Facile Synthesis of Ag-Doped Urchin-like MnO2 on Carbon Cloth for Supercapacitors
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Preparation of Ag-Doped MnO2 at Room Temperature (RTMOA)
2.3. Preparation of Ag@MnO2/CC Composite Materials at Room Temperature (CRTMOA)
2.4. Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
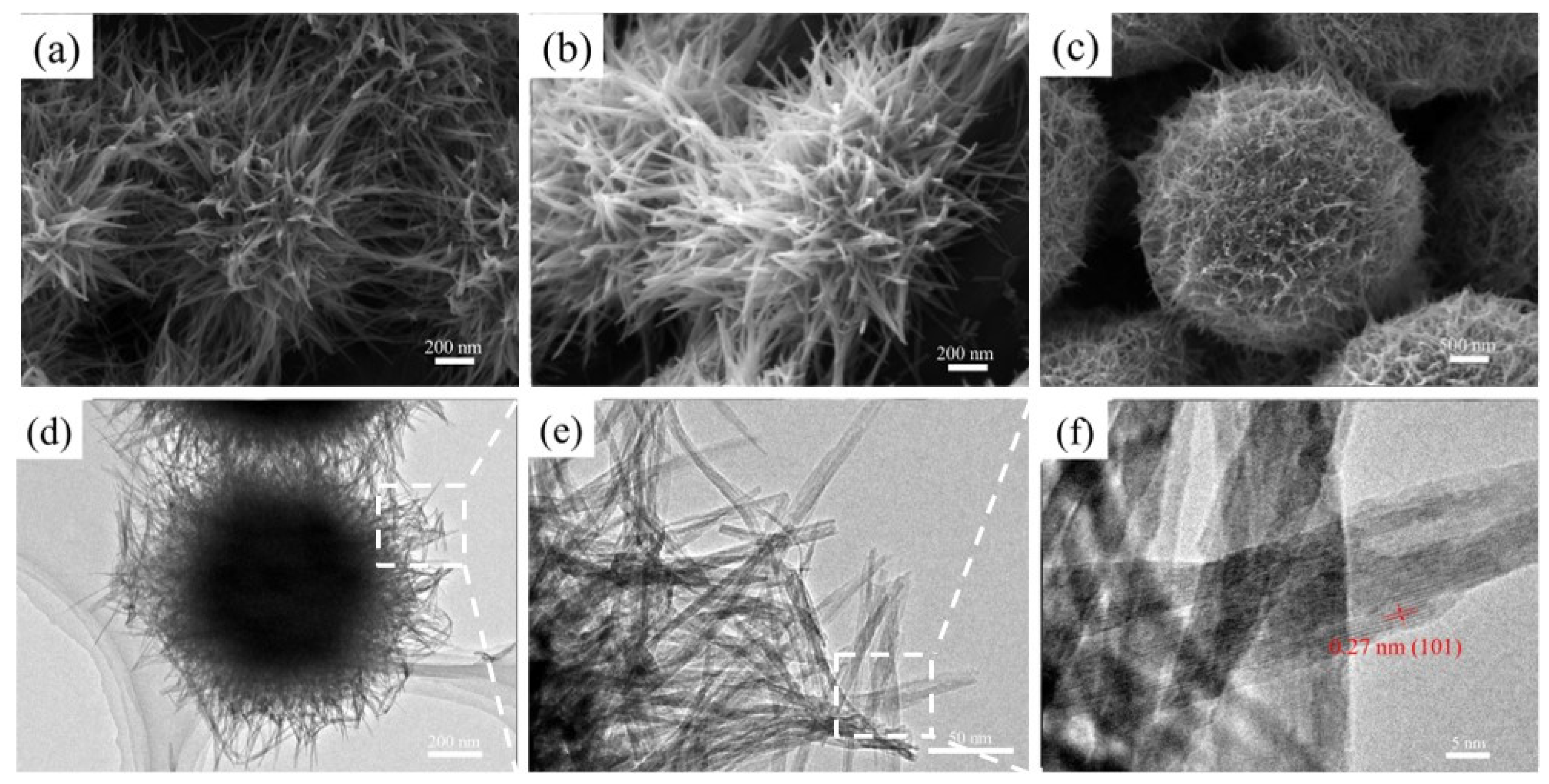
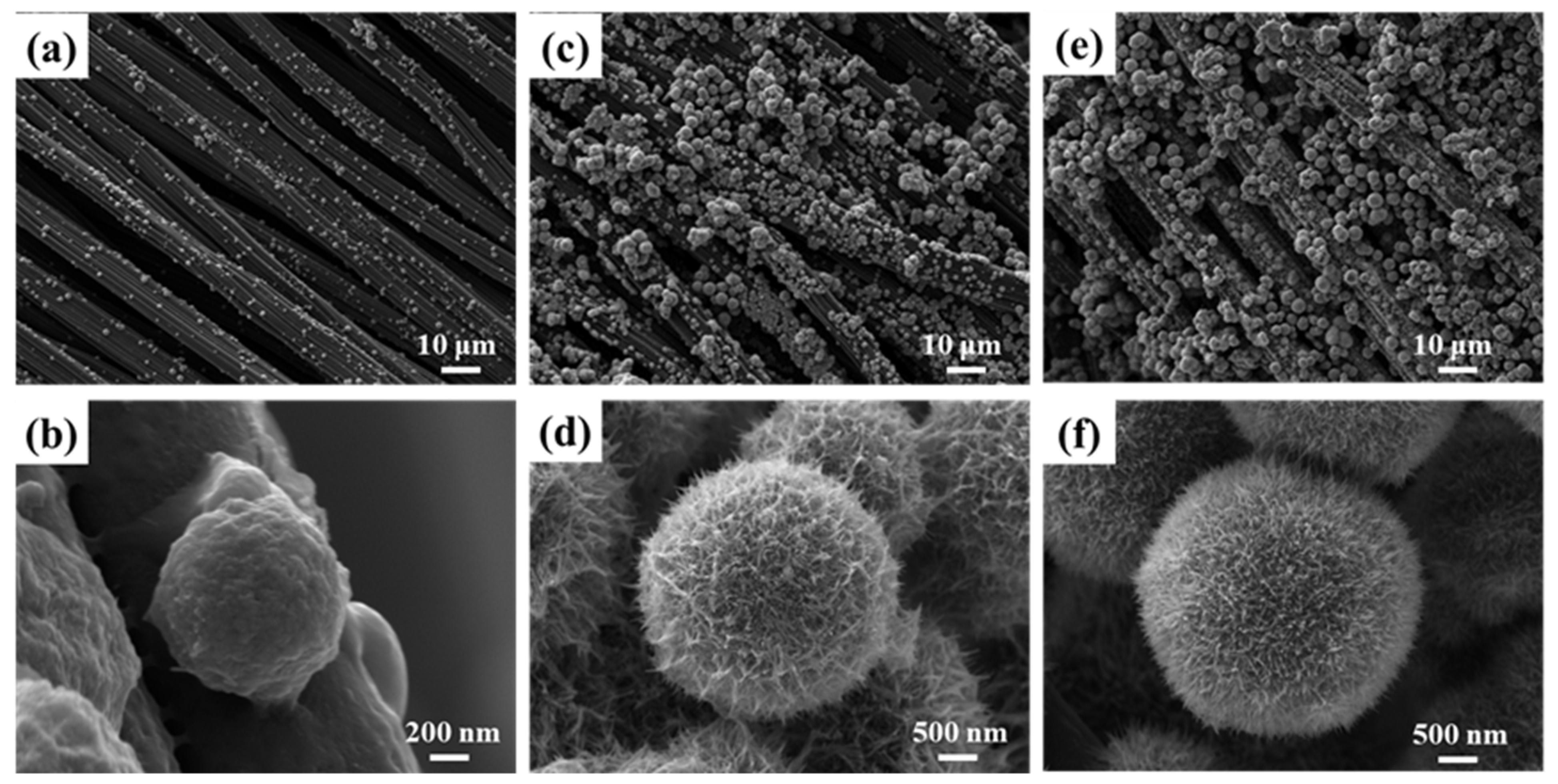
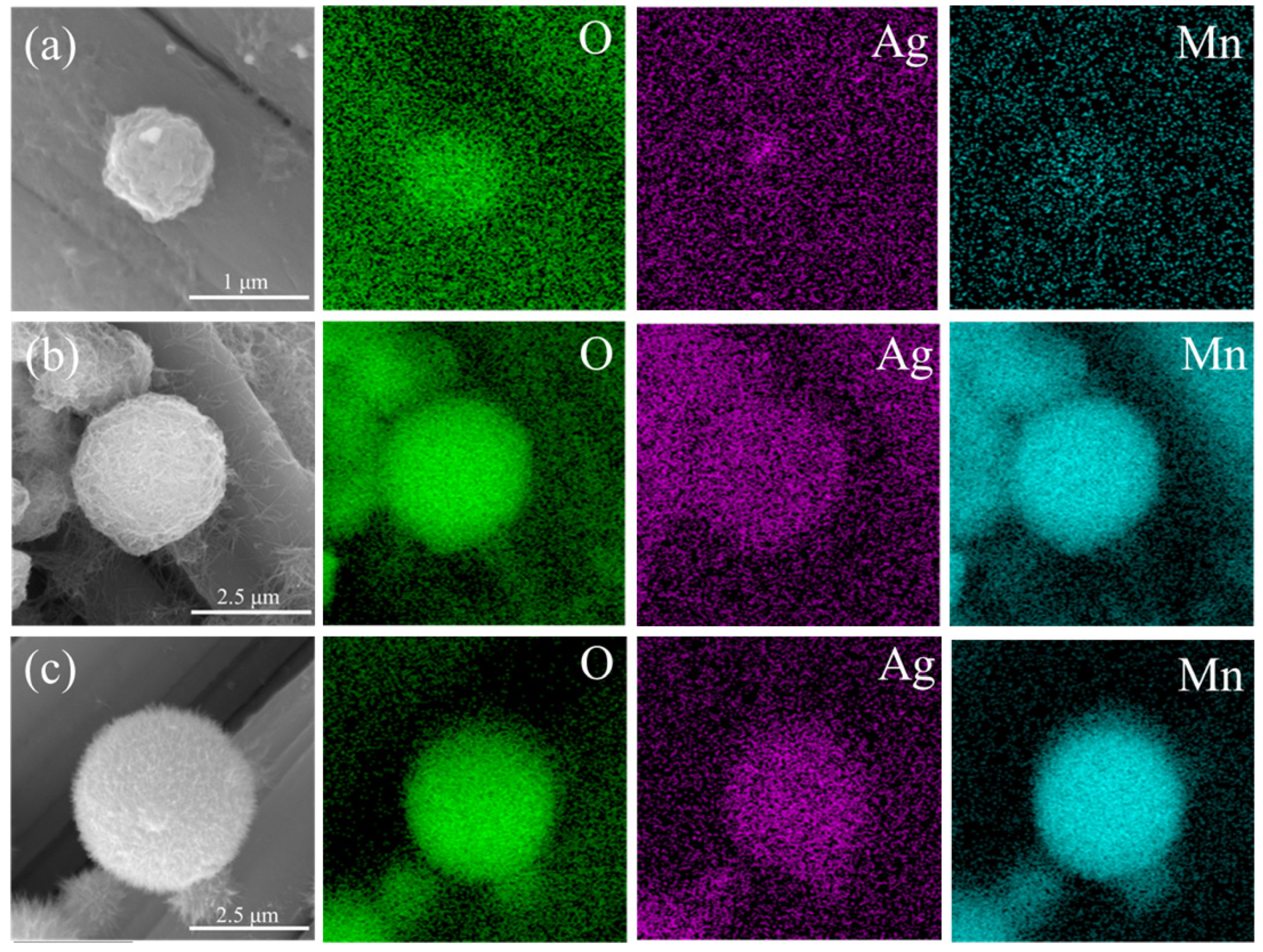
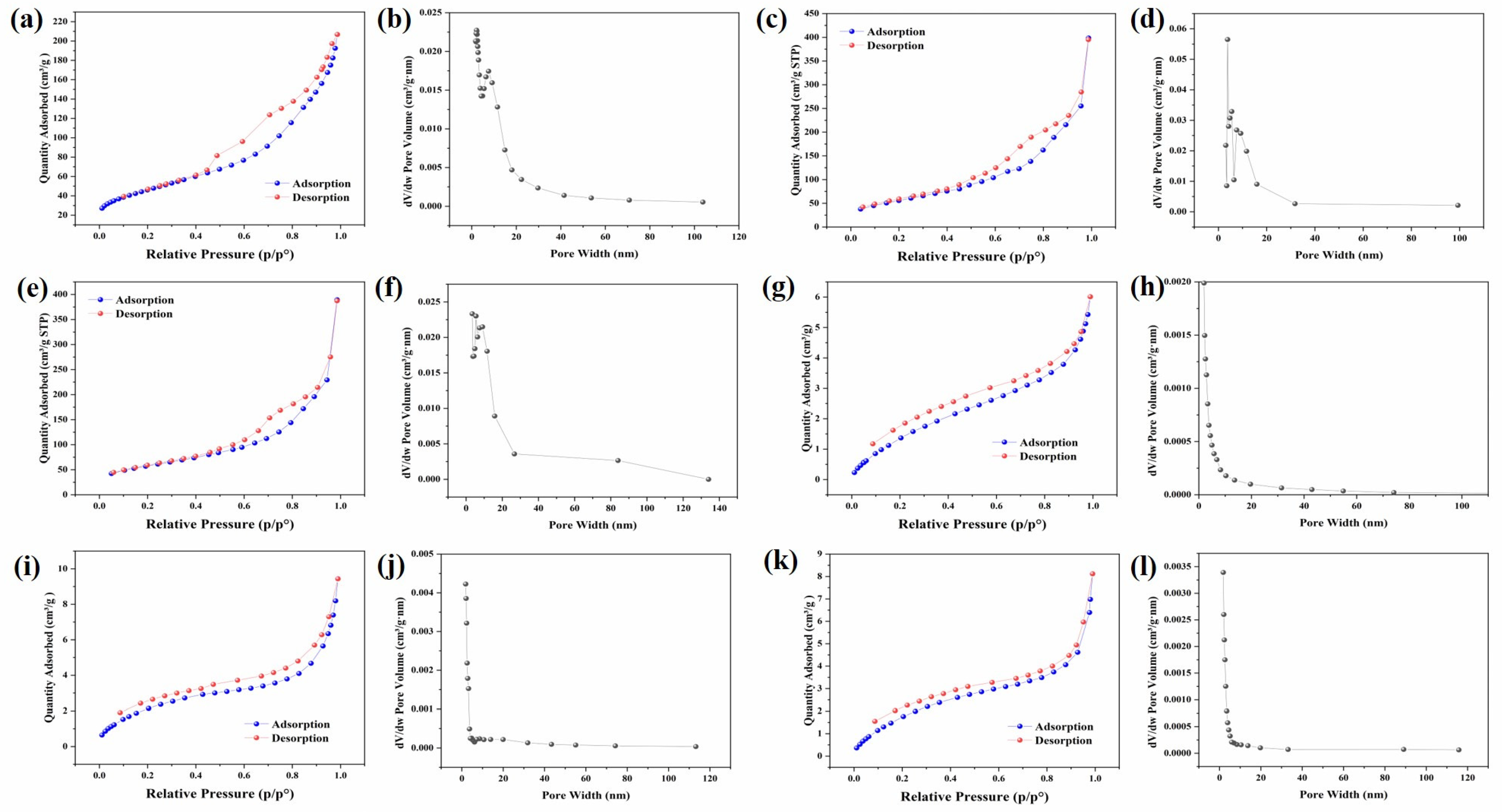
3.1. Surface Morphology Analysis
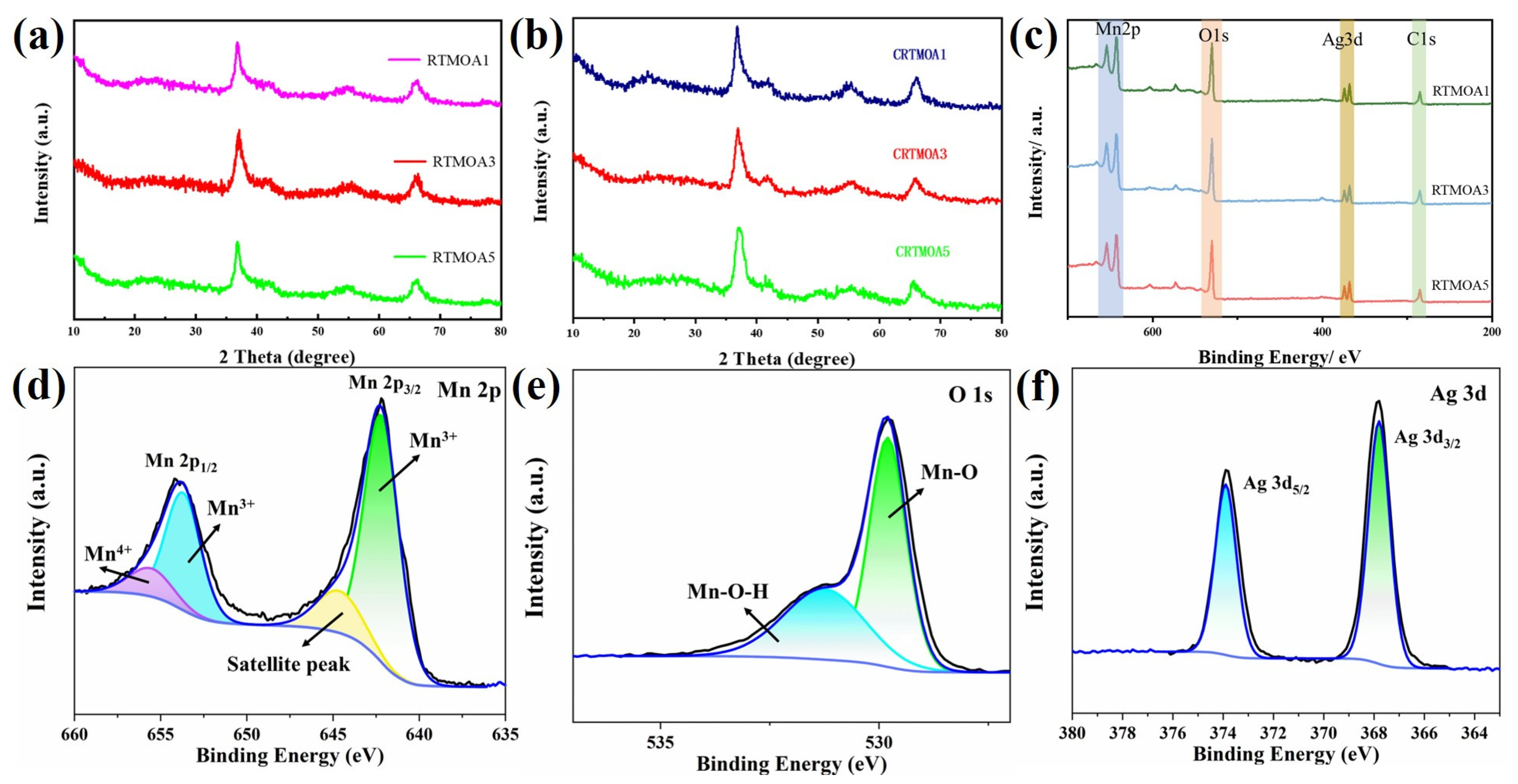
3.2. Chemical Structure Analysis
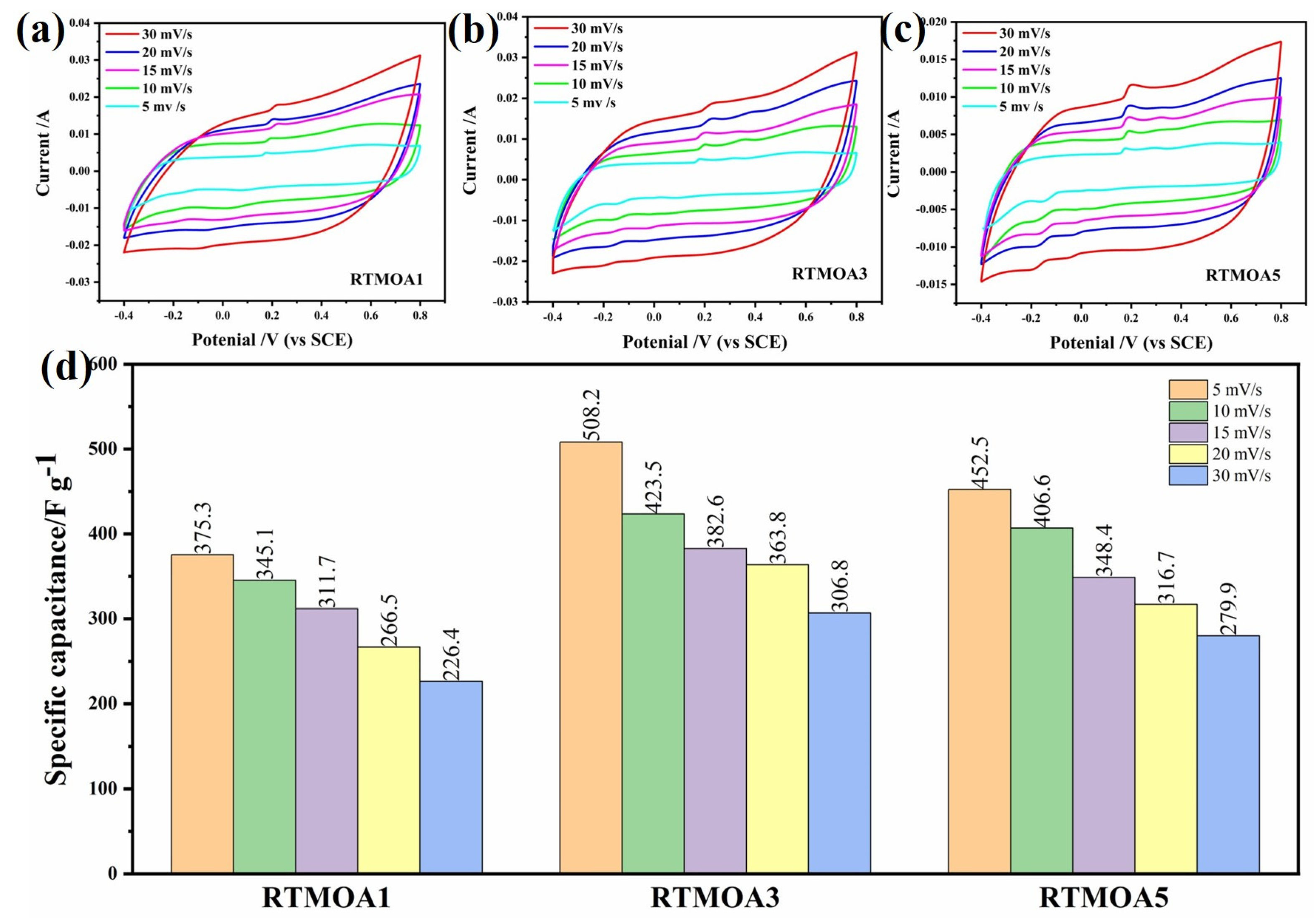
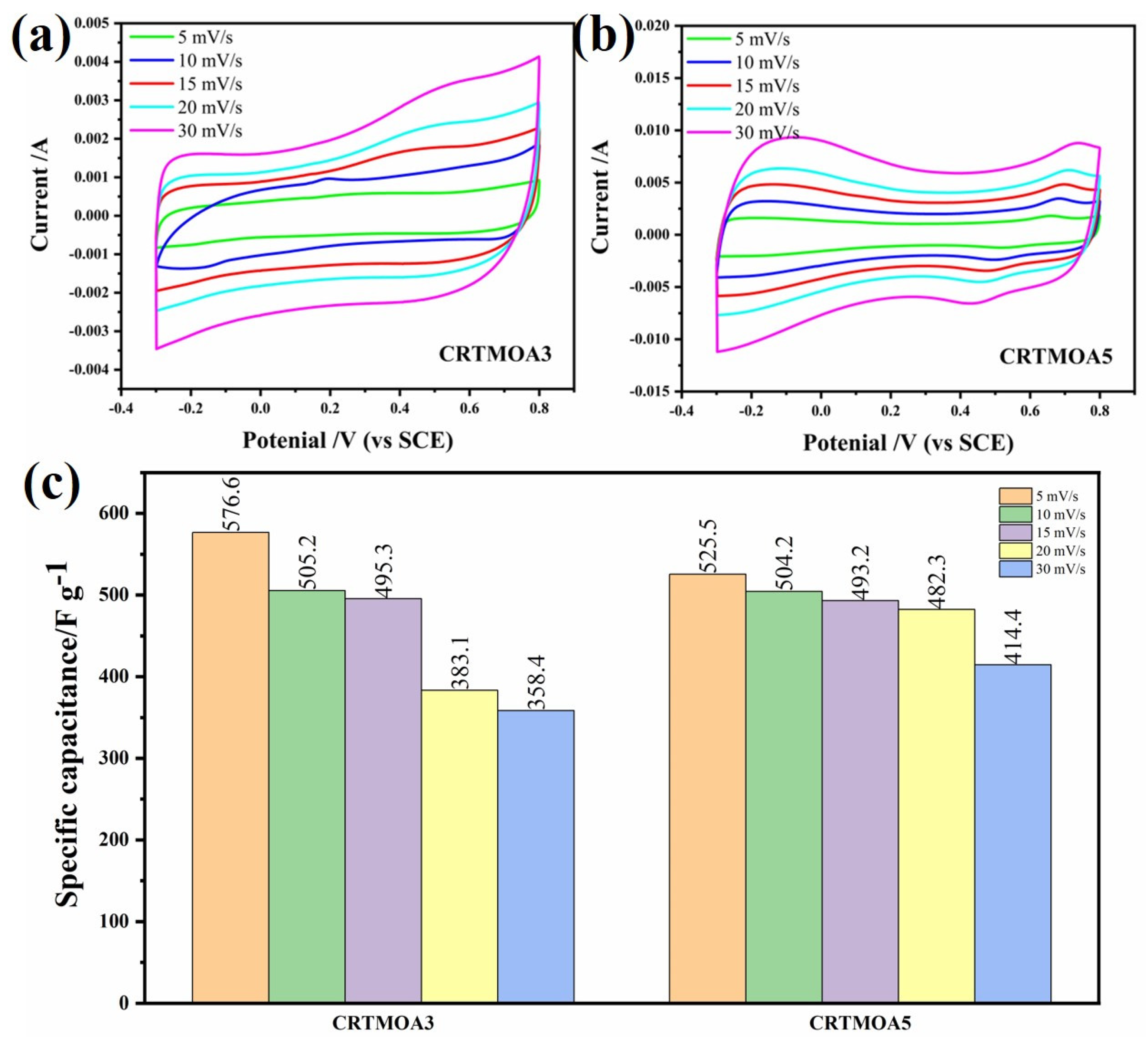
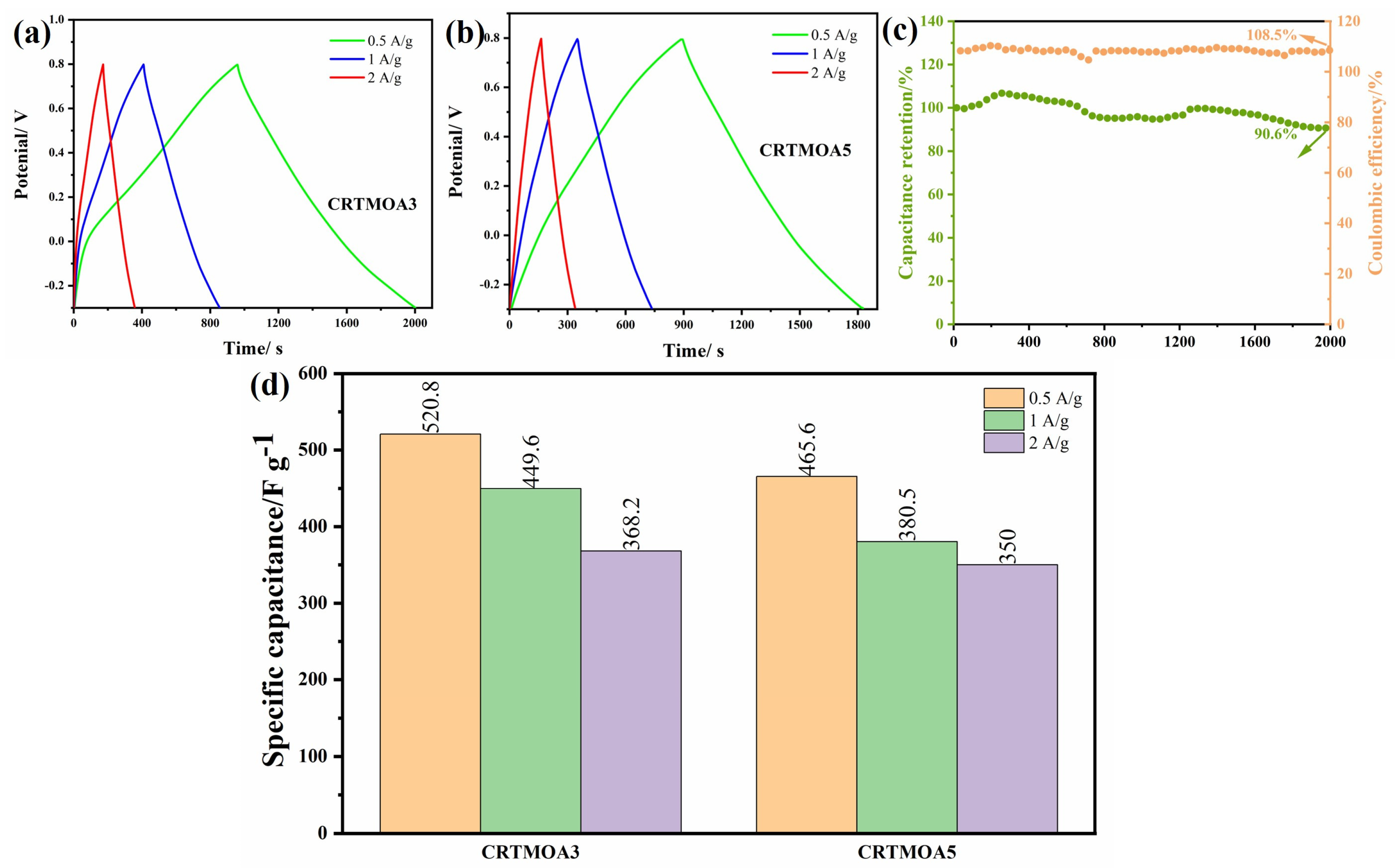
3.3. Electrochemical Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sayed, E.T.; Olabi, A.G.; Alami, A.H.; Radwan, A.; Mdallal, A.; Rezk, A.; Abdelkareem, M.A. Renewable Energy and Energy Storage Systems. Energies 2023, 16, 1415. [Google Scholar] [CrossRef]
- Molaei, M.; Rostami, G.R.; Zardkhoshoui, A.M.; Davarani, S.S.H. In situ tellurization strategy for crafting nickel ditelluride/cobalt ditelluride hierarchical nanostructures: A leap forward in hybrid supercapacitor electrode materials. J. Colloid Interface Sci. 2024, 653 Pt B, 1683–1693. [Google Scholar] [CrossRef]
- Gao, H.N.; Gallant, B.M. Advances in the chemistry and applications of alkali-metal-gas batteries. Nat. Rev. Chem. 2020, 4, 566–583. [Google Scholar] [CrossRef]
