Glucose-Assisted Synthesis of Porous, Urchin-like Co3O4 Hierarchical Structures for Low-Concentration Hydrogen Sensing Materials
Abstract
:1. Introduction
2. Experimental Section
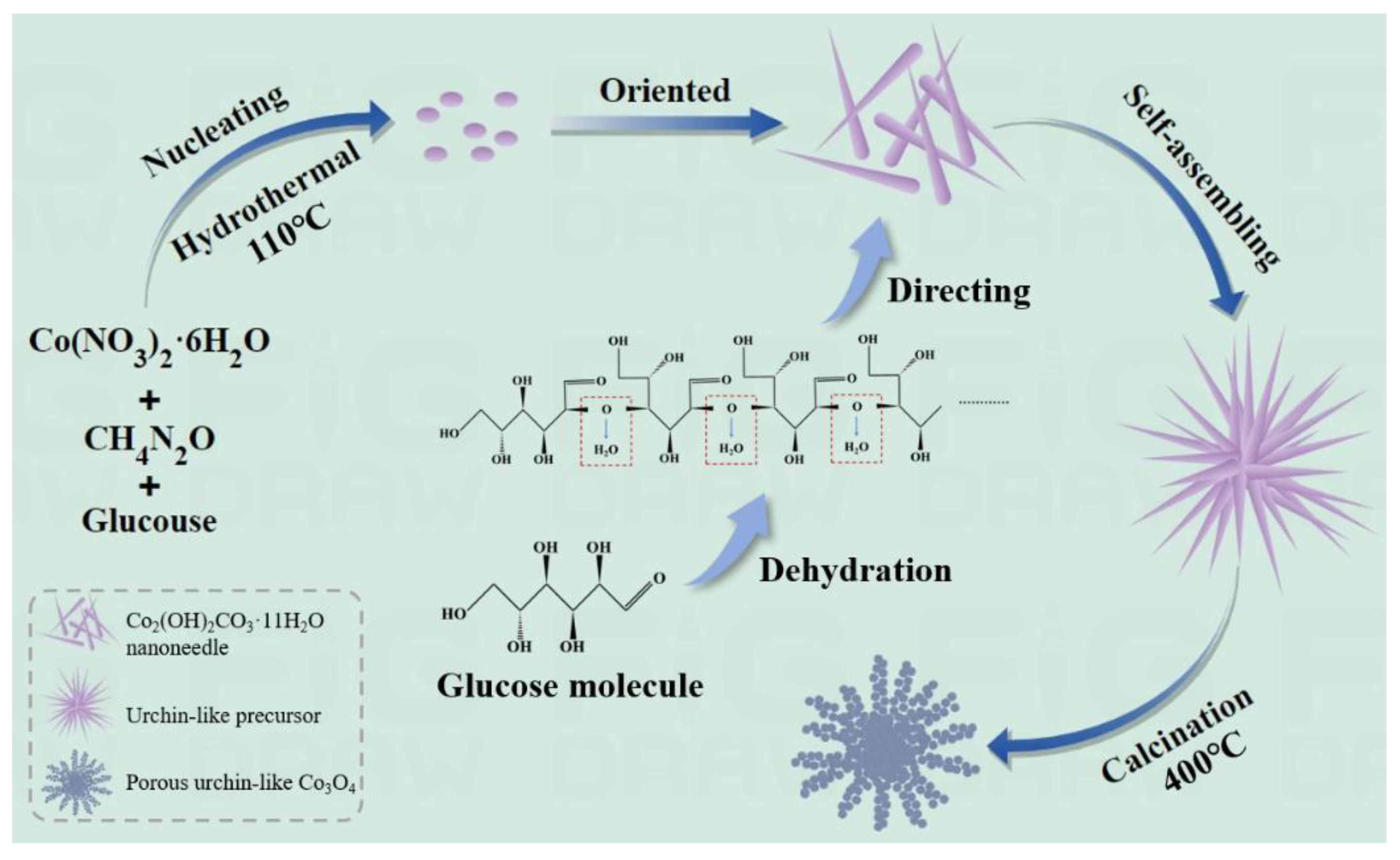
2.1. Preparation of Porous, Urchin-like Co3O4 Hierarchical Structure
2.2. Characterization Methods
2.3. Measurement of Gas-Sensing Performance
3. Results and Discussion
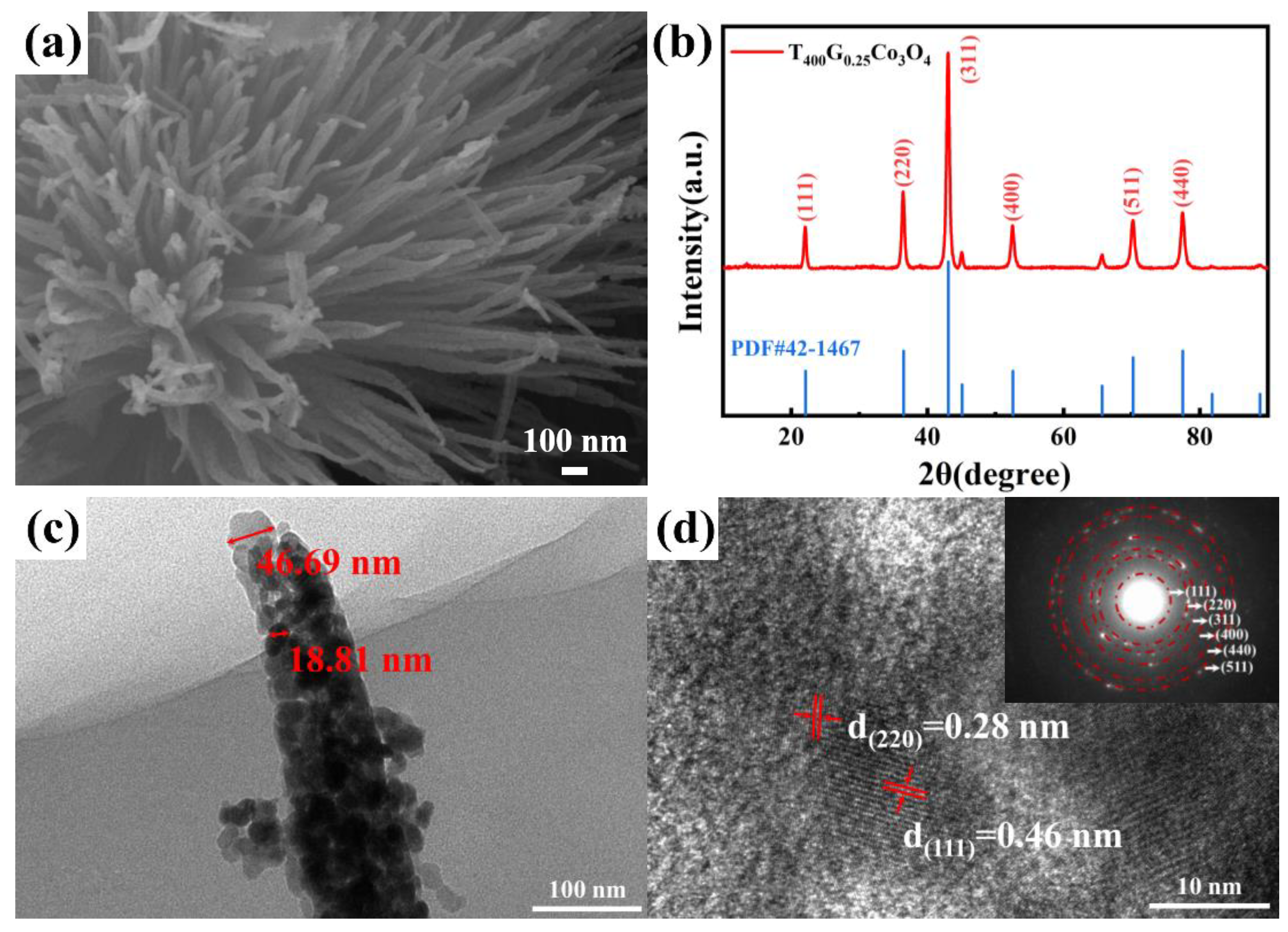
3.1. Morphology and Structure of Porous, Urchin-like Co3O4
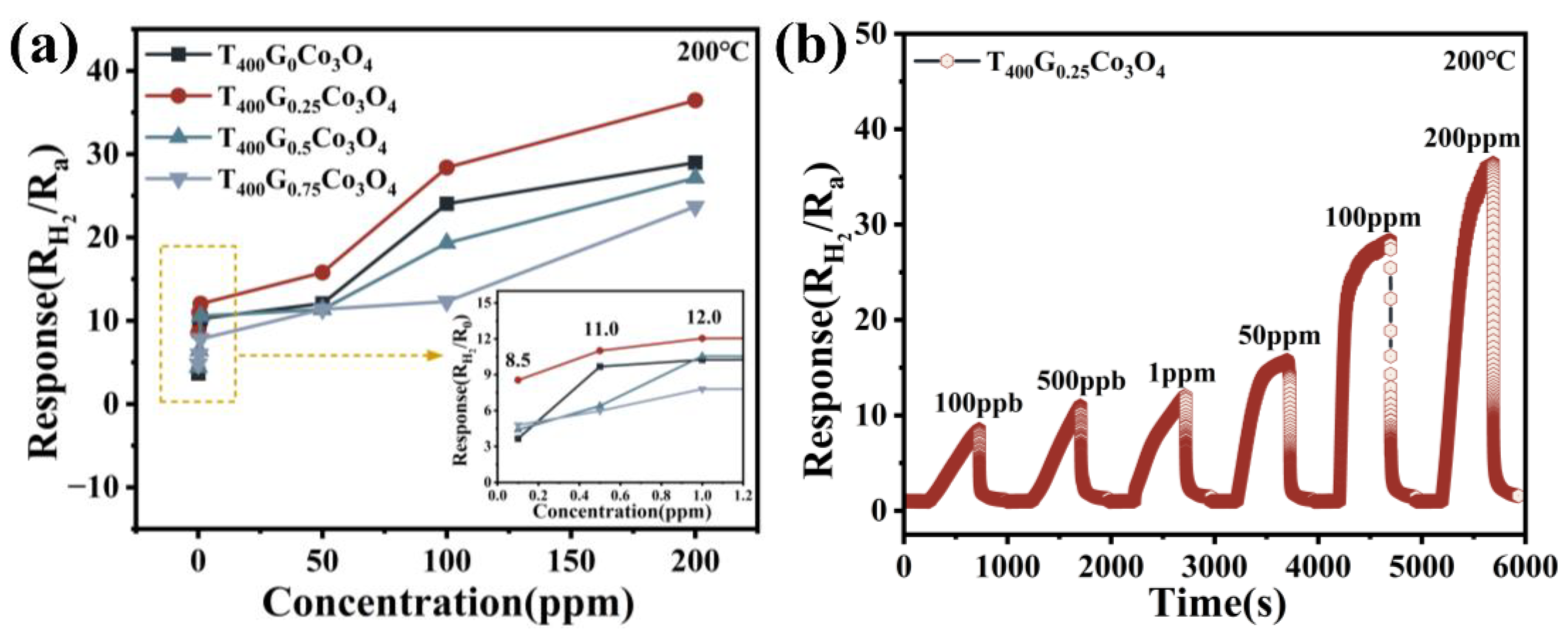
3.2. Hydrogen-Sensing Performance of Porous, Urchin-like Co3O4
3.3. Hydrogen-Sensing Mechanism and Performance Analysis of Porous, Urchin-like Co3O4
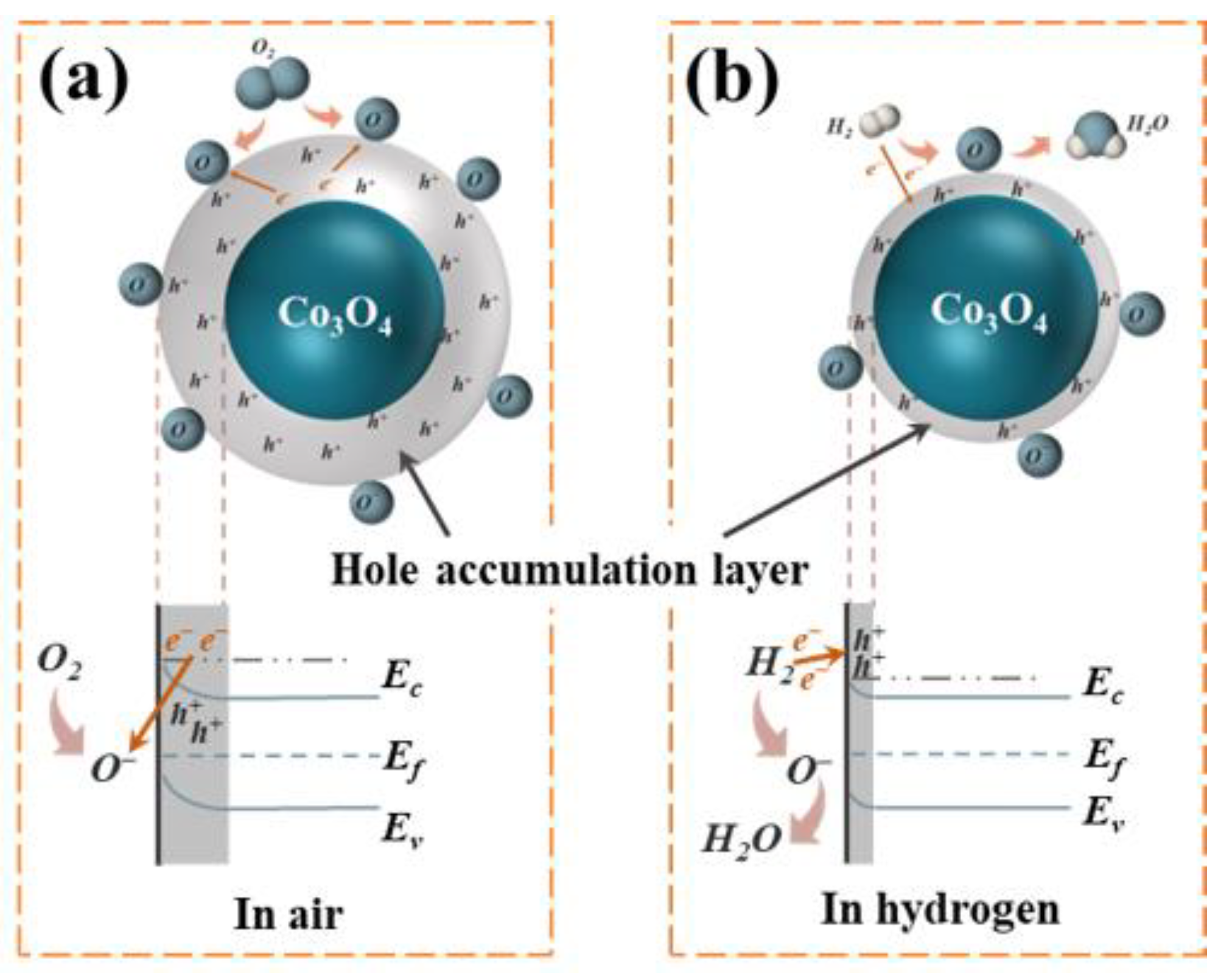
3.3.1. Hydrogen-Sensing Mechanism of Co3O4
3.3.2. Analysis of Hydrogen-Sensing Performance of Porous, Urchin-like Co3O4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrog. Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Rasul, M.G.; Hazrat, M.A.; Sattar, M.A.; Jahirul, M.I.; Shearer, M.J. The future of hydrogen: Challenges on production, storage and applications. Energy Convers. Manag. 2022, 272, 116326. [Google Scholar] [CrossRef]
- Hübert, T.; Boon-Brett, L.; Black, G.; Banach, U. Hydrogen sensors—A review. Sens. Actuators B Chem. 2011, 157, 329–352. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen production for energy: An overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Kruefu, V.; Inpan, U.; Leangtanom, P.; Arkarvipath, C.; Kongpark, P.; Phokharatkul, D.