Influence of Zr Addition on the Microstructure and Hydrogenation Kinetics of Ti50−xV25Cr25Zrx (x = 0, 5, 7, and 9) Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
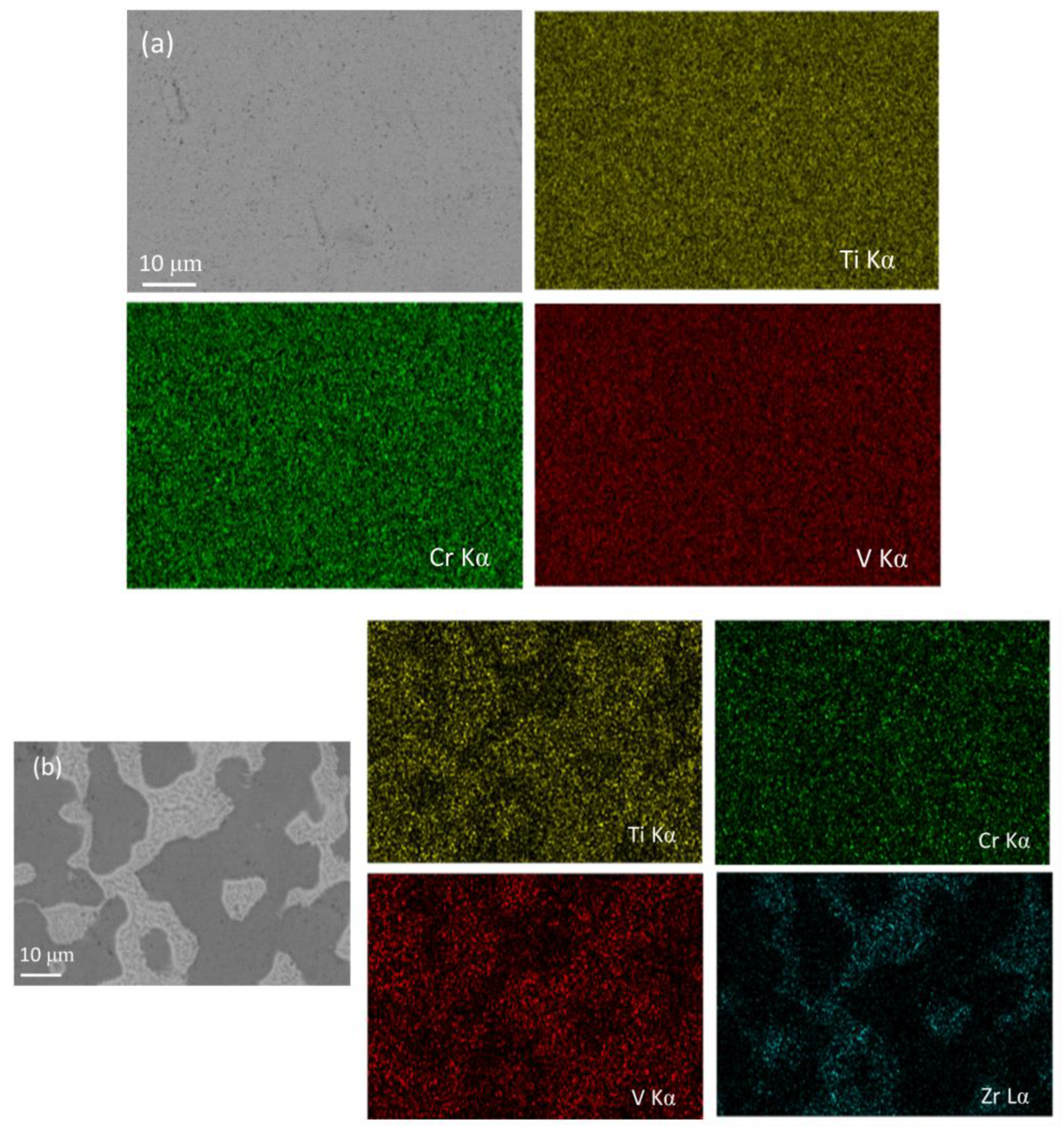
3.1. Structural Characterizations
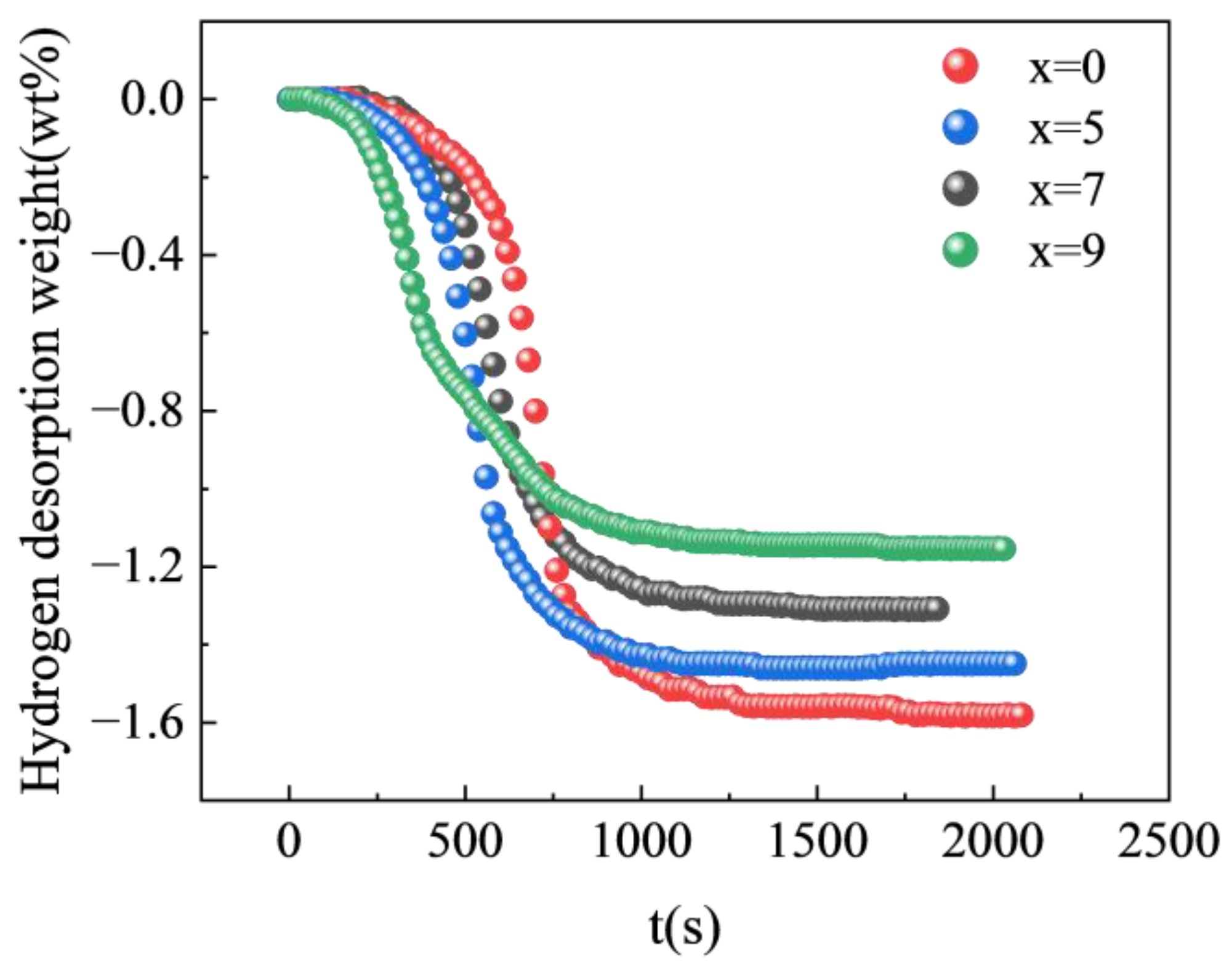
3.2. Absorption and Desorption Properties
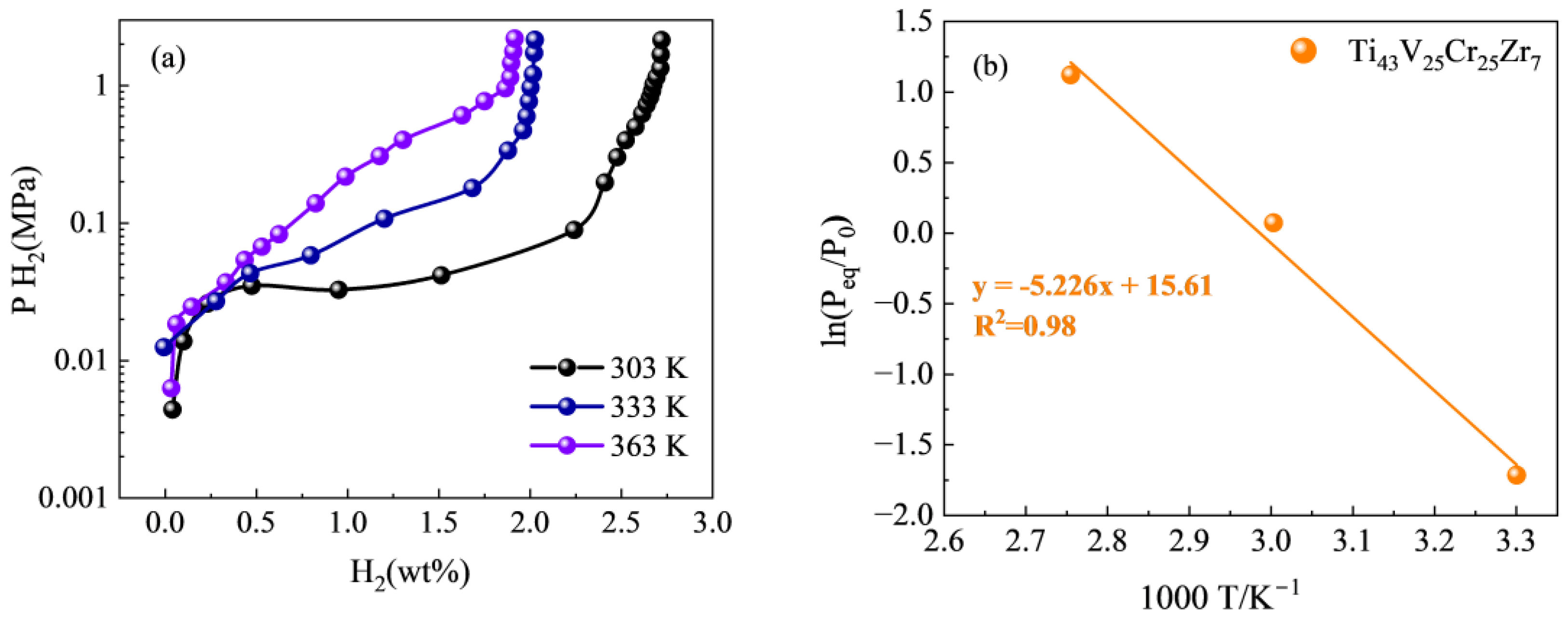
3.3. PCT Curve Analyze
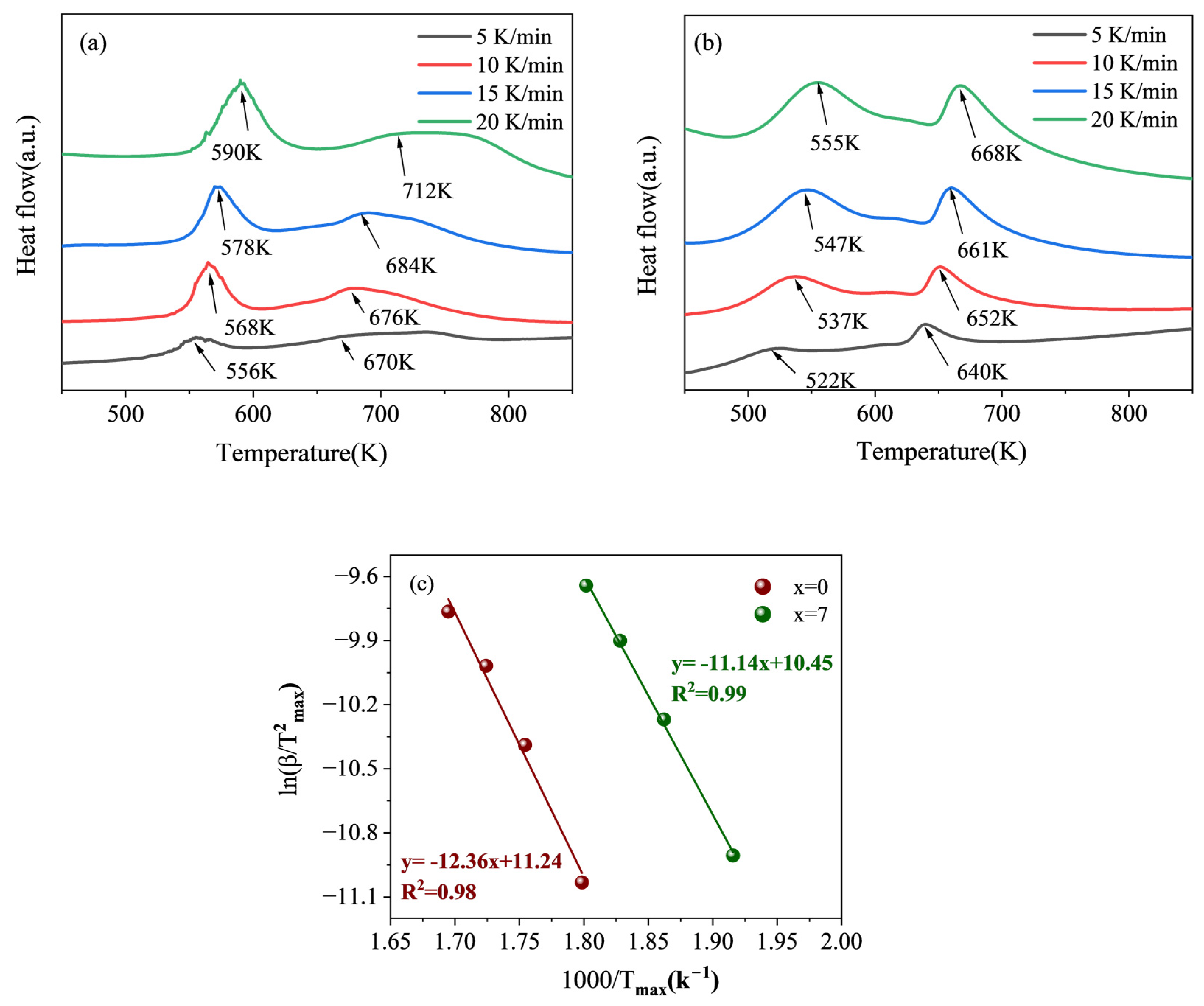
3.4. DSC
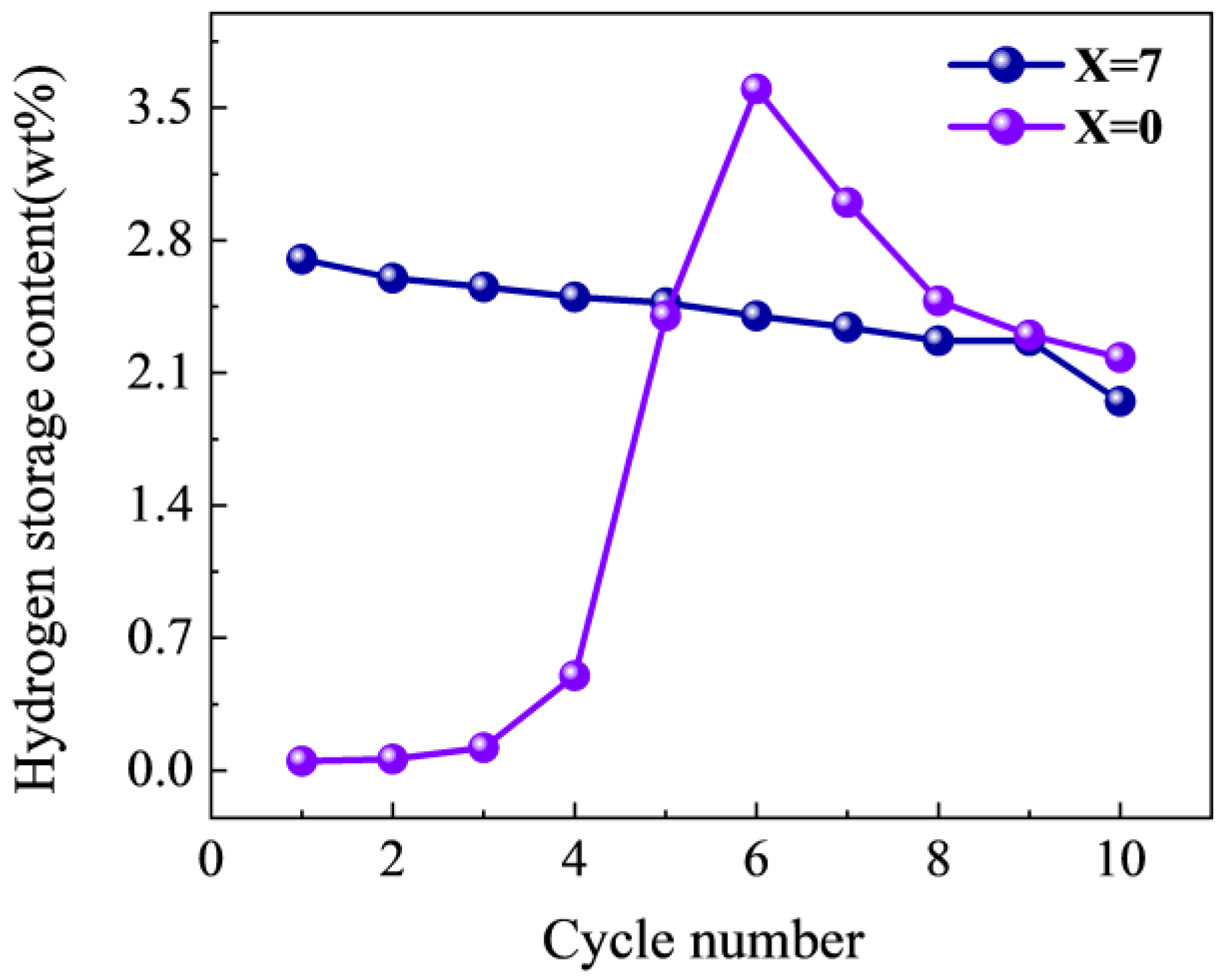
3.5. Cycling and Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faisal, M.; Kim, J.-H.; Cho, Y.W.; Jang, J.; Suh, J.-Y.; Shim, J.-H.; Lee, Y.-S. Design of V-Substituted TiFe-Based Alloy for Target Pressure Range and Easy Activation. Materials 2021, 14, 4829. [Google Scholar] [CrossRef] [PubMed]
- Dixit, V.; Huot, J. Investigation of the Microstructure, Crystal Structure and Hydrogenation Kinetics of Ti-V-Cr Alloy with Zr Addition. J. Alloys Compd. 2019, 785, 1115–1120. [Google Scholar] [CrossRef]
- Patel, A.K.; Tougas, B.; Sharma, P.; Huot, J. Effect of Cooling Rate on the Microstructure and Hydrogen Storage Properties of TiFe with 4 Wt% Zr as an Additive. J. Mater. Res. Technol. 2019, 8, 5623–5630. [Google Scholar] [CrossRef]
