Optimizing FeSiCr-Based Soft Magnetic Composites Using the Deionized Water as the Phosphating Solvent
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Effects of Solvent on the Microstructure and Magnetic Properties of Phosphated Powder and SMCs
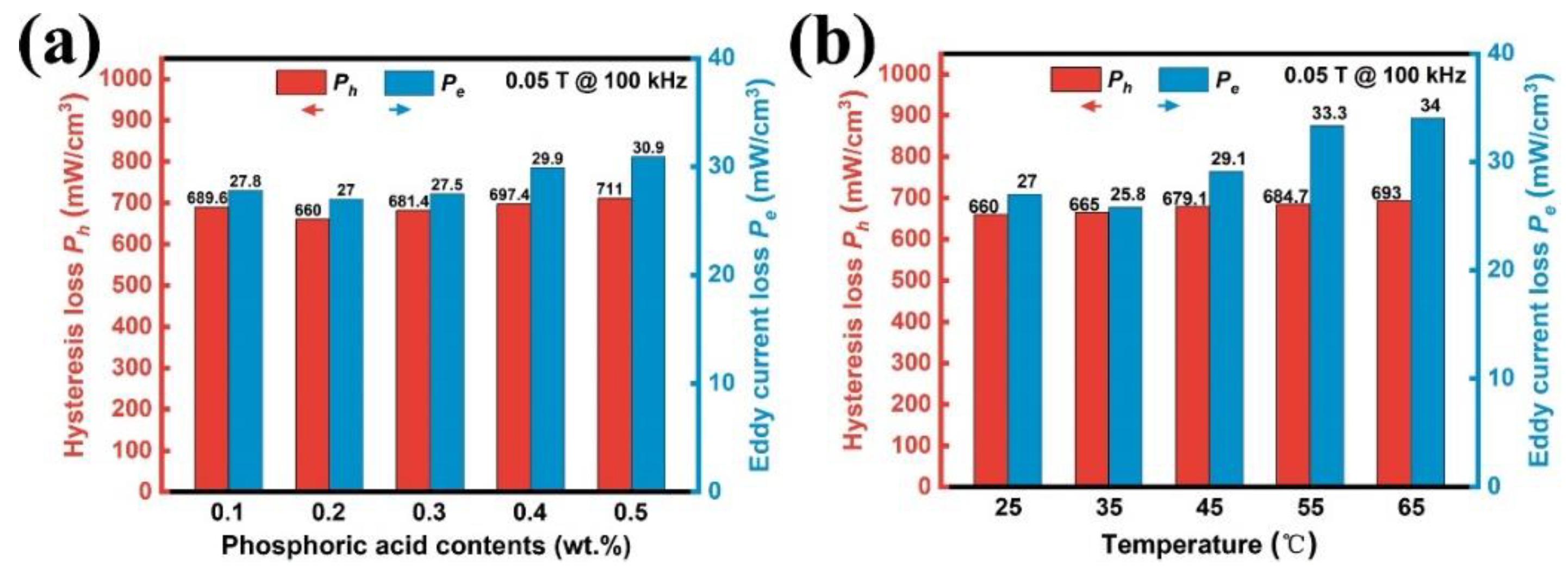
3.2. Process Optimization for SMCs Prepared in Deionized Water
3.3. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, J.Y.; Yuan, H.; Nie, M.; Guo, H.; Yu, H.Y.; Liu, Z.W.; Sun, R. Soft magnetic materials for power inductors: State of art and future development. Mater. Today Electron. 2023, 6, 100066. [Google Scholar] [CrossRef]
- Silveyra, J.M.; Ferrara, E.; Huber, D.L.; Monson, T.C. Soft magnetic materials for a sustainable and electrified world. Science 2018, 362, eaao0195. [Google Scholar] [CrossRef]
- Perigo, E.A.; Weidenfeller, B.; Kollar, P.; Fuzer, J. Past, present, and future of soft magnetic composites. Appl. Phys. Rev. 2018, 5, 031301. [Google Scholar] [CrossRef]
- Dosoudil, R.; Franek, J.; Sláma, J.; Usakova, M.; Gruskova, A. Electromagnetic wave absorption performances of metal alloy/spinel ferrite/polymer composites. IEEE Trans. Magn. 2012, 48, 1524–1527. [Google Scholar] [CrossRef]
- Sunday, K.J.; Taheri, M.L. Soft magnetic composites: Recent advancements in the technology. Metal Powder Rep. 2017, 72, 425–429. [Google Scholar] [CrossRef]
- Poskovic, E.; Franchini, F.; Ferraris, L.; Fracchia, E.; Bidulska, J.; Carosio, F.; Bidulsky, R.; Grande, M. Recent advances in multi-functional coatings for soft magnetic composites. Materials 2021, 14, 6844. [Google Scholar] [CrossRef]
- Taghvaei, A.H.; Shokrollahi, H.; Janghorban, K.; Abiri, H. Eddy current and total power loss separation in the iron–phosphate–polyepoxy soft magnetic composites. Mater. Des. 2009, 30, 3989–3995. [Google Scholar] [CrossRef]
- Ren, X.T.; Corcolle, R.; Daniel, L. Bounds and estimates on eddy current losses in soft magnetic composites. J. Appl. Phys. 2018, 123, 235109. [Google Scholar] [CrossRef]
- Vesa, J.; Hyvarinen, L.; Rasilo, P. Eddy-current loss model for soft magnetic composite materials considering particle size distribution. IEEE Trans. Magn. 2023, 59, 6300508. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, M.; Kim, J. Magnetic properties of pure iron soft magnetic composites coated by manganese phosphates. IEEE Trans. Magn. 2017, 53, 2003304. [Google Scholar] [CrossRef]
- Long, H.M.; Wu, X.J.; Lu, Y.K.; Zhang, H.F.; Hao, J.J. Effect of polyimide-phosphating double coating and annealing on the magnetic properties of Fe-Si-Cr SMCs. Materials 2022, 15, 3350. [Google Scholar] [CrossRef]
- Neamtu, B.V.; Nasui, M.; Cupa, G.; Ware, E.; Popa, F.; Marinca, T.F.; Chicinas, I. Effects of adding carbonyl Fe or Mn-Zn ferrite powders to fibre-based soft magnetic composites prepared via hybrid cold sintering/spark plasma sintering. J. Mater. Res. Technol. 2024, 28, 2969–2979. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.; Bon, C.Y.; Lee, K.H.; Choi, S.J.; Yoo, S.I. Novel fabrication method for a high-performance soft-magnetic composite composed of alumina-coated Fe-based metal powder. J. Electron. Mater. 2021, 50, 664–674. [Google Scholar] [CrossRef]
- Sunday, K.J.; Hanejko, F.G.; Taheri, M.L. Magnetic and microstructural properties of Fe3O4-coated Fe powder soft magnetic composites. J. Magn. Magn. Mater. 2017, 423, 164–170. [Google Scholar] [CrossRef]
- Bures, R.; Streckova, M.; Faberova, M.; Kollar, P.; Fuszer, J. Advances in powder metallurgy soft magnetic composite materials. Arch. Metall. Mater. 2021, 150, 109841. [Google Scholar]
- Lu, H.; Dong, Y.Q.; Liu, X.C.; Liu, Z.H.; Ma, Y.; Wu, Y.; He, A.N.; Li, J.W.; Wang, X.M. Enhanced magnetic properties of FeSiAl soft magnetic composites prepared by utilizing phosphate: PSA as insulating layer. J. Mater. Sci. Mater. Electron. 2022, 33, 10131–10141. [Google Scholar] [CrossRef]
- Li Ndjock, B.I.D.; Baenla, J.; Yanne, E.; Mbah, J.B.B.; Elimbi, A. Effects of Al and Fe powders on the formation of foamed cement obtained by phosphoric acid activation of volcanic ash. Mater. Lett. 2022, 308, 131147. [Google Scholar] [CrossRef]
- Saldanha, R.L.; Gomes, B.C.; Torres, G.D.; De Lima, B.R.; De Castro, J.A.; Da Silva, L.; Ferreira, E.A. Inhibition of the oxygen evolution reaction during titanium passivation in aqueous phosphoric acid solution. J. Solid State Electron. 2020, 24, 1991–1998. [Google Scholar] [CrossRef]
- Nguyen, M.P.; Yoshida, S.; Okamoto, S.; Miyazaki, T.; Endo, Y. Fe-based amorphous powder cores with low core loss and improvement of permeability. AIP Adv. 2024, 14, 025113. [Google Scholar] [CrossRef]
- Hsiang, H.I.; Fan, L.F.; Ho, K.T. Minor yttrium nitrate addition effect on FeSiCr alloy powder core electromagnetic properties. J. Magn. Magn. Mater. 2017, 444, 1–6. [Google Scholar] [CrossRef]
- Yu, H.C.; Zhou, S.X.; Zhang, G.Q.; Dong, B.S.; Meng, L.B.; Li, Z.Z.; Dong, Y.Q.; Cao, X. The phosphating effect on the properties of FeSiCr alloy powder. J. Magn. Magn. Mater. 2022, 552, 168741. [Google Scholar] [CrossRef]
- Hsiang, H.I.; Fan, L.F.; Hung, J.J. Phosphoric acid addition effect on the microstructure and magnetic properties of iron-based soft magnetic composites. J. Magn. Magn. Mater. 2018, 447, 1–8. [Google Scholar] [CrossRef]