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Enhanced Gas-Sensing Performances of Ru-Loaded p-Type Co3O4 Nanoparticles. Physica Status Solidi (a) 2018, 215, 1701015. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Akhond, M.R.; Islam, M.J.; Rahaman, M.; Alam, R.B.; Ul-hamid, A.; Islam, M.R. A combined experimental and theoretical study on the structural, optical and electronic properties of hetero interface-functionalized MoS2/Co3O4 nanocomposite. Surf. Interfaces 2023, 37, 102750. [Google Scholar] [CrossRef]
- Makole, R.; Tshabalala, Z.P.; Swart, H.C.; Coetsee-Hugo, L.; Leshabane, N.; Motaung, D.E. Fabrication of one-dimensional porous p-type Co3O4 rods-based sensors for ultra-high sensitivity and selectivity towards benzene vapour. Mater. Today Commun. 2024, 38, 108426. [Google Scholar] [CrossRef]
- Kumarage, G.W.C.; Zappa, D.; Mihalcea, C.G.; Maraloiu, V.-A.; Stefan, M.; Comini, E. Revolutionizing n-type Co3O4 Nanowire for Hydrogen Gas Sensing. Adv. Energy Sustain. Res. 2023, 4, 2300067. [Google Scholar] [CrossRef]
- Bhalerao, K.D.; Khan, M.; Nakate, Y.T.; Kadam, R.M.; Manzoor, S.; Masrat, S.; Mishra, P.; Nakate, U.T.; Ahmad, R. Co3O4 hexagonal nanodisks: Synthesis and efficient ethanol gas sensing application. Surf. Interfaces 2023, 42, 103350. [Google Scholar] [CrossRef]
- Hu, J.; Xiong, X.; Guan, W.; Long, H. Controllable fabrication of hierarchical bubble-like Co3O4 tubes with enhanced trimethylamine sensing performance. Sens. Actuators B Chem. 2023, 389, 133886. [Google Scholar] [CrossRef]