- Jena, P. Materials for Hydrogen Storage: Past, Present, and Future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Garzón Baquero, J.E.; Bellon Monsalve, D. From Fossil Fuel Energy to Hydrogen Energy: Transformation of Fossil Fuel Energy Economies into Hydrogen Economies through Social Entrepreneurship. Int. J. Hydrogen Energy 2024, 54, 574–585. [Google Scholar] [CrossRef]
- Yong, H.; Ji, Y.; Hu, J.; Zhao, D.; Wang, S. Absorption and Desorption Hydrogen Kinetic of Mg-Y-Ni Based Hydrogen Storage Alloy. Chin. J. Rare Met. 2022, 46, 1021–1030. [Google Scholar] [CrossRef]
- Sleiman, S.; Huot, J. Microstructure and Hydrogen Storage Properties of Ti1V0.9Cr1.1 Alloy with Addition of × Wt % Zr (x = 0, 2, 4, 8, and 12). Inorganics 2017, 5, 86. [Google Scholar] [CrossRef]
- Martínez, A.; dos Santos, D.S. Hydrogen Absorption/Desorption Properties in the TiCrV Based Alloys. Mat. Res. 2012, 15, 809–812. [Google Scholar] [CrossRef]
- Aranda, V.; Leiva, D.R.; Huot, J.; Botta, W.J.; Zepon, G. Hydrogen Storage Properties of the TiVFeZr Multicomponent Alloy with C14-Type Laves Phase Structure. Intermetallics 2023, 162, 108020. [Google Scholar] [CrossRef]
- Gao, M.; Miao, H.; Zhao, Y.; Liu, Y.; Pan, H. Effects of Rare Earth Elements Substitution for Ti on the Structure and Electrochemical Properties of a Fe-Doped Ti–V-Based Hydrogen Storage Alloy. J. Alloys Compd. 2009, 484, 249–255. [Google Scholar] [CrossRef]
