Study on the High-Temperature Interaction between Coke and Iron Ores with Different Layer Thicknesses
Abstract
:1. Introduction
2. Materials and Experiment
2.1. Raw Iron Ores and Coke
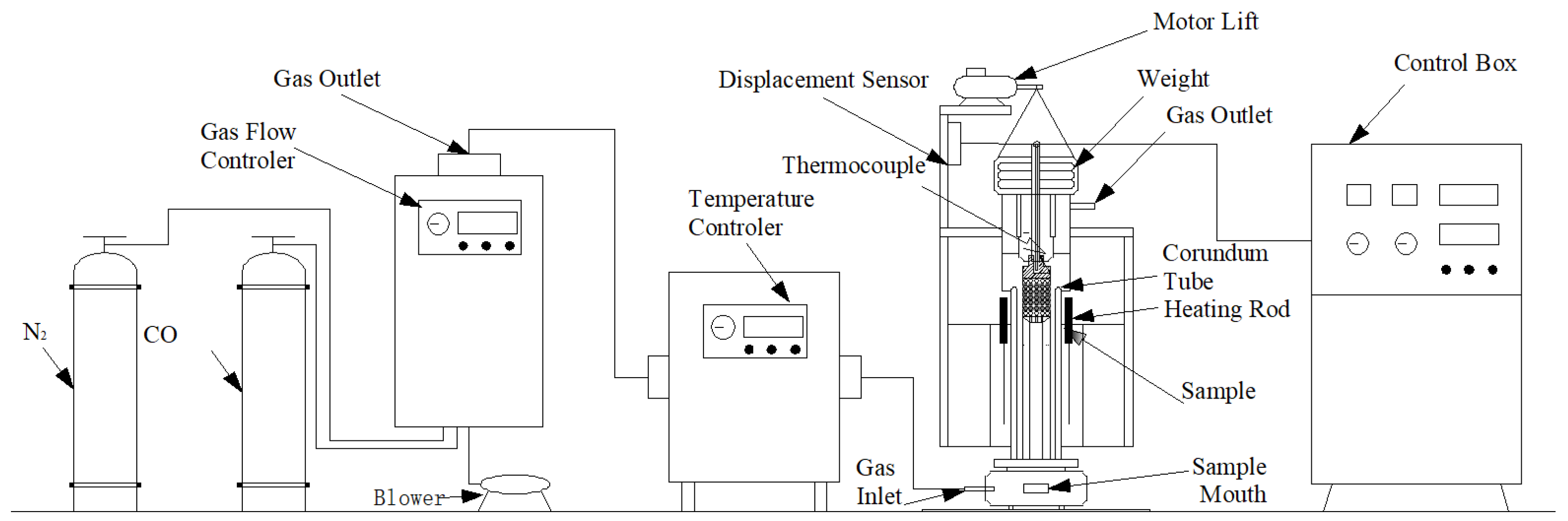
2.2. Development of Melting-Drop Furnace
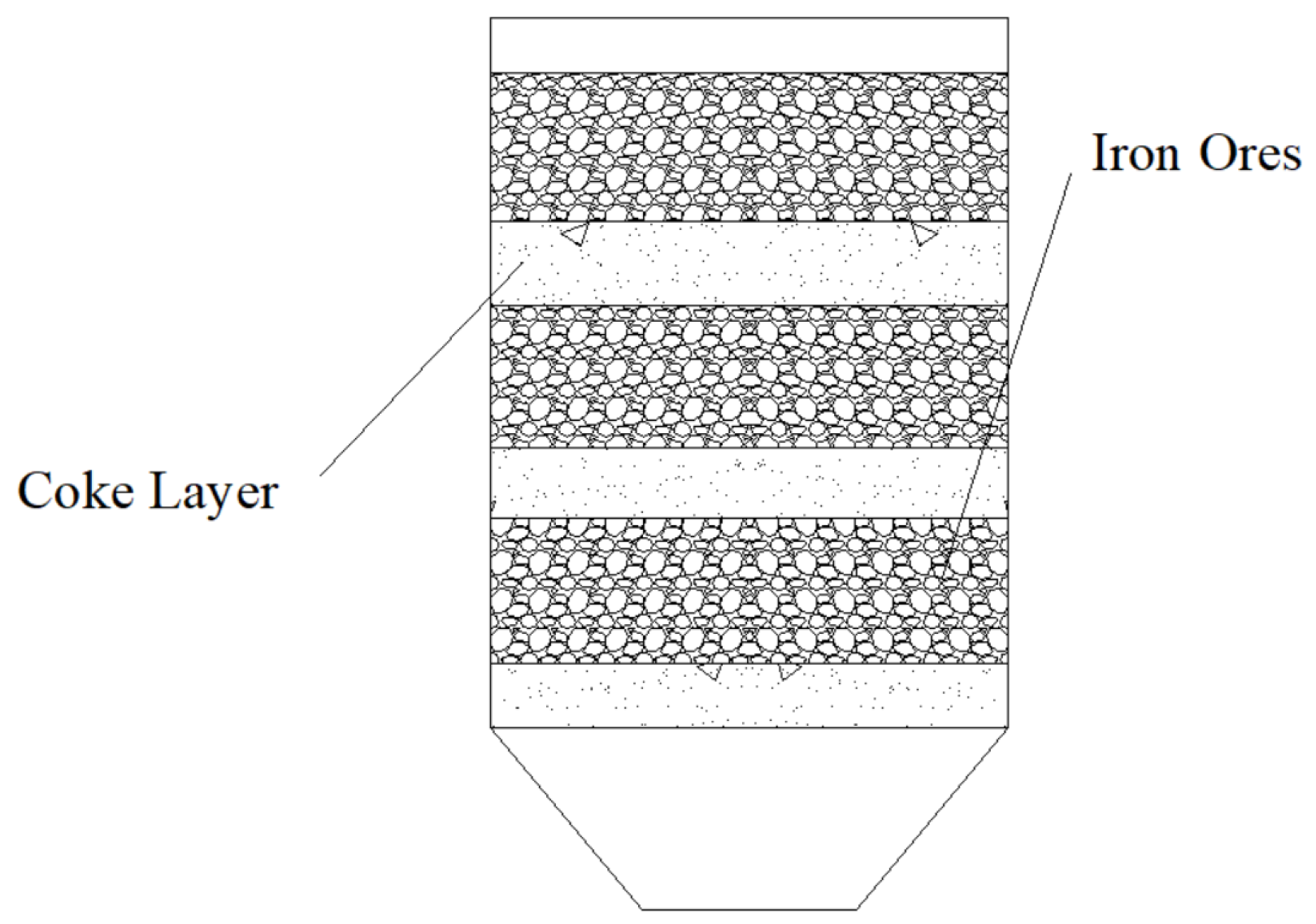
