The Influence of Ferrule Design and Pulpal Extensions on the Accuracy of Fit and the Fracture Resistance of Zirconia-Reinforced Lithium Silicate Endocrowns
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens Preparation
Teeth Selection, Preparation, and Grouping
2.2. Marginal and Internal Adaptation Assessment
2.3. Fracture Resistance Test
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hyeon Kim, D.D.; Tawil, D.P.; Jean-Pierre Albouy, D.; Duqum, D.I. Retrospective Assessment of Endodontically Teeth Replaced by Dental Implants. J. Endod. 2024, 50, 310–315. [Google Scholar] [CrossRef]
- Williams, J.V.; Williams, L.R. Is coronal restoration more important than root filling for ultimate endodontic success? Dent. Update 2010, 37, 187–193. [Google Scholar] [CrossRef]
- Zhu, Z.; Dong, X.Y.; He, S.; Pan, X.; Tang, L. Effect of Post Placement on the Restoration of Endodontically Treated Teeth: A Systematic Review. Int. J. Prosthodont. 2015, 28, 475–483. [Google Scholar] [CrossRef]
- Kato, T.; Fujiwara, N.; Kuraji, R.; Numabe, Y. Relationship between periodontal parameters and non-vital pulp in dental clinic patients: A cross-sectional study. BMC Oral Health 2020, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Setzer, F.C.; Boyer, K.R.; Jeppson, J.R.; Karabucak, B.; Kim, S. Long-Term Prognosis of Endodontically Treated Teeth: A Retrospective Analysis of Preoperative Factors in Molars. J. Endod. 2011, 37, 21–25. [Google Scholar] [CrossRef]
- Awawdeh, L.; Hemaidat, K.; Al-Omari, W. Higher Maximal Occlusal Bite Force in Endodontically Treated Teeth versus Vital Contralateral Counterparts. J. Endod. 2017, 43, 871–875. [Google Scholar] [CrossRef]
- Hajaj, T.; Negrutiu, M.L.; Rominu, M.; Barbuzan, A.; Sinescu, C. 82—Evaluation of Different Coronal Sealing Materials on Endodontically Treated Teeth. Dent. Mater. 2022, 38, e51. [Google Scholar] [CrossRef]
- Atlas, A.; Grandini, S.; Martignoni, M. Evidence-based treatment planning for the restoration of endodontically treated single teeth: Importance of coronal seal, post vs no post, and indirect vs direct restoration. Quintessence Int. 2019, 50, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Mangold, J.T.; Kern, M. Influence of glass-fiber posts on the fracture resistance and failure pattern of endodontically treated premolars with varying substance loss: An in vitro study. J. Prosthet. Dent. 2011, 105, 387–393. [Google Scholar] [CrossRef]
- Sarkis-Onofre, R.; Fergusson, D.; Cenci, M.S.; Moher, D.; Pereira-Cenci, T. Performance of Post-retained Single Crowns: A Systematic Review of Related Risk Factors. J. Endod. 2017, 43, 175–183. [Google Scholar] [CrossRef]