- Mazzolai, G.; Coluzzi, B.; Biscarini, A.; Mazzolai, F.M.; Tuissi, A.; Agresti, F.; Lo Russo, S.; Maddalena, A.; Palade, P.; Principi, G. Hydrogen-Storage Capacities and H Diffusion in Bcc TiVCr Alloys. J. Alloys Compd. 2008, 466, 133–139. [Google Scholar] [CrossRef]
- Serrano, L.; Moussa, M.; Yao, J.-Y.; Silva, G.; Bobet, J.-L.; Santos, S.F.; Cardoso, K.R. Development of Ti-V-Nb-Cr-Mn High Entropy Alloys for Hydrogen Storage. J. Alloys Compd. 2023, 945, 169289. [Google Scholar] [CrossRef]
- Dixit, V.; van Eijck, L.; Huot, J. Investigation of Dehydrogenation of Ti–V–Cr Alloy by Using in-Situ Neutron Diffraction. J. Alloys Compd. 2020, 844, 156130. [Google Scholar] [CrossRef]
- Fuda, T.; Matsumoto, K.; Tominaga, Y.; Tamura, T.; Kuriiwa, T.; Kamegawa, A.; Okada, M. Effects of Additions of BCC Former Elements on Protium Absorbing Properties of Cr–Ti–V Alloys. Mater. Trans. JIM 2000, 41, 577–580. [Google Scholar] [CrossRef]
- Young, K.-H.; Ouchi, T.; Nei, J.; Moghe, D. The Importance of Rare-Earth Additions in Zr-Based AB2 Metal Hydride Alloys. Batteries 2016, 2, 25. [Google Scholar] [CrossRef]
- Kumar, A.; Banerjee, S.; Bharadwaj, S.R. Hydrogen Storage Properties of Ti0.32Cr0.43V0.25 Alloy and Its Composite with TiMn2. J. Alloys Compd. 2015, 649, 801–808. [Google Scholar] [CrossRef]
- Liu, J. A Study on the Microstructures and Hydrogen Storage Characteristics of Ti-Cr-V Based Hydrogen Storage Alloys. Master’s Thesis, Zhejiang University, Hangzhou, China, 2005. [Google Scholar]
