High-Temperature Reactive Wetting of Natural Quartz by Liquid Magnesium
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
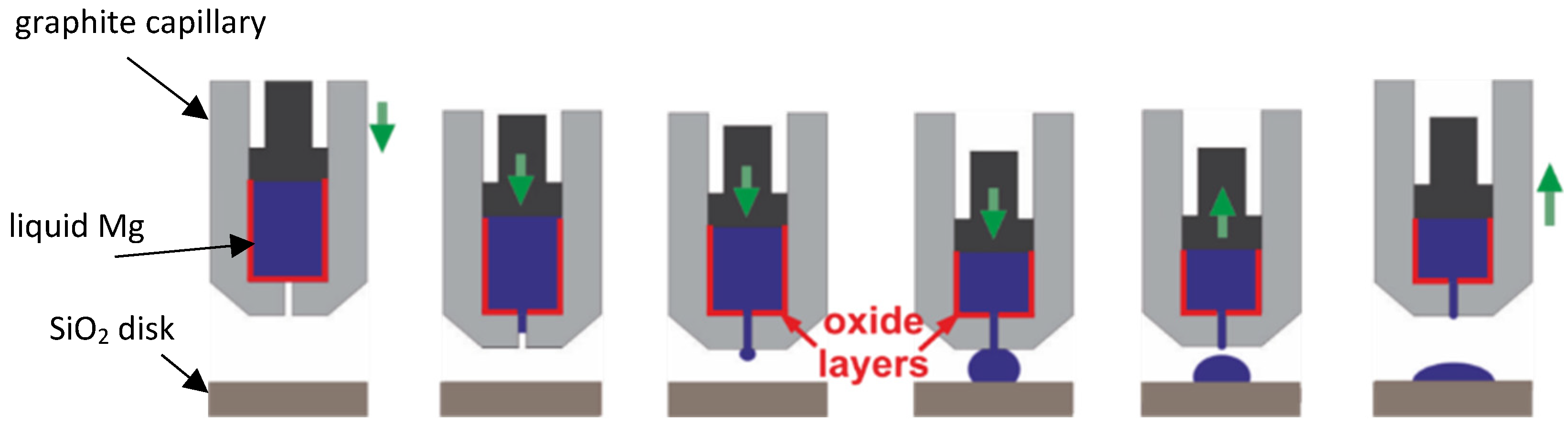
2.2. Procedure
