Changing Template/Al2O3 Ratio in Reaction Gel—An Effective Way to Regulate Nature of Intermediate Phases and Properties of SAPO-11 Molecular Sieves during Crystallization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Silicoaluminophosphate Gels
2.2. Crystallization of Silicoaluminophosphate Molecular Sieves SAPO-11
2.3. Characterization
2.4. Hydroisomerization of n-Hexadecane
3. Results and Discussion
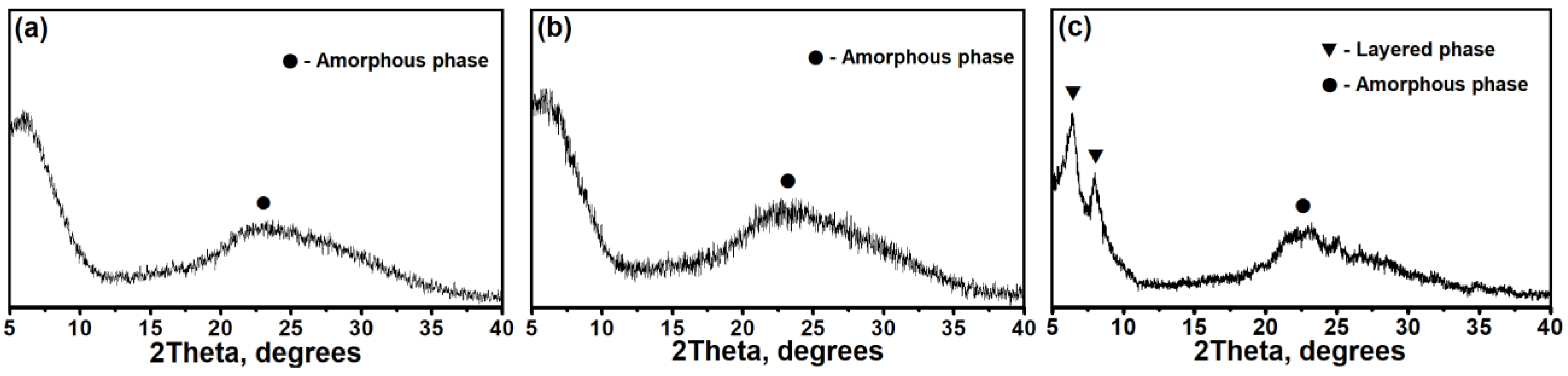
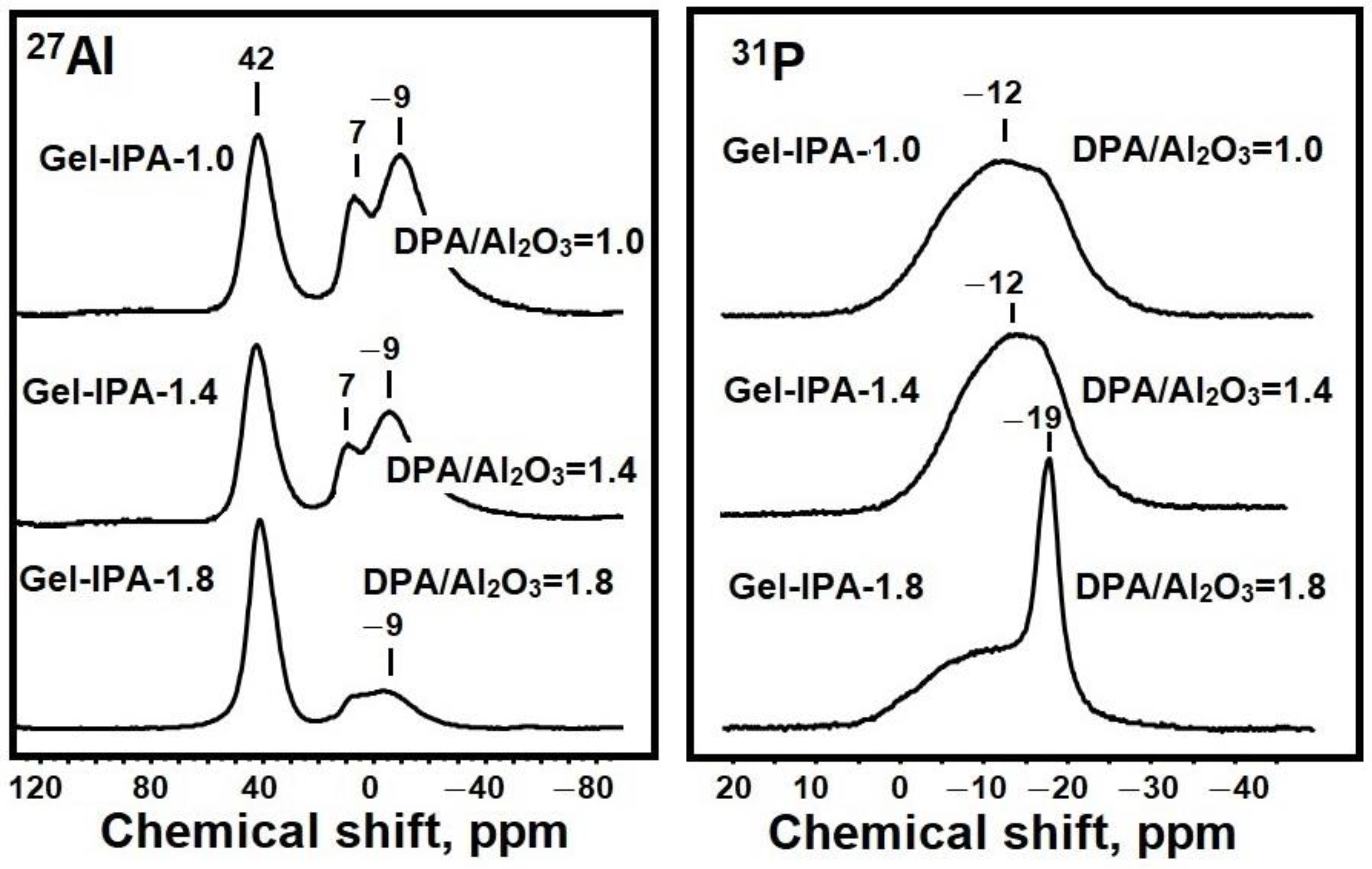
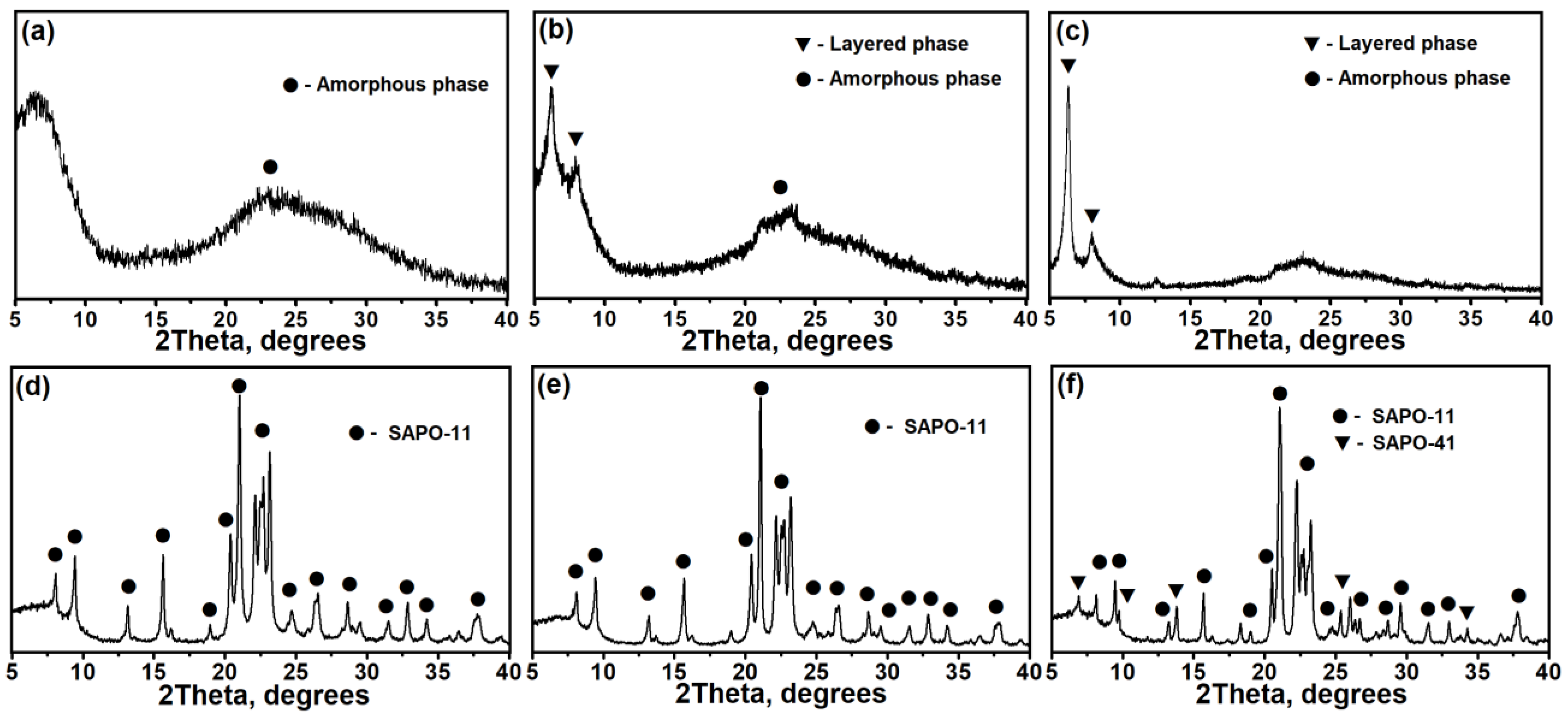
