Multistage Porous Carbon Derived from Enzyme-Treated Waste Walnut Green Husk and Polyethylene Glycol for Phase Change Energy Storage
Abstract
1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Preparation of PC (Porous Carbon)
2.3. Preparation of PEG/PC SSPCM
2.4. Characterization Methods
3. Results and Discussion
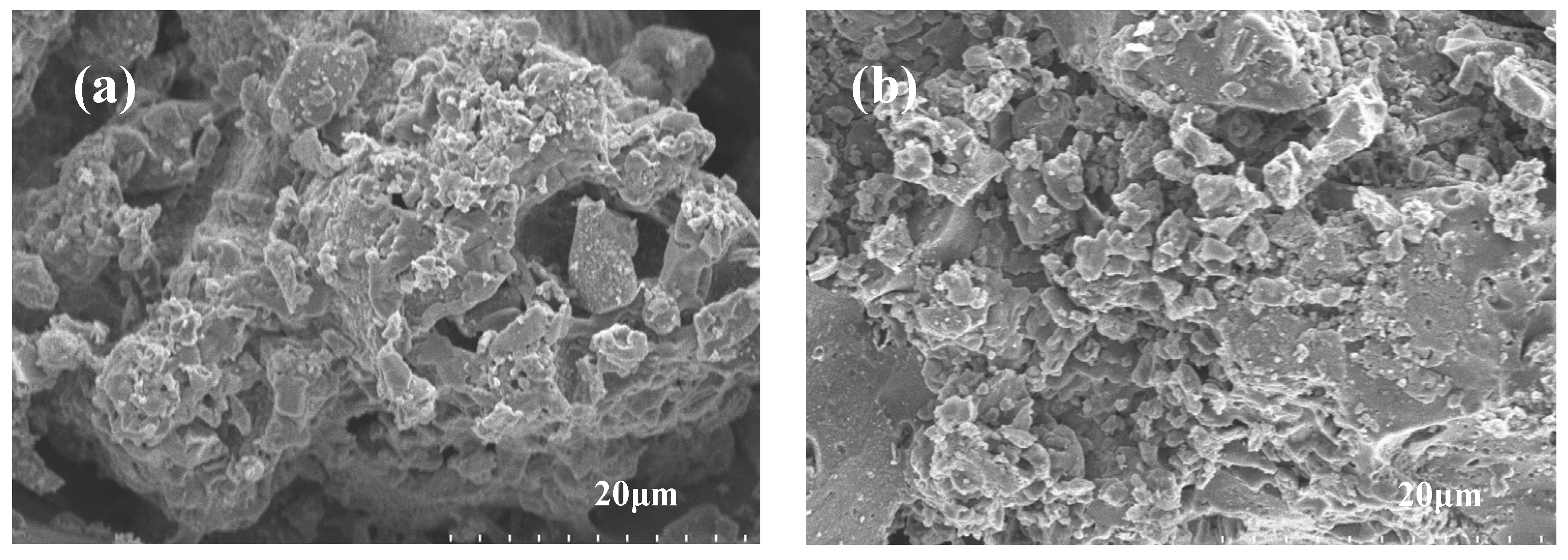
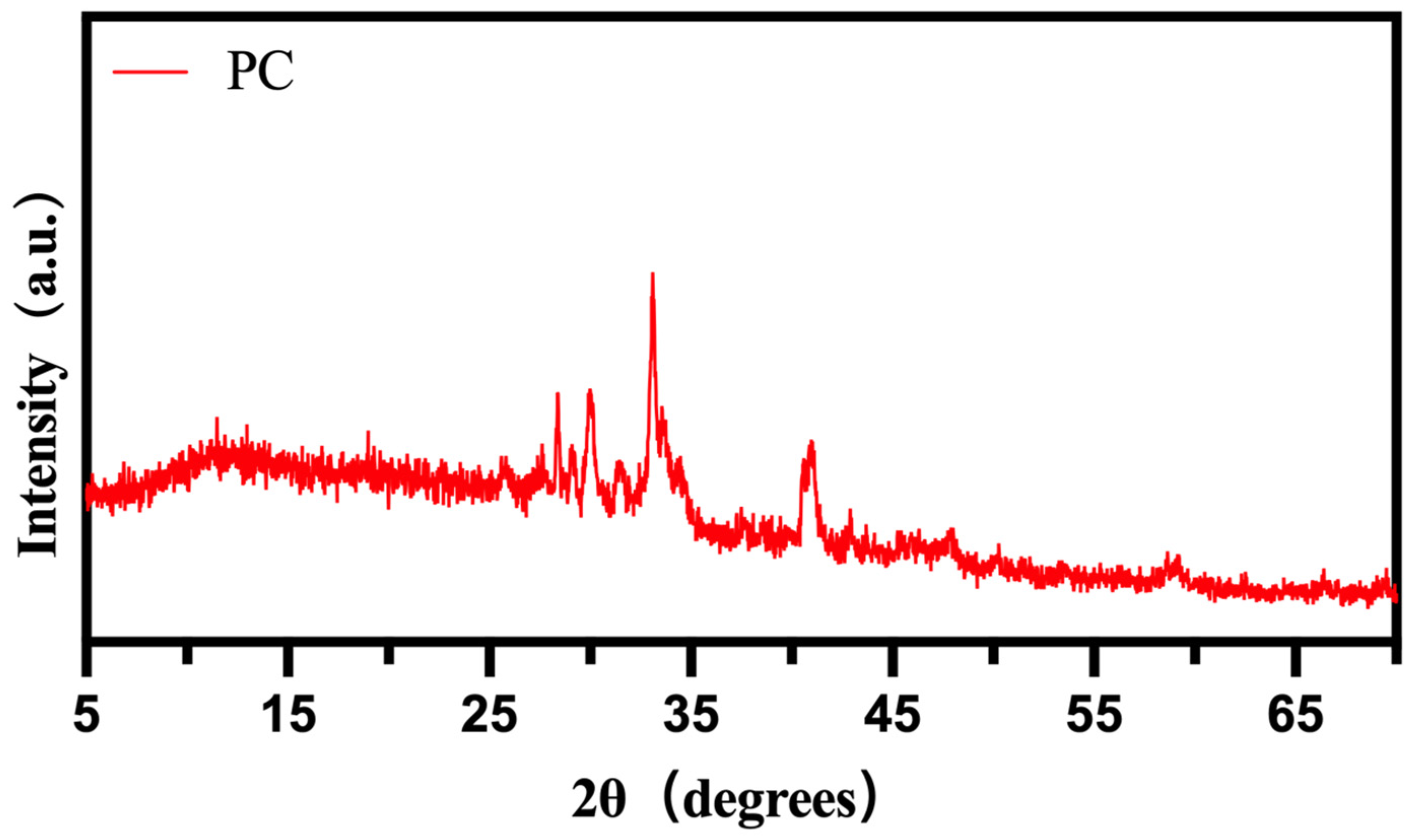
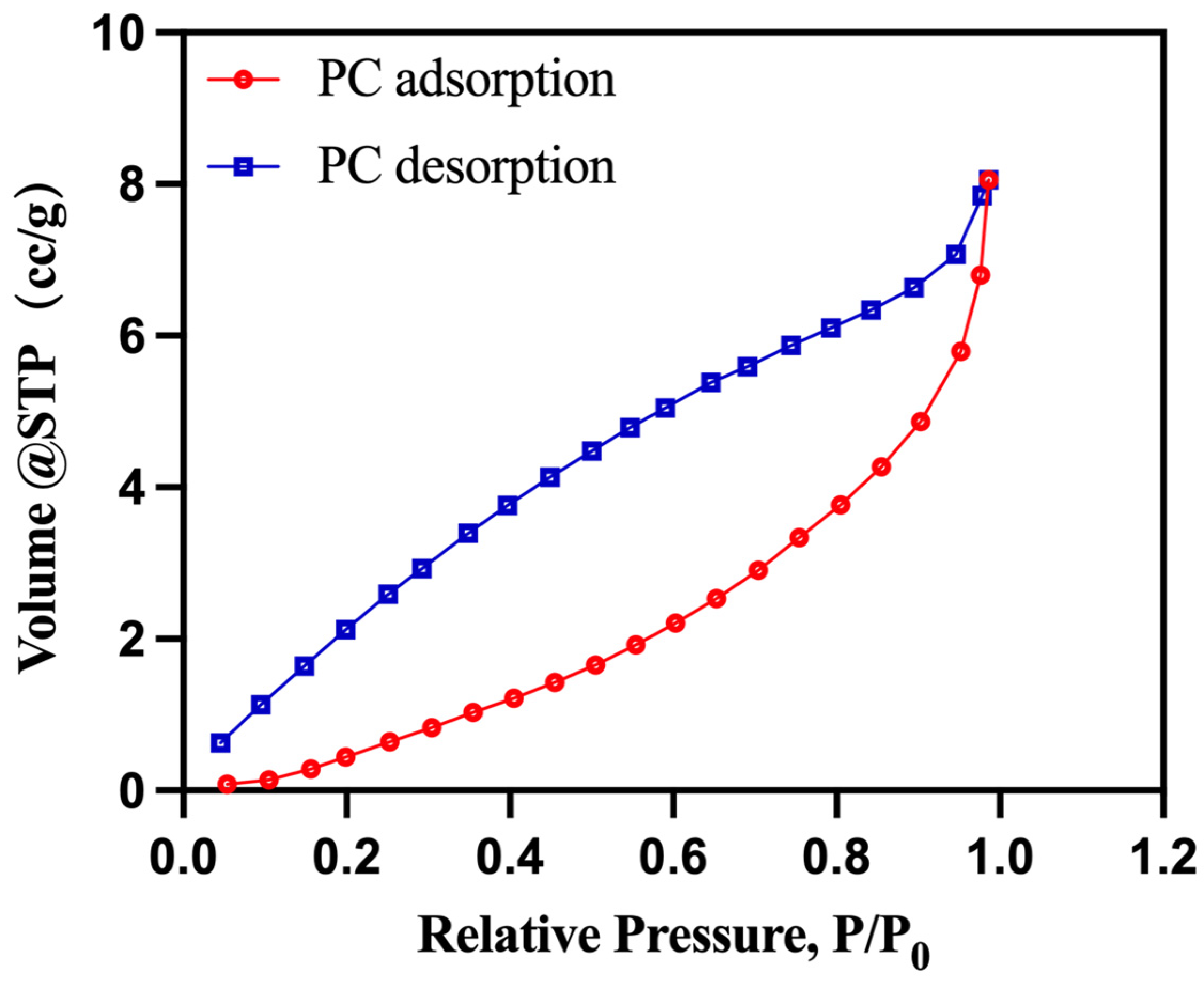
3.1. Structure of PEG/PC SSPCM
3.2. Thermal Energy Storage Properties of PEG/PC SSPCM
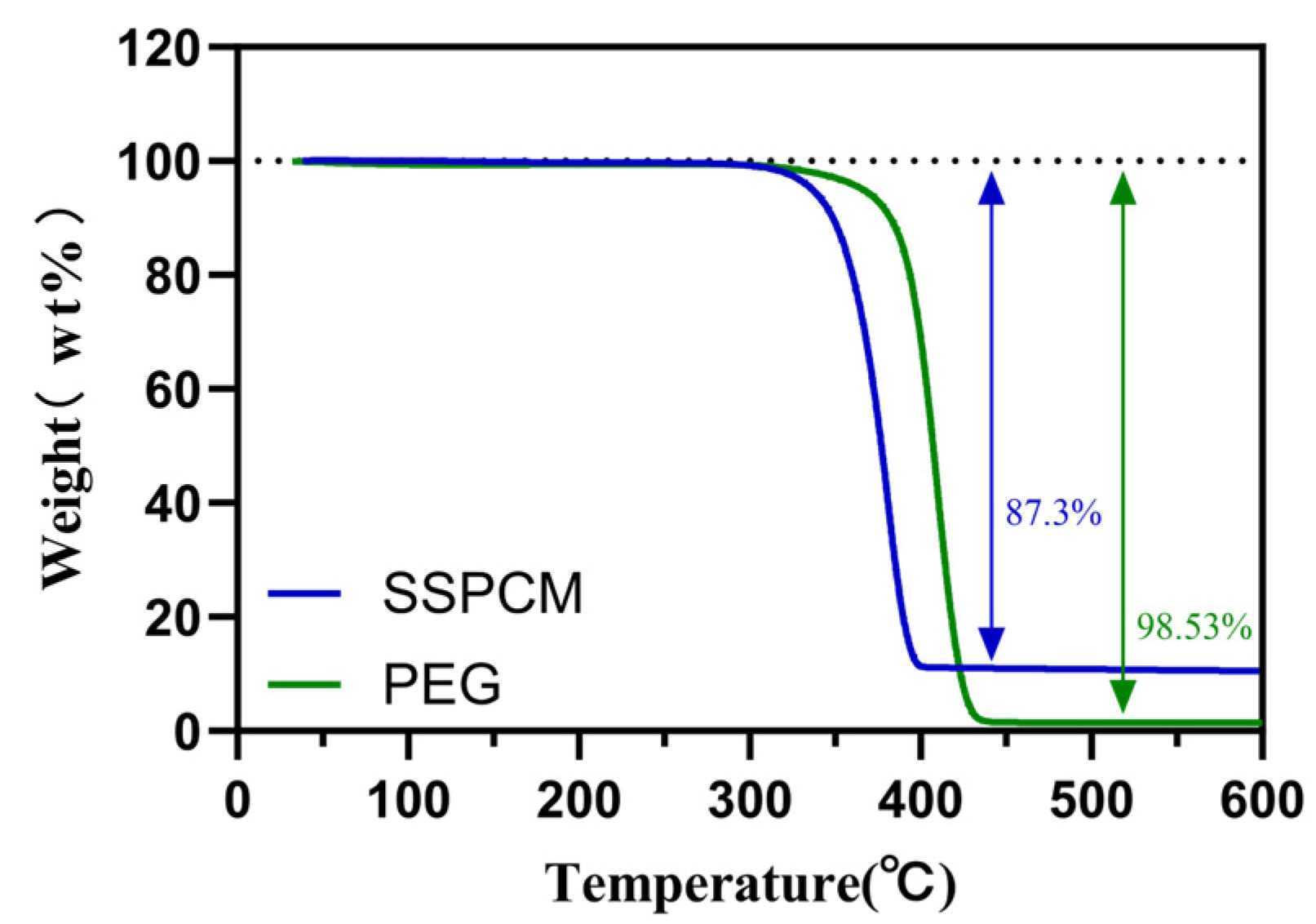
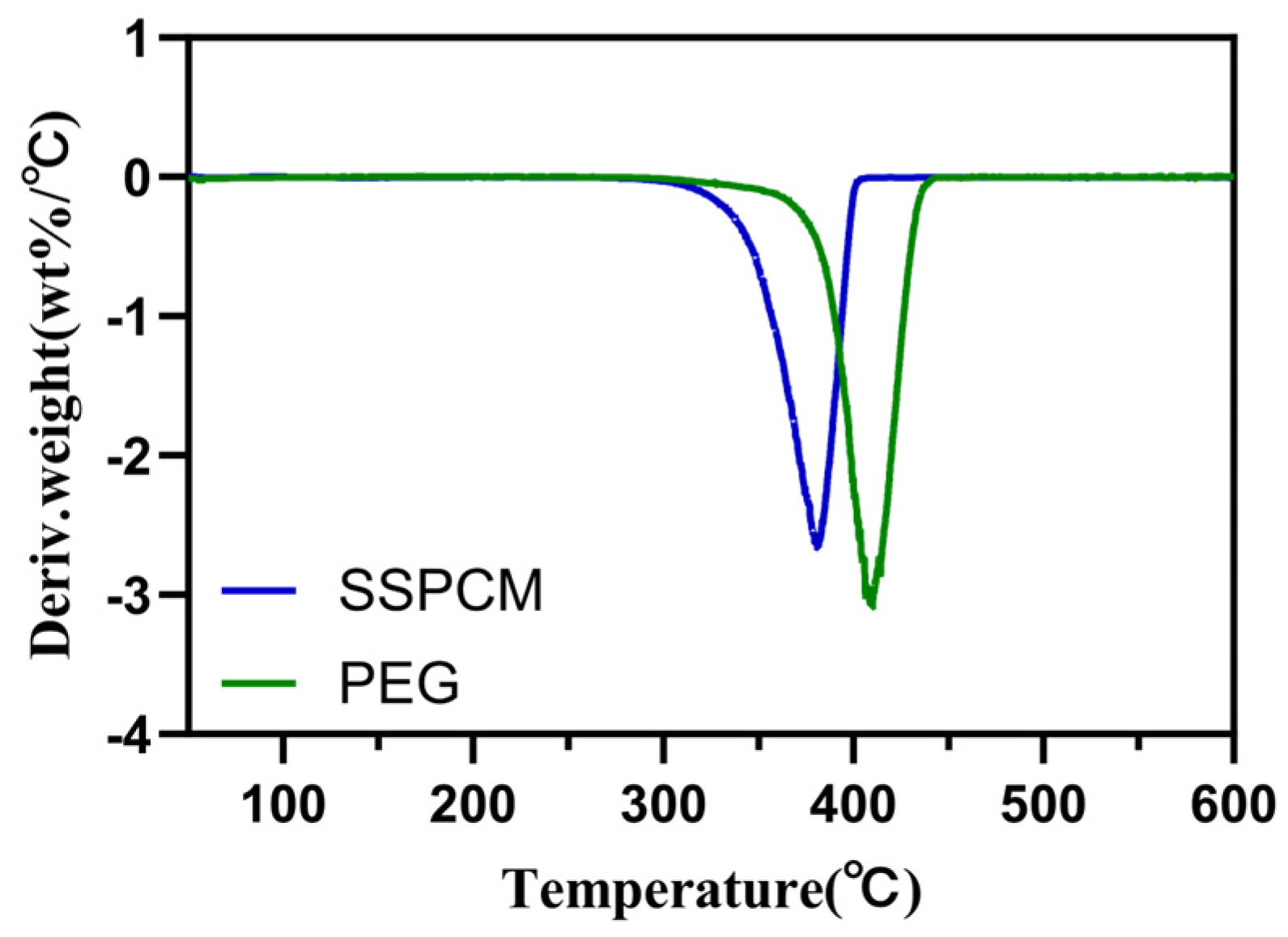
3.3. The Thermal Stability of PEG/PC SSPCM
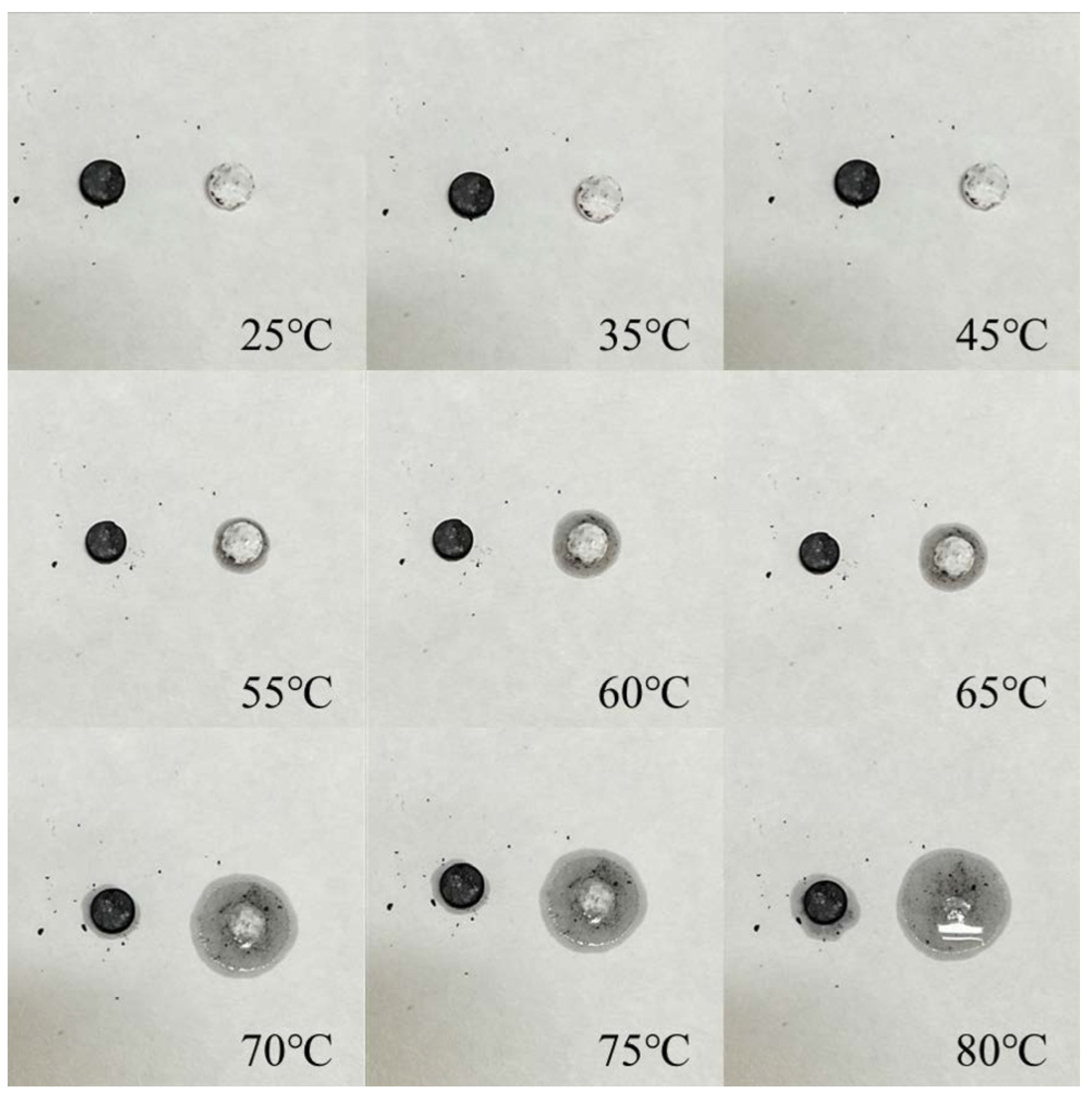
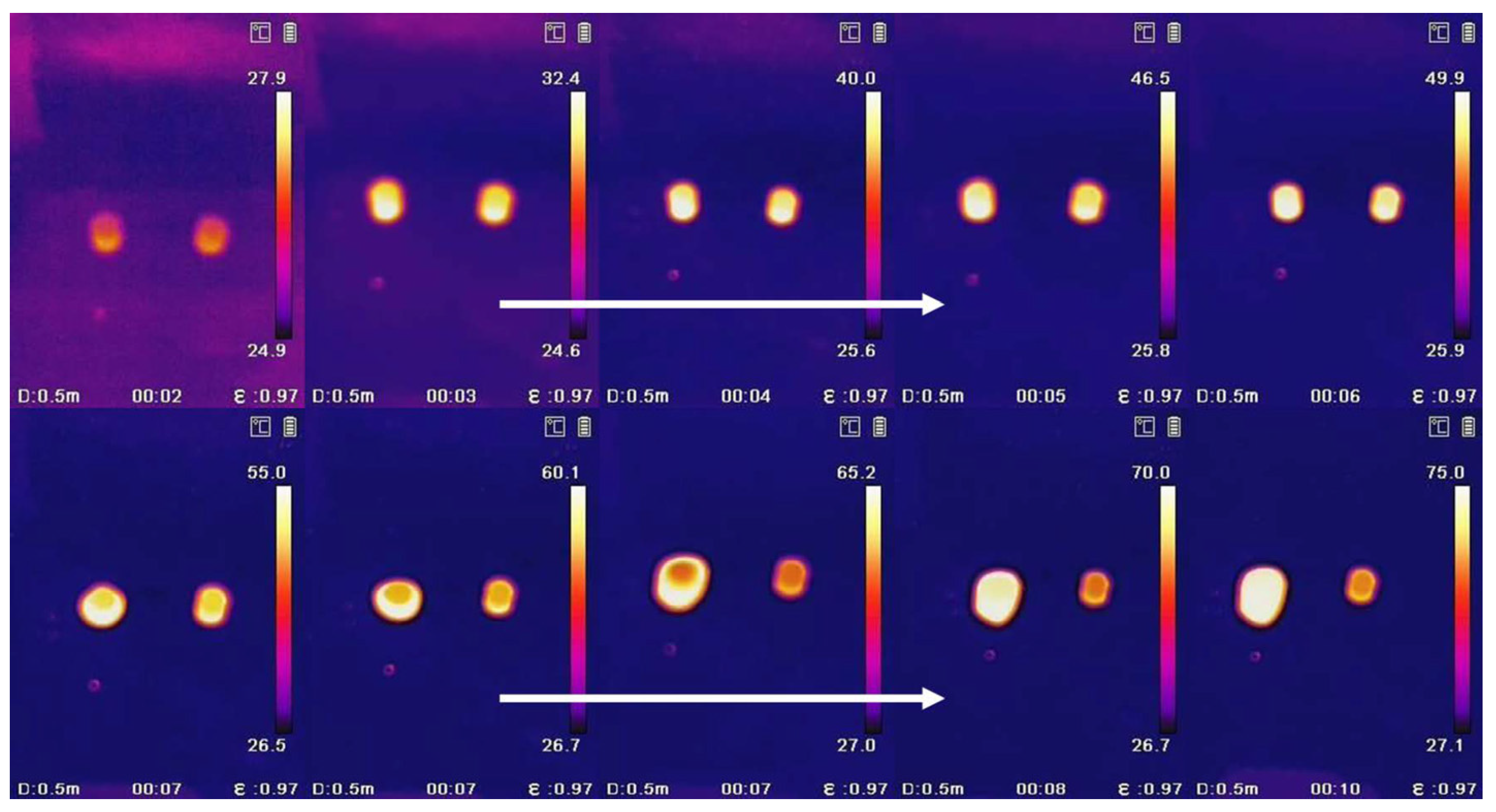
3.4. The Waste Heat Recovery Behavior of PEG/PC SSPCM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Full name |
| WGH | walnut green husk |
| PC | porous carbon |
| PEG | polyethylene glycol |
| PCM | phase-change material |
| SSPCM | solid–solid phase-change material |
References
- Faraj, K.; Khaled, M.; Faraj, J.; Hachem, F.; Castelain, C. Phase change material thermal energy storage systems for cooling applications in buildings: A review. Renew. Sustain. Energy Rev. 2020, 119, 109579. [Google Scholar] [CrossRef]
- Hassan, F.; Jamil, F.; Hussain, A.; Ali, H.M.; Janjua, M.M.; Khushnood, S.; Farhan, M.; Altaf, K.; Said, Z.; Li, C. Recent advancements in latent heat phase change materials and their applications for thermal energy storage and buildings: A state of the art review. Sustain. Energy Technol. Assess. 2022, 49, 101646. [Google Scholar] [CrossRef]
- Khudhair, A.M.; Farid, M. A review on energy conservation in building applications with thermal storage by latent heat using phase change materials. Therm. Energy Storage Phase Chang. Mater. 2021, 45, 162–175. [Google Scholar]
- Alva, G.; Lin, Y.; Fang, G. An overview of thermal energy storage systems. Energy 2018, 144, 341–378. [Google Scholar] [CrossRef]