- Abdul, J.M.; Kolawole, S.K.; Salawu, G.A. Microstructure and Hydrogen Storage Characteristics of Rhodium Substituted Ti-V-Cr Alloys. JOM 2021, 73, 4112–4118. [Google Scholar] [CrossRef]
- Sakaki, K.; Kim, H.; Majzoub, E.H.; Machida, A.; Watanuki, T.; Ikeda, K.; Otomo, T.; Mizuno, M.; Matsumura, D.; Nakamura, Y. Displacement of Hydrogen Position in Di-Hydride of V-Ti-Cr Solid Solution Alloys. Acta Mater. 2022, 234, 118055. [Google Scholar] [CrossRef]
- Singh, B.K.; Shim, G.; Cho, S.-W. Effects of Mechanical Milling on Hydrogen Storage Properties of Ti0.32Cr0.43V0.25 Alloy. Int. J. Hydrogen Energy 2007, 32, 4961–4965. [Google Scholar] [CrossRef]
- Pei, P.; Zhang, P.; Zhang, B.; Song, X. V Based Hydrogen Storage Alloys and Alloying Research. Mater. Rev. 2006, 20, 123–127. [Google Scholar] [CrossRef]
- Santos, S.F.; Costa, A.L.M.; Castro, J.F.R.D.; Dos Santos, D.S.; Botta Filho, W.J.; Ishikawa, T.T. Mechanical and Reactive Milling of a TiCrV BCC Solid Solution. J. Metastable Nanocrystalline Mater. 2004, 20–21, 291–296. [Google Scholar] [CrossRef]
- Kamble, A.; Sharma, P.; Huot, J. Effect of Addition of Zr, Ni, and Zr-Ni Alloy on the Hydrogen Absorption of Body Centred Cubic 52Ti-12V-36Cr Alloy. Int. J. Hydrogen Energy 2018, 43, 7424–7429. [Google Scholar] [CrossRef]