- Su, C.; Zhang, L.; Han, Y.; Chen, X.; Wang, S.; Zeng, M.; Hu, N.; Su, Y.; Zhou, Z.; Wei, H.; et al. Glucose-assisted synthesis of hierarchical flower-like Co3O4 nanostructures assembled by porous nanosheets for enhanced acetone sensing. Sens. Actuators B Chem. 2019, 288, 699–706. [Google Scholar] [CrossRef]
- Yusof, N.M.; Rozali, S.; Ibrahim, S.; Siddick, S.Z. Synthesis of hybridized fireworks-like go-Co3O4 nanorods for acetone gas sensing applications. Mater. Today Commun. 2023, 35, 105516. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, Y. Controllable synthesis of porous Co3O4 nanorods and their ethanol-sensing performance. Ceram. Int. 2022, 48, 29659–29668. [Google Scholar] [CrossRef]
- Fang, B.; Yao, H.; Xiao, X.; Zhang, Q.; Zhang, X.; Yang, W. Metal-organic frameworks-derived hollow Co3O4 nanotubes for efficient detection of toluene vapor. J. Alloys Compd. 2023, 937, 168535. [Google Scholar] [CrossRef]
- Zhang, R.; Gao, S.; Zhou, T.; Tu, J.; Zhang, T. Facile preparation of hierarchical structure based on p-type Co3O4 as toluene detecting sensor. Appl. Surf. Sci. 2020, 503, 144167. [Google Scholar] [CrossRef]
- Yang, B.; Myung, N.V.; Tran, T. 1D Metal Oxide Semiconductor Materials for Chemiresistive Gas Sensors: A Review. Adv. Electron. Mater. 2021, 7, 2100271. [Google Scholar] [CrossRef]
- Chang, B.; Gu, Z.; Guo, Y.; Li, Z.; Yang, B. Glucose-assisted synthesis of Co3O4 nanostructure with controllable morphologies from nanosheets to nanowires. J. Alloys Compd. 2016, 676, 26–36. [Google Scholar] [CrossRef]
- Xu, J.M.; Zhang, J.; Wang, B.B.; Liu, F. Shape-regulated synthesis of cobalt oxide and its gas-sensing property. J. Alloys Compd. 2015, 619, 361–367. [Google Scholar] [CrossRef]
