraction Method for Its Recovery from Chromium-Tanned Leather Waste
Abstract
1. Introduction
2. Materials and Methods
2.1. Chromium-Tanned Leather Shavingsand Reagents
2.2. Substitution Extraction Experimental Procedure
2.2.1. Extraction Solution Preparation
2.2.2. Experimental Procedure
2.3. Analytical Methods for Cr and N Content Determination in CTLS
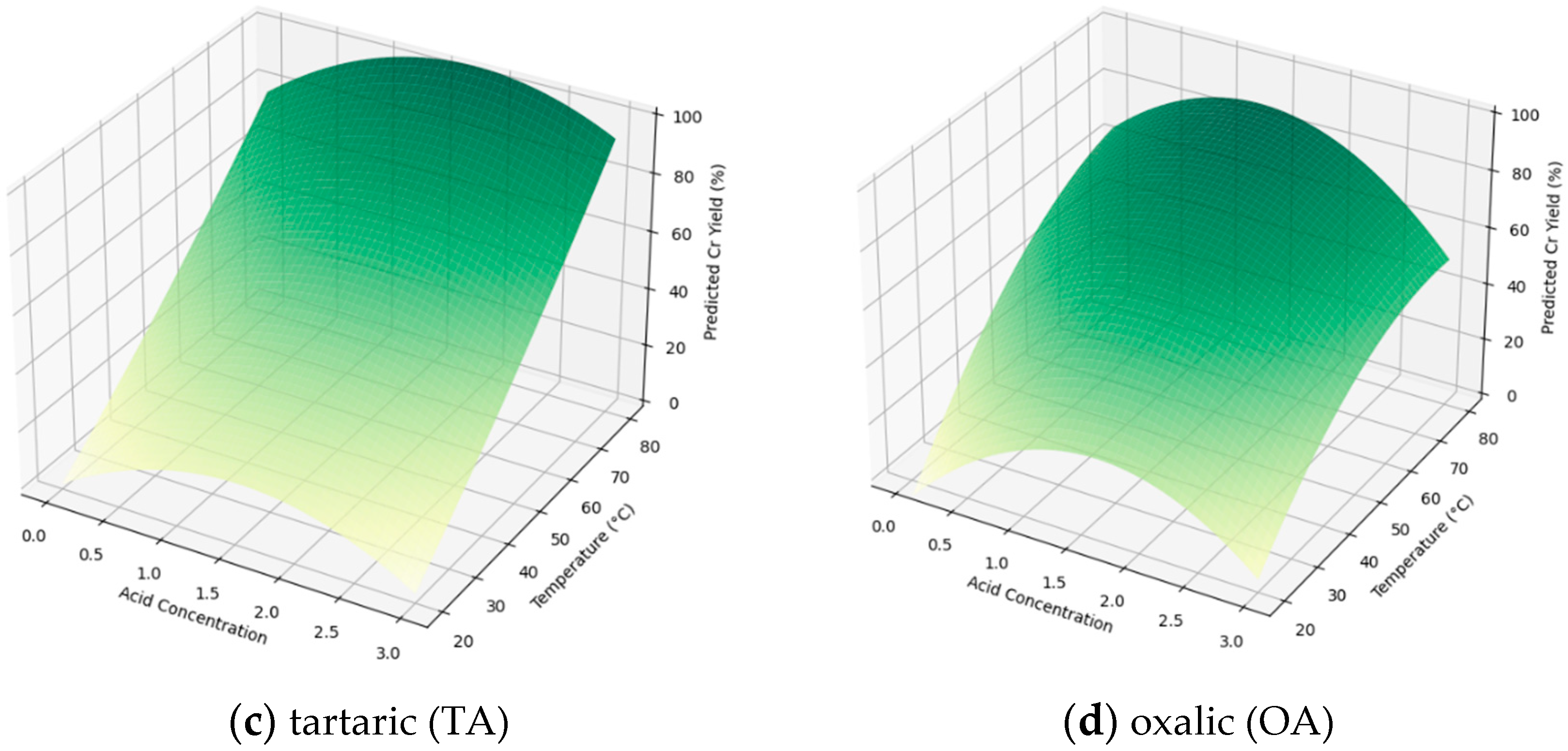
2.4. Optimization of Substitution Extraction Process Using Response Surface Methodology (RSM)
2.5. Data Predictions and Analysis
3. Results and Discussion
3.1. Substrate Characterization
3.2. Chromium Complexing Ability by Organic Acids
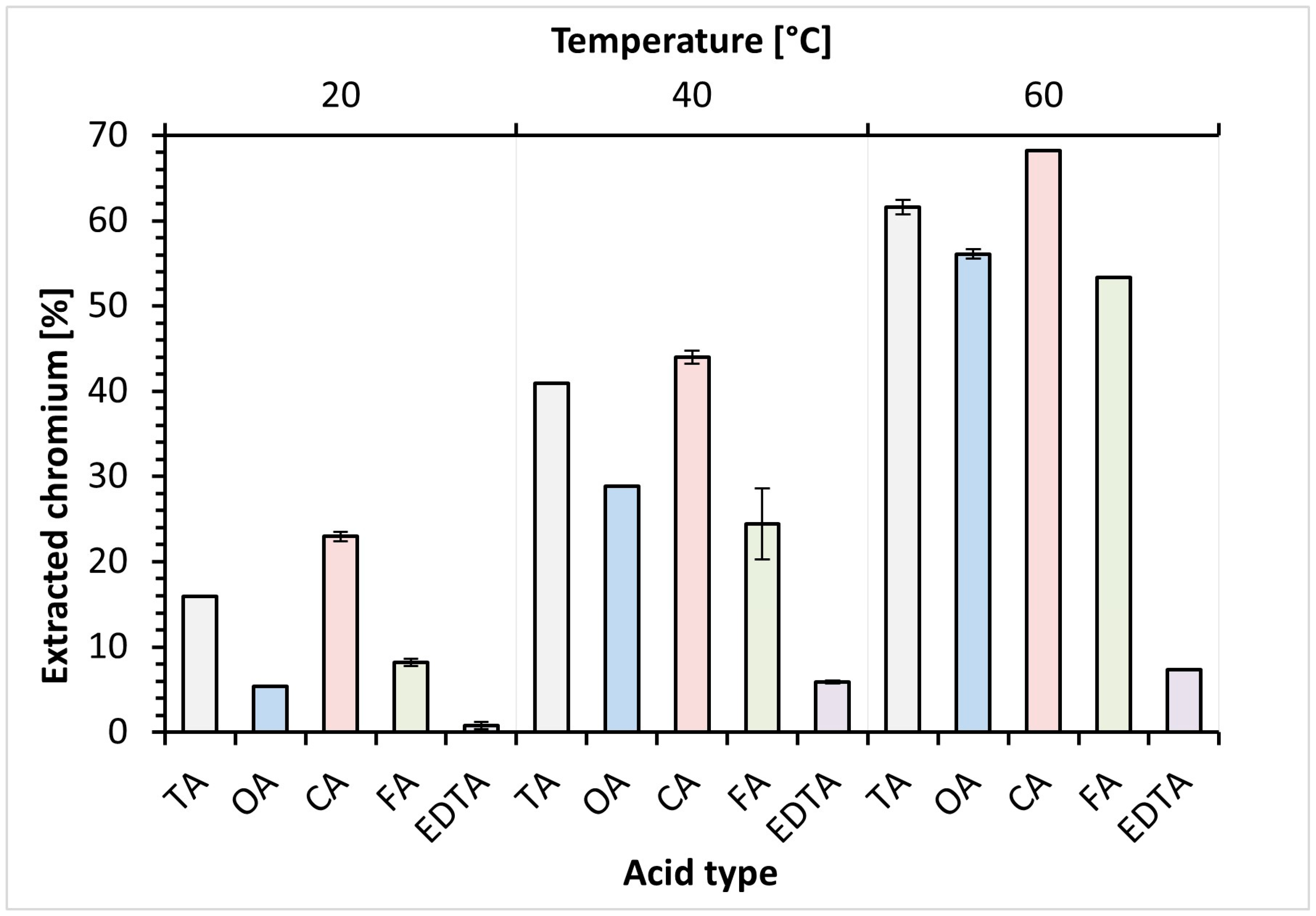
3.3. Chromium Removal Efficiency by Substitution Extraction
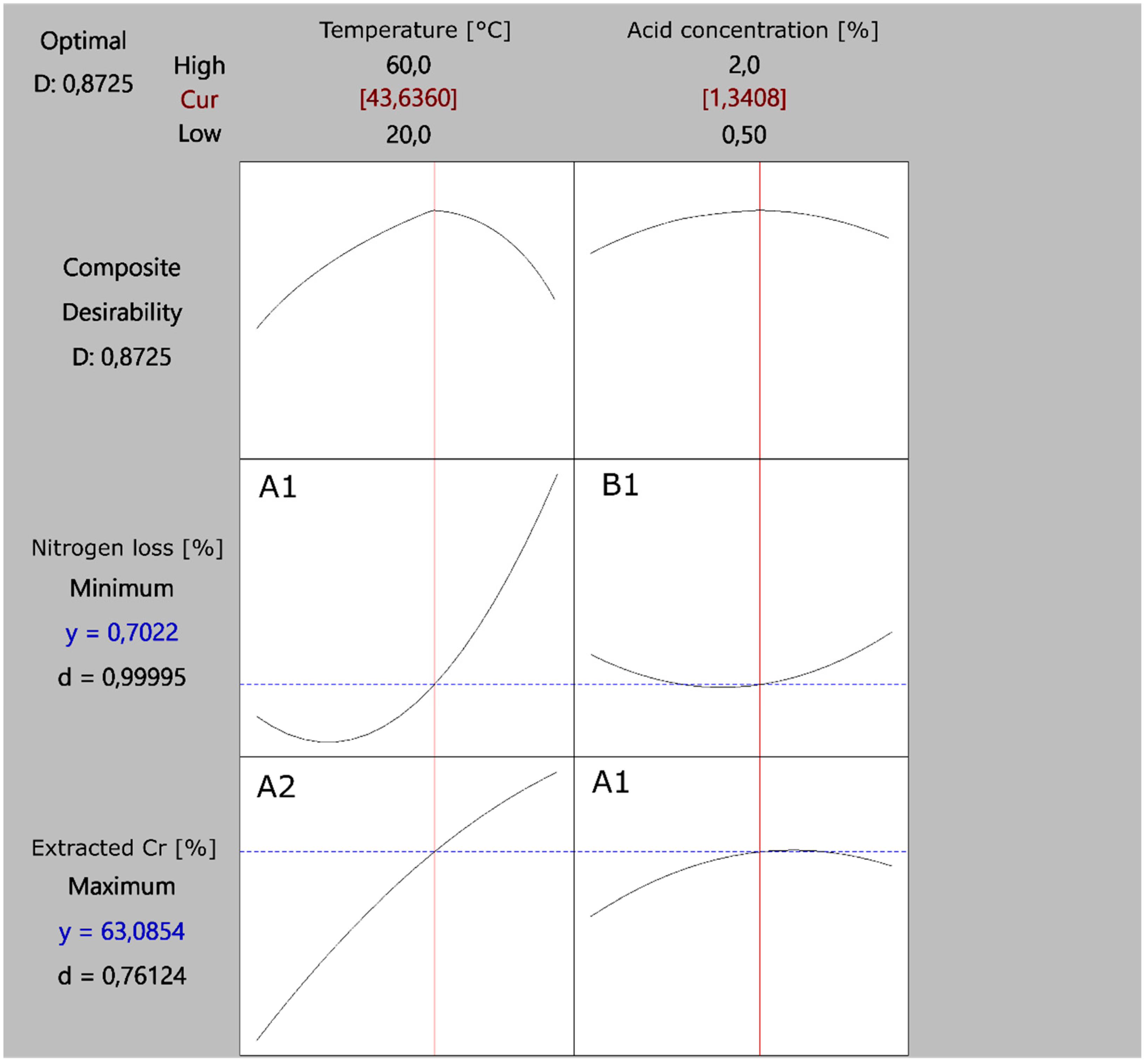
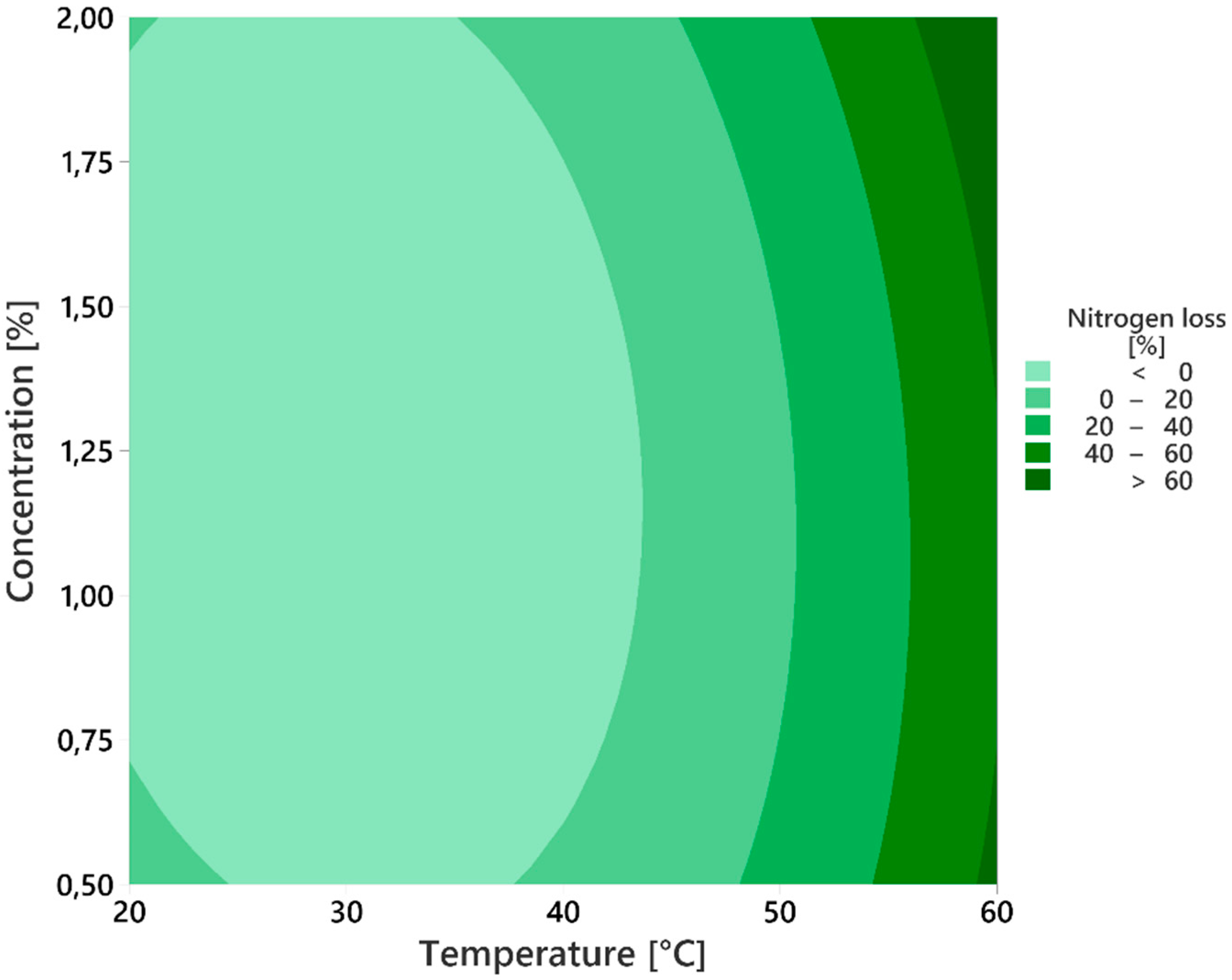
3.4. Process Optimization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pati, A.; Chaudhary, R.; Subramani, S. A Review on Management of Chrome-Tanned Leather Shavings: A Holistic Paradigm to Combat the Environmental Issues. Environ. Sci. Pollut. Res. 2014, 21, 11266–11282. [Google Scholar] [CrossRef] [PubMed]
- Muralidharan, V.; Palanivel, S.; Balaraman, M. Turning Problem into Possibility: A Comprehensive Review on Leather Solid Waste Intra-Valorization Attempts for Leather Processing. J. Clean. Prod. 2022, 367, 133021. [Google Scholar] [CrossRef]
- Luo, F.; Liu, Z.; Zhao, Q.; Wang, S.; He, L.; Wu, Y.; Chen, Z. An Innovative Ionic Liquid-Aqueous Biphasic System for Simultaneously Highly Efficient Dechroming and Collagen Recovery from Chrome-Tanned Leather Shavings at Room Temperature. Sep. Purif. Technol. 2024, 349, 127861. [Google Scholar] [CrossRef]
