Solidification/Stabilization of Chromium-Contaminated Soils by Polyurethane during Freeze–Thaw Cycles: Mechanical, Leaching and Microstructure Characterization
Abstract
1. Introduction
2. Materials
2.1. Soil Material
2.2. Polyurethane Materials
2.3. Specimen Preparation
2.4. Test Program
2.4.1. Freeze–Thaw Cycle
2.4.2. UCS Test
2.4.3. TCLP Test
2.4.4. SEM Test
2.4.5. NMR Test
2.4.6. FTIR Test
3. Results and Discussion
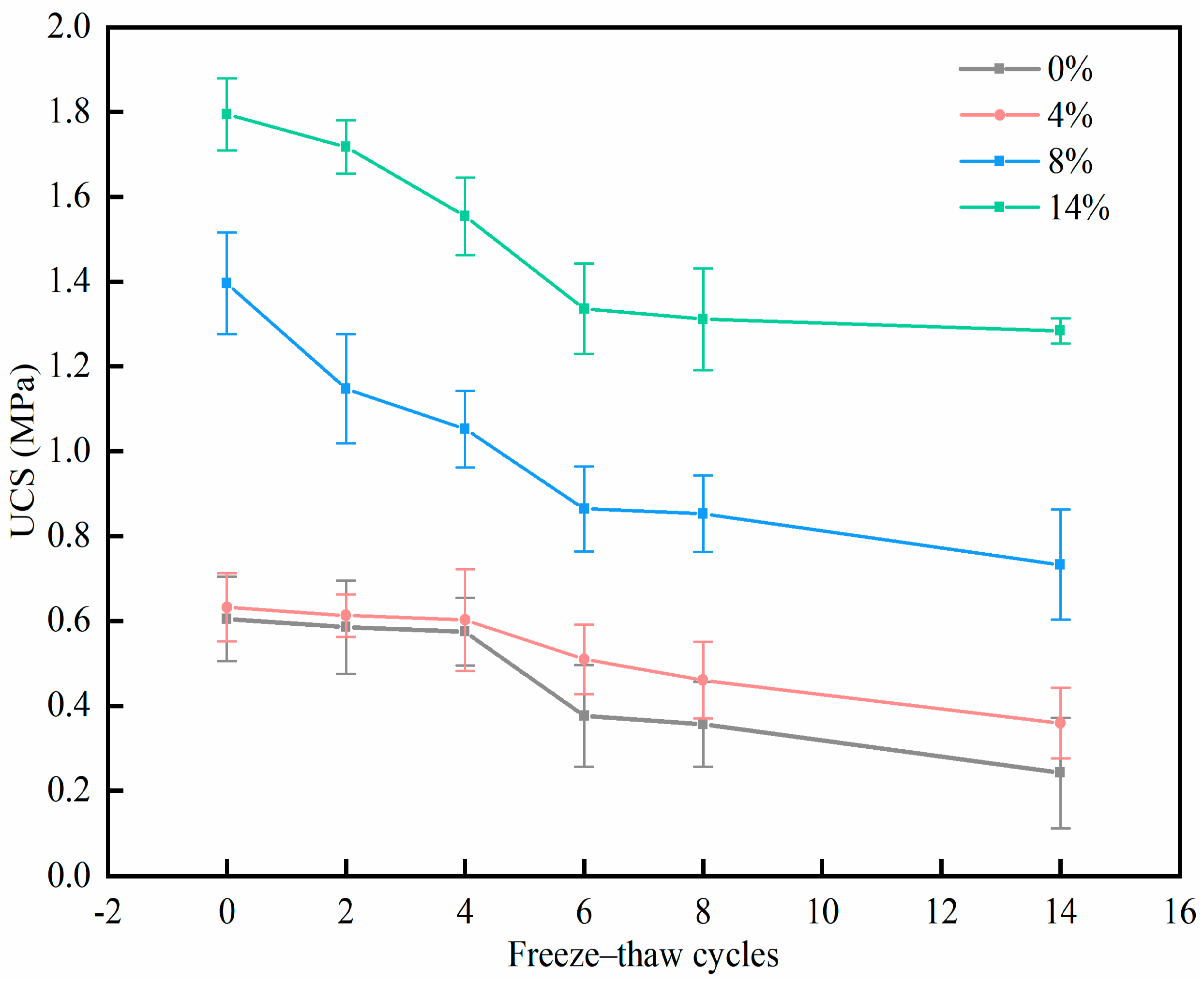
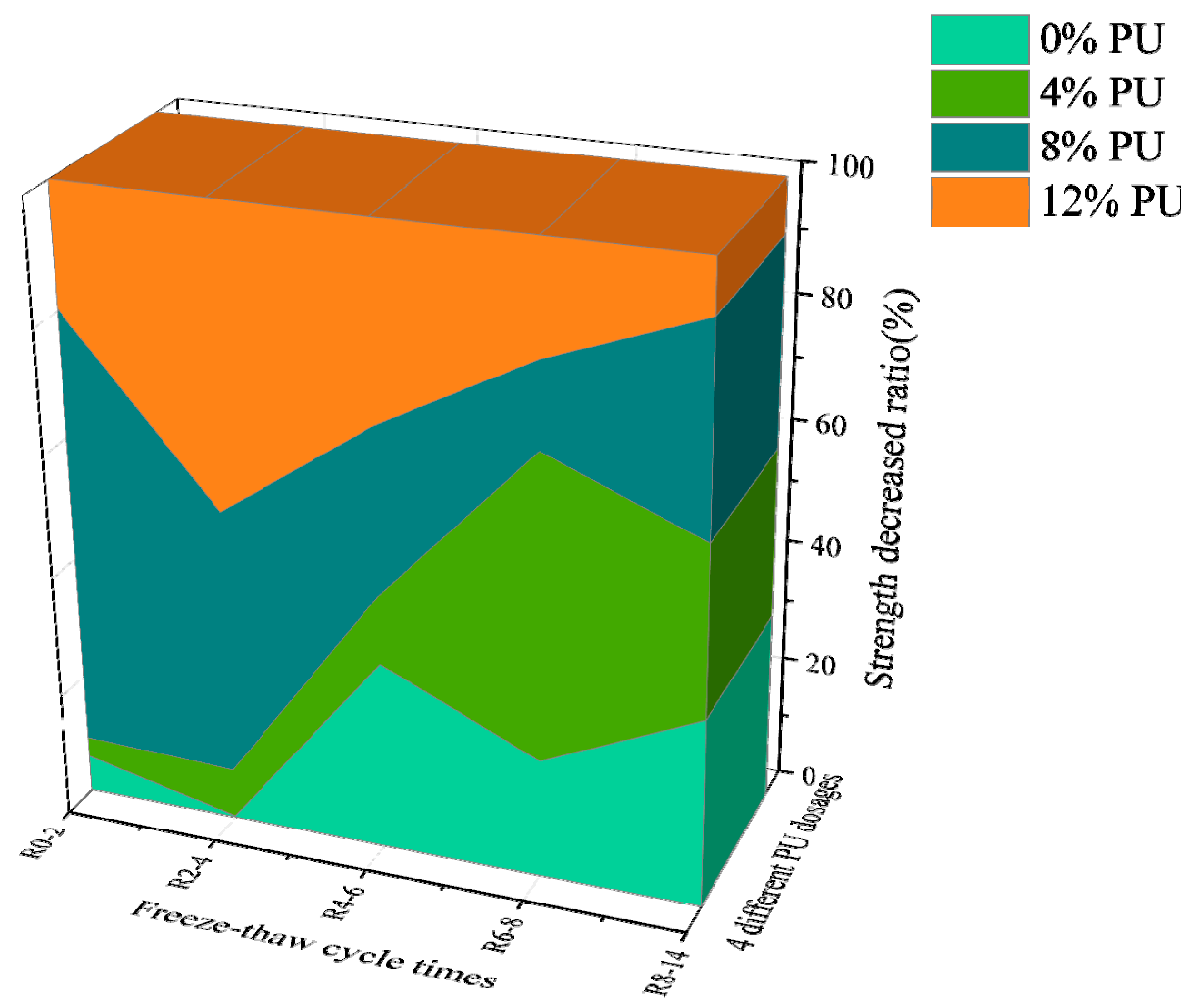
