pH-Responsive Pesticide-Loaded Hollow Mesoporous Silica Nanoparticles with ZnO Quantum Dots as a Gatekeeper for Control of Rice Blast Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Regents
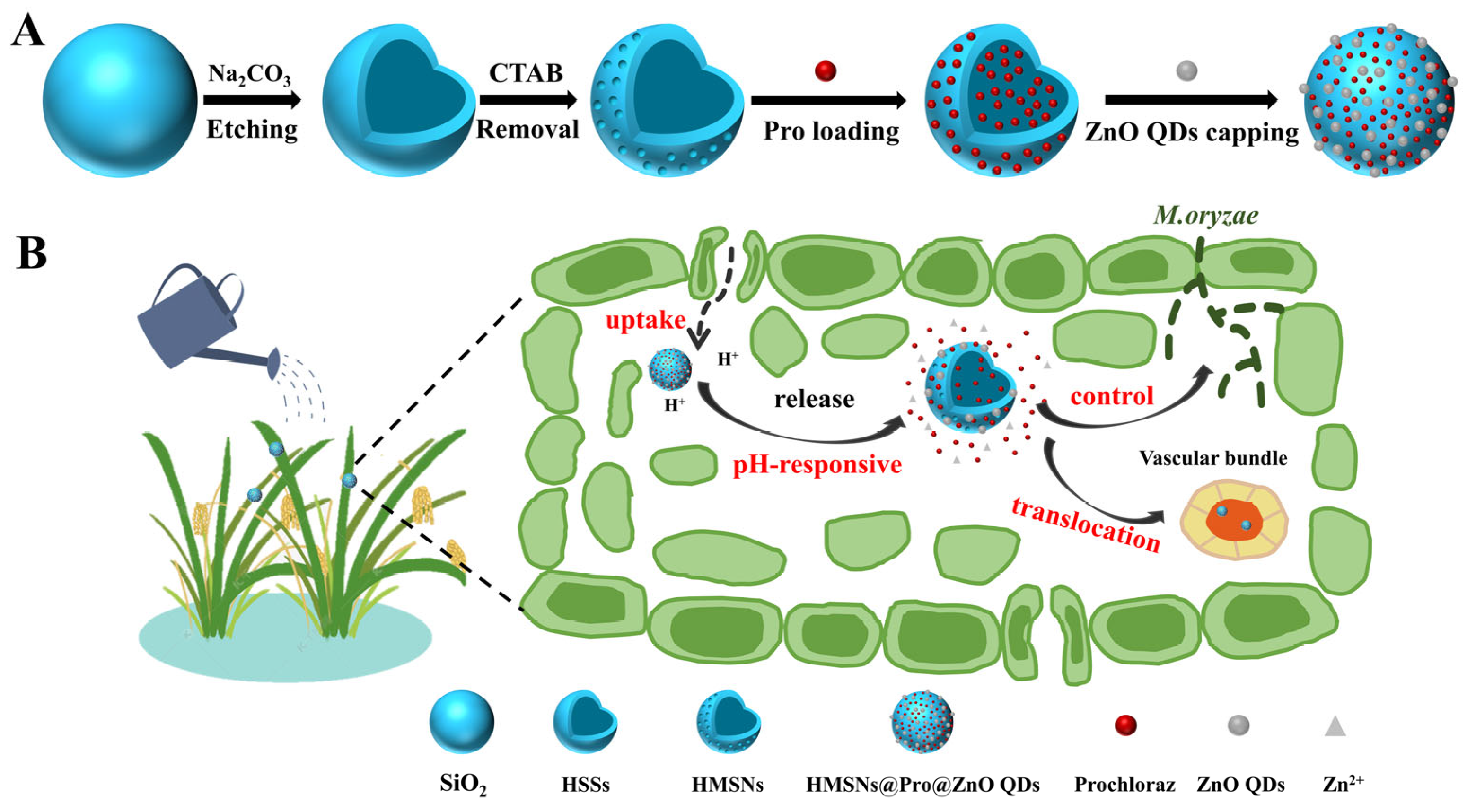
2.2. Synthesis and Modification of HMSNs
2.3. Synthesis of ZnO QDs
2.4. Preparation of ZnO QDs Capped HMSNs@Pro
2.5. Characterization of NPs
2.6. Evaluation of Pro Loading
2.7. Characterization of Controlled Release Behavior
2.8. Evaluation of In Vitro Fungicidal Activity
2.9. Evaluation of HMSNs@Pro@ZnO QDs for Rice Blast Disease Control
2.10. Photostability of HMSNs@Pro@ZnO QDs
2.11. Investigation of Distribution and Translocation of HMSNs@FITC in Plant Tissue
3. Results and Discussion
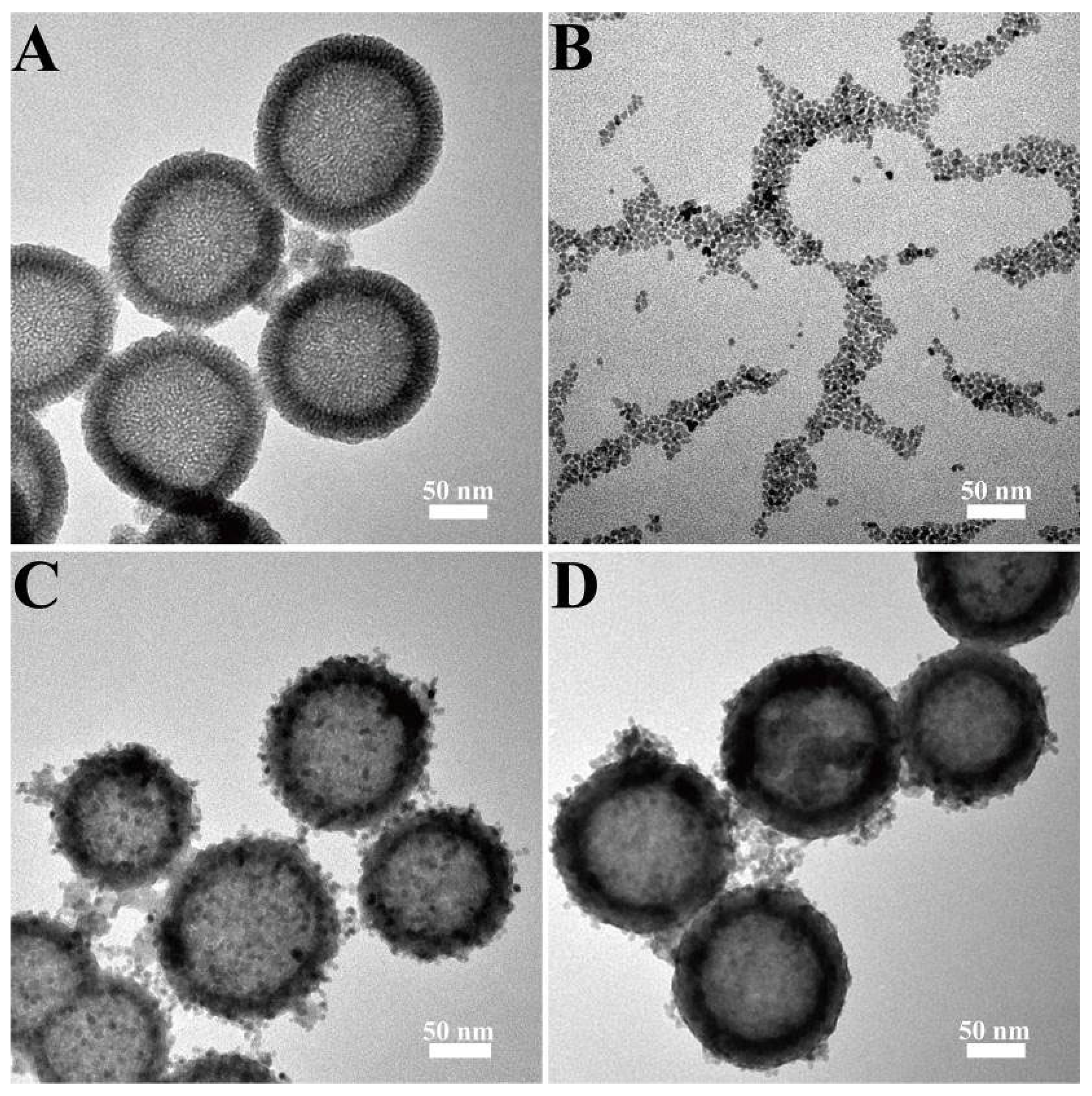
3.1. Synthesis and Characterization of NMs
3.2. Release Performance of HMSNs@Pro and HMSNs@Pro@ZnO QDs
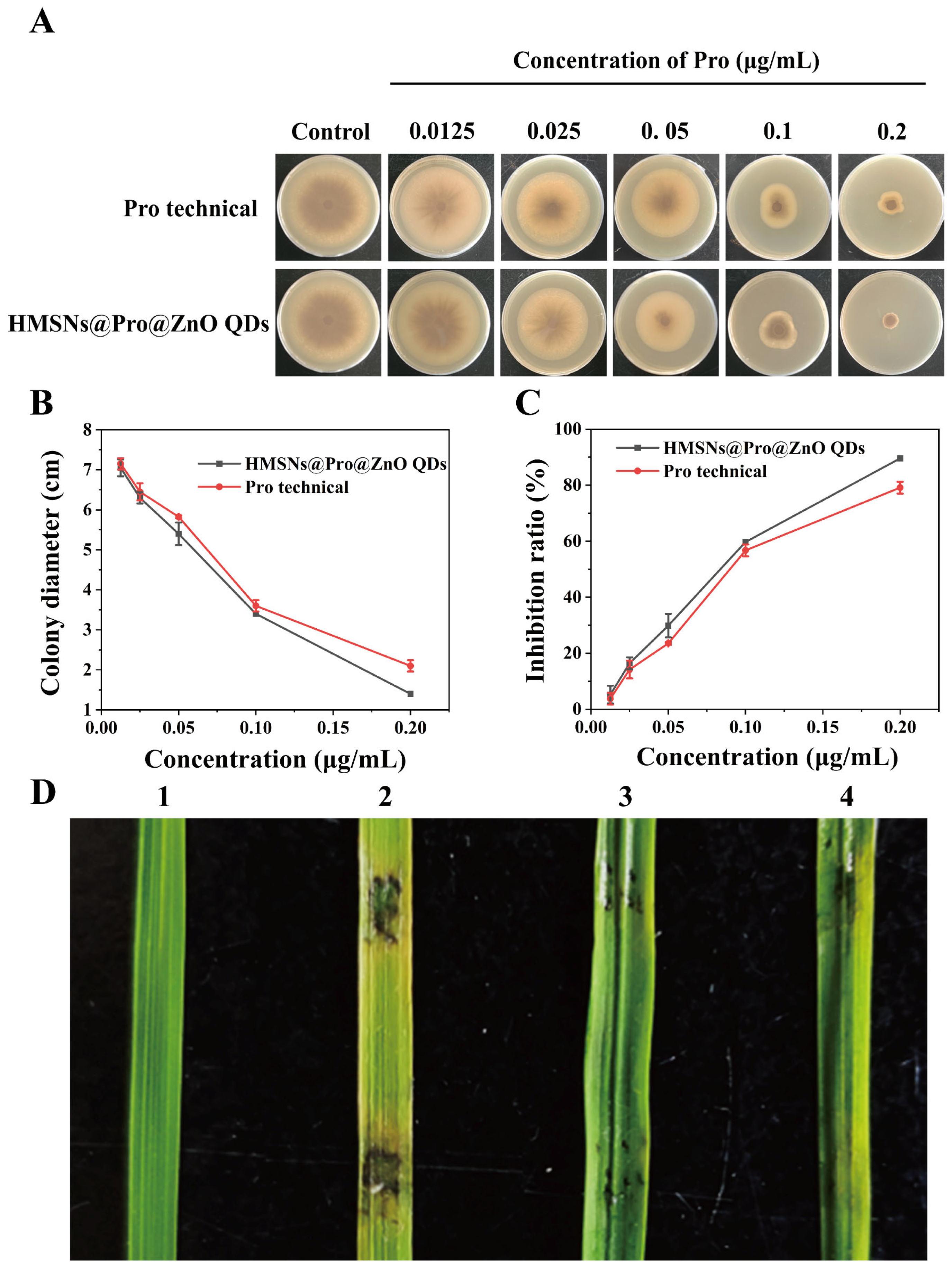
3.3. Control Efficiency of HMSNs@Pro@ZnO QDs against M. oryzae
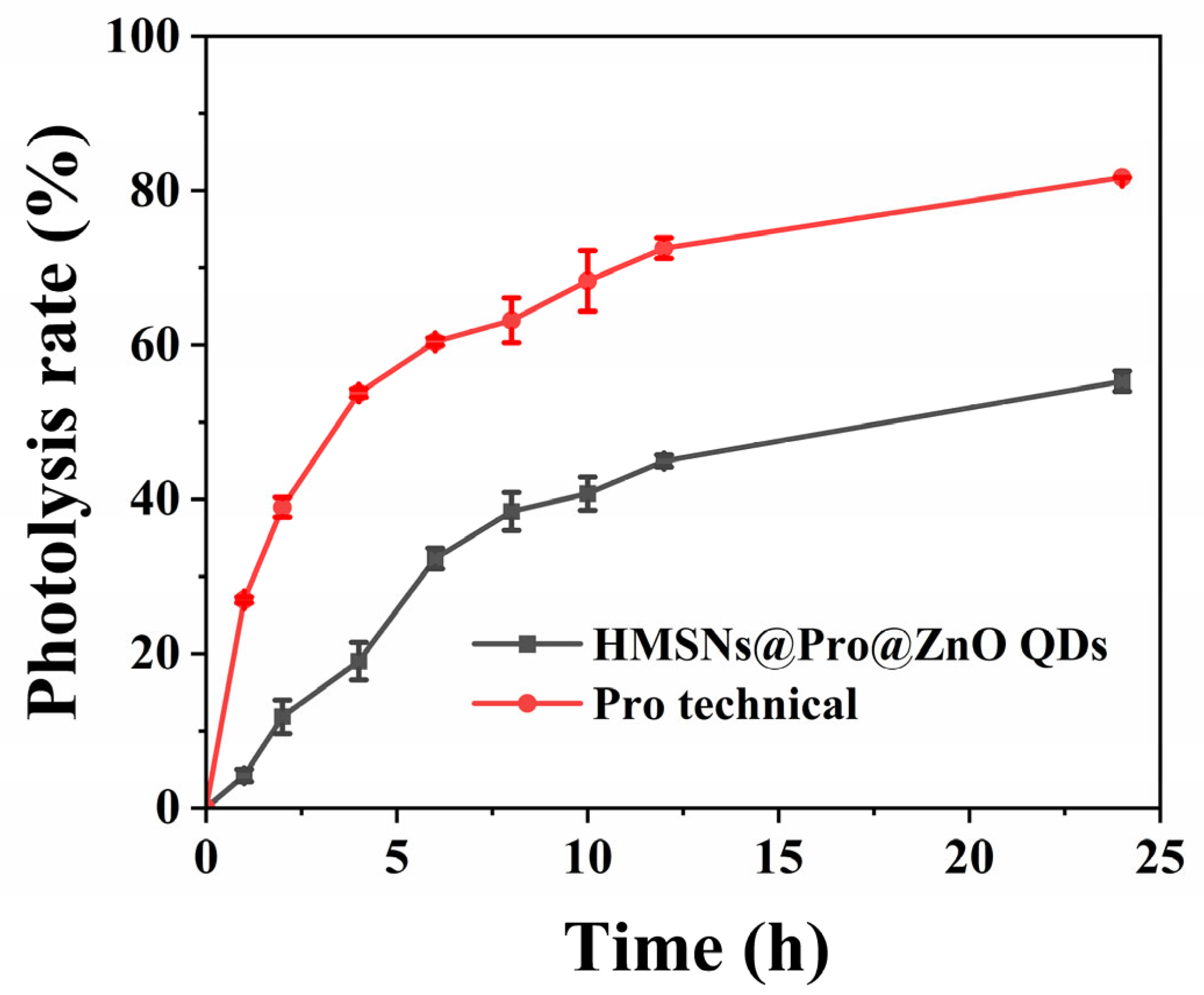
3.4. Evaluation of the Photostability of the Pesticide
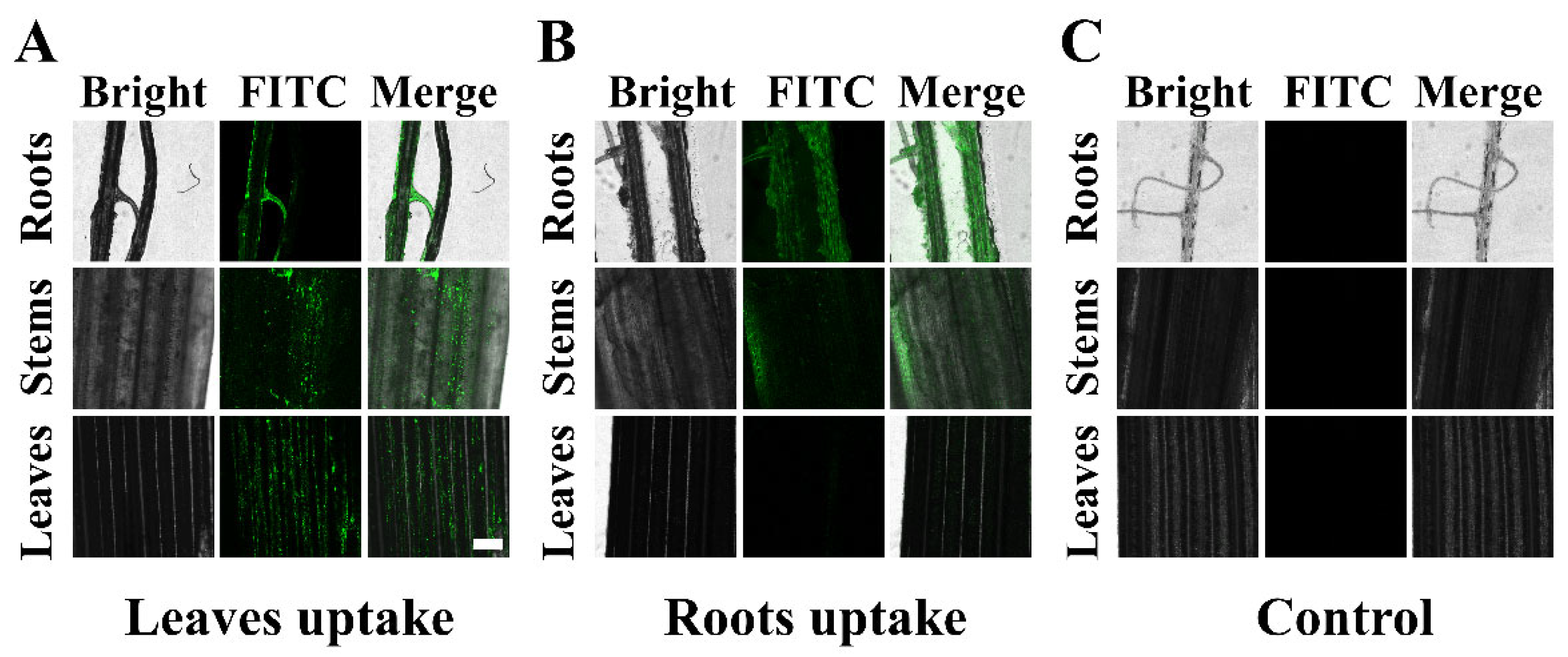
3.5. Study on the Translocation of HMSNs@FITC in Plants
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sasaki, T. The map-based sequence of the rice genome. Nature 2005, 436, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Hashim, N.; Ali, M.M.; Mahadi, M.R.; Abdullah, A.F.; Wayayok, A.; Mohd Kassim, M.S.; Jamaluddin, A. Smart Farming for Sustainable Rice Production: An Insight into Application, Challenge, and Future Prospect. Rice Sci. 2024, 31, 47–61. [Google Scholar] [CrossRef]
- Dauda, W.P.; Singh Rana, V.; Solanke, A.U.; Krishnan, G.; Bashya, B.M.; Aggarwal, R.; Shanmugam, V. Metabolomic analysis of sheath blight disease of rice (Oryza sativa L.) induced by Rhizoctonia solani phytotoxin. J. Appl. Microbiol. 2022, 133, 3215–3227. [Google Scholar] [CrossRef] [PubMed]