- Kamble, A.; Sharma, P.; Huot, J. Effect of Doping and Particle Size on Hydrogen Absorption Properties of BCC Solid Solution 52Ti-12V-36Cr. Int. J. Hydrogen Energy 2017, 42, 11523–11527. [Google Scholar] [CrossRef]
- Feng, Z.; Zhong, H.; Li, D.; Li, X.; Yang, B.; Li, S. Microstructure and Hydrogen Storage Properties of Ti–V–Mn Alloy with Zr, Ni, and Zr7Ni10 Addition. J. Mater. Res. 2022, 37, 1591–1601. [Google Scholar] [CrossRef]
- Hang, Z.; Xiao, X.; Weng, W.; Bing, Z.; Huang, S.; Xu, H.; Chen, L. Microstructure and Hydrogen Storage Properties of Ti–V–Cr Based BCC-Type High Entropy Alloys. J. Mater. Sci. Eng. 2016, 34, 874–877+1003. [Google Scholar] [CrossRef]
- Hang, Z.; Xiao, X.; Tan, D.; He, Z.; Li, W.; Li, S.; Chen, C.; Chen, L. Microstructure and Hydrogen Storage Properties of Ti10V84−xFe6Zrx (X=1–8) Alloys. Int. J. Hydrogen Energy 2010, 35, 3080–3086. [Google Scholar] [CrossRef]
- Shashikala, K.; Banerjee, S.; Kumar, A.; Pai, M.R.; Pillai, C.G.S. Improvement of Hydrogen Storage Properties of TiCrV Alloy by Zr Substitution for Ti. Int. J. Hydrogen Energy 2009, 34, 6684–6689. [Google Scholar] [CrossRef]
- Bellon Monsalve, D.; Ulate-Kolitsky, E.; Martínez-Amariz, A.-D.; Huot, J. Effect of Zr3Fe Addition on Hydrogen Storage Behaviour of Ti2CrV Alloys. Heliyon 2023, 9, e22537. [Google Scholar] [CrossRef]
- Kumar, A.; Banerjee, S.; Ruz, P.; Sudarsan, V. Hydrogen Storage Properties of Al-Containing Ti2CrV Alloys. Bull. Mater. Sci. 2023, 47, 8. [Google Scholar] [CrossRef]