- Liu, D.; Pervaiz, E.; Adimi, S.; Thomas, T.; Qu, F.; Huang, C.; Wang, R.; Jiang, H.; Yang, M. Theoretical study on W-Co3O4 (111) surface: Acetone adsorption and sensing mechanism. Appl. Surf. Sci. 2021, 566, 150642. [Google Scholar] [CrossRef]
- Ullah, M.; Bai, X.; Chen, J.; Lv, H.; Liu, Z.; Zhang, Y.; Wang, J.; Sun, B.; Li, L.; Shi, K. Metal-organic framework material derived Co3O4 coupled with graphitic carbon nitride as highly sensitive NO2 gas sensor at room temperature. Colloids Surf. A Physicochem. Eng. Asp. 2021, 612, 125972. [Google Scholar] [CrossRef]
- Kishore, K.R.; Teddu, L.B.; Balamurugan, D.; Gopalakrishnan, J.B. Electrospun Co3O4 nanoparticles and its methanol detection property. Appl. Nanosci. 2021, 11, 637–655. [Google Scholar] [CrossRef]
- Manoharan, M.; Govindharaj, K.; Muthumalai, K.; Kumaravel, S.; Haldorai, Y.; Rajendra Kumar, R.T. Interface Oxygen Vacancy-Enhanced Co3O4/WO3 Nanorod Heterojunction for Sub-ppm Level Detection of NOx. Adv. Eng. Mater. 2023, 25, 2300727. [Google Scholar] [CrossRef]
- Jiao, Q.; Fu, M.; You, C.; Zhao, Y.; Li, H. Preparation of Hollow Co3O4 Microspheres and Their Ethanol Sensing Properties. Inorg. Chem. 2012, 51, 11513–11520. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.K.N.; Brown, J.J. Hydrogen sensors using 2-dimensional materials: A review. Chem. Sel. 2020, 5, 7277–7297. [Google Scholar] [CrossRef]
