Chemistry of Polythiols and Their Industrial Applications
Abstract
:1. History of Sulfur Compounds
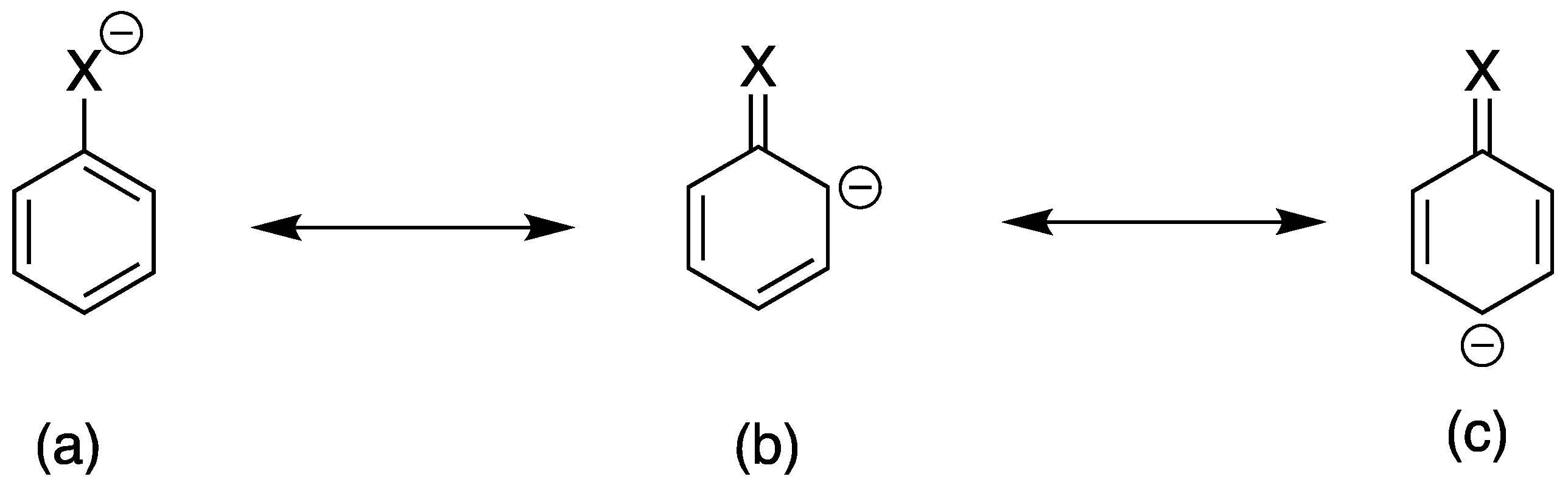
2. Comparison of the Physical Properties of Thiols and Alcohols
3. Synthesis of Thiol Compounds
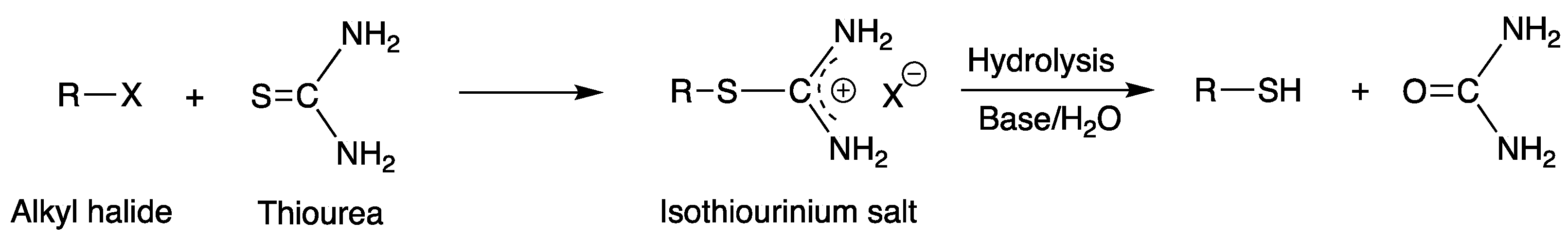
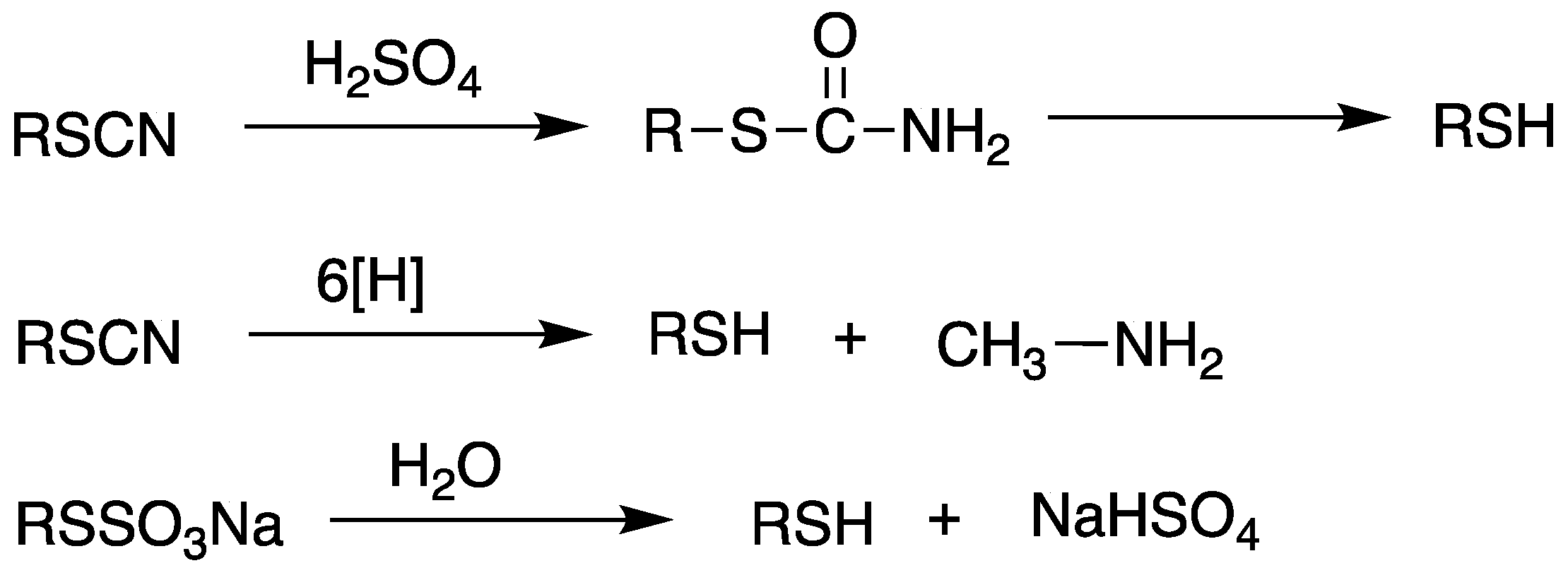
3.1. Nucleophilic Substitution
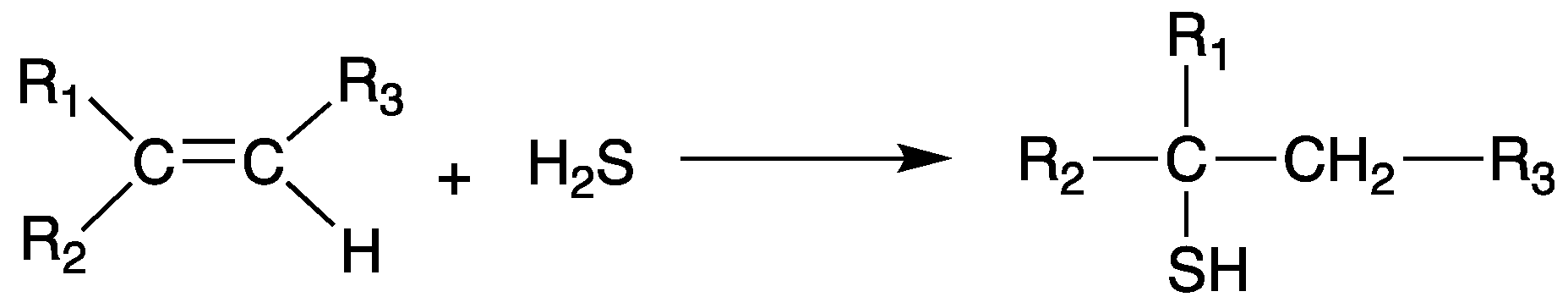
3.2. Addition to Olefins
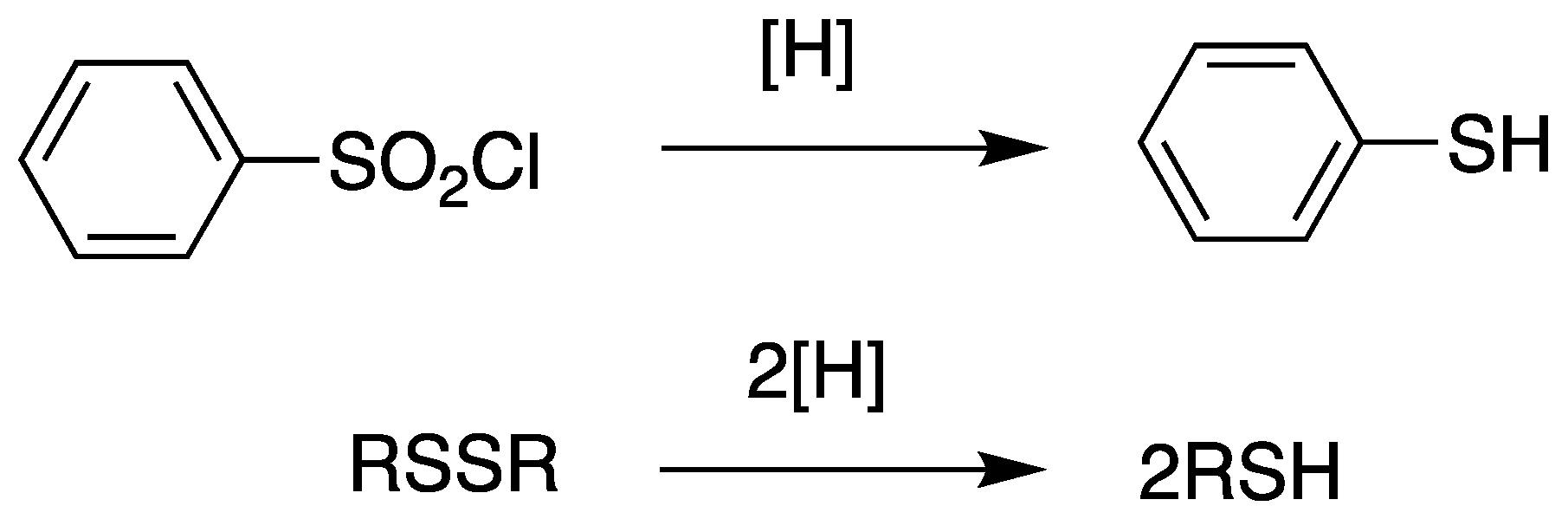
3.3. Reduction Reaction
3.4. From Organometallics
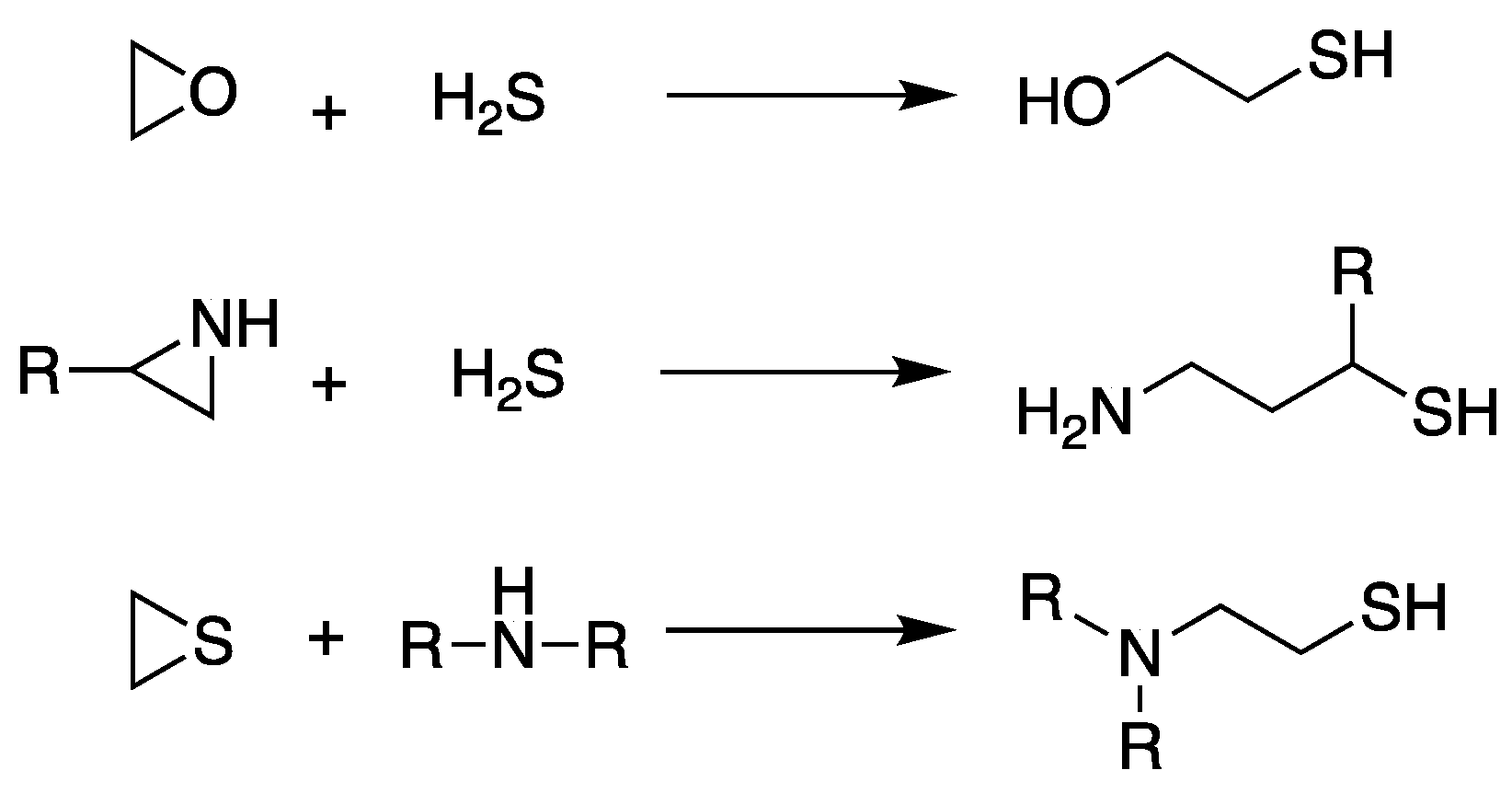
3.5. Other Methods
4. Reaction of Thiol Compounds
5. Polythiol Compounds
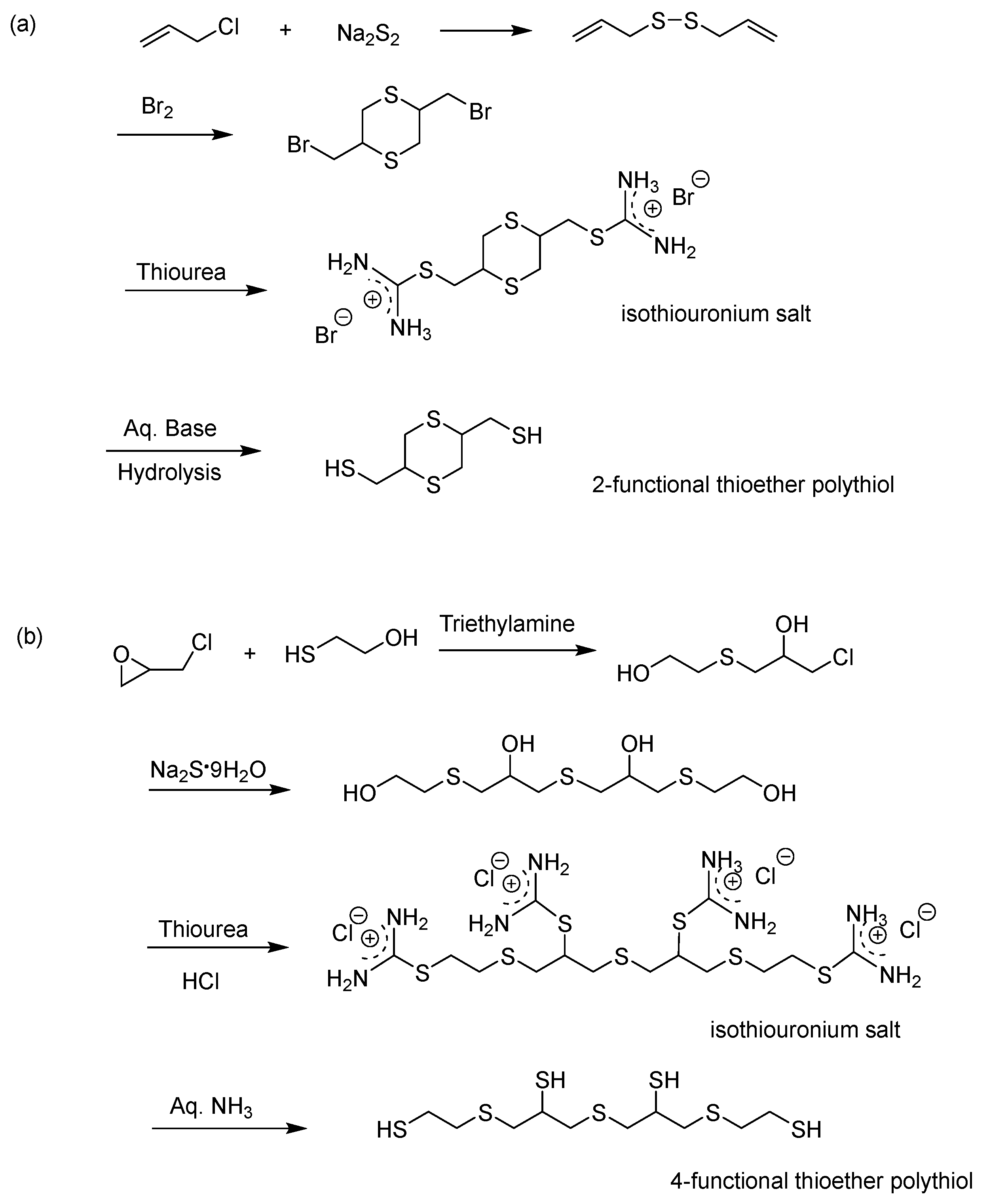
5.1. Thioether Polythiol Compounds
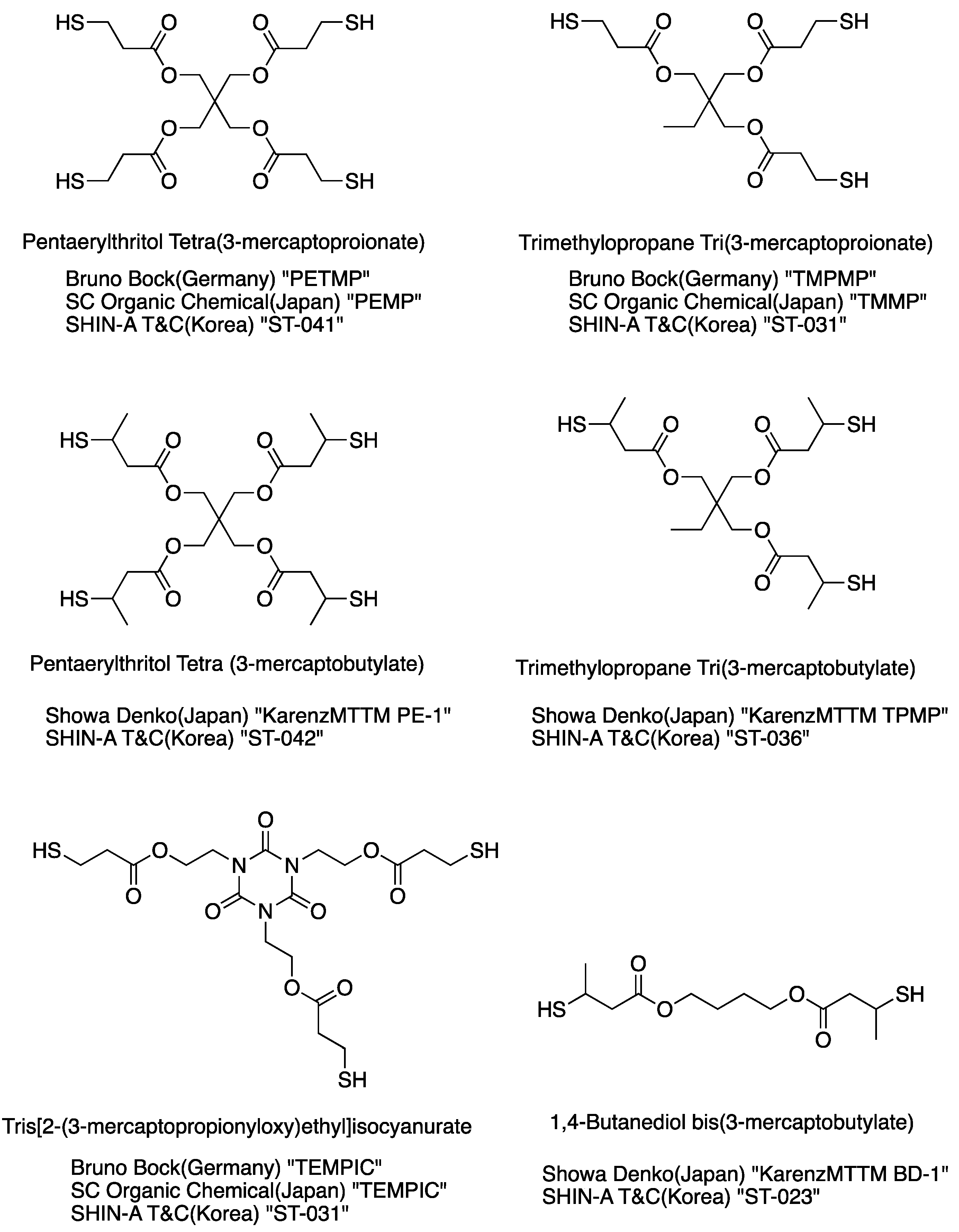
5.2. Ester Polythiol
6. Applications of Polythiol Compounds
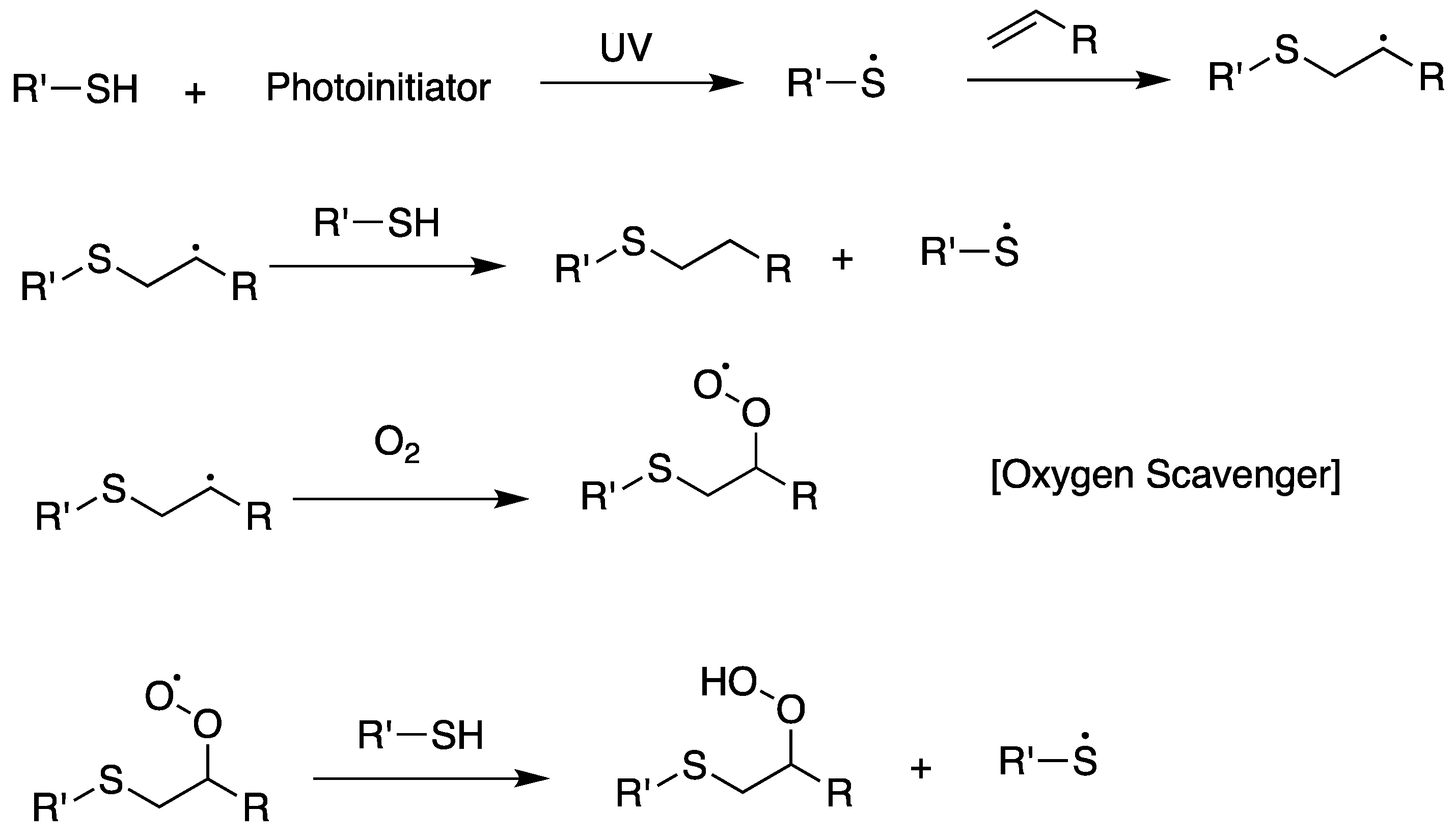
6.1. Ultraviolet Curing Area
6.2. High Refractive Index Plastic Spectacle Lenses for Vision Correction
6.3. Area of Epoxy Resin Hardener
7. Conclusions and Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Graf, H.F.; Feichter, J.; Langmann, B. Volcanic sulfur emissions: Estimates of source strength and its contribution to the global sulfate distribution. J. Geophys. Res. Atmos. 1997, 102, 10727–10738. [Google Scholar] [CrossRef]
- Lim, J.; Pyun, J.; Char, K. Recent approaches for the direct use of elemental sulfur in the synthesis and processing of advanced materials. Angew. Chem. Int. Ed. 2015, 54, 3249–3258. [Google Scholar] [CrossRef]
- Powell, R.E.; Eyring, H. The properties of liquid sulfur. J. Am. Chem. Soc. 1943, 6, 648–654. [Google Scholar] [CrossRef]
- Fairbrother, F.; Gee, G.; Merrall, G. The polymerization of sulfur. J. Polym. Sci. 1955, 16, 459–469. [Google Scholar] [CrossRef]
- Kutney, G. Sulfur: History, Technology, Applications & Industry; ChemTec Pub.: Toronto, ON, Canada, 2007. [Google Scholar]
- Eisenberg, A.; Tobolsky, A.V. Equilibrium polymerization of selenium. J. Polym. Sci. 1960, 46, 19–28. [Google Scholar] [CrossRef]
- Tobolsky, A.V. Polymeric sulfur and related polymers. J. Polym. Sci. Polym. Symp. 1966, 12, 71–78. [Google Scholar] [CrossRef]
- Rauchfuss, T. Under sulfur’s spell. Nat. Chem. 2011, 3, 648. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B. Elemental sulfur. Chem. Rev. 1976, 76, 367–388. [Google Scholar] [CrossRef]
- Meyer, B.; Oommen, T.; Jensen, D. Color of liquid sulfur. J. Phys. Chem. 1971, 75, 912–917. [Google Scholar] [CrossRef]
- Hendry, R.F. Lavoisier and Mendeleev on the elements. Found. Chem. 2005, 7, 31–48. [Google Scholar] [CrossRef]
- Holm, R.H. Synthetic approaches to the active sites of iron-sulfur proteins. Acc. Chem. Res. 1977, 10, 427–434. [Google Scholar] [CrossRef]
- Venkateswara Rao, P.; Holm, R. Synthetic analogues of the active sites of iron−sulfur proteins. Chem. Rev. 2004, 104, 527–560. [Google Scholar] [CrossRef]
- Cooper, R.C.; Lee, C.; Marianetti, C.A.; Wei, X.; Hone, J.; Kysar, J.W. Nonlinear elastic behavior of two-dimensional molybdenum disulfide. Phys. Rev. B 2013, 87, 035423. [Google Scholar] [CrossRef]
- Zhou, W.; Zou, X.; Najmaei, S.; Liu, Z.; Shi, Y.; Kong, J.; Lou, J.; Ajayan, P.M.; Yakobson, B.I.; Idrobo, J.-C. Intrinsic structural defects in monolayer molybdenum disulfide. Nano Lett. 2013, 13, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Van Der Zande, A.M.; Huang, P.Y.; Chenet, D.A.; Berkelbach, T.C.; You, Y.; Lee, G.-H.; Heinz, T.F.; Reichman, D.R.; Muller, D.A.; Hone, J.C. Grains and grain boundaries in highly crystalline monolayer molybdenum disulphide. Nat. Mater. 2013, 12, 554–561. [Google Scholar] [CrossRef] [PubMed]
- McCullough, R.D. The chemistry of conducting polythiophenes. Adv. Mater. 1998, 10, 93–116. [Google Scholar] [CrossRef]
- McQuade, D.T.; Pullen, A.E.; Swager, T.M. Conjugated polymer-based chemical sensors. Chem. Rev. 2000, 100, 2537–2574. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.J.; Griebel, J.J.; Kim, E.T.; Yoon, H.; Simmonds, A.G.; Ji, H.J.; Dirlam, P.T.; Glass, R.S.; Wie, J.J.; Nguyen, N.A. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 2013, 5, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Tatchell, A.R. Fundamental Aliphatic Chemistry: Organic Chemistry for General Degree Students; Pergamon Press: Oxford, UK, 1965. [Google Scholar]
- Cottrell, T. The Strength of Chemical Bonds; Butterworth: London, UK, 1958. [Google Scholar]
- Franklin, J.L.; Lumpkin, H.E. Some CS, HS and SS bond strengths by the electron impact method. J. Am. Chem. Soc. 1952, 74, 1023–1026. [Google Scholar] [CrossRef]
- Pauling, L. The Nature of the Chemical Bond; Cornell University Press: New York, NY, USA, 1960. [Google Scholar]
