High-Performance Organic Field-Effect Transistors of Liquid Crystalline Organic Semiconductor by Laser Mapping Annealing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
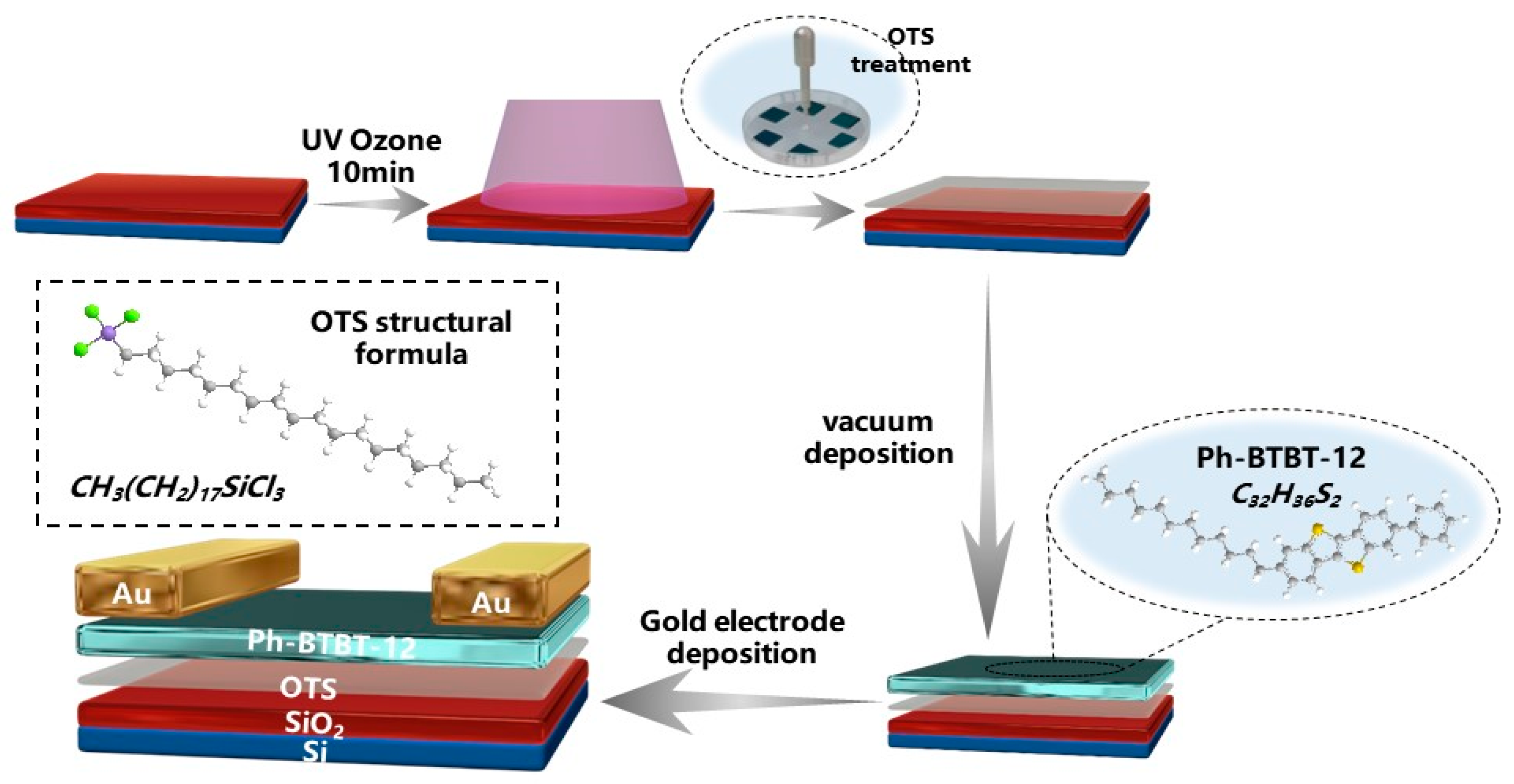
2.2. Preparation of Thin Films
2.3. Device Fabrication
2.4. Characterizations
3. Results
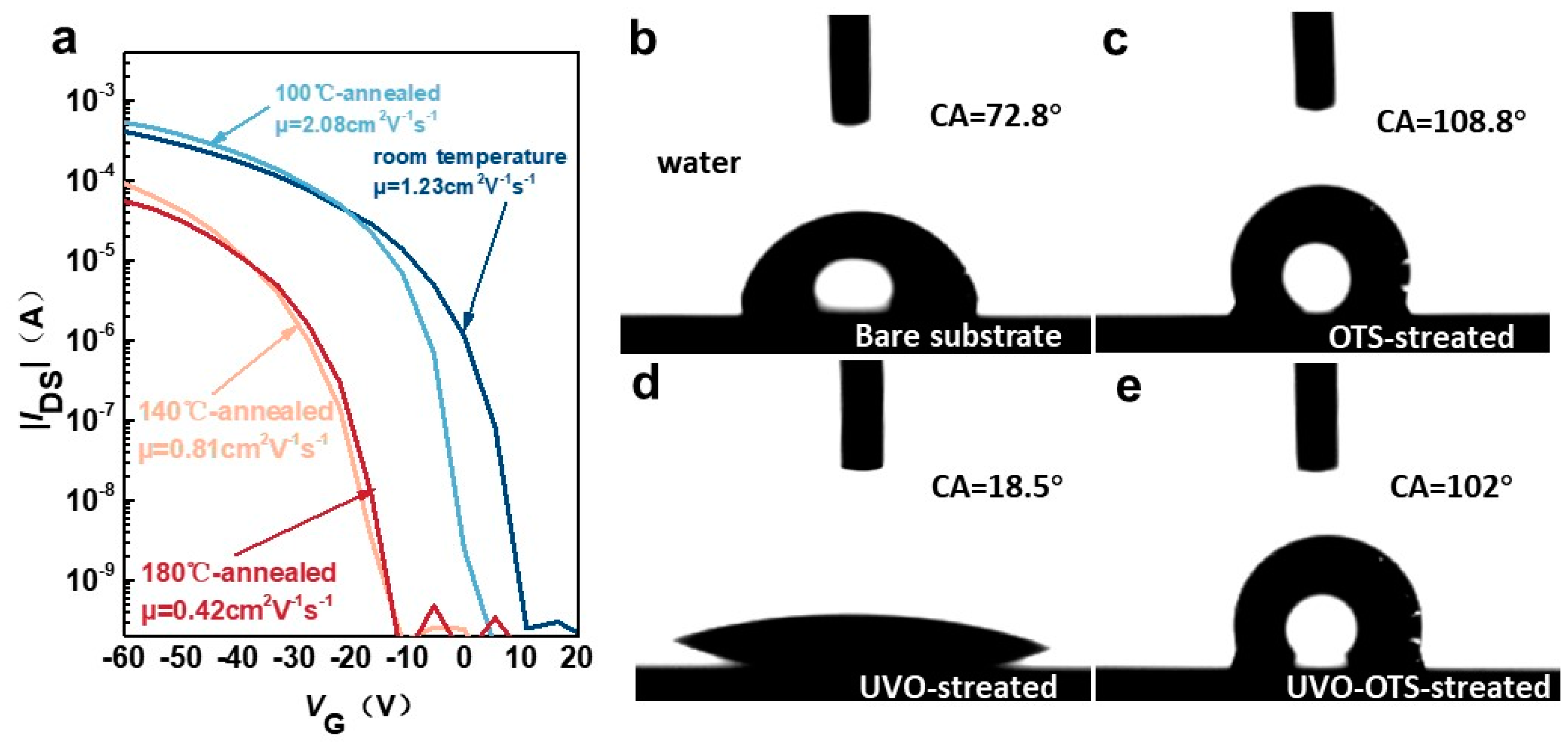
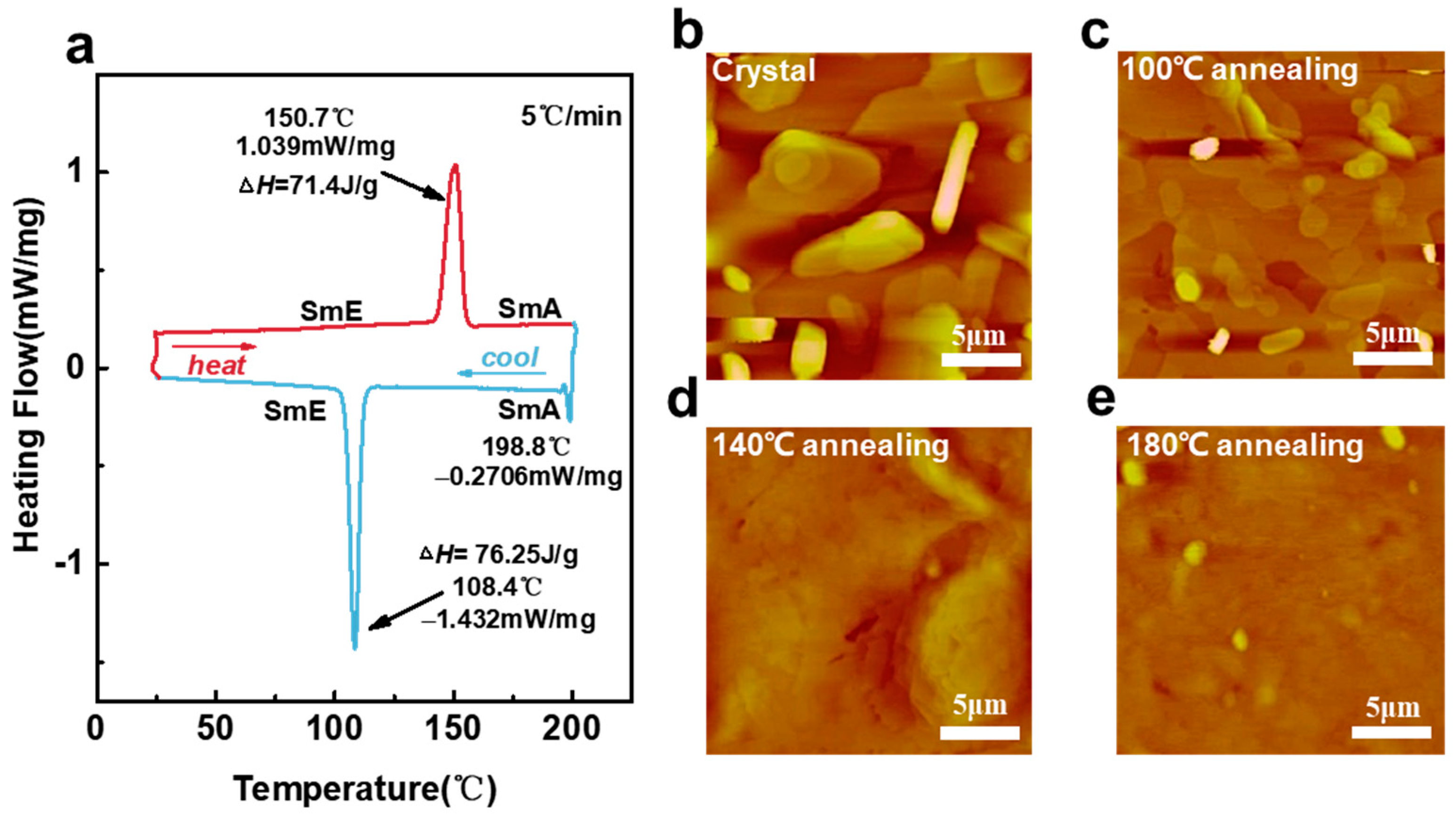
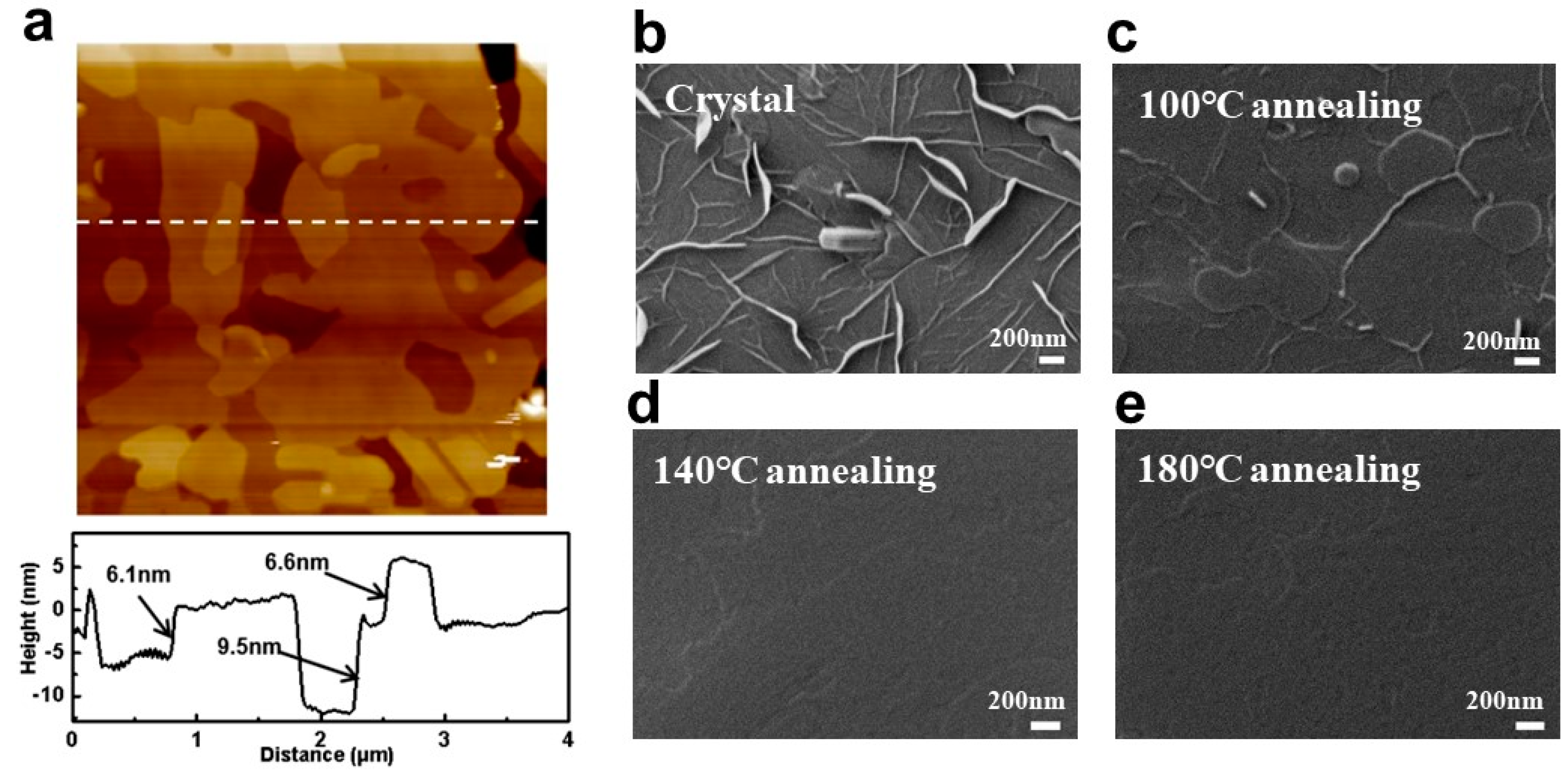
3.1. Interpretation of Ph-BTBT-12 Material Properties Combined with the Liquid Crystal Phase and Annealing Properties
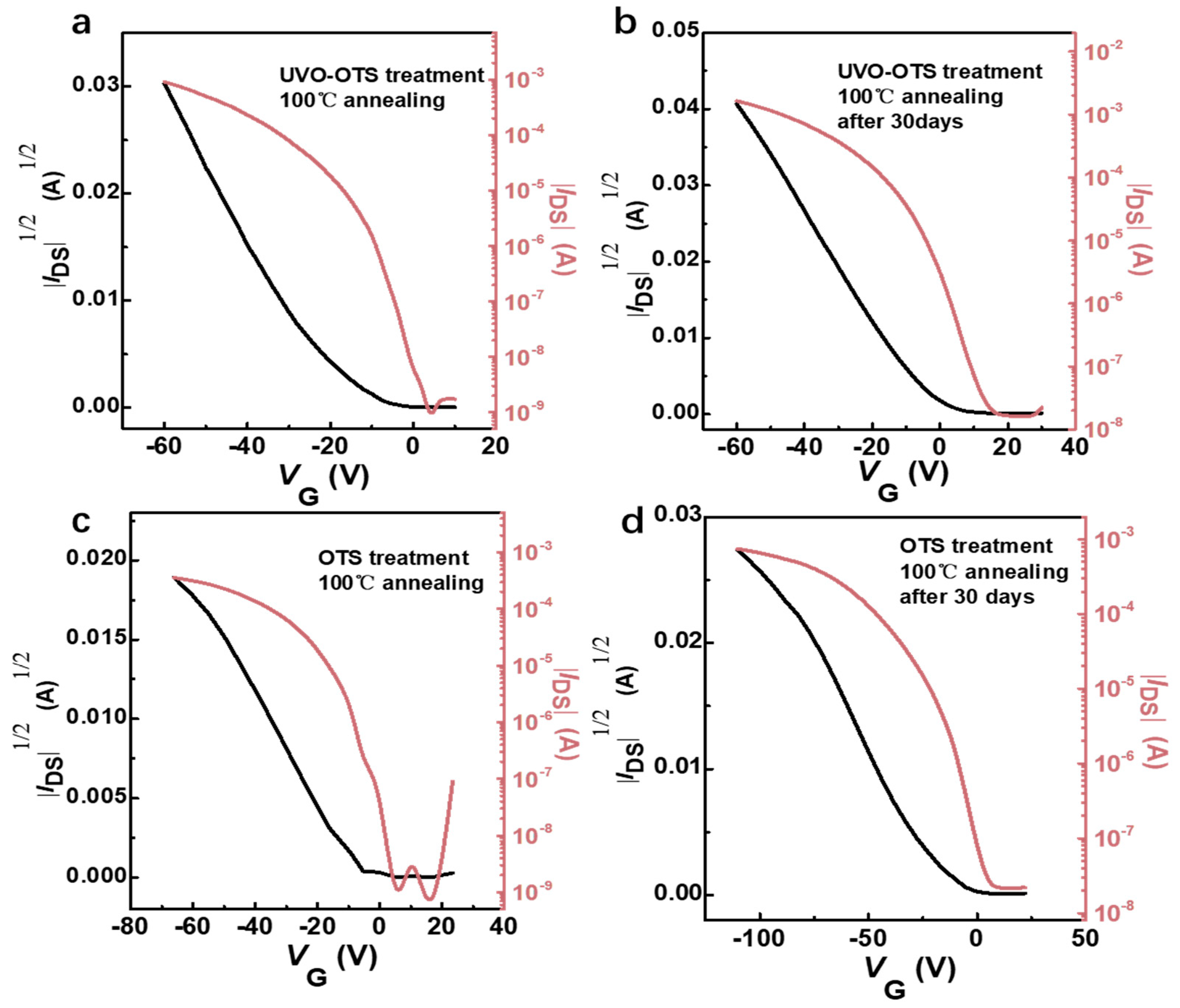
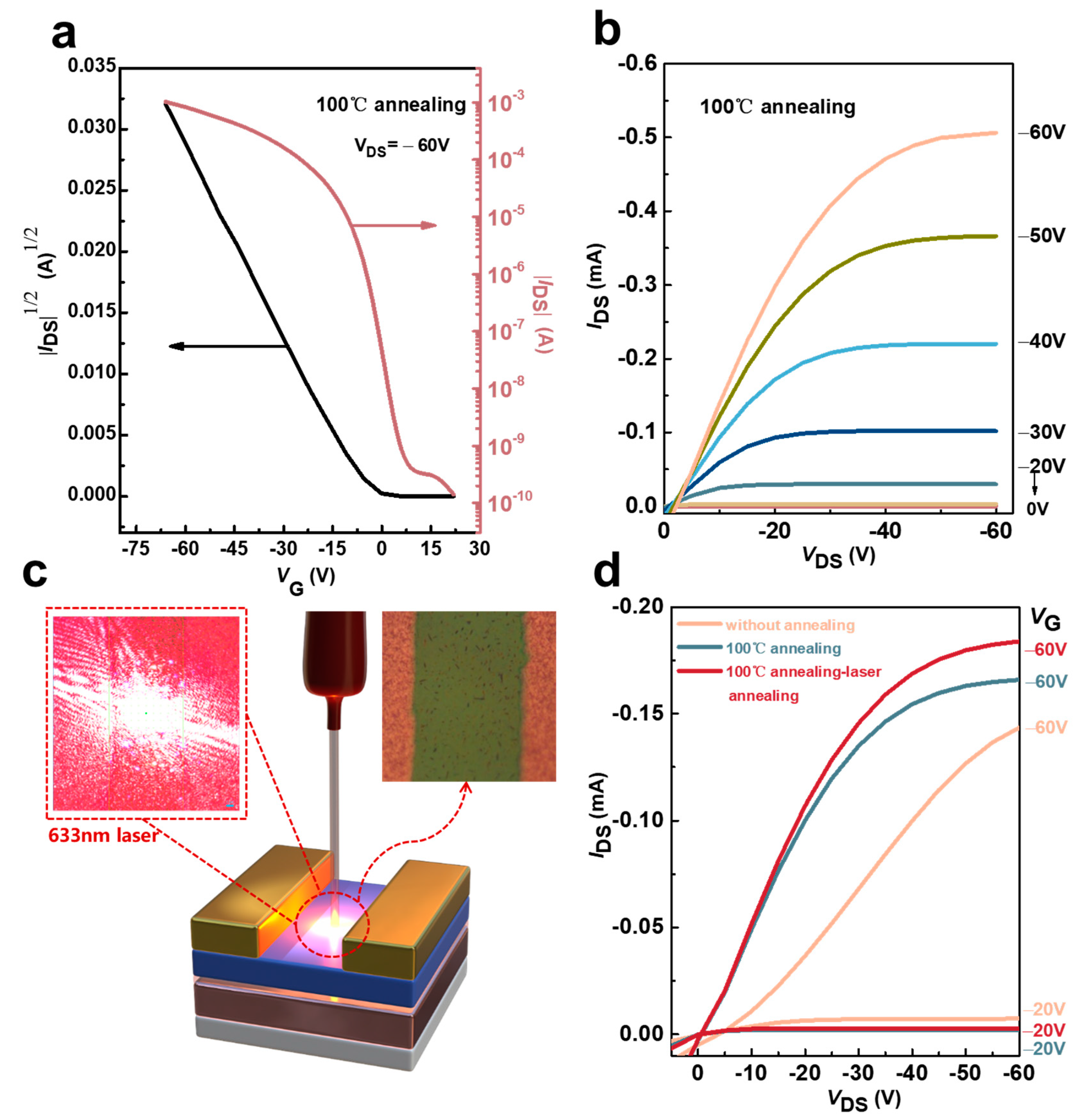
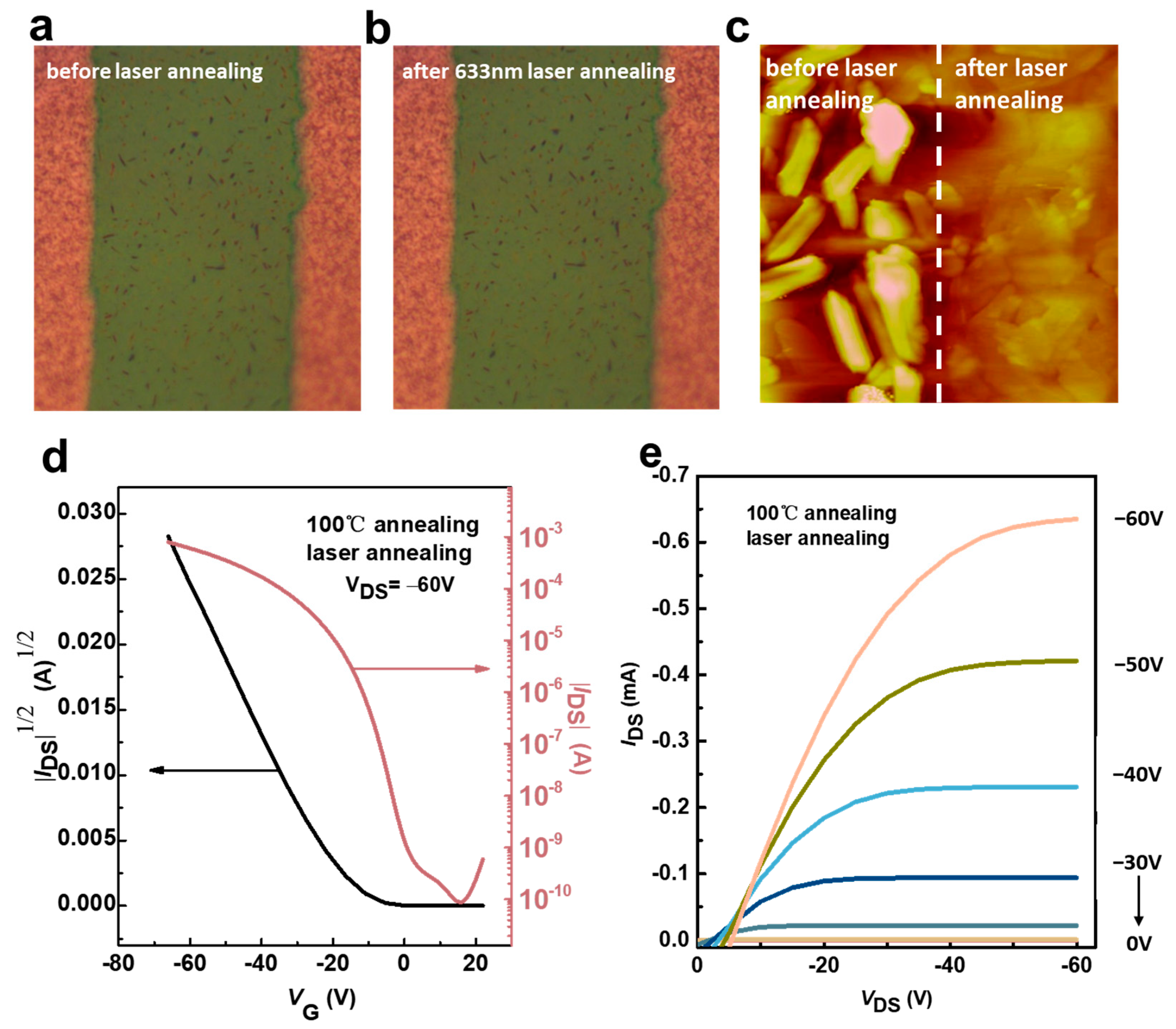
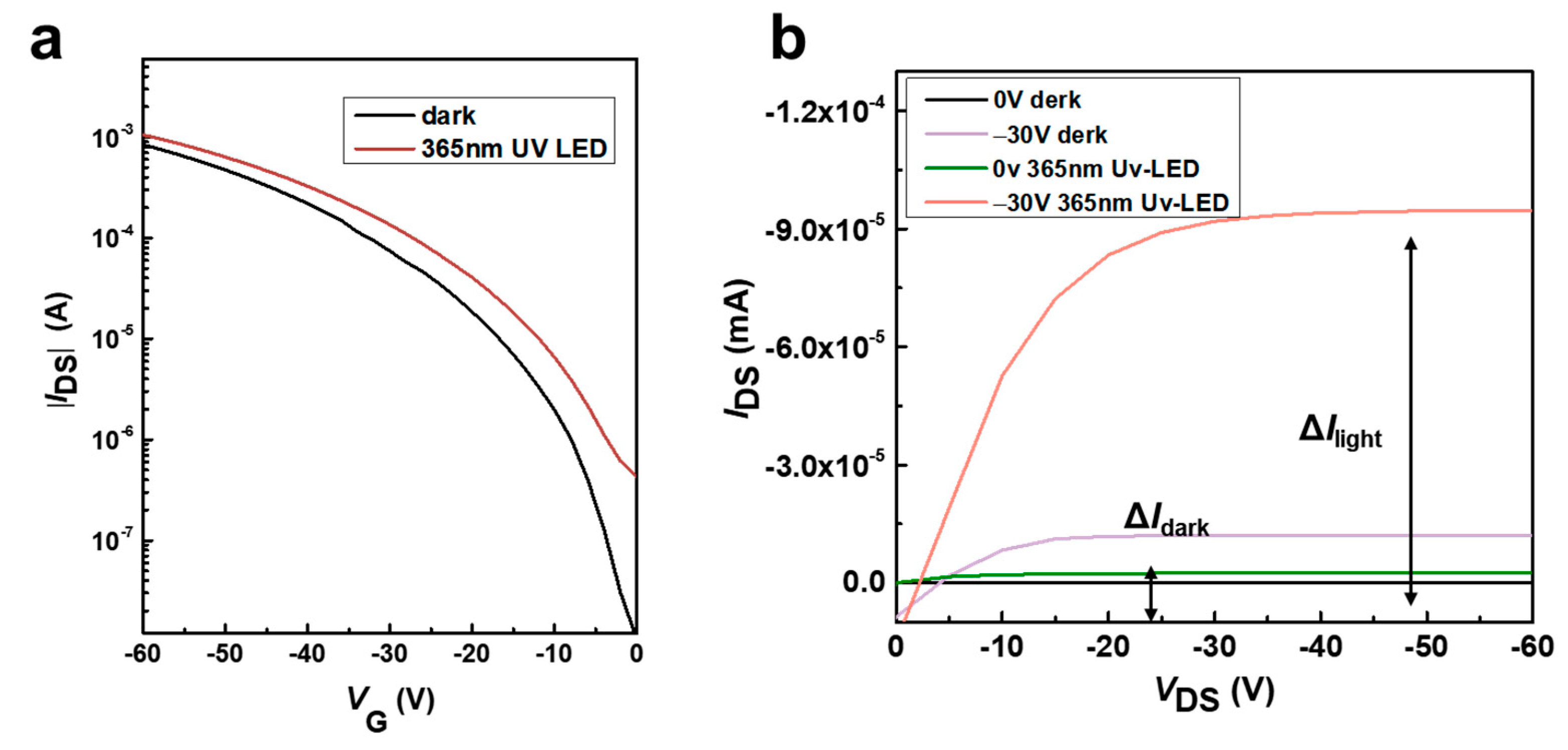
3.2. Study on the Change in Laser Annealing on the Properties of Devices Prepared Based on Ph-BTBT-12 Material and Its Optoelectronic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, C.; Dong, H.; Jiang, L.; Hu, W. Organic semiconductor crystals. Chem. Soc. Rev. 2018, 47, 422–500. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Liu, Y.; Zhu, D. π-Conjugated molecules with fused rings for organic field-effect transistors: Design, synthesis and applications. Chem. Soc. Rev. 2010, 39, 1489–1502. [Google Scholar] [CrossRef] [PubMed]
- Han, J.L.; Rong, X.; Xu, C.H.; Deng, Y.F.; Geng, Y.H.; Dong, G.F.; Duan, L. Complementary inverter based on n-type and p-type ofets with the same ambipolar organic semiconductor and ITO S/D electrodes. Adv. Electron. Mater. 2023, 9, 2201288. [Google Scholar] [CrossRef]
- Fichou, D.; Horowitz, G. Molecular and Polymer Semiconductors, Conductors, and Superconductors: Overview. In Encyclopedia of Materials: Science and Technology; Buschow, K.H.J., Cahn, R.W., Flemings, M.C., Ilschner, B., Kramer, E.J., Mahajan, S., Veyssière, P., Eds.; Elsevier: Oxford, UK, 2001; pp. 5748–5757. [Google Scholar]
- Price, M.B.; Hume, P.A.; Ilina, A.; Wagner, I.; Tamming, R.R.; Thorn, K.E.; Jiao, W.; Goldingay, A.; Conaghan, P.J.; Lakhwani, G.; et al. Free charge photogeneration in a single component high photovoltaic efficiency organic semiconductor. Nat. Commun. 2022, 13, 2827. [Google Scholar] [CrossRef]
- Poriel, C.; Rault-Berthelot, J. Dihydroindenofluorenes as building units in organic semiconductors for organic electronics. Chem. Soc. Rev. 2023, 52, 6754–6805. [Google Scholar] [CrossRef]