- Sarker, P.C.; Islam, M.R.; Guo, Y.G.; Zhu, J.G.; Lu, H.Y. State-of-the-art technologies for development of high frequency transformers with advanced magnetic materials. IEEE Trans. Appl. Supercond. 2019, 29, 7000111. [Google Scholar] [CrossRef]
- Li, J.M.; Yu, H.Y.; Luo, P.; Yuan, H.; Liu, Z.W.; Wang, Y.; Yang, L.; Wu, W.J. Effects of phosphating treatment on the growth of a phosphate layer and the magnetic properties of Fe-based amorphous magnetic powder cores. J. Electron. Mater. 2023, 52, 5412–5421. [Google Scholar] [CrossRef]
- Li, K.L.; Cheng, D.N.; Yu, H.Y.; Liu, Z.W. Process optimization and magnetic properties of soft magnetic composite cores based on phosphated and mixed resin coated Fe powders. J. Magn. Magn. Mater. 2020, 501, 166455. [Google Scholar] [CrossRef]
- Luo, P.; Yu, H.Y.; Wang, C.; Yuan, H.; Liu, Z.W.; Wang, Y.; Yang, L.; Wu, W.J. Properties optimization of soft magnetic composites based on the amorphous powders with double layer inorganic coating by phosphating and sodium silicate treatment. Metals 2023, 13, 560. [Google Scholar] [CrossRef]
- Kakde, A.S.; Belekar, R.M.; Wakde, G.C.; Borikar, M.A.; Rewatkar, K.G.; Shingade, B.A. Evidence of magnetic dilution due to unusual occupancy of zinc on B-site in NiFe2O4 spinel nano-ferrite. J. Solid State Chem. 2021, 300, 122279. [Google Scholar] [CrossRef]
- Choi, K.D.; Lee, S.Y.; Hwang, J.S.; Yang, S.; Huh, J.Y.; Yi, K.W.; Byun, J.Y. Magnetic properties of Fe soft magnetic composites with a double-insulating layer comprising Fe3O4 and silicone resin. J. Alloys Compd. 2023, 936, 168255. [Google Scholar] [CrossRef]
- Goodall, A.D.; Chechik, L.; Mitchell, R.L.; Jewell, G.W.; Todd, I. Cracking of soft magnetic FeSi to reduce eddy current losses in stator cores. Addit. Manuf. 2023, 70, 103555. [Google Scholar] [CrossRef]
- Wang, P.; Zhu, Z.Q.; Liu, J.Q.; Wang, C.F.; Pang, J.; Zhang, J.Q. Industrial-scale fabrication of FeSiCr magnetic powder cores with high magnetic permeability and low loss. J. Alloys Compd. 2023, 962, 171095. [Google Scholar] [CrossRef]
- Xia, C.; Peng, Y.D.; Yi, Y.; Deng, H.; Zhu, Y.Y.; Hu, G. The magnetic properties and microstructure of phosphated amorphous FeSiCr/silane soft magnetic composite. J. Magn. Magn. Mater. 2019, 3, 424–433. [Google Scholar] [CrossRef]
- Miskovic, D.M.; Pohl, K.; Birbilis, N.; Laws, K.J.; Ferry, M. Formation of a phosphate conversion coating on bioresorbable Mg-based metallic glasses and its effect on corrosion performance. Corros. Sci. 2017, 129, 214–225. [Google Scholar] [CrossRef]
- Bircakova, Z.; Kollar, P.; Fuzer, J.; Bures, R.; Faberova, M.; Vojtek, V. Wide induction range analysis of DC magnetic properties and magnetization processes of Fe-based soft magnetic composites. J. Phys. D Appl. Phys. 2023, 56, 425003. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Weir, G.; Leveneur, J.; Long, N.C. Magnetic susceptibility of soft magnetic composite materials. J. Magn. Magn. Mater. 2022, 551, 169103. [Google Scholar] [CrossRef]
- Oikonomou, C.; Hryha, E.; Nyborg, L. An XPS investigation on the thermal stability of the insulating surface layer of soft magnetic composite powder. Surf. Interface Anal. 2016, 48, 445–450. [Google Scholar] [CrossRef]
- Ouda, K.; Danninger, H.; Gierl-Mayer, C.; Hellein, R.; Mueller, A. Ferrothermal reduction of iron (III) phosphate insulating layers in soft magnetic composites. Powder Metall. 2021, 64, 351–359. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Park, J.Y.; Park, K.; Fadonougbo, J.O.; Jang, K.R.; Park, S.D.; Park, C.S.; Mo, C.B.; Hwang, N.M.; Park, H.K. Enhancement of the magnetic properties of Fe-Si-Cr soft magnetic composite by selective oxidation annealing. J. Magn. Magn. Mater. 2022, 561, 169697. [Google Scholar] [CrossRef]
- Chen, Z.H.; Liu, X.S.; Kan, X.C.; Wang, Z.; Zhu, R.W.; Yang, W.; Wu, Q.Y.; Shezad, M. Phosphate coatings evolution study and effects of ultrasonic on soft magnetic properties of FeSiAl by aqueous phosphoric acid solution passivation. J. Alloys Compd. 2019, 783, 434–440. [Google Scholar] [CrossRef]
- Yuan, H.; Hu, J.W.; Yu, H.Y.; Luo, P.; Li, J.Z.; Liu, Z.W. Temperature resistance of soft magnetic composites based on carbonyl iron powder for molding inductor. Mater. Chem. Phys. 2024, 314, 128808. [Google Scholar] [CrossRef]
- Li, W.C.; Cai, H.W.; Kang, Y.; Ying, Y.; Yu, J.; Zheng, J.W.; Qiao, L.; Jiang, Y.; Che, S.L. High permeability and low loss bioinspired soft magnetic composites with nacre-like structure for high frequency applications. Acta Mater. 2019, 167, 267–274. [Google Scholar] [CrossRef]
- Shokrollahi, H.; Janghorban, K. Soft magnetic composite materials (SMCs). J. Mater. Process. Technol. 2007, 189, 1–12. [Google Scholar] [CrossRef]
- Wang, L.L.; Qiao, L.; Zheng, J.W.; Cai, W.; Ying, Y.; Li, W.C.; Che, S.L.; Yu, J. Microstructure and properties of FeSiCr/PA6 composites by injection molding using FeSiCr powders by phosphating and coupling treatment. J. Magn. Magn. Mater. 2018, 452, 210–218. [Google Scholar] [CrossRef]
- Wu, X.J.; Chen, C.G.; Hao, J.J.; Zhao, T.C.; Ma, H.; Lu, Y.K.; Ren, Z.K. Effect of phosphating and heat treatment on magnetic properties of Fe-3.3Si-6.5Cr soft magnetic composites. J. Supercond. Nov. Magn. 2020, 33, 1889–1897. [Google Scholar] [CrossRef]
- ASTM B895-16; Evaluating the Corrosion Resistance of Stainless Steel Powder Metallurgy (PM) Parts/Specimens by Immersion in a Sodium Chloride Solution. American Society for Testing and Materials: West Conshohocken, PA, USA, 2016.