- Chen, Q.; Jin, J.L.; Song, M.D.; Zhang, X.Y.; Li, H.; Zhang, J.L.; Hou, G.Y.; Tang, Y.P.; Mai, L.Q.; Zhou, L. High-Energy Aqueous Ammonium-Ion Hybrid Supercapacitors. Adv. Mater. 2022, 34, 8. [Google Scholar] [CrossRef]
- Loganathan, N.N.; Perumal, V.; Pandian, B.R.; Atchudan, R.; Edison, T.; Ovinis, M. Recent studies on polymeric materials for supercapacitor development. J. Energy Storage 2022, 49, 22. [Google Scholar] [CrossRef]
- Chowdhury, A.; Shukla, R.; Bhattacharyya, K.; Tyagi, A.K.; Chandra, A.; Grover, V. Electrochemical performance of K+-intercalated MnO2 nano-cauliflowers and their Na-ion-based pseudocapacitors. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2023, 295, 12. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.Y.; Zhang, H.; Shen, M.X.; Baker, F.; Liu, Y.X.; Liu, L.Q.; Yang, J.; Cao, H.B.; Xu, Q.L.; et al. Houttuynia-derived nitrogen-doped hierarchically porous carbon for high-performance supercapacitor. Carbon 2020, 161, 62–70. [Google Scholar] [CrossRef]
- Wei, Y.D.; Luo, W.L.; Li, X.; Lin, Z.T.; Hou, C.P.; Ma, M.L.; Ding, J.X.; Li, T.X.; Ma, Y. PANI-MnO2 and Ti3C2Tx (MXene) as electrodes for high-performance flexible asymmetric supercapacitors. Electrochim. Acta 2022, 406, 10. [Google Scholar] [CrossRef]
- Chin, S.X.; Lau, K.S.; Ginting, R.T.; Tan, S.T.; Khiew, P.S.; Chia, C.H.; Wongchoosuk, C. Facile Preparation of Carbon Nanotubes/Cellulose Nanofibrils/Manganese Dioxide Nanowires Electrode for Improved Solid-Sate Supercapacitor Performances. Polymers 2023, 15, 3758. [Google Scholar] [CrossRef] [PubMed]