- Pedrotti, M.F.; Santos, D.; Cauduro, V.H.; Bizzi, C.A.; Flores, E.M.M. Ultrasound-Assisted Extraction of Chromium from Tanned Leather Shavings: A Promising Continuous Flow Technology for the Treatment of Solid Waste. Ultrason. Sonochem. 2022, 89, 106124. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zou, H.; Cheng, Y.; Tao, E. Immobilizing Chromium in Tannery Sludge via Adding Collagen Protein Waste: An in-Depth Study on Mechanism. Environ. Sci. Pollut. Res. 2022, 29, 30337–30347. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.B.; Johansen, J.D.; Menné, T. Chromium Allergy: Significance of Both Cr(III) and Cr(VI). Contact Dermatitis 2003, 49, 206–212. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Bani Mfarrej, M.F.; Alam, P.; Rinklebe, J.; Ahmad, P. Accumulation of Chromium in Plants and Its Repercussion in Animals and Humans. Environ. Pollut. 2022, 301, 119044. [Google Scholar] [CrossRef] [PubMed]
- Kolomaznik, K.; Adamek, M.; Andel, I.; Uhlirova, M. Leather Waste-Potential Threat to Human Health, and a New Technology of Its Treatment. J. Hazard. Mater. 2008, 160, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, M.; Clement, Y.; Blanc, N.; Demesmay, C. Hexavalent Chromium Release from Leather over Time Natural Ageing vs. Accelerated Ageing According to a Multivariate Approach. J. Hazard. Mater. 2019, 368, 811–818. [Google Scholar] [CrossRef]
- Wright, A.; Laundry-Mottiar, L.; Hedberg, Y.S. The Ability of Sweat and Buffer Solutions to Reduce Hexavalent Chromium of Relevance for Leather Extraction. Regul. Toxicol. Pharmacol. 2022, 133, 105222. [Google Scholar] [CrossRef]
- Mikula, K.; Konieczka, M.; Taf, R.; Skrzypczak, D.; Izydorczyk, G.; Moustakas, K.; Kułażyński, M.; Chojnacka, K.; Witek-Krowiak, A. Tannery Waste as a Renewable Source of Nitrogen for Production of Multicomponent Fertilizers with Biostimulating Properties. Environ. Sci. Pollut. Res. 2023, 30, 8759–8777. [Google Scholar] [CrossRef] [PubMed]
- Maliha, M.; Rashid, T.U.; Rahman, M.M. A Green Strategy for Collagen Extraction from Tannery Raw Trimmings Using Papain Enzyme: Process Optimization by MW-TOPSIS for Enhanced Yield. Int. J. Biol. Macromol. 2024, 262, 130040. [Google Scholar] [CrossRef]