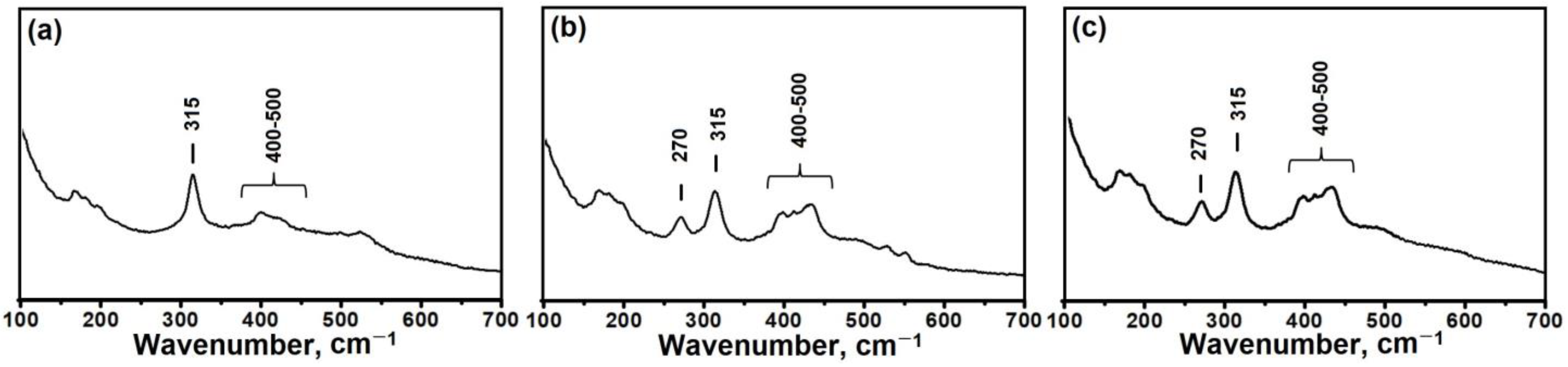
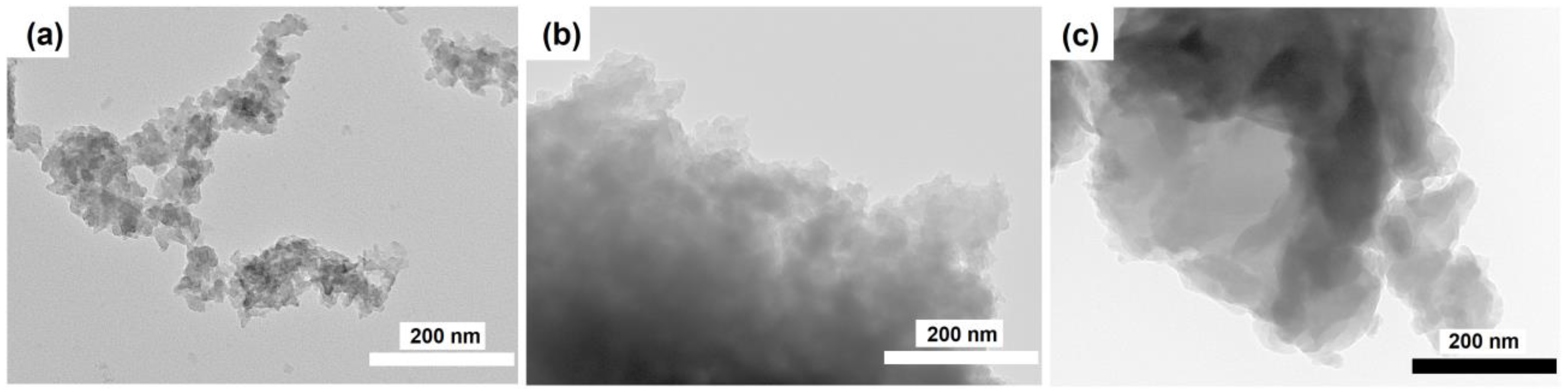
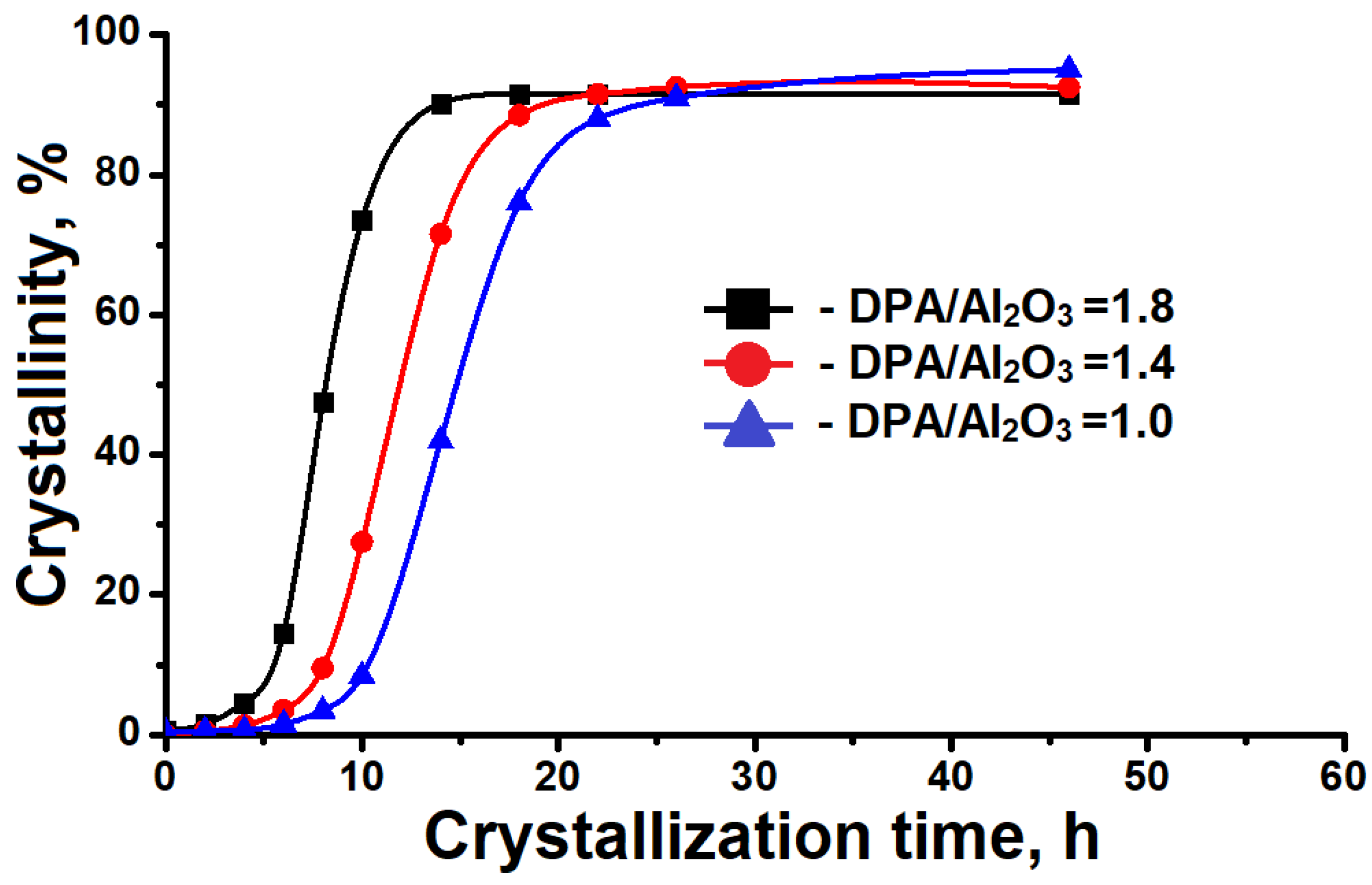
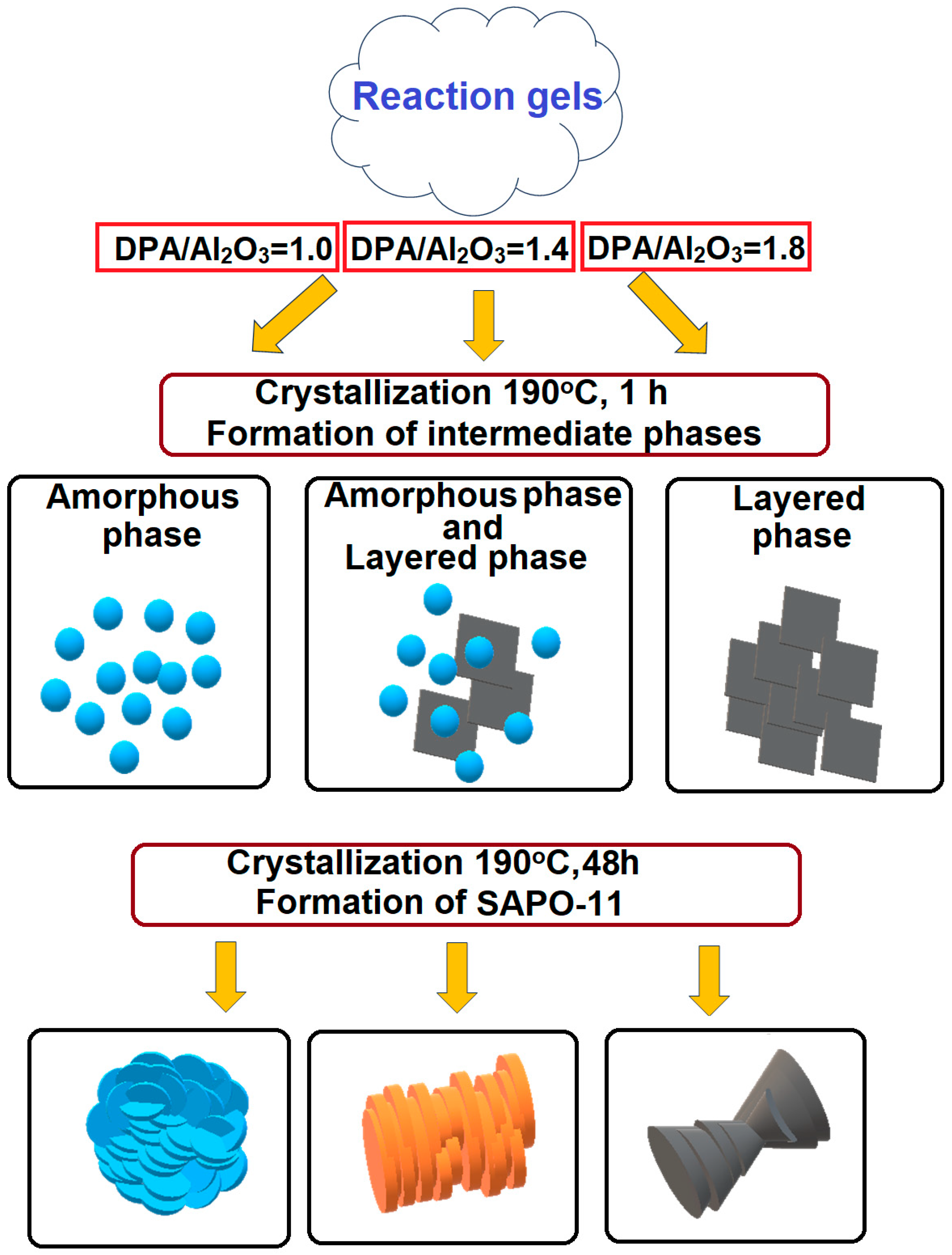
3.1. The Effect of the DPA/Al2O3 Ratio on the Formation of Intermediate Phases and Crystallization of SAPO-11