- Motoyama, T.; Kondoh, Y.; Shimizu, T.; Hayashi, T.; Honda, K.; Uchida, M.; Osada, H. Identification of Scytalone Dehydratase Inhibitors Effective against Melanin Biosynthesis Dehydratase Inhibitor-Resistant Pyricularia oryzae. J. Agric. Food Chem. 2022, 70, 3109–3116. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, A.; Ghosh, S.; Sahoo, D.; Jha, G. Fungal effectors, the double edge sword of phytopathogens. Curr. Genet. 2021, 67, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Hogan, D.A.; Vylkova, S. Environmental pH modulation by pathogenic fungi as a strategy to conquer the host. PLoS Pathog. 2017, 13, e1006149. [Google Scholar]
- Masudulla, K.; Azhar, U.K.; Mohd Abul, H.; Krishna Kumar, Y.; Marina, M.C.P.; Nazia, M.; Virendra Kumar, Y.; Afzal Husain, K.; Saiful, I.; Gulshan Kumar, S. Agro-Nanotechnology as an Emerging Field: A Novel Sustainable Approach for Improving Plant Growth by Reducing Biotic Stress. Appl. Sci. 2021, 11, 2282. [Google Scholar]
- Zabkiewicz, J.A.; Pethiyagoda, R.; Forster, W.A.; van Leeuwen, R.; Moroney, T.J.; McCue, S.W. Simulating spray droplet impaction outcomes: Comparison with experimental data. Pest. Manag. Sci. 2020, 76, 3469–3476. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public. Health 2021, 18, 1112. [Google Scholar] [CrossRef]
- Yao, J.; Zhi, H.; Shi, Q.; Zhang, Y.; Feng, J.; Liu, J.; Huang, H.; Xie, X. Tannic Acid Interfacial Modification of Prochloraz Ethyl Cellulose Nanoparticles for Enhancing the Antimicrobial Effect and Biosafety of Fungicides. ACS Appl. Mater. Interfaces 2023, 15, 41324–41336. [Google Scholar] [CrossRef]