3. High-Temperature Interaction between Coke and Iron Ores
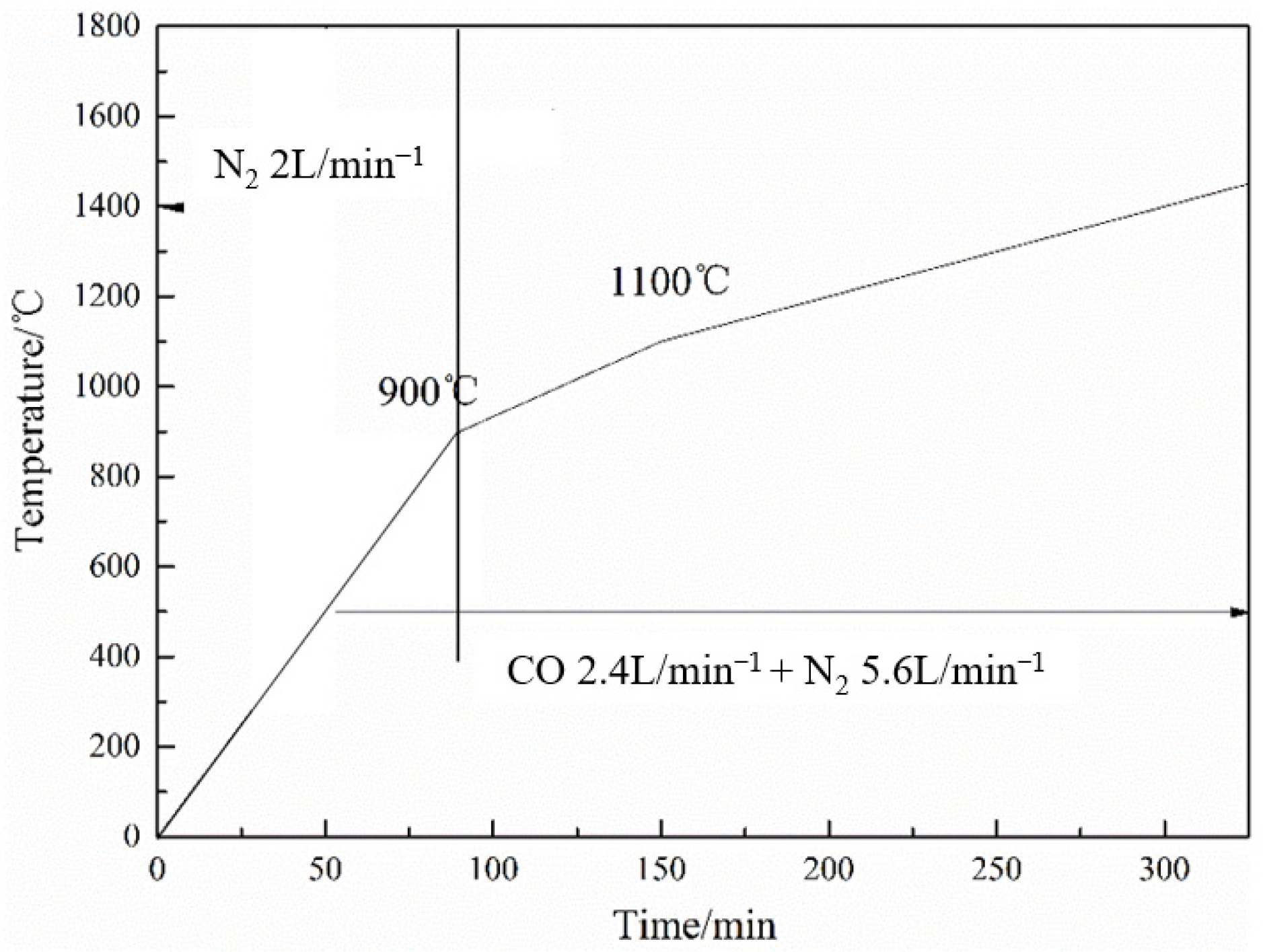
3.1. Experimental Conditions
3.2. Experimental Results
3.2.1. Dripping Properties of Molten Metal
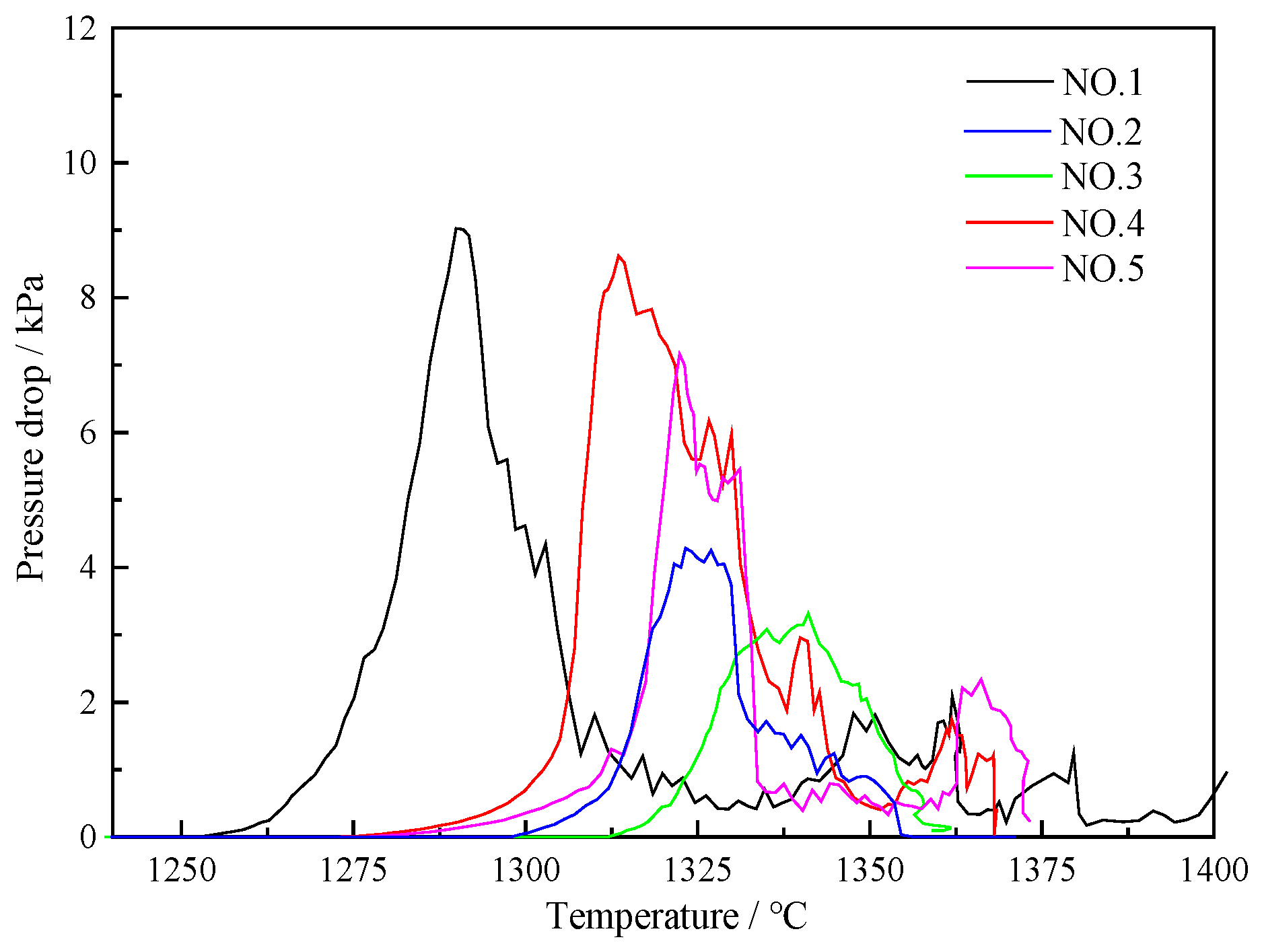
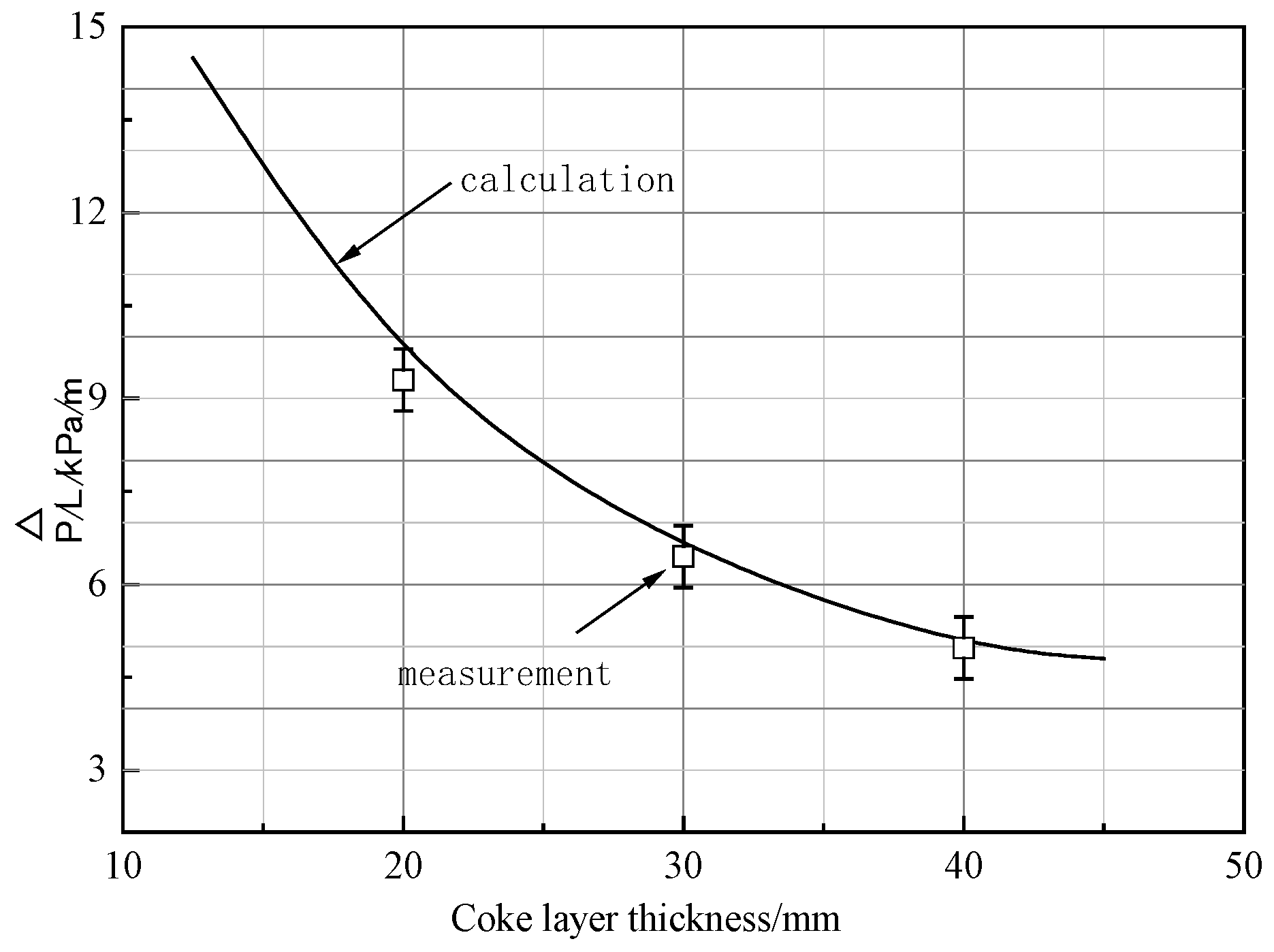