2.3. Product Characterization
3. Results and Discussion
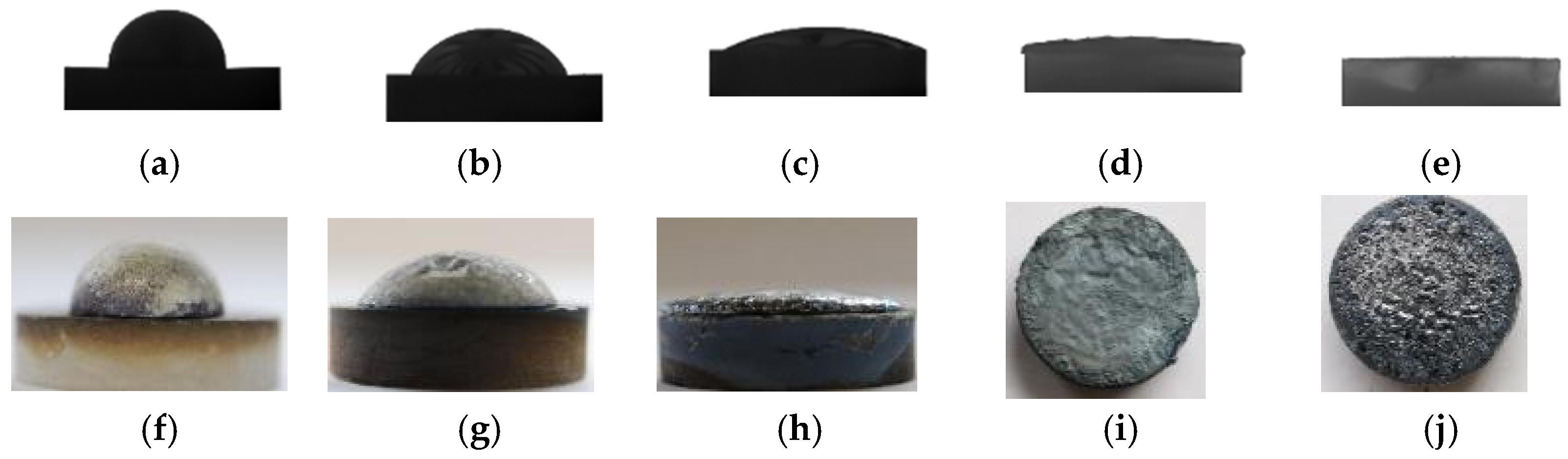