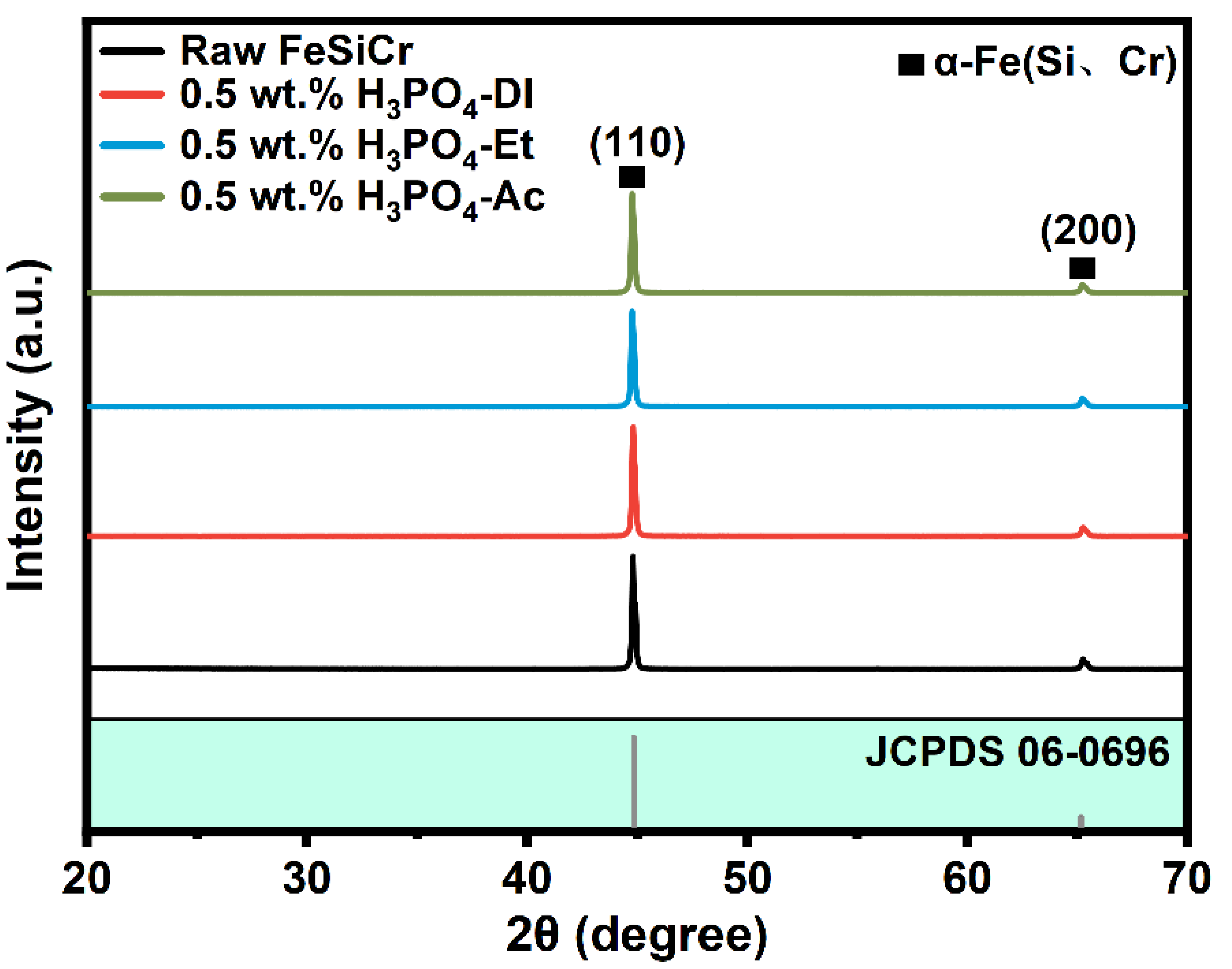

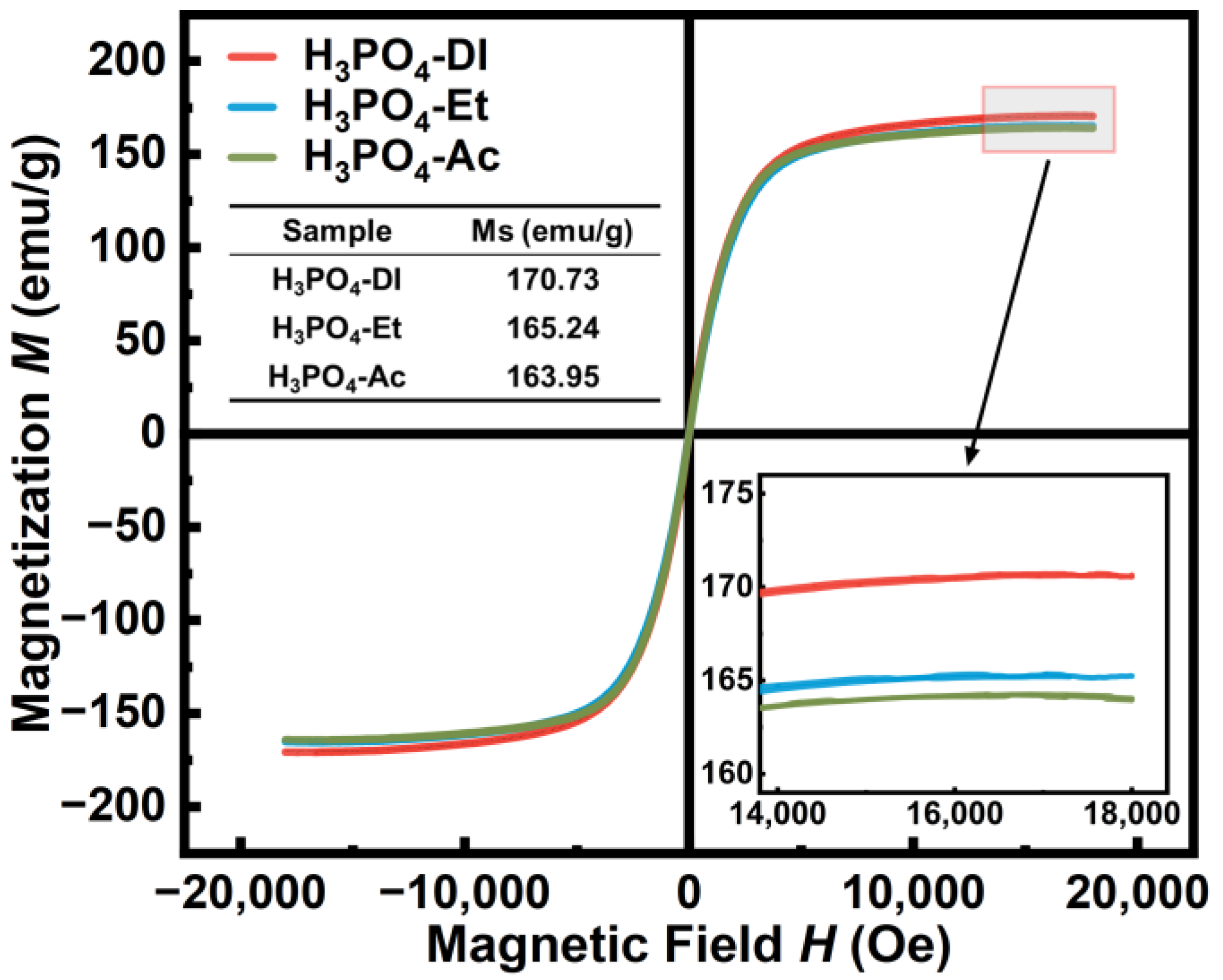
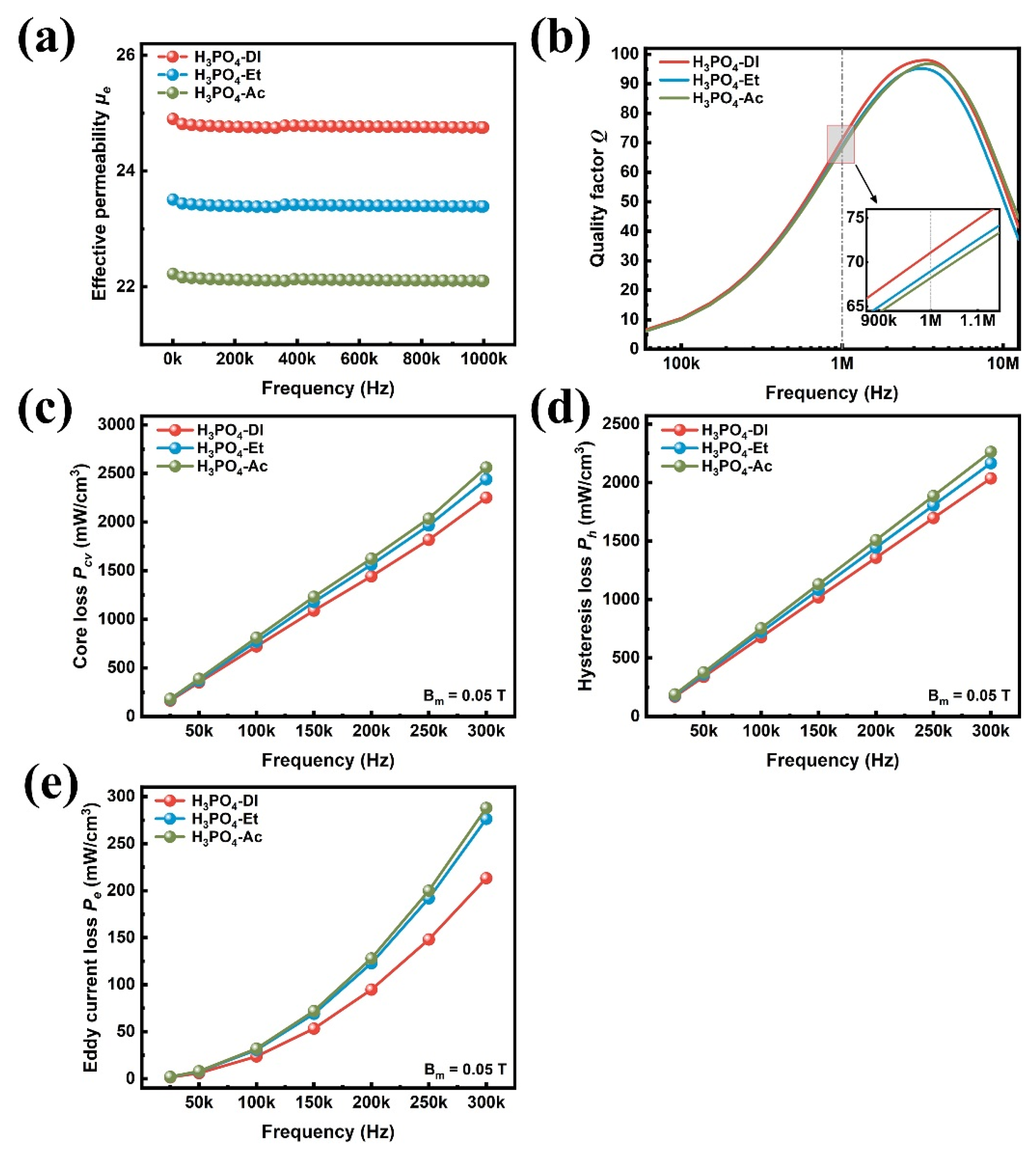
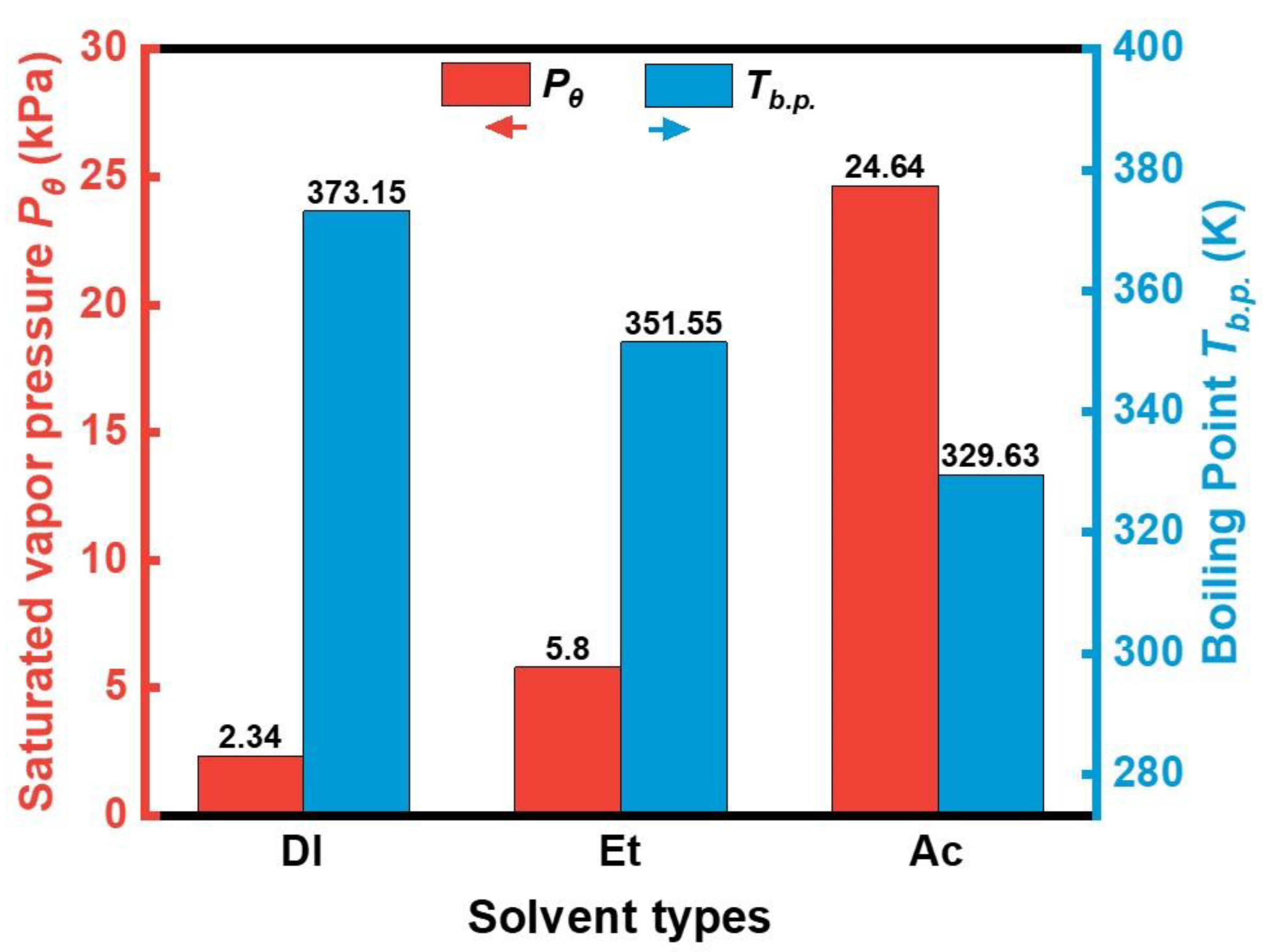
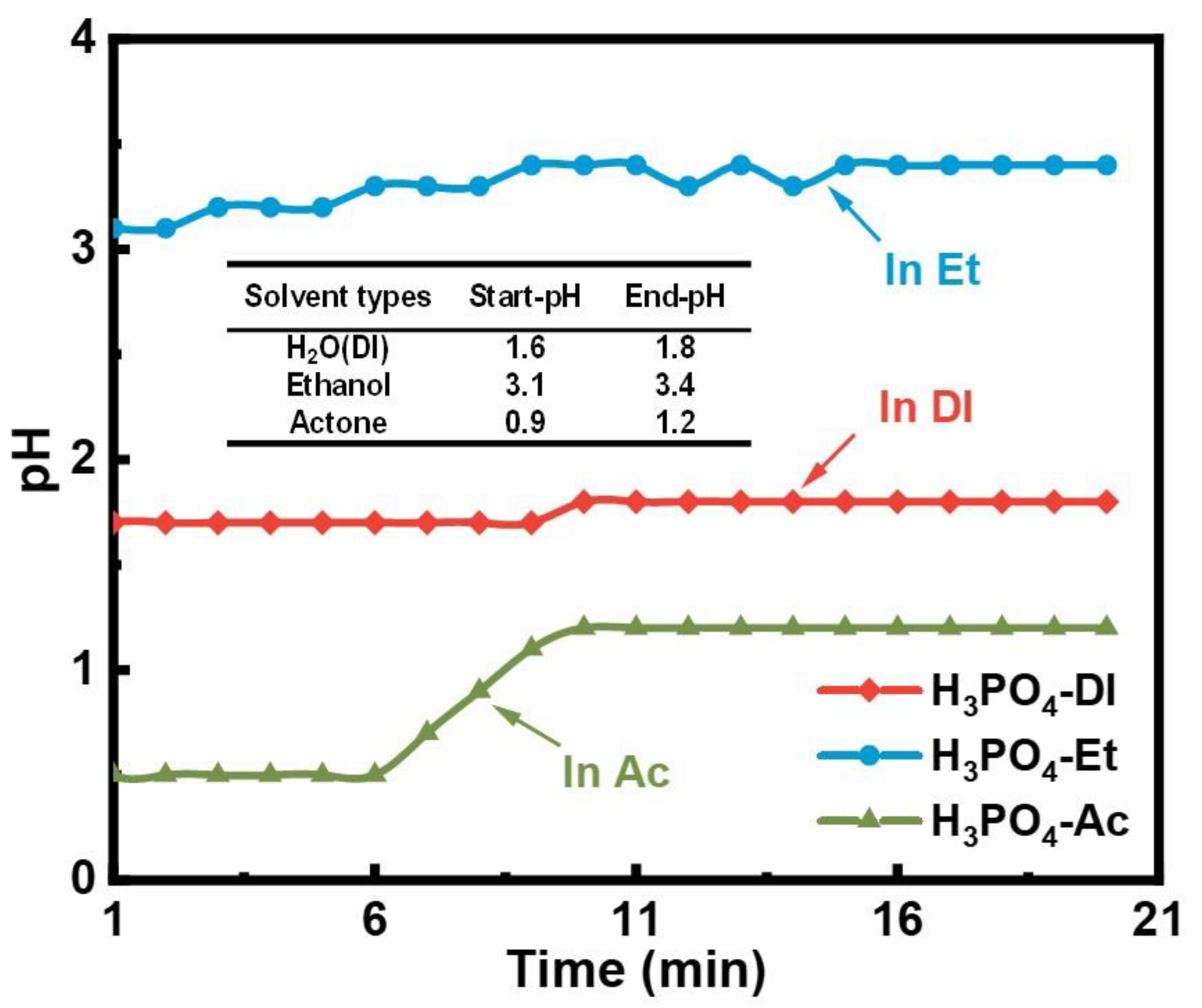
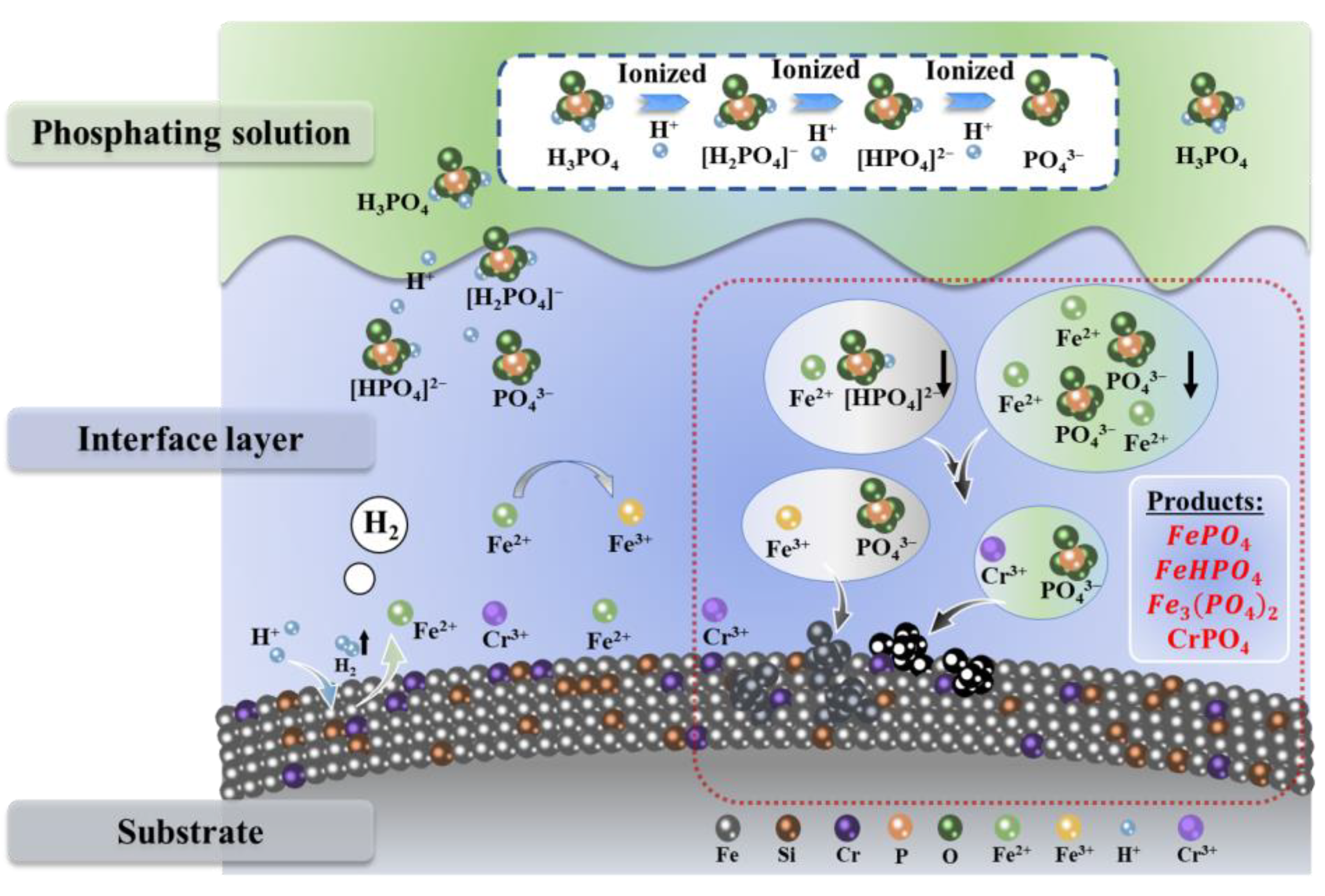
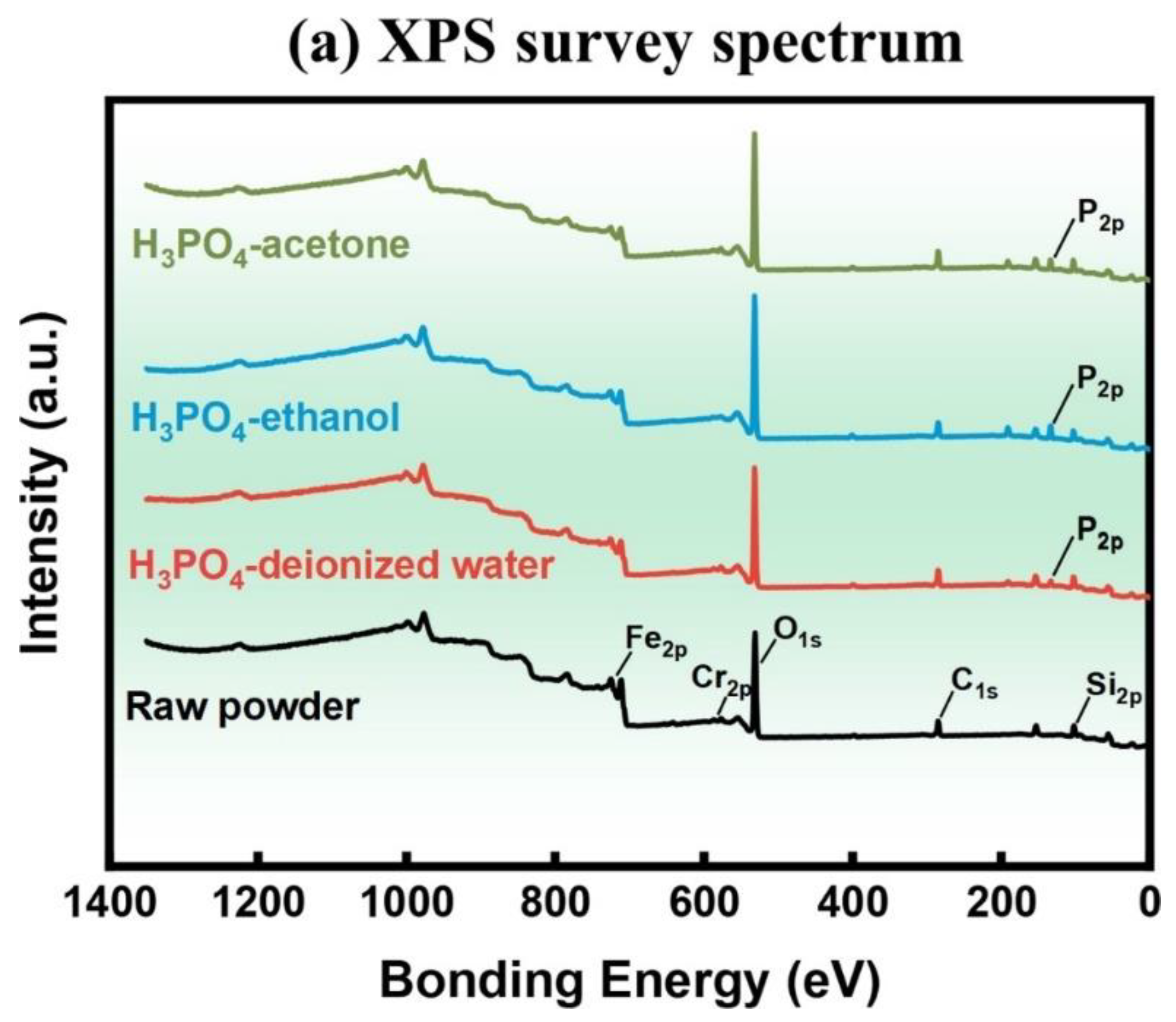
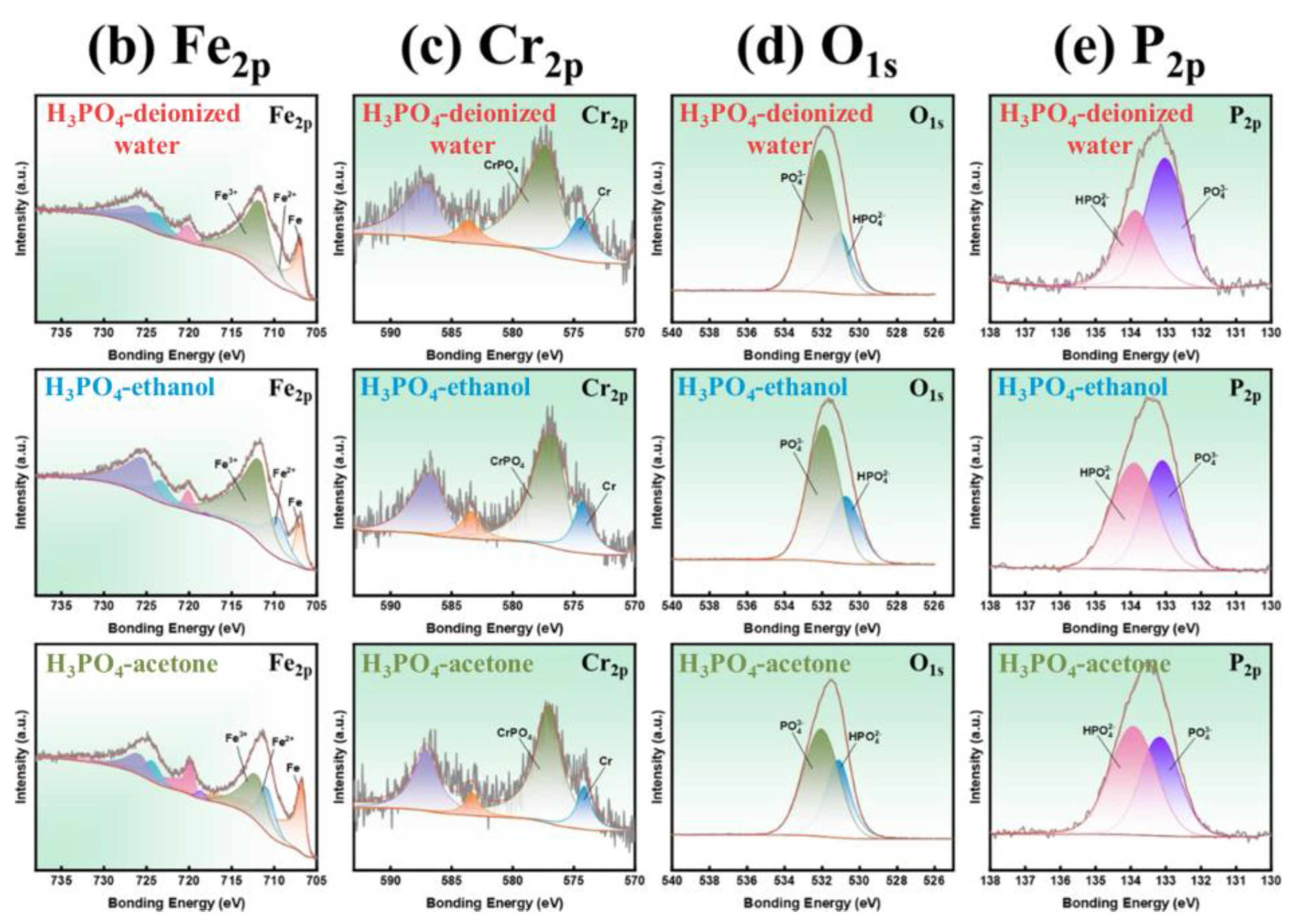
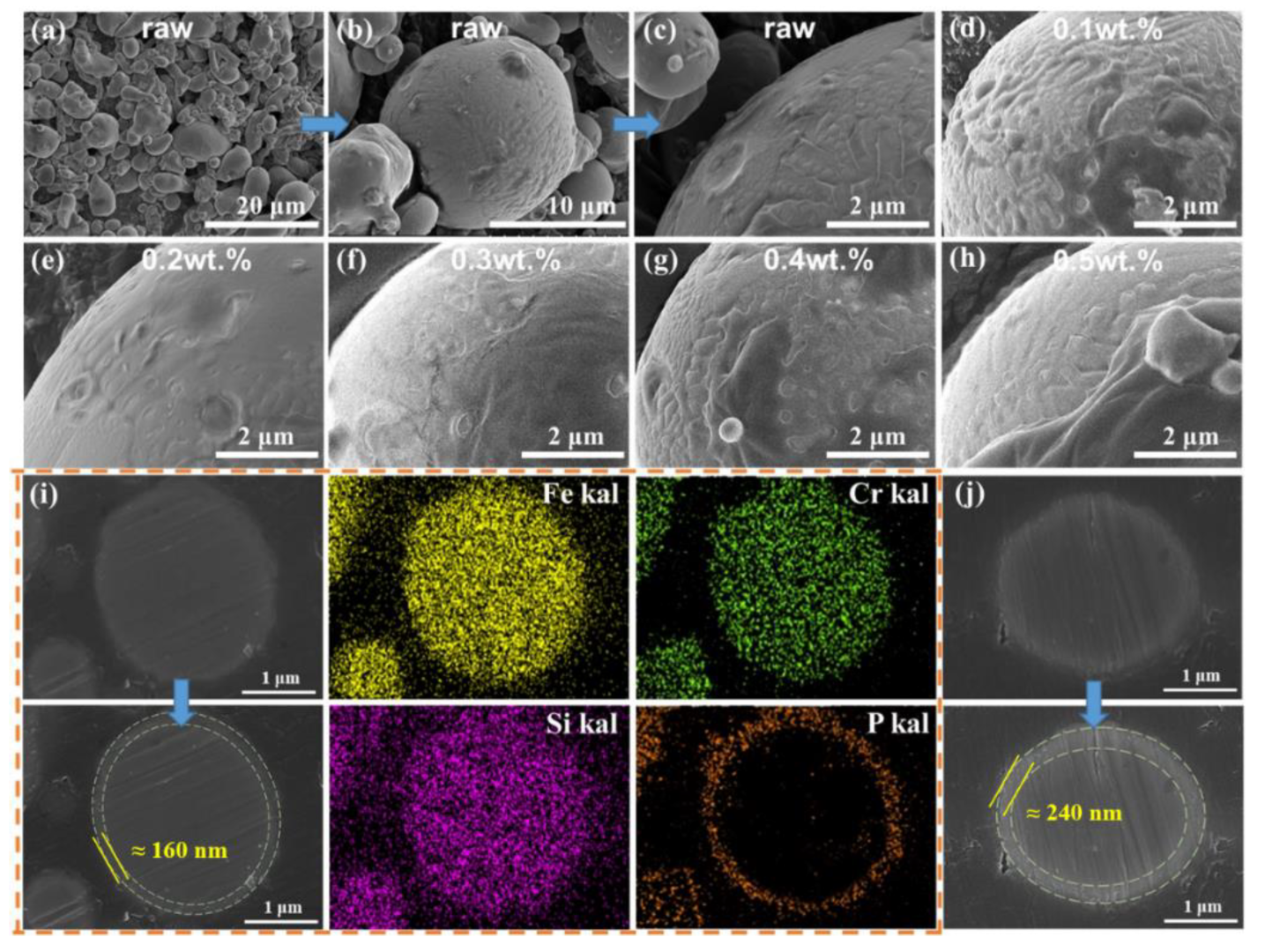
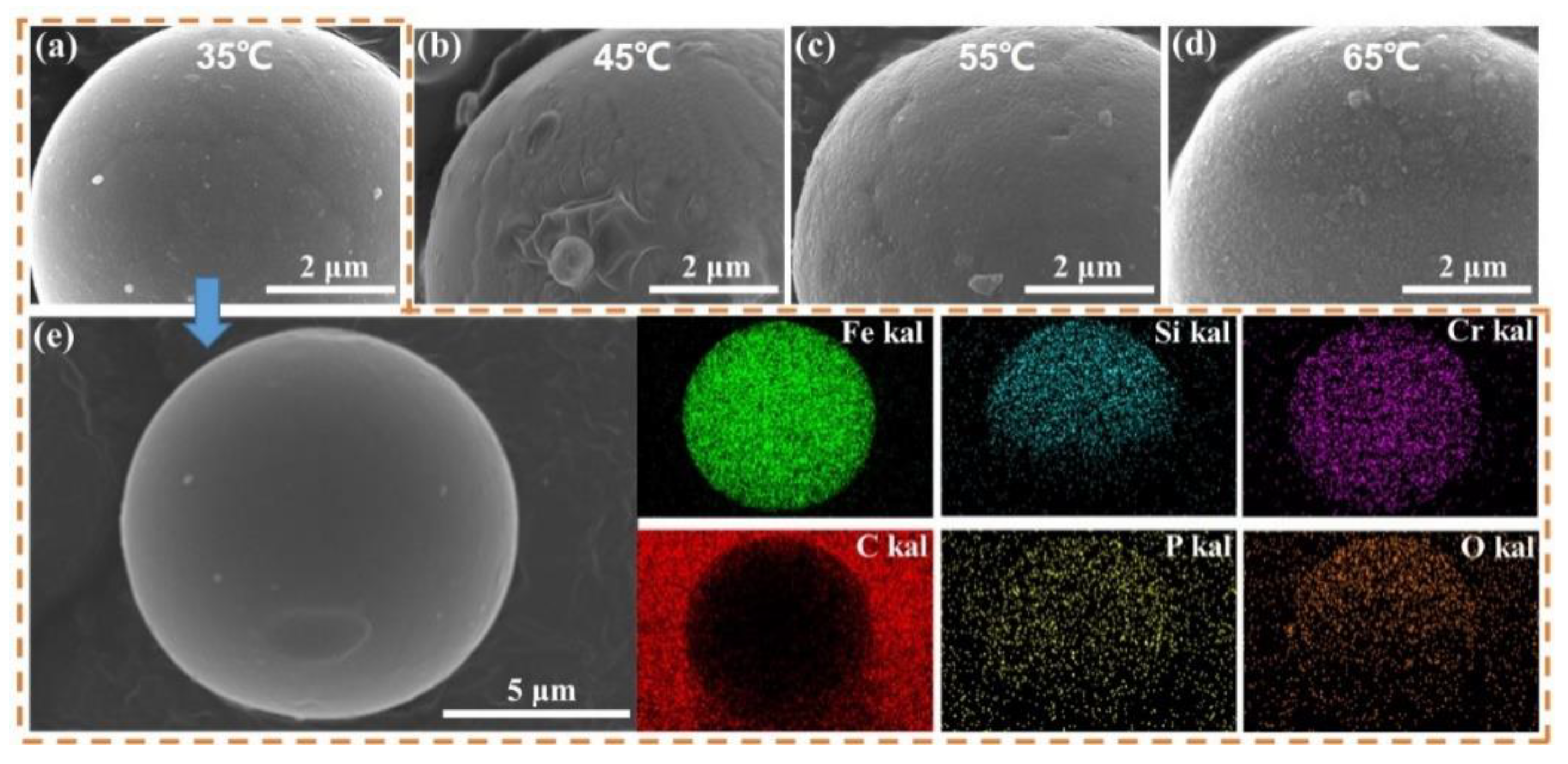
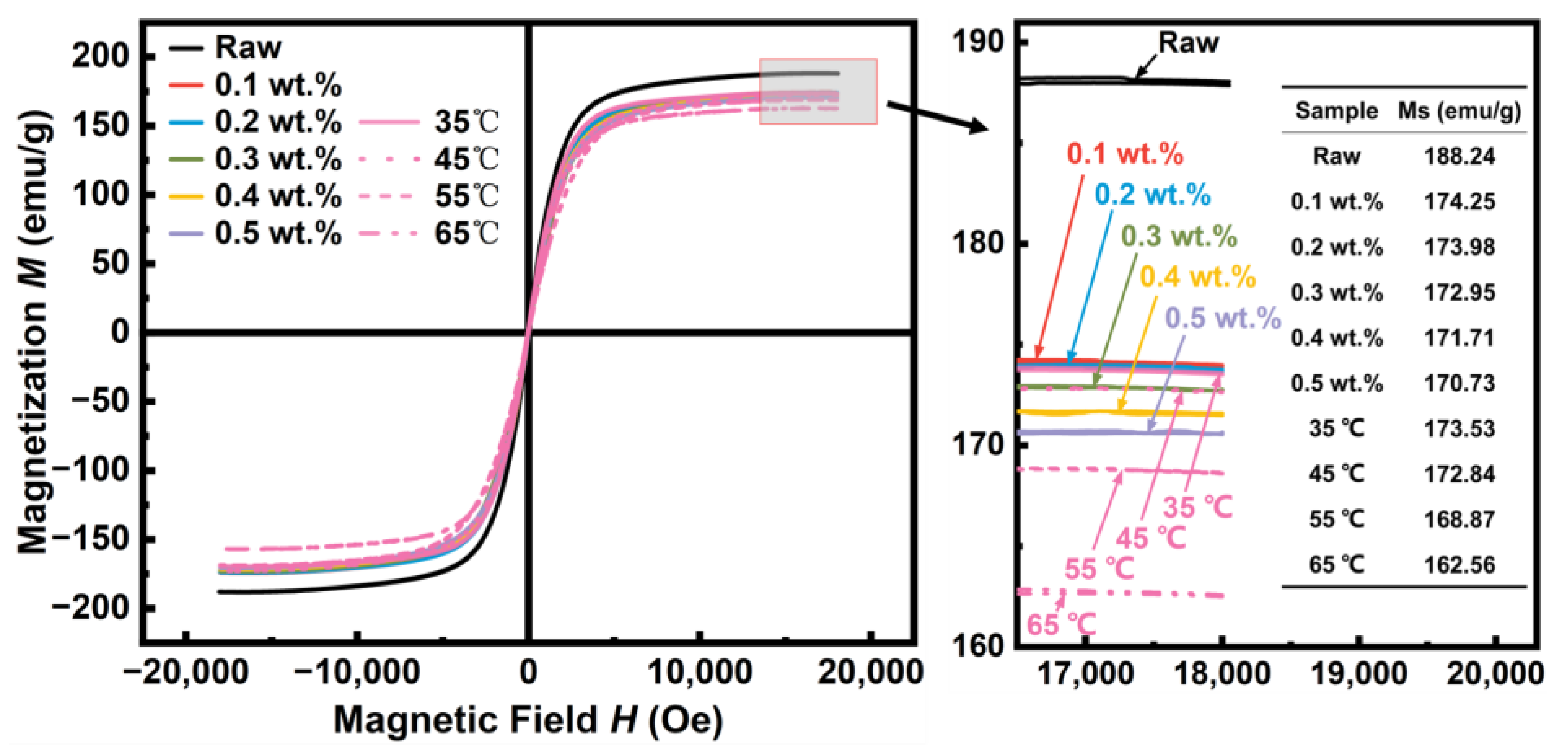
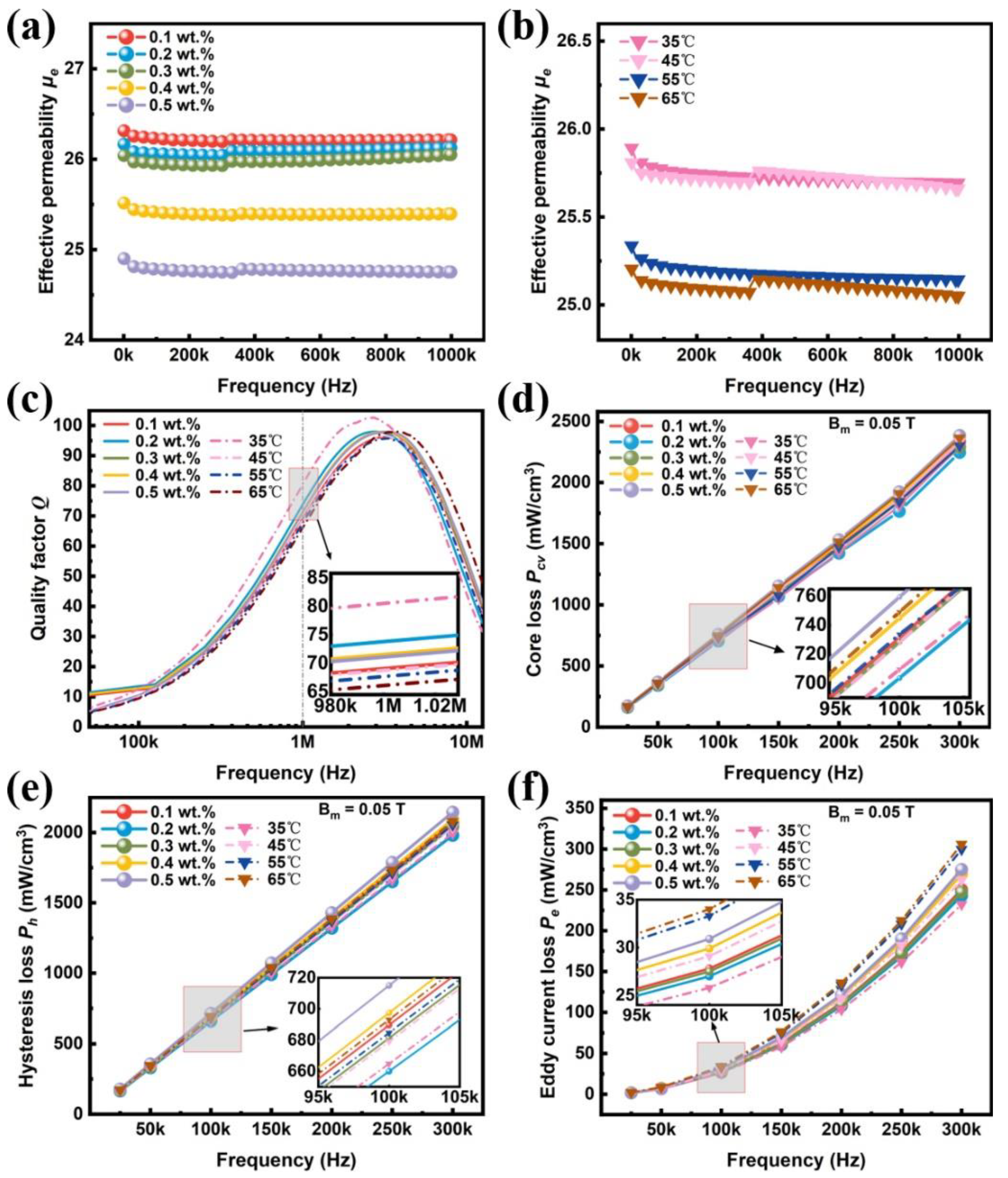
| Sample | Density | µe (1 MHz) | Q (1 MHz) | Pcv/(mW·cm−3) @ 0.05 T | Withstanding Voltage (V) | |
|---|---|---|---|---|---|---|