3.2.2. Pressure Drop in Layer Thickness Condition
3.2.3. Shrinkage Rate in Layer Thickness Condition
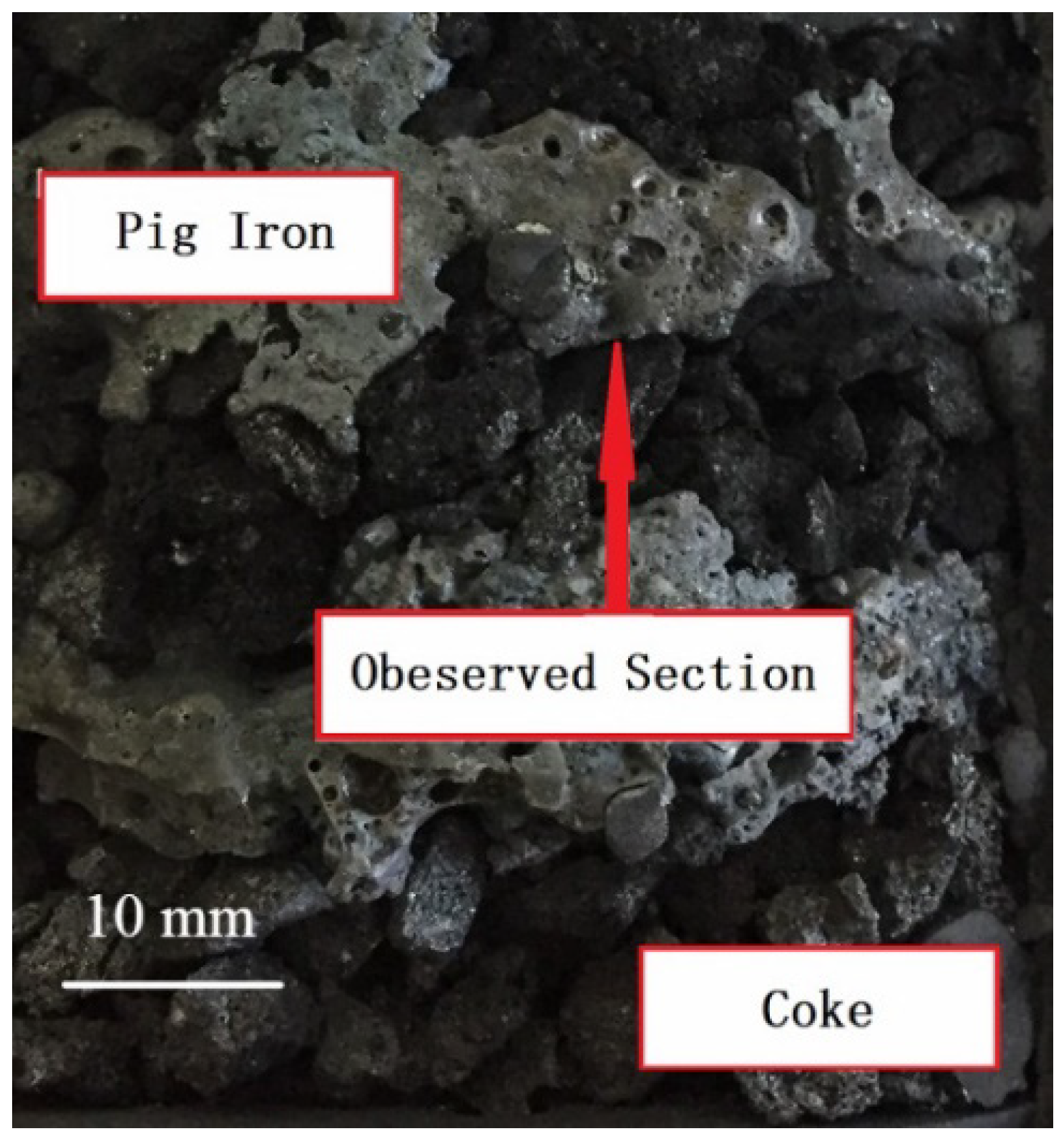
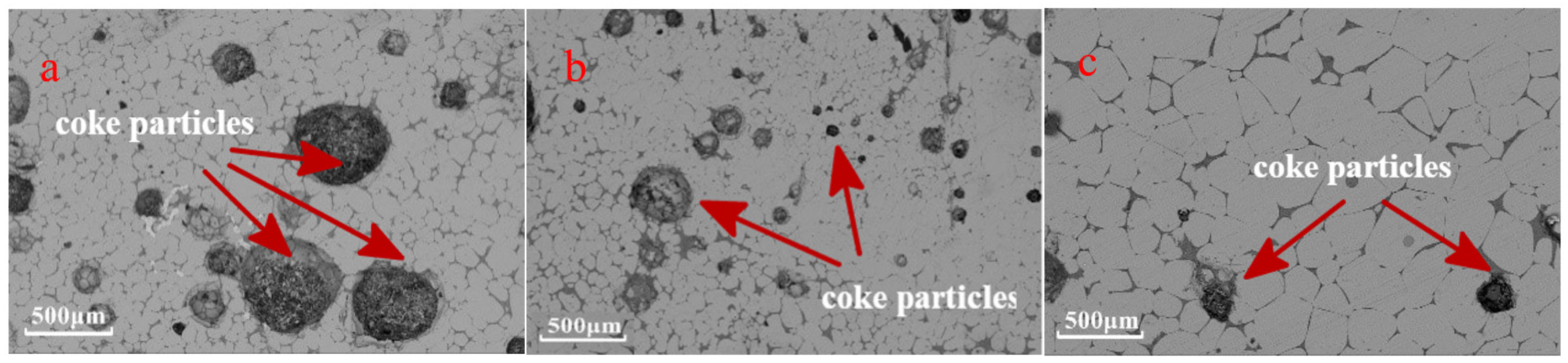
3.2.4. Effect of Coke Solution Loss
4. Discussion
4.1. Solution Loss Reaction of Coke
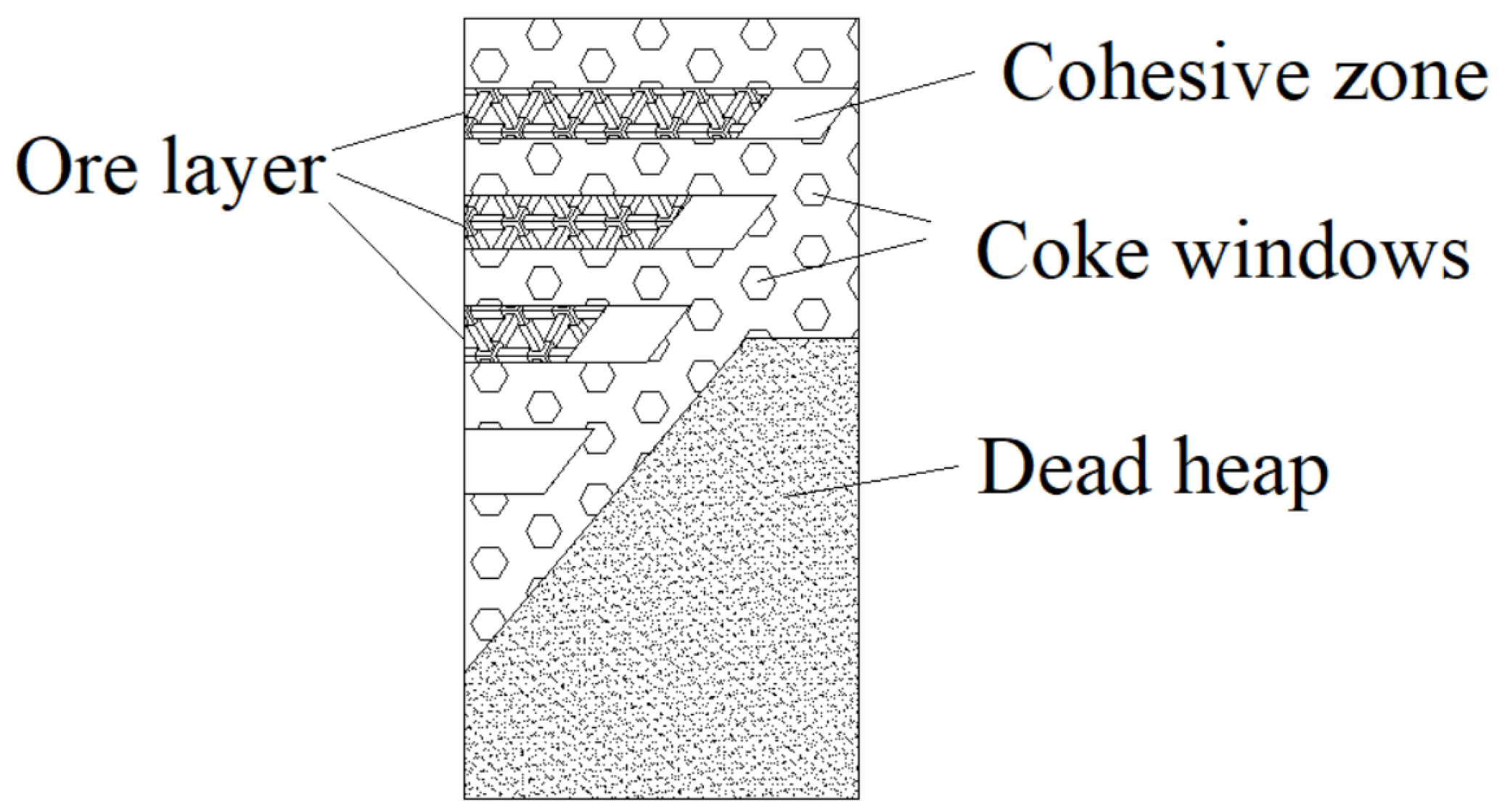