- Awwad, M.M.; Taha, S.M.; Khalil, M.M.H.; Salem, A.M.; Chovelon, J.-M. The simultaneous degradation of prochloraz and tebuconazole in water with monitoring their degradation products using liquid chromatography-tandem mass spectrometry. Environ. Sci. Pollut. Res. Int. 2023, 30, 83810–83820. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Cao, L.; Ma, D.; Zhou, Z.; Huang, Q.; Pan, C. Translocation, distribution and degradation of prochloraz-loaded mesoporous silica nanoparticles in cucumber plants. Nanoscale 2018, 10, 1798–1806. [Google Scholar] [CrossRef]
- Dengjun, W.; Navid, B.S.; Andrew, B.; Richard, Z.; Endalkachew, S.-D.; Todd, P.L.; Kay, T.H.; Robert, M.B.; Markus, F.; Jason, C.W.; et al. Nano-enabled pesticides for sustainable agriculture and global food security. Nat. Nanotechnol. 2022, 17, 347–360. [Google Scholar]
- Bueno, V.; Gao, X.; Abdul Rahim, A.; Wang, P.; Bayen, S.; Ghoshal, S. Uptake and Translocation of a Silica Nanocarrier and an Encapsulated Organic Pesticide Following Foliar Application in Tomato Plants. Environ. Sci. Technol. 2022, 56, 6722–6732. [Google Scholar] [CrossRef]
- Vijayakumar, M.D.; Surendhar, G.J.; Natrayan, L.; Patil, P.P.; Ram, P.M.B.; Paramasivam, P. Evolution and Recent Scenario of Nanotechnology in Agriculture and Food Industries. J. Nanomater. 2022, 2022, 1280411. [Google Scholar] [CrossRef]
- Pan, X.; Guo, X.; Zhai, T.; Zhang, D.; Rao, W.; Cao, F.; Guan, X. Nanobiopesticides in sustainable agriculture: Developments, challenges, and perspectives. Environ. Sci. Nano 2023, 10, 41–61. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nanopesticides: State of Knowledge, Environmental Fate, and Exposure Modeling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Kah, M.; Hofmann, T. Nanopesticide research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Rehman, A.; Feng, J.; Qunyi, T.; Korma, S.A.; Assadpour, E.; Usman, M.; Han, W.; Jafari, S.M. Pesticide-loaded colloidal nanodelivery systems; preparation, characterization, and applications. Adv. Colloid Interface Sci. 2021, 298, 102552. [Google Scholar] [CrossRef]