- Hu, H.; Ma, C.; Chen, Q. Improved Hydrogen Storage Properties of Ti2CrV Alloy by Mo Substitutional Doping. Int. J. Hydrogen Energy 2022, 47, 11929–11937. [Google Scholar] [CrossRef]
- Kumar, A.; Shashikala, K.; Banerjee, S.; Nuwad, J.; Das, P.; Pillai, C.G.S. Effect of Cycling on Hydrogen Storage Properties of Ti2CrV Alloy. Int. J. Hydrogen Energy 2012, 37, 3677–3682. [Google Scholar] [CrossRef]
- Kumar, A.; Banerjee, S.; Pillai, C.G.S.; Bharadwaj, S.R. Hydrogen Storage Properties of Ti2−xCrVMx (M = Fe, Co, Ni) Alloys. Int. J. Hydrogen Energy 2013, 38, 13335–13342. [Google Scholar] [CrossRef]
- Sleiman, S.; Aliouat, A.; Huot, J. Enhancement of First Hydrogenation of Ti1V0.9Cr1.1 BCC Alloy by Cold Rolling and Ball Milling. Materials 2020, 13, 3106. [Google Scholar] [CrossRef]
- Chen, X. Microstructure and Hydrogen Absorption/Desorption Properties of Ti-V-Mn Multicomponent Alloys. Ph.D. Thesis, Harbin Institute of Technology, Harbin, China, 2019. [Google Scholar]
- Toby, B.H.; Von Dreele, R.B. GSAS-II: The Genesis of a Modern Open-Source All Purpose Crystallography Software Package. J. Appl. Cryst. 2013, 46, 544–549. [Google Scholar] [CrossRef]
- Mouri, T.; Iba, H. Hydrogen-Absorbing Alloys with a Large Capacity for a New Energy Carrier. Mater. Sci. Eng. A 2002, 329–331, 346–350. [Google Scholar] [CrossRef]