4.2. Coke Layer Function in Cohesive Zone
4.3. Mathematical Model of Permeability
5. Conclusions
- The solution loss reaction of coke and reduction reaction of iron oxide could be a recycling of CO and CO2. Raising the thickness of the coke layer could promote this coupling reaction and increase the CO concentration, which is beneficial for indirect reduction. The mechanical strength of the coke layer was seriously damaged when the thickness of the coke layer became thinner. However, further quantitative research is still needed.
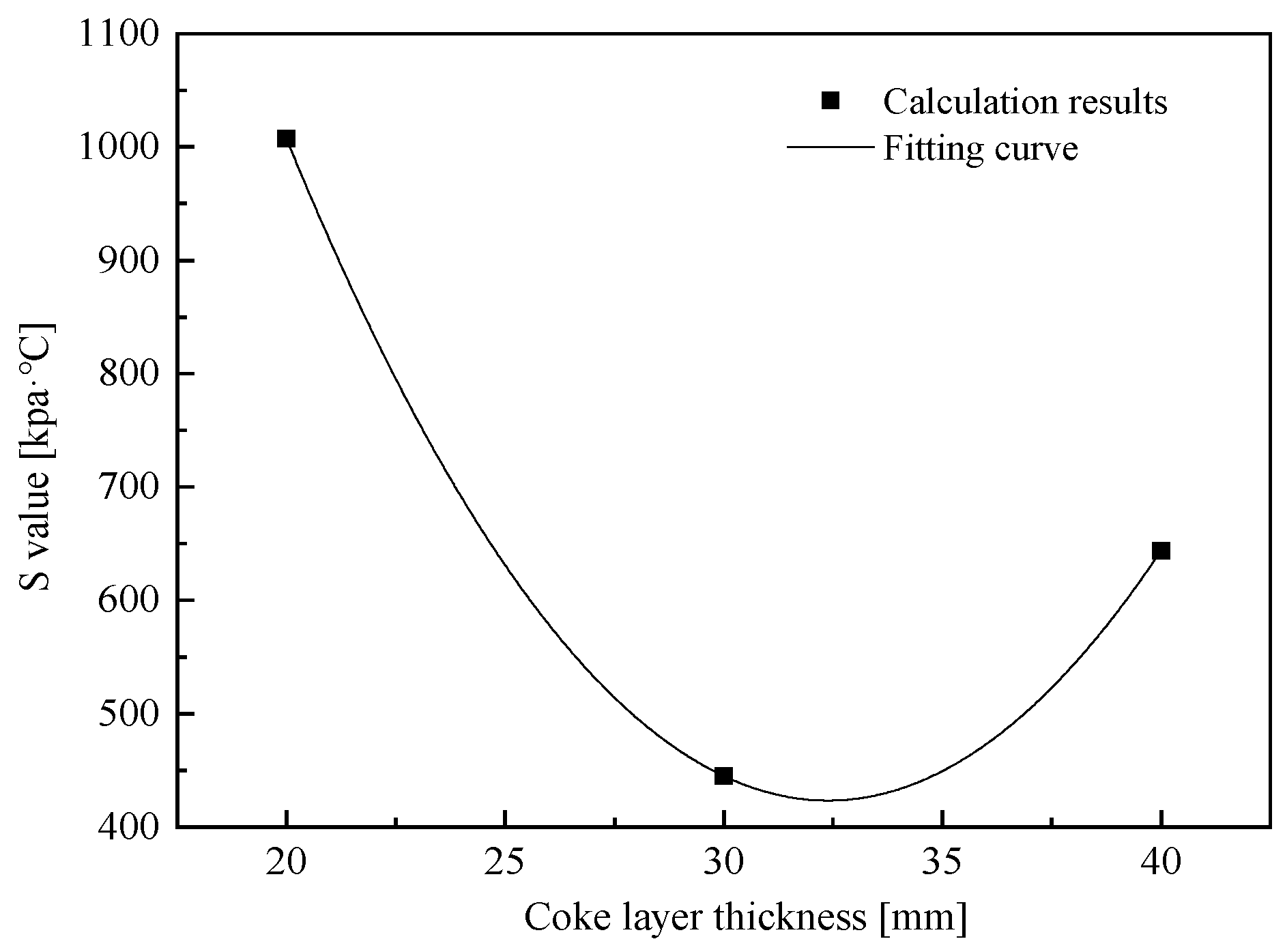
- Coke layers with higher thickness are propitious to the development of the dripping properties of molten iron. In this study, the layer thickness of coke was adjusted from 20 mm to 30 mm and 40 mm, where the permeability of the cohesive was improved to some extent, but the best is 30 mm.