- Young, M.; Ozcan, A.; Myers, M.E.; Johnson, E.G.; Graham, J.H.; Santra, S. Multimodal Generally Recognized as Safe ZnO/Nanocopper Composite: A Novel Antimicrobial Material for the Management of Citrus Phytopathogens. J. Agric. Food Chem. 2017, 66, 6604–6608. [Google Scholar] [CrossRef]
- Maity, D.; Gupta, U.; Saha, S. Biosynthesized metal oxide nanoparticles for sustainable agriculture: Next-generation nanotechnology for crop production, protection and management. Nanoscale 2022, 14, 13950–13989. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Bhakuni, P.; Barman, S.R.; Nayak, B. RSM-CCD optimized hollow mesoporous silica nanospheres encapsulating sorafenib induce mitochondrial membrane potential mediated apoptotic cell death in non-small cell lung cancer. Microporous Mesoporous Mater. 2024, 370, 113032. [Google Scholar] [CrossRef]
- Šoltys, M.; Balouch, M.; Kašpar, O.; Lhotka, M.; Ulbrich, P.; Zadražil, A.; Kovačík, P.; Štĕpánek, F. Evaluation of scale-up strategies for the batch synthesis of dense and hollow mesoporous silica microspheres. Chem. Eng. J. 2018, 334, 1135–1147. [Google Scholar] [CrossRef]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli-Responsive Nano-Architecture Drug-Delivery Systems to Solid Tumor Micromilieu: Past, Present, and Future Perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef] [PubMed]
- Camara, M.C.; Campos, E.V.R.; Monteiro, R.A.; do Espirito Santo Pereira, A.; de Freitas Proença, P.L.; Fraceto, L.F. Development of stimuli-responsive nano-based pesticides: Emerging opportunities for agriculture. J. Nanobiotechnol. 2019, 17, 100. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Y.; Dong, H.; Niu, J.; Tang, J.; Yang, J.; Tang, G.; Zhou, Z.; Tang, R.; Shi, X.; et al. A Bioresponsive System Based on Mesoporous Organosilica Nanoparticles for Smart Delivery of Fungicide in Response to Pathogen Presence. ACS Sustain. Chem. Eng. 2020, 8, 5716–5723. [Google Scholar] [CrossRef]