- Poudel, M.B.; Kim, A.R.; Ramakrishan, S.; Logeshwaran, N.; Ramasamy, S.K.; Kim, H.J.; Yoo, D.J. Integrating the essence of metal organic framework-derived ZnCoTe-N-C/MoS2 cathode and ZnCo-NPS-N-CNT as anode for high-energy density hybrid supercapacitors. Compos. Pt. B-Eng. 2022, 247, 14. [Google Scholar] [CrossRef]
- Thakur, V.N.; Chetana, S.; Gajraj, V.; Kumar, N.; Joshi, N.C.; Basavakumar, K.G. Chemical vapour deposition synthesized novel LaFe2O3/Al2O3/Fe/CNT heterostructure for enhanced super-capacitive performance. J. Mater. Sci.-Mater. Electron. 2023, 34, 13. [Google Scholar] [CrossRef]
- Pligovka, A.; Lazavenka, A.; Zakhlebayeva, A. Electro-Physical Properties of Niobia Columnlike Nanostructures via the Anodizing of Al/Nb Layers. In Proceedings of the 2018 IEEE 18th International Conference on Nanotechnology (IEEE-NANO), Cork, Ireland, 23–26 July 2018; pp. 1–5. [Google Scholar]
- Panmand, R.; Sethi, Y.; Jha, A.; Kale, B. The synthesis and super capacitive characterization of microwave-assisted highly crystalline α-Fe2O3/Fe3O4 nanoheterostructures. RSC Adv. 2023, 13, 20951–20957. [Google Scholar] [CrossRef]
- Tang, C.; Wang, X.; Ma, M.Z.; Wang, Z.L.; Li, Y.; Li, H.; Li, B.; Zhang, Y.X.; Zhu, X.B. Optimizing the electrons/ions diffusion kinetics in δ-MnO2 for realizing an ultra-high rate-capability supercapacitor. Chem. Eng. J. 2023, 471, 11. [Google Scholar] [CrossRef]
- Worku, A.K.; Ayele, D.W.; Habtu, N.G.; Ambaw, M.D. Engineering nanostructured Ag doped α-MnO2 electrocatalyst for highly efficient rechargeable zinc-air batteries. Heliyon 2022, 8, 12. [Google Scholar] [CrossRef]