- Kimble, P.; Stuhr, S.; McDonald, N.; Venugopalan, A.; Campos, M.S.; Cavalcanti, B. Decision Making in the Restoration of Endodontically Treated Teeth: Effect of Biomimetic Dentistry Training. Dent. J. 2023, 11, 159. [Google Scholar] [CrossRef]
- Sedrez-Porto, J.A.; Rosa, W.L.; da Silva, A.F.; Münchow, E.A.; Pereira-Cenci, T. Endocrown restorations: A systematic review and meta-analysis. J. Dent. 2016, 52, 8–14. [Google Scholar] [CrossRef]
- Pissis, P. Fabrication of a metal-free ceramic restoration utilizing the monobloc technique. Pract. Periodontics Aesthet. Dent. 1995, 7, 83–94. [Google Scholar]
- Bindl, A.; Mörmann, W.H. Clinical evaluation of adhesively placed Cerec endo-crowns after 2 years—Preliminary results. J. Adhes. Dent. 1999, 1, 255–265. [Google Scholar]
- Bindl, A.; Richter, B.; Mörmann, W.H. Survival of ceramic computer-aided design/manufacturing crowns bonded to preparations with reduced macroretention geometry. Int. J. Prosthodont. 2005, 18, 219–224. [Google Scholar] [CrossRef]
- Zou, Y.; Zhan, D.; Xiang, J.; Li, L. Clinical research on restorations using CAD/CAM-fabricated monolithic zirconia endocrowns and post and core crowns after up to 5 years. Int. J. Comput. Dent. 2022, 25, 287–294. [Google Scholar] [CrossRef]
- Ahmed, M.A.A.; Kern, M.; Mourshed, B.; Wille, S.; Chaar, M.S. Fracture resistance of maxillary premolars restored with different endocrown designs and materials after artificial ageing. J. Prosthodont. Res. 2022, 66, 141–150. [Google Scholar] [CrossRef]
- Einhorn, M.; DuVall, N.; Wajdowicz, M.; Brewster, J.; Roberts, H. Preparation Ferrule Design Effect on Endocrown Failure Resistance. J. Prosthodont. 2019, 28, e237–e242. [Google Scholar] [CrossRef]
- Alqutaibi, A.Y.; Ghulam, O.; Krsoum, M.; Binmahmoud, S.; Taher, H.; Elmalky, W.; Zafar, M.S. Revolution of Current Dental Zirconia: A Comprehensive Review. Molecules 2022, 27, 1699. [Google Scholar] [CrossRef]
- Kanat-Ertürk, B.; Saridağ, S.; Köseler, E.; Helvacioğlu-Yiğit, D.; Avcu, E.; Yildiran-Avcu, Y. Fracture strengths of endocrown restorations fabricated with different preparation depths and CAD/CAM materials. Dent. Mater. J. 2018, 37, 256–265. [Google Scholar] [CrossRef]
- da Cunha, L.F.; Gonzaga, C.C.; Pissaia, J.F.; Correr, G.M. Lithium silicate endocrown fabricated with a CAD-CAM system: A functional and esthetic protocol. J. Prosthet. Dent. 2017, 118, 131–134. [Google Scholar] [CrossRef]
- Mavriqi, L.; Valente, F.; Murmura, G.; Sinjari, B.; Macrì, M.; Trubiani, O.; Caputi, S.; Traini, T. Lithium disilicate and zirconia reinforced lithium silicate glass-ceramics for CAD/CAM dental restorations: Biocompatibility, mechanical and microstructural properties after crystallization. J. Dent. 2022, 119, 104054. [Google Scholar] [CrossRef] [PubMed]