3.1. UCS Analysis
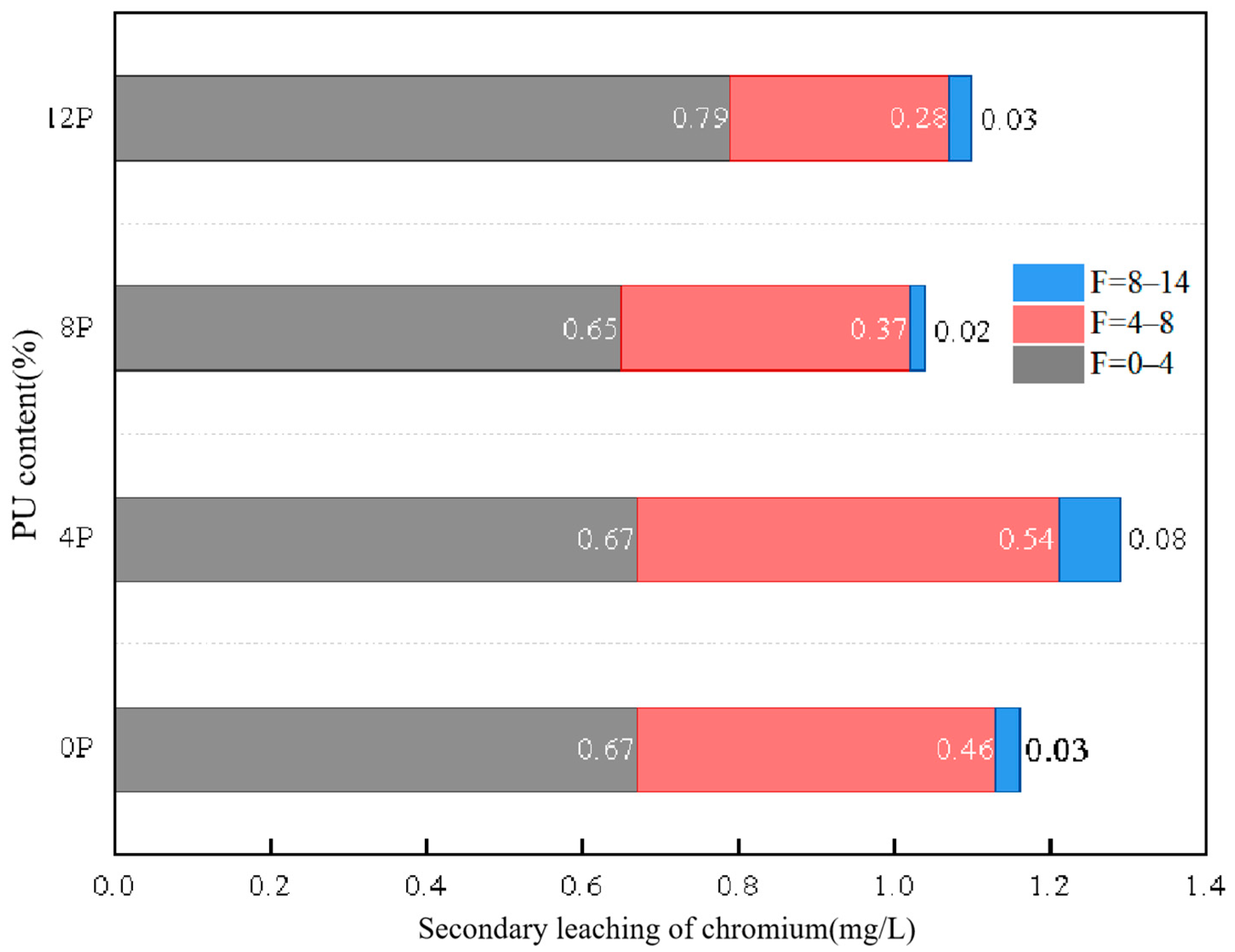
3.2. The Leaching Behavior of Hexavalent Chromium
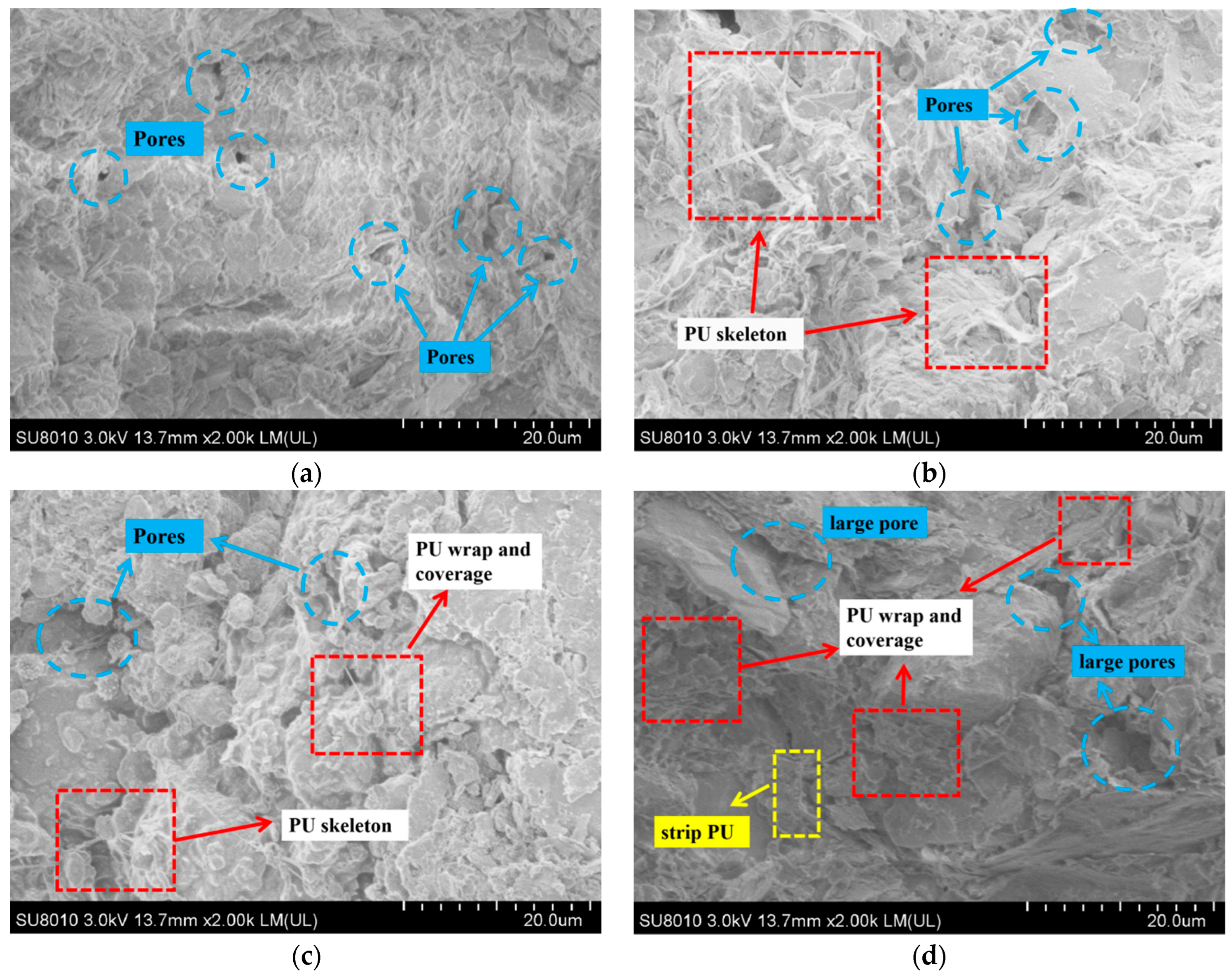
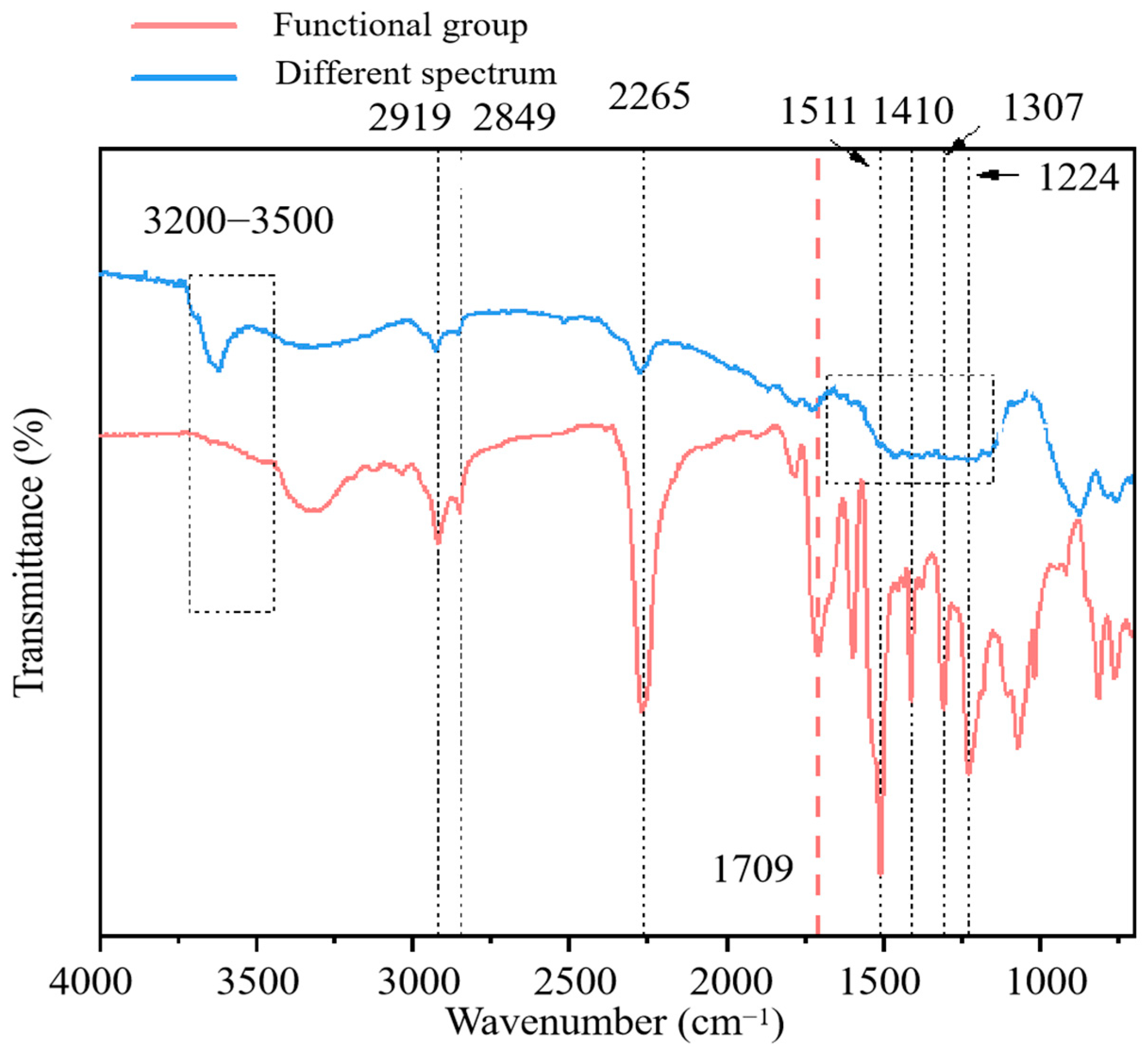
3.3. Micromorphology Analysis of PU
