3.1. Analysis of Bronze Samples
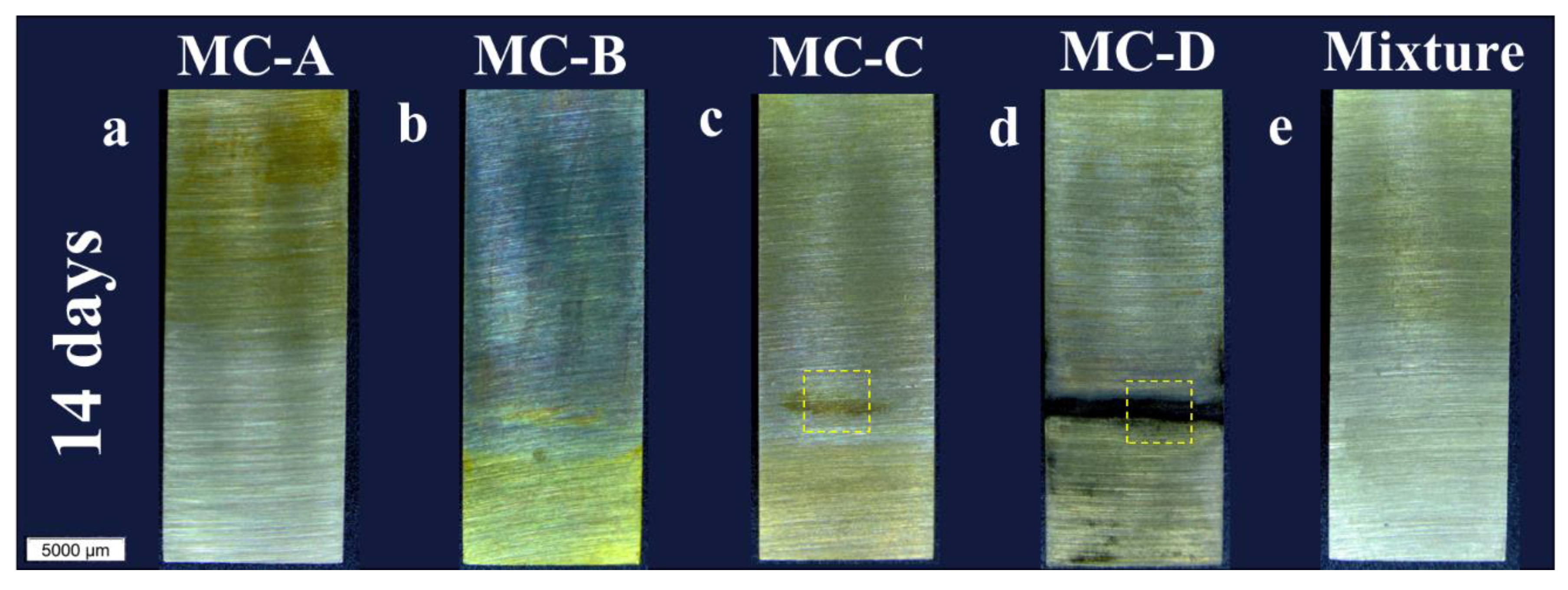
Figure 2 displays images of the pieces in their end-of-test condition at 14, 21, and 28 days. Approximately one-third of each piece’s surface, specifically the lower region shown in
Figure 2, was submerged (called inside region in
Figure 1). All samples exhibited color changes during the test, varying in intensity and location. The color change was particularly noticeable in the MC-A samples (
Figure 2a,f,k) and the mixture (
Figure 2e,j,o) in the non-submerged areas, whereas the MC-B sample (
Figure 2b,g,l) showed color changes throughout the entire piece. Microscopic observations do not conclusively determine whether there was corrosion in the MC-A, MC-B, and mixture samples throughout the duration of the test.
On the other hand, the samples tested in MC-D showed corrosion at the interface between the lubricant and the air from 14 days (
Figure 2d), which then propagated until the end of the test from the interface (
Figure 2n). Regarding the MC-C samples, corrosion began at the lower end after 21 days (
Figure 2h, marked by a dashed yellow square) and by the end of the test it had extended throughout the entire submerged region (
Figure 2m).