- Vetrikarasan, B.T.; Nair, A.R.; Karthick, T.; Shinde, S.K.; Kim, D.Y.; Sawant, S.N.; Jagadale, A.D. Co-precipitation synthesis of pseudocapacitive λ-MnO2 for 2D MXene (Ti3C2Tx) based asymmetric flexible supercapacitor. J. Energy Storage 2023, 72, 12. [Google Scholar] [CrossRef]
- de la Cruz, I.J.; Rodríguez, S.J.L.; Fuentes, I.; Tiznado, H.; Vazquez-Arce, J.L.; Romero-Ibarra, I.; Guzmán, C.J.I.; Gutiérrez, H.M. Effect of crystalline phase of MnO2 on the degradation of Bisphenol A by catalytic ozonation. J. Environ. Chem. Eng. 2023, 11, 14. [Google Scholar] [CrossRef]
- Alsaif, N.A.M.; Atta, A.; Abdeltwab, E.; Abdel-Hamid, M.M. Synthesis, structural characterization, and optical properties of PVA/MnO2 materials for optoelectronics applications. Macromol. Res. 2024, 32, 35–44. [Google Scholar] [CrossRef]
- Cao, Z.G.; Yang, Y.B.; Qin, J.L.; Su, Z.X. A core-shell porous MnO2/Carbon nanosphere composite as the anode of lithium-ion batteries. J. Power Sources 2021, 491, 9. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Kumar, Y.A.; Pallavolu, M.R.; Arbi, H.M.; Alzahmi, S.; Obaidat, I.M. Two-Dimensional Core-Shell Structure of Cobalt-Doped@MnO2 Nanosheets Grown on Nickel Foam as a Binder-Free Battery-Type Electrode for Supercapacitor Application. Nanomaterials 2022, 12, 3187. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Zhao, L.; Lei, Y.H.; Li, Z.; Wang, G. N-doped MnO2 with abundant oxygen vacancies achieves high-capacity and stable ammonium ion capture by capacitive deionization. Sep. Purif. Technol. 2024, 329, 10. [Google Scholar] [CrossRef]