- Parisi, M.; Nanni, A.; Colonna, M. Recycling of Chrome-Tanned Leather and Its Utilization as Polymeric Materials and in Polymer-Based Composites: A Review. Polymers 2021, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Munir, D.; Santos, R.M. Beneficial Use of Animal Hides for Abattoir and Tannery Waste Management: A Review of Unconventional, Innovative, and Sustainable Approaches. Environ. Sci. Pollut. Res. 2022, 29, 1807–1823. [Google Scholar] [CrossRef]
- Khatoon, M.; Kashif, S.; Saad, S.; Umer, Z.; Rasheed, A. Extraction of Amino Acids and Proteins from Chrome Leather Waste. J. Waste Recycl. 2017, 2, 6. [Google Scholar]
- Velusamy, M.; Chakali, B.; Ganesan, S.; Tinwala, F.; Shanmugham Venkatachalam, S. Investigation on Pyrolysis and Incineration of Chrome-Tanned Solid Waste from Tanneries for Effective Treatment and Disposal: An Experimental Study. Environ. Sci. Pollut. Res. 2020, 27, 29778–29790. [Google Scholar] [CrossRef]
- Nasr, A.; Gaber, M.; Ali, H.; Eissa, M. Treating Leather Shaving Waste To Decrease Its Environmental Impact. Egypt. J. Desert Res. 2018, 68, 75–88. [Google Scholar] [CrossRef]
- Abdulla-Al-Mamun, M.; Hossain, N.; Hossain, M.I.; Sultana, R. Conversion of Leather Industry Solid Waste to Organic Fertilizer by Vermicomposting: Use for Plant Growth. Text. Leather Rev. 2023, 6, 37–56. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Lorea, B.; González-Gaitano, G. Biological Solubilisation of Leather Industry Waste in Anaerobic Conditions: Effect of Chromium (III) Presence, Pre-Treatments and Temperature Strategies. Int. J. Mol. Sci. 2022, 23, 13647. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, D.T.; Kathir, I.B.; Adeyi, O.A.; Ejila, A. De-Chroming of Chrome Tanned Leather Solid Waste Using Modified Alkaline Hydrolysis Process. Int. J. Eng. Res. Technol. 2014, 3, 620–623. [Google Scholar]