- Rao, A.; Gillett, A.J.; Friend, R.H. Engineering the spin-exchange interaction in organic semiconductors. Nat. Mater. 2022, 21, 976–978. [Google Scholar] [CrossRef]
- Van Gompel, W.T.M.; Herckens, R.; Denis, P.H.; Mertens, M.; Gelvez-Rueda, M.C.; Van Hecke, K.; Ruttens, B.; D’Haen, J.; Grozema, F.C.; Lutsen, L.; et al. 2D layered perovskite containing functionalised benzothieno-benzothiophene molecules: Formation, degradation, optical properties and photoconductivity. J. Mater. Chem. C 2020, 8, 7181–7188. [Google Scholar] [CrossRef]
- Roche, G.H.; Flot, D.; Moreau, J.J.E.; Dautel, O.J.; Filhol, J.S.; van der Lee, A. Packing polymorphism affecting the optoelectronic properties of a π-conjugated organic compound. Cryst. Growth Des. 2021, 21, 3850–3863. [Google Scholar] [CrossRef]
- Shivaji, B.S.; Boddula, R.; Singh, S.P. [1]Benzothieno[3,2-b][1] benzothiophene-based dyes: Effect of the ancillary moiety on mechanochromism and aggregation-induced emission. Phys. Chem. Chem. Phys. 2022, 24, 15110–15120. [Google Scholar] [CrossRef]
- Kaya, I.C.; Ozdemir, R.; Usta, H.; Sonmezoglu, S. A dopant-free 2,7-dioctyl [1]benzothieno[3,2-b][1] benzothiophene (C8-BTBT)-based hole transporting layer for highly stable perovskite solar cells with efficiency over 22%. J. Mater. Chem. A 2022, 10, 12464–12472. [Google Scholar] [CrossRef]
- Takimiya, K.; Osaka, I.; Mori, T.; Nakano, M. Organic semiconductors based on [1]benzothieno[3,2-b][1]benzothiophene substructure. Acc. Chem. Res. 2014, 47, 1493–1502. [Google Scholar] [CrossRef]
- He, D.; Zhang, Y.; Wu, Q.; Xu, R.; Nan, H.; Liu, J.; Yao, J.; Wang, Z.; Yuan, S.; Li, Y.; et al. Two-dimensional quasi-freestanding molecular crystals for high-performance organic field-effect transistors. Nat. Commun. 2014, 5, 5162. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, D.; Shuai, Z. High-performance organic thermoelectric materials: Theoretical insights and computational design. Adv. Electron. Mater. 2019, 5, 1800882. [Google Scholar] [CrossRef]
- Chung, H.; Chen, S.; Sengar, N.; Davies, D.W.; Garbay, G.; Geerts, Y.H.; Clancy, P.; Diao, Y. Single atom substitution alters the polymorphic transition mechanism in organic electronic crystals. Chem. Mater. 2019, 31, 9115–9126. [Google Scholar] [CrossRef]