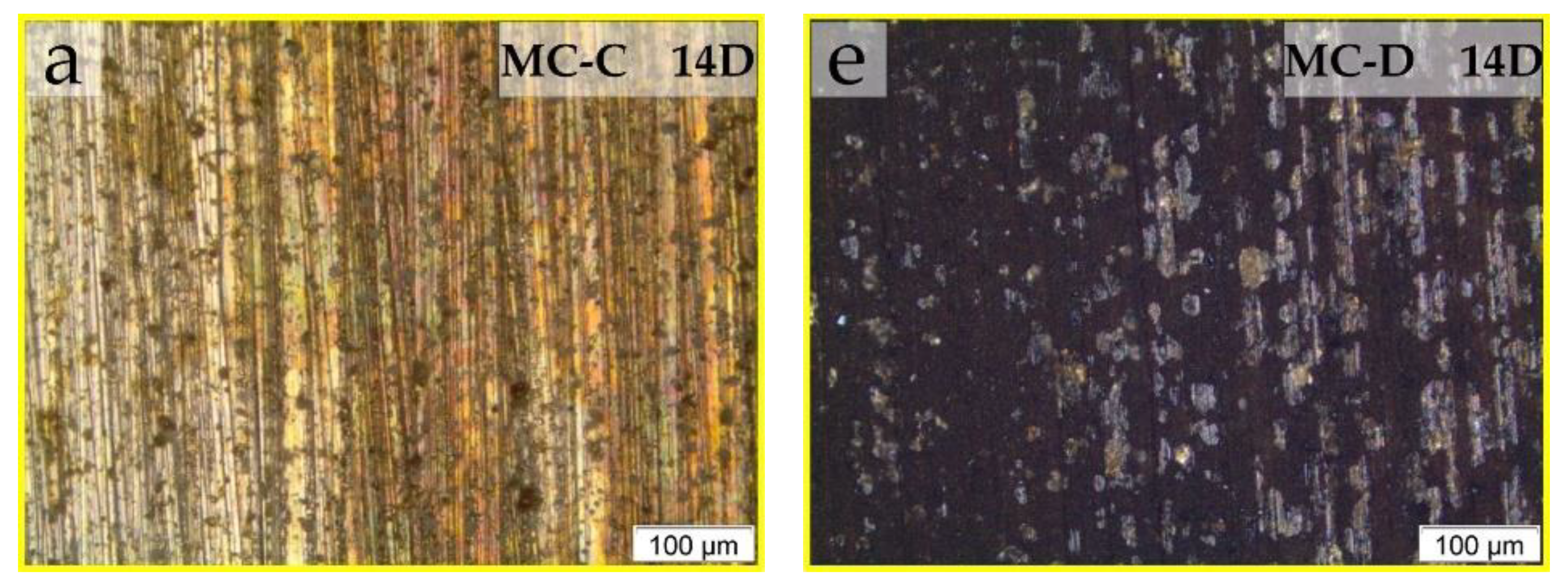
Higher magnification optical micrographs within the dashed squares of
Figure 2 were taken to detail the corrosion of the samples tested in MC-C and MC-D.
Figure 3 shows the magnified views of these dashed regions for all evaluation periods. On the surfaces in contact with MC-C (
Figure 3a–d), an evolution of corrosion products was observed throughout the testing period. This progression appears to have begun at 21 days at the lower end (
Figure 2h and
Figure 3b) and subsequently spread uniformly across the immersed region (
Figure 2m and
Figure 3c,d). The surface texture of the piece tested in MC-C (
Figure 3b–d) seems to be the result of the growth of a layer of corrosive deposits emerging from the surface.
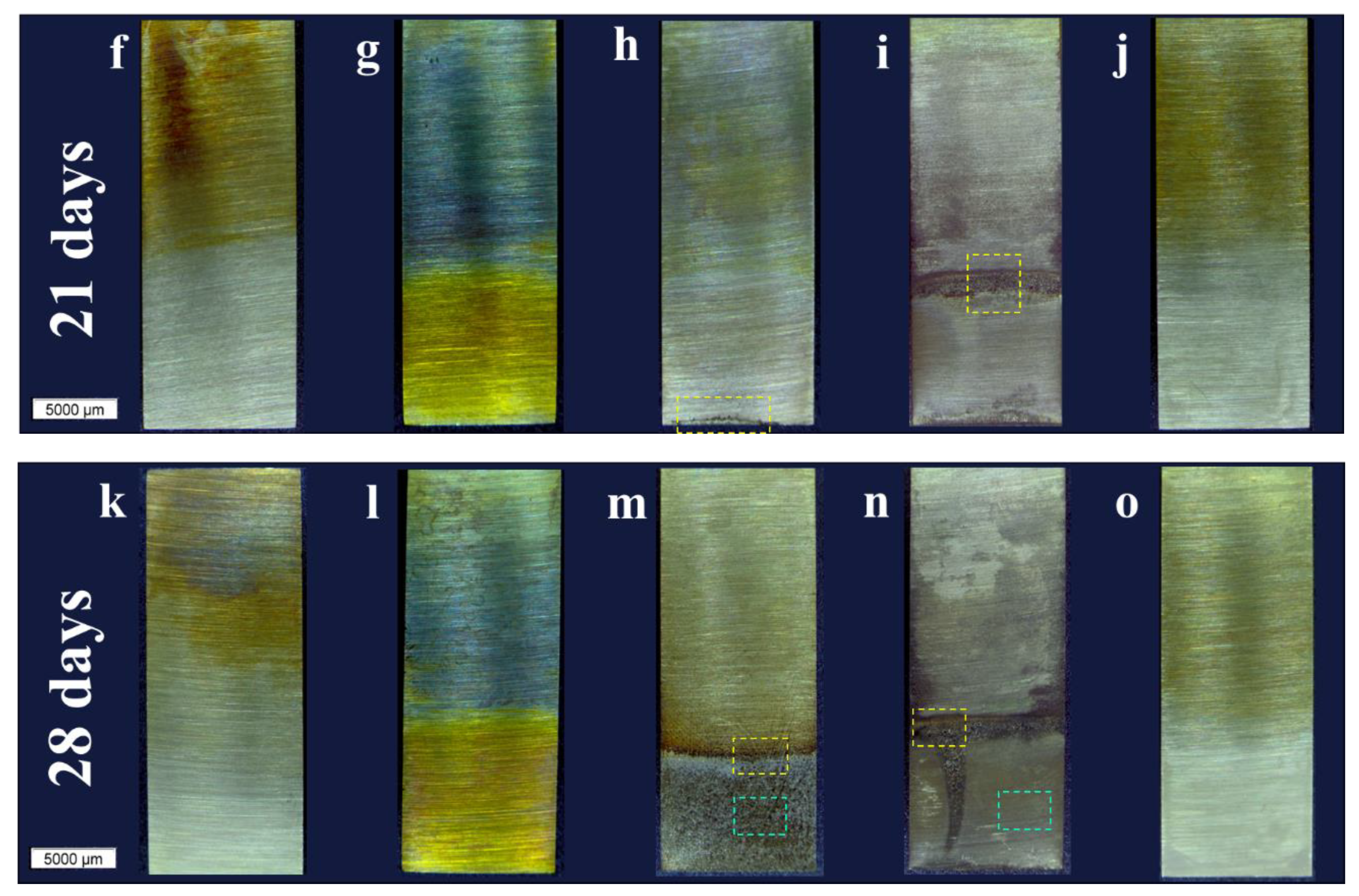
In MC-D, the evolution of corrosion followed a different mechanism, beginning and spreading from the interface between the lubricant and air (
Figure 2d). In the magnified view of this interface in
Figure 3e (14 days), regions of the sanded surface that remained unattacked are observable, while the extensive matrix underwent corrosion (dark areas). At 21 days, at the interface (
Figure 3f), regions of the sanded surface are still visible along with the growth of a layer of corrosion products projecting from the surface. By the end of the test, the corrosion morphology differed between the interface (yellow square in
Figure 2n) and the immersed region (blue square in
Figure 2n). At the interface (
Figure 3g), the surface texture results from corrosion products with varying shades. In the immersed region (
Figure 3h), the original sanded surface marks are still visible alongside areas of localized attack as well as widespread surface corrosion.