- Kotake, M. Constants of Organic Compounds; Asakura Publishing Company: Tokyo, Japan, 1963. [Google Scholar]
- Rousselot, M. Étude par résonance magnétique nucléaire d’effets intermoléculaires dans quelques thiols aliphatiques. C. R. Hebd. Seances Acad. Sci. Sér. C 1966, 262, 1–26. [Google Scholar]
- Marcus, S.H.; Miller, S.I. Hydrogen Bonding in Thiols1. J. Am. Chem. Soc. 1966, 88, 3719–3724. [Google Scholar] [CrossRef]
- Fletcher, W.H. Ionization Constants of Some Weak Acids in Aqueous Tertiary Butyl Alcohol. J. Am. Chem. Soc. 1946, 68, 2726–2728. [Google Scholar] [CrossRef]
- Lumbroso, H. II.-Sur les facteurs déterminant la grandeur de la conjugaison entre le soufre et les cycles aromatiques. J. Chim. Phys. 1952, 49, 394–398. [Google Scholar] [CrossRef]
- Hammett, L.P. Physical Organic Chemistry; McGraw-Hill: New York, NY, USA, 1940. [Google Scholar]
- Golumbic, C.; Orchin, M.; Weller, S. Partition studies on phenols. I. Relation between partition coefficient and ionization constant. J. Am. Chem. Soc. 1949, 71, 2624–2627. [Google Scholar] [CrossRef]
- Loy, H.; Himmelblau, D. The first ionization constant of hydrogen sulfide in water. J. Phys. Chem. 1961, 65, 264–267. [Google Scholar] [CrossRef]
- Kreevoy, M.M.; Harper, E.T.; Duvall, R.E.; Wilgus, H.S., III; Ditsch, L.T. Inductive Effects on the Acid Dissociation Constants of Mercaptans. J. Am. Chem. Soc. 1960, 82, 4899–4902. [Google Scholar] [CrossRef]
- Swain, C.G.; Scott, C.B. Quantitative correlation of relative rates. Comparison of hydroxide ion with other nucleophilic reagents toward alkyl halides, esters, epoxides and acyl halides. J. Am. Chem. Soc. 1953, 75, 141–147. [Google Scholar] [CrossRef]
- Schoberl, A.; Wagner, A. Methoden der Organischen Chemie, 4th ed.; George Thieme, Verlag: Stuttgart, Germany, 1955; Volume 9, p. 221. [Google Scholar]
- Wagner, R.B.; Zook, H.D. Synthetic Organic Chemistry; John Wiley & Son: New York, NY, USA, 1953. [Google Scholar]
- Sandler, S.R.; Karo, W. Organic Functional Group Preparations; Academic Press: Boston, MA, USA, 2013. [Google Scholar]
- Ellis, L., Jr.; Reid, E.E. The preparation and properties of a double series of aliphatic mercaptans. J. Am. Chem. Soc. 1932, 54, 1674–1687. [Google Scholar] [CrossRef]
- Alt, G.; Speziale, A. Reactions of enamines ii: A novel conversion of an enaminoketone to a chloroiminium chloride. Tetrahedron Lett. 1963, 4, 111–113. [Google Scholar] [CrossRef]
- Lofod, H. Furfuryl mercaptan. Org. Synth. Collect. 1963, 4, 66–67. [Google Scholar]
- Foster, H.S. HR, 4-Methyl-6-hydroxypyrimidine. Org. Synth. Collect. 1955, 4, 638–639. [Google Scholar]
- Urquhart, G.G.; Gates, J.W., Jr.; Connor, R. n-Dodecyl (lauryl) mercaptan. Org. Synth. Collect. 1955, 3, 363–369. [Google Scholar]
- Levitt, L.S.; Levitt, B.W. Inductive effects on molecular ionization potentials. IV. Hydrogen sulfide and mercaptans. J. Org. Chem. 1972, 37, 332–333. [Google Scholar] [CrossRef]
- Levitt, L.S. The alkyl inductive effect. I. Relations between σI*, σ* and ρI ρ* for H and Alkyl Groups; σI Values from the Size and Branching of R. Z. Naturforsch. B 1979, 34, 81–85. [Google Scholar] [CrossRef]
- Bestian, H. Über einige Reaktionen des Äthylen-imins. Justus Liebigs Ann. Chem. 1950, 566, 210–244. [Google Scholar] [CrossRef]
- Gilman, H.; Woods, L.A. Some 6-methoxy-8-quinolylamino sulfides. J. Am. Chem. Soc. 1945, 67, 1843–1845. [Google Scholar] [CrossRef]
- Snyder, H.; Stewart, J.M.; Ziegler, J. The Synthesis of amino mercaptans from olefin sulfides. J. Am. Chem. Soc. 1947, 69, 2672–2674. [Google Scholar] [CrossRef] [PubMed]
- Ipatieff, V.; Friedman, B. Reaction of thiol compounds with aliphatic olefins. J. Am. Chem. Soc. 1939, 61, 71–74. [Google Scholar] [CrossRef]
- Jones, S.; Reid, E.E. The addition of sulfur, hydrogen sulfide and mercaptans to unsaturated hydrocarbons. J. Am. Chem. Soc. 1938, 60, 2452–2455. [Google Scholar] [CrossRef]
- Bordwell, F.; Hewett, W. The free radical addition of thiolacetic acid to some cyclic olefins. J. Am. Chem. Soc. 1957, 79, 3493–3496. [Google Scholar] [CrossRef]
- Uchiro, H.; Kobayashi, S. Non-aqueous reduction of aromatic sulfonyl chlorides to thiols using a dichlorodimethylsilane-zinc-dimethylacetamide system. Tetrahedron Lett. 1999, 40, 3179–3182. [Google Scholar] [CrossRef]
- Litvay, O.; Riesz, E.; Landau, L. Über schwefel-haltige chinon-und hydrochinon-derivate. Ber. Dtsch. Chem. Ges. 1929, 62, 1863–1870. [Google Scholar] [CrossRef]
- Strating, J.; Backer, H. La réaction du tétrahydrure de lithiumaluminium sur quelques composés organiques soufrés. Recl. Trav. Chim. Pays-Bas 1950, 69, 638–648. [Google Scholar] [CrossRef]