- Qiu, J.; Huo, D.; Xia, Y. Phase-change materials for controlled release and related applications. Adv. Mater. 2020, 32, 2000660. [Google Scholar] [CrossRef] [PubMed]
- Javadi, F.; Metselaar, H.; Ganesan, P. Performance improvement of solar thermal systems integrated with phase change materials (PCM), a review. Sol. Energy 2020, 206, 330–352. [Google Scholar] [CrossRef]
- Yuan, K.; Shi, J.; Aftab, W.; Qin, M.; Usman, A.; Zhou, F.; Lv, Y.; Gao, S.; Zou, R. Engineering the thermal conductivity of functional phase-change materials for heat energy conversion, storage, and utilization. Adv. Funct. Mater. 2020, 30, 1904228. [Google Scholar] [CrossRef]
- Putra, N.; Rawi, S.; Amin, M.; Kusrini, E.; Kosasih, E.A.; Mahlia, T.M.I. Preparation of beeswax/multi-walled carbon nanotubes as novel shape-stable nanocomposite phase-change material for thermal energy storage. J. Energy Storage 2019, 21, 32–39. [Google Scholar] [CrossRef]
- Jeon, J.; Park, J.H.; Wi, S.; Kim, K.-H.; Kim, S. Thermal performance enhancement of a phase change material with expanded graphite via ultrasonication. J. Ind. Eng. Chem. 2019, 79, 437–442. [Google Scholar] [CrossRef]
- Allahbakhsh, A.; Arjmand, M. Graphene-based phase change composites for energy harvesting and storage: State of the art and future prospects. Carbon 2019, 148, 441–480. [Google Scholar] [CrossRef]
- Solangi, N.H.; Mubarak, N.M.; Karri, R.R.; Mazari, S.A.; Jatoi, A.S.; Koduru, J.R.; Dehghani, M.H. MXene-based phase change materials for solar thermal energy storage. Energy Convers. Manag. 2022, 273, 116432. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, H.; Dou, B.; Zhang, G.; Wu, W.; Zhou, X. Effect of copper metal foam proportion on heat transfer enhancement in the melting process of phase change materials. Appl. Therm. Eng. 2022, 201, 117778. [Google Scholar] [CrossRef]
