Investigation of Multi-Factor Stress Corrosion Cracking Failure of Safe-End Feedwater Lines of Submarine Power System
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Solution
2.2. Electrochemical and Immersion Testing
2.3. Fluent Model Simulation
2.4. Residual Stress Testing
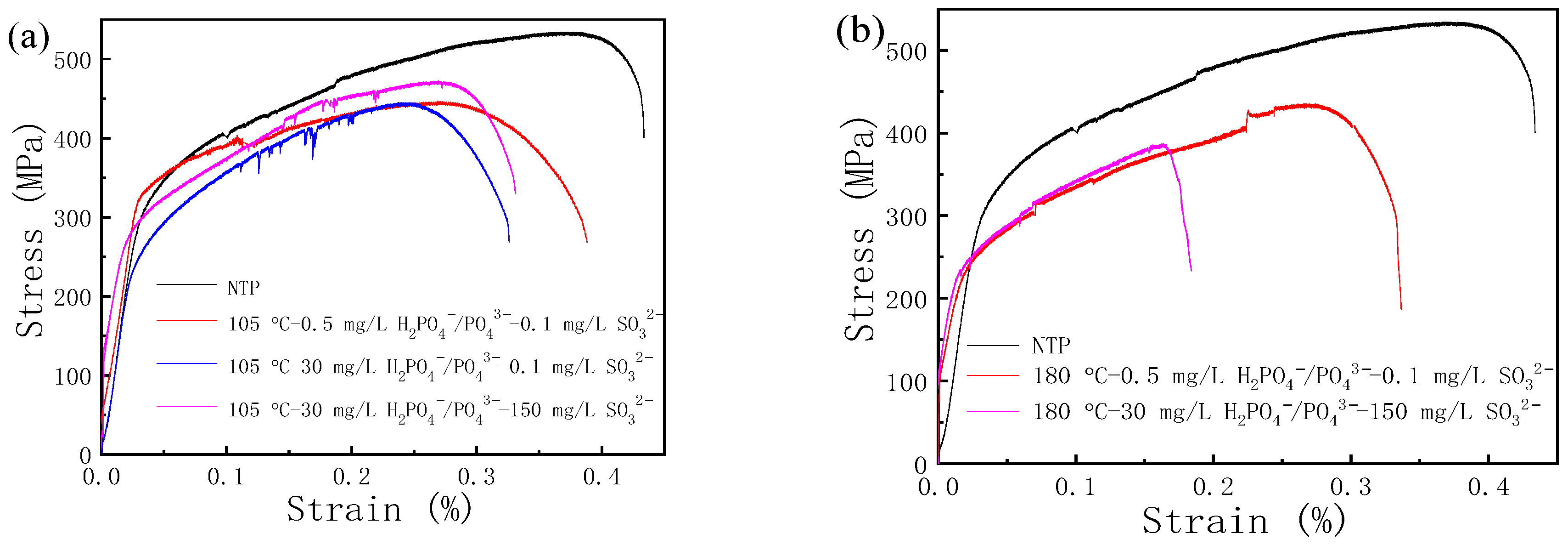
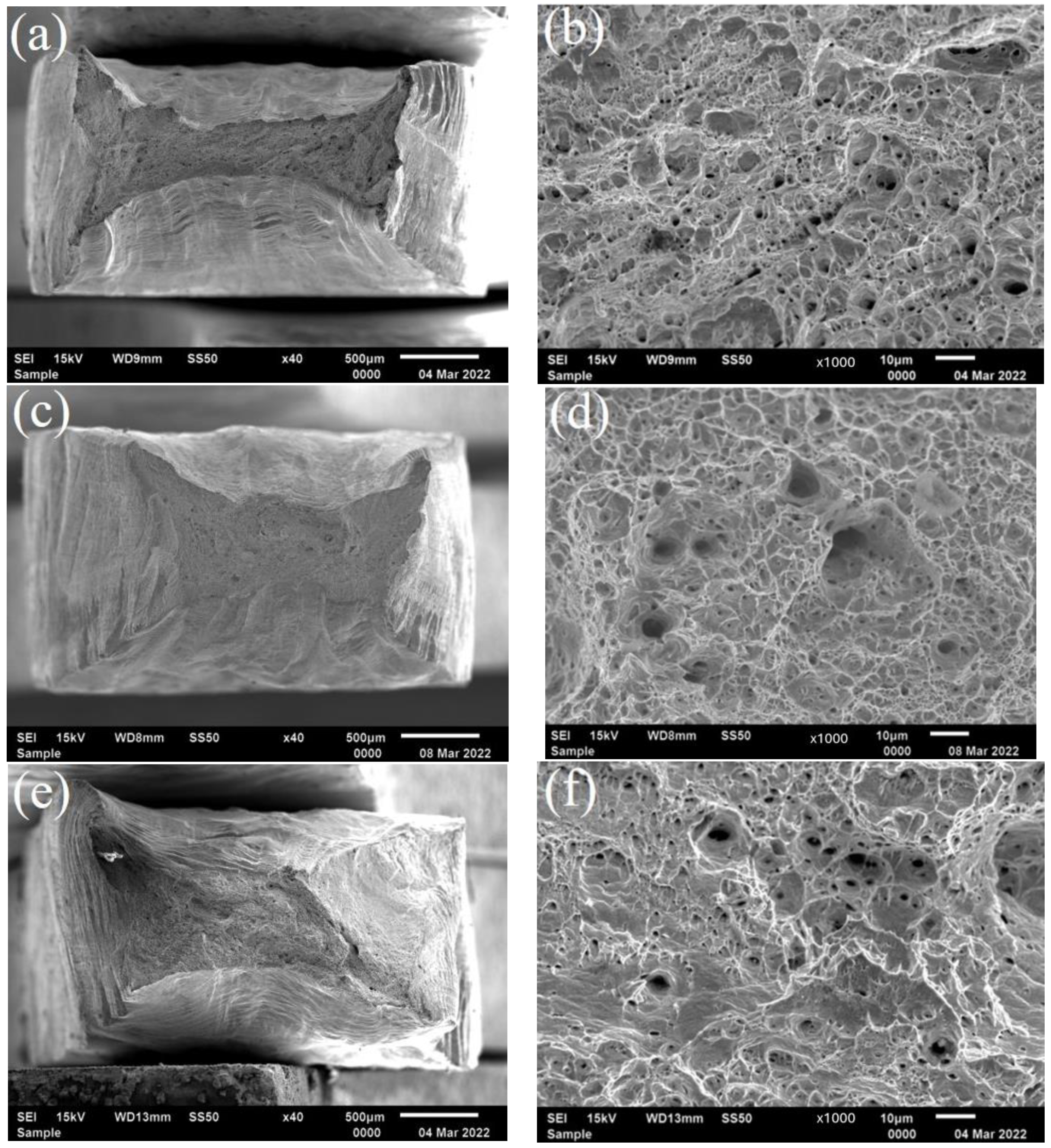
2.5. Slow Strain Rate Test
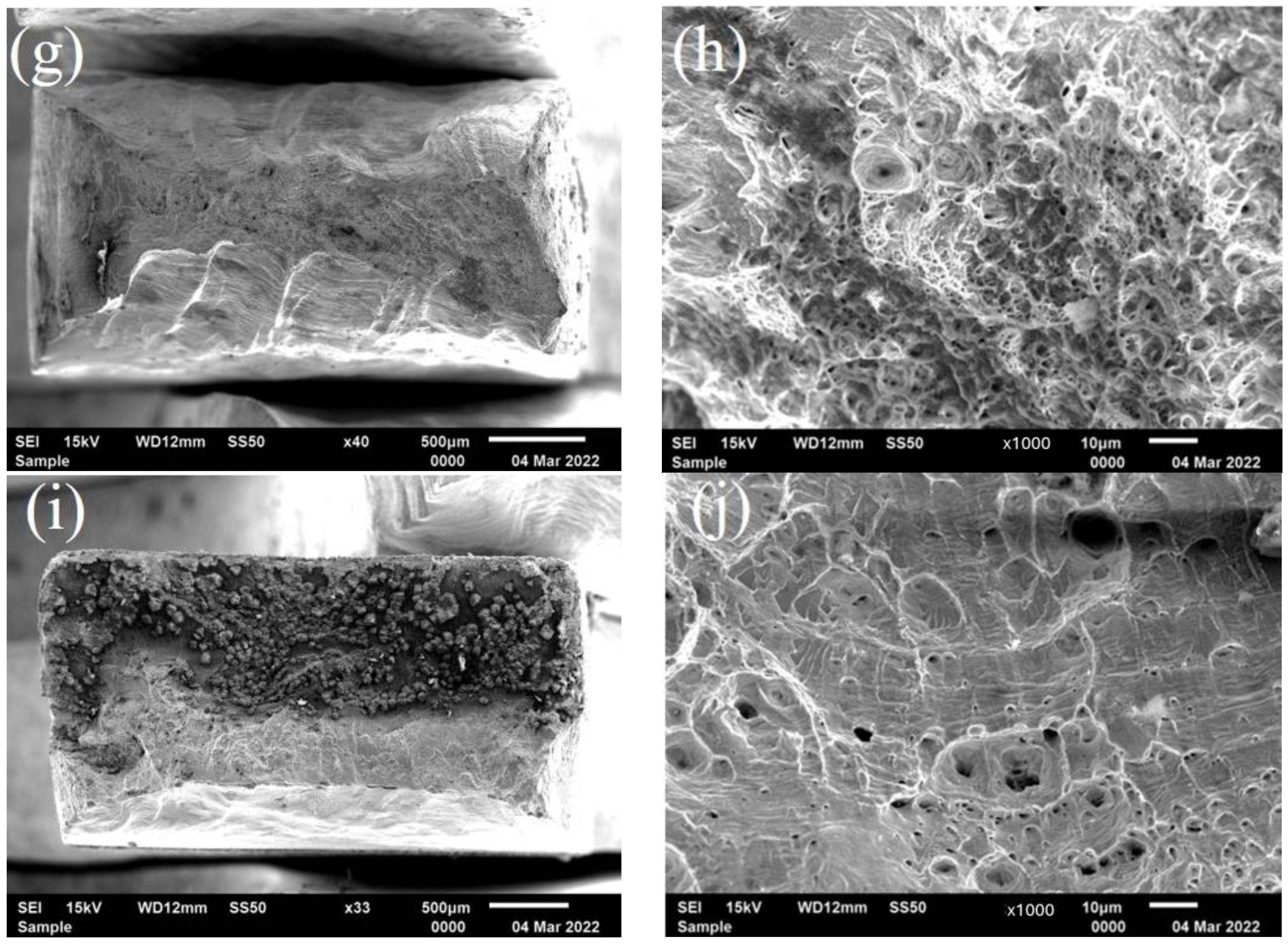
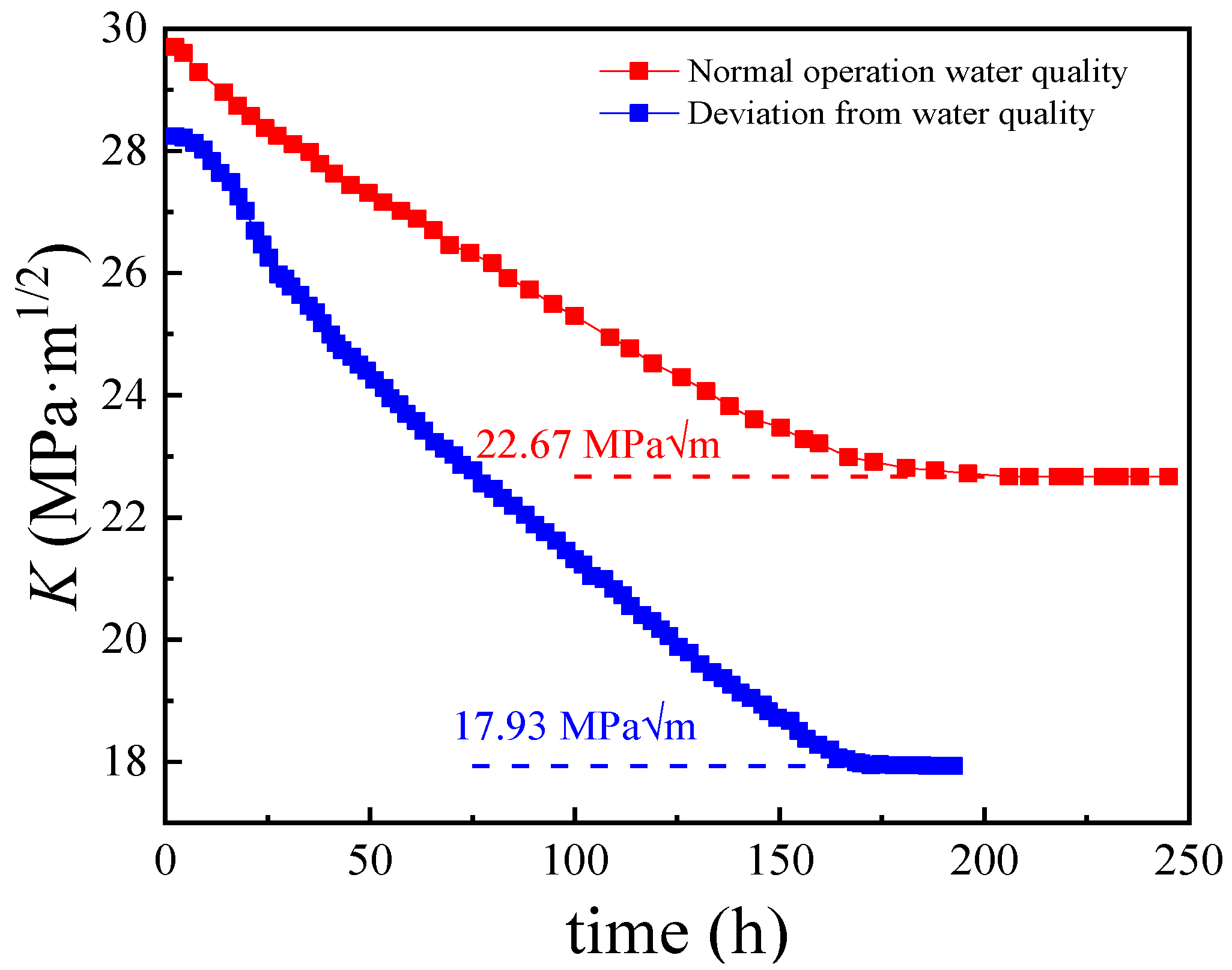
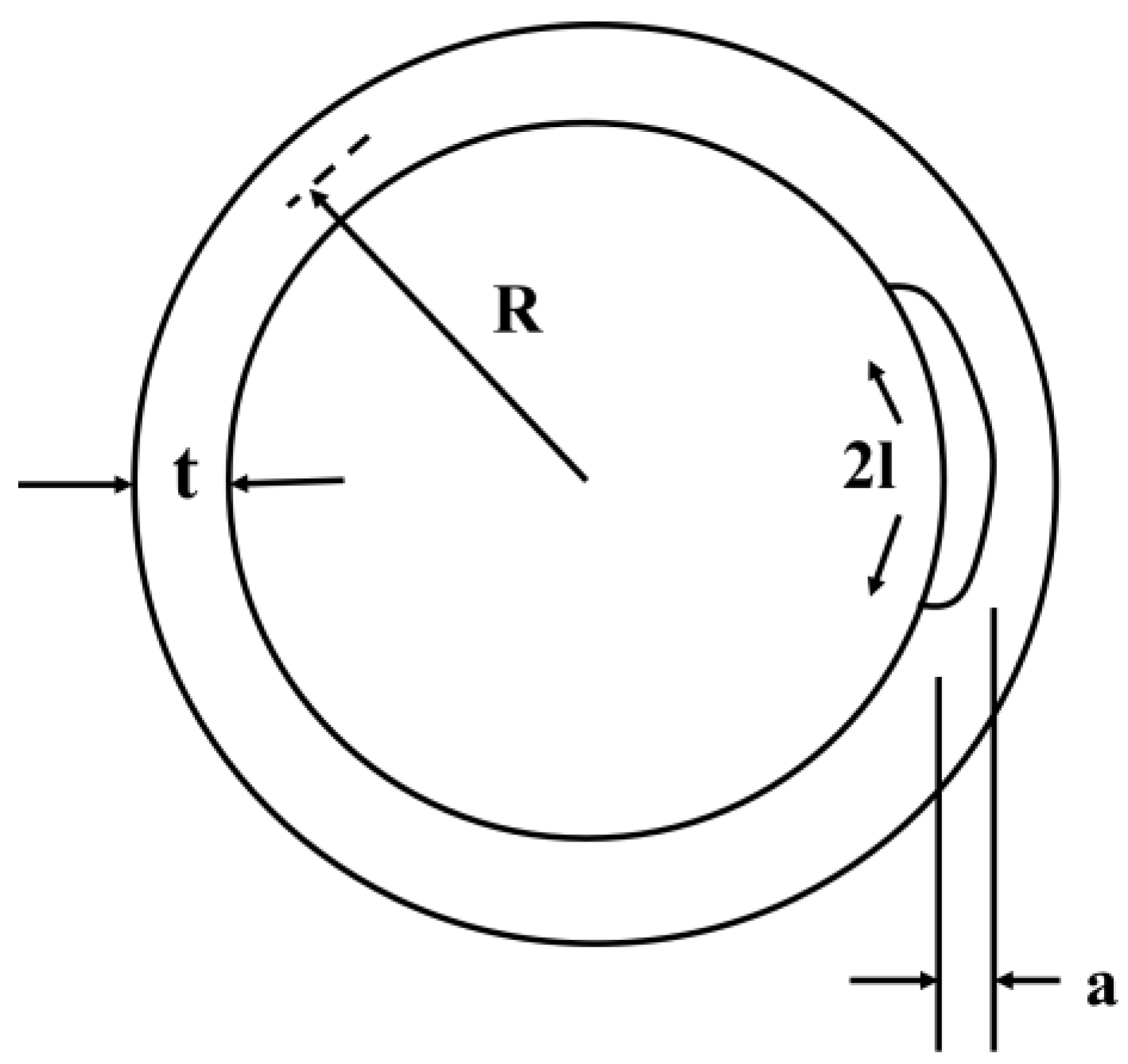
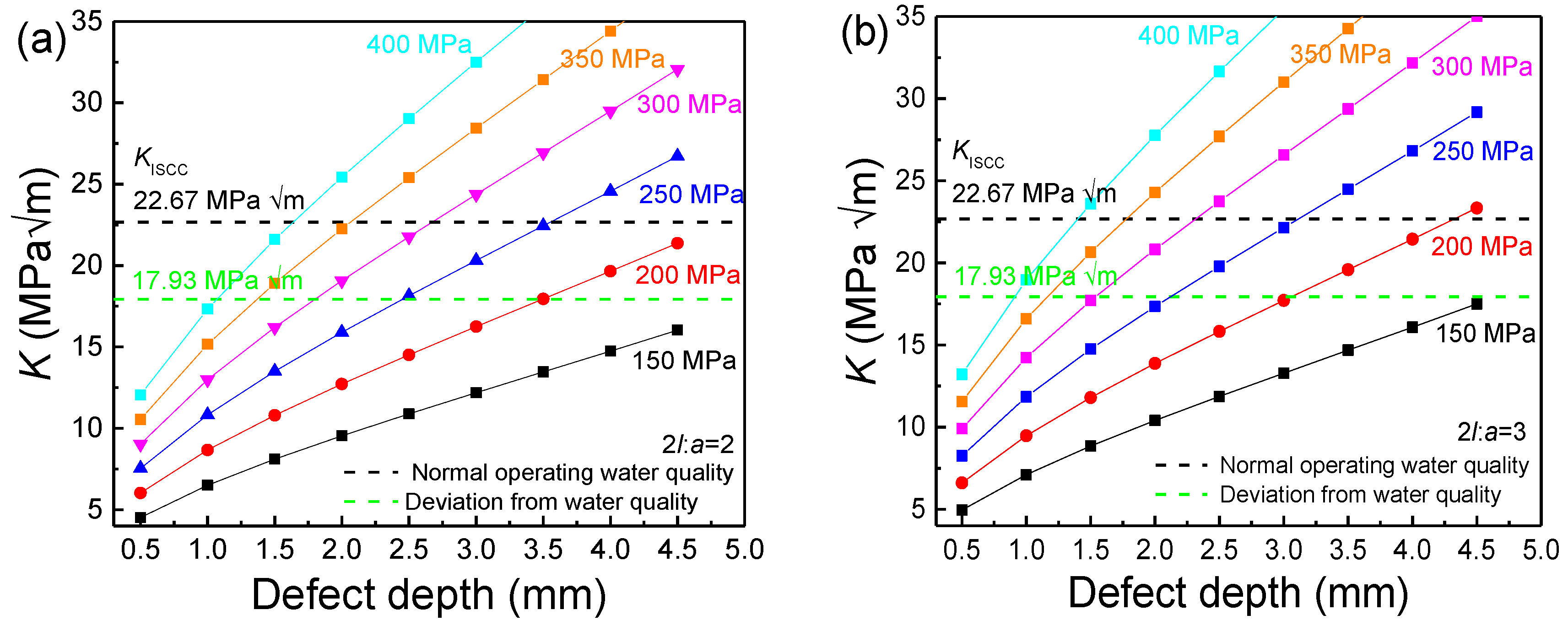
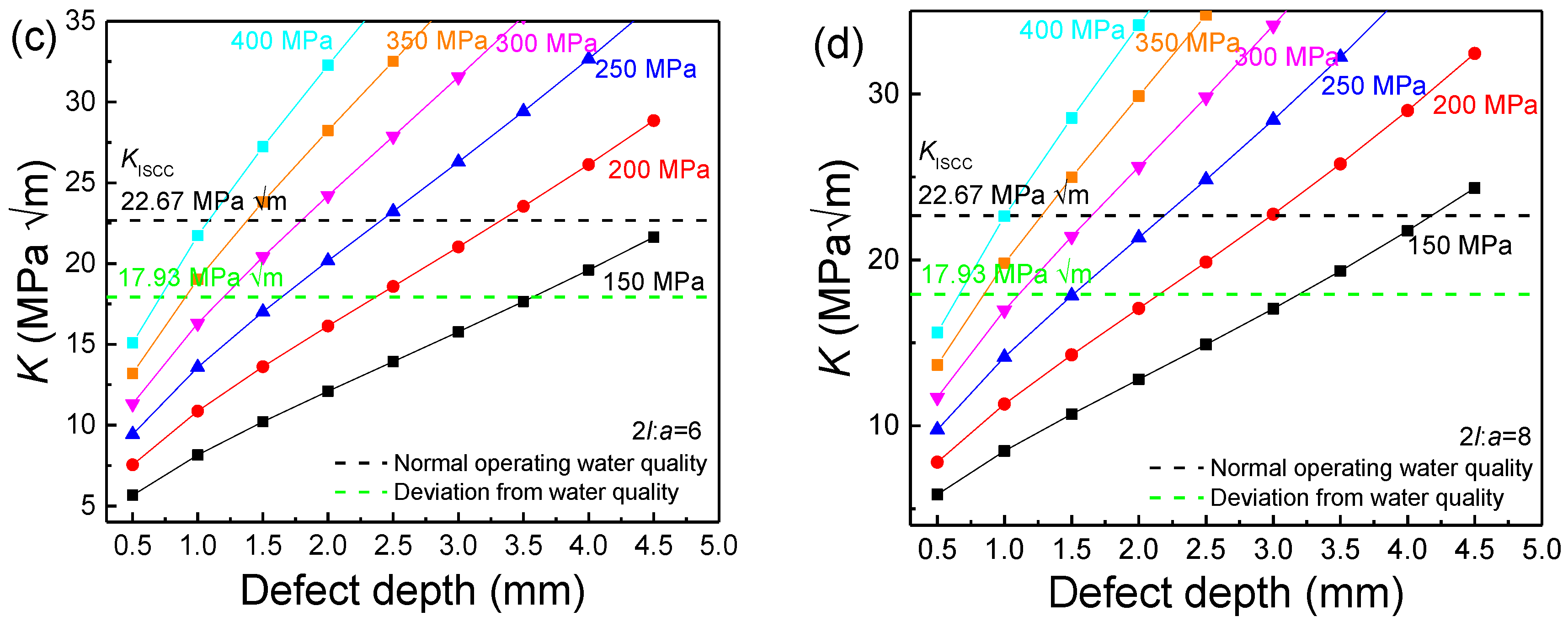
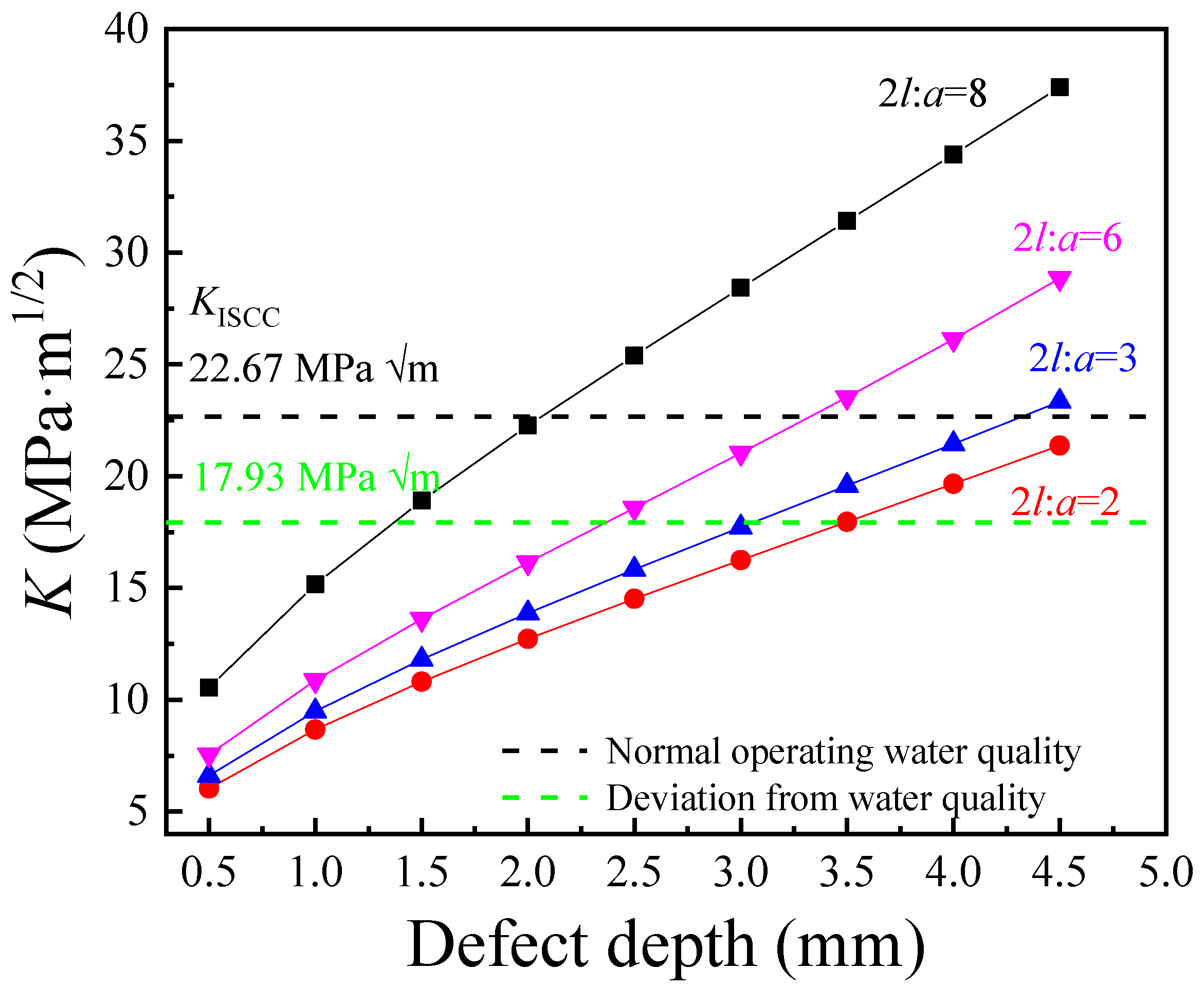
2.6. Critical Stress Intensity Factor for Stress Corrosion Cracking (KISCC)
3. Result and Discussion: From Normal to Deviation Water Quality
3.1. The Galvanic Corrosion of 20 Steel and 316L
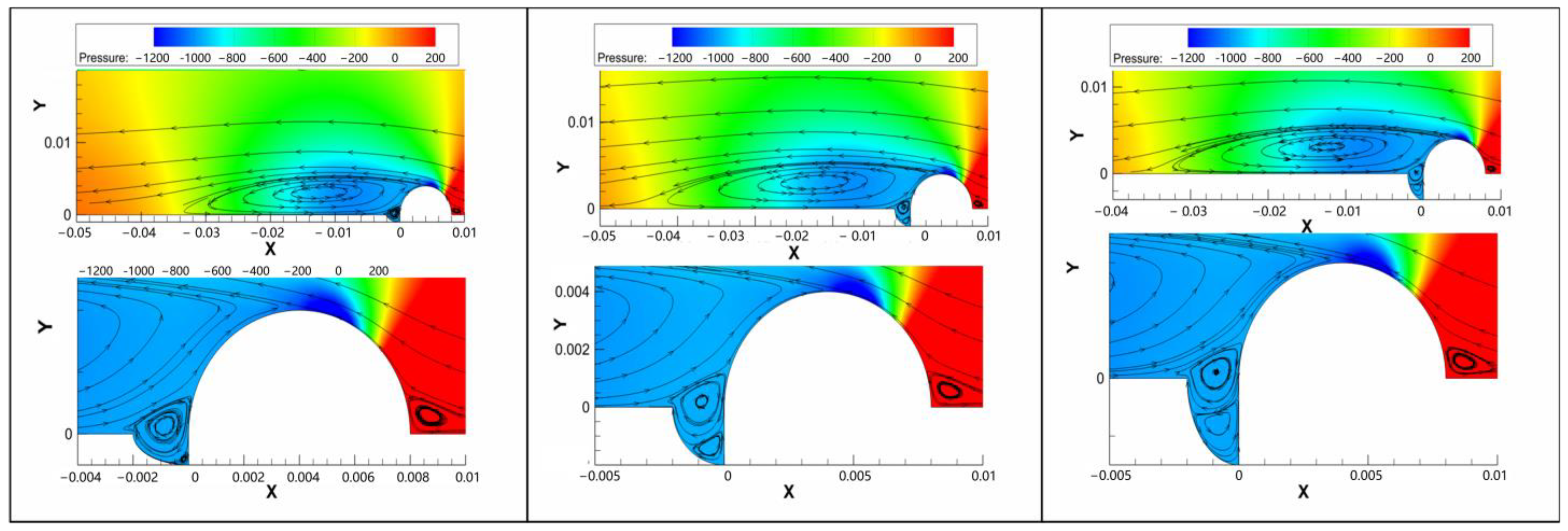
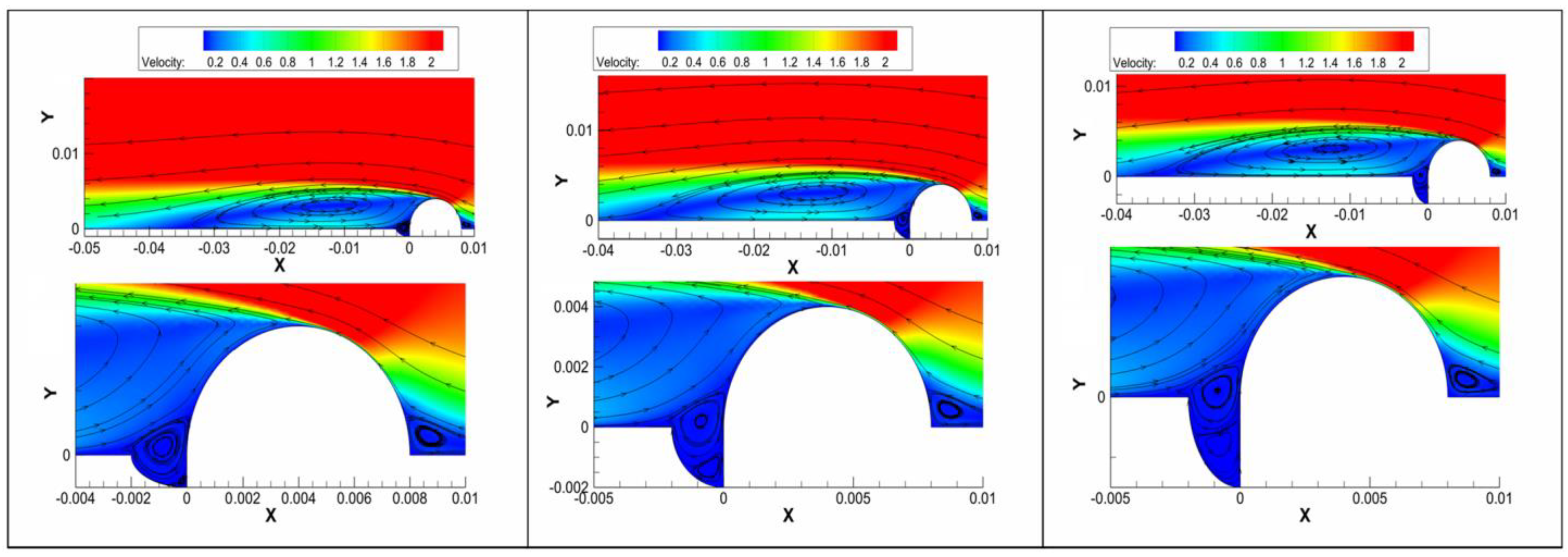
3.2. Flow Results of the Safe-End Feedwater Lines
3.3. Residual Stress and SCC Susceptibility
4. Discussion
4.1. Effect of Vortex Flow
4.2. The Stress Corrosion Cracking Process
5. Conclusions
- (1)
- The failure of safe-end feedwater lines was controlled by the combined action of galvanic corrosion, residual stress, and flow. When the 20 steel was welded with 316L stainless steel, the 20 steel with low potential existed along with the galvanic corrosion. The obvious pitting corrosion and cracks could be observed on the 20 steel side of the weld joint. Combined with the field data, this was consistent with the hypotheses, and galvanic corrosion was indeed the critical failure factor.