- Lee, H.J.; Noor, N.; Gumeci, C.; Dale, N.; Parrondo, J.; Higgins, D.C. Understanding the Impact of the Morphology, Phase Structure, and Mass Fraction of MnO2 within MnO2/Reduced Graphene Oxide Composites for Supercapacitor Applications. J. Phys. Chem. C 2022, 11, 13004–130014. [Google Scholar] [CrossRef]
- Kadam, S.L.; Ingole, R.S.; Tiwari, N.G.; Nakate, U.T.; Nakate, Y.T.; Kamat, R.K.; Ok, J.G.; Kulkarni, S.B. Facile synthesis of nanourchin like manganese oxide electrode material for high performance symmetric supercapacitor. Surf. Interfaces 2023, 42, 9. [Google Scholar] [CrossRef]
- Xavier, J.R.; Vinodhini, S.P. Flexible and high-energy density asymmetrical supercapacitors based on polyindole/GCN/MnO2 nanocomposite for energy storage applications. J. Mater. Sci. 2023, 22, 18147–18168. [Google Scholar] [CrossRef]
- Arkhipova, E.A.; Ivanov, A.S.; Isaikina, O.Y.; Novotortsev, R.Y.; Stolbov, D.N.; Xia, H.; Savilov, S.V. Application of MnO2/MWCNT composite in supercapacitors. Mater. Today Proc. 2022, 60, 1008–1011. [Google Scholar] [CrossRef]
- Cakici, M.; Kakarla, R.R.; Alonso-Marroquin, F. Advanced electrochemical energy storage supercapacitors based on the flexible carbon fiber fabric-coated with uniform coral-like MnO2 structured electrodes. Chem. Eng. J. 2017, 309, 151–158. [Google Scholar] [CrossRef]