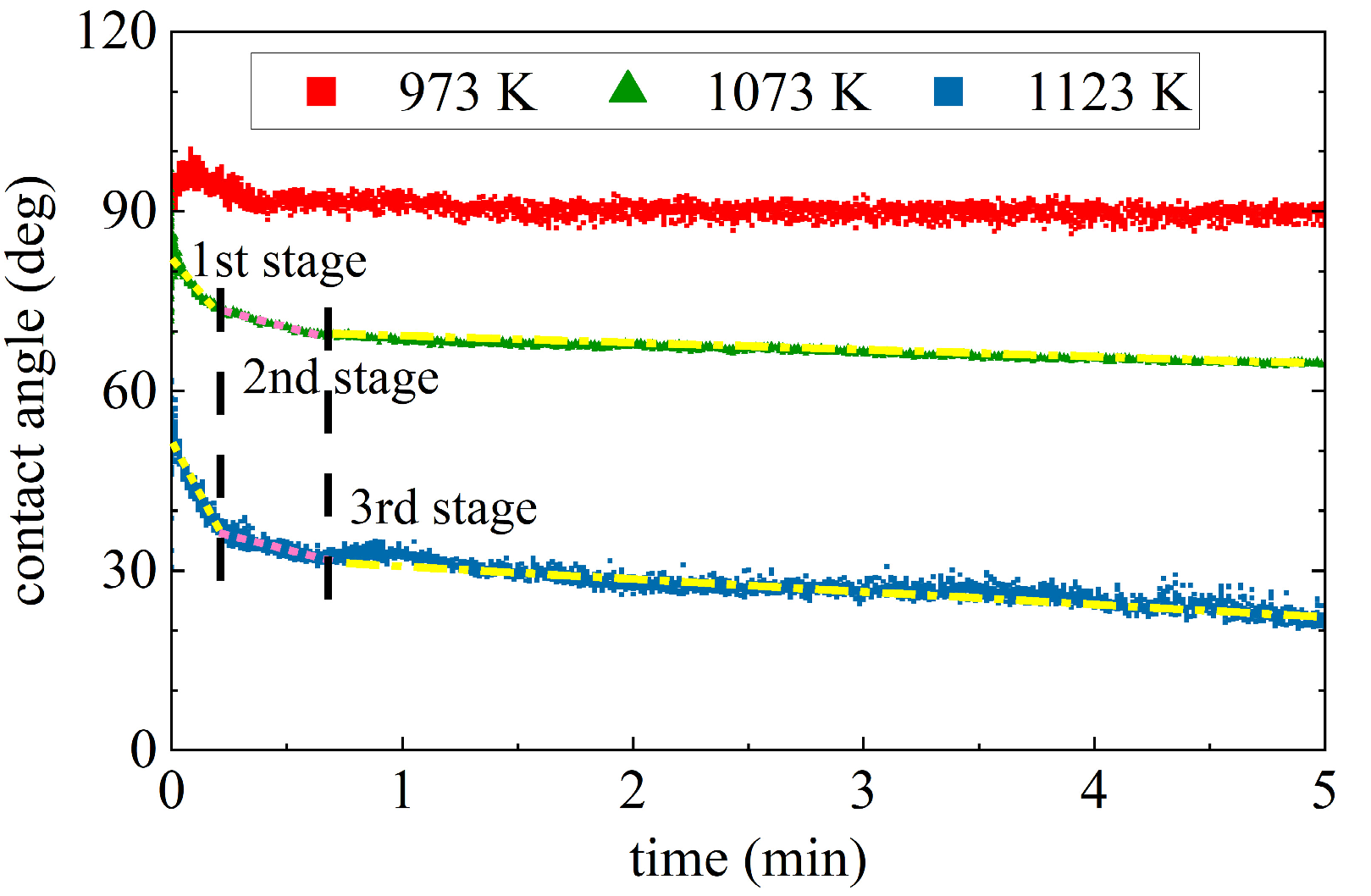
3.1. Wetting Behavior
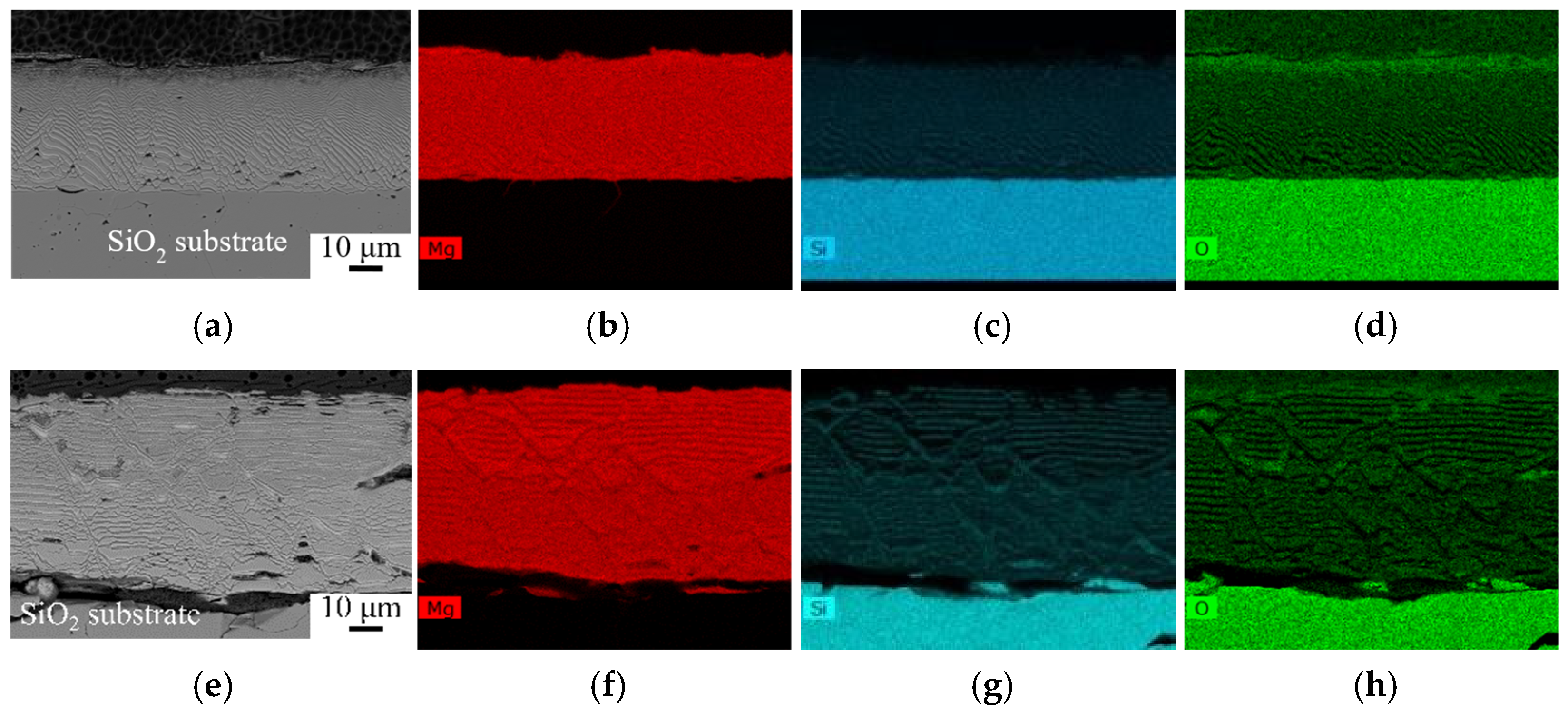