- (2)
- The vortex flow was detected around the welding bump and within the pits. The H+ ion concentration was shifted toward the upstream portion of the pitting. Then, the growth of the pits was accelerated in the horizontal direction, forming shallow-shaped pitting. Finally, the flow velocity promoted the pitting growth rate in both the vertical and horizontal directions. The pitting area provided stress concentration under residual stress conditions, which was consistent with the shape of the pitting observed. Therefore, it can be assumed that vortex flow existed in the safe-end feedwater lines.
- (3)
- The increase in the width–depth ratio (2l/a) enhanced the stress concentration at the defect, with K exceeding KISCC at a smaller defect depth and the quick transformation of pitting into a crack. The deviation water quality significantly reduced the KISCC value of the weld joint. The stress corrosion cracking was initiated from the localized corrosion area and propagated along with the failures of the safe-end feedwater lines.
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibrahim, A.O.; Ighodaro, O.O.; Fasogbon, S.K. Failure investigation of the tube of a dual fired steam boiler in a western nigerian food and beverage manufacturing plant. Eng. Fail. Anal. 2022, 10, 6906. [Google Scholar] [CrossRef]
- Ke, X.; Zhang, Y.; Liu, X. Development of biomass-fired circulating fluidized bed boiler with high steam parameters based on theoretical analysis and industrial practices. J. Energy Inst. 2022, 10, 011. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Yang, C.; Pehg, F. Analysis of boiler water treatment for ships and offshore platforms. Sci. Technol. Inf. 2013, 14, 58–59. [Google Scholar] [CrossRef]