- Krishna, K.G.; Parne, S.; Pothukanuri, N.; Kathirvelu, V.; Gandi, S.; Joshi, D. Nanostructured metal oxide semiconductor-based gas sensors: A comprehensive review. Sens. Actuators A Phys. 2022, 341, 113578. [Google Scholar] [CrossRef]
- Kumar, S.; Lawaniya, S.D.; Agarwal, S.; Yu, Y.T.; Nelamarri, S.R.; Kumar, M.; Mishra, Y.K.; Awasthi, K. Optimization of Pt nanoparticles loading in ZnO for highly selective and stable hydrogen gas sensor at reduced working temperature. Sens. Actuators B Chem. 2023, 375, 132943. [Google Scholar] [CrossRef]
- Raju, P.; Li, Q. Review—Semiconductor Materials and Devices for Gas Sensors. J. Electrochem. Soc. 2022, 169, 057518. [Google Scholar] [CrossRef]
- Lee, J.H.; Kim, J.Y.; Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Co3O4-loaded ZnO nanofibers for excellent hydrogen sensing. Int. J. Hydrog. Energy 2019, 44, 27499–27510. [Google Scholar] [CrossRef]
- Makole, R.; Tshabalala, Z.P.; Jozela, M.; Cummings, F.R.; Swart, H.C.; Motaung, D.E. Facile synthesis of high surface area of p-n Co3O4-In2O3 heterostructure-based sensor for improved xylene detection at low temperature. Inorg. Chem. Commun. 2023, 158, 111652. [Google Scholar] [CrossRef]
- Kumarage, G.W.C.; Comini, E. Low-Dimensional Nanostructures Based on Cobalt Oxide (Co3O4) in Chemical-Gas Sensing. Chemosensors 2021, 9, 197. [Google Scholar] [CrossRef]
- Kim, H.-J.; Lee, J.-H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Doan, T.L.H.; Kim, J.Y.; Lee, J.H.; Nguyen, L.H.T.; Dang, Y.T.; Bui, K.B.T.; Pham, A.T.T.; Mirzaei, A.; Phan, T.B.; Kim, S.S. Preparation of n-ZnO/p-Co3O4 heterojunctions from zeolitic imidazolate frameworks (ZIF-8/ZIF-67) for sensing low ethanol concentrations. Sens. Actuators B Chem. 2021, 348, 130684. [Google Scholar] [CrossRef]