- Ghahremannezhad, A.; Xu, H.; Salimpour, M.R.; Wang, P.; Vafai, K. Thermal performance analysis of phase change materials (PCMs) embedded in gradient porous metal foams. Appl. Therm. Eng. 2020, 179, 115731. [Google Scholar] [CrossRef]
- Bai, L.; Su, X.; Feng, J.; Ma, S. Preparation of sugarcane bagasse biochar/nano-iron oxide composite and mechanism of its Cr (VI) adsorption in water. J. Clean. Prod. 2021, 320, 128723. [Google Scholar] [CrossRef]
- Ghazi Sarwat, S.; Philip, T.M.; Chen, C.T.; Kersting, B.; Bruce, R.L.; Cheng, C.W.; Li, N.; Saulnier, N.; BrightSky, M.; Sebastian, A. Projected mushroom type phase-change memory. Adv. Funct. Mater. 2021, 31, 2106547. [Google Scholar] [CrossRef]
- Hadebe, L.; Cele, Z.; Gumbi, B. Properties of porous carbon electrode material derived from biomass of coffee waste grounds for capacitive deionization. Mater. Today Proc. 2022, 56, 2178–2183. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A.; Kar, T.; Keleş, S.; Kaygusuz, K.; Yıldırım, N.; Tyagi, V.V.; Sharma, R.K.; Saleh, T.A. Carbonized waste hazelnut wood-based shape-stable composite phase change materials for thermal management implementations. Int. J. Energy Res. 2021, 45, 10271–10284. [Google Scholar] [CrossRef]
- Kishore, R.A.; Bianchi, M.V.; Booten, C.; Vidal, J.; Jackson, R. Enhancing building energy performance by effectively using phase change material and dynamic insulation in walls. Appl. Energy 2021, 283, 116306. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A.; Kar, T.; Keleş, S.; Kaygusuz, K.; Tyagi, V.; Sharma, R.; Al-Ahmed, A.; Al-Sulaiman, F.A.; Saleh, T.A. Walnut shell derived bio-carbon/methyl palmitate as novel composite phase change material with enhanced thermal energy storage properties. J. Energy Storage 2021, 35, 102288. [Google Scholar] [CrossRef]
- Wan, Y.-C.; Chen, Y.; Cui, Z.-X.; Ding, H.; Gao, S.-F.; Han, Z.; Gao, J.-K. A promising form-stable phase change material prepared using cost effective pinecone biochar as the matrix of palmitic acid for thermal energy storage. Sci. Rep. 2019, 9, 11535. [Google Scholar] [CrossRef]