- Taboury, M. Action du soufre et du selenium sur lea combinaisons organomagnesiennes des hydrocarbyres aromatigues mono-et dihalogenes dans le’noyau. C.R. Acad. Sci. Ser. IIc Chim. 1904, 138, 982–985. [Google Scholar]
- Wuyts, H. Sur le mécanisme de l’action du soufre et du sélénium sur les organomagnésiens. Bull. Soc. Chim. Fr. 1909, 5, 405–412. [Google Scholar]
- Mailhe, A.; Murat, M. Action du soufre et du selenium dur le chlorure de cyclohexylmagnesium. Bull. Soc. Chem. 1910, 7, 288–291. [Google Scholar]
- Gilman, H.; Fullhart, L. Some substituted β-hydroxyethyl sulfides. J. Am. Chem. Soc. 1949, 71, 1478–1481. [Google Scholar] [CrossRef]
- Murphy, M.T.; Duggan, A.C. Pyrolysis of butadiene. J. Am. Chem. Soc. 1949, 71, 3347–3349. [Google Scholar] [CrossRef]
- Hofmann, A. Ueber die dem senföl entsprechenden isomeren der schwefelcyanwasserstoffäther. Ber. Dtsch. Chem. Ges. 1868, 1, 169–184. [Google Scholar] [CrossRef]
- Gibson, D.T. Significant studies in the organic chemistry of sulfur. Chem. Rev. 1934, 14, 431–457. [Google Scholar] [CrossRef]
- Alcalay, W. Mono-alcoylmercapto-quinones. Méthode de synthèse simple. Helv. Chim. Acta 1947, 30, 578–584. [Google Scholar] [CrossRef]
- Koval, I. Synthesis, structure, and physicochemical characteristics of thiols. Russ. J. Org. Chem. 2005, 41, 631–648. [Google Scholar] [CrossRef]
- Moad, G.; Solomon, D.H. The Chemistry of Radical Polymerization; Elsevier Science: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: Chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lowe, A.B.; Bowman, C.N. Thiol-click chemistry: A multifaceted toolbox for small molecule and polymer synthesis. Chem. Soc. Rev. 2010, 39, 1355–1387. [Google Scholar] [CrossRef] [PubMed]
- Griesbaum, K. Problems and possibilities of the free-radical addition of thiols to unsaturated compounds. Angew. Chem. Int. Ed. 1970, 9, 273–287. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Bowman, C.N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Kade, M.J.; Burke, D.J.; Hawker, C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 743–750. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Lowe, A.B.; Hoyle, C.E.; Bowman, C.N. Thiol-yne click chemistry: A powerful and versatile methodology for materials synthesis. J. Mater. Chem. 2010, 2, 4745–4750. [Google Scholar] [CrossRef]
- Naik, S.S.; Chan, J.W.; Comer, C.; Hoyle, C.E.; Savin, D.A. Thiol–yne ‘click’chemistry as a route to functional lipid mimetics. Polym. Chem. 2011, 2, 303–305. [Google Scholar] [CrossRef]
- Nguyen, L.-T.T.; Gokmen, M.T.; Du Prez, F.E. Kinetic comparison of 13 homogeneous thiol–X reactions. Polym. Chem. 2013, 4, 5527–5536. [Google Scholar] [CrossRef]
- Fringuelli, F.; Pizzo, F.; Tortoioli, S.; Vaccaro, L. Thiolysis of 1, 2-epoxides by thiophenol catalyzed under solvent-free conditions. Tetrahedron Lett. 2003, 44, 6785–6787. [Google Scholar] [CrossRef]
- Dyer, E.; Glenn, J.F.; Lendrat, E.G. The kinetics of the reactions of phenyl isocyanate with thiols. J. Org. Chem. 1961, 26, 2919–2925. [Google Scholar] [CrossRef]
- Li, H.; Yu, B.; Matsushima, H.; Hoyle, C.E.; Lowe, A.B. The thiol−isocyanate click reaction: Facile and quantitative access to ω-end-functional poly (N,N-diethylacrylamide) synthesized by RAFT radical polymerization. Macromolecules 2009, 42, 6537–6542. [Google Scholar] [CrossRef]
- Nair, D.P.; Podgorski, M.; Chatani, S.; Gong, T.; Xi, W.; Fenoli, C.R.; Bowman, C.N. The thiol-Michael addition click reaction: A powerful and widely used tool in materials chemistry. Chem. Mater. 2014, 26, 724–744. [Google Scholar] [CrossRef]
- Chan, J.W.; Hoyle, C.E.; Lowe, A.B.; Bowman, M. Nucleophile-initiated thiol-Michael reactions: Effect of organocatalyst, thiol, and ene. Macromolecules 2010, 43, 6381–6388. [Google Scholar] [CrossRef]
- Li, G.-Z.; Randev, R.K.; Soeriyadi, A.H.; Rees, G.; Boyer, C.; Tong, Z.; Davis, T.P.; Becer, C.R.; Haddleton, D.M. Investigation into thiol-(meth) acrylate Michael addition reactions using amine and phosphine catalysts. Polym. Chem. 2010, 1, 1196–1204. [Google Scholar] [CrossRef]
- Mather, B.D.; Viswanathan, K.; Miller, K.M.; Long, T.E. Michael addition reactions in macromolecular design for emerging technologies. Prog. Polym. Sci. 2006, 31, 487–531. [Google Scholar] [CrossRef]
- Chatani, S.; Nair, D.P.; Bowman, C.N. Relative reactivity and selectivity of vinyl sulfones and acrylates towards the thiol–Michael addition reaction and polymerization. Polym. Chem. 2013, 4, 1048–1055. [Google Scholar] [CrossRef]
- Patai, S. The Chemistry of Thiol Group; Part 1 & Part 2; John Wiley & Sons: London, UK, 1984. [Google Scholar]
- Le Neindre, M.; Nicolaÿ, R. Polythiol copolymers with precise architectures: A platform for functional materials. Polym. Chem. 2014, 5, 4601–4611. [Google Scholar] [CrossRef]