- Arias, N.; Jaramillo, F. Highly reflective aluminum films on polycarbonate substrates by physical vapor deposition. Appl. Surf. Sci. 2020, 505, 144596. [Google Scholar] [CrossRef]
- Murali, G.; Lee, M.; Modigunta, J.K.R.; Kang, B.; Kim, J.; Park, E.; Kang, H.; Lee, J.; Park, Y.H.; Park, S.Y. Ultraviolet–ozone-activation-driven Ag nanoparticles grown on plastic substrates for antibacterial applications. ACS Appl. Nano Mater. 2022, 5, 8767–8774. [Google Scholar] [CrossRef]
- Raveendran, R.; Namboothiry, M.A.G. Surface-treated poly(dimethylsiloxane) as a gate dielectric in solution-processed organic field-effect transistors. ACS Omega 2018, 3, 11278–11285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.S.; Zhou, A.G.; Sun, B.R.; Chen, K.S.; Yu, H.Z. Functional and versatile superhydrophobic coatings via stoichiometric silanization. Nat. Commun. 2021, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Xiaosong, C.; Zeyang, X.; Kunjie, W.; Suna, Z.; Hongwei, L.; Yancheng, M.; Zhongwu, W.; Liqiang, L.; Xueming, M. Facile peeling method as a post-remedy strategy for producing an ultrasmooth self-assembled monolayer for high-performance organic transistors. Langmuir 2016, 32, 9492–9500. [Google Scholar]
- Chen, M.; Zhu, Y.N.; Yao, C.; Zhang, D.W.; Zeng, X.W.; Murtaza, I.; Chen, H.B.; Kasai, S.; Meng, H.; Goto, O. Intrinsic charge carrier mobility in single-crystal OFET by “fast trapping vs. slow detrapping” model. Org. Electron. 2018, 54, 237–244. [Google Scholar] [CrossRef]
- Liu, F.; Gu, Y.; Hu, Y.; Wang, Z.; Ning, Y.; Bradley, R.; Lou, D.; Zhao, B.; Wu, W. Freestanding ultrathin precisely structured hierarchical porous carbon blackbody film for efficient solar interfacial evaporation. Sol. RRL 2023, 7, 2200803. [Google Scholar] [CrossRef]
- Pylypova, O.; Antonin, S.; Fedorenko, L.; Muryi, Y.; Skryshevsky, V.; Evtukh, A. Influence of laser annealing of silicon enriched siox films on their electrical conductivity. Silicon 2022, 14, 12599–12605. [Google Scholar] [CrossRef]
- Uedono, A.; Nogami, T.; Gluschenkov, O.; Sulehria, Y.; Liu, J.; Tabata, T.; Lu, L.; Mitsuda, K.; Brown, I.; Okuno, Y. Impact of nanosecond laser annealing on vacancies in electroplated cu films studied by monoenergetic positron beams. J. Appl. Phys. 2023, 134, 135103. [Google Scholar] [CrossRef]
- Shimamune, Y.; Nagumo, R.; Jimbo, K. Cu2ZnSnS4 formation by laser annealing in controlled atmosphere. Jpn. J. Appl. Phys. 2024, 63, 02SP16. [Google Scholar] [CrossRef]