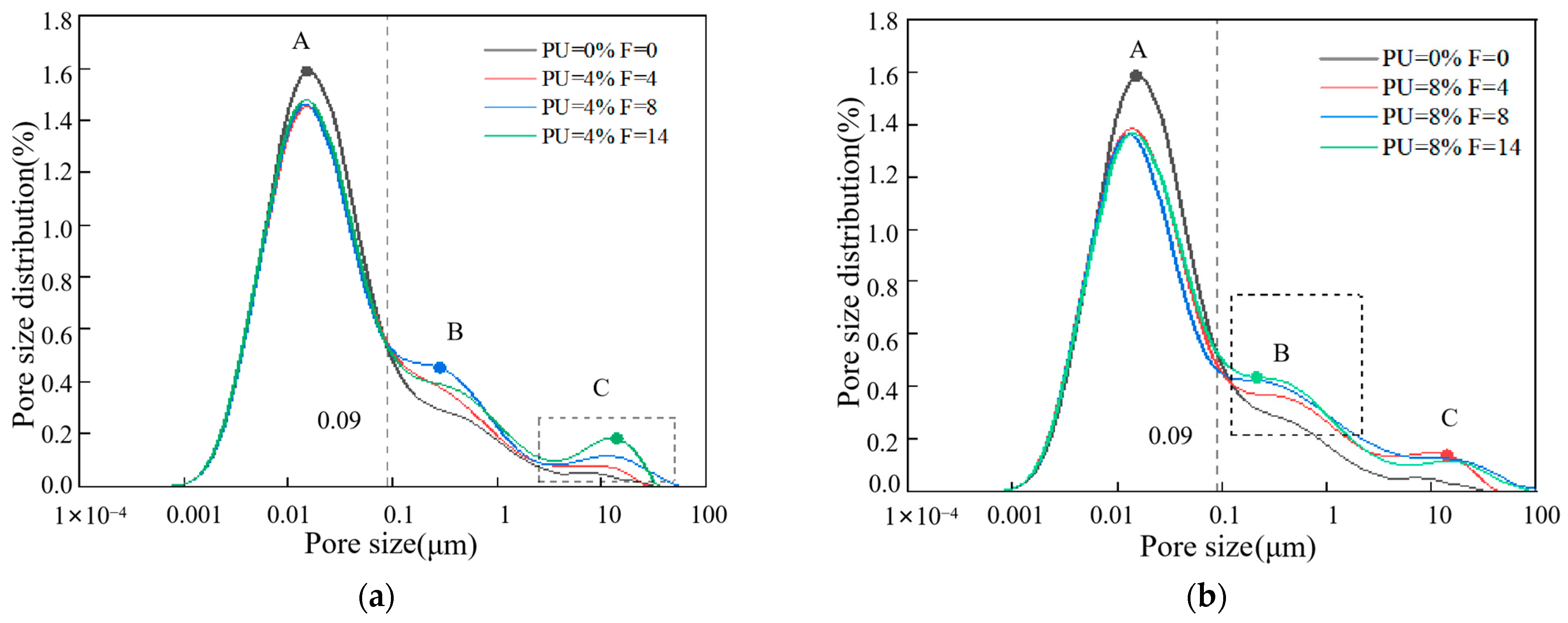
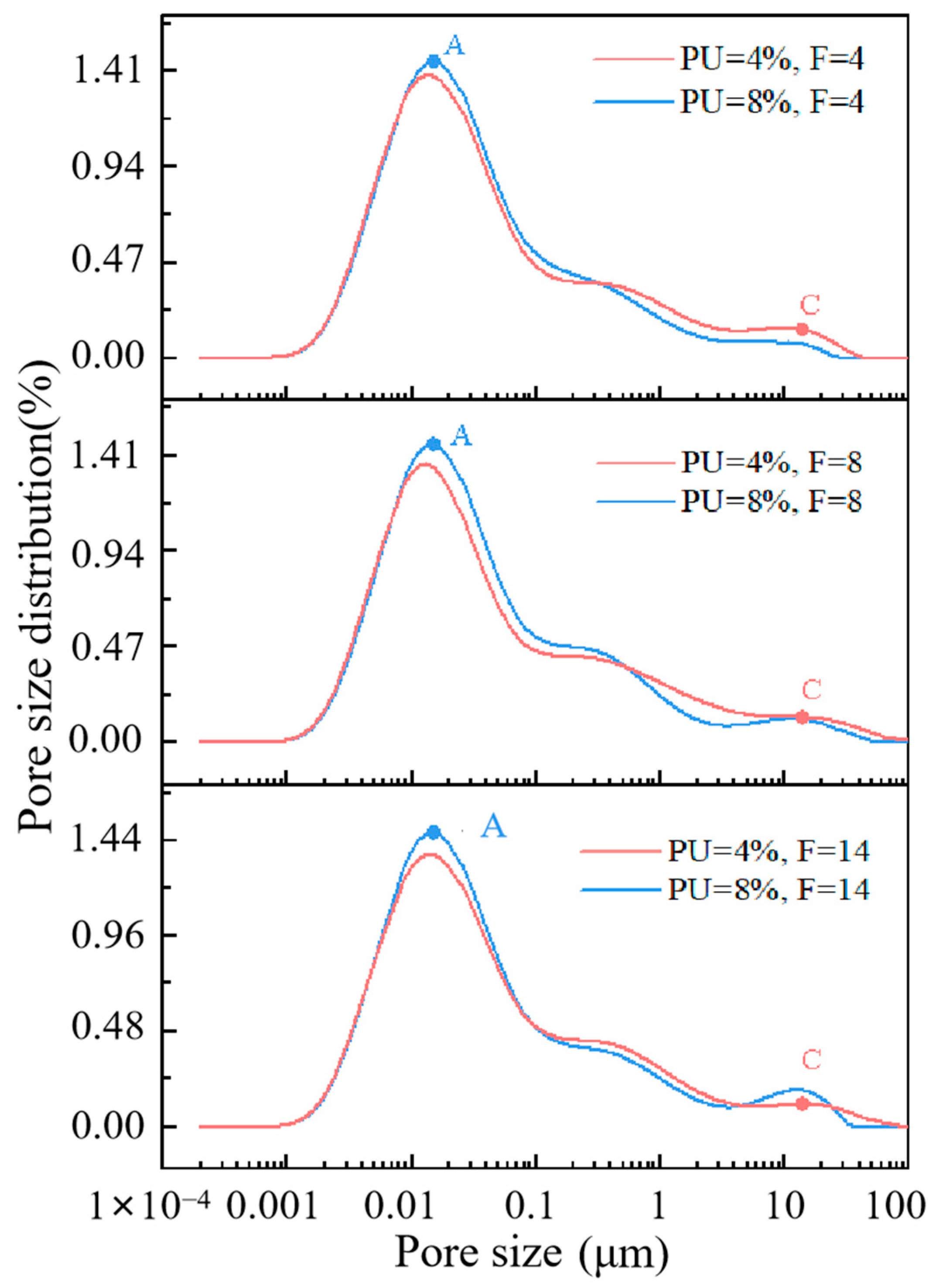
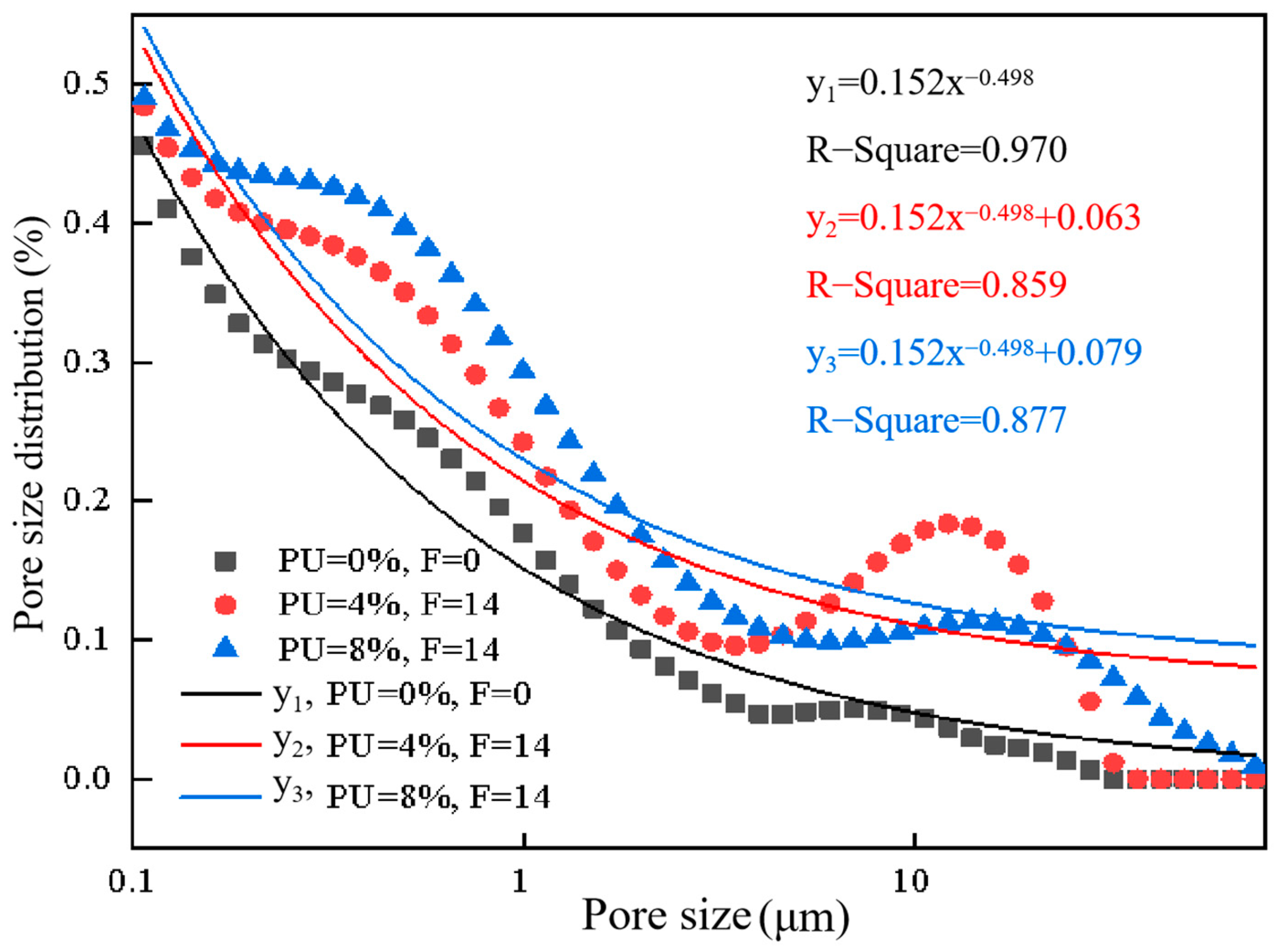
3.4. Analysis of Pore Structure Characteristics
3.5. Further Solidification Mechanism Analysis
4. Conclusions
- (1)
- PU successfully mitigates the strength degradation of chromium-contaminated soil after freeze–thaw cycles. The UCS of specimens with 12% PU reaches 1.284 MPa after 14 freeze–thaw cycles, which is 5.2 times higher than specimens without PU.