| (g/cm3) | 100 kHz | 200 kHz | ||||
| 0.5 wt.% H3PO4-DI | 5.47 | 24.7 | 71.6 | 759.4 | 1533.0 | 241 |
| 0.5 wt.% H3PO4-Et | 5.40 | 23.4 | 69.5 | 771.0 | 1561.0 | 225 |
| 0.5 wt.% H3PO4-Ac | 5.34 | 22.1 | 69.1 | 811.9 | 1624.0 | 217 |
| Sample | µe (1 MHz) | Q (1 MHz) | Pcv/(mW·cm−3) @ 0.05 T | Withstanding Voltage (V) | |
|---|---|---|---|---|---|
| 100 kHz | 200 kHz | ||||
| 0.1 wt.% H3PO4-DI | 26.2 | 69.4 | 731.0 | 1476.0 | 259 |
| 0.2 wt.% H3PO4-DI | 26.1 | 74.7 | 703.5 | 1423.0 | 269 |
| 0.3 wt.% H3PO4-DI | 26.0 | 72.0 | 728.4 | 1461.0 | 250 |
| 0.4 wt.% H3PO4-DI | 25.4 | 72.5 | 744.7 | 1503.0 | 246 |
| 0.5 wt.% H3PO4-DI | 24.7 | 71.6 | 759.4 | 1533.0 | 241 |
| 35 °C @ H3PO4-DI | 25.7 | 80.2 | 709.5 | 1431.0 | 276 |
| 45 °C @ H3PO4-DI | 25.6 | 68.7 | 728.1 | 1463.0 | 281 |
| 55 °C @ H3PO4-DI | 25.1 | 67.2 | 733.6 | 1471.0 | 287 |
| 65 °C @ H3PO4-DI | 25.0 | 65.5 | 749.0 | 1515.0 | 291 |
| References | Powder | Phosphating Solvent | Molding Pressure (MPa) | µe | Pcv/(mW·cm−3) a | |
|---|---|---|---|---|---|---|
| 100 kHz | 200 kHz | |||||
| [22] | CIP | Acetone | 580 | 14.0 | -- | -- |
| [23] | FeSiCr | Ethanol | 600 | 20.6 | -- | 2087 |
| [44] | FeSiCr | Ethanol | 265 | 16.3 | 1500 | 3550 |
| [45] | FeSiCr | Acetone | 600 | 44.5 | 780 | -- |
