Design and In Vitro Activity of Furcellaran/Chitosan Multilayer Microcapsules for the Delivery of Glutathione and Empty Model Multilayer Microcapsules Based on Polysaccharides
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials for Preparing Capsules
2.2. Preparation of Biopolymer Solution
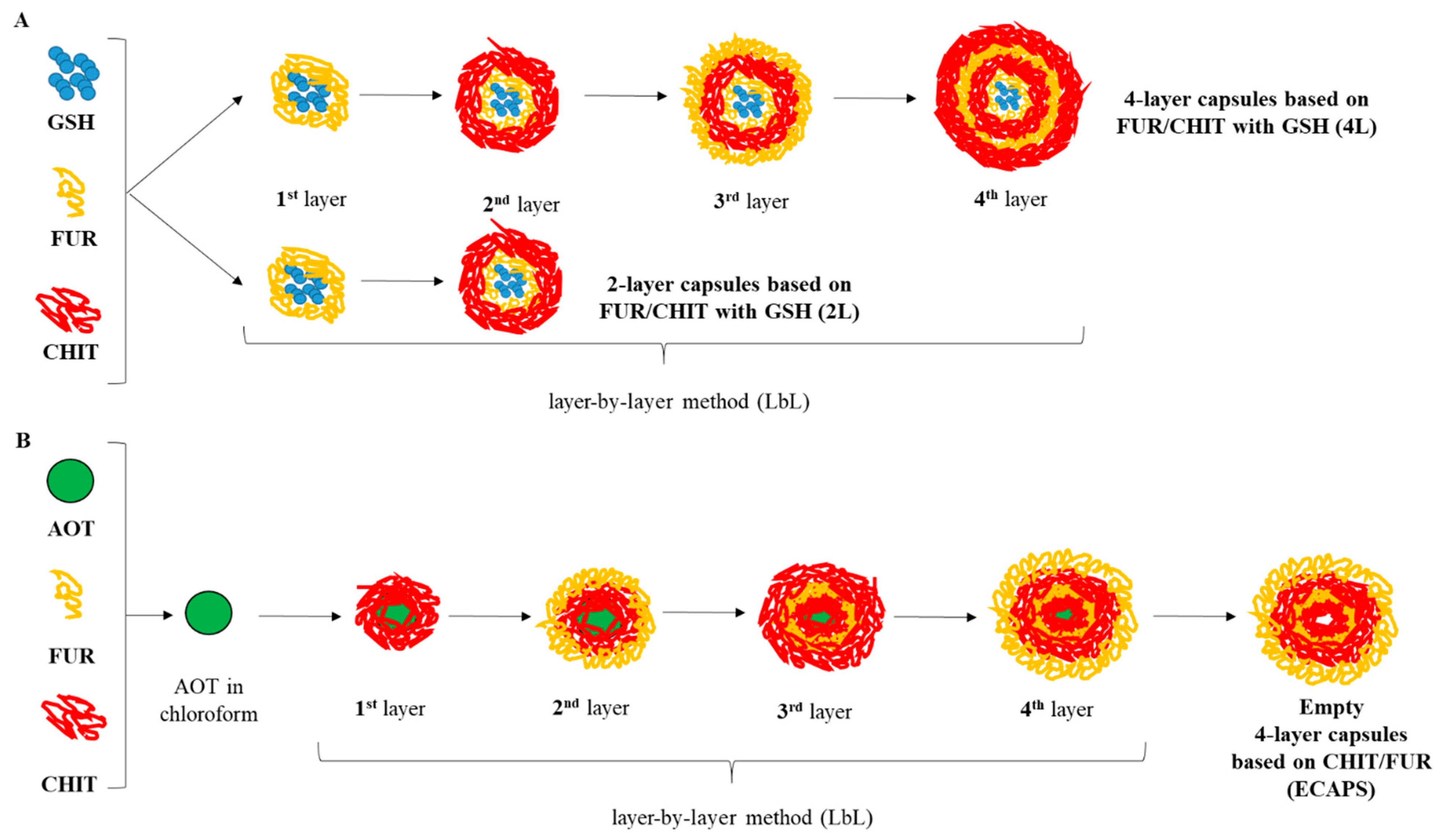
2.3. Preparation of Capsules with Different Number of Layers Enclosed with Glutathione
2.4. Preparation of Empty Capsules with Four Layers
- -
- FUR/CHIT two-layer capsule enriched in GSH (5 mg or 25 mg/mL)—2L5 and 2L25
- -
- (FUR/CHIT)2 four-layer capsule enriched in GSH (5 mg or 25 mg/mL)—4L5 and 4L25
- -
- (CHIT/FUR)2 empty capsule—ECAPS
2.5. Particle Size and Zeta Potential
2.6. SEM
2.7. Simulated In Vitro Digestion Model of Gastrointestinal Tract
2.8. Simulated In Vitro Absorption Model of Gastrointestinal Tract
2.9. Cell Culture
2.10. Cell Treatment
2.11. Cell Proliferation Assessment
2.12. Cytotoxicity Assay
2.13. Muse Flow Cytometer Analysis
2.14. Statistical Analysis
3. Results and Discussion
3.1. Preparation of Capsules
3.2. Impact on Cell Proliferation
3.3. Cytotoxicity
3.4. PI3K/MAPK Activity Assay
3.5. Detection of Early and Late Markers of Biochemical Apoptosis
3.6. Cell Cycle Phase Distribution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Milosavljevic, V.; Jamróz, E.; Gagic, M.; Haddad, Y.; Michalkova, H.; Balkova, R.; Tesarova, B.; Moulick, A.; Heger, Z.; Richtera, L. Encapsulation of doxorubicin in furcellaran/chitosan nanocapsules by layer-by-layer technique for selectively controlled drug delivery. Biomacromolecules 2020, 21, 418–434. [Google Scholar] [CrossRef]
- Wang, C.; Ye, S.; Dai, L.; Tong, Z. Enzymatic desorption of layer-by-layer assembled multilayer films and effects on the release of encapsulated indomethacin microcrystals. Carbohydr. Res. 2007, 342, 2237–2243. [Google Scholar] [CrossRef]
- Xuan, M.; Zhao, J.; Shao, J.; Du, C.; Cui, W.; Duan, L.; Qi, W.; Li, J. Recent progresses in layer-by-layer assembled biogenic capsules and their applications. J. Colloid Interface Sci. 2017, 487, 107–117. [Google Scholar] [CrossRef]
- Jamróz, E.; Para, G.; Jachimska, B.; Szczepanowicz, K.; Warszyński, P.; Para, A. Albumin–furcellaran complexes as cores for nanoencapsulation. Colloids Surf. A Physicochem. Eng. Asp. 2014, 441, 880–884. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.M.; Assadpour, E. Development of a nutraceutical nano-delivery system through emulsification/internal gelation of alginate. Food Chem. 2017, 229, 286–295. [Google Scholar] [CrossRef]