- Huet, K. Chapter 4—Laser annealing applications for semiconductor devices manufacturing. In Laser Annealing Processes in Semiconductor Technology; Cristiano, F., La Magna, A., Eds.; Woodhead Publishing: Sawston, UK, 2021; pp. 137–173. [Google Scholar]
- Arduino, D.; Stassi, S.; Spano, C.; Scaltrito, L.; Ferrero, S.; Bertana, V. Silicon and silicon carbide recrystallization by laser annealing: A review. Materials 2023, 16, 7674. [Google Scholar] [CrossRef] [PubMed]
- Sirota, B.; Glavin, N.; Voevodin, A.A. Room temperature magnetron sputtering and laser annealing of ultrathin MoS2 for flexible transistors. Vacuum 2019, 160, 133–138. [Google Scholar] [CrossRef]
- Liu, X.; Islam, A.; Yang, N.; Odhner, B.; Tupta, M.A.; Guo, J.; Feng, P.X.L. Atomic layer MoTe2 field-effect transistors and monolithic logic circuits configured by scanning laser annealing. ACS Nano 2021, 15, 19733–19742. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lin, C.C.; Lin, Y.C. Transparent conducting Nb-doped TiO2 electrodes activated by laser annealing for inexpensive flexible organic solar cells. Jpn. J. Appl. Phys. 2011, 51, 015502. [Google Scholar] [CrossRef]
- Shin, H.I.; Kim, K.H.; Kim, T.W.; Kim, H.K. Fiber laser annealing of brush-painted ITO nanoparticles for use as transparent anode for organic solar cells. Ceram. Int. 2016, 42, 13983–13989. [Google Scholar] [CrossRef]
- Chen, Y.; Okada, T.; Noguchi, T.; Mazzamuto, F.; Huet, K. Excimer laser annealing for low-voltage power MOSFET. Jpn. J. Appl. Phys. 2016, 55, 086503. [Google Scholar] [CrossRef]
- Cao, B.L.; Mu, Y.Y.; Zhang, X.P. Direct laser annealing of surface-enhanced raman scattering substrates. Adv. Eng. Mater. 2019, 21, 1900779. [Google Scholar] [CrossRef]
- Zou, C.; Yanahashi, N.; Wu, Y.; Wang, J.; Zhang, C.; Xiong, G.; Yang, H.; Jiang, L.; Ikeda, T. Patterning smectic liquid crystals for OFETs at low temperature. Adv. Funct. Mater. 2019, 29, 1804838. [Google Scholar] [CrossRef]
- Renkert, S.; Fall, S.; Motamen, S.; Jarrosson, T.; Serein-Spirau, F.; Heiser, T.; Simon, L.; Reiter, G.; Bubendorff, J.L. A new growth process for crystalline ultra-thin layers of conjugated oligomers used in field-effect transistor applications. Appl. Surf. Sci. 2021, 539, 148024. [Google Scholar] [CrossRef]