- Chaudhuri, H.; Karak, N. Heterostructured Hybrid rGO@α-MnO2/rGO@δ-MnO2 Nanoflower: An Efficient Catalyst for Aerobic Solvent-Free N-Alkylation Reactions and Energy Storage Material. ChemCatChem 2020, 12, 1617–1629. [Google Scholar] [CrossRef]
- He, X.J.; Feng, J.X.; Ren, Q.; Li, G.R. Ni nanoparticle-decorated-MnO2 nanodendrites as highly selective and efficient catalysts for CO2 electroreduction (vol 6, pg 19438, 2018). J. Mater. Chem. A 2019, 7, 26641–26642. [Google Scholar] [CrossRef]
- Chao, D.L.; Zhou, W.H.; Ye, C.; Zhang, Q.H.; Chen, Y.G.; Gu, L.; Davey, K.; Qiao, S.Z. An Electrolytic Zn-MnO2 Battery for High-Voltage and Scalable Energy Storage. Angew. Chem.-Int. Edit. 2019, 58, 7823–7828. [Google Scholar] [CrossRef]
- Zong, Q.J.; Zhang, Q.C.; Mei, X.; Li, Q.L.; Zhou, Z.Y.; Li, D.; Chen, M.Y.; Shi, F.Y.; Sun, J.; Yao, Y.G.; et al. Facile Synthesis of Na-Doped MnO2 Nanosheets on Carbon Nanotube Fibers for Ultrahigh-Energy-Density All-Solid-State Wearable Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 37233–37241. [Google Scholar] [CrossRef]
- Chomkhuntod, P.; Hantanasirisakul, K.; Duangdangchote, S.; Phattharasupakun, N.; Sawangphruk, M. The charge density of intercalants inside layered birnessite manganese oxide nanosheets determining Zn-ion storage capability towards rechargeable Zn-ion batteries. J. Mater. Chem. A 2022, 10, 5561–5568. [Google Scholar] [CrossRef]