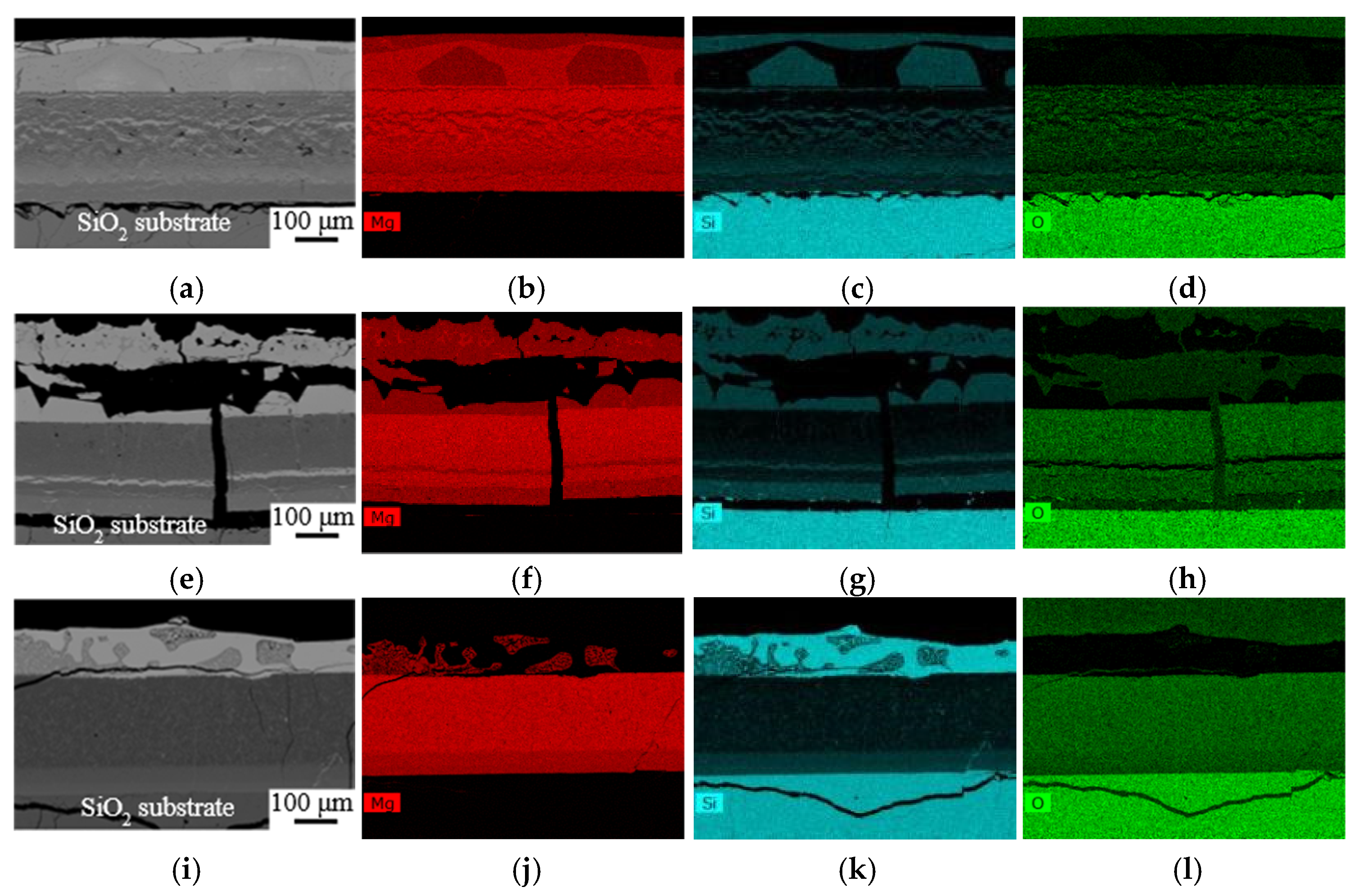
3.2. Microstructure Evaluation of the SiO2/Mg Interface