- Seemann, R.; Herminghaus, S.; Jacobs, K. Dewetting patterns and molecular forces: A reconciliation. Phys. Rev. Lett. 2001, 86, 5534–5537. [Google Scholar] [CrossRef] [PubMed]
- Xue, L.; Han, Y. Pattern formation by dewetting of polymer thin film. Prog. Polym. Sci. 2011, 36, 269–293. [Google Scholar] [CrossRef]
- Drakopoulou, S.; Murgia, M.; Albonetti, C.; Benaglia, S.; Borgatti, F.; Di Lauro, M.; Bianchi, M.; Greco, P.; Papo, D.; Garcia, R.; et al. Nanoscale quantized oscillations in thin-film growth greatly enhance transconductance in organic transistors. Adv. Electron. Mater. 2023, 9, 2300320. [Google Scholar] [CrossRef]
- Roy, P.K.; Frenkel, M.; Legchenkova, I.; Shoval, S.; Binks, B.P.; Bormashenko, E. Liquid Marble-Induced Dewetting. J. Phys. Chem. C 2020, 124, 9345–9349. [Google Scholar] [CrossRef]
- Haque, A.; Liu, Y.; Taqy, S.; Narayan, J. Cost-Effective synthesis of diamond nano-/microstructures from amorphous and graphitic carbon materials: Implications for nanoelectronics. ACS Appl. Nano Mater. 2023, 6, 6488–6495. [Google Scholar] [CrossRef]
- Stock, F.; Antoni, F.; Aubel, D.; Hajjar-Garreau, S.; Muller, D. Pure carbon conductive transparent electrodes synthetized by a full laser deposition and annealing process. Appl. Surf. Sci. 2020, 505, 144505. [Google Scholar] [CrossRef]
- Xue-pei, W.; Lu-wei, Z.; Xue-bing, B.; Xian-bin, M.; Xiao-shuan, Z. Infrared spectral characterization of ultraviolet ozone treatment on substrate surface for flexible electronics. Spectrosc. Spect. Anal. 2022, 42, 1867–1873. [Google Scholar]
- Sattari-Esfahlan, S.M.; Lee, E.C.; Kim, C.H. Synergistic effects of self-assembled monolayers in solution-processed 6,13-bis(triisopropylsilylethynyl) pentacene transistors. ChemPhysChem 2021, 22, 1706–1711. [Google Scholar] [CrossRef]
- Riera-Galindo, S.; Chen, L.; Serena Maglione, M.; Zhang, Q.; Bromley, S.T.; Rovira, C.; Mas-Torrent, M. Functionalising the gate dielectric of organic field-effect transistors with self-assembled monolayers: Effect of molecular electronic structure on device performance. Appl. Phys. A-Mater. 2022, 128, 322. [Google Scholar] [CrossRef]
- Park, M.; Kang, C.M.; Park, S.; Jo, H.; Roh, J. Effect of variations in the alkyl chain lengths of self-assembled monolayers on the crystalline-phase-mediated electrical performance of organic field-effect transistors. ACS Omega 2021, 6, 33639–33644. [Google Scholar] [CrossRef] [PubMed]