- Yin, X.T.; Yang, Z.Y.; Dastan, D.; Liu, Y.; Tan, X.-M.; Gao, X.C.; Zhou, Y.-W.; Li, J.; Ma, X.-G. Sensitivity and selectivity Pt loaded SnO2–Co3O4 gas sensor for hydrogen detection. Ceram. Int. 2023, 49, 38717–38725. [Google Scholar] [CrossRef]
- Pai, S.H.S.; Mondal, A.; Barathy, T.R.; Ajitha, B.; Samuel, E.J.J.; Reddy, Y.A.K. Effect of calcination temperature on NiO for hydrogen gas sensor performance. Int. J. Hydrog. Energy 2024, 50, 928–941. [Google Scholar] [CrossRef]
- Ai, T.; Zhang, J.; Li, J.; Zhang, Y.; Yin, Y.; Lu, J. Ultrafast response of Pt functionalized Fe2(MoO4)3 nanoflower gas sensors for ultra-low ppm level H2 gas detection. J. Alloys Compd. 2024, 970, 172567. [Google Scholar] [CrossRef]
- Roso, S.; Bittencourt, C.; Umek, P.; González, O.; Güell, F.; Urakawa, A.; Llobet, E. Synthesis of single crystalline In2O3 octahedra for the selective detection of NO2 and H2 at trace levels. J. Mater. Chem. C 2016, 4, 9418–9427. [Google Scholar] [CrossRef]
- Wang, Y.T.; Whang, W.T.; Chen, C.-H. Hollow V2O5 Nanoassemblies for High-Performance Room-Temperature Hydrogen Sensors. ACS Appl. Mater. Interfaces 2015, 7, 8480–8487. [Google Scholar] [CrossRef] [PubMed]
- Maji, B.; Barik, B.; Sahoo, S.J.; Achary, L.S.K.; Kumar Sahoo, K.; Kar, J.P.; Dash, P. Shape selective comprehensive gas sensing study of different morphological manganese-cobalt oxide based nanocomposite as potential room temperature hydrogen gas sensor. Sens. Actuators B Chem. 2023, 380, 133348. [Google Scholar] [CrossRef]