- Liu, S.; Wu, H.; Zhao, Q. Corrosion failure analysis of the heat exchanger in a hot water heating boiler. Eng. Fail. Anal. 2022, 10, 6847. [Google Scholar] [CrossRef]
- Sathish, T.; Mohanavel, V.; Afzal, A. Advancement of steam generation process in water tube boiler using Taguchi design of experiments. Case Stud. Therm. Eng. 2021, 10, 1247. [Google Scholar] [CrossRef]
- Khedr, M.; Abd, W.; Newishy, M. Metallurgical analysis of ASME SA213 T12 boiler vertical water-wall tubes failure. Eng. Fail. Anal. 2023, 10, 7016. [Google Scholar] [CrossRef]
- Da Silveira, R.M.S.; Guimarães, A.V.; Oliveira, G. Failure of an ASTM A213 T12 steel tube of a circulating fluidized bed boiler. Eng. Fail. Anal. 2023, 10, 7188. [Google Scholar] [CrossRef]
- Suwarno, S.; I’jazurrohman, A.J.; Yudanto, F.D. Failure analysis of waste heat boiler tubing caused by a high local heat flux. Eng. Fail. Anal. 2022, 10, 6147. [Google Scholar] [CrossRef]
- Wasim, M.; Djukic, M.B.B. External corrosion of oil and gas pipelines: A review of failure mechanisms and predictive preventions. J. Nat. Gas Sci. Eng. 2022, 100, 104467. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of Metallic Biomaterials: A Review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Kadowaki, M.; Katayama, H.; Yamamoto, M. Corrosion behavior of AA6016/SM490 galvanic couple in NaCl-containing droplets: Effect of Fe species on galvanic corrosion acceleration. Corros. Sci. 2023, 218, 111190. [Google Scholar] [CrossRef]