- (2)
- PU can effectively reduce the leaching concentration of chromium-contaminated soil and prevent the deterioration of solidification ability after freeze–thaw cycles. The chromium secondary leaching concentration of specimen 12P-14F was only 1.09 mg/L. This is in line with China’s chromium pollution standards.
- (3)
- PU-solidified chromium-contaminated soil in seasonal frozen regions has great potential. The polyurethane film can effectively resist the extrusion of ice crystal volume expansion. In addition, PU can fill soil pores (0.01–0.09 μm), which reduces the secondary leaching risk of heavy metal chromium after freeze–thaw cycles in frozen soil areas. Although freeze–thaw cycles disperse the PU structure, increasing the number of pores sized 1–10 μm, heavy metal chromium remains encapsulated within the PU film, maintaining chromium concentration in toxic leachate within acceptable limits.
- (4)
- PU solidified chromium through physical encapsulation and complexation reaction. PU wrapped on the surface of soil particles, sealing soil particles to reduce the diffusion of chromium. On the other hand, the amide functional groups, –CH3 of methyl and the isocyanate-group in PU played significant roles in the complexity with chromium.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, R.; Wang, L.; Shen, Z.; Alessi, D.S.; Hou, D. Simultaneous reduction and immobilization of Cr (VI) in seasonally frozen areas: Remediation mechanisms and the role of ageing. J. Hazard. Mater. 2021, 415, 125650. [Google Scholar] [CrossRef]
- Sricharoenvech, P.; Siebecker, M.G.; Tappero, R.; Landrot, G.; Fischel, M.H.H.; Sparks, D.L. Chromium speciation and mobility in contaminated coastal urban soils affected by water salinity and redox conditions. J. Hazard. Mater. 2024, 462, 132661. [Google Scholar] [CrossRef]
- Rai, A.; Kumar Sharma, N.; Kumar Singh, V.; Rai, A.; Kumar, V.; Kumar, A.; Shankar Singh, J.; Kudesia, S.; Kumar Rai, P. Use of biowaste to ameliorate chromium-contaminated soils to improve crop productivity. Waste. Manag. Bull. 2024, 2, 276–288. [Google Scholar] [CrossRef]
- Liu, J.; Sun, S.; Zhang, H.; Kong, Q.; Li, Q.; Yao, X.J.E.R. Remediation materials for the immobilization of hexavalent chromium in contaminated soil: Preparation, applications, and mechanisms. Environ. Res. 2023, 237, 116918. [Google Scholar] [CrossRef]
- Derk, L.; Unold, F. Effect of temperature gradients on water migration, frost heave and thaw-settlement of a clay during freezing-thaw process. Exp. Heat. Transfer. 2022, 36, 585–596. [Google Scholar] [CrossRef]
- Dayarathne, R.; Hawlader, B.; Phillips, R. Centrifuge modelling of gas pipelines undergoing freeze–thaw cycles. Can. Geotech. J. 2022, 59, 485–497. [Google Scholar] [CrossRef]
- Acharya, R.; Lenka, A.; Parida, K. Magnetite modified amino group based polymer nanocomposites towards efficient adsorptive detoxification of aqueous Cr (VI): A review. J. Mol. Liq. 2021, 337, 116487. [Google Scholar] [CrossRef]
- Komaei, A.; Noorzad, A.; Ghadir, P. Stabilization and solidification of arsenic contaminated silty sand using alkaline activated slag. J. Environ. Manag. 2023, 344, 118395. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, X.; Xu, D.; Yang, W.; Bai, S.J.N.R. Application of nanoscale zero-valent iron in hexavalent chromium-contaminated soil: A review. Nanotechnol. Rev. 2020, 9, 736–750. [Google Scholar] [CrossRef]
- Nag, M.; Shimaoka, T. A novel and sustainable technique to immobilize lead and zinc in MSW incineration fly ash by using pozzolanic bottom ash. J. Environ. Manag. 2023, 329, 117036. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, B.; Chang, J.; Shen, L.; Li, T.; Han, X.; Liu, Z. Effect of curing temperatures on geopolymerization and heavy metal solidification in alkali-activated zeolite/MSWI fly ash specimens. Constr. Build. Mater. 2023, 393, 132152. [Google Scholar] [CrossRef]
- Pallav, V.; Teppand, T.; Leinpuu, A.; Shanskiy, M.; Mets, M.; Mändar, H.; Rikmann, E.; Liiv, J. Stabilization of Road Embankments on Peat Soils Using Oil Shale Ash and Pozzolanic Additives. Appl. Sci. 2023, 13, 8366. [Google Scholar] [CrossRef]
- Samadi, P.; Ghodrati, A.; Ghadir, P.; Javadi, A.A. Effect of Seawater on the Mechanical Strength of Geopolymer/Cement Stabilized Sandy Soils. In Proceedings of the TMIC 2022 Slope Stability Conference (TMIC 2022); Springer Nature: Berlin/Heidelberg, Germany, 2023; pp. 121–129. [Google Scholar]