- The excessively higher coke layer extended the dropping distance of iron as well as the contact time between molten iron and coke, resulting in huge resistance for the dripping process of molten iron. Then, the dripping properties of molten metal could be wrecked. Therefore, it is necessary to choose the appropriate coke layer thickness based on the volume of the blast furnace and the requirements for production.
- Increasing the thickness of the coke layer is one of the main measures to strengthen smelting, reduce the coke ratio, and stabilize the upper gas flow. A reasonable thickness of the coke layer plays a crucial role in the rational distribution of the furnace charge and upper gas flow, as well as in improving the utilization of gas heat energy, improving the permeability of the blocky zone, and ensuring the smooth descent of the furnace charge, uniform, and active operation of the furnace charge column, and the reasonable distribution of initial gas flow.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rahmatmand, B.; Tahmasebi, A.; Lomas, H.; Honeyands, T.; Koshy, P.; Hockings, K.; Jayasekara, A. A technical review on coke rate and quality in low-carbon blast furnace ironmaking. Fuel 2023, 336, 127077. [Google Scholar] [CrossRef]
- Diez, M.A.; Centeno, T.A.; Amado-Fierro, Á. 15—Coal use for iron and steel production in low-carbon transition scenarios. In Towards Cleaner Coal Utilization; Woodhead Publishing: Sawston, UK, 2023; pp. 493–546. [Google Scholar] [CrossRef]
- Roeplal, R.; Pang, Y.; Adema, A.; van der Stel, J.; Schott, D. Modelling of phenomena affecting blast furnace burden permeability using the Discrete Element Method (DEM)—A review. Powder Technol. 2023, 415, 118161. [Google Scholar] [CrossRef]
- Natsui, S.; Hirai, A.; Terui, K.; Kashihara, Y.; Murao, A.; Miki, Y.; Nogami, H. Method for simulating gas permeability of a coke bed including fines based on 3D imaging on the coke particle morphology. ISIJ Int. 2021, 61, 1814–1825. [Google Scholar] [CrossRef]
- Lundgren, M.; Ökvist, L.S.; Björkman, B. Coke Reactivity under Blast Furnace Conditions and in the CSR/CRI Test. Steel Res. Int. 2009, 80, 396–401. [Google Scholar] [CrossRef]
- Liu, Q.-H.; Yang, S.-P.; Wang, C.; Ji, Y.-L. Effect of carbon solution-loss reaction on properties of coke in blast furnace. J. Iron Steel Res. Int. 2020, 27, 489–499. [Google Scholar] [CrossRef]
- Kumar, D.; Saxena, V.K.; Tiwari, H.P.; Nandi, B.K.; Verma, A.; Tiwary, V.K. Variability in Metallurgical Coke Reactivity Index (CRI) and Coke Strength after Reaction (CSR): An Experimental Study. ACS Omega 2022, 7, 1703–1711. [Google Scholar] [CrossRef] [PubMed]
- Numazawa, Y.; Matsukawa, Y.; Hara, Y.; Matsushita, Y.; Aoki, H. Large-scale simulation of CO2 gasification reaction with mass transfer for metallurgical coke: Comparison with lab-scale experiment at 1373 K in early stage. Results Eng. 2023, 19, 101212. [Google Scholar] [CrossRef]
- Ono, Y.; Fukuda, Y.; Sumitani, Y.; Matsukawa, Y.; Saito, Y.; Matsushita, Y.; Aoki, H.; Shishido, T.; Okuyama, N. Experimental and numerical study on degradation behavior of coke with CO2 or H2O gasification reaction at high temperature. Fuel 2022, 309, 122061. [Google Scholar] [CrossRef]
- Rodero, J.I.; Sancho-Gorostiaga, J.; Ordiales, M.; Fernández-González, D.; Mochón, J.; Ruiz-Bustinza, I.; Fuentes, A.; Verdeja, L.F. Blast furnace and metallurgical coke’s reactivity and its determination by thermal gravimetric analysis. Ironmak. Steelmak. 2015, 42, 618–625. [Google Scholar] [CrossRef]
- Nogueira, P.F.; Fruehan, R.J. Blast furnace burden softening and melting phenomena: Part I. Pellet bulk interaction observation. Met. Mater. Trans. B 2004, 35, 829–838. [Google Scholar] [CrossRef]
- Hoque, M.M.; Doostmohammadi, H.; Mitra, S.; O’dea, D.; Liu, X.; Honeyands, T. High Temperature Softening and Melting Interactions Between Newman Blend Lump and Sinter. ISIJ Int. 2021, 61, 2944–2952. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y. Large scale production practice of Shougang Jingtang No.2 blast furnace. Ironmaking 2012, 31, 6–9. [Google Scholar]
- Du, H.; Du, G. Study on the suitable thickness of ore and coke layer for blast furnaces. Iron Steel 1981, 16, 34–42. [Google Scholar]
- Ichikawa, K.; Kashihara, Y.; Oyama, N.; Hirosawa, T.; Ishii, J.; Sato, M.; Matsuno, H. Evaluating effect of coke layer thickness on permeability by pressure drop estimation model. ISIJ Int. 2017, 57, 254–261. [Google Scholar] [CrossRef]
- GB/T 6730.87-2023; Iron Ores—Determination of Total Iron and Other Multi-Element Content—Wavelength Dispersive X-ray Fluorescence Spectrometry (Cobalt Internal Standard Method). Standardization Administration of China: Beijing, China, 2023.