- Fattah-Alhosseini, A.; Vafaeian, S. Comparison of electrochemical behavior between coarse-grained and fine-grained AISI 430 ferritic stainless steel by Mott-Schottky analysis and EIS measurements. J. Alloys Compd. 2015, 639, 301–307. [Google Scholar] [CrossRef]
- Hu, Z.; Meng, Y.; Ma, X. Experimental and theoretical studies of benzothiazole derivatives as corrosion inhibitors for carbon steel in 1 M HCl. Corros. Sci. 2016, 112, 563–575. [Google Scholar] [CrossRef]
- Zhang, G.A.; Zeng, L.; Huang, H.L. A study of flow accelerated corrosion at elbow of carbon steel pipeline by array electrode and computational fluid dynamics simulation. Corros. Sci. 2013, 77, 334–341. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Kamali, R.; Sotoudeh, F. Large eddy simulation of pseudo shock structure in a convergent-long divergent duct. Comput. Math. Appl. 2021, 81, 823–837. [Google Scholar] [CrossRef]
- Ochoa, N.; Vega, C.; Pébère, N. CO2 corrosion resistance of carbon steel in relation with microstructure changes. Mater. Chem. Phys. 2015, 156, 198–205. [Google Scholar] [CrossRef]
- Fushimi, K.; Naganuma, A.; Azumi, K. Current distribution during galvanic corrosion of carbon steel welded with type-309 stainless steel in NaCl solution. Corros. Sci. 2008, 50, 903–911. [Google Scholar] [CrossRef]
- Ardy, H.; Putra, Y.P.; Anggoro, A.D. Failure analysis of primary waste heat boiler tube in ammonia plant. Heliyon 2021, 7, e06151. [Google Scholar] [CrossRef]