- Jang, D.G.; Kwon, M.J.; Lee, S.M.; Kim, J.H.; Jin, W.Y.; Seo, J.M. Synthesis of novel polythiol for plastic optical lens and its ophthalmic lens. Bull. Korean Chem. Soc. 2009, 30, 2227–2232. [Google Scholar]
- Okazaki, K.; Yoshinobu, K.; Teruyuki, N. Polythiol, Process for Producing Same, Sulfur-Containing Urethane-Base Resin Prepared from the Polythiol, Process for Producing the Resin, and Lens. European Application No. 0665219, 22 April 1998. [Google Scholar]
- Kawaguchi, M.; Nishimura, T. Method for Producing Polythiol Compound, Polymerizable Composition for Optical Material, and Uses Thereof. U.S. Patent Application No. 2015/0133692, 2 May 2015. [Google Scholar]
- Hong, S.M.; Seo, H.M.; Shin, J.H. Method for Preparing Polythiol Having Improved Storage Stability. KR Patent Application No. 10-203421, 25 April 2018. [Google Scholar]
- Tanaka, M.M.; Kanamura, Y.B.; Funae, A.H.; Yamamoto, H.D.; Kobayashi, S.C. Manufacturing Method of 2,5-bis(Mercaptomethyl)1,4-dithuanes. JP Patent Application No. 4217018, 29 October 2001. [Google Scholar]
- Kemmerer, R.R.; Johson, M.A.; McConnel, J.A.; Willard, F.K. Performance and potential UV/EB application of a new class of polyester acrylate. In Proceedings of the Conference on Radiation Curing Asia, CRCA’88, Tokyo, Japan, 17–19 October 1988; pp. 523–535. [Google Scholar]
- Roberts, J.D.; Caserio, M.C. Basic Principles of Organic Chemistry; WA Benjamin, Inc.: Menlo Park, CA, USA, 1977. [Google Scholar]
- Kloosterboer, J.G. Network Formation by Chain Crosslinking Photopolymerization and Its Applications in Electronics. In Electronic Application; Advances in Polymer Science; Springer: Berlin, Switzerland, 1988; Volume 84. [Google Scholar]
- Decker, C. New Developments in UV-radiation curing of protective coatings. Surf. Coat. Int. Part B Coat. Trans. 2005, 88, 9–18. [Google Scholar] [CrossRef]
- Lee, T.; Guymon, C.; Jönsson, E.S.; Hoyle, C.E. The effect of monomer structure on oxygen inhibition of (meth)acrylates photopolymerization. Polymer 2004, 45, 6155–6162. [Google Scholar] [CrossRef]
- Bowman, C.N.; Kloxin, C.J. Toward an enhanced understanding and implementation of photopolymerization reactions. AIChE J. 2008, 54, 2775–2795. [Google Scholar] [CrossRef]
- Roper, T.M.; Lee, T.Y.; Guymon, C.A.; Hoyle, C.E. In situ characterization of photopolymerizable systems using a thin-film calorimeter. Macromolecules 2005, 38, 10109–10116. [Google Scholar] [CrossRef]
- Bowman, C.N.; Peppas, N.A. Coupling of kinetics and volume relaxation during polymerizations of multiacrylates and multimethacrylates. Macromolecules 1991, 24, 1914–1920. [Google Scholar] [CrossRef]
- Lu, H.; Stansbury, J.W.; Bowman, C.N. Towards the elucidation of shrinkage stress development and relaxation in dental composites. Dent. Mater. 2004, 20, 979–986. [Google Scholar] [CrossRef]
- Anseth, K.S.; Wang, C.M.; Bowman, C.N. Kinetic evidence of reaction diffusion during the polymerization of multi (meth) acrylate monomers. Macromolecules 1994, 27, 650–655. [Google Scholar] [CrossRef]
- Decker, C. Kinetic study and new applications of UV radiation curing. Macromol. Rapid Commun. 2002, 23, 1067–1093. [Google Scholar] [CrossRef]
- Lee, T.Y.; Roper, T.M.; Jönsson, E.S.; Guymon, C.; Hoyle, C.E. Influence of hydrogen bonding on photopolymerization rate of hydroxyalkyl acrylates. Macromolecules 2004, 37, 3659–3665. [Google Scholar] [CrossRef]
- Simon, G.; Allen, P.; Bennett, D.; Williams, D.; Williams, E. Nature of residual unsaturation during cure of dimethacrylates examined by CPPEMAS carbon-13 NMR and simulation using a kinetic gelation model. Macromolecules 1989, 22, 3555–3561. [Google Scholar] [CrossRef]
- Kannurpatti, A.R.; Anseth, J.W.; Bowman, C.N. A study of the evolution of mechanical properties and structural heterogeneity of polymer networks formed by photopolymerizations of multifunctional (meth) acrylates. Polymer 1998, 39, 2507–2513. [Google Scholar] [CrossRef]
- Kannurpatti, A.R.; Anderson, K.J.; Anseth, J.W.; Bowman, C.N. Use of “living” radical polymerizations to study the structural evolution and properties of highly crosslinked polymer networks. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 2297–2307. [Google Scholar] [CrossRef]
- Posner, T. Beiträge zur Kenntniss der ungesättigten Verbindungen. II. Ueber die Addition von Mercaptanen an ungesättigte Kohlenwasserstoffe. Ber. Dtsch. Chem. Gesell. 1905, 38, 646–657. [Google Scholar] [CrossRef]
- Chiou, B.-S.; Khan, S.A. Real-time FTIR and in situ rheological studies on the UV curing kinetics of thiol-ene polymers. Macromolecules 1997, 30, 7322–7328. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.; Choi, H.; Hong, J. Photocuring of a thiol-ene system based on an unsaturated polyester. J. Appl. Polym. Sci. 2005, 95, 342–350. [Google Scholar] [CrossRef]