- El Ghoul, W.; Özcan, M.; Silwadi, M.; Salameh, Z. Fracture resistance and failure modes of endocrowns manufactured with different CAD/CAM materials under axial and lateral loading. J. Esthet. Restor. Dent. 2019, 31, 378–387. [Google Scholar] [CrossRef]
- Jalalian, E.; Zarbakhsh, A.; Khorshidi, S.; Golalipour, S.; Mohammadnasl, S.; Sayyari, M. Comparative analysis of endocrown fracture resistance and marginal adaptation: CAD/CAM technology using lithium disilicate vs. zirconia-reinforced lithium silicate ceramics. Saudi Dent. J. 2024, 36, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Alshali, S.; Attar, E. Fracture Strength of Endocrowns Fabricated from Three Different Computer-Aided Design/Computer-Aided Manufacturing Ceramic Materials: An In-Vitro Study. Cureus 2023, 15, e41531. [Google Scholar] [CrossRef] [PubMed]
- Ghajghouj, O.; Taşar-Faruk, S. Evaluation of Fracture Resistance and Microleakage of Endocrowns with Different Intracoronal Depths and Restorative Materials Luted with Various Resin Cements. Materials 2019, 12, 2528. [Google Scholar] [CrossRef] [PubMed]
- Krishan, R.; Paqué, F.; Ossareh, A.; Kishen, A.; Dao, T.; Friedman, S. Impacts of conservative endodontic cavity on root canal instrumentation efficacy and resistance to fracture assessed in incisors, premolars, and molars. J. Endod. 2014, 40, 1160–1166. [Google Scholar] [CrossRef]
- Teixeira, E.S.; Rizzante, F.A.; Ishikiriama, S.K.; Mondelli, J.; Furuse, A.Y.; Mondelli, R.F.; Bombonatti, J.F. Fracture strength of the remaining dental structure after different cavity preparation designs. Gen. Dent. 2016, 64, 33–36. [Google Scholar]
- Mostafavi, A.S.; Allahyari, S.; Niakan, S.; Atri, F. Effect of Preparation Design on Marginal Integrity and Fracture Resistance of Endocrowns: A Systematic Review. Front. Dent. 2022, 19, 37. [Google Scholar] [CrossRef]
- Vianna, A.; Prado, C.J.D.; Bicalho, A.A.; Pereira, R.; Neves, F.D.D.; Soares, C.J. Effect of cavity preparation design and ceramic type on the stress distribution, strain and fracture resistance of CAD/CAM onlays in molars. J. Appl. Oral Sci. 2018, 26, e20180004. [Google Scholar] [CrossRef]
- Gurpinar, B.; Tak, O. Effect of pulp chamber depth on the accuracy of endocrown scans made with different intraoral scanners versus an industrial scanner: An in vitro study. J. Prosthet. Dent. 2022, 127, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Topkara, C.; Keleş, A. Examining the adaptation of modified endocrowns prepared with CAD-CAM in maxillary and mandibular molars: A microcomputed tomography study. J. Prosthet. Dent. 2022, 127, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Gong, Q.; Huang, L.; Luo, J.; Zhang, Y.; Meng, Q.; Quan, J.; Tong, Z. The practicability of different preparation of mandibular molar restored by modified endocrown with intracanal extension: Computational analysis using finite element models. Comput. Methods Programs Biomed. 2022, 226, 107178. [Google Scholar] [CrossRef] [PubMed]