- Zhou, T.; Sang, Y.; Wang, X.; Wu, C.; Zeng, D.; Xie, C. Pore size dependent gas-sensing selectivity based on ZnO@ZIF nanorod arrays. Sens. Actuators B Chem. 2018, 258, 1099–1106. [Google Scholar] [CrossRef]
- Ambardekar, V.; Bandyopadhyay, P.P.; Majumder, S.B. Atmospheric plasma sprayed SnO2 coating for ethanol detection. J. Alloys Compd. 2018, 752, 440–447. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, W.; Han, J.; Gao, Y.; Gao, Y.; Zhao, L.; Sun, P.; Lu, G. Mesoporous Co3O4 nanosheets with exposed Co2+-rich crystal facets for improved toluene detection. Appl. Surf. Sci. 2023, 619, 156714. [Google Scholar] [CrossRef]
- Qin, C.; Wang, B.; Wu, N.; Han, C.; Wu, C.; Zhang, X.; Tian, Q.; Shen, S.; Li, P.; Wang, Y. Metal-organic frameworks derived porous Co3O4 dodecahedeons with abundant active Co3+ for ppb-level CO gas sensing. Appl. Surf. Sci. 2020, 506, 144900. [Google Scholar] [CrossRef]
- Giri, S.; Anantharamaiah, P.N.; Sahoo, B. Sensing of oxidizing and reducing gases by sensors prepared using nanoscale Co3O4 powders: A study through Cu substitution. Adv. Powder Technol. 2022, 33, 103529. [Google Scholar] [CrossRef]

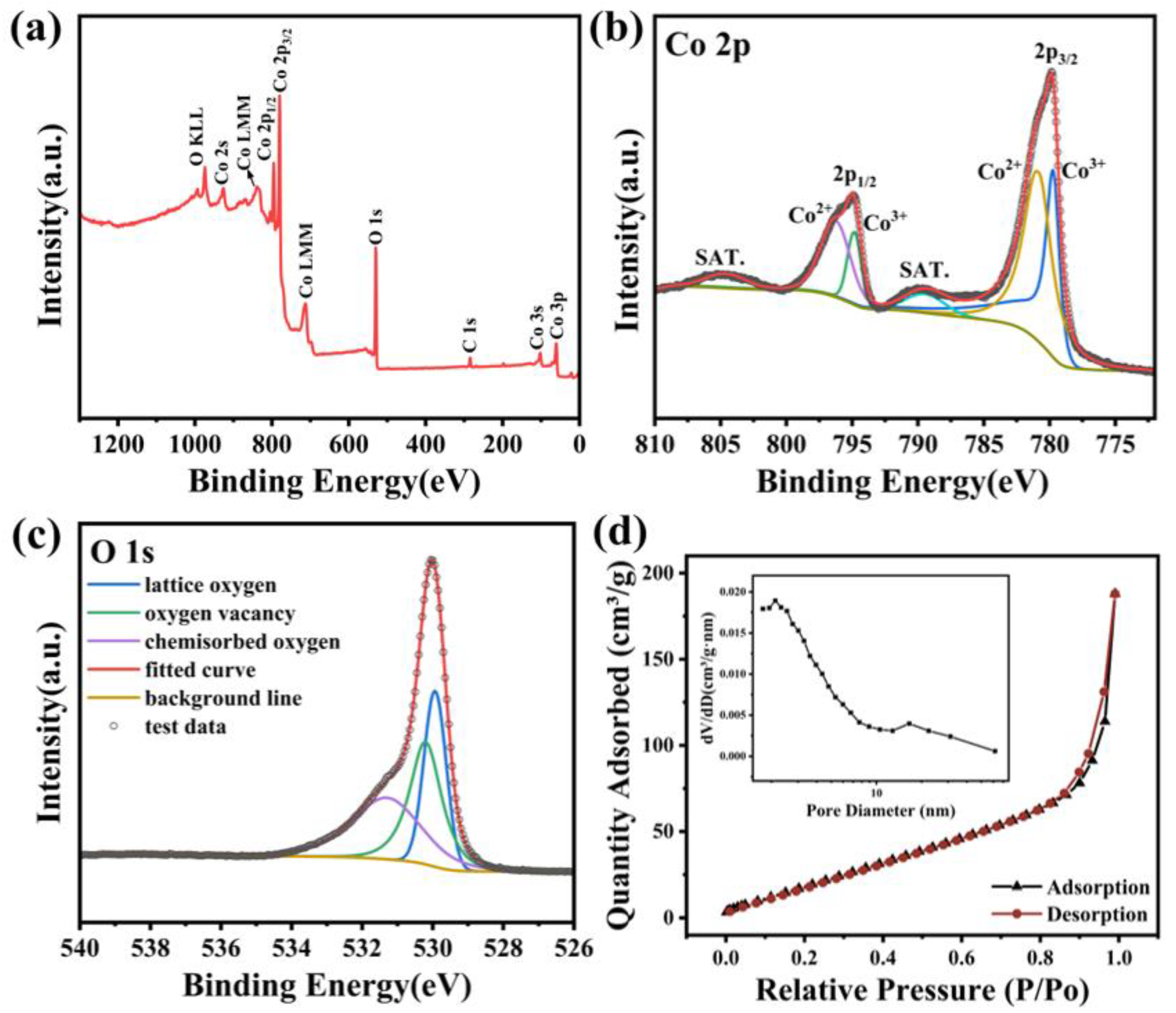

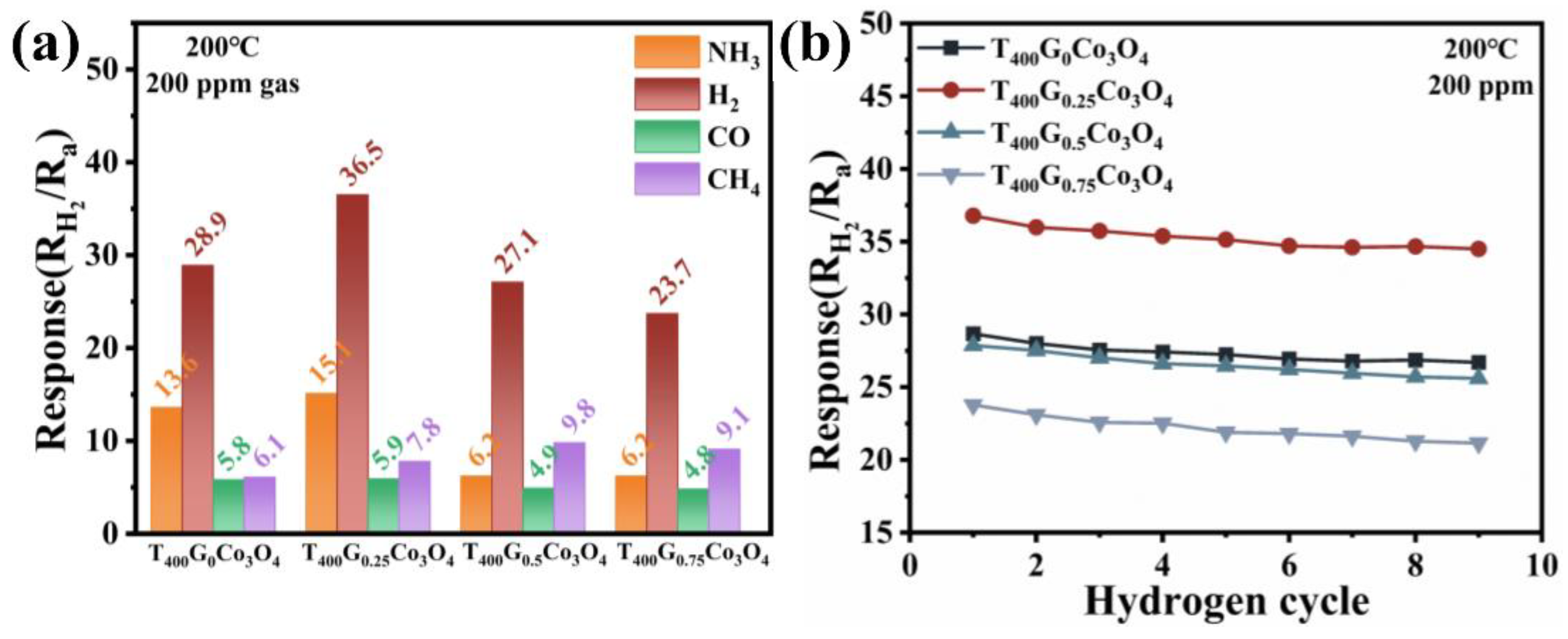
| Sample | Specific Surface Areas (m2/g) | Average Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| T400G0Co3O4 | 42.7 | 32.7 | 0.285 |
| T400G0.25Co3O4 | 81.4 | 11.0 | 0.449 |
| T400G0.5Co3O4 | 57.5 | 20.3 | 0.379 |
| T400G0.75Co3O4 | 55.3 | 22.6 | 0.407 |
| Sample | NH3 | CO | CH4 |