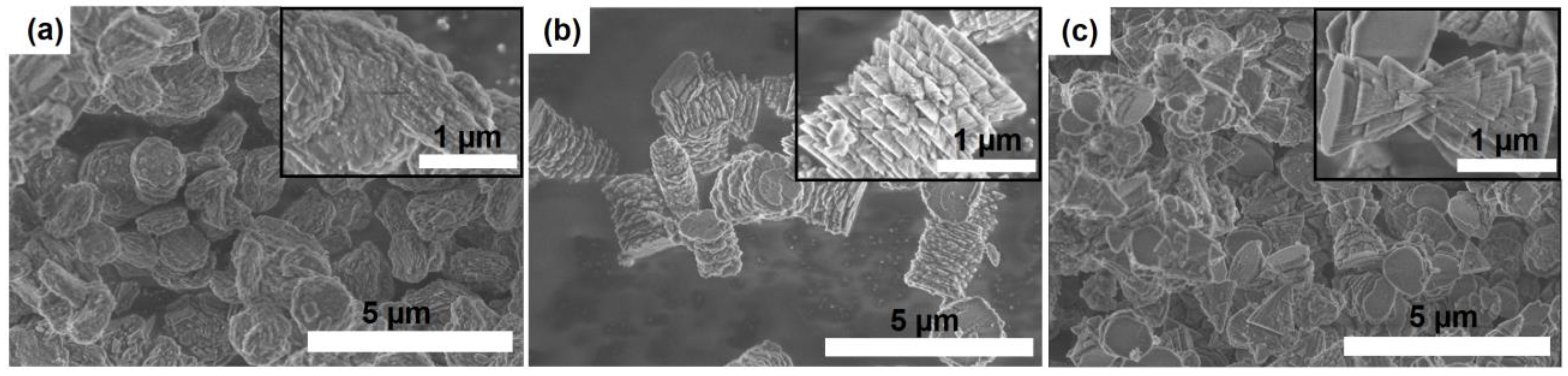
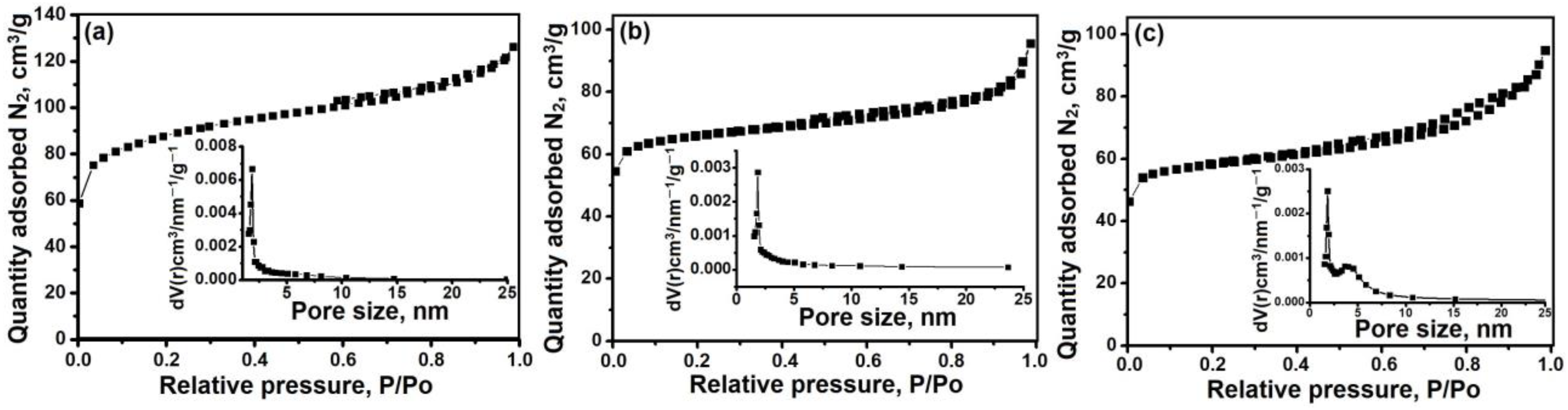
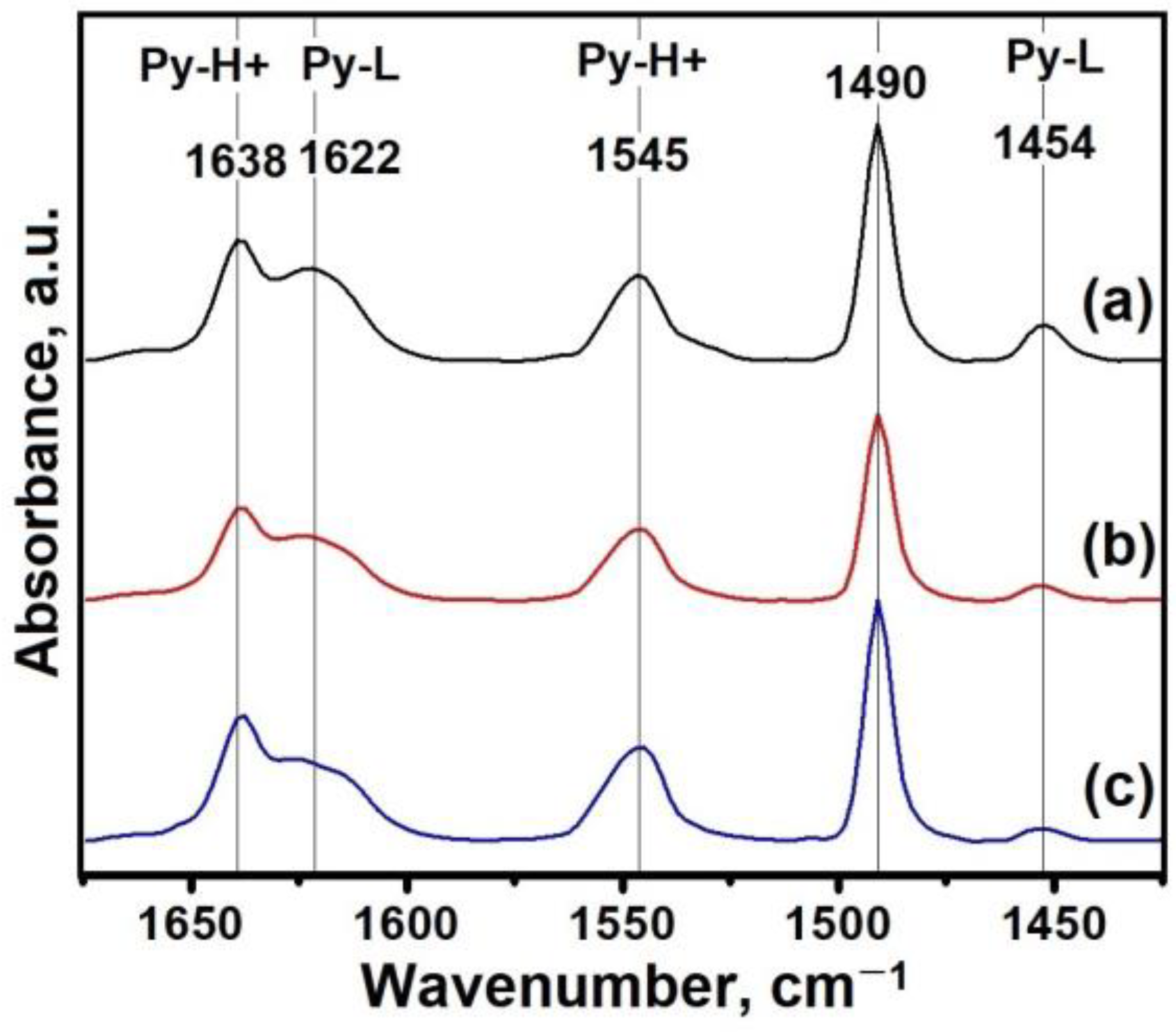
3.2. The Effect of Intermediate Phases on the Properties of SAPO-11 Molecular Sieves
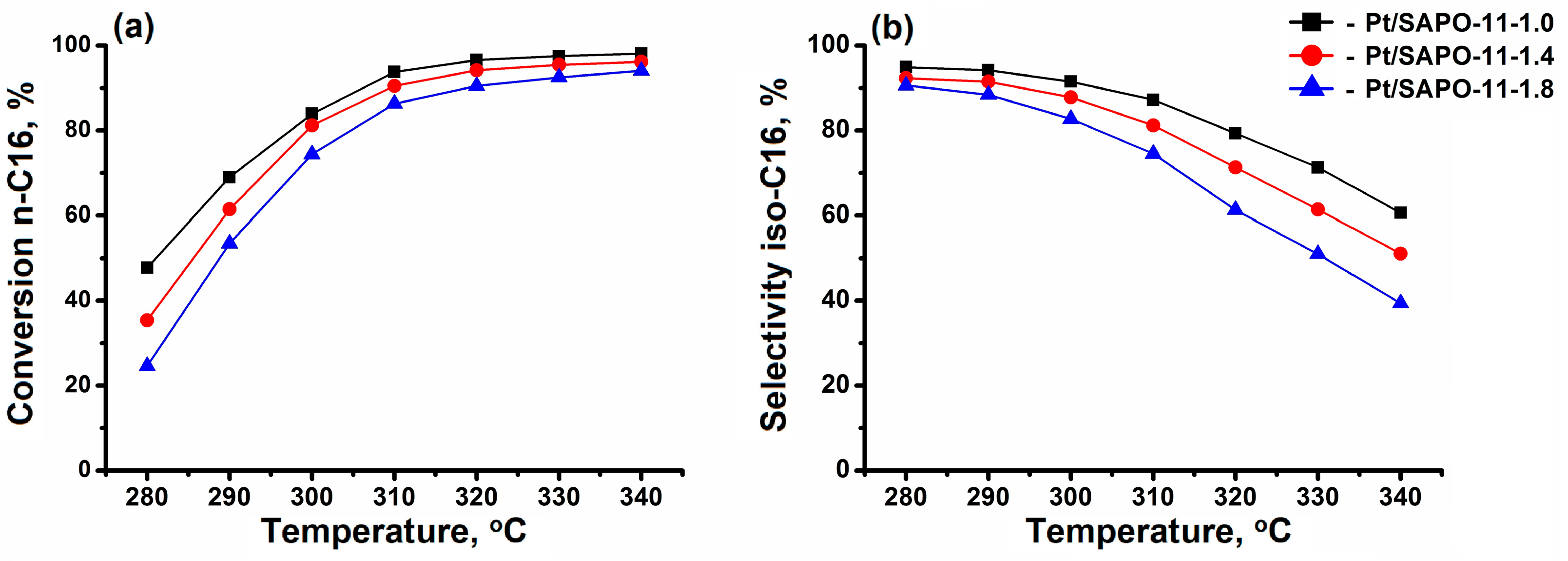
3.3. The Catalytic Properties of SAPO-11 in the Hydroisomerization of n-C16
4. Conclusions
- -
- Intermediate phases with a layered structure crystallize into SAPO-11 molecular sieves faster than amorphous systems due to the closeness of their structure to molecular sieves.