- Truschner, M.; Janda, A.; Bodner, S.C. Effect of cold deformation on the stress corrosion cracking resistance of a high-strength stainless steel. J. Mater. Sci. 2022, 57, 20447–20461. [Google Scholar] [CrossRef]
- Tan, Z.W.; Zhang, D.L.; Yang, L.Y.; Wang, Z.; Cheng, F.; Zhang, M.; Jin, Y.; Zhu, S. Development mechanism of local corrosion pit in X80 pipeline steel under flow conditions. Tribol. Int. 2020, 146, 145–156. [Google Scholar] [CrossRef]
- Pang, L.; Wang, Z.B.; Zheng, Y.G.; Lai, X.; Han, X. On the localized corrosion of carbon steel induced by the in-situ local damage of porous corrosion products. J. Mater. Sci. Technol. 2020, 54, 95–104. [Google Scholar] [CrossRef]
- Schoell, R.; Xi, L.; Zhao, Y.C. Mechanism of chlorine-induced stress corrosion cracking of two 304 SS heats in simulated marine environment through in situ X-ray tomography and diffraction: Role of deformation induced martensite and crack branching. Mater. Charact. 2022, 190, 112020. [Google Scholar] [CrossRef]
- Seok, K.Y.; Lee, K.; Man, S.D. Factors Affecting Stress Corrosion Cracking Susceptibility of Alloy 600 MA Steam Generator Tubes. Corros. Sci. Technol.-K. 2021, 20, 22–25. [Google Scholar] [CrossRef]
- Yoo, Y.R.; Choi, S.H.; Kim, Y.S. Effect of Laser Shock Peening on the Stress Corrosion Cracking of 304L Stainless Steel. Metals 2023, 12, 516. [Google Scholar] [CrossRef]
- Ai, L.; Soltangharaei, V.; Greer, B. Structural health monitoring of stainless-steel nuclear fuel storage canister using acoustic emission. Dev. Built Environ. 2024, 17, 100294. [Google Scholar] [CrossRef]