- Majee, S.; Halder, G.; Krishnaraj, R.N.; Mandal, T. Development and Formulation of an Organic Fertilizer from Industrial and Agricultural Waste to Study the Growth of Marigold (Tagetes) Plant. Int. J. Math. Eng. Manag. Sci. 2020, 5, 395–404. [Google Scholar] [CrossRef]
- Mwondu, J.M.; Ombui, J.N.; Kironchi, G.; Onyuka, A. Development of an Eco-Friendly and Sustainable Method of Dechroming Leather Wastes. Text. Leather Rev. 2021, 4, 364–391. [Google Scholar] [CrossRef]
- Kubilius, K.; Biškauskaitė, R.; Valeika, V. Enzymatic Hydrolysis of Chromed Leather Wastes and Application of Hydrolysate. Int. J. Sci. Eng. Manag. 2023, 10, 5–8. [Google Scholar]
- Wang, L.; Li, J.; Jin, Y.; Chen, M.; Luo, J.; Zhu, X.; Zhang, Y. Study on the Removal of Chromium(III) from Leather Waste by a Two-Step Method. J. Ind. Eng. Chem. 2019, 79, 172–180. [Google Scholar] [CrossRef]
- Gendaszewska, D.; Lasoń-Rydel, M.; Ławińska, K.; Grzesiak, E.; Pipiak, P. Characteristics of Collagen Preparations from Leather Wastes by the High Pressure Liquid Chromatography Method. Fibres Text. East. Eur. 2021, 29, 75–79. [Google Scholar] [CrossRef]
- Beltrán-Prieto, J.C.; Veloz-Rodríguez, R.; Pérez-Pérez, M.C.; Navarrete-Bolaños, J.L.; Vázquez-Nava, E.; Jiménez-Islas, H.; Botello-Álvarez, J.E. Chromium Recovery from Solid Leather Waste by Chemical Treatment and Optimisation by Response Surface Methodology. Chem. Ecol. 2012, 28, 89–102. [Google Scholar] [CrossRef]
- Belkacemi, H.; Benhadji, A.; Taleb Ahmed, M. Recovery of Chromium from Wet Blue Shavings and Its Use as a Semiconductor for Wastewater Treatment. Int. J. Environ. Sci. Technol. 2023, 20, 6319–6338. [Google Scholar] [CrossRef]