- -
- The di-n-propylamine/Al2O3 ratio in the reaction gels affects the morphology and size of primary SAPO-11 crystals. It was found that using isopropoxide as an aluminum source allows the preparation of SAPO-11 with a hierarchical porous structure. SAPO-11 samples synthesized at a DPA/Al2O3 ratio = 1.0 are characterized by SBET = 286 m2/g and Vmeso = 0.13 cm3/g and are spherical-like aggregates (1–1.5 μm) of primary crystals in the form of thin plates of ~100 nm. The SAPO-11 samples prepared at DPA/Al2O3 = 1.4 represent conglomerates in the form of cylinders of ~1–2 μm formed from mated cones ~200–300 nm and are characterized by SBET = 209 m2/g and Vmeso = 0.08 cm3/g. With a further increase in the DPA/Al2O3 ratio of up to =1.8, crystals in the form of conjoined pyramids of ~1–1.5 μm in size are formed and characterized by SBET = 184 m2/g and Vmeso = 0.09 cm3/g.
- -
- The synthesized DPA/Al2O3 ratio = 1.0 sample SAPO-11, with nanocrystals of cubic morphology, provides higher values of n-hexadecane conversion and selectivity for C16 isomers due to smaller crystal sizes, which reduce the diffusion limitations and decrease the residence time of the reaction products inside the channels compared to the samples with larger crystals.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Primo, A.; Garcia, H. Zeolites as catalysts in oil refining. Chem. Soc. Rev. 2014, 43, 7548–7561. [Google Scholar] [CrossRef]
- Vermeiren, W.; Gilson, J.P. Impact of zeolites on the petroleum and petrochemical industry. Top. Catal. 2009, 52, 1131–1161. [Google Scholar] [CrossRef]
- Potter, M.E. Down the microporous rabbit hole of silicoaluminophosphates: Recent developments on synthesis, characterization, and catalytic applications. ACS Catal. 2020, 10, 9758–9789. [Google Scholar] [CrossRef]
- Hartmann, M.; Elangovan, S. Catalysis with Microporous Aluminophosphates and Silicoaluminophosphates Containing Transition Metals. Adv. Nanoporous Mater. 2010, 1, 237–312. [Google Scholar]
- Tian, P.; Wei, Y.; Ye, M.; Liu, Z. Methanol to olefins (MTO): From fundamentals to commercialization. ACS Catal. 2015, 5, 1922–1938. [Google Scholar] [CrossRef]
- Yadav, R.; Sakthivel, A. Silicoaluminophosphate molecular sieves as potential catalysts for hydroisomerization of alkanes and alkenes. Appl. Catal. A Gen. 2014, 481, 143–160. [Google Scholar] [CrossRef]
- Database of Zeolite Structure. Available online: http://www.iza-structure.org/databases (accessed on 20 January 2024).
- Barthomeuf, D. Topological model for the compared acidity of SAPOs and SiAl zeolites. Zeolites 1994, 14, 394–401. [Google Scholar] [CrossRef]
- Wang, W.; Liu, C.-J.; Wu, W. Bifunctional catalysts for the hydroisomerization of n-alkanes: The effects of metal-acid balance and textural structure. Catal. Sci. Technol. 2019, 9, 4162–4187. [Google Scholar] [CrossRef]
- Deldari, H. Suitable catalysts for hydroisomerization of long-chain normal paraffins. Appl. Catal. A Gen. 2005, 293, 1–10. [Google Scholar] [CrossRef]
- Halim, E.; Lee, C.-P.; Wang, W.-C.; Lin, J.-K.; Lin, Y.-C. Production of hydro-processed renewable jet fuel over SAPO-11-based catalyst. Int. J. Energy Res. 2022, 46, 1059–1076. [Google Scholar] [CrossRef]
- Ma, J.; Liu, X.; Yuan, H. In situ synthesis of mesoporous Pt/SAPO–11 for the preparation of biological aviation kerosene. J. Porous Mater. 2022, 29, 1387–1398. [Google Scholar] [CrossRef]
- Shamanaev, I.V.; Shamanaeva, I.A.; Parkhomchuk, E.V.; Bukhtiyarova, G.A. Hydrodeoxygenation–Isomerization of Methyl Palmitate over SAPO-11-Supported Ni-Phosphide Catalysts. Catalysts 2022, 12, 1486. [Google Scholar] [CrossRef]
- Ding, Y.; Lin, J.; Yu, P.; Zhang, H.; Ma, Y.; Cai, Z.; Cao, Y.; Huang, K.; Jiang, L. An in-situ combo Mo-based ionic liquid and SAPO-11 catalyst for efficient biolipids hydrodeoxygenation and isomerization. Fuel 2024, 362, 130827. [Google Scholar] [CrossRef]
- Khan, S.; Qureshi, K.M.; Lup, A.N.K.; Patah, M.F.A.; Daud, W.M.A.W. Role of Ni–Fe/ZSM-5/SAPO-11 bifunctional catalyst on hydrodeoxygenation of palm oil and triolein for alternative jet fuel production. Biomass Bioenergy 2022, 164, 106563. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Arzumanov, S.S.; Gerasimov, E.Y.; Grigorieva, N.G.; Bikbaeva, V.R.; Serebrennikov, D.V.; Khalilov, L.M.; Kutepov, B.I. Crystal engineering of SAPO-11 sieves by forming intermediate phases. CrystEngComm 2023, 25, 3096–3107. [Google Scholar] [CrossRef]
- Singh, P.S.; Bandyopadhyay, R.; Hegde, S.G.; Rao, B.S. Vapour phase beckmann rearrangement of cyclohexanone oxime over SAPO-11 molecular sieve. Appl. Catal. A Gen. 1996, 136, 249–263. [Google Scholar] [CrossRef]
- Yang, Z.; Li, J.; Liu, Y.; Liu, C. Effect of silicon precursor on silicon incorporation in SAPO-11 and their catalytic performance for hydroisomerization of n-octane on Pt-based catalysts. J. Energy Chem. 2017, 26, 688–694. [Google Scholar] [CrossRef]