- GB/T 6730.8-2016; Iron ores—Determination of Iron(II) Content—Potassium Dichromate Titrimetric Method. Standardization Administration of China: Beijing, China, 2016.
- GB/T 2001-2013; Coke-Determination of Proximate Analysis. Standardization Administration of China: Beijing, China, 2013.
- GB/T 2006-2008; Coke for Metallurgy—Determination of Mechanical Strength. Standardization Administration of China: Beijing, China, 2008.
- GB/T 4000-2017; Determination of Coke Reactivity Index (CRI) and Coke Strength after Reaction (CSR). Standardization Administration of China: Beijing. China, 2017.
- ISO 728-2021; Coke—Size Analysis by Sieving. International Organization for Standardization: Geneva, Switzerland, 2021.
- GB/T 34211-2017; Iron Ores—Method for Determination of Iron Reduction Softening Drippinger Performance under Load. Standardization Administration of China: Beijing, China, 2017.
- Chakrabarty, A.; Ohri, R.; Chaudhari, U.; Sahoo, T.; Nag, S.; Gupta, I.P. Influence of above burden probes in blast furnace on centre coke charging and subsequent operational stability. J. Sustain. Met. 2023, 9, 1790–1802. [Google Scholar] [CrossRef]
- Ren, Y.; Wang, Y.; Bao, J.; Chen, H. Study on high-temperature thermal properties of coke. Fuel Chem. Process 2023, 54, 11–15. [Google Scholar]
- Ergun, S. Fluid flow through packed columns. Chem. Eng. Prog. 1952, 48, 89–94. [Google Scholar]
- Yamada, T.; Sato, M.; Miyazaki, N.; Shimamura, H.; Taguchi, S. Distribution of burden materials and gas permeability in a large volume blast furnace. Kawasaki Steel Giho 1974, 6, 16–37. [Google Scholar]
- Pan, Y.; Zuo, H.; Wang, J.; Xue, Q.G.; Wang, G.; She, X.F. Review on improving gas permeability of blast furnace. J. Iron Steel Res. Int. 2020, 27, 121–131. [Google Scholar] [CrossRef]

| Form | T[Fe] | FeO | SiO2 | CaO | Al2O3 | MgO | TiO2 | MnO |
|---|---|---|---|---|---|---|---|---|
| S-1 | 58.52 | 9.33 | 4.85 | 9.04 | 1.69 | 1.47 | 0.11 | 0.22 |
| P-1 | 66.53 | 0.32 | 2.02 | 1.49 | 0.59 | 0.16 | 0.04 | 0.13 |
| O-1 | 63.39 | 0.48 | 3.14 | 0.04 | 1.25 | 0.06 | 0.04 | 0.13 |
| O-2 | 62.47 | 0.32 | 2.96 | 0.04 | 1.26 | 0.05 | 0.04 | 0.27 |
| Index | M40 | CSR | Ms | A | S | C |
|---|---|---|---|---|---|---|
| coke | 86.19 | 65.35 | 47.6 | 12.8 | 0.98 | 85.24 |
| No. | Coke Layer Thickness/mm | Packed Bed Thickness mm | Furnace Burden |
|---|---|---|---|
| ① | 20 | 104.2 | 65% S-1 + 20% P-1 + 15% O-1 |
| ② | 30 | 172.5 | 65% S-1 + 20% P-1 + 15% O-1 |
| ③ | 40 | 241.0 | 65% S-1 + 20% P-1 + 15% O-1 |
| ④ | 20 | 104.5 | 65% S-1 + 20% P-1 + 15% O-2 |
| ⑤ | 40 | 248.4 | 65% S-1 + 20% P-1 + 15% O-2 |
| T10/°C | T40/°C | ΔT | T40/°C | Pmax | Td/°C | S/kPa·°C | |
|---|---|---|---|---|---|---|---|
| ① | 1191 | 1278 | 87 | 1285 | 9322 | 1403 | 1007 |
| ② | 1170 | 1271 | 101 | 1296 | 6559 | 1364 | 445 |
| ③ | 1169 | 1278 | 109 | 1289 | 4970 | 1347 | 644 |
| ④ | 1183 | 1282 | 99 | 1285 | 11,090 | 1360 | 936 |
| ⑤ | 1165 | 1280 | 115 | 1300 | 10,884 | 1365 | 624 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-H.; Du, P.; Diao, J.; Xie, B.; Zhu, M.-H. Study on the High-Temperature Interaction between Coke and Iron Ores with Different Layer Thicknesses. Materials 2024, 17, 1358. https://doi.org/10.3390/ma17061358
Wang Y-H, Du P, Diao J, Xie B, Zhu M-H. Study on the High-Temperature Interaction between Coke and Iron Ores with Different Layer Thicknesses. Materials. 2024; 17(6):1358. https://doi.org/10.3390/ma17061358
Chicago/Turabian StyleWang, Yong-Hong, Ping Du, Jiang Diao, Bing Xie, and Ming-Hua Zhu. 2024. "Study on the High-Temperature Interaction between Coke and Iron Ores with Different Layer Thicknesses" Materials 17, no. 6: 1358. https://doi.org/10.3390/ma17061358