- Habtu, N.G.; Worku, A.K.; Ayele, D.W.; Teshager, M.A.; Workineh, Z.G. Facile Preparation and Electrochemical Investigations of Copper-Ion Doped alpha-MnO2 Nanoparticles. In Advances of Science and Technology: 9th EAI International Conference, ICAST 2021, Proceedings; Lecture Notes of the Institute for Computer Sciences, Social Informatics and Telecommunications Engineering; Springer: Cham, Switzerland, 2022; pp. 543–553. [Google Scholar]
- Khan, A.; Toufiq, A.M.; Tariq, F.; Khan, Y.; Hussain, R.; Akhtar, N.; Rahman, S.U. Influence of Fe doping on the structural, optical and thermal properties of α-MnO2 nanowires. Mater. Res. Express 2019, 6, 9. [Google Scholar] [CrossRef]
- Adewinbi, S.A.; Maphiri, V.M.; Taleatu, B.A.; Marnadu, R.; Shkir, M.; Hakami, J.; Kim, W.K.; Gedi, S. Binder-less fabrication, some surface studies, and enhanced electrochemical performance of Co, Cu-embedded MnO2 thin film electrodes for supercapacitor application. Ceram. Int. 2022, 48, 26312–26325. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, N.; Yao, M.Q.; Yang, C.T.; Hu, W.C.; Komarneni, S. Porous Ag-doped MnO2 thin films for supercapacitor electrodes. J. Porous Mat. 2017, 24, 1717–1723. [Google Scholar] [CrossRef]
- Mazinani, B.; Kazazi, M.; Mobarhan, G.; Shokouhimehr, M. The Combustion Synthesis of Ag-Doped MnCo2O4 Nanoparticles for Supercapacitor Applications. JOM 2019, 71, 1499–1506. [Google Scholar] [CrossRef]
- He, C.E.; Liu, Z.X.; Peng, H.Y.; Yang, Y.K.; Shi, D.A.; Xie, X.L. Room-temperature catalytic growth of hierarchical urchin-like MnO2 spheres on graphene to achieve silver-doped nanocomposites with improved supercapacitor performance. Electrochim. Acta 2016, 222, 1393–1401. [Google Scholar] [CrossRef]