- Dartora, N.R.; de Conto Ferreira, M.B.; Moris, I.C.M.; Brazão, E.H.; Spazin, A.O.; Sousa-Neto, M.D.; Silva-Sousa, Y.T.; Gomes, E.A. Effect of Intracoronal Depth of Teeth Restored with Endocrowns on Fracture Resistance: In Vitro and 3-dimensional Finite Element Analysis. J. Endod. 2018, 44, 1179–1185. [Google Scholar] [CrossRef]
- Farghal, A.; Dewedar, K.; AbdElaziz, M.H.; Saker, S.; Hassona, M.; Algabri, R.; Alqutaibi, A.Y. Effect of ceramic materials and tooth preparation design on computer-aided design and computer-aided manufacturing endocrown adaptation and retentive strength: An in vitro study. Clin. Exp. Dent. Res. 2024, 10, e843. [Google Scholar] [CrossRef]
- Kles, P.; Bar-On, H.; Zabrovsky, A.; Kles, K.; Eldad, S.; Ben-Gal, G. Number of consecutive procedures after endodontic treatment to extraction: A 28-year retrospective study. J. Prosthet. Dent. 2023. [Google Scholar] [CrossRef]
- Dioguardi, M.; Alovisi, M.; Troiano, G.; Caponio, C.V.A.; Baldi, A.; Rocca, G.T.; Comba, A.; Lo Muzio, L.; Scotti, N. Clinical outcome of bonded partial indirect posterior restorations on vital and non-vital teeth: A systematic review and meta-analysis. Clin. Oral Investig. 2021, 25, 6597–6621. [Google Scholar] [CrossRef]
- de Kuijper, M.; Meisberger, E.W.; Rijpkema, A.G.; Fong, C.T.; De Beus, J.H.W.; Özcan, M.; Cune, M.S.; Gresnigt, M.M.M. Survival of molar teeth in need of complex endodontic treatment: Influence of the endodontic treatment and quality of the restoration. J. Dent. 2021, 108, 103611. [Google Scholar] [CrossRef]
- Beji Vijayakumar, J.; Varadan, P.; Balaji, L.; Rajan, M.; Kalaiselvam, R.; Saeralaathan, S.; Ganesh, A. Fracture resistance of resin based and lithium disilicate endocrowns. Which is better?—A systematic review of in-vitro studies. Biomater. Investig. Dent. 2021, 8, 104–111. [Google Scholar] [CrossRef]
- Bozkurt, D.A.; Buyukerkmen, E.B.; Terlemez, A. Comparison of the pull-out bond strength of endodontically treated anterior teeth with monolithic zirconia endocrown and post-and-core crown restorations. J. Oral Sci. 2023, 65, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Emam, Z.N.; Elsayed, S.M.; Abu-Nawareg, M.; Zidan, A.Z.; Abuelroos, E.M.; Shokier, H.M.R.; Fansa, H.A.; Elsisi, H.A.; ElBanna, K.A. Retention of different all ceramic endocrown materials cemented with two different adhesive techniques. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 2232–2240. [Google Scholar] [CrossRef]