- Ji, F.; Li, J.; Qin, Z.; Yang, B.; Zhang, E.; Dong, D.; Wang, J.; Wen, Y.; Tian, L.; Yao, F. Engineering pectin-based hollow nanocapsules for delivery of anticancer drug. Carbohydr. Polym. 2017, 177, 86–96. [Google Scholar] [CrossRef]
- Vilela, C.; Figueiredo, A.R.; Silvestre, A.J.; Freire, C.S. Multilayered materials based on biopolymers as drug delivery systems. Expert Opin. Drug Deliv. 2017, 14, 189–200. [Google Scholar] [CrossRef]
- No, K.; Park, N.Y.; Lee, S.H.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan 549 and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the physical properties, antioxidant and antimicrobial activity of ternary potato starch-furcellaran-gelatin films incorporated with lavender essential oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Lu, L. Polydopamine and Its derivative materials: Synthesis and promising applications in energy, environmental, and biomedical fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar]
- Liu, Y.; Yang, J.; Zhao, Z.; Li, J.; Zhang, R.; Yao, F. Formation and characterization of natural polysaccharide hollow nanocapsules via template layer-by-layer self-assembly. J. Colloid Interface Sci. 2012, 379, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, N.; Narang, J.; Meena; Pundir, C.S. An amperometric glutathione biosensor based on chitosan–iron coated gold nanoparticles modified Pt electrode. Int. J. Biol. Macromol. 2010, 51, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.; Bradley, R.D. Effects of oral glutathione supplementation on systemic oxidative stress biomarkers in human volunteers. J. Altern. Complement. Med. 2011, 17, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Dronka-Góra, D.; Para, G.; Warszyński, P. Encapsulation of liquid cores by layer-by-layer adsorption of polyelectrolytes. J. Microencapsul. 2010, 27, 198–204. [Google Scholar] [CrossRef]
- Guzey, D.; McClements, D.J. Formation, stability and properties of multilayer emulsions for application in the food industry. Adv. Colloid Interface Sci. 2006, 128–130, 227–248. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D. A standardized static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Piasna-Słupecka, E.; Leszczyńska, T.; Drozdowska, M.; Dziadek, K.; Domagała, B.; Domagała, D.; Koronowicz, A. Young shoots of red beet and the root at full maturity inhibit proliferation and induce apoptosis in breast cancer cell lines. Int. J. Mol. Sci. 2023, 24, 6889. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Bourbon, A.I.; Cerqueira, M.A.; Maricato, É.; Nunes, C.; Coimbra, M.A.; Vicente, A.A. Chitosan/fucoidan multilayer nanocapsules as a vehicle for controlled release of bioactive compounds. Carbohydr. Polym. 