The described differences between the pieces tested in MC-C and MC-D suggest that certain additives in the formulations are corrosive to the bronze alloy. These additives are likely of different chemical nature, as they act through distinct corrosion mechanisms. Based on the images in
Figure 2e,j,o, it is important to highlight that such additives from MC-D, when mixed with the commercial oil 10W-30, did not produce apparent corrosion.
3.2. Analysis of Lubricants
Since oxidation and corrosion are inseparable phenomena, the drained lubricants were analyzed using FTIR to assess their degradation and ICP-OES to quantify corrosive wear metals and additive consumption.
Table 3 shows the variation between the new and tested products in the region associated with oxidation (1800–1670 cm
−1) according to ASTM E2412 (FTIR, Ref. [
17]).
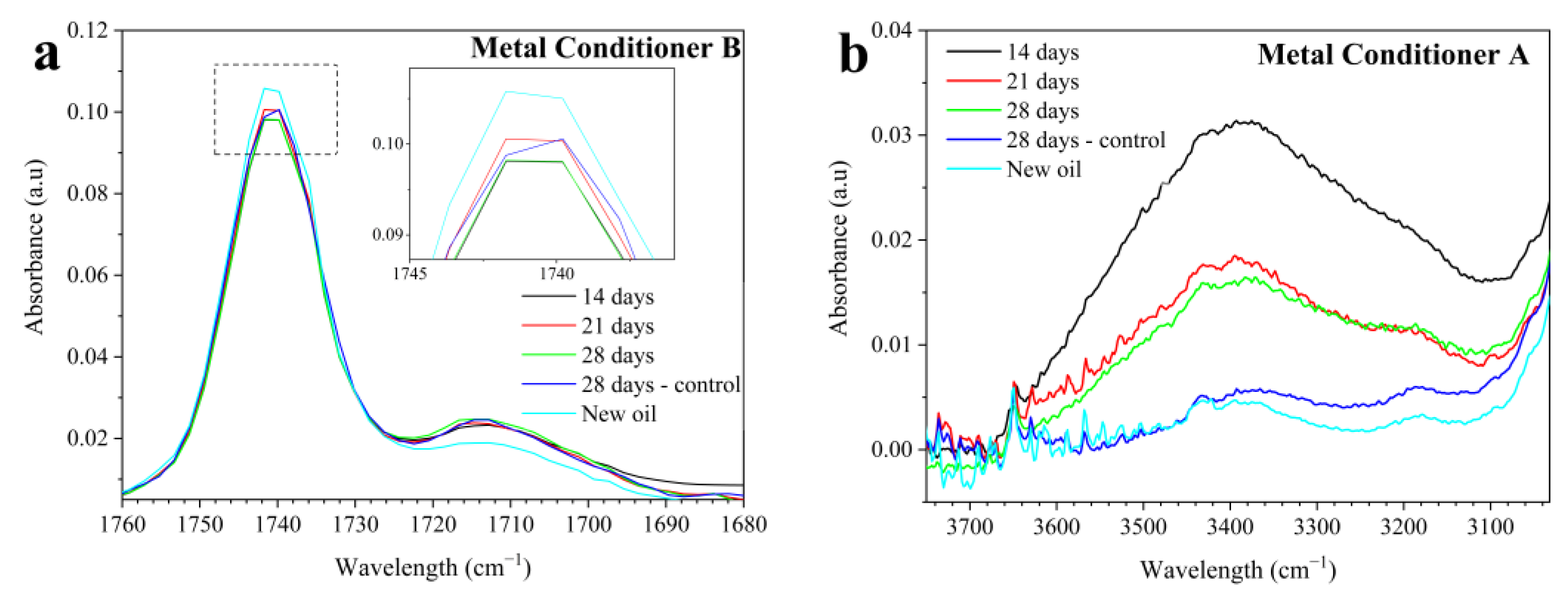
Figure 4a representatively presents the results of one of the conditioners in the oxidation region, as the others showed similar trends.