- Hasanzade, M.; Sahebi, M.; Zarrati, S.; Payaminia, L.; Alikhasi, M. Comparative Evaluation of the Internal and Marginal Adaptations of CAD/CAM Endocrowns and Crowns Fabricated from Three Different Materials. Int. J. Prosthodont. 2021, 34, 341–347. [Google Scholar] [CrossRef]
- Wong, J.L.; Chew, C.L. CRNC11: One-Year Follow-up of a Maxillary First Molar Restored with A Endocrown. J. Indian. Prosthodont. Soc. 2018, 18, S49–S50. [Google Scholar] [CrossRef]
- Sahebi, M.; Ghodsi, S.; Berahman, P.; Amini, A.; Zeighami, S. Comparison of retention and fracture load of endocrowns made from zirconia and zirconium lithium silicate after aging: An in vitro study. BMC Oral Health 2022, 22, 41. [Google Scholar] [CrossRef]
- El-Damanhoury, H.M.; Haj-Ali, R.N.; Platt, J.A. Fracture resistance and microleakage of endocrowns utilizing three CAD-CAM blocks. Oper. Dent. 2015, 40, 201–210. [Google Scholar] [CrossRef]
- de Kuijper, M.; Cune, M.S.; Tromp, Y.; Gresnigt, M.M.M. Cyclic loading and load to failure of lithium disilicate endocrowns: Influence of the restoration extension in the pulp chamber and the enamel outline. J. Mech. Behav. Biomed. Mater. 2020, 105, 103670. [Google Scholar] [CrossRef]
- Hayes, A.; Duvall, N.; Wajdowicz, M.; Roberts, H. Effect of Endocrown Pulp Chamber Extension Depth on Molar Fracture Resistance. Oper. Dent. 2017, 42, 327–334. [Google Scholar] [CrossRef]
- Banditmahakun, S.; Kuphausuk, W.; Kanchanavasita, W.; Kuphasuk, C. The effect of base materials with different elastic moduli on the fracture loads of machinable ceramic inlays. Oper. Dent. 2006, 31, 180–187. [Google Scholar] [CrossRef]
- Lee, S.K.; Wilson, P.R. Fracture strength of all-ceramic crowns with varying core elastic moduli. Aust. Dent. J. 2000, 45, 103–107. [Google Scholar]
- Gan, H.; Sun, S.; Tian, R.; Liu, F.; Li, J.; Xie, X. In vitro analysis of the influence of different tooth positions and retention depths of the pulp cavity on the accuracy of digital impression of the endocrown. Nan Fang Yi Ke Da Xue Xue Bao = J. South. Med. Univ. 2023, 43, 1941–1946. [Google Scholar]
- Elsaid, S.T.; Ahmed, A.F.; Hassan, S.M. Fracture Resistance and Retention of CAD/CAM Endo-Crowns Using Different Preparation Designs. Al-Azhar Dent. J. Girls 2020, 7, 203–211. [Google Scholar] [CrossRef]
- Kokubo, Y.; Nagayama, Y.; Tsumita, M.; Ohkubo, C.; Fukushima, S.; Vult von Steyern, P. Clinical marginal and internal gaps of In-Ceram crowns fabricated using the GN-I system. J. Oral Rehabil. 2005, 32, 753–758. [Google Scholar] [CrossRef]