2015, 115, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Liang, M.; Dou, X.; Feng, C.; Pang, J.; Cheng, X.; Liu, H.; Liu, T.; Wang, Y.; Chen, X. Development of alginate hydrogel/gum Arabic/gelatin based composite capsules and their application as oral delivery carriers for antioxidant. Int. J. Biol. Macromol. 2019, 132, 1090–1097. [Google Scholar] [CrossRef]
- Such, A.; Wisła-Świder, A.; Węsierska, E.; Nowak, E.; Szatkowski, P.; Kopcińska, J.; Koronowicz, A. Edible chitosan-alginate based coatings enriched with turmeric and oregano additives: Formulation, antimicrobial and non-cytotoxic properties. Food Chem. 2023, 426, 136662. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.Y.; Jang, M.K.; Nah, J.W. Influence of molecular weight on oral absorption of water soluble chitosans. J. Control. Release 2005, 102, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Cheung, R.C.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An update on potential biomedical and pharmaceutical applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2002, 282, 119132. [Google Scholar] [CrossRef]
- Jasek-Gajda, E.; Jurkowska, H.; Jasińska, M.; Lis, G.J. Targeting the MAPK/ERK and PI3K/AKT signaling pathways affects NRF2, Trx and GSH antioxidant systems in leukemia cells. Antioxidants 2020, 9, 633. [Google Scholar] [CrossRef]
- Bonel-Pérez, G.C.; Pérez-Jiménez, A.; Gris-Cárdenas, I.; Parra-Pérez, A.M.; Lupiáñez, J.A.; Reyes-Zurita, F.J.; Siles, E.; Csuk, R.; Peragón, J.; Rufino-Palomares, E.E. Antiproliferative and pro-apoptotic effect of uvaol in human hepatocarcinoma HepG2 cells by affecting G0/G1 cell cycle arrest, ROS production and AKT/PI3K signaling pathway. Molecules 2020, 25, 4254. [Google Scholar] [CrossRef] [PubMed]
- Moelling, K.; Schad, K.; Bosse, M.; Zimmermann, S.; Schweneker, M. Regulation of Raf-Akt cross-talk. J. Biol. Chem. 2002, 277, 31099–31106. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, A.P. Beyond annexin V: Fluorescence response of cellular membranes to apoptosis. Cytotechnology 2013, 65, 157–172. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, Y.T. Glutathione and apoptosis. Free Radic. Res. 2008, 42, 689–706. [Google Scholar] [CrossRef] [PubMed]
- Gamcsik, M.P.; Kasibhatla, M.S.; Teeter, S.D.; Colvin, O.M. Glutathione levels in human tumors. Biomarkers 2012, 17, 671–691. [Google Scholar] [CrossRef] [PubMed]