Given the short test period (28 days) and the absence of tribological contact, a proportional evolution of oxidation over time was not observed (
Table 3). This fact is also associated with the presence of compounds with C=O bonds verified in the spectra of all new oils, as seen in
Figure 4a for MC-B. These compounds may come from an ester base oil or ester-based additives. Therefore, the oxidation analysis should be comparative to the new oil rather than absolute, as is usually enacted for engine oils. Thus,
Figure 4a shows a decrease in the intensity of the major C=O bond peak (~1740 cm
−1) in the tested oils compared to the new oil. This decrease occurred in all tested lubricants and is associated with the reduction in additives containing the C=O bond, either due to their migration to the piece’s surface (adsorption) or their thermal degradation. On the other hand, during the test, an increase in absorbance at the peak of ~1712 cm
−1 compared to the new condition (
Figure 4a) was noted, possibly due to mild oil oxidation, increasing the measured area in the region between 1800 and 1670 cm
−1 (
Table 3).
Calabokis et al. (2022) [
15] and Macián et al. (2012) [
19] also observed variations in oxidation values that did not align with the degradation time of used oils. They attributed these changes to the presence of esters, both in the additive load [
15] and in the formulation of ester-based oils [
19]. Specifically, Calabokis et al. (2022) reported percentage changes in oxidation in used motorcycle crankcase oils containing metal conditioners, ranging from −23% to +220%, with an average oxidation increase of 27% over three oil changes [
15]. In the tests presented here, the maximum variation associated with the consumption or degradation of C=O compounds in the mixture was −6.41% (new vs. 14D), and the maximum variation due to oil oxidation was +4.36% (new vs. 28D). This indicates that the test results (
Table 3) are consistent with the values observed in real-world conditions.
Additionally, for all lubricants, an increase in absorbance was observed in the region between 3600 and 3150 cm
−1 (
Figure 4b,
Table 3), compared to the condition of new (fresh) oil.
Figure 4b representatively shows the results for one of the conditioners, as similar trends were observed in the others. The 3600–3150 cm
−1 region corresponds to the intermolecular O-H bond [
17]. Therefore, an increase in this region is commonly observed due to water contamination [
17] or the formation of degradation products in the oil, such as alcohols, carboxylic acids, and OH- radicals [
9]. Considering that the tests were conducted under ‘closed-cap’ conditions (
Figure 1) with limited air exposure, and that the spectra of lubricants in contact with the parts (over all time periods) showed higher absorbances than the 28-day control oil in the 3600–3150 cm
−1 region (
Table 3), it is likely that the observed increase predominantly relates to the formation of oil degradation products. Some of these products may have originated from the decomposition of ester-type additives, as indicated by the decreased absorbance at the ~1740 cm
−1 peak observed in
Figure 4a, a trend observed across all lubricants.
Table 4 displays the concentrations in parts per million (ppm) of corrosive wear metals (Cu, Pb, and Zn) and the elements from the additive load, as determined by ICP-OES. The analysis revealed zero (0) ppm for other components of the alloy: Fe, Ni, and Sn.
Table 4 reveals that there was a detachment of copper in the tests conducted with MC-A, MC-C, and MC-D. In the case of MC-A, the values were only 1 ppm higher than the new oil, while in MC-C and MC-D oils, increases of 3.3 and 32.5 ppm were observed, respectively. For MC-C, copper was detected only at 28 days, which aligns with the visual observations presented in
Figure 2m, where extensive corrosion in the immersed region is only observed on day 28. MC-D caused corrosive wear with detachment of Cu and Zn particles at all evaluation intervals. The amount of Cu in MC-C and MC-D after 28 days seems small compared to the extensive corrosion they caused in the bronze parts (
Figure 2 and
Figure 3). This is due to the limitations of ICP-OES spectroscopy, whose results are sensitive to particle size [
18,
20]. Indeed, in the oils drained from MC-C and MC-D after 28 days, clusters of corrosion products of millimeter sizes were observed.
An unexpected result was the Pb content in the drained MC-A and the mixture (
Table 4). This reveals that they caused selective corrosion of lead in the high-leaded tin bronze parts during all evaluation periods. In these lubricants, the Pb content increased over the duration of the test. These results are surprising since neither the macrographs in
Figure 2 nor the micrographs exhibit evident signs of corrosion. The only notable change was the loss of color and brightness in the immersed region of the pieces tested with MC-A (
Figure 2a,f,k) and the mixture(
Figure 2e,j,o).
Table 4 also presents the ICP-OES results for some elements of the additive package (P, Ca, Mg, B, and Na). A general trend observed for all lubricants is the reduction in the additive load compared to the new oil. This reduction was always greater for the 14D condition compared to the 21D and 28D conditions. This result is associated with the adsorption of additives on the virgin metal surface, leading to their depletion in the oil. Over time, due to the oxidation of the surfaces, these additives probably return to the oil. Additionally, the 28D-control condition has a lower content of elements compared to the new condition, a result that could be linked to the thermal degradation of the additives or the lubricant, as observed in field tests on vehicles by Agocs et al. [
20]. Specifically, they noted that the presence of oil degradation products accelerated the depletion of additives, especially antioxidants [
20].