- Kaziem, A.E.; Gao, Y.; He, S.; Li, J. Synthesis and Insecticidal Activity of Enzyme-Triggered Functionalized Hollow Mesoporous Silica for Controlled Release. J. Agric. Food Chem. 2017, 65, 7854–7864. [Google Scholar] [CrossRef]
- Shrestha, P.K.; Chun, Y.T.; Chu, D. A high-resolution optically addressed spatial light modulator based on ZnO nanoparticles. Light Sci. Appl. 2015, 4, e259. [Google Scholar] [CrossRef]
- Sarma, B.; Sarma, B.K. Fabrication of Ag/ZnO heterostructure and the role of surface coverage of ZnO microrods by Ag nanoparticles on the photophysical and photocatalytic properties of the metal-semiconductor system. Appl. Surf. Sci. 2017, 410, 557–565. [Google Scholar] [CrossRef]
- Sobhani, Z.; Khalifeh, R.; Banizamani, M.; Rajabzadeh, M. Water-soluble ZnO quantum dots modified by polyglycerol: The pH-sensitive and targeted fluorescent probe for delivery of an anticancer drug. J. Drug Delivery Sci. Technol. 2022, 76, 103452. [Google Scholar] [CrossRef]
- Singh, P.; Singh, R.K.; Kumar, R. Journey of ZnO quantum dots from undoped to rare-earth and transition metal-doped and their applications. RSC Adv. 2021, 11, 2512–2545. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, Y.; Wang, Y.; Gong, H.; Zhu, H.; Liu, M. Redox/pH dual stimuli-responsive ZnO QDs-gated mesoporous silica nanoparticles as carriers in cancer therapy. IET Nanobiotechnol. 2019, 13, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.-B.; Du, M.-R.; Liu, K.-K.; Zhou, R.; Ma, R.-N.; Jiao, Z.; Zhao, Q.; Shan, C.-X. Hydrophilic ZnO Nanoparticles@Calcium Alginate Composite for Water Purification. ACS Appl. Mater. Interfaces 2020, 12, 13305–13315. [Google Scholar] [CrossRef]
- Qiu, J.; Chen, Y.; Liu, Z.; Wen, H.; Jiang, N.; Shi, H.; Kou, Y. The application of zinc oxide nanoparticles: An effective strategy to protect rice from rice blast and abiotic stresses. Environ. Pollut. 2023, 331, 121925. [Google Scholar] [CrossRef]