- Wu, T.; Xue, X.; Zhang, T.; Hu, R.; Kou, H.; Li, J. Microstructures and Hydrogenation Properties of (ZrTi)(V1−xAlx)2 Laves Phase Intermetallic Compounds. J. Alloys Compd. 2015, 645, 358–368. [Google Scholar] [CrossRef]
- Ruz, P.; Banerjee, S.; Halder, R.; Kumar, A.; Sudarsan, V. Thermodynamics, Kinetics and Microstructural Evolution of Ti0.43Zr0.07Cr0.25V0.25 Alloy upon Hydrogenation. Int. J. Hydrogen Energy 2017, 42, 11482–11492. [Google Scholar] [CrossRef]
- Nygård, M.M.; Ek, G.; Karlsson, D.; Sørby, M.H.; Sahlberg, M.; Hauback, B.C. Counting Electrons—A New Approach to Tailor the Hydrogen Sorption Properties of High-Entropy Alloys. Acta Mater. 2019, 175, 121–129. [Google Scholar] [CrossRef]
- Yukawa, H.; Takagi, M.; Teshima, A.; Morinaga, M. Alloying Effects on the Stability of Vanadium Hydrides. J. Alloys Compd. 2002, 330, 105–109. [Google Scholar] [CrossRef]
- Peng, L.; Jiang, C.; Xu, Q.; Wu, X. Hydrogen-Induced Disproportionation Characteristics of Zr(1−x)Hf(x)Co(X=0, 0.1, 0.2 and 0.3) Alloys. Fusion Eng. Des. 2013, 88, 299–303. [Google Scholar] [CrossRef]
- Bouzidi, A.; Laversenne, L.; Nassif, V.; Elkaim, E.; Zlotea, C. Hydrogen Storage Properties of a New Ti-V-Cr-Zr-Nb High Entropy Alloy. Hydrogen 2022, 3, 270–284. [Google Scholar] [CrossRef]
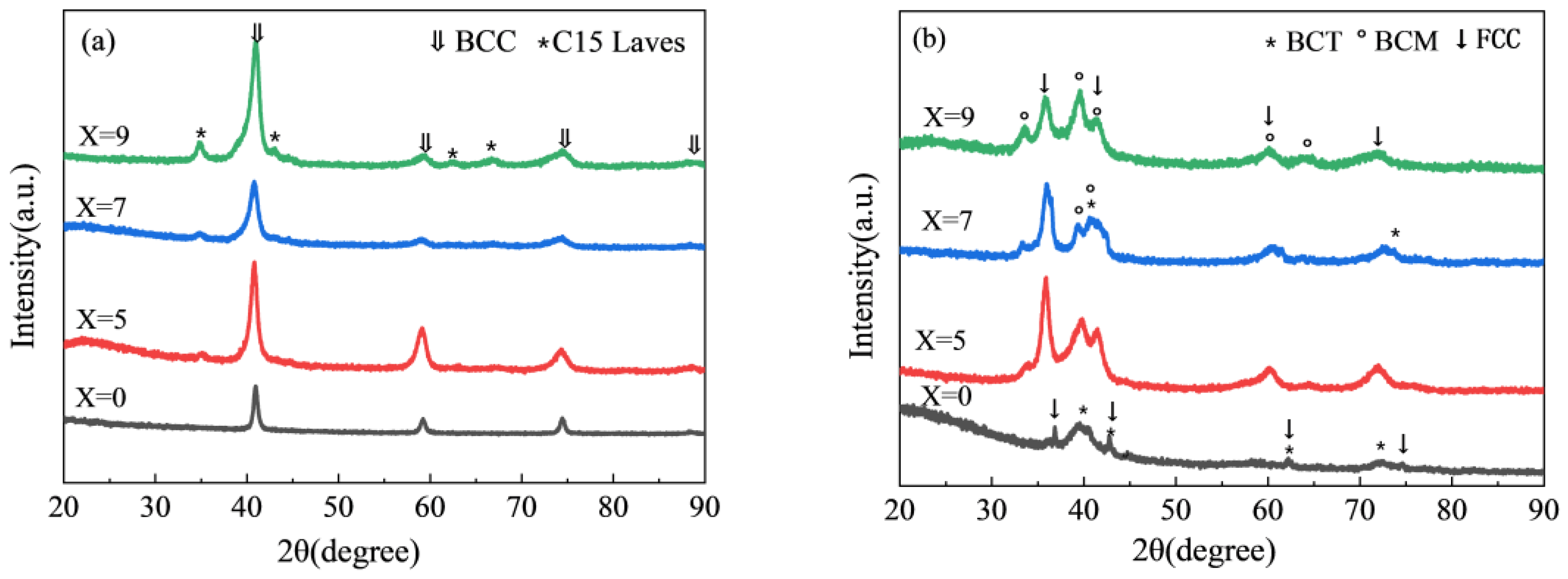
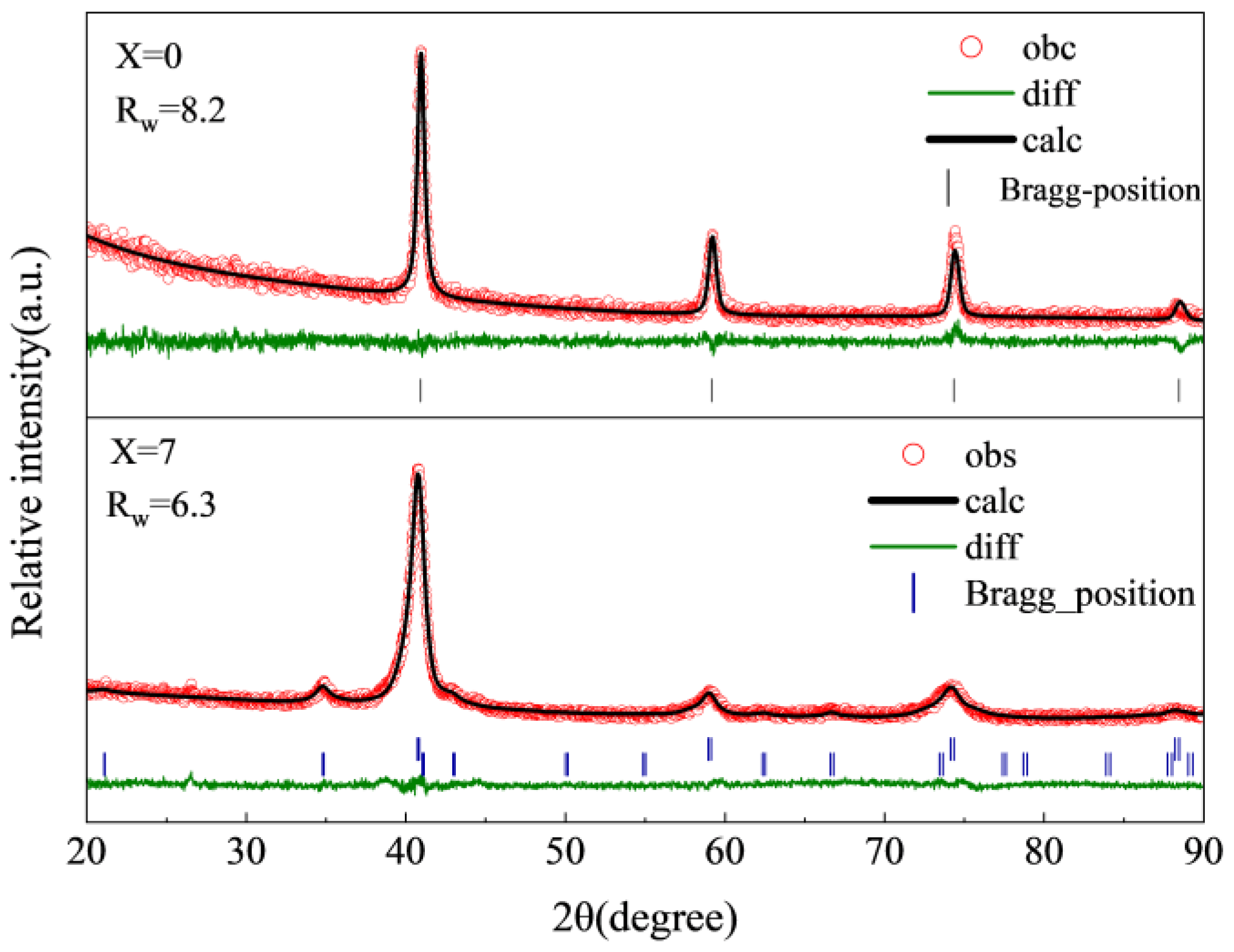



| X | Rw | Phase | Lattice Parameter (Å) | Phase Abundance (wt%) |