- Mamo, M.D.; Shin, E.S.; Noh, Y.Y. Self-aligned patterning of conductive films on plastic substrates for electrodes of flexible electronics. J. Mater. Chem. C 2017, 5, 10900–10906. [Google Scholar] [CrossRef]
- Londhe, P.; Chaure, N.B.; Athawale, A. Interface engineering of gate dielectrics with multifunctional self-assembled monolayers in copper phthalocyanine based organic field-effect transistors. Mater. Sci. Eng. B-Adv. 2021, 273, 115397. [Google Scholar] [CrossRef]
- Chen, K.T.; Hsu, C.H.; Jiang, S.C.; Liang, L.S.; Gao, P.; Qiu, Y.; Wu, W.Y.; Zhang, S.; Zhu, W.Z.; Lien, S.Y. Effect of annealing temperature on tantalum-doped TiO2 as electron transport layer in perovskite solar cells. IEEE. Trans. Electron. Devices 2022, 69, 1149–1154. [Google Scholar] [CrossRef]
- Yun, S.; Yun, C.; Ho, D.; Chae, W.; Earmme, T.; Kim, C.; Seo, S.Y. Side chain engineering of [1]benzothieno[3,2-b]benzothiophene (BTBT)-based semiconductors for organic field-effect transistors. Synth. Met. 2022, 285, 117022. [Google Scholar] [CrossRef]
- Zhong, Y.; Ping, D.H.; Song, X.Y.; Yin, F.X. Determination of grain size by XRD profile analysis and TEM counting in nano-structured Cu. J. Alloys Compd. 2009, 476, 113–117. [Google Scholar] [CrossRef]
- Liu, X.; Su, X.L.; Livache, C.; Chamoreau, L.M.; Sanaur, S.; Sosa-Vargas, L.; Ribierre, J.C.; Kreher, D.; Lhuillier, E.; Lacaze, E.; et al. Investigation of charge transport properties of [1]Benzothieno[3,2-b] [1]-benzothiophene single-crystals in field-effect transistor configuration. Org. Electron. 2020, 78, 105605. [Google Scholar] [CrossRef]
- Ebata, H.; Izawa, T.; Miyazaki, E.; Takimiya, K.; Ikeda, M.; Kuwabara, H.; Yui, T. Highly soluble [1]benzothieno[3,2-b]benzothiophene (BTBT) derivatives for high-performance, solution-processed organic field-effect transistors. J. Am. Chem. Soc. 2007, 129, 15732–15733. [Google Scholar] [CrossRef]
- Kim, S.; Kim, A.; Jang, K.S.; Yoo, S.; Ka, J.W.; Kim, J.; Yi, M.H.; Won, J.C.; Hong, S.K.; Kim, Y.H. The effect of thermal annealing on the layered structure of smectic liquid crystalline organic semiconductor on polyimide gate insulator and its OFET performance. Synth. Met. 2016, 220, 311–317. [Google Scholar] [CrossRef]