- Schnelldorfer, T.; Gansauge, S.; Gansauge, F.; Schlosser, S.; Beger, H.G.; Nussler, A.K. Glutathione depletion causes cell growth inhibition and enhanced apoptosis in pancreatic cancer cells. Cancer 2000, 89, 1440–1447. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Sinha, I.; Calcagnotto, A.; Trushin, N.; Haley, J.S.; Schell, T.D.; Richie, J.P., Jr. Oral supplementation with liposomal glutathione elevates body stores of glutathione and markers of immune function. Eur. J. Clin. Nutr. 2018, 72, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Marini, H.R.; Facchini, B.A.; di Francia, R.; Freni, J.; Puzzolo, D.; Montella, L.; Facchini, G.; Ottaiano, A.; Berretta, M.; Minutoli, L. Glutathione: Lights and shadows in patients. Biomedicines 2023, 11, 2226. [Google Scholar] [CrossRef] [PubMed]
- Bertoni, S.; Albertini, B.; Facchini, C.; Prata, C.; Passerini, N. Glutathione-loaded solid lipid microparticles as innovative delivery system for oral antioxidant therapy. Pharmaceutics 2019, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Oestreicher, J.; Morgan, B. Glutathione: Subcellular distribution and membrane transport. Biochem. Cell Biol. 2019, 97, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Perego, P.; Gatti, L.; Carenini, N.; Dal Bo, L.; Zunino, F. Apoptosis induced by extracellular glutathione is mediated by H2O2 production and DNA damage. Int. J. Cancer 2000, 87, 343–348. [Google Scholar] [CrossRef]
- Shen, H.M.; Yang, C.F.; Liu, J.; Ong, C.N. Dual role of glutathione in selenite-induced oxidative stress and apoptosis in human hepatoma cells. Free Radic. Biol. Med. 2000, 28, 1115–1124. [Google Scholar] [CrossRef]
- Duan, J.; Zhang, Y.; Han, S.; Chen, Y.; Li, B.; Liao, M.; Huang, B. Synthesis and In Vitro/In Vivo anti-cancer evaluation of curcumin-loaded chitosan/poly(butyl cyanoacrylate) nanoparticles. Int. J. Pharm. 2010, 400, 211–220. [Google Scholar]
- Hasegawa, M.; Yagi, K.; Iwakawa, S.; Hirai, M. Chitosan induces apoptosis via caspase-3 activation in bladder tumor cells. Jpn. J. Cancer Res. 2001, 92, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Chitosan promotes ROS-mediated apoptosis and S phase cell cycle arrest in triple-negative breast cancer cells: Evidence for intercalative interaction with genomic DNA. RSC Adv. 2017, 7, 43141–43150. [Google Scholar] [CrossRef]
- Wimardhani, Y.S. Chitosan exerts anticancer activity through induction of apoptosis and cell cycle arrest in oral cancer cells. J. Oral Sci. 2014, 56, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Estevez, H.; Garcia-Lidon, J.C.; Luque-Garcia, J.L.; Camara, C. Effects of chitosan-stabilized selenium nanoparticles on cell proliferation, apoptosis and cell cycle pattern in HepG2 cells: Comparison with other selenospecies. Colloids Surf. B 2014, 122, 184–193. [Google Scholar] [CrossRef] [PubMed]