- Popiolski, A.S.; Dallago, R.M.; Steffens, J.; Mignoni, M.L.; Venquiaruto, L.D.; Santos, D.; Duarte, F.A. Ultrasound-Assisted Extraction of Cr from Residual Tannery Leather: Feasibility of Ethylenediaminetetraacetic Acid as the Extraction Solution. ACS Omega 2018, 3, 16074–16080. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, M.; Li, J.; Jin, Y.; Zhang, Y.; Wang, Y. A Novel Substitution-Based Method for Effective Leaching of Chromium (III) from Chromium-Tanned Leather Waste: The Thermodynamics, Kinetics and Mechanism Studies. Waste Manag. 2020, 103, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; Glass, W.K.; Rasul Jan, M.; Mulders, R. Investigation of Hydroxamic Acids for the Extraction of Chromium(III) from Leather Waste and the Possible Re-Use of the Extracted Chromium in the Tanning Industry. Environ. Technol. Lett. 1986, 7, 283–288. [Google Scholar] [CrossRef]
- Chen, H.; Li, Y.; Dai, H.; Chen, L.; Ding, X.; Hu, Z. Collagen Hydrolyzed Extract Derived from Leather Waste as a Multifunctional Additive for the Preparation of Granular Fertilizer. Sustain. Chem. Pharm. 2023, 36, 101327. [Google Scholar] [CrossRef]
- Popiolski, A.S.; Demaman Oro, C.E.; Steffens, J.; Bizzi, C.A.; Santos, D.; Alessio, K.O.; Flores, É.M.M.; Duarte, F.A.; Dallago, R.M. Microwave-Assisted Extraction of Cr from Residual Tanned Leather: A Promising Alternative for Waste Treatment from Tannery Industry. J. Environ. Chem. Eng. 2022, 10, 107081. [Google Scholar] [CrossRef]
- Bizzi, C.A.; Zanatta, R.C.; Santos, D.; Giacobe, K.; Dallago, R.M.; Mello, P.A.; Flores, E.M.M. Ultrasound-Assisted Extraction of Chromium from Residual Tanned Leather: An Innovative Strategy for the Reuse of Waste in Tanning Industry. Ultrason. Sonochem. 2020, 64, 104682. [Google Scholar] [CrossRef]
- Malek, A.; Hachemi, M.; Didier, V. New Approach of Depollution of Solid Chromium Leather Waste by the Use of Organic Chelates. Economical and Environmental Impacts. J. Hazard. Mater. 2009, 170, 156–162. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, Y.; Wang, H.; Zhang, K. Regeneration of Native Collagen from Hazardous Waste: Chrome-Tanned Leather Shavings by Acid Method. Environ. Sci. Pollut. Res. 2020, 27, 31300–31310. [Google Scholar] [CrossRef]