- Branson, Y.; Soltl, S.; Buchmann, C.; Wei, R.; Schaffert, L.; Badenhorst, C.P.S.; Reisky, L.; Jager, G.; Bornscheuer, U.T. Urethanases for the Enzymatic Hydrolysis of Low Molecular Weight Carbamates and the Recycling of Polyurethanes. Angew. Chem. Int. Ed. Engl. 2023, 62, e202216220. [Google Scholar] [CrossRef]
- Unnikrishna Pillai, U.; Anand, K.B. Performance evaluation of polyurethane-nano silica modified cement mortar. Mater. Today Proc. 2021, 46, 4788–4794. [Google Scholar] [CrossRef]
- Li, Y.; Kong, K.; Wang, R.; Yang, X. Mechanical properties and abrasion resistance of polyurethane mortar subjected to freeze–thaw cycles and sulfate attack. J. Build. Eng. 2023, 78, 107760. [Google Scholar] [CrossRef]
- Wei, W.; Bi, Y.; Bi, G. Study of viscoelastic damage and micromechanism of polyurethane in freeze-thaw cycle environment. Case Stud. Constr. Mater. 2023, 19, e02551. [Google Scholar] [CrossRef]
- Asif, U.A.; Mahmood, K.; Naqvi, S.R.; Mehran, M.T.; Noor, T. Development of high-capacity surface-engineered MXene composite for heavy metal Cr (VI) removal from industrial wastewater. Chemosphere 2023, 326, 138448. [Google Scholar] [CrossRef] [PubMed]
- ASTM D4959-16; Standard Test Method for Determination of Water Content of Soil By Direct Heating. American Society for Testing and Materials: West Conshohocken, PA, USA, 2016.
- ASTM D698-12(2021); Standard Test Methods for Laboratory Compaction Characteristics of Soil Using Standard Effort. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ASTM D4972-01; Standard Test Method for pH of Soils. American Society for Testing and Materials: West Conshohocken, PA, USA, 2017.
- Ma, Q.; Chen, J.; Li, W.; Wu, N. Studying the Properties of Chromium-Contaminated Soil Solidified by Polyurethane. Polymers 2023, 15, 2118. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Lin, Z.; Wang, C.; Fu, Y.; Li, J.; Li, Y.; Chen, C. Study on remediation of chromium contaminated soil by an improved electrokinetic extraction device with auxiliary liquid chamber. Chem. Eng. J. 2024, 482, 148814. [Google Scholar] [CrossRef]
- Zheng, P.; Li, W.; Ma, Q.; Xi, L. Mechanical properties of phosphogypsum-soil stabilized by lime activated ground granulated blast-furnace slag. Constr. Build. Mater. 2023, 402, 132994. [Google Scholar] [CrossRef]
- ASTM D2166-06; Standard Test Method for Unconfined Compressive Strength of Cohesive Soil. American Society for Testing and Materials:: West Conshohocken, PA, USA, 2010.
- SW-846 Test Method 1311; Toxicity Characteristic Leaching Procedure. United States Environmental Protection Agency: Columbia, WA, USA, 1992.
- ASTM C1308-21; Standard Test Method for Accelerated Leach Test for Measuring Contaminant Releases from Solidified Waste. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- Fisher, K.S.; Vreugdenhil, A.J. Adsorption of Chromium (VI) Using an Activated Carbon Derived from Petroleum Coke Feedstock. Int. J. Mol. Sci 2022, 23, 16172. [Google Scholar] [CrossRef]
- Liu, P.; Liu, H.L.; Xiao, Y.; Yang, G. Experimental study on static characteristics of polymer cemented rockfill. Chin. J. Rock Soil Mech. 2015, 36, 749–754. [Google Scholar] [CrossRef]
- Hao, X.; Ma, W.; Feng, W.; Wen, Z.; Zhang, L.; Chen, S. Investigation on the frost heave-induced pressure and hydro-thermal processes in freezing soil under rigid constraint and hydraulic pressure. Eng. Geol. 2023, 323, 107238. [Google Scholar] [CrossRef]
- Amirshahi, S.; Jorjani, E. Preliminary Flowsheet Development for Mixed Rare Earth Elements Production from Apatite Leaching Aqueous Solution Using Biosorption and Precipitation. Minerals 2023, 13, 909. [Google Scholar] [CrossRef]
- Tang, L.; Sun, S.; Zheng, J.; Jin, L.; Yu, Y.; Luo, T.; Duan, X. Pore evolution and shear characteristics of a soil-rock mixture upon freeze-thaw cycling. Res. Cold Arid. Reg. 2023, 15, 179–190. [Google Scholar] [CrossRef]
- Farooq, M.A.; Nimbalkar, S. Monotonic and cyclic triaxial testing of untreated and polyurethane-treated soil and soil-rubber mixtures. Acta Geotech. 2023. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Doroganov, A.P.; Larin, D.E.; Smirnova, M.E.; Cherkaev, G.V.; Kabaeva, N.M.; Kitaeva, D.K.; Buyanovskaya, A.G.; Philippova, O.E. Hydrogels of Polysaccharide Carboxymethyl Hydroxypropyl Guar Crosslinked by Multivalent Metal Ions. Polym. Sci. Ser. A 2021, 63, 24–33. [Google Scholar] [CrossRef]
- Iqhrammullah, M.; Marlina, M.; Khalil, H.P.S.A.; Kurniawan, K.H.; Suyanto, H.; Hedwig, R.; Karnadi, I.; Olaiya, N.G.; Abdullah, C.K.; Abdulmadjid, S.N. Characterization and Performance Evaluation of Cellulose Acetate–Polyurethane Film for Lead II Ion Removal. Polymers 2020, 12, 1317. [Google Scholar] [CrossRef] [PubMed]