- Liu, P.; Ren, J.; Sun, Y. Synthesis, characterization and catalytic properties of SAPO-11 with high silicon dispersion. Catal. Commun. 2008, 9, 1804–1809. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, W.; Guo, S.; Zhao, L.; Xiang, H. Optimization of the synthesis of SAPO-11 for the methylation of naphthalene with methanol by varying templates and template content. J. Braz. Chem. Soc. 2013, 24, 1180–1187. [Google Scholar] [CrossRef]
- Fernandes, A.; Ribeiro, F.; Lourenço, J.; Gabelica, Z. An elegant way to increase acidity in SAPOs: Use of methylamine as co-template during synthesis. Stud. Surf. Sci. Catal. 2008, 174, 281–284. [Google Scholar]
- Liu, P.; Ren, J.; Sun, Y. Acidity and Isomerization Activity of SAPO-11 Synthesized by an Improved Hydrothermal Method. Chin. J. Catal. 2008, 29, 379–384. [Google Scholar] [CrossRef]
- Blasco, T.; Chica, A.; Corma, A.; Murphy, W.J.; Agúndez-Rodríguez, J.; Pérez-Pariente, J. Changing the Si distribution in SAPO-11 by synthesis with surfactants improves the hydroisomerization/dewaxing properties. J. Catal. 2006, 242, 153–161. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, Y.; Sun, Q.; Dai, Z.; Gies, H.; Wu, Q.; Pan, S.; Bian, C.; Tian, Z.; Meng, X.; et al. Design and preparation of efficient hydroisomerization catalysts by the formation of stable SAPO-11 molecular sieve nanosheets with 10–20 nm thickness and partially blocked acidic sites. Chem. Commun. 2017, 53, 4942–4945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Song, W.; Zhu, S.; Lai, W.; Yi, X.; Fang, W. Synthesis of a multi-branched dandelion-like SAPO-11 by an in situ inoculating seed-induced-steam-assisted conversion method (SISAC) as a highly effective hydroisomerization support. RSC Adv. 2017, 7, 4656–4666. [Google Scholar] [CrossRef]
- Majano, G.; Raltchev, K.; Vicente, A.; Mintova, S. High-yield nanosized (Si) AlPO-41 using ethanol polarity equalization and co-templating synthesis approach. Nanoscale 2015, 7, 5787–5793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, W.; Wang, J.; Yang, W.; Zhu, X.; Zhu, K. Interface mediated crystallization of plate-like SAPO-41 crystals to promote catalytic hydroisomerization. Appl. Catal. A Gen. 2020, 602, 117738. [Google Scholar] [CrossRef]
- Jin, D.; Ye, G.; Zheng, J.; Yang, W.; Zhu, K.; Coppens, M.-O.; Zhou, X. Hierarchical Silicoaluminophosphate Catalysts with Enhanced Hydroisomerization Selectivity by Directing the Orientated Assembly of Premanufactured Building Blocks. ACS Catal. 2017, 7, 5887–5902. [Google Scholar] [CrossRef]
- Guo, L.; Bao, X.; Fan, Y.; Shi, G.; Liu, H.; Bai, D. Impact of cationic surfactant chain length during SAPO-11 molecular sieve synthesis on structure, acidity, and n-octane isomerization to di-methyl hexanes. J. Catal. 2012, 294, 161–170. [Google Scholar] [CrossRef]
- Huang, Y.; Demko, B.A.; Kirby, C.W. Investigation of the evolution of intermediate phases of ALPO4-18 molecular sieve synthesis. Chem. Mater. 2003, 15, 2437–2444. [Google Scholar] [CrossRef]
- Albuquerque, A.; Coluccia, S.; Marchese, L.; Pastore, H.O. Synthesis of SAPO-34 from the lamellar AlPO-Kanemite. Stud. Surf. Sci. Catal. 2004, 154, 966–970. [Google Scholar]
- Vistad, Ø.B.; Akporiaye, D.E.; Lillerud, K.P. Identification of a Key Precursor Phase for Synthesis of SAPO-34 and Kinetics of Formation Investigated by in Situ X-ray Diffraction. J. Phys. Chem. B. 2001, 105, 12437–12447. [Google Scholar] [CrossRef]
- Venkatathri, N.; Hegde, S.G.; Ramaswamy, V.; Sivasanker, S. Isolation and characterization of a novel lamellar-type aluminophosphate, AlPO4-L, a common precursor for AlPO4 molecular sieves. Micropor. Mesopor. Mater. 1998, 23, 277–285. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Kolyagin, Y.G.; Serebrennikov, D.V.; Grigor’eva, N.G.; Dmitrenok, A.S.; Maistrenko, V.N.; Dib, E.; Mintova, S.; Kutepov, B.I. Acid properties and morphology of SAPO-11 molecular sieve controled by silica source. Micropor. Mesopor. Mater. 2022, 338, 111962. [Google Scholar] [CrossRef]
- Tamura, M.; Shimizu, K.I.; Satsuma, A. Comprehensive IR study on acid/base properties of metal oxides. Appl. Catal. A Gen. 2012, 433, 135–145. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Y. Examining the self-assembly of microporous material AlPO4-11 by dry-gel conversion. J. Phys. Chem. C. 2007, 111, 15236–15243. [Google Scholar] [CrossRef]
- Zhang, B.; Xu, J.; Fan, F.; Guo, Q.; Tong, X.; Yan, W.; Yu, J.; Deng, F.; Li, C.; Xu, R. Molecular engineering of microporous crystals: (III) The influence of water content on the crystallization of microporous aluminophosphate AlPO4-11. Micropor. Mesopor. Mater. 2012, 147, 212–221. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, W.; Xu, J.; Tian, Z.; Deng, F.; Han, X.; Bao, X. Multinuclear Solid-State NMR Studies on the Formation Mechanism of Aluminophosphate Molecular Sieves in Ionic Liquids. J. Phys. Chem. C. 2013, 117, 5848–5854. [Google Scholar] [CrossRef]