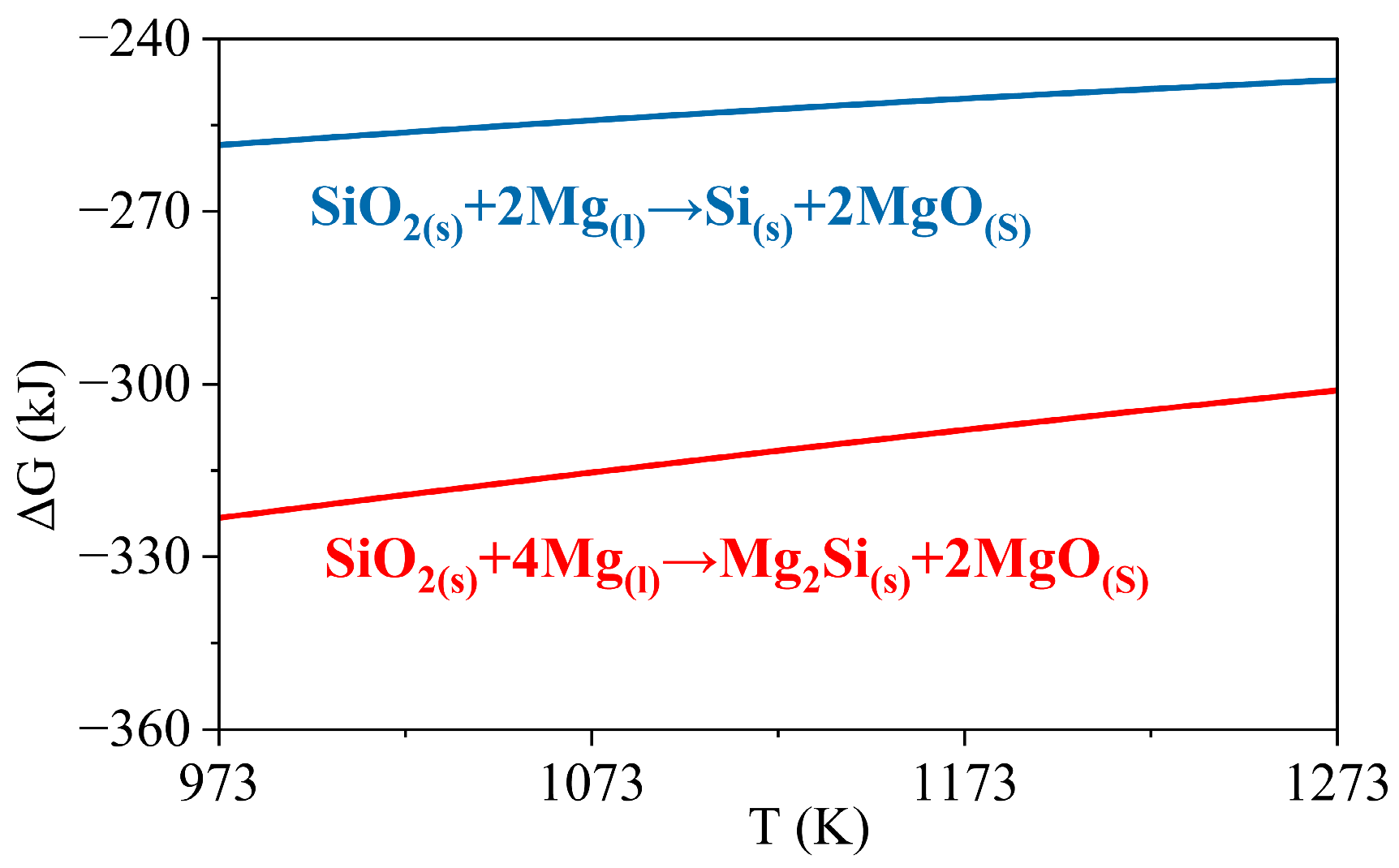
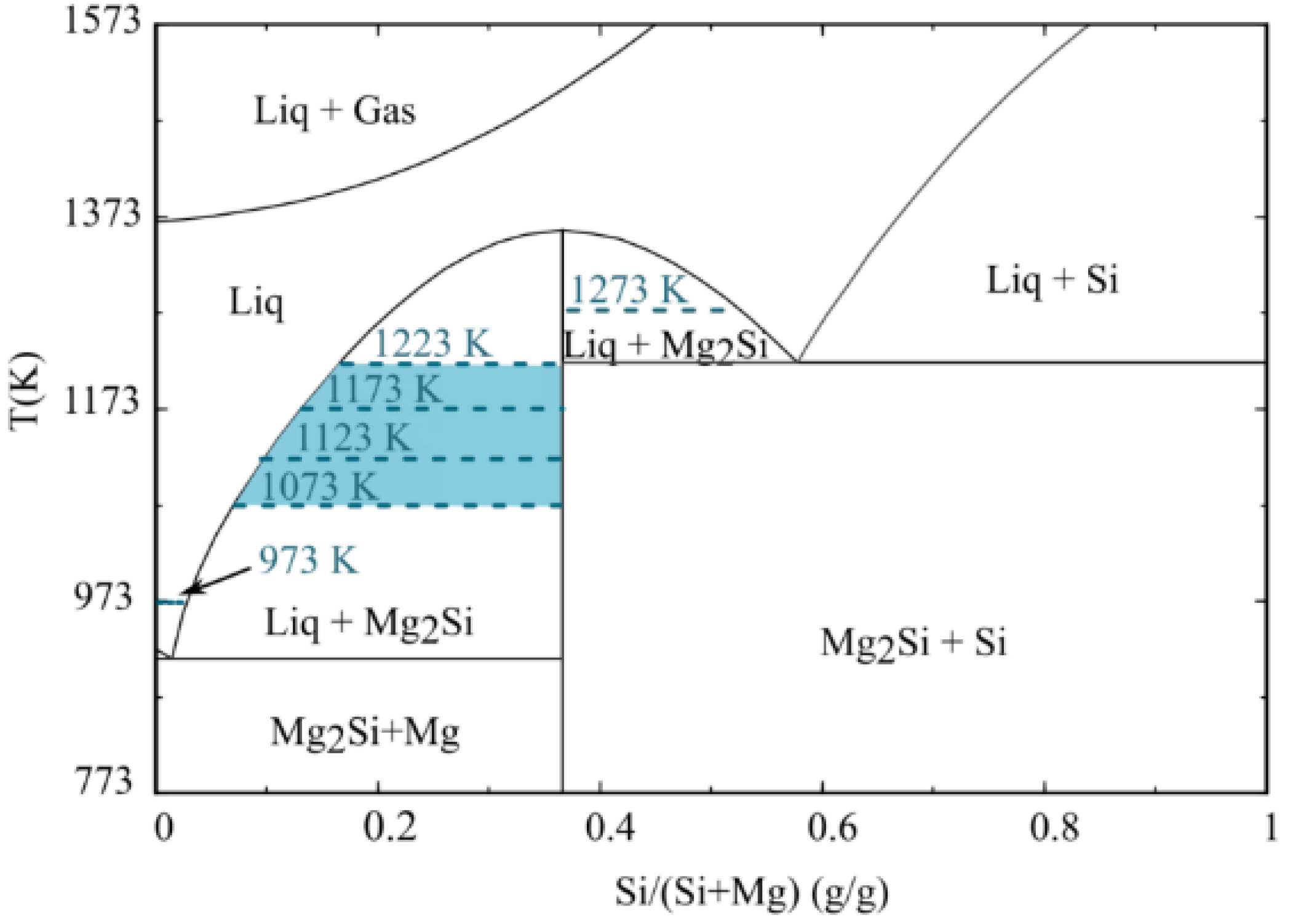
3.3. Wetting Mechanism