| This work | FeSiCr | Deionized water | 600 | 25.7 | 709.5 | 1431.0 |
| Phosphated in different solvents (0.5 wt.% H3PO4 concentration; 25 °C) | Solvents | Raw | Acetone | Ethanol | Water | / |
| Photograph after corrosion resistance test |  |  |  |  | / | |
| Corrosion area ratio (%) | >96% | <1.0% | <0.5% | <0.3% | / | |
| Phosphated with different H3PO4 concentrations (in deionized water; 25 °C) | H3PO4 concentrations | 0.1 wt.% | 0.2 wt.% | 0.3 wt.% | 0.4 wt.% | 0.5 wt.% |
| Photograph after corrosion resistance test |  |  |  |  |  | |
| Corrosion area ratio (%) | <1.0% | <0.3% | <0.5% | <0.5% | <0.1% | |
| Phosphated at different temperatures (in deionized water; 0.2 wt.% H3PO4 concentration) | Temperatures | 25 °C | 35 °C | 45 °C | 55 °C | 65 °C |
| Photograph after corrosion resistance test |  |  |  |  |  | |
| Corrosion area ratio (%) | <0.3% | <0.1% | <1.0% | <5.0% | <10.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Yu, H.; Wang, H.; Yuan, T.; Liu, Z. Optimizing FeSiCr-Based Soft Magnetic Composites Using the Deionized Water as the Phosphating Solvent. Materials 2024, 17, 1631. https://doi.org/10.3390/ma17071631
Li X, Yu H, Wang H, Yuan T, Liu Z. Optimizing FeSiCr-Based Soft Magnetic Composites Using the Deionized Water as the Phosphating Solvent. Materials. 2024; 17(7):1631. https://doi.org/10.3390/ma17071631
Chicago/Turabian StyleLi, Xiangdong, Hongya Yu, Hongxiang Wang, Tongxin Yuan, and Zhongwu Liu. 2024. "Optimizing FeSiCr-Based Soft Magnetic Composites Using the Deionized Water as the Phosphating Solvent" Materials 17, no. 7: 1631. https://doi.org/10.3390/ma17071631
APA StyleLi, X., Yu, H., Wang, H., Yuan, T., & Liu, Z. (2024). Optimizing FeSiCr-Based Soft Magnetic Composites Using the Deionized Water as the Phosphating Solvent. Materials, 17(7), 1631. https://doi.org/10.3390/ma17071631