- Yorgancioglu, A.; Başaran, B.; Sancakli, A. Value Addition to Leather Industry Wastes and By-Products: Hydrolyzed Collagen and Collagen Peptides. In Waste in Textile and Leather Sectors; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Popita, G.E.; Rosu, C.; Manciula, D.; Corbu, O.; Popovici, A.; Nemes, O.; Sandu, A.V.; Proorocu, M.; Dan, S.B. Industrial Tanned Leather Waste Embedded in Modern Composite Materials. Mater. Plast. 2016, 53, 308–311. [Google Scholar]
- Nalyanya, K.M.; Rop, R.K.; Onyuka, A.S.; Birech, Z.; Okonda, J.J. Variation of Elemental Concentration in Leather during Post-Tanning Operation Using Energy Dispersive X-Ray Fluorescence Spectroscopy: Principal Component Analysis Approach. Int. J. Environ. Anal. Chem. 2022, 102, 1935–1947. [Google Scholar] [CrossRef]
- Yang, J.; Shan, Z.; Zhang, Y.; Chen, L. Stabilization and Cyclic Utilization of Chrome Leather Shavings. Environ. Sci. Pollut. Res. 2019, 26, 4680–4689. [Google Scholar] [CrossRef] [PubMed]
- Scopel, B.S.; Ribeiro, M.E.; Dettmer, A.; Baldasso, C. Cornstarch-Gelatin Films: Commercial Gelatin Versus Chromed Leather Waste Gelatin and Evaluation of Drying Conditions. J. Polym. Environ. 2018, 26, 1998–2006. [Google Scholar] [CrossRef]
- Wolf, K.; Gilbert, P.A. EDTA—Ethylenediaminetetraacetic Acid. In Detergents; Springer: Berlin/Heidelberg, Germany, 1992; pp. 243–259. [Google Scholar]
- Ding, W.; Liao, X.; Zhang, W.; Shi, B.I. Dechroming of Chromium-Containing Leather Waste with Low Hydrolysis Degree of Collagen. J. Soc. Leather Technol. Chem. 2015, 99, 129–133. [Google Scholar]
- Vaiopoulou, E.; Gikas, P. Regulations for Chromium Emissions to the Aquatic Environment in Europe and Elsewhere. Chemosphere 2020, 254, 126876. [Google Scholar] [CrossRef] [PubMed]
- Tao, E.; Cheng, Y.; Yang, S.; Zou, H. Recovering Cr(III) from Chromium-Containing Waste: An in-Depth Study on Mechanism via Retaining Organic Matters. J. Environ. Chem. Eng. 2021, 9, 105598. [Google Scholar] [CrossRef]