The analysis of Zn is more complex since it is found both in the lubricating oil (additive package) and in the tested bronze alloy (
Table 1). Consequently, the presence of Zn in the used oils could originate from either or both sources. The observed reduction in Zn content—relative to its initial state—in tests involving MC-A, MC-B, and the mixture, correlates with the consumption or degradation of Zn-containing additives over time, as was observed for the other additive elements (P, Ca, B, and Na). In the case of MC-C, the formulation does not include Zn, which is consistent with its absence in the drained oil. Conversely, despite also not including Zn in its formulation, the drained MC-D exhibited Zn concentrations beginning at 21 days, suggesting a process of selective corrosion of Zn.
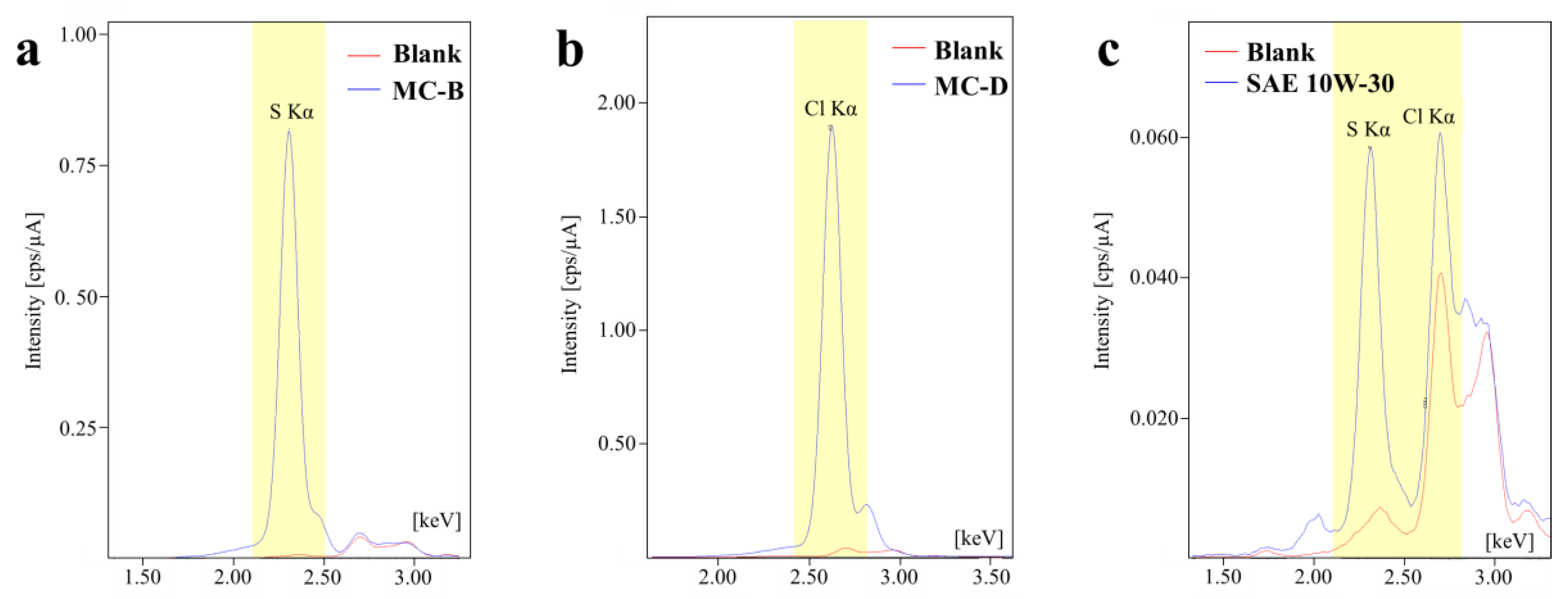
The presence of S- and Cl-containing additives in the formulation of the metal conditioners is expected, given their role in extreme pressure and anti-wear functions. In this context, the presence of chlorine and sulfur in new oils was qualitatively assessed using EDXRF. Characteristic fluorescence spectra for this analysis are displayed in
Figure 5, but only for the samples MC-B, MC-D, and SAE 10W-30 API SL JASO MA. The S-Kα and Cl-Kα were used as analysis lines, highlighted in yellow (
Figure 5). The red curves denote the background interference from scattered X-rays originating from the X-ray tube, which in this case employed a Rhodium (Rh) target. The blue curves in
Figure 5 correspond to the sample’s spectra.
Notably, the spectra of MC-B (
Figure 5a), MC-A (not included in the figure), and SAE 10W-30 (
Figure 5c) revealed a distinct peak in the S-Kα region. Whereas for MC-C and MC-D (
Figure 5b), no prominent signal appeared in the S-Kα region but rather in the Cl-Kα region. Additionally, the SAE 10W-30 sample (
Figure 5c) also exhibited a peak for Cl-Kα. However, the analysis of chlorine posed a challenge due to background interference. The characteristic X-rays from the X-ray tube overlapped with the target peak in the spectra of MC-A and MC-B (
Figure 5a), particularly in the range of 2.5–3.0 keV. This overlap complicates the confirmation of chlorine compounds in these samples.