- Pandey, L. Surface engineering of nano-sorbents for the removal of heavy metals: Interfacial aspects. J. Environ. Chem. Eng. 2021, 9, 104586. [Google Scholar] [CrossRef]
- Pan, Z.; Zhang, T.; Qian, X.; Zhao, Y. Probing the fast transformation mechanism of Cr (VI) on carbon dots with structural defects and surface oxygen functional groups. Appl. Catal. B 2023, 330, 122571. [Google Scholar] [CrossRef]
- Moawed, E.A.; El-Hagrasy, M.A.; Farhat, A.A.M. Application of magnetic isothiouronium polyurethane sorbent for the removal of acidic and basic dyes from wastewater. J. Clean. Prod. 2017, 157, 232–242. [Google Scholar] [CrossRef]
- Yadav, M.; Saha, J.K.; Ghosh, S.K. Surface, Chemical, and Mechanical Properties of Polyurethane-Coated Galvanized Steel Sheets. J. Mater. Eng. Perform. 2024. [Google Scholar] [CrossRef]
- Bashir, A.; Manzoor, T.; Malik, L.A.; Qureashi, A.; Pandith, A.H. Enhanced and Selective Adsorption of Zn(II), Pb(II), Cd(II), and Hg(II) Ions by a Dumbbell- and Flower-Shaped Potato Starch Phosphate Polymer: A Combined Experimental and DFT Calculation Study. Acs Omega 2020, 5, 4853–4867. [Google Scholar] [CrossRef] [PubMed]
- Famurewa, A.C.; Renu, K.; Eladl, M.A.; Chakraborty, R.; Myakala, H.; El-Sherbiny, M.; Elsherbini, D.M.A.; Vellingiri, B.; Madhyastha, H.; Ramesh Wanjari, U.; et al. Hesperidin and hesperetin against heavy metal toxicity: Insight on the molecular mechanism of mitigation. Biomed. Pharmacother. 2022, 149, 112914. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Ajaj, Y.; Mahmoud, Z.H.; Kamil Ghadir, G.; Khalid Alani, Z.; Hussein, M.M.; Abed Hussein, S.; Morad Karim, M.; Al-khalidi, A.; Abbas, J.K.; et al. Adsorption of heavy metal ions use chitosan/graphene nanocomposites: A review study. Results Chem. 2024, 7, 101332. [Google Scholar] [CrossRef]
- Yang, Z.; Li, X.; Li, D.; Wang, Y.; Liu, X. Effects of Long-Term Repeated Freeze-Thaw Cycles on the Engineering Properties of Compound Solidified/Stabilized Pb-Contaminated Soil: Deterioration Characteristics and Mechanisms. Int. J. Environ. Res. Public Health 2020, 17, 1798. [Google Scholar] [CrossRef]
- Hayashi, N.; Matsumura, D.; Hoshina, H.; Ueki, Y.; Tsuji, T.; Chen, J.H.; Seko, N. Chromium(VI) adsorption-reduction using a fibrous amidoxime-grafted adsorbent. Sep. Purif. Technol. 2021, 277, 119536. [Google Scholar] [CrossRef]



| Properties | Value |
|---|---|
| Dry density (g/cm3) | 1.78 |
| Specific gravity (g/cm3) | 2.69 |
| Optimal moisture concentration (%) | 19.5 |
| Initial water content (%) | 32.00 |
| Liquid limit (%) | 48.20 |
| Plastic limit (%) | 20.10 |
| pH | 7.43 |
| Element | SiO2 | MgO | CaO | Al2O3 | Fe2O3 | K2O | Na2O | TiO2 |
| Soil | 63.90 | 2.19 | 3.66 | 18.69 | 6.45 | 2.59 | 0.74 | 0.95 |
| Experiment | Freeze–Thaw Cycle Times | ||||||
|---|---|---|---|---|---|---|---|
| UCS | PU dosage | chromium concentration 2000 mg/kg | |||||
| 0% | 0P-0F | 0P-2F | 0P-4F | 0P-6F | 0P-8F | 0P-14F | |
| 4% | 4P-0F | 4P-2F | 4P-4F | 4P-6F | 4P-8F | 4P-14F | |
| 8% | 8P-0F | 8P-2F | 8P-4F | 8P-6F | 8P-8F | 8P-14F | |
| 12% | 12P-0F | 12P-2F | 12P-4F | 12P-6F | 12P-8F | 12P-14F | |
| TCLP | chromium concentration 2000 mg/kg | ||||||
| 0% | 0P-0F | 0P-4F | 0P-8F | 0P-14F | |||
| 4% | 4P-0F | 4P-4F | 4P-8F | 4P-14F | |||
| 8% | 8P-0F | 8P-4F | 8P-8F | 8P-14F | |||
| 12% | 12P-0F | 12P-4F | 12P-8F | 12P-14F | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Q.; Zheng, P.; Chen, J.; Lu, X. Solidification/Stabilization of Chromium-Contaminated Soils by Polyurethane during Freeze–Thaw Cycles: Mechanical, Leaching and Microstructure Characterization. Materials 2024, 17, 1347. https://doi.org/10.3390/ma17061347
Ma Q, Zheng P, Chen J, Lu X. Solidification/Stabilization of Chromium-Contaminated Soils by Polyurethane during Freeze–Thaw Cycles: Mechanical, Leaching and Microstructure Characterization. Materials. 2024; 17(6):1347. https://doi.org/10.3390/ma17061347
Chicago/Turabian StyleMa, Qiang, Pangkun Zheng, Junjie Chen, and Xuesong Lu. 2024. "Solidification/Stabilization of Chromium-Contaminated Soils by Polyurethane during Freeze–Thaw Cycles: Mechanical, Leaching and Microstructure Characterization" Materials 17, no. 6: 1347. https://doi.org/10.3390/ma17061347
APA StyleMa, Q., Zheng, P., Chen, J., & Lu, X. (2024). Solidification/Stabilization of Chromium-Contaminated Soils by Polyurethane during Freeze–Thaw Cycles: Mechanical, Leaching and Microstructure Characterization. Materials, 17(6), 1347. https://doi.org/10.3390/ma17061347