- Liao, J.; Wang, H.; Wu, J. Addition of niobium in Fe-13Cr-4.5Al-2Mo alloy used as ATF cladding: Effect on high temperature water corrosion and in-situ electrochemistry. Mater. Des. 2022, 220, 110854. [Google Scholar] [CrossRef]
- Galakhova, A.; Prattes, K.; Mori, G. High-temperature high-pressure SCC testing of corrosion-resistant alloys. Mater. Corros. 2021, 72, 1831–1842. [Google Scholar] [CrossRef]
- Vecchiato, L.; Campagnolo, A.; Meneghetti, G. Numerical calibration and experimental validation of the direct current potential drop (DCPD) method for fracture mechanics fatigue testing of single-edge-crack round bars. Int. J. Fatigue 2021, 150, 106316. [Google Scholar] [CrossRef]
- Ba, F.; Li, K.; Xu, L.; Li, S. Analysis of residual stress calculation equations in national standard GB/T 7704-2017. Nondestruct. Test. 2020, 42, 4–11. [Google Scholar] [CrossRef]
- Meneghetti, G.; Vecchiato, L.; Campagnolo, A. Numerical calibration of the direct current potential drop (DCPD) method in fracture mechanics fatigue tests. Procedia Struct. Integr. 2020, 28, 1536–1550. [Google Scholar] [CrossRef]
- Sun, D.; Li, H.; Feng, H. Calibrating Johnson’s formula for applying DCPD method to an axial through-wall crack in a pipe. Eng. Fract. Mech. 2021, 242, 107461. [Google Scholar] [CrossRef]
- Shrestha, S.; Kannan, M.; Morscher, G.N. In-situ fatigue life analysis by modal acoustic emission, direct current potential drop and digital image correlation for steel. Int. J. Fatigue 2021, 142, 105924. [Google Scholar] [CrossRef]