- Wutticharoenwong, K.; Soucek, M.D. Influence of the Thiol Structure on the Kinetics of Thiol-ene Photopolymerization with Time-Resolved Infrared Spectroscopy. Macromol. Mater. Eng. 2008, 293, 45–56. [Google Scholar] [CrossRef]
- Crivello, J.V.; Reichmanis, E. Photopolymer materials and processes for advanced technologies. Chem. Mater. 2014, 26, 533–548. [Google Scholar] [CrossRef]
- Andrzejewska, E. Photopolymerization kinetics of multifunctional monomers. Prog. Polym. Sci. 2001, 26, 605–665. [Google Scholar] [CrossRef]
- Takada, K.; Sun, H.-B.; Kawata, S. Improved spatial resolution and surface roughness in photopolymerization-based laser nanowriting. Appl. Phys. Lett. 2005, 86, 071122. [Google Scholar] [CrossRef]
- Fouassier, J.-P. Photoinitiation, Photopolymerization, and Photocuring: Fundamentals and Applications; Hanser Publisher Inc.: Munich, Germany, 1995. [Google Scholar]
- Roffey, C. Photopolymerization of Surface Coatings; Wiley-Inter-Science: New York, NY, USA, 1982. [Google Scholar]
- O’Brien, A.K.; Cramer, N.B.; Bowman, C.N. Oxygen inhibition in thiol–acrylate photopolymerizations. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 2007–2014. [Google Scholar] [CrossRef]
- Ligon, S.C.; Husár, B.; Wutzel, H.; Holman, R.; Liska, R. Strategies to reduce oxygen inhibition in photoinduced polymerization. Chem. Rev. 2014, 114, 557–589. [Google Scholar] [CrossRef]
- Jacobine, A.F.; Glaser, D.M.; Nakos, S.T. Curable Norbornenyl Functional Silicone Formulations. AU Patent Application No. 627835B2, 24 August 1990. [Google Scholar]
- Cramer, N.B.; Reddy, S.K.; Cole, M.; Hoyle, C.E.; Bowman, C.N. Initiation and kinetics of thiol–ene photopolymerizations without photoinitiators. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5817–5826. [Google Scholar] [CrossRef]
- Woods, J.G.; Rakas, M.A.; Jacobine, A.F.; Alberino, L.M.; Kropp, P.L.; Sutkaitis, D.M.; Glaser, D.M.; Nakos, S.T. Optical Fiber PrimaryCoatings and Fibers Coated Therewith. U.S. Patent Application No. 94/07061, 22 June 1994. [Google Scholar]
- Wei, H.; Senyurt, A.F.; Jönsson, S.; Hoyle, C.E. Photopolymerization of ternary thiol–ene/acrylate systems: Film and network properties. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 822–829. [Google Scholar] [CrossRef]
- Kazybayeva, D.S.; Irmukhametova, G.S.; Khutoryanskiy, V.V. Thiol-ene “click reactions” as a promising approach to polymer materials. Polym Sci. Ser. B 2021, 64, 1–16. [Google Scholar] [CrossRef]
- Cakmakci, E. Recent advances in flame retardant polymers via thiol-ene click reaction. J. Macromol. Sci. A Pure Appl. Chem. 2023, 60, 817–840. [Google Scholar] [CrossRef]
- Cramer, N.B.; Bowman, C.N. Kinetics of thiol–ene and thiol–acrylate photopolymerizations with real-time fourier transform infrared. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 3311–3319. [Google Scholar] [CrossRef]
- Fouassier, J.-P.; Rabek, J.F. Radiation Curing in Polymer Science and Technology; Springer: Dordrecht, The Netherlands, 1993. [Google Scholar]
- Cramer, N.B.; Scott, J.P.; Bowman, C.N. Photopolymerizations of thiol−ene polymers without photoinitiators. Macromolecules 2002, 35, 5361–5365. [Google Scholar] [CrossRef]
- Carioscia, J.A.; Lu, H.; Stanbury, J.W.; Bowman, C.N. Thiol-ene oligomers as dental restorative materials. Dent. Mater. 2005, 21, 1137–1143. [Google Scholar] [CrossRef]
- Rydholm, A.E.; Bowman, C.N.; Anseth, K.S. Degradable thiol-acrylate photopolymers: Polymerization and degradation behavior of an in situ forming biomaterial. Biomaterials 2005, 26, 4495–4506. [Google Scholar] [CrossRef]
- Resetco, C.; Bendriks, B.; Badi, N.; Du Prez, F. Thiol-ene chemistry for polymer coatings and surface modification-building in sustainability and performance. Mater. Horiz. 2017, 4, 1041–1053. [Google Scholar] [CrossRef]
- Fu, Q.; Liu, J.; Shi, W. Preparation and photopolymerization behavior of multifunctional thiol–ene systems based on hyperbranched aliphatic polyesters. Prog. Org. Coat. 2008, 63, 100–109. [Google Scholar] [CrossRef]
- Morgan, C.; Magnotta, F.; Ketley, A. Thiol/ene photocurable polymers. J. Polym. Sci. Polym. Chem. Ed. 1977, 15, 627–645. [Google Scholar] [CrossRef]
- Cramer, N.B.; Reddy, S.K.; O’Brien, A.K.; Bowman, C.N. Thiol−ene photopolymerization mechanism and rate limiting step changes for various vinyl functional group chemistries. Macromolecules 2003, 36, 7964–7969. [Google Scholar] [CrossRef]