- Liu, S.; Peng, S.; Zhang, B.; Xue, B.; Yang, Z.; Wang, S.; Xu, G. Effects of biochar pyrolysis temperature on thermal properties of polyethylene glycol/biochar composites as shape-stable biocomposite phase change materials. RSC Adv. 2022, 12, 9587–9598. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Yun, B.Y.; Kim, Y.U.; Wi, S.; Kim, S. Introduction of eicosane into biochar derived from softwood and wheat straw: Influence of porous structure and surface chemistry. Chem. Eng. J. 2021, 415, 128887. [Google Scholar] [CrossRef]
- Seow, Y.X.; Tan, Y.H.; Mubarak, N.; Kansedo, J.; Khalid, M.; Ibrahim, M.L.; Ghasemi, M. A review on biochar production from different biomass wastes by recent carbonization technologies and its sustainable applications. J. Environ. Chem. Eng. 2022, 10, 107017. [Google Scholar] [CrossRef]
- Frigione, M.; Lettieri, M.; Sarcinella, A. Phase change materials for energy efficiency in buildings and their use in mortars. Materials 2019, 12, 1260. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Xiang, W.; Abdelmotalleib, M.; Zhao, X. Efficient NiO impregnated walnut shell-derived carbon for dye-sensitized solar cells. ACS Appl. Electron. Mater. 2022, 4, 1063–1071. [Google Scholar] [CrossRef]
- Kaur, P.; Verma, G.; Sekhon, S. Biomass derived hierarchical porous carbon materials as oxygen reduction reaction electrocatalysts in fuel cells. Prog. Mater. Sci. 2019, 102, 1–71. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, Y.; Liu, W.; Yang, L.; Zhang, B.; Wang, L.P.; Ji, G.; Xu, Z.J. Biomass-derived porous carbon-based nanostructures for microwave absorption. Nano-Micro Lett. 2019, 11, 1–17. [Google Scholar] [CrossRef]