- Lambourg, A.; Henaff, G.; Nadot, Y. Optimization of the DCPD technique for monitoring the crack propagation from notch root in localized plasticity. Int. J. Fatigue 2020, 130, 105228. [Google Scholar] [CrossRef]
- Li, Y.Y.; Wang, Z.Z.; Guo, X.P. Galvanic corrosion between N80 carbon steel and 13Cr stainless steel under supercritical CO2 conditions. Corros. Sci. 2019, 147, 260–272. [Google Scholar] [CrossRef]
- Refait, P.; Grolleau, A.M.; Jeannin, M. Localized corrosion of carbon steel in marine media: Galvanic coupling and heterogeneity of the corrosion product layer. Corros. Sci. 2016, 111, 583–595. [Google Scholar] [CrossRef]
- Liew, Y.; Örnek, C.; Pan, J. Towards understanding micro-galvanic activities in localised corrosion of AA2099 aluminium alloy. Electrochim. Acta 2021, 13, 9005. [Google Scholar] [CrossRef]
- Elias, A.L.P.; Koizumi, M.S.; Ortiz, E.L.; Rodrigues, J.F.Q.; Bortolozo, A.D. Corrosion behavior of an Al-Si casting and a sintered Al/Si composite immersed into biodiesel and blends. Fuel Process. Technol. 2020, 202, 106360. [Google Scholar] [CrossRef]
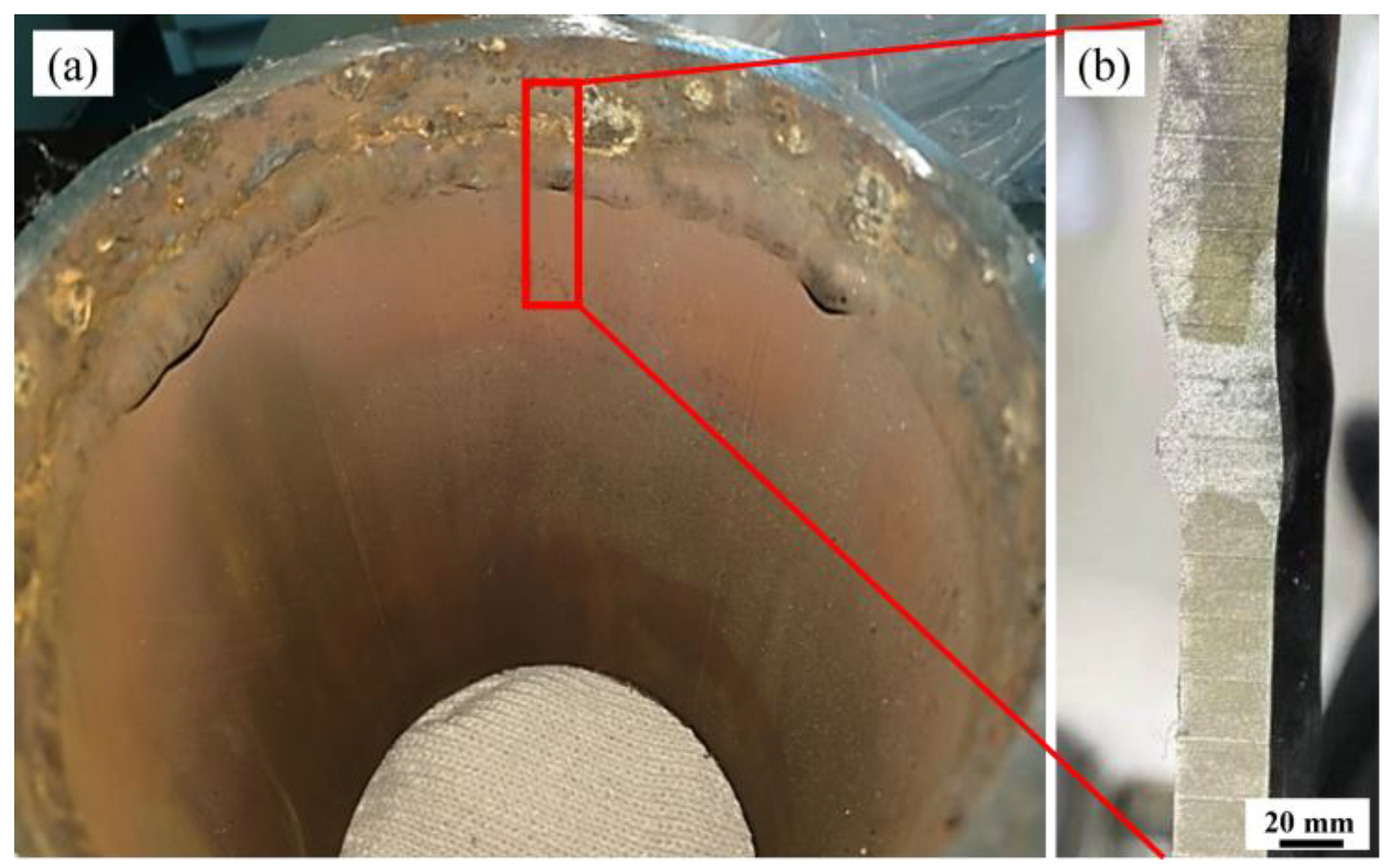
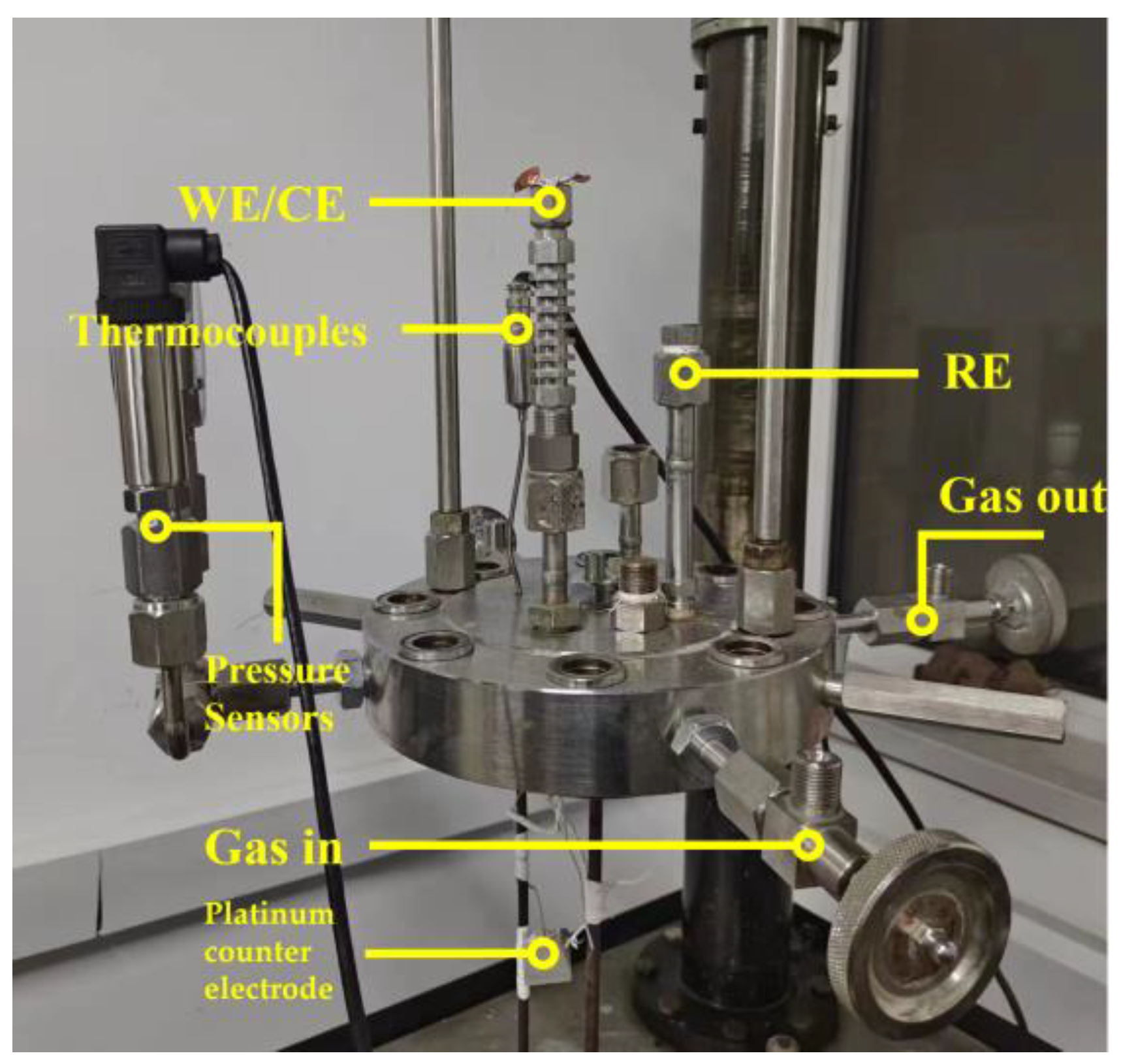
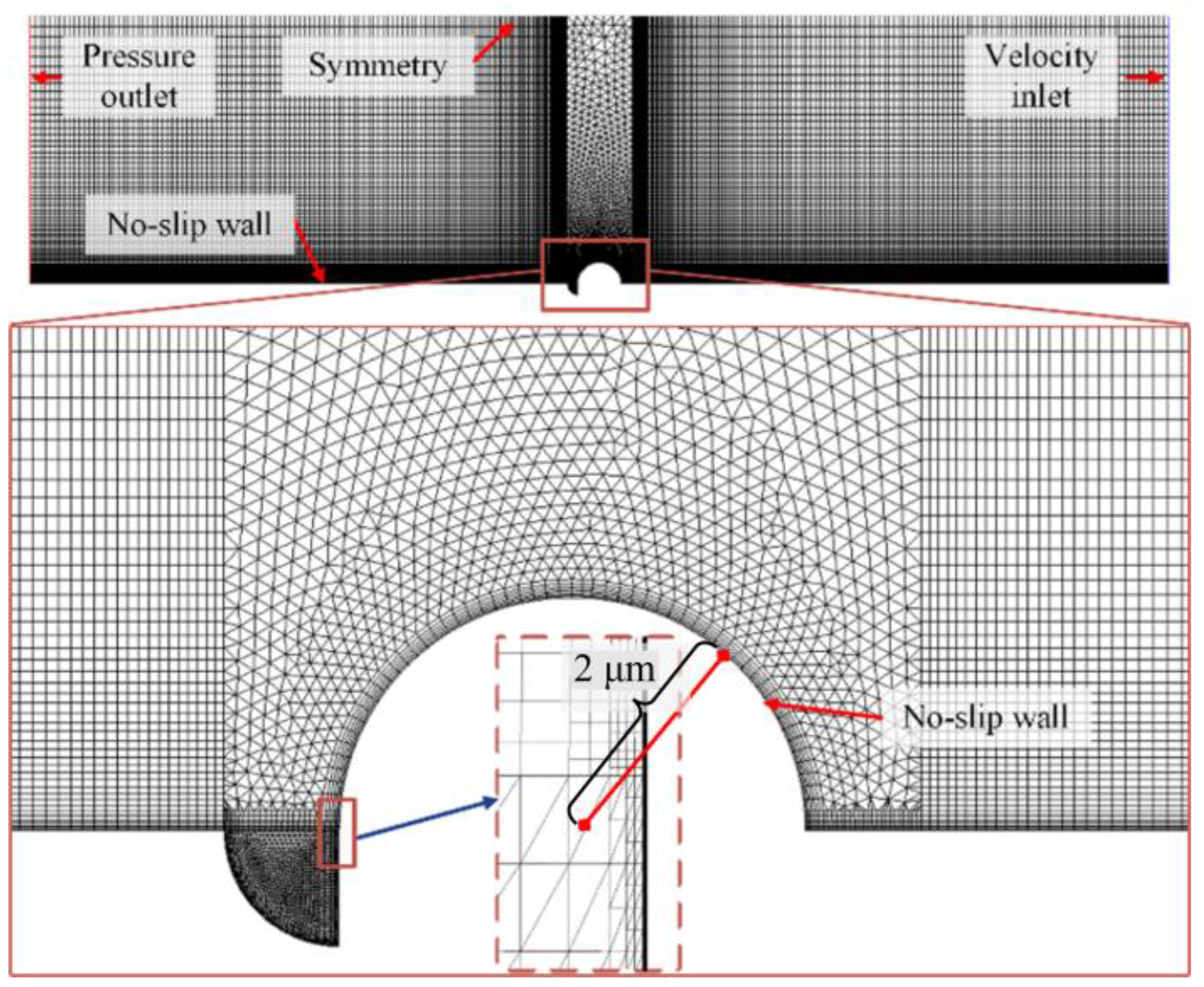
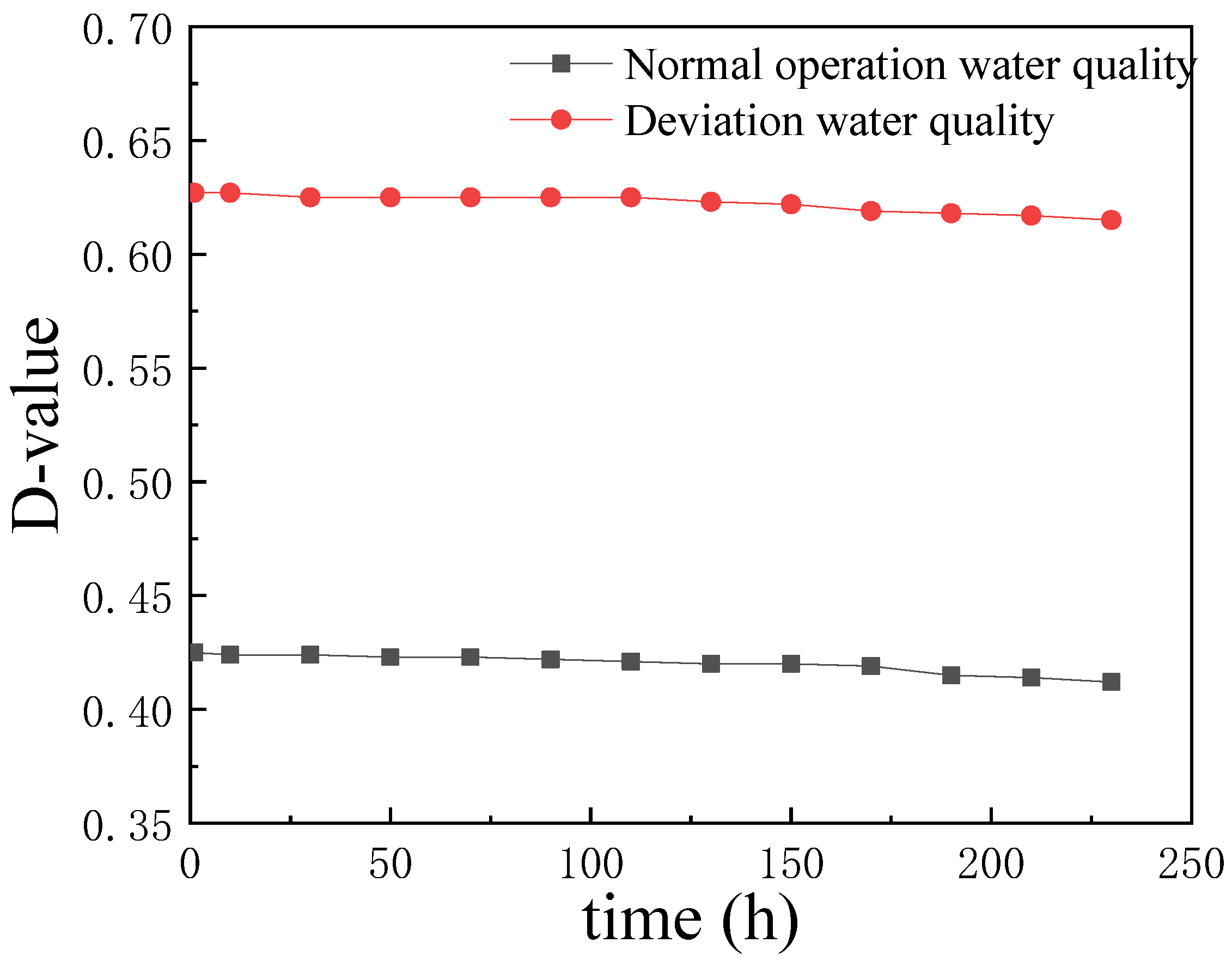
| Steel | C | Si | Mn | S | P | Cr | Ni | Cu | Fe |
|---|---|---|---|---|---|---|---|---|---|
| 20 | 0.220 | 0.260 | 0.570 | 0.019 | 0.098 | 0.019 | 0.006 | 0.006 | Bal. |
| 316L | 0.018 | 0.220 | 1.420 | 0.002 | 0.023 | 17.200 | 12.950 | 0.033 | Bal. |
| 316L welding wire | 0.022 | 0.420 | 1.890 | 0.002 | 0.002 | 19.120 | 12.620 | 0.340 | Bal. |