| PI3K/MAPK Activation | ||||||||
|---|---|---|---|---|---|---|---|---|
| UC | STS | GSH5 | GSH25 | 2L5 | 2L25 | 4L25 | ECAPS | |
| MAPK activation | 0.97 c ± 0.46 | 0.05 a ± 0.07 | 0.73 b,c ± 0.25 | 0.83 c ± 0.25 | 0.83 c ± 1.21 | 0.3 a,c ± 0.35 | 0.2 a ± 0.2 | 0.5 a,b,c ± 0.17 |
| PI3K activation | 25.27 a ± 1.79 | 56.85 d ± 0.64 | 45.0 b ± 1.14 | 42.87 b ± 1.1 | 42.4 b ± 0.96 | 52.07 c ± 1.99 | 54.4 c,d ± 1.47 | 53.9 c ± 2.14 |
| dual pathway activation | 64.67 e ± 0.96 | 16.17 a ± 0.57 | 39.63 c ± 2.0 | 40.07 c ± 2.39 | 43.73 d ± 2.63 | 30.3 b ± 1.44 | 29.0 b ± 2.8 | 29.13 b ± 0.46 |
| negative | 9.1 a ± 0.35 | 26.4 d ± 1.13 | 14.63 b,c ± 1.12 | 16.23 b,c ± 3.0 | 13.03 b ± 1.8 | 17.33 c ± 1.0 | 16.4 c ± 1.81 | 16.47 c ± 2.65 |
| Apoptosis Activity | ||||||||
| UC | STS | GSH5 | GSH25 | 2L5 | 2L25 | 4L25 | ECAPS | |
| Live | 85.98 e ± 0.88 | 55.93 a ± 0.93 | 62.67 c ± 1.62 | 62.65 c ± 1.4 | 59.15 b ± 0.13 | 60.13 b,c ± 1.92 | 66.3 d ± 3.29 | 67.58 d ± 0.72 |
| Early apoptotic | 7.78 a ± 0.43 | 22.78 c ± 1.58 | 16.98 b ± 1.19 | 14.52 b ± 1.58 | 17.95 b ± 0.75 | 15.10 b ± 3.88 | 9.9 a ± 2.08 | 10.0 a ± 1.16 |
| Late apoptotic | 3.40 a ± 2.0 | 19.55 b,c ± 0.2 | 17.60 b ± 0.78 | 19.47 b,c ± 0.76 | 19.87 b,c ± 0.43 | 23.62 d ± 1.73 | 21.80 c,d ± 1.73 | 20.75 c ± 1.57 |
| Dead | 1.15 a ± 0.0 | 1.4 a,b ± 0.1 | 2.75 c ± 0.41 | 3.37 d ± 0.4 | 3.03 c,d ± 0.33 | 1.15 a ± 0.52 | 2.0 b ± 0.1 | 1.67 a,b ± 0.43 |
| Total apoptotic | 12.88 a ± 0.88 | 42.68 e ± 1.03 | 34.58 c ± 1.85 | 37.82 b,c ± 0.38 | 37.82 d ± 0.38 | 38.72 d ± 2.44 | 31.70 b,c ± 3.23 | 30.75 b ± 1.13 |
| Caspase-3/7 Activity | ||||||||
| UC | STS | GSH5 | GSH25 | 2L5 | 2L25 | 4L25 | ECAPS | |
| Live | 89.8 e ± 0.35 | 46.47 b,c ± 2.8 | 43.17 b ± 9.56 | 48.28 b,c ± 1.51 | 36.50 a ± 1.26 | 41.78 a,b ± 1.08 | 51.44 c,d ± 0.72 | 57.37 d ± 0.86 |
| Early apoptotic | 2.93 a ± 0.53 | 31.87 e ± 4.59 | 19.58 c,d ± 5.25 | 7.18 a ± 0.35 | 21.15 d ± 1.26 | 16.32 b,c ± 0.85 | 17.16 b,c,d ± 0.96 | 14.27 b ± 0.15 |
| Late apoptotic | 7.13 a ± 0.68 | 21.55 b ± 2.07 | 30.47 c ± 7.57 | 39.43 d ± 2.32 | 39.83 d ± 1.89 | 40.05 d ± 1.03 | 29.31 c ± 0.7 | 26.43 b,c ± 1.05 |
| Dead | 0.13 a ± 0.14 | 0.12 a ± 0.08 | 6.78 b ± 7.73 | 4.43 a,b ± 0.51 | 2.52 a,b ± 0.21 | 1.85 a,b ± 0.31 | 2.09 a,b ± 0.14 | 1.99 a,b ± 0.15 |
| Total apoptotic | 10.07 a ± 0.3 | 53.45 d,e ± 2.70 | 50.05 c,d ± 6.08 | 46.62 c ± 2.16 | 60.68 f ± 1.05 | 56.37 e ± 0.8 | 46.47 c ± 0.64 | 40.69 b ± 0.99 |
| BCL-2 Activation | ||||||||
| UC | STS | GSH5 | GSH25 | 2L5 | 2L25 | 4L25 | ECAPS | |