- Lecamp, L.; Houllier, F.; Youssef, B.; Bunel, C. Photoinitiated cross-linking of a thiol–methacrylate system. Polymer 2001, 42, 2727–2736. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Nishimura, T. Polythiol Composition, Polymerizable Composition for Optical Material and Use Thereof. U.S. Patent Application No. 10858473B2, 8 December 2020. [Google Scholar]
- Nishimori, Y.; Kamura, T.; Horikoshi, H. Polythiol Compound and Method for Producing Same. United States Patent U.S. No. 10071959, 11 September 2018. [Google Scholar]
- Higashihara, T.; Ueda, M. Recent progress in high refractive index polymers. Macromolecules 2015, 48, 1915–1929. [Google Scholar] [CrossRef]
- Griebel, J.J.; Namnabat, S.; Kim, E.T.; Himmelhuber, R.; Moronta, D.H.; Chung, W.J.; Simmonds, A.G.; Kim, K.J.; van der Laan, J.; Nguyen, N.A. New infrared transmitting material via inverse vulcanization of elemental sulfur to prepare high refractive index polymers. Adv. Mater. 2014, 26, 3014–3018. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Liu, J.-G.; Zhao, X.-J.; Yang, H.-X.; Yang, S.-Y. Synthesis and characterization of organo-soluble thioether-bridged polyphenylquinoxalines with ultra-high refractive indices and low birefringences. Polymer 2010, 51, 3851–3858. [Google Scholar] [CrossRef]
- Minns, R.A.; Gaudiana, R.A. Design and synthesis of high refractive index polymers. II. J. Macromol. Sci. Pure Appl. Chem. 1992, 29, 19–30. [Google Scholar] [CrossRef]
- Kim, H.; Yeo, H.; Goh, M.; Ku, B.-C.; Hahn, J.R.; You, N.-H. Preparation of UV-curable acryl resin for high refractive index based on 1, 5-bis (2-acryloylenethyl)-3, 4-ethylenedithiothiophene. Eur. Polym. J. 2016, 75, 303–309. [Google Scholar] [CrossRef]
- Fushimi, T.; Allcock, H.R. Cyclotriphosphazenes with sulfur-containing side groups: Refractive index and optical dispersion. Dalton Trans. 2009, 2477–2481. [Google Scholar] [CrossRef]
- Liu, J.G.; Nakamura, Y.; Shibasaki, Y.; Ando, S.; Ueda, M. Synthesis and characterization of highly refractive polyimides from 4, 4′-thiobis [(p-phenylenesulfanyl) aniline] and various aromatic tetracarboxylic dianhydrides. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 5606–5617. [Google Scholar] [CrossRef]
- Fukuzaki, N.; Higashihara, T.; Ando, S.; Ueda, M. Synthesis and characterization of highly refractive polyimides derived from thiophene-containing aromatic diamines and aromatic dianhydrides. Macromolecules 2010, 43, 1836–1843. [Google Scholar] [CrossRef]
- You, N.-H.; Higashihara, T.; Oishi, Y.; Ando, S.; Ueda, M. Highly refractive poly (phenylene thioether) containing triazine unit. Macromolecules 2010, 43, 4613–4615. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Suzuki, Y.; Higashihara, T.; Ando, S.; Ueda, M. Synthesis of highly refractive poly (phenylene thioether) derived from 2, 4-dichloro-6-alkylthio-1, 3, 5-triazines and aromatic dithiols. Macromolecules 2011, 44, 9180–9186. [Google Scholar] [CrossRef]
- Suzuki, Y.; Higashihara, T.; Ando, S.; Ueda, M. Synthesis and characterization of high refractive index and high Abbe’s number poly (thioether sulfone) s based on tricyclo [5.2. 1.02, 6] decane moiety. Macromolecules 2012, 45, 3402–3408. [Google Scholar] [CrossRef]
- Dislich, H. Plastics as optical materials. Angew. Chem. Int. Ed. 1979, 18, 49–59. [Google Scholar] [CrossRef]
- Yang, C.-J.; Jenekhe, S.A. Group contribution to molar refraction and refractive index of conjugated polymers. Chem. Mater. 1995, 7, 1276–1285. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, B.; Jin, J.; Li, J. High refractive index polythiourethane networks with high mechanical property via thiol-isocyanate click reaction. Polymer 2019, 180, 121746. [Google Scholar] [CrossRef]
- Keita, G.; Obordo, J.O.; McClimans, P.A.; Turshani, Y.Y. Method for Making Transparent Polythiourethane Substrates in Particular Optical Substrates. U.S. Patent 10/012727, 3 May 2005. [Google Scholar]
- Dai, Y.; Chen, X.; Zhang, X. Recent advances in stimuli-responsive polymeric micelles via click chemistry. Polym. Chem. 2019, 10, 34–44. [Google Scholar] [CrossRef]
- Li, Y.; Dong, X.-H.; Zou, Y.; Wang, Z.; Yue, K.; Huang, M.; Liu, H.; Feng, X.; Lin, Z.; Zhang, W. Polyhedral oligomeric silsesquioxane meets “click” chemistry: Rational design and facile preparation of functional hybrid materials. Polymer 2017, 125, 303–329. [Google Scholar] [CrossRef]
- Li, P.-Z.; Wang, X.-J.; Zhao, Y. Click chemistry as a versatile reaction for construction and modification of metal-organic frameworks. Coord. Chem. Rev. 2019, 380, 484–518. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, L.; Yang, L.; Zhu, F.; Ding, M.; Lin, F.; Wang, Z.; Li, Y. “Click” chemistry in polymeric scaffolds: Bioactive materials for tissue engineering. J. Control. Release 2018, 273, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Lee, J.; Jeong, H.M. Properties of polythiourethanes prepared by thiol–isocyanate click reaction. J. Appl. Polym. Sci. 2018, 135, 46070. [Google Scholar] [CrossRef]