- Rahman, A.U.; Zarshad, N.; Wu, J.H.; Faiz, F.; Raziq, F.; Ali, A.; Li, G.G.; Ni, H.M. Fabrication of Ag-doped MnO2 nanosheets@carbon cloth for energy storage device. Mater. Sci. Eng. B-Adv. Funct. Solid-State Mater. 2021, 269, 11. [Google Scholar] [CrossRef]
- Tan, Y.L.; Yang, C.X.; Qian, W.W.; Teng, C. Flower-like MnO2 on layered carbon derived from sisal hemp for asymmetric supercapacitor with enhanced energy density. J. Alloys Compd. 2020, 826, 9. [Google Scholar] [CrossRef]
- Shen, L.L.; Peng, L.H.; Fu, R.F.; Liu, Z.C.; Jiang, X.C.; Wang, D.X.; Kamali, A.R.; Shi, Z.N. Synthesis of flower-like MnO2 nanostructure with freshly prepared Cu particles and electrochemical performance in supercapacitors. PLoS ONE 2022, 17, 16. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, Y.; Qu, H.; Wang, Y.; Wang, L.; Wang, Y.; Yang, D.; Ding, B.; Sun, Y.; Guo, J.; Dai, S. Facile Synthesis of Ag-Doped Urchin-like MnO2 on Carbon Cloth for Supercapacitors. Materials 2024, 17, 1312. https://doi.org/10.3390/ma17061312
Feng Y, Qu H, Wang Y, Wang L, Wang Y, Yang D, Ding B, Sun Y, Guo J, Dai S. Facile Synthesis of Ag-Doped Urchin-like MnO2 on Carbon Cloth for Supercapacitors. Materials. 2024; 17(6):1312. https://doi.org/10.3390/ma17061312
Chicago/Turabian StyleFeng, Yanqiu, Henghui Qu, Yanxiang Wang, Lanzhong Wang, Yongbo Wang, Deli Yang, Bohan Ding, Yue Sun, Jinghe Guo, and Shichao Dai. 2024. "Facile Synthesis of Ag-Doped Urchin-like MnO2 on Carbon Cloth for Supercapacitors" Materials 17, no. 6: 1312. https://doi.org/10.3390/ma17061312