- Beuer, F.; Naumann, M.; Gernet, W.; Sorensen, J.A. Precision of fit: Zirconia three-unit fixed dental prostheses. Clin. Oral Investig. 2009, 13, 343–349. [Google Scholar] [CrossRef]
- McLean, J.W.; von Fraunhofer, J.A. The estimation of cement film thickness by an in vivo technique. Br. Dent. J. 1971, 131, 107–111. [Google Scholar] [CrossRef]
- Gaintantzopoulou, M.D.; El-Damanhoury, H.M. Effect of Preparation Depth on the Marginal and Internal Adaptation of Computer-aided Design/Computer-assisted Manufacture Endocrowns. Oper. Dent. 2016, 41, 607–616. [Google Scholar] [CrossRef]
- Soliman, M.; Alzahrani, G.; Alabdualataif, F.; Eldwakhly, E.; Alsamady, S.; Aldegheishem, A.; Abdelhafeez, M.M. Impact of Ceramic Material and Preparation Design on Marginal Fit of Endocrown Restorations. Materials 2022, 15, 5592. [Google Scholar] [CrossRef]
- Montagner, A.F.; Opdam, N.J.; Ruben, J.L.; Bronkhorst, E.M.; Cenci, M.S.; Huysmans, M.C. Behavior of failed bonded interfaces under in vitro cariogenic challenge. Dent. Mater. 2016, 32, 668–675. [Google Scholar] [CrossRef]
- Maske, T.T.; Hollanders, A.C.C.; Kuper, N.K.; Bronkhorst, E.M.; Cenci, M.S.; Huysmans, M. A threshold gap size for in situ secondary caries lesion development. J. Dent. 2019, 80, 36–40. [Google Scholar] [CrossRef]
- Abualsaud, R.; Alalawi, H. Fit, Precision, and Trueness of 3D-Printed Zirconia Crowns Compared to Milled Counterparts. Dent. J. 2022, 10, 215. [Google Scholar] [CrossRef]
- Al Hamad, K.Q.; Al-Rashdan, R.B.; Al-Rashdan, B.A.; Baba, N.Z. Effect of Milling Protocols on Trueness and Precision of Ceramic Crowns. J. Prosthodont. 2021, 30, 171–176. [Google Scholar] [CrossRef]
- Contrepois, M.; Soenen, A.; Bartala, M.; Laviole, O. Marginal adaptation of ceramic crowns: A systematic review. J. Prosthet. Dent. 2013, 110, 447–454.e10. [Google Scholar] [CrossRef]
- Li, Y.Q.; Wang, H.; Wang, Y.J.; Chen, J.H. Effect of different grit sizes of diamond rotary instruments for tooth preparation on the retention and adaptation of complete coverage restorations. J. Prosthet. Dent. 2012, 107, 86–93. [Google Scholar] [CrossRef]
- Winkelmeyer, C.; Wolfart, S.; Marotti, J. Analysis of tooth preparations for zirconia-based crowns and fixed dental prostheses using stereolithography data sets. J. Prosthet. Dent. 2016, 116, 783–789. [Google Scholar] [CrossRef]
- Renne, W.; Wolf, B.; Kessler, R.; McPherson, K.; Mennito, A.S. Evaluation of the Marginal Fit of CAD/CAM Crowns Fabricated Using Two Different Chairside CAD/CAM Systems on Preparations of Varying Quality. J. Esthet. Restor. Dent. 2015, 27, 194–202. [Google Scholar] [CrossRef]
- Shim, J.S.; Lee, J.S.; Lee, J.Y.; Choi, Y.J.; Shin, S.W.; Ryu, J.J. Effect of software version and parameter settings on the marginal and internal adaptation of crowns fabricated with the CAD/CAM system. J. Appl. Oral Sci. 2015, 23, 515–522. [Google Scholar] [CrossRef]