- Catalina, M.M.; Antunes, A.P.M.; Attenburrow, G.; Cot, J.; Covington, A.D.; Phillips, P.S.; Antunes, P.; Attenburrow, G.; Cot, J.; Covington, A.D.; et al. Sustainable Management of Waste-Reduction of the Chromium Content of Tannery Solid Waste as a Step in the Cleaner Production of Gelatin. J. Solid Waste Technol. Manag. 2007, 33, 43–50. [Google Scholar]


| Experimental Procedure | Organic Acid | Concentration | pH | Temperature [°C] | Stirring Speed [RPM] | Extraction Time [min] |
|---|---|---|---|---|---|---|
| Complexing ability experiments Concentration in [mol/dm3] | Tartaric acid (TA) | 0.225 | 1.728 | 20, 40, 60 | 500 | 300 |
| Oxalic acid (OA) | 0.019 | 1.820 | ||||
| Citric acid (CA) | 0.32 | 1.694 | ||||
| Formic acid (FA) | 1.29 | 1.631 | ||||
| EDTA acid (EDTA) | 0.0034 | 2.847 | ||||
| Substitution extraction experiments Concentration in [%] | TA | 0.5 | 2.505 | 20, 40, 60 | 500 | 300 |
| 1.0 | 2.168 | |||||
| 2.0 | 1.937 | |||||
| OA | 0.5 | 1.239 | ||||
| 1.0 | 1.488 | |||||
| 2.0 | 1.206 | |||||
| CA | 0.5 | 2.368 | ||||
| 1.0 | 2.301 | |||||
| 2.0 | 2.130 | |||||
| FA | 0.5 | 2.532 | ||||
| 1.0 | 2.232 | |||||
| 2.0 | 2.001 | |||||
| EDTA | 0.05 | 3.542 |
| Organic Acid | Acid Concentration [mol/dm3] | Acid Concentration [%] | pH Calculated | pH Measured |
|---|---|---|---|---|
| TA | 0.20 | 3.0 | 1.823 | 1.728 |
| OA | 0.019 | 0.17 | 1.823 | 1.820 |
| CA | 0.31 | 5.9 | 1.824 | 1.794 |
| FA | 1.3 | 5.8 | 1.823 | 1.731 |
| EDTA * | 0.026 | 0.75 | 1.823 | 2.847 1 |
| Organic Acid | Acid Concentration [%] | Temperature [%] | Nt Loss [%] | Cr Yield [%] |
|---|---|---|---|---|
| TA | 0.50% | 60 | 8.0 | 60 |
| TA | 1.00% | 60 | 6.9 | 72 |
| TA | 2.00% | 60 | 15 | 72 |
| OA | 0.50% | 60 | 72 | 68 |
| OA | 1.00% | 40 | 1.1 | 57 |
| OA | 2.00% | 40 | 1.8 | 57 |
| OA | 1.00% | 60 | 44 | 79 |
| OA | 2.00% | 60 | 85 | 73 |