| Activated | 82.6 d ± 1.51 | 7.53 a ± 0.84 | 14.03 a,b ± 1.89 | 12.9 a,b ± 1.68 | 15.3 b ± 2.19 | 15.47 b ± 1.81 | 26.8 c ± 9.07 | 24.72 c ± 3.51 |
| Inactivated | 17.3 a ± 1.51 | 91.87 d ± 0.67 | 85.2 c,d ± 1.65 | 86.2 c,d ± 1.9 | 83.5 c ± 2.29 | 83.5 c ± 1.61 | 72.23 b ± 9.26 | 74.92 b ± 3.42 |
| Non-expressing | 0.1 a ± 0.0 | 0.53 a,b,c ± 0.21 | 0.67 b,c,d ± 0.38 | 0.83 c,d ±0.31 | 1.13 d ± 0.5 | 0.93 c,d ± 0.31 | 0.63 a,b,c,d ± 0.15 | 0.21 a,b ± 0.1 |
| Cell Cycle Phase Distribution | ||||||||
|---|---|---|---|---|---|---|---|---|
| UC | STS | GSH5 | GSH25 | 2L5 | 2L25 | 4L25 | ECAPS | |
| G0/G1 | 41.80 c ± 0.79 | 26.7 a ± 2.19 | 42.47 c ± 3.26 | 36.67 b ± 1.72 | 37.3 b ± 1.68 | 42.17 c ± 1.95 | 39.37 b,c ± 2.55 | 37.57 b ± 2.22 |
| S | 29.33 c ± 0.12 | 24.33 b ± 1.46 | 21.27 a ± 1.0 | 22.1 a,b ± 0.87 | 22.77 a,b ± 0.72 | 21.6 a ± 0.0 | 24.33 b ± 2.15 | 23.70 a,b ± 2.36 |
| G2/M | 15.73 a ± 0.86 | 29.03 b ± 0.32 | 30.6 b,c ± 1.76 | 34.33 e ± 1.38 | 32.8 d,e ± 0.95 | 30.43 b,c ± 1.43 | 30.67 b,c ± 0.93 | 31.9 c,d ± 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drozdowska, M.; Piasna-Słupecka, E.; Such, A.; Dziadek, K.; Krzyściak, P.; Kruk, T.; Duraczyńska, D.; Morawska-Tota, M.; Jamróz, E. Design and In Vitro Activity of Furcellaran/Chitosan Multilayer Microcapsules for the Delivery of Glutathione and Empty Model Multilayer Microcapsules Based on Polysaccharides. Materials 2024, 17, 2047. https://doi.org/10.3390/ma17092047
Drozdowska M, Piasna-Słupecka E, Such A, Dziadek K, Krzyściak P, Kruk T, Duraczyńska D, Morawska-Tota M, Jamróz E. Design and In Vitro Activity of Furcellaran/Chitosan Multilayer Microcapsules for the Delivery of Glutathione and Empty Model Multilayer Microcapsules Based on Polysaccharides. Materials. 2024; 17(9):2047. https://doi.org/10.3390/ma17092047
Chicago/Turabian StyleDrozdowska, Mariola, Ewelina Piasna-Słupecka, Aleksandra Such, Kinga Dziadek, Paweł Krzyściak, Tomasz Kruk, Dorota Duraczyńska, Małgorzata Morawska-Tota, and Ewelina Jamróz. 2024. "Design and In Vitro Activity of Furcellaran/Chitosan Multilayer Microcapsules for the Delivery of Glutathione and Empty Model Multilayer Microcapsules Based on Polysaccharides" Materials 17, no. 9: 2047. https://doi.org/10.3390/ma17092047
APA StyleDrozdowska, M., Piasna-Słupecka, E., Such, A., Dziadek, K., Krzyściak, P., Kruk, T., Duraczyńska, D., Morawska-Tota, M., & Jamróz, E. (2024). Design and In Vitro Activity of Furcellaran/Chitosan Multilayer Microcapsules for the Delivery of Glutathione and Empty Model Multilayer Microcapsules Based on Polysaccharides. Materials, 17(9), 2047. https://doi.org/10.3390/ma17092047