- Ghorbani, M.; Konvalina, P.; Neugschwandtner, R.W.; Soja, G.; Bárta, J.; Chen, W.-H.; Amirahmadi, E. How do different feedstocks and pyrolysis conditions effectively change biochar modification scenarios? A critical analysis of engineered biochars under H2O2 oxidation. Energy Convers. Manag. 2024, 300, 117924. [Google Scholar] [CrossRef]
- Sun, K.; Kou, Y.; Zhang, Y.; Liu, T.; Shi, Q. Photo-triggered hierarchical porous carbon-based composite phase-change materials with superior thermal energy conversion capacity. ACS Sustain. Chem. Eng. 2020, 8, 3445–3453. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Li, W.; Zheng, J.; Tian, W.; Li, X. Shape-stabilized phase change materials based on polyethylene glycol/porous carbon composite: The influence of the pore structure of the carbon materials. Sol. Energy Mater. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
- Liu, Z.; Zang, C.; Ju, Z.; Hu, D.; Zhang, Y.; Jiang, J.; Liu, C. Consistent preparation, chemical stability and thermal properties of a shape-stabilized porous carbon/paraffin phase change materials. J. Clean. Prod. 2020, 247, 119565. [Google Scholar] [CrossRef]
- Kalyani, P.; Anitha, A. Biomass carbon & its prospects in electrochemical energy systems. Int. J. Hydrog. Energy 2013, 38, 4034–4045. [Google Scholar]
- Muddasar, M.; Mushtaq, M.; Beaucamp, A.; Kennedy, T.; Culebras, M.; Collins, M.N. Synthesis of sustainable lignin precursors for hierarchical porous carbons and their efficient performance in energy storage applications. ACS Sustain. Chem. Eng. 2024, 12, 2352–2363. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Junior, O.F.C.; Sreńscek-Nazzal, J. Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage. Carbon 2023, 201, 633–647. [Google Scholar] [CrossRef]
- Albatrni, H.; Qiblawey, H.; Al-Marri, M.J. Walnut shell based adsorbents: A review study on preparation, mechanism, and application. J. Water Process Eng. 2022, 45, 102527. [Google Scholar] [CrossRef]