According to [
21], matrix effects significantly impact the quantitative determination of sulfur in petroleum-derived products when using EDXRF. Such effects arise from the fluid’s composition, like the oxygen content or the carbon/hydrogen ratio [
21]. In this study, no corrective procedures to account for matrix effects or calibration curves were implemented. Therefore, the quantitative data provided by the equipment (expressed as intensity in cps/µA) that is directly related to the content of that element, should be interpreted only for comparative ranking purposes, given that all samples were analyzed under the same measurement conditions. The intensity results (cps/µA) for the S-Kα line were as follows: 10.1053 (MC-B) > 2.6267 (MC-A) > 0.6969 (SAE 10W-30) > 0.2292 (MC-D) > 0.1868 (MC-C). Regarding the Cl-Kα line, the intensities were ranked as follows: 24.354 (MC-D) > 15.2156 (MC-C) > 0.3031 (SAE 10W-30) > 0.2176 (MC-A) > 0.1086 (MC-B). Based on the mentioned considerations and the obtained results, it is only possible to affirm the presence of sulfur in the MC-A and MC-B additives, and in the SAE 10W-30 oil. The presence of chlorine is confirmed in the MC-C and MC-D, and also, in a lower amount, in the SAE 10W-30 oil. These results are crucial for the analyses of the surfaces presented in the following section.
In the analysis of the drained oils, a slight degradation was observed by FTIR, which is expected considering the short testing period (28 days) and the absence of tribological contact. All lubricants showed a reduction in the intensity of the ester peak and an increase in the absorption of the intermolecular O-H bond. These changes are indicative of the consumption of additives containing the C=O bond and overall lubricant degradation. Moreover, the level of additive elements in the drained oils showed a decrease across all testing periods compared to their initial conditions. These reductions can be attributed to thermal degradation or adsorption onto surfaces. On the other hand, the detection of metals from the bronze alloy, such as copper, zinc, and lead, in the drained oils points to the corrosive effect of some products on these specific metals.
3.3. Analysis of Metal Surfaces
The chemical composition of the bronze surfaces was analyzed using FTIR and SEM-EDS. It is crucial to highlight that these characterizations were performed following the cleaning of the samples. As a result, the findings exclusively represent the corrosion products and additives that remained adsorbed onto the surfaces.
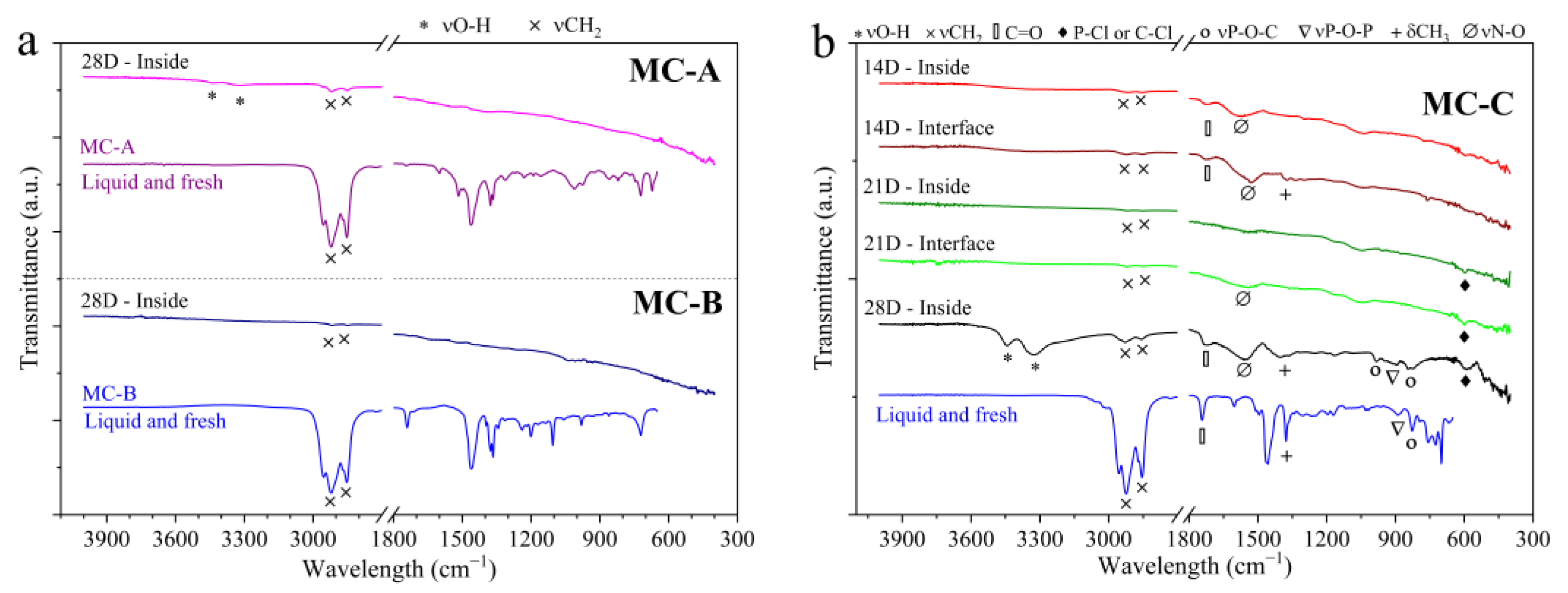