- Hamza, T.A.; Ezzat, H.A.; El-Hossary, M.M.; Katamish, H.A.; Shokry, T.E.; Rosenstiel, S.F. Accuracy of ceramic restorations made with two CAD/CAM systems. J. Prosthet. Dent. 2013, 109, 83–87. [Google Scholar] [CrossRef]
- Hamza, T.A.; Sherif, R.M. In vitro evaluation of marginal discrepancy of monolithic zirconia restorations fabricated with different CAD-CAM systems. J. Prosthet. Dent. 2017, 117, 762–766. [Google Scholar] [CrossRef]
- Li, R.; Chen, H.; Wang, Y.; Sun, Y. Performance of stereolithography and milling in fabricating monolithic zirconia crowns with different finish line designs. J. Mech. Behav. Biomed. Mater. 2021, 115, 104255. [Google Scholar] [CrossRef]
- Takaki, P.; Vieira, M.; Bommarito, S. Maximum bite force analysis in different age groups. Int. Arch. Otorhinolaryngol. 2014, 18, 272–276. [Google Scholar] [CrossRef]
- Comba, A.; Baldi, A.; Carossa, M.; Michelotto Tempesta, R.; Garino, E.; Llubani, X.; Rozzi, D.; Mikonis, J.; Paolone, G.; Scotti, N. Post-fatigue fracture resistance of lithium disilicate and polymer-infiltrated ceramic network indirect restorations over endodontically-treated molars with different preparation designs: An in-vitro study. Polymers 2022, 14, 5084. [Google Scholar] [CrossRef]
- Frankenberger, R.; Winter, J.; Dudek, M.-C.; Naumann, M.; Amend, S.; Braun, A.; Krämer, N.; Roggendorf, M.J. Post-fatigue fracture and marginal behavior of endodontically treated teeth: Partial crown vs. full crown vs. endocrown vs. fiber-reinforced resin composite. Materials 2021, 14, 7733. [Google Scholar] [CrossRef]
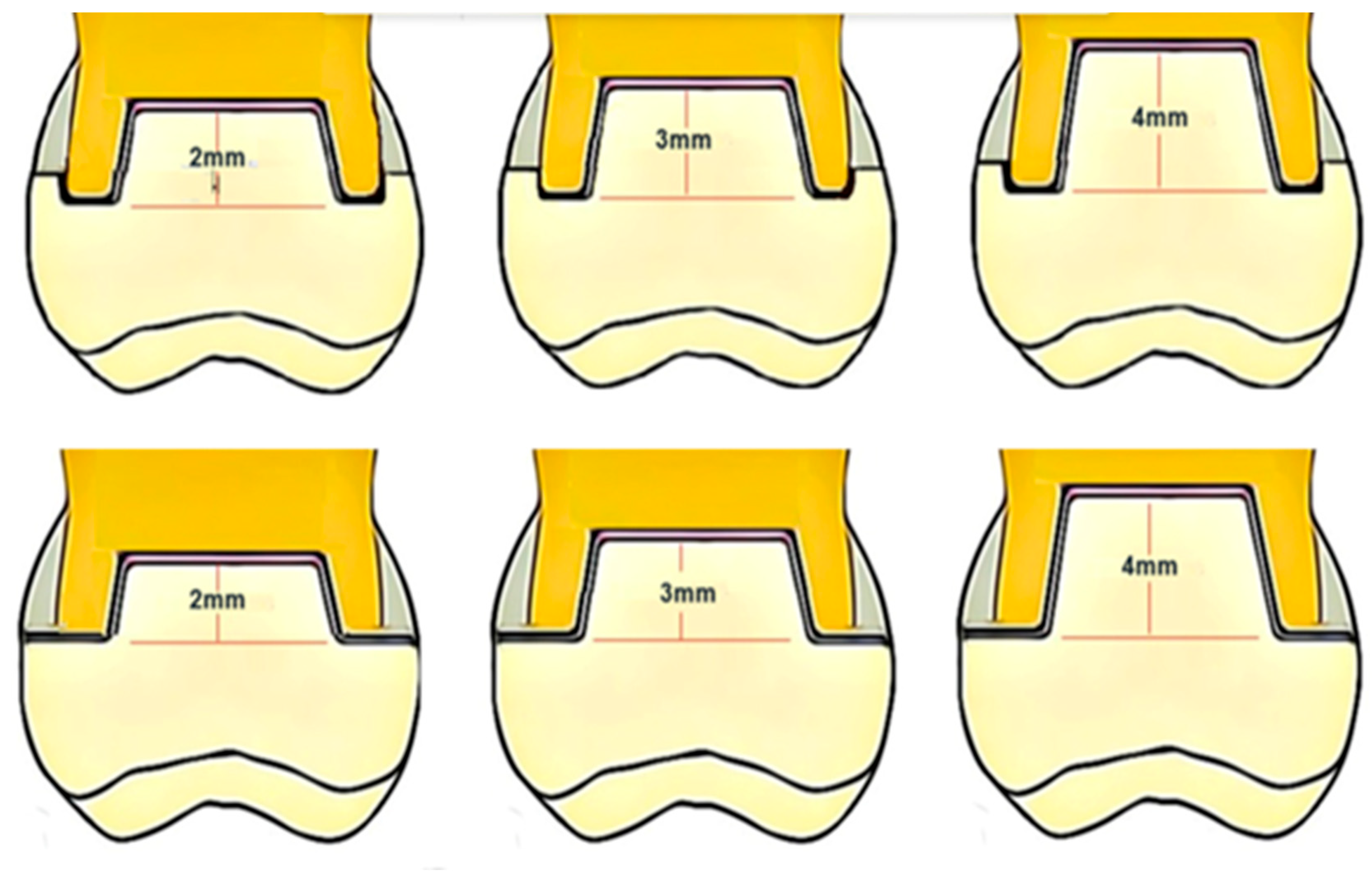

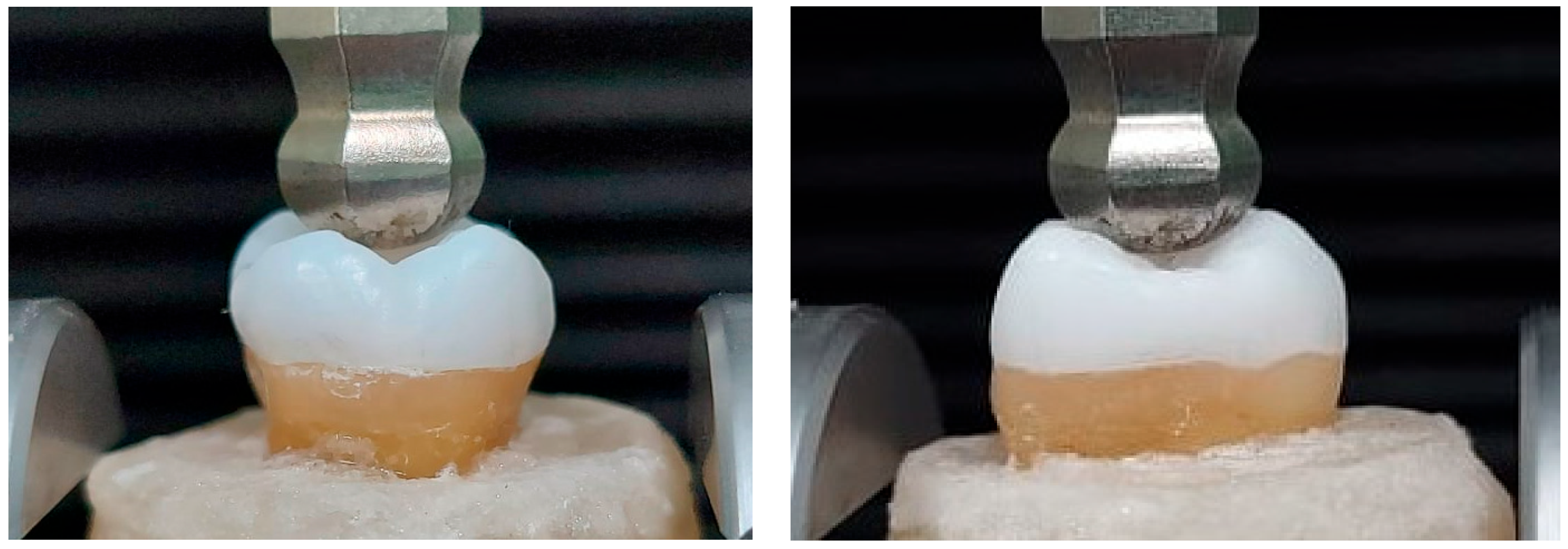

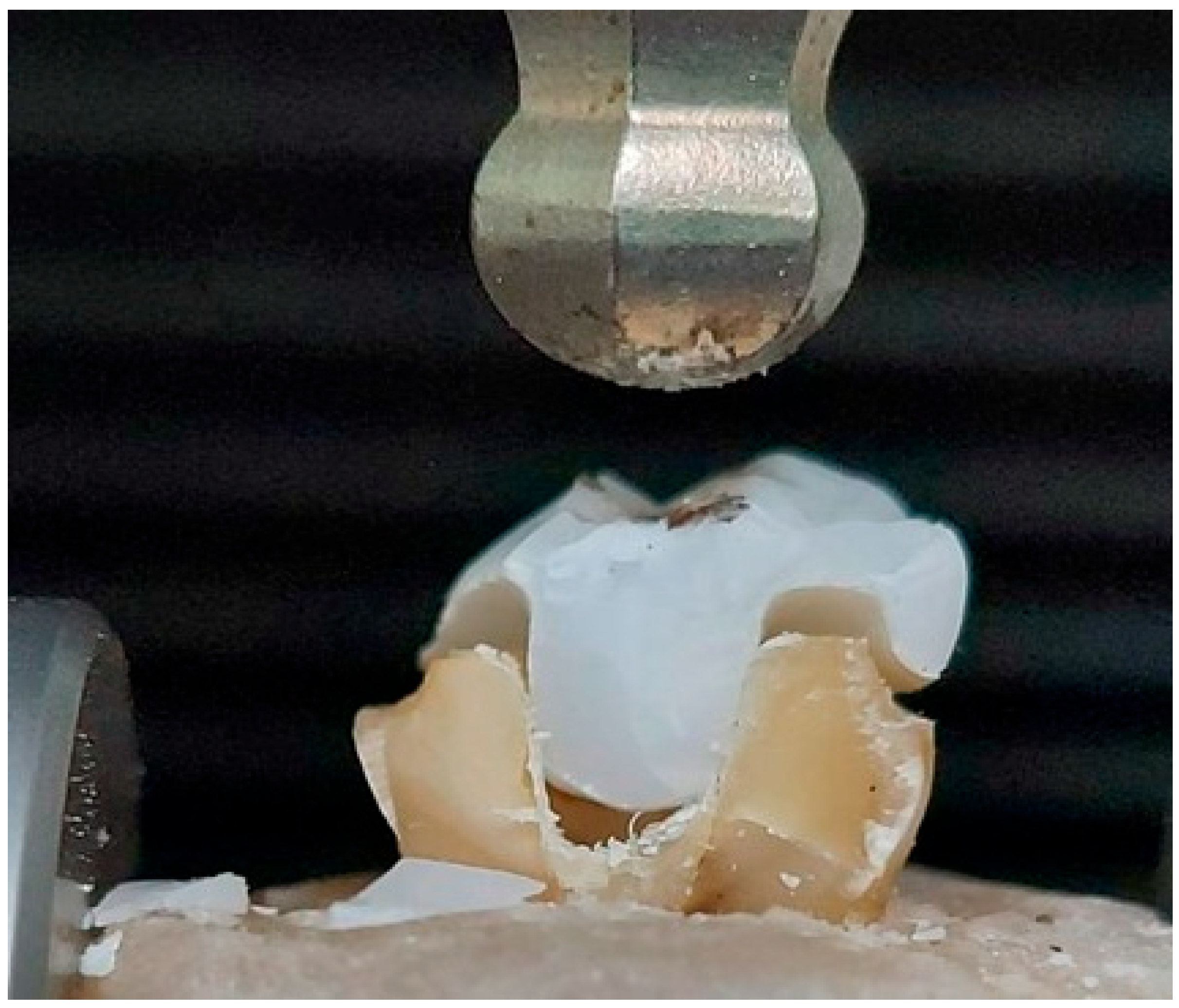
| Trade Name | Scientific Name | Composition | Productive Company |
|---|---|---|---|
| Vita Suprinity | Zirconia-reinforced lithia silicate | SiO2 56–64 wt%, Li2O 15–21 wt%, K2O 1–4 wt%, P2O5 3–8 wt%, Al2O3 1–4 wt%, ZrO2 8–12 wt%, CeO2 0–4 wt%, La2O3 0–1 wt%, Pigments 0–6 wt%. | VITA Zahnfabrik, Germany |
| Gradia Core | Dual-cured composite core build-up material | Resin; Bis-GMA, TEGDMA. Filler: Silanated glass, silica Filler loading: (74 wt%) 52 vol% The particle size of 0.04 μm to 23 μm Camphorquinone, Benzoylperoxide | GC America |
| Clearfill SE | Two-step, self-etch adhesive | Primer: MDP, HEMA, dimethacrylate monomer, water, catalyst Bond: MDP, HEMA, dimethacrylate monomer, microfiller, catalyst | Kuraray America, Houston, TX, USA |
| Rely X Unicem | Self-adhesive resin cement powder | Glass powder, initiator, silica, substituted pyrimidine, calcium hydroxide, peroxy compound, pigment | 3 M ESPE, Seefeld, Germany. |