- Okolie, O.; Kumar, A.; Edwards, C.; Lawton, L.A.; Oke, A.; McDonald, S.; Thakur, V.K.; Njuguna, J. Bio-Based Sustainable Polymers and Materials: From Processing to Biodegradation. J. Compos. Sci. 2023, 7, 213. [Google Scholar] [CrossRef]
- Vinod, A.; Pulikkalparambil, H.; Jagadeesh, P.; Rangappa, S.M.; Siengchin, S. Recent advancements in lignocellulose biomass-based carbon fiber: Synthesis, properties, and applications. Heliyon 2023, 9, e13614. [Google Scholar] [CrossRef]
- Lu, X.; Fang, C.; Sheng, X.; Zhang, L.; Qu, J. One-step and solvent-free synthesis of polyethylene glycol-based polyurethane as solid–solid phase change materials for solar thermal energy storage. Ind. Eng. Chem. Res. 2019, 58, 3024–3032. [Google Scholar] [CrossRef]
- Tan, B.; Huang, Z.; Yin, Z.; Min, X.; Liu, Y.G.; Wu, X.; Fang, M. Preparation and thermal properties of shape-stabilized composite phase change materials based on polyethylene glycol and porous carbon prepared from potato. RSC Adv. 2016, 6, 15821–15830. [Google Scholar] [CrossRef]
- Das, D.; Bordoloi, U.; Muigai, H.H.; Kalita, P. A novel form stable PCM based bio composite material for solar thermal energy storage applications. J. Energy Storage 2020, 30, 101403. [Google Scholar] [CrossRef]
- Zhao, C.; Guo, P.; Sheng, N.; Zhu, C.; Rao, Z. Cloth-derived anisotropic carbon scroll attached with 2D oriented graphite layers for supporting phase change material with efficient thermal storage. Chem. Eng. J. 2023, 454, 139999. [Google Scholar] [CrossRef]
- Pérez-Mayoral, E.; Matos, I.; Bernardo, M.; Fonseca, I.M. New and advanced porous carbon materials in fine chemical synthesis. Emerging precursors of porous carbons. Catalysts 2019, 9, 133. [Google Scholar] [CrossRef]
- Chen, X.; Gao, H.; Tang, Z.; Dong, W.; Li, A.; Wang, G. Optimization strategies of composite phase change materials for thermal energy storage, transfer, conversion and utilization. Energy Environ. Sci. 2020, 13, 4498–4535. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S.; Luo, J.; Sun, K.; Zhang, J.; Tan, Z.; Shi, Q. Thermal analysis and heat capacity study of polyethylene glycol (PEG) phase change materials for thermal energy storage applications. J. Chem. Thermodyn. 2019, 128, 259–274. [Google Scholar] [CrossRef]