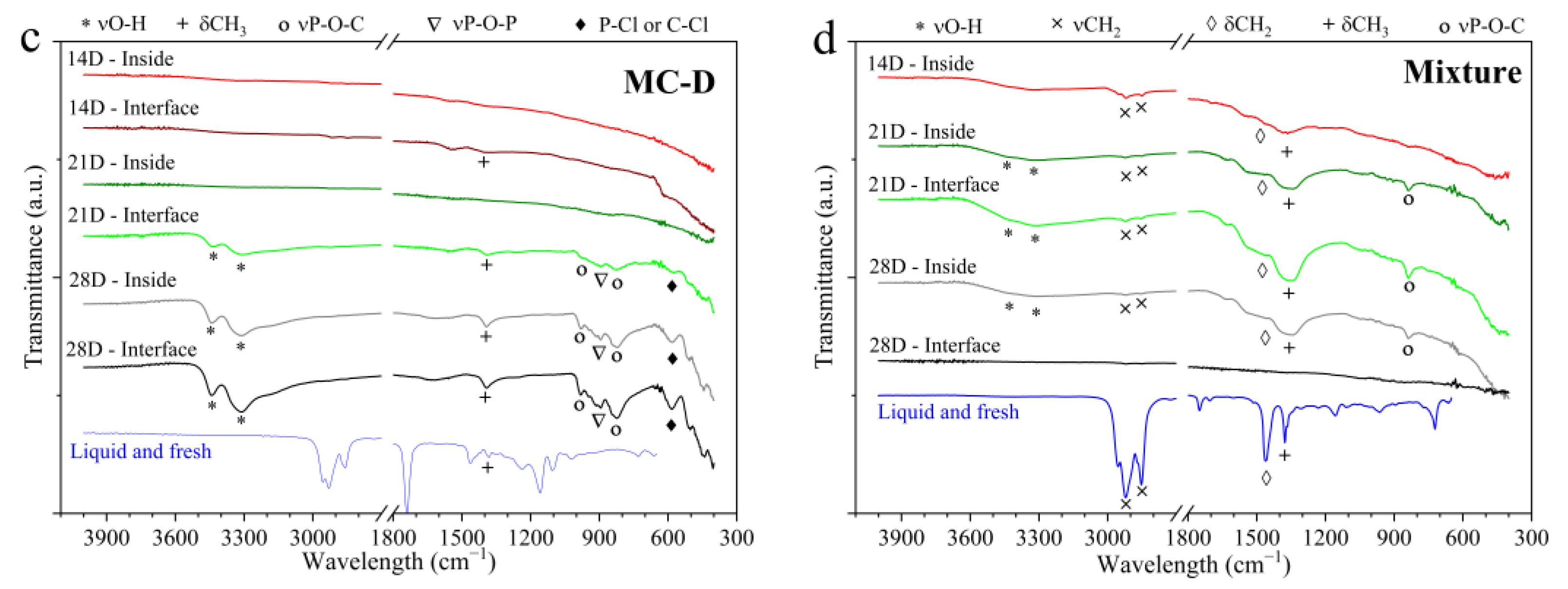
Figure 6 presents the FTIR spectra of the surfaces. For comparative purposes, the spectrum of each oil in its new condition is also presented, with the analysis conducted in the liquid state. Overall,
Figure 6 demonstrates that a limited number of organic groups remained adsorbed on the metal surfaces after the cleaning procedure. The characteristic peaks of the surface spectra are broader and less intense compared to the spectrum of the new oils, this observation aligns with the findings of Piras, Rossi, and Spencer (2003) [
13].
In the case of MC-A and MC-B (
Figure 6a), results are only shown for the end of the test in the immersed region, which show absorbance attributable to the aliphatic groups of hydrocarbons (νCH
2). For other evaluation times, the spectra did not exhibit any absorbance for the parts in MC-A and MC-B, and hence are not presented. For the MC-C (
Figure 6b), MC-D (
Figure 6c), and the mixture (
Figure 6d), the spectra are provided for all evaluation times, both in the immersed region and at the interface. These spectra clearly demonstrate the adsorption of various organic compounds, including the stretching and bending vibrations of C-H, such as νCH
2, δCH
2, and δCH
3. Interestingly, the adsorption of the carbonyl group (C=O) on the surfaces was only confirmed for the MC-C samples (
Figure 6b), despite all the new additives having this characteristic peak in their spectra.
In the spectra of the parts tested in MC-C (
Figure 6b), MC-D (
Figure 6c) and in the mixture (
Figure 6d), peaks identified in the region between 1000 and 775 cm
−1 were associated with stretching vibrations of P-O-P and P-O-C (νP-O-P, νP-O-C). According to [
13], these bonds originate from the degradation of antiwear additives such as Zinc dialkyldithiophosphates (ZDTPs). Furthermore, it is noteworthy that from 21 days onwards in MC-C and MC-D, the peak located at 585 cm
−1 could possibly be associated with the stretching of halo compounds such as P-Cl or C-Cl. This is supported by the detection of chlorine by EDXRF and in the surface analyses performed by SEM-EDS, as will be presented subsequently.
Finally, one or two broad bands in the 3500–3250 cm
−1 region associated with the O-H stretching vibration (νO-H) are identified in the spectra of the surfaces in MC-A, MC-C, MC-D, and the mixture (
Figure 6). The presence of the O-H group on the surfaces confirms the hypothesis raised regarding the spectra of the drained oils (
Figure 4b and
Table 3): partially degraded oil products have formed and remain adsorbed on the bronze pieces.