- Chandrinos, A. Review of polymers and plastic high index optical materials. J. Mater. Sci. Res. Rev. 2021, 7, 1–14. [Google Scholar]
- de Pariza, X.L.; Fanlo, P.; Fonseca, L.P.; Luzuriaga, A.R.; Sardon, H. Polythiourethanes: Synthesis, applications, and opportunities. Prog. Polym. Sci. 2023, 145, 101735. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, Z.; Tang, G.; Wei, L.; Du, H.; Du, W. Balancing optical property and enhancing stability for high-refractive index polythiourethane with assistance of cubic thiol-functionalized silsesquioxanes. ACS Appl. Polym. Mater. 2021, 3, 153–161. [Google Scholar] [CrossRef]
- Silva, A.L.; Bordado, J.C. Recent developments in polyurethane catalysis: Catalytic mechanisms review. Catal. Rev. 2004, 46, 31–51. [Google Scholar] [CrossRef]
- Bacaloglu, R.; Cotarca, L.; Marcu, N.; Tölgyi, S. Kinetics and mechanism of isocyanate reactions. II. Reactions of aryl isocyanates with alcohols in the presence of tertiary amines. J. Prakt. Chem. 1988, 330, 530–540. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Chen, Y.; Yang, Z.; Chen, M.; Qin, Z. High-refractive index polythiourethane resin based on 2.3-bis((2-mercatoethyl)thio)-1-propanethiol and 1,3-bis(isocyanantomethyl)cyclohexane using tertiary amine catalyst. J. Appl. Polym. Sci. 2021, 138, e50278. [Google Scholar] [CrossRef]
- Sardon, H.; Pascual, A.; Mecerreyes, D.; Taton, D.; Cramail, H.; Hedrick, J.L. Synthesis of polyurethanes using organocatalysis: A perspective. Macromolecules 2015, 48, 3153–3165. [Google Scholar] [CrossRef]
- Concalves, F.A.M.M.; Santos, M.; Cernadas, T.; Alves, P.; Ferreira, P. Influence of fillers on epoxy resins properties: A review. J. Mater. Sci. 2022, 57, 15183–15212. [Google Scholar] [CrossRef]
- Aziz, T.; Haq, F.; Farid, A.; Cheng, L.; Chuah, L.F.; Bokhari, A.; Mubashir, M.; Tang, D.Y.Y.; Show, P.L. The epoxy resin system: Function and role of curing agents. Carbon Lett. 2024, 34, 477–494. [Google Scholar] [CrossRef]
- Mika, T.F.B.; Ronald, S. Curing Agents and Modifiers. In Epoxy Resins: Chemistry and Technology; May, C.A., Ed.; Marcel Dekker: New York, NY, USA, 1988. [Google Scholar]
- Pascault, J.-P.; Wiliams, R.J.J. Thermosetting Polymers. In Handbook of Polymer Synthesis, Characterization, and Processing; Saldívar-Guerra, E., Eduardo, V.-L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar]
- Eloundou, J. Étude diélectrique de systèmes époxy-amine. 2. Cinétique chimique et conductivité. Eur. Polym. J. 1999, 35, 1481–1489. [Google Scholar] [CrossRef]
- Shechter, L.; Wynstra, J.; Kurkjy, R.P. Glycidyl ether reactions with amines. Ind. Eng. Chem. 1956, 48, 94–97. [Google Scholar] [CrossRef]
- Rozenberg, B.A. Kinetics, thermodynamics and mechanism of reactions of epoxy oligomers with amines. In Epoxy Resins and Composites II, Advances in Polymer Science; Dusek, K., Ed.; Springer: Berlin, Switzerland, 1986; Volume 75. [Google Scholar]
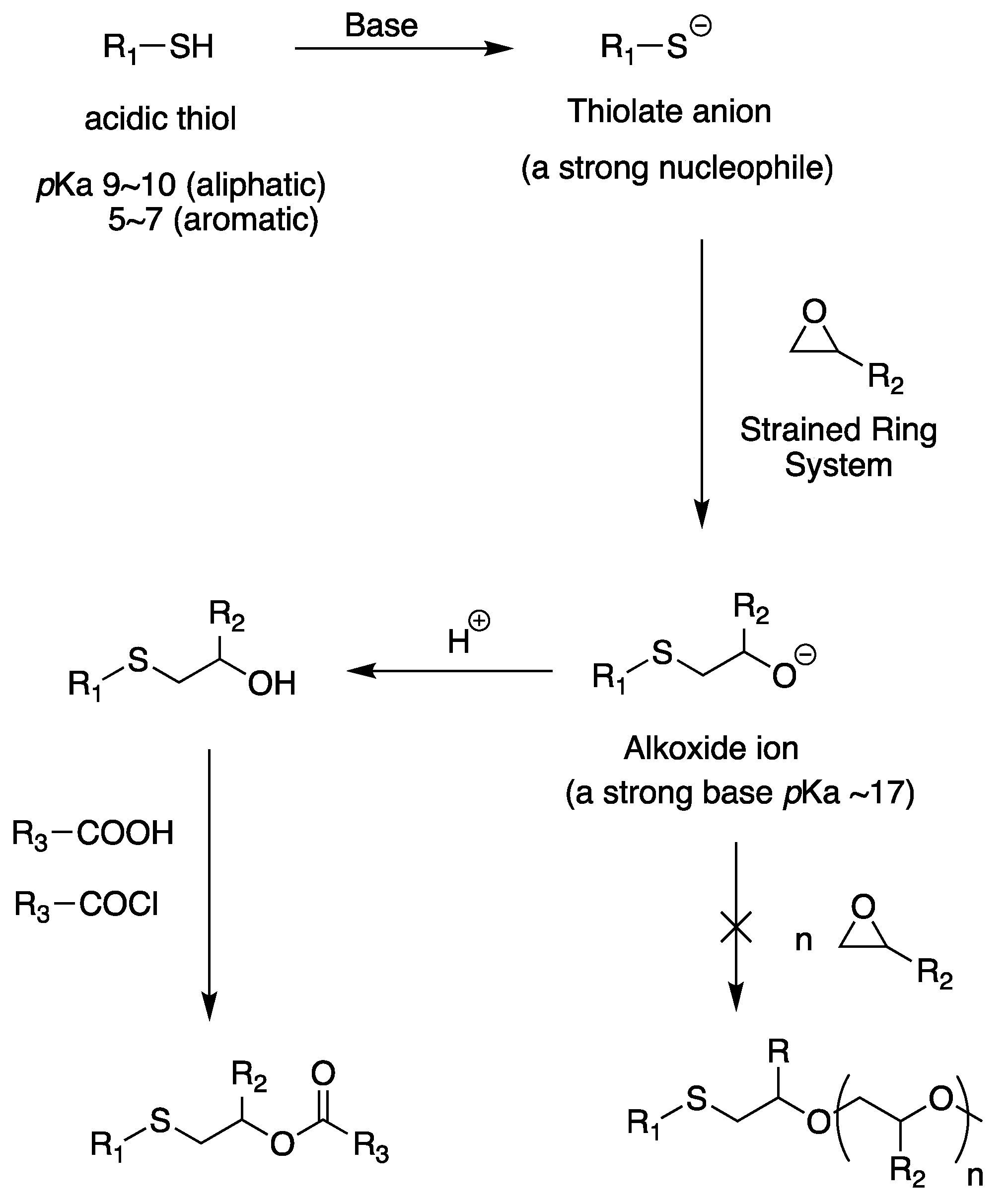
- Behrens, C.H.; Ko, S.Y.; Sharpless, K.B.; Walker, F.J. Selective transformation of 2, 3-epoxy alcohols and related derivatives. Strategies for nucleophilic attack at carbon-1. J. Org. Chem. 1985, 50, 5687–5696. [Google Scholar] [CrossRef]
- Azizi, N.; Khajeh-Amiri, A.; Ghafuri, H.; Bolourtchian, M. LiOH-Catalyzed simple ring opening of epoxides under solvent-free conditions. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 1550–1557. [Google Scholar] [CrossRef]
- Abbey, K.J.P.; Mark, W. Thiol-Cured Epoxy Composition. U.S. Patent Application No. 6153719A, 28 November 2000. [Google Scholar]
- Gough, J.D.; Gargano, J.M.; Donofrio, A.E.; Lees, W.J. Aromatic Thiol p K a Effects on the Folding Rate of a Disulfide Containing Protein. Biochemistry 2003, 42, 11787–11797. [Google Scholar] [CrossRef] [PubMed]
- Hupe, D.; Jencks, W. Nonlinear structure-reactivity correlations. Acyl transfer between sulfur and oxygen nucleophiles. J. Am. Chem. Soc. 1977, 99, 451–464. [Google Scholar] [CrossRef]
- Kin Loch, A.; Shaw, S. The fracture resistance of a toughened epoxy adhesive. J. Adhes. 1981, 12, 59–77. [Google Scholar] [CrossRef]
- Ocaña, R.; Arenas, J.; Alía, C.; Narbón, J. Evaluation of degradation of structural adhesive joints in functional automotive applications. Procedia Eng. 2015, 132, 716–723. [Google Scholar] [CrossRef]
- Banea, M.; Da Silva, L.; Carbas, R. Debonding on command of adhesive joints for the automotive industry. Int. J. Adhes. Adhes. 2015, 59, 14–20. [Google Scholar] [CrossRef]