- Mitran, R.-A.; Ioniţǎ, S.; Lincu, D.; Berger, D.; Matei, C. A review of composite phase change materials based on porous silica nanomaterials for latent heat storage applications. Molecules 2021, 26, 241. [Google Scholar] [CrossRef]
- Pan, H.; Li, T.; Xu, L.; Li, K.; Shen, Y. Shape-stable composite phase change materials encapsulated by lignin-based ordered porous carbon for thermal energy storage. J. Wood Chem. Technol. 2023, 43, 92–102. [Google Scholar] [CrossRef]
- Paberit, R.; Rilby, E.; Gohl, J.; Swenson, J.; Refaa, Z.; Johansson, P.; Jansson, H. Cycling stability of poly(ethylene glycol) of six molecular weights: Influence of thermal conditions for energy applications. ACS Appl. Energy Mater. 2020, 3, 10578–10589. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, X.; Zhang, X.; Yin, Z.; Liu, Y.; Fang, M.; Wu, X.; Min, X.; Huang, Z. Lauric-stearic acid eutectic mixture/carbonized biomass waste corn cob composite phase change materials: Preparation and thermal characterization. Thermochim. Acta 2019, 674, 21–27. [Google Scholar] [CrossRef]
- Gondora, W.; Doudin, K.; Nowakowski, D.J.; Xiao, B.; Ding, Y.; Bridgwater, T.; Yuan, Q. Encapsulation of phase change materials using rice-husk-char. Appl. Energy 2016, 182, 274–281. [Google Scholar] [CrossRef]
- Hekimoğlu, G.; Sarı, A.; Gencel, O.; Önal, Y.; Ustaoğlu, A.; Erdogmus, E.; Harja, M.; Tyagi, V. Thermal energy storage performance evaluation of bio-based phase change material/apricot kernel shell derived activated carbon in lightweight mortar. J. Energy Storage 2023, 73, 109122. [Google Scholar] [CrossRef]