The results of the semi-quantitative elemental chemical composition analyzed by SEM-EDS are displayed in
Table 5. Considering the necessity for rigorous sample cleaning in SEM-EDS analysis, the following results are confined to examining the elemental composition of compounds formed between the additives and the metallic matrix, which remained adsorbed despite ultrasonic cleaning. The EDS analyses of the surfaces tested in MC-A and MC-B (
Table 5) corroborate the results shown in
Figure 6a, indicating limited adsorption of additives. Chlorine was detected on the surfaces analyzed with MC-A and MC-B, displaying low concentrations and a wide range of results. Given that its determination is semi-quantitative, such results indicate that its presence is due to contamination rather than being an additive, which is corroborated by the findings obtained through EDXRF (
Figure 5). Specifically, in the surfaces with MC-A, the presence of sulfur was confirmed across the entire piece. In the pieces with MC-B, calcium was identified in the immersed region.
The oxygen content of the surfaces before testing was 3.52 wt.%. Thus, the results in
Table 5 suggest that the formation of surface oxides in MC-A and MC-B was minimal compared to the high oxygen content observed in conditions MC-C, MC-D, and the mixture. Notably,
Table 5 presents results in the interface region for MC-D, as this was the only condition that exhibited preferential corrosion in this area (refer to
Figure 2d,i,n). Chlorine was the sole additive element detected on the surfaces tested in MC-C and MC-D. The surface region with the highest chlorine content appears to coincide with the incidence of corrosion: a more significant attack was observed with MC-C in the immersed region (
Figure 2m and
Figure 3d) and with MC-D at the interface (
Figure 2n and
Figure 3g). The parts tested in the mixture showed the greatest diversity of additive elements (Ca, Na, Cl). This outcome is a result of the lubricant used, SAE 10W-30, being a fully formulated oil.
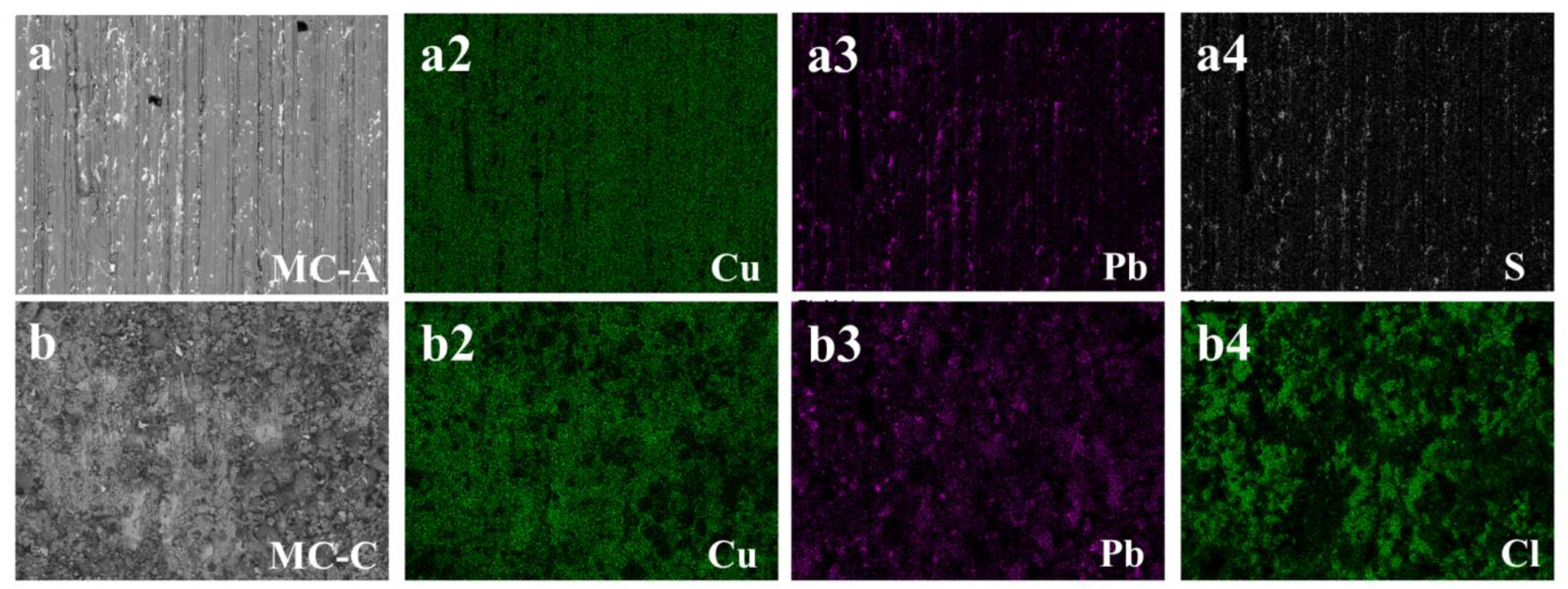
The analyses conducted using SEM-EDS enabled the identification of a preferential distribution of the additive elements sulfur (S) and chlorine (Cl) in the microstructure of the bronze, as demonstrated in
Figure 7. Under the conditions in which sulfur or chlorine are adsorbed onto immersed surfaces (
Table 5), a representative case for each of these elements was selected for presentation in
Figure 7a4,b4, respectively.
In
Figure 7a, a significant correlation emerges between the intensity of the sulfur element (
Figure 7a4) and the presence of lead islands (
Figure 7a3) within the microstructure. Conversely, the analysis presents a different scenario for chlorine, as depicted in
Figure 7b. The map for chlorine (
Figure 7b4) displays extensive coverage across the matrix of the microstructure, predominantly composed of copper. These findings indicate a selective affinity: sulfur for regions rich in lead, and chlorine for the copper matrix. This pattern is likely attributable to the formation of sulfide and chloride compounds, respectively, in these specific areas.