- Liang, Y.; Duan, Y.; Fan, C.; Dong, H.; Yang, J.; Tang, J.; Tang, G.; Wang, W.; Jiang, N.; Cao, Y. Preparation of kasugamycin conjugation based on ZnO quantum dots for improving its effective utilization. Chem. Eng. J. 2019, 361, 671–679. [Google Scholar] [CrossRef]
- Xie, Q.; Lu, H.; Wang, X.; Zhang, Y.; Zhou, N. Functionalized hollow mesoporous silica for detection of Staphylococcus aureus and sterilization. J. Environ. Chem. Eng. 2021, 9, 105892. [Google Scholar] [CrossRef]
- Chen, W.; Cheng, C.-A.; Cosco, E.D.; Ramakrishnan, S.; Lingg, J.G.P.; Bruns, O.T.; Zink, J.I.; Sletten, E.M. Shortwave Infrared Imaging with J-Aggregates Stabilized in Hollow Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2019, 141, 12475–12480. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Cai, R.; Zhang, Y.; Wang, X.; Zhou, N. A pH-Gated Functionalized Hollow Mesoporous Silica Delivery System for Photodynamic Sterilization in Staphylococcus aureus Biofilm. Materials 2022, 15, 2815. [Google Scholar] [CrossRef]
- Chen, M.; Hu, J.; Bian, C.; Zhu, C.; Chen, C.; Guo, Z.; Zhang, Z.; Agyekum, G.A.; Zhang, Z.; Cao, X. pH-Responsive and Biodegradable ZnO-Capped Mesoporous Silica Composite Nanoparticles for Drug Delivery. Materials 2020, 13, 3950. [Google Scholar] [CrossRef]
- Moyen, E.; Kim, J.H.; Kim, J.; Jang, J. ZnO Nanoparticles for Quantum-Dot-Based Light-Emitting Diodes. ACS Appl. Nano Mater. 2020, 3, 5203–5211. [Google Scholar] [CrossRef]
- Shi, L.; Liang, Q.; Zang, Q.; Lv, Z.; Meng, X.; Feng, J. Construction of Prochloraz-Loaded Hollow Mesoporous Silica Nanoparticles Coated with Metal–Phenolic Networks for Precise Release and Improved Biosafety of Pesticides. Nanomaterials 2022, 12, 2885. [Google Scholar] [CrossRef]