- Kalidasan, B.; Pandey, A.; Saidur, R.; Aljafari, B.; Yadav, A.; Samykano, M. Green synthesized 3D coconut shell biochar/polyethylene glycol composite as thermal energy storage material. Sustain. Energy Technol. Assess. 2023, 60, 103505. [Google Scholar] [CrossRef]
- Zhai, S.; Zhang, L.; Zhao, X.; Wang, Q.; Yan, Y.; Li, C.; Zhang, X. Enzymatic synthesis of a novel solid–liquid phase change energy storage material based on levulinic acid and 1,4-butanediol. Bioresour. Bioprocess. 2022, 9, 12. [Google Scholar] [CrossRef]

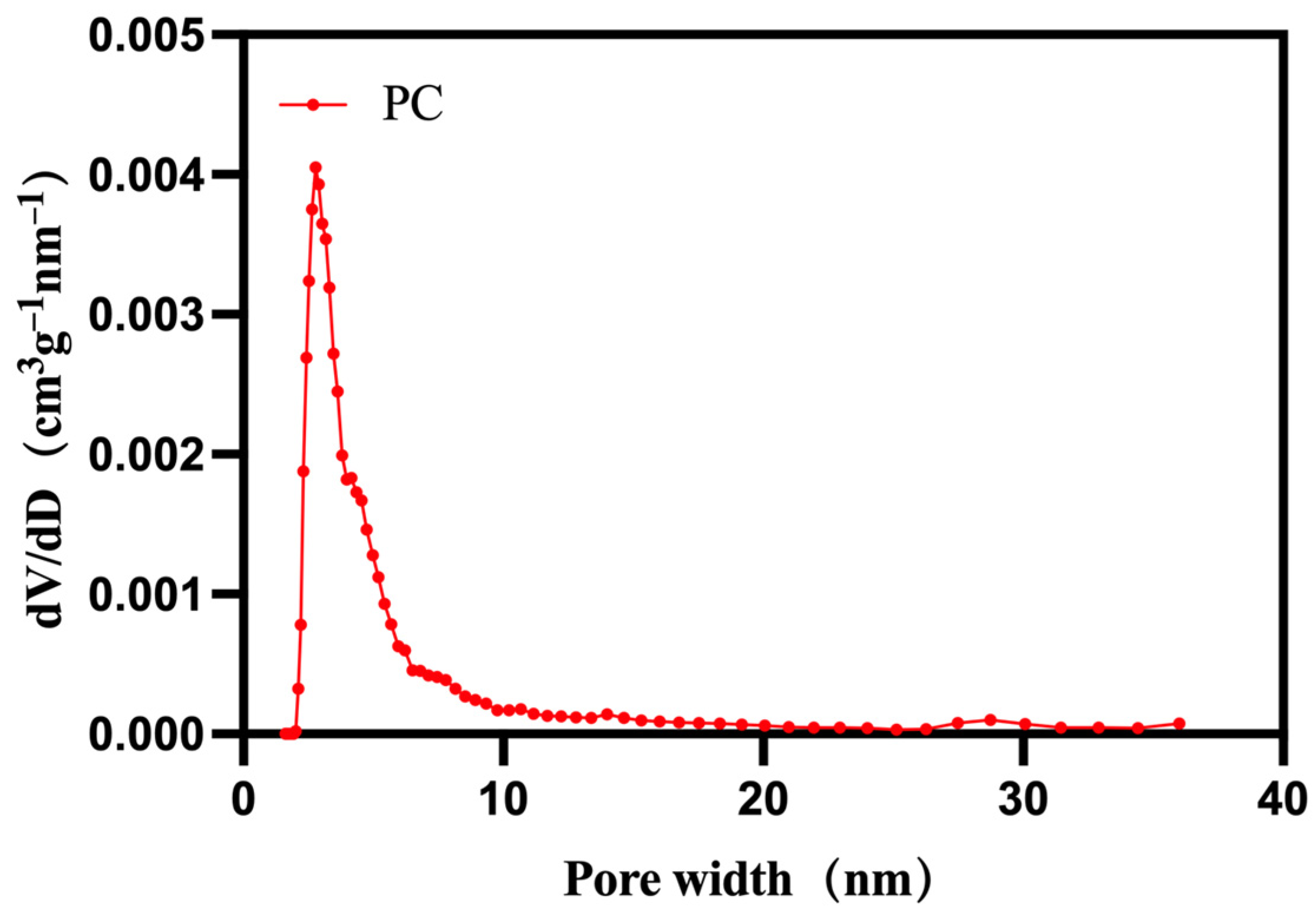
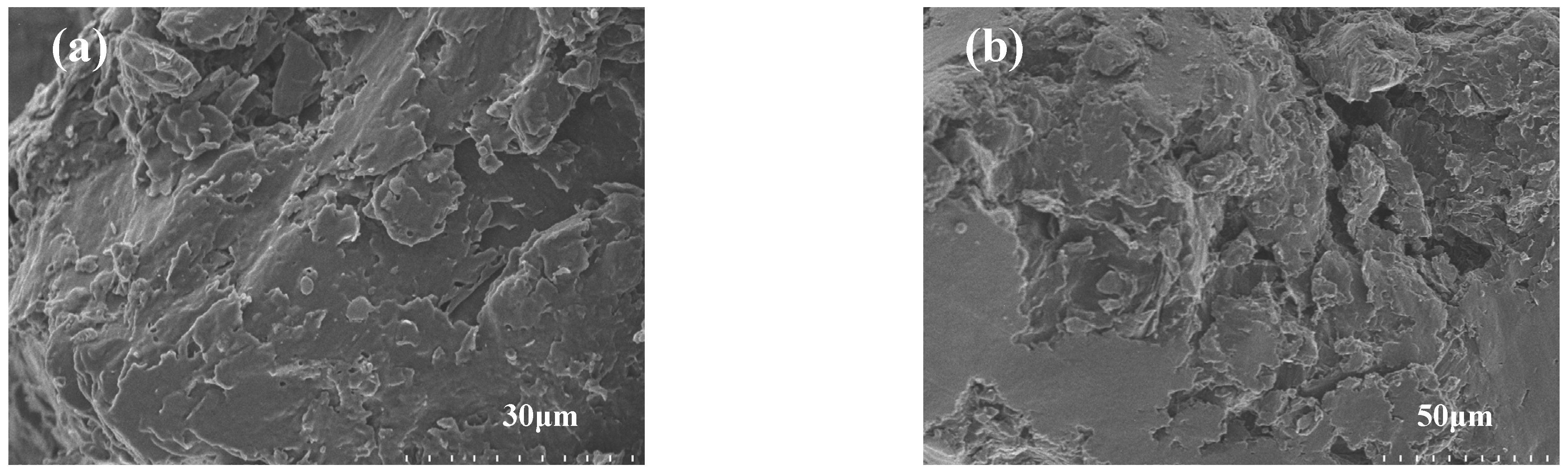
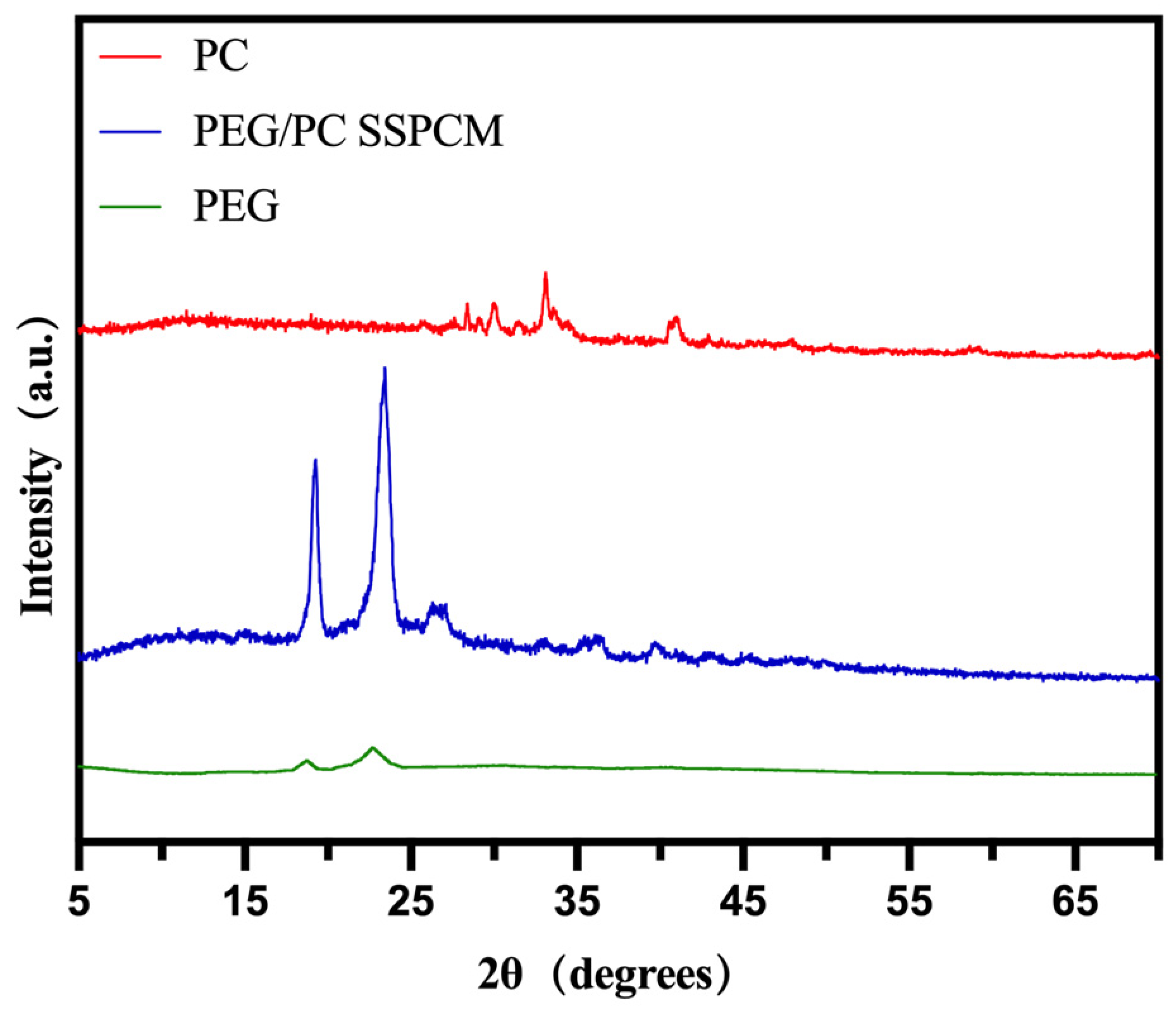
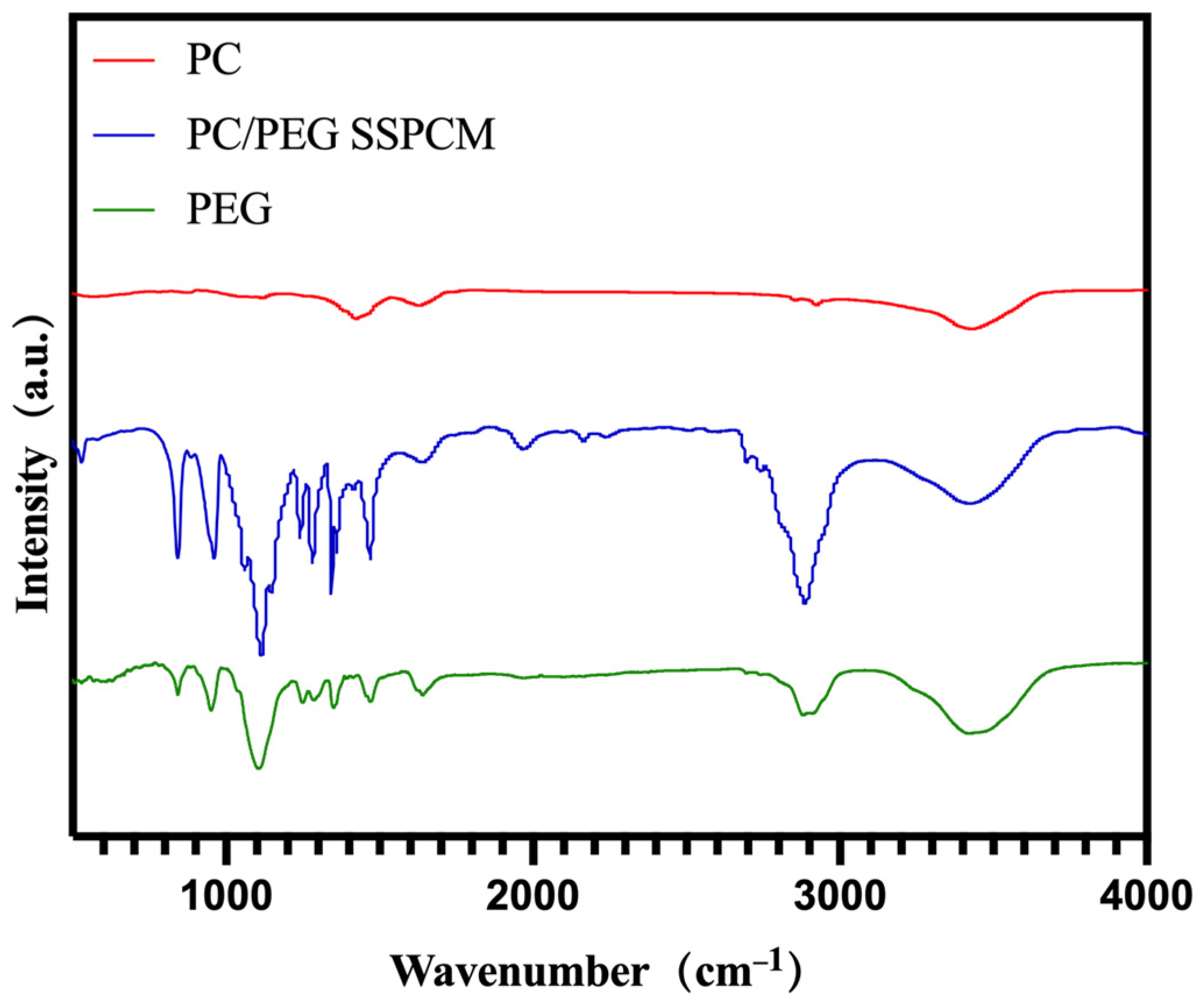
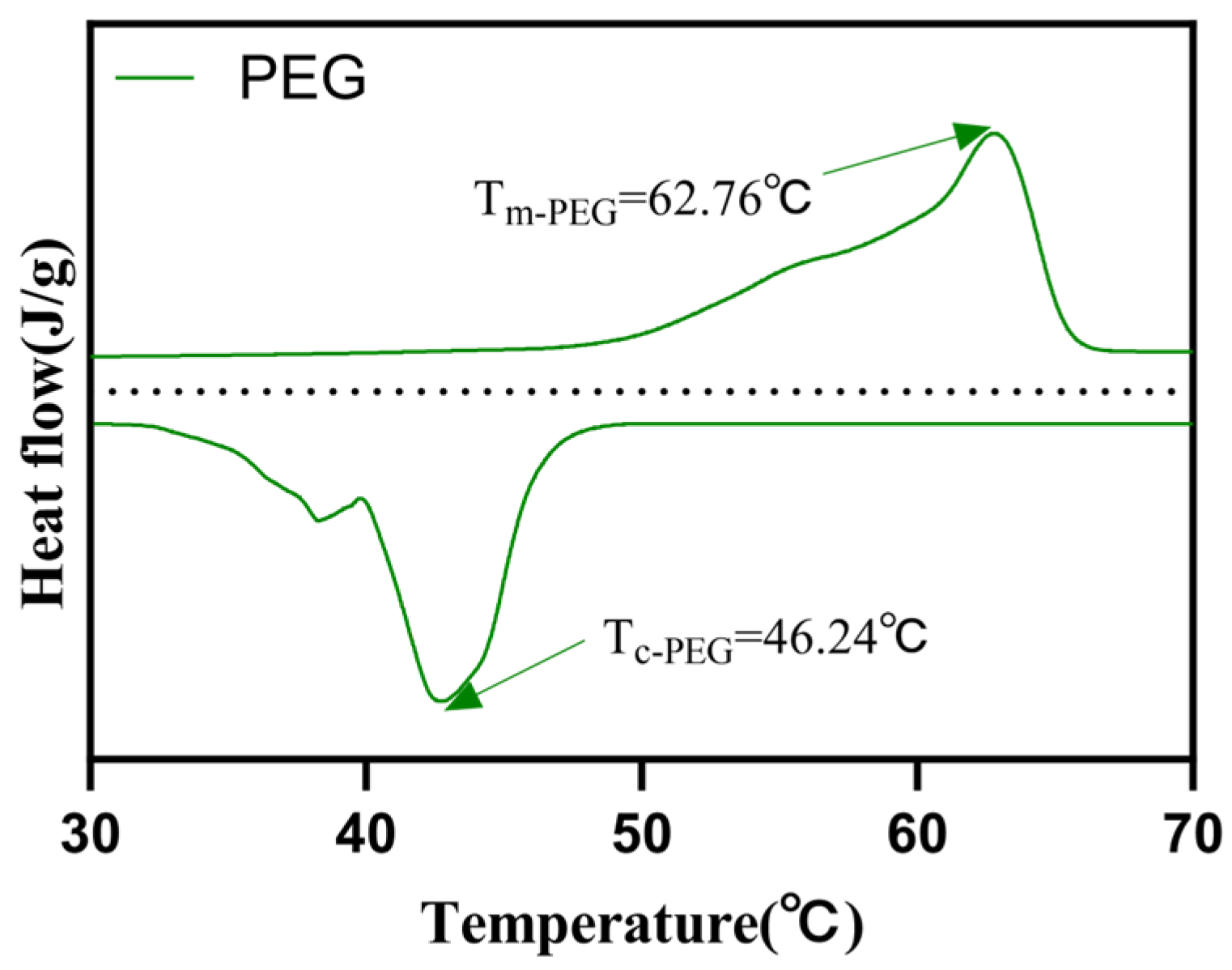
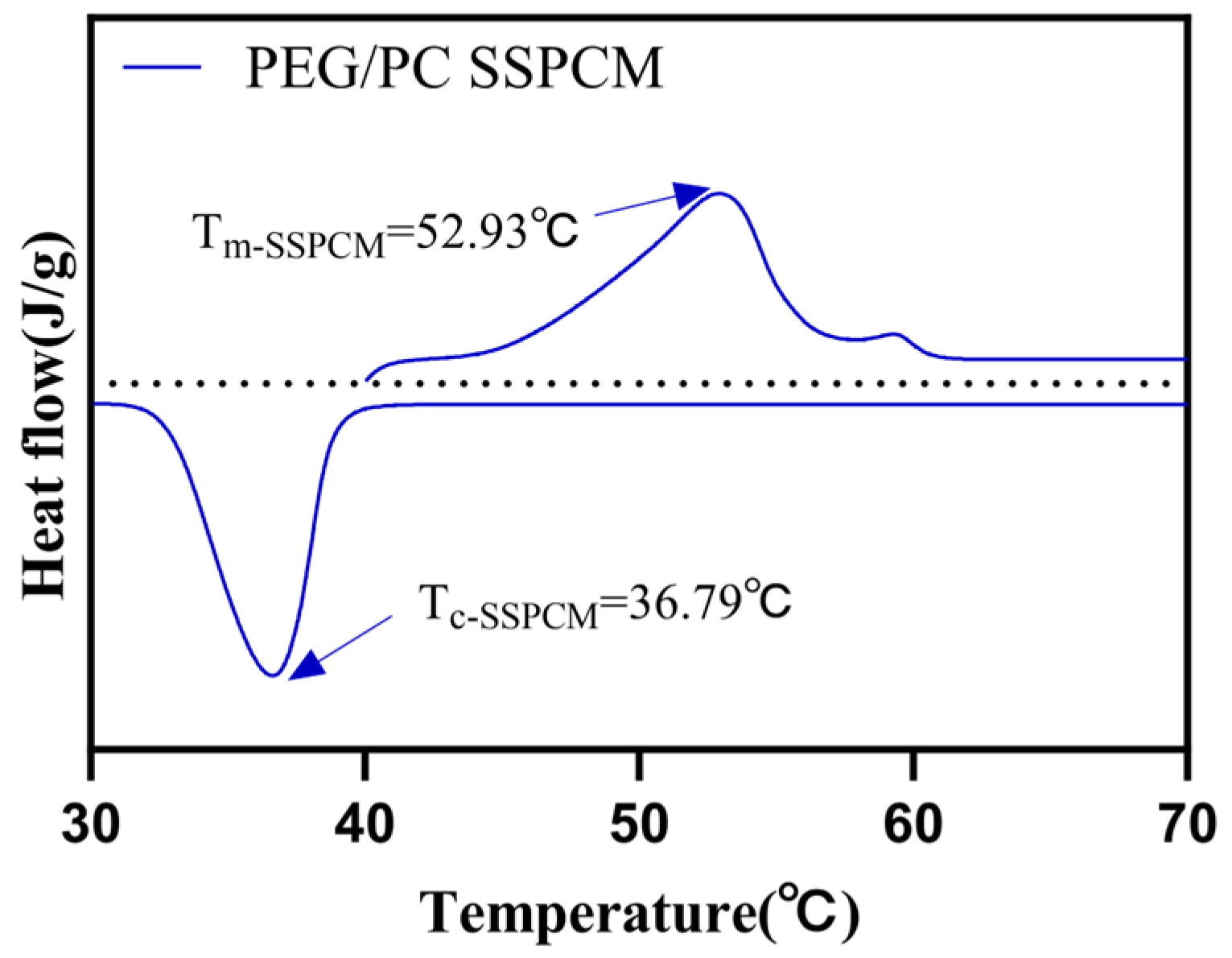
| Samples | Tm/°C | Tc/°C | ∆Hm/(J g−1) | ∆Hc/(J g−1) |
|---|---|---|---|---|
| PEG | 62.76 | 46.24 | 270.16 | 213.73 |
| PEG/PC SSPCM | 52.93 | 36.79 | 194.76 | 155.77 |
| Samples | R/% | E% |
|---|---|---|
| PEG/PC SSPCM | 72.09 | 72.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, L.; Cao, H. Multistage Porous Carbon Derived from Enzyme-Treated Waste Walnut Green Husk and Polyethylene Glycol for Phase Change Energy Storage. Materials 2024, 17, 1379. https://doi.org/10.3390/ma17061379
Wang Z, Liu L, Cao H. Multistage Porous Carbon Derived from Enzyme-Treated Waste Walnut Green Husk and Polyethylene Glycol for Phase Change Energy Storage. Materials. 2024; 17(6):1379. https://doi.org/10.3390/ma17061379
Chicago/Turabian StyleWang, Ziming, Luo Liu, and Hui Cao. 2024. "Multistage Porous Carbon Derived from Enzyme-Treated Waste Walnut Green Husk and Polyethylene Glycol for Phase Change Energy Storage" Materials 17, no. 6: 1379. https://doi.org/10.3390/ma17061379
APA StyleWang, Z., Liu, L., & Cao, H. (2024). Multistage Porous Carbon Derived from Enzyme-Treated Waste Walnut Green Husk and Polyethylene Glycol for Phase Change Energy Storage. Materials, 17(6), 1379. https://doi.org/10.3390/ma17061379