|---|---|---|---|
| T400G0Co3O4 | 2.13 | 4.98 | 4.74 |
| T400G0.25Co3O4 | 2.42 | 6.19 | 4.68 |
| T400G0.5Co3O4 | 4.37 | 5.53 | 2.77 |
| T400G0.75Co3O4 | 3.82 | 4.94 | 2.60 |
| Materials | Structure | Working Temperature (°C) | Concentration (ppm) | Response | Detection Limit (ppm) | Ref. |
|---|---|---|---|---|---|---|
| Pd-SnO2/Co3O4 | nanoparticle | 300 | 100 | 57.9 | 10 | [34] |
| NiO | nanofilm | 250 | 200 | 35.7 * | 50 | [35] |
| 1 at.% Pt-ZnO | pencil-like microrods | 150 | 100 | 2.8 * | 10 | [27] |
| Pt-Fe2(MoO4)3 | nanoflower | 300 | 10 | 3.1 * | 1 | [36] |
| In2O3 | octahedra | 260 | 500 | 14 | 4 | [37] |
| V2O5 | hollow structure | 25 | 200 | 2.9 | 10 | [38] |
| MnCo2O4/r-GO | flower | 160 | 250 | 1.1 | 100 | [39] |
| T400G0.25Co3O4 | urchin-like | 200 | 200 | 36.5 | 0.1 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Zhang, X.; Long, X.; Liu, X. Glucose-Assisted Synthesis of Porous, Urchin-like Co3O4 Hierarchical Structures for Low-Concentration Hydrogen Sensing Materials. Materials 2024, 17, 1364. https://doi.org/10.3390/ma17061364
Deng X, Zhang X, Long X, Liu X. Glucose-Assisted Synthesis of Porous, Urchin-like Co3O4 Hierarchical Structures for Low-Concentration Hydrogen Sensing Materials. Materials. 2024; 17(6):1364. https://doi.org/10.3390/ma17061364
Chicago/Turabian StyleDeng, Xin, Xiao Zhang, Xiaochuan Long, and Xiaopeng Liu. 2024. "Glucose-Assisted Synthesis of Porous, Urchin-like Co3O4 Hierarchical Structures for Low-Concentration Hydrogen Sensing Materials" Materials 17, no. 6: 1364. https://doi.org/10.3390/ma17061364