- Viana, G.; Machado, J.; Carbas, R.; Costa, M.; Da Silva, L.; Vaz, M.; Banea, M. Strain rate dependence of adhesive joints for the automotive industry at low and high temperatures. J. Adhes. Sci. Technol. 2018, 32, 2162–2179. [Google Scholar] [CrossRef]
- Ji, Y.-H.; Liu, Y.; Huang, G.-W.; Shen, X.-J.; Xiao, H.-M.; Fu, S.-Y. Ternary Ag/epoxy adhesive with excellent overall performance. ACS Appl. Mater. Interfaces 2015, 7, 8041–8052. [Google Scholar] [CrossRef] [PubMed]
- Aradhana, R.; Mohanty, S.; Nayak, S.K. A review on epoxy-based electrically conductive adhesives. Int. J. Adhes. Adhes. 2020, 96, 102596. [Google Scholar] [CrossRef]
- Encinas, N.; Oakley, B.; Belcher, M.; Blohowiak, K.; Dillingham, R.; Abenojar, J.; Martínez, M. Surface modification of aircraft used composites for adhesive bonding. Int. J. Adhes. Adhes. 2014, 50, 157–163. [Google Scholar] [CrossRef]
- Brockmann, W.; Hennemann, O.-D.; Kollek, H.; Matz, C. Adhesion in bonded aluminium joints for aircraft construction. Int. J. Adhes. Adhes. 1986, 6, 115–143. [Google Scholar] [CrossRef]
- Higgins, A. Adhesive bonding of aircraft structures. Int. J. Adhes. Adhes. 2000, 20, 367–376. [Google Scholar] [CrossRef]
- Sousa, J.M.; Correia, J.R.; Cabral-Fonseca, S. Durability of an epoxy adhesive used in civil structural applications. Constr. Build. Mater. 2018, 161, 618–633. [Google Scholar] [CrossRef]
- Raetzke, K.; Shaikh, M.; Faupel, F.; Noeske, P.-L. Shelf stability of reactive adhesive formulations: A case study for dicyandiamide-cured epoxy systems. Int. J. Adhes. Adhes. 2010, 30, 105–110. [Google Scholar] [CrossRef]
- Jojibabu, P.; Zhang, Y.X.; Prusty, B.G. A review of research advances in epoxy-based nanocomposites as adhesives materials. Int. J. Adhes. Adhes. 2020, 96, 102454. [Google Scholar] [CrossRef]
- Yorkgitis, E.; Marhevka, V.; Lamon, A. Bonding of Aluminum Structures with Advanced 1K and 2K Adhesives; SAE Technical Paper; SAE International: Warrendale, PA, USA, 1995. [Google Scholar]
- Strzelec, K.; Leśniak, E.; Janowska, G. New polythiourethane hardeners for epoxy resins. Polym. Int. 2005, 54, 1337–1344. [Google Scholar] [CrossRef]
- Konuray, A.O.; Fernandez-Francos, X.; Ramis, X. Latent curing of epoxy-thiol thermosets. Polymer 2017, 116, 191–203. [Google Scholar] [CrossRef]
- Eom, S.Y.; Seo, S.B.; Lee, K.Y. Study on cure behavior of low temperature and fast cure epoxy with mercaptan hardener. Polym. Korea 2013, 37, 240–248. [Google Scholar] [CrossRef]
- Clark, L.J.; Cosman, M.A. Use of Permapol® P3. 1 polymers and epoxy resins in the formulation of aerospace sealants. Int. J. Adhes. Adhes. 2003, 23, 343–348. [Google Scholar] [CrossRef]
- Kim, W.Y.; Eom, S.Y.; Seo, S.B.; Lee, K.Y. Mechanical Properties of Low Temperature and Fast Cure Epoxy with Various Mercaptans. Polym. Korea 2013, 37, 557–562. [Google Scholar] [CrossRef]
- Hong, S.M.; Hwang, S.H. Synthesis and characteristics of novel 2-hydroxy-3-mercaptopropyl terminated polyoxypropylene glyceryl ether as an epoxy hardener of epoxy-based adhesives. Polym. Test. 2022, 110, 107593. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, F.; Li, L.; Wei, L.; Su, Z.; Li, H.; Liu, Y.; Liu, H.; Liu, Y. Performance study of an epoxy–polythiol curing system for local insulating spraying based on the imbalance stoichiometric ratio. Mater. Lett. 2023, 351, 135123. [Google Scholar] [CrossRef]
- Capricho, J.C.; Fox, B.; Hameed, N. Multifunctionality in epoxy resins. Polym. Rev. 2020, 60, 1. [Google Scholar] [CrossRef]

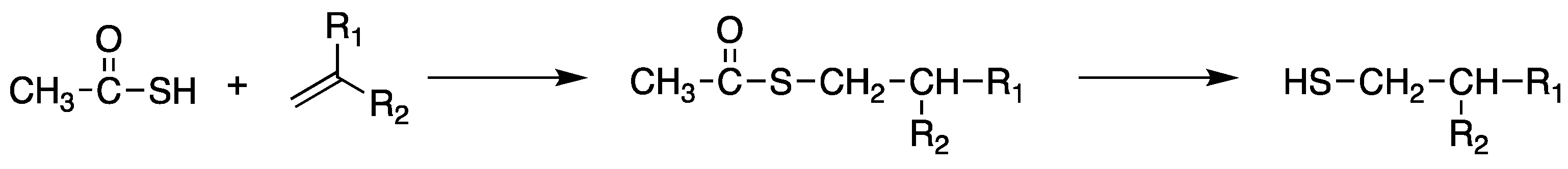





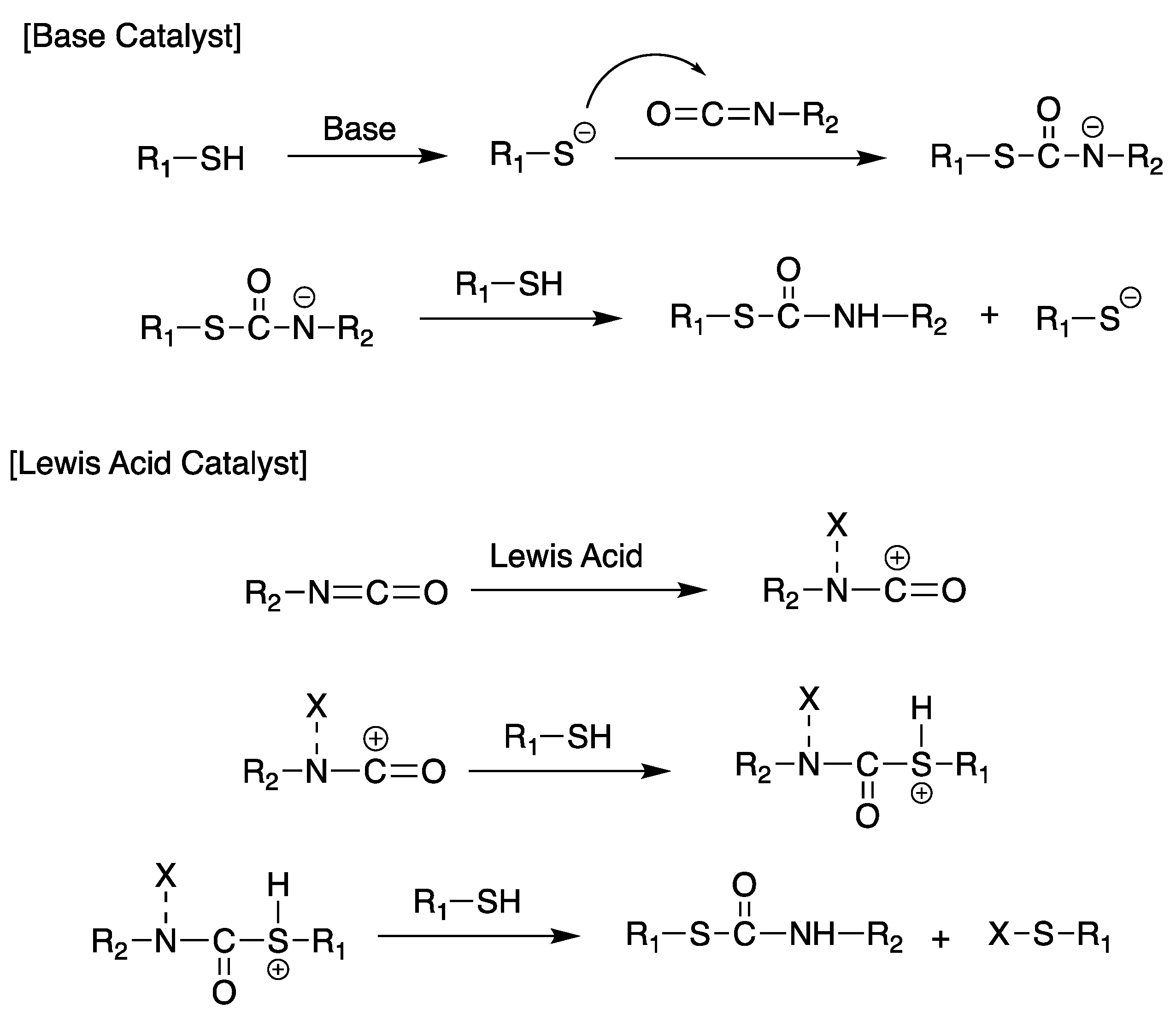
| Product Name | Chemical Name | Chemical Structure | Refractive Index (n20) | Abbe Number (ve20) |
|---|---|---|---|---|
| MRTM-8 | NBDI 1) | 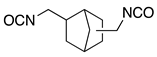 | 1.60 | 41 |
| GST 2) | 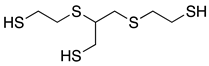 | |||
| PETMP 3) | 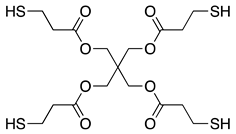 | |||
| MRTM-7 | m-XDI 4) | 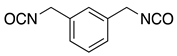 | 1.67 | 31 |
| GST 2) |  | |||
| MRTM-10 | m-XDI 4) | 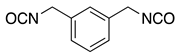 | 1.67 | 31 |
| HMMP 5) | 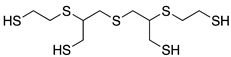 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, S.-M.; Kim, O.Y.; Hwang, S.-H. Chemistry of Polythiols and Their Industrial Applications. Materials 2024, 17, 1343. https://doi.org/10.3390/ma17061343
Hong S-M, Kim OY, Hwang S-H. Chemistry of Polythiols and Their Industrial Applications. Materials. 2024; 17(6):1343. https://doi.org/10.3390/ma17061343
Chicago/Turabian StyleHong, Seung-Mo, Oh Young Kim, and Seok-Ho Hwang. 2024. "Chemistry of Polythiols and Their Industrial Applications" Materials 17, no. 6: 1343. https://doi.org/10.3390/ma17061343