- Dong, Y.; Li, H.; Liu, J.; Zhang, J.; Shi, X.; Shi, Y.; Li, C.; Liu, Z.; Li, T.; Jiang, L. Asymmetrical [1]benzothieno[3,2-b][1]benzothiophene (BTBT) derivatives for organic thin-film and single-crystal transistors. Org. Electron. 2020, 77, 105537. [Google Scholar] [CrossRef]
- Roche, G.H.; Tsai, Y.T.; Clevers, S.; Thuau, D.; Castet, F.; Geerts, Y.H.; Moreau, J.J.E.; Wantz, G.; Dautel, O.J. The role of H-bonds in the solid state organization of [1]benzothieno[3,2-b][1]benzothiophene (BTBT) structures: Bis(hydroxy-hexyl)-BTBT, as a functional derivative offering efficient air stable organic field effect transistors (OFETs). J. Mater. Chem. C 2016, 4, 6742–6749. [Google Scholar] [CrossRef]
- Trul, A.A.; Sizov, A.S.; Chekusova, V.P.; Borshchev, O.V.; Agina, E.V.; Shcherbina, M.A.; Bakirov, A.V.; Chvalun, S.N.; Ponomarenko, S.A. Organosilicon dimer of BTBT as a perspective semiconductor material for toxic gas detection with monolayer organic field-effect transistors. J. Mater. Chem. C 2018, 6, 9649–9659. [Google Scholar] [CrossRef]
- Zhao, W.; Jie, J.; Wei, Q.; Lu, Z.; Jia, R.; Deng, W.; Zhang, X.; Zhang, X. A Facile method for the growth of organic semiconductor single crystal arrays on polymer dielectric toward flexible field-effect transistors. Adv. Funct. Mater. 2019, 29, 1902494. [Google Scholar] [CrossRef]
- Iino, H.; Usui, T.; Hanna, J. Liquid crystals for organic thin-film transistors. Nat. Commun. 2015, 6, 6828. [Google Scholar] [CrossRef] [PubMed]
- Dohr, M.; Ehmann, H.M.A.; Jones, A.O.F.; Salzmann, I.; Shen, Q.; Teichert, C.; Ruzie, C.; Schweicher, G.; Geerts, Y.H.; Resel, R.; et al. Reversibility of temperature driven discrete layer-by-layer formation of dioctyl-benzothieno-benzothiophene films. Soft. Matter. 2017, 13, 2322–2329. [Google Scholar] [CrossRef] [PubMed]
- Iino, H.; Hanna, J.I. Availability of liquid crystallinity in solution processing for polycrystalline thin films. Adv. Mater. 2011, 23, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- He, Y.W.; Sezen, M.; Zhang, D.W.; Li, A.Y.; Yan, L.J.; Yu, H.T.; He, C.; Goto, O.; Loo, Y.L.; Meng, H. High performance OTFTs fabricated using a calamitic liquid crystalline material of 2-(4-dodecyl phenyl)[1]benzothieno[3,2-b][1]benzothiophene. Adv. Electron. Mater. 2016, 2, 1600179. [Google Scholar] [CrossRef]