- Wu, L.; Pan, H.; Huang, W.; Hu, Z.; Wang, M.; Zhang, F. pH and Redox Dual-Responsive Mesoporous Silica Nanoparticle as Nanovehicle for Improving Fungicidal Efficiency. Materials 2022, 15, 2207. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Y.; Lou, X.-Y.; Cai, Z.; Zhang, M.-Z.; Jia, C.; Qin, J.-C.; Yang, Y.-W. Supramolecular Nanoplatform Based on Mesoporous Silica Nanocarriers and Pillararene Nanogates for Fungus Control. ACS Appl. Mater. Interfaces 2021, 13, 32295–32306. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Xie, Z.; Cheng, J.; Xiao, D.; Xiong, Q.; Wang, Q.; Zhao, J.; Gui, W. A Light-Triggered pH-Responsive Metal–Organic Framework for Smart Delivery of Fungicide to Control Sclerotinia Diseases of Oilseed Rape. ACS Nano 2021, 15, 6987–6997. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, L.; Zhao, R.; Sun, Z.; Wang, Y.; Yu, M.; Pan, S.; Guo, X.; Xu, Y.; Wang, H.; et al. Nanoencapsulation-based fabrication of eco-friendly pH-responsive pyraclostrobin formulations with enhanced photostability and adhesion to leaves. J. Environ. Chem. Eng. 2023, 11, 109688. [Google Scholar] [CrossRef]
- Abdelrahman, T.M.; Qin, X.; Li, D.; Senosy, I.A.; Mmby, M.; Wan, H.; Li, J.; He, S. Pectinase-responsive carriers based on mesoporous silica nanoparticles for improving the translocation and fungicidal activity of prochloraz in rice plants. Chem. Eng. J. 2021, 404, 126440. [Google Scholar] [CrossRef]
- Muhammad, F.; Guo, M.; Qi, W.; Sun, F.; Wang, A.; Guo, Y.; Zhu, G. pH-Triggered controlled drug release from mesoporous silica nanoparticles via intracelluar dissolution of ZnO nanolids. J. Am. Chem. Soc. 2011, 133, 8778–8781. [Google Scholar] [CrossRef]
- Askarizadeh, M.; Esfandiari, N.; Honarvar, B.; Sajadian, S.A.; Azdarpour, A. Kinetic Modeling to Explain the Release of Medicine from Drug Delivery Systems. ChemBioEng Rev. 2023, 10, 1006–1049. [Google Scholar] [CrossRef]