|---|---|---|---|---|
| X = 0 | 8.2 | BCC | 3.128 (0) | 100 |
| X = 7 | 6.3 | BCC | 3.133 (8) | 85.9 |
| C15 Laves | 7.288 (8) | 14.1 |
| Zr Content | Phase | Compositions (at%) | |||
|---|---|---|---|---|---|
| Ti | V | Cr | Zr | ||
| X = 0 | BCC | 50.93 | 24.7 | 24.36 | - |
| X = 5 | BCC | 46.17 | 26.69 | 24.43 | 2.69 |
| C15 Laves | 38.67 | 17.5 | 25.68 | 18.13 | |
| X = 7 | BCC | 43.82 | 27.28 | 25.75 | 3.15 |
| C15 Laves | 39.17 | 17.08 | 24.72 | 19.42 | |
| x = 9 | BCC | 41.46 | 30.14 | 25.40 | 3.3 |
| C15 Laves | 34.75 | 17.96 | 26.69 | 20.61 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, Q.; Wang, F.; Li, Z.; Rong, M.; Wang, J.; Wang, Z. Influence of Zr Addition on the Microstructure and Hydrogenation Kinetics of Ti50−xV25Cr25Zrx (x = 0, 5, 7, and 9) Alloys. Materials 2024, 17, 1366. https://doi.org/10.3390/ma17061366
Zeng Q, Wang F, Li Z, Rong M, Wang J, Wang Z. Influence of Zr Addition on the Microstructure and Hydrogenation Kinetics of Ti50−xV25Cr25Zrx (x = 0, 5, 7, and 9) Alloys. Materials. 2024; 17(6):1366. https://doi.org/10.3390/ma17061366
Chicago/Turabian StyleZeng, Qianying, Feng Wang, Zhengxi Li, Maohua Rong, Jiang Wang, and Zhongmin Wang. 2024. "Influence of Zr Addition on the Microstructure and Hydrogenation Kinetics of Ti50−xV25Cr25Zrx (x = 0, 5, 7, and 9) Alloys" Materials 17, no. 6: 1366. https://doi.org/10.3390/ma17061366