- Gbabode, G.; Dohr, M.; Niebel, C.; Balandier, J.Y.; Ruzie, C.; Negrier, P.; Mondieig, D.; Geerts, Y.H.; Resel, R.; Sferrazza, M. X-ray Structural Investigation of Nonsymmetrically and Symmetrically Alkylated [1]benzothieno[3,2-b][1]benzothiophene Derivatives in Bulk and Thin Films. ACS Appl. Mater. Inter. 2014, 6, 13413–13421. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.; Tews, H.; Legros, R. Oxygen Diffusion in ZnTe During Laser Annealing. J. Cryst. 1982, 59, 293–296. [Google Scholar] [CrossRef]
- Gedda, M.; Yengel, E.; Faber, H.; Paulus, F.; Kress, J.A.; Tang, M.C.; Zhang, S.; Hacker, C.A.; Kumar, P.; Naphade, D.R.; et al. Ruddlesden-popper-phase hybrid halide perovskite/small-molecule organic blend memory transistors. Adv. Mater. 2021, 33, 2003137. [Google Scholar] [CrossRef]
| T/°C | μsat [cm2 V−1 s−1] | Ion/Ioff | Vth [V] | |
|---|---|---|---|---|
| Bare | 100 | 1.08 * | 2.34 × 105 | −23.13 |
| OTS | 100 | 2.07 ** | 3.28 × 106 | −19.31 |
| UVO-OTS | 100 | 2.31 *** | 4.43 × 106 | −18.81 |
| Materials | Preparation Method | μsat [cm2 V−1 s−1] |
|---|---|---|
| Ph-BTBT-10 [53] | spin coating | 2.27 |
| TBTBT1 [54] | thermal evaporation | 0.24 |
| bis(hydroxy-hexyl)-BTBT [55] | thermal evaporation | 0.17 |
| D2-Und-BTBT-Hex [56] | spin coating | 0.07 |
| BTBT single-crystals [51] | physical vapor transport deposition | 0.032 |
| C8-BTBT [57] | thermal evaporation | 2.25 |
| Ph-BTBT-12 | thermal evaporation | 4.80 |
| μsat [cm2 V−1 s−1] | Ion/Ioff | Vth [V] | |
|---|---|---|---|
| Room temperature | 0.13 | 2.34 × 105 | −27.3 |
| 100 °C annealing | 2.08 | 3.28 × 106 | −21.2 |
| Laser annealing | 2.80 | 4.43 × 106 | −4.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.; Liu, F.; Bao, J.; Li, X.; Wu, W. High-Performance Organic Field-Effect Transistors of Liquid Crystalline Organic Semiconductor by Laser Mapping Annealing. Materials 2024, 17, 1395. https://doi.org/10.3390/ma17061395
Huang L, Liu F, Bao J, Li X, Wu W. High-Performance Organic Field-Effect Transistors of Liquid Crystalline Organic Semiconductor by Laser Mapping Annealing. Materials. 2024; 17(6):1395. https://doi.org/10.3390/ma17061395
Chicago/Turabian StyleHuang, Luying, Fenghua Liu, Jiachen Bao, Xiaoman Li, and Weiping Wu. 2024. "High-Performance Organic Field-Effect Transistors of Liquid Crystalline Organic Semiconductor by Laser Mapping Annealing" Materials 17, no. 6: 1395. https://doi.org/10.3390/ma17061395