- Pooresmaeil, M.; Namazi, H. Facile preparation of pH-sensitive chitosan microspheres for delivery of curcumin; characterization, drug release kinetics and evaluation of anticancer activity. Int. J. Biol. Macromol. 2020, 162, 501–511. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Rani, S.; Kumari, N.; Sharma, V. Uptake, translocation, transformation and physiological effects of nanoparticles in plants. Arch. Agron. Soil. Sci. 2022, 69, 1579–1599. [Google Scholar] [CrossRef]
- Afzal, S.; Aftab, T.; Singh, N.K. Impact of Zinc Oxide and Iron Oxide Nanoparticles on Uptake, Translocation, and Physiological Effects in Oryza sativa L. J. Plant Growth Regul. 2022, 41, 1445–1461. [Google Scholar] [CrossRef]


| Sample | Models | pH | Fitting Equation | R2 |
|---|---|---|---|---|
| 5.0 | y = 64.74(1−e−0.13 x) | 0.9960 | ||
| HMSNs@Pro | First order | 6.5 | y = 70.08(1−e−0.13 x) | 0.9842 |
| 7.4 | y = 58.08(1−e−0.26 x) | 0.9982 | ||
| 5.0 | y = 49.19(1−e−0.10 x) | 0.9925 | ||
| HMSNs@Pro@ZnO QDs | First order | 6.5 | y = 34.37(1−e−0.09 x) | 0.9659 |
| 7.4 | y = 16.28(1−e−0.32 x) | 0.9524 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Zhang, Y.; Yan, Y.; Huang, Z.; Zhang, Y.; Wang, X.; Zhou, N. pH-Responsive Pesticide-Loaded Hollow Mesoporous Silica Nanoparticles with ZnO Quantum Dots as a Gatekeeper for Control of Rice Blast Disease. Materials 2024, 17, 1344. https://doi.org/10.3390/ma17061344
Zhao Y, Zhang Y, Yan Y, Huang Z, Zhang Y, Wang X, Zhou N. pH-Responsive Pesticide-Loaded Hollow Mesoporous Silica Nanoparticles with ZnO Quantum Dots as a Gatekeeper for Control of Rice Blast Disease. Materials. 2024; 17(6):1344. https://doi.org/10.3390/ma17061344
Chicago/Turabian StyleZhao, Yi, Yanning Zhang, Yilin Yan, Zunyao Huang, Yuting Zhang, Xiaoli Wang, and Nandi Zhou. 2024. "pH-Responsive Pesticide-Loaded Hollow Mesoporous Silica Nanoparticles with ZnO Quantum Dots as a Gatekeeper for Control of Rice Blast Disease" Materials 17, no. 6: 1344. https://doi.org/10.3390/ma17061344