| Environmental Parameter | Water Quality | |
|---|---|---|
| Normal Operation Water Quality | Deviation Water Quality | |
| Oxygen content (mg/L) | ≤0.007 | ≤0.1 |
| pH (25 °C) | 7~8 | 9.6~10.3 |
| Cl− (mg/L) | ≤0.1 | ≤5 |
| Pressure (MPa) | 2.6~3.2 | 2.6~3.2 |
| Temperature (°C) | 105 | ~180 |
| Conductivity (μS/cm) | ~50 | ~1600 |
| H2PO4−/PO43− (mg/L) | 0.1 | 30 |
| SO32− (mg/L) | 0.5 | 150 |
| Conditions | Corrosion Rate (Mm/Yr) | |
|---|---|---|
| 20 Steel | 20 Steel at Weld Couple | |
| Normal operating water quality | 0.053 ± 0.012 | 0.061 ± 0.009 |
| Deviation water quality | 0.39 ± 0.02 | 0.48 ± 0.03 |
| Residual Stress (MPa) | ||
|---|---|---|
| Inner Surface | Outer Surface | |
| Sample 1 | 149.1 ± 11.7 | −126.7 ± 30.3 |
| Sample 2 | 156.9 ± 21.3 | −169.1 ± 45.3 |
| Sample 3 | 150.5 ± 17.9 | −159.1 ± 44.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, C.; Zheng, Z.; Qin, Z.; Xue, H. Investigation of Multi-Factor Stress Corrosion Cracking Failure of Safe-End Feedwater Lines of Submarine Power System. Materials 2024, 17, 1381. https://doi.org/10.3390/ma17061381
Ji C, Zheng Z, Qin Z, Xue H. Investigation of Multi-Factor Stress Corrosion Cracking Failure of Safe-End Feedwater Lines of Submarine Power System. Materials. 2024; 17(6):1381. https://doi.org/10.3390/ma17061381
Chicago/Turabian StyleJi, Chenlong, Zhongliang Zheng, Ziming Qin, and Hao Xue. 2024. "Investigation of Multi-Factor Stress Corrosion Cracking Failure of Safe-End Feedwater Lines of Submarine Power System" Materials 17, no. 6: 1381. https://doi.org/10.3390/ma17061381