| CA | 0.50% | 60 | 8.3 | 54 |
| CA | 1.00% | 60 | 3.9 | 53 |
| CA | 2.00% | 60 | 23 | 63 |
| FA | 2.00% | 60 | 0.9 | 50 |
| Method Reference | Temperature (°C) | Time | Type of Acid | Cr extraction Efficiency | Collagen Loss | Cr(VI) Monitoring |
|---|---|---|---|---|---|---|
| Substitution Extraction [30] | 50 | 8 h | Citric and oxalic acids | 78% | High decomposition above 60 °C | Not specified |
| Microwave-Assisted Extraction [28] | 60 | 3 min | EDTA acid (3 mol/L) | 99% | Not considered in this study | Not specified |
| Acid extraction [35] | 40 | 12 h | Sulfuric and oxalic acid (1:1 ratio) | 96% | High-purity collagen obtained | Not specified |
| Ultrasound-Assisted Extraction [33] | 50 | 3 min + 3 × 3-min washing | EDTA (Cr/EDTA molar ratio 1:3) | >98% | Not specified | Not specified |
| Substitution extraction (this study) | 43.6 | 5 h | Oxalic acid 1.3 wt% | 63.1% | 0.70% | Monitored (0.65 mg/L) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świerczek, L.; Hercel, P.; Konkol, I.; Kuligowski, K.; Cenian, A. Chromium Substitution Extraction Method for Its Recovery from Chromium-Tanned Leather Waste. Materials 2025, 18, 118. https://doi.org/10.3390/ma18010118
Świerczek L, Hercel P, Konkol I, Kuligowski K, Cenian A. Chromium Substitution Extraction Method for Its Recovery from Chromium-Tanned Leather Waste. Materials. 2025; 18(1):118. https://doi.org/10.3390/ma18010118
Chicago/Turabian StyleŚwierczek, Lesław, Paulina Hercel, Izabela Konkol, Ksawery Kuligowski, and Adam Cenian. 2025. "Chromium Substitution Extraction Method for Its Recovery from Chromium-Tanned Leather Waste" Materials 18, no. 1: 118. https://doi.org/10.3390/ma18010118
APA StyleŚwierczek, L., Hercel, P., Konkol, I., Kuligowski, K., & Cenian, A. (2025). Chromium Substitution Extraction Method for Its Recovery from Chromium-Tanned Leather Waste. Materials, 18(1), 118. https://doi.org/10.3390/ma18010118