| Self-adhesive resin cement liquid | Methacrylated phosphoric ester, dimethacrylate, acetate, stabilizer, initiator. |
| Measurements Location | Marginal | Cervical | Axial | Pulpal | Internal | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocrown Design | Mean | St. Dev. | Mean | St. Dev. | Mean | St. Dev. | Mean | St. Dev. | Mean | St. Dev. | |
| Butt joint design | 2 mm inlay | 90.700 Aa | 11.3925 | 85.800 Aa | 8.9790 | 90.290 Aa | 4.9328 | 123.000 Ab | 14.7573 | 99.6980 Aa | 5.64745 |
| 3 mm inlay | 105.300 Bb | 13.2082 | 88.300 Aa | 9.3101 | 94.730 Ba | 5.4766 | 138.300 Bc | 10.2095 | 107.1110 Bb | 4.29440 | |
| 4 mm inlay | 107.100 Bb | 16.3670 | 94.500 Ba | 11.6357 | 97.360 Ba | 5.2243 | 143.400 Cc | 12.1582 | 111.7540 Cb | 5.07847 | |
| Ferrule design | 2 mm inlay | 102.300 Bb | 13.5158 | 94.600 Ba | 10.2220 | 90.270 Aa | 2.7105 | 126.400 Ac | 11.4426 | 103.7590 Bb | 6.82651 |
| 3 mm inlay | 116.500 Cb | 12.7126 | 109.000 Cb | 12.8841 | 95.120 Ba | 6.7690 | 138.500 Bc | 10.2095 | 115.4080 Cb | 8.28399 | |
| 4 mm inlay | 119.500 Cb | 15.8902 | 118.100 Db | 13.6092 | 102.590 Ca | 13.4518 | 147.000 Cb | 12.1582 | 122.5640 Db | 10.56441 | |
| Endocrown Design | Butt Joint Design | Ferrule Design | ||
|---|---|---|---|---|
| Mean | St. Dev. | Mean | St. Dev. | |
| 2 mm inlay | 1371.0900 Aa | 105.48131 | 1162.1600 Bb | 375.71287 |
| 3 mm inlay | 1409.6600 Aa | 49.95278 | 1246.6100 Ab | 104.55067 |
| 4 mm inlay | 1396.4833 Aa | 81.54658 | 1215.3867 Ab | 225.40423 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saker, S.; Alqutaibi, A.Y.; Alghauli, M.A.; Hashem, D.; Borzangy, S.; Farghal, A.E.; Alnazzawi, A.A.; Ainoosah, S.; AbdElaziz, M.H. The Influence of Ferrule Design and Pulpal Extensions on the Accuracy of Fit and the Fracture Resistance of Zirconia-Reinforced Lithium Silicate Endocrowns. Materials 2024, 17, 1411. https://doi.org/10.3390/ma17061411
Saker S, Alqutaibi AY, Alghauli MA, Hashem D, Borzangy S, Farghal AE, Alnazzawi AA, Ainoosah S, AbdElaziz MH. The Influence of Ferrule Design and Pulpal Extensions on the Accuracy of Fit and the Fracture Resistance of Zirconia-Reinforced Lithium Silicate Endocrowns. Materials. 2024; 17(6):1411. https://doi.org/10.3390/ma17061411
Chicago/Turabian StyleSaker, Samah, Ahmed Yaseen Alqutaibi, Mohammed Ahmed Alghauli, Danya Hashem, Sary Borzangy, Ahmed E. Farghal, Ahmad A. Alnazzawi, Sultan Ainoosah, and Mohammed H. AbdElaziz. 2024. "The Influence of Ferrule Design and Pulpal Extensions on the Accuracy of Fit and the Fracture Resistance of Zirconia-Reinforced Lithium Silicate Endocrowns" Materials 17, no. 6: 1411. https://doi.org/10.3390/ma17061411