- Agliullin, M.R.; Yakovenko, R.E.; Kolyagin, Y.G.; Serebrennikov, D.V.; Vildanov, F.S.; Prosochkina, T.R.; Kutepov, B.I. Relation between Morphology and Porous Structure of SAPO-11 Molecular Sieves and Chemical and Phase Composition of Silicoaluminophosphate Gels. Gels 2022, 8, 142. [Google Scholar] [CrossRef]
- Fan, F.; Feng, Z.; Sun, K.; Guo, M.; Guo, Q.; Song, Y.; Li, W.; Li, C. In Situ UV Raman Spectroscopic Study on the Synthesis Mechanism of AlPO-5. Angew. Chem. 2009, 121, 8899–8903. [Google Scholar] [CrossRef]
- Holmes, A.J.; Kirkby, S.J.; Ozin, G.A.; Young, D. Raman Spectra of the Unidimensional Aluminophosphate Molecular Sieves AlPO4-11, AlPO4-5, AlPO4-8, and VPI-5. J. Phys. Chem. 1994, 98, 4677–4682. [Google Scholar] [CrossRef]
- De Yoreo, J.J.; Gilbert, P.U.P.A.; Sommerdijk, N.A.J.M.; Lee Penn, R.; Whitelam, S.; Joester, D.; Zhang, H.; Rimer, J.D.; Navrotsky, A.; Banfield, J.F.; et al. Crystallization by particle attachment in synthetic, biogenic, and geologic environments. Science 2015, 349, aaa6760. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, M.; Goldwasser, J.; Lopez, C.M.; Machado, F.J.; Matjushin, M.; Mendez, B.; Ramirez de Agudelo, M.M. Effect of the synthesis conditions on the crystallinity and surface acidity of SAPO-11. J. Mol. Catal. A Chem. 1995, 98, 35–48. [Google Scholar] [CrossRef]
- Höchtl, M.; Jentys, A.; Vinek, H. Hydroisomerization of heptane isomers over Pd/SAPO molecular sieves: Influence of the acid and metal site concentration and the transport properties on the activity and selectivity. J. Catal. 2000, 190, 419–432. [Google Scholar] [CrossRef]
- Akhmedov, V.M.; Al-Khowaiter, S.H. Recent advances and future aspects in the selective isomerization of high n-alkanes. Catal. Rev. 2007, 49, 33–139. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, Y.; Liu, T.; Mao, L.; Wei, Q.; Zhou, Y. Deep Hydroisomerization of n-Hexadecane over NiWS/SAPO-11 Catalyst: Size Effect of the Support and Revelation of the Reaction Network. Energy Fuel 2023, 37, 15995–16010. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Chen, G.; Yao, J.; Yan, B.; Yi, W.; Cheng, Z.; Tian, C.; Zao, H. Enhanced conversion of syngas to high-quality gasoline hydrocarbons over ZnZrOx coupled SAPO-11 nanosheets bifunctional catalyst. Fuel Process. Technol. 2023, 252, 107967. [Google Scholar] [CrossRef]
- Dai, X.; Cheng, Y.; Liu, T.; Mao, L.; Wei, Q.; Zhou, Y. Novel construction and synthesis mechanism of b-axis oriented flower-like SAPO-11 molecular sieve in OSC-containing system for efficient hydroisomerization of long-chain n-alkanes. Chem. Eng. J. 2023, 475, 146412. [Google Scholar] [CrossRef]

| Sample | SBET, m2/g | SEX, m2/g | Vmicro, cm3/g | Vmeso, cm3/g |
|---|---|---|---|---|
| SAPO-11-1.0 | 286 | 172 | 0.06 | 0.13 |
| SAPO-11-1.4 | 209 | 66 | 0.07 | 0.08 |
| SAPO-11-1.8 | 184 | 65 | 0.06 | 0.09 |
| Sample | Chemical Composition, Gel | Chemical Composition, AEL |
|---|---|---|
| SAPO-11-1.0 | Al1.00P0.98Si0.09 | Al1.00P0.98Si0.06 |
| SAPO-11-1.4 | Al1.00P0.98Si0.10 | Al1.00P0.98Si0.07 |
| SAPO-11-1.8 | Al1.00P0.99Si0.09 | Al1.00P0.99Si0.08 |
| Sample | Acidity (μmol/g) | |||||
|---|---|---|---|---|---|---|
| BAS | LAS | |||||
| 150 °C | 250 °C | 350 °C | 150 °C | 250 °C | 350 °C | |
| SAPO-11-1.0 | 155 | 90 | 30 | 43 | 13 | 6 |
| SAPO-11-1.4 | 130 | 89 | 27 | 19 | 7 | 6 |
| SAPO-11-1.8 | 173 | 127 | 37 | 13 | 6 | 5 |
| Catalyst | Pt/SAPO-11-1.0 | Pt/SAPO-11-1.4 | Pt/SAPO-11-1.8 |
|---|---|---|---|
| Conversion, wt % | 83.9 | 81.2 | 74.4 |
| Selectivity by iso-C16, wt % | 94.2 | 87.8 | 82.7 |
| Iso-C16 yield, wt %: | |||
| 2-MeC15 | 8.0 | 5.5 | 6.2 |
| 3-MeC15 | 8.5 | 5.9 | 7.4 |
| 4-MeC15 | 6.8 | 4.8 | 5.8 |
| 5-MeC15 | 6.2 | 4.8 | 4.8 |
| 6+-MeC15 | 22.4 | 15.6 | 21.5 |
| (CH3)2–C14 | 23.0 | 34.5 | 15.7 |
| Yield ΣC1–C4, wt % | 0.5 | 0.5 | 0.6 |
| Yield ΣC5–C15, wt % | 7.1 | 9.4 | 12.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agliullin, M.R.; Serebrennikov, D.V.; Kutepov, B.I. Changing Template/Al2O3 Ratio in Reaction Gel—An Effective Way to Regulate Nature of Intermediate Phases and Properties of SAPO-11 Molecular Sieves during Crystallization. Materials 2024, 17, 1359. https://doi.org/10.3390/ma17061359
Agliullin MR, Serebrennikov DV, Kutepov BI. Changing Template/Al2O3 Ratio in Reaction Gel—An Effective Way to Regulate Nature of Intermediate Phases and Properties of SAPO-11 Molecular Sieves during Crystallization. Materials. 2024; 17(6):1359. https://doi.org/10.3390/ma17061359
Chicago/Turabian StyleAgliullin, Marat R., Dmitry V. Serebrennikov, and Boris I. Kutepov. 2024. "Changing Template/Al2O3 Ratio in Reaction Gel—An Effective Way to Regulate Nature of Intermediate Phases and Properties of SAPO-11 Molecular Sieves during Crystallization" Materials 17, no. 6: 1359. https://doi.org/10.3390/ma17061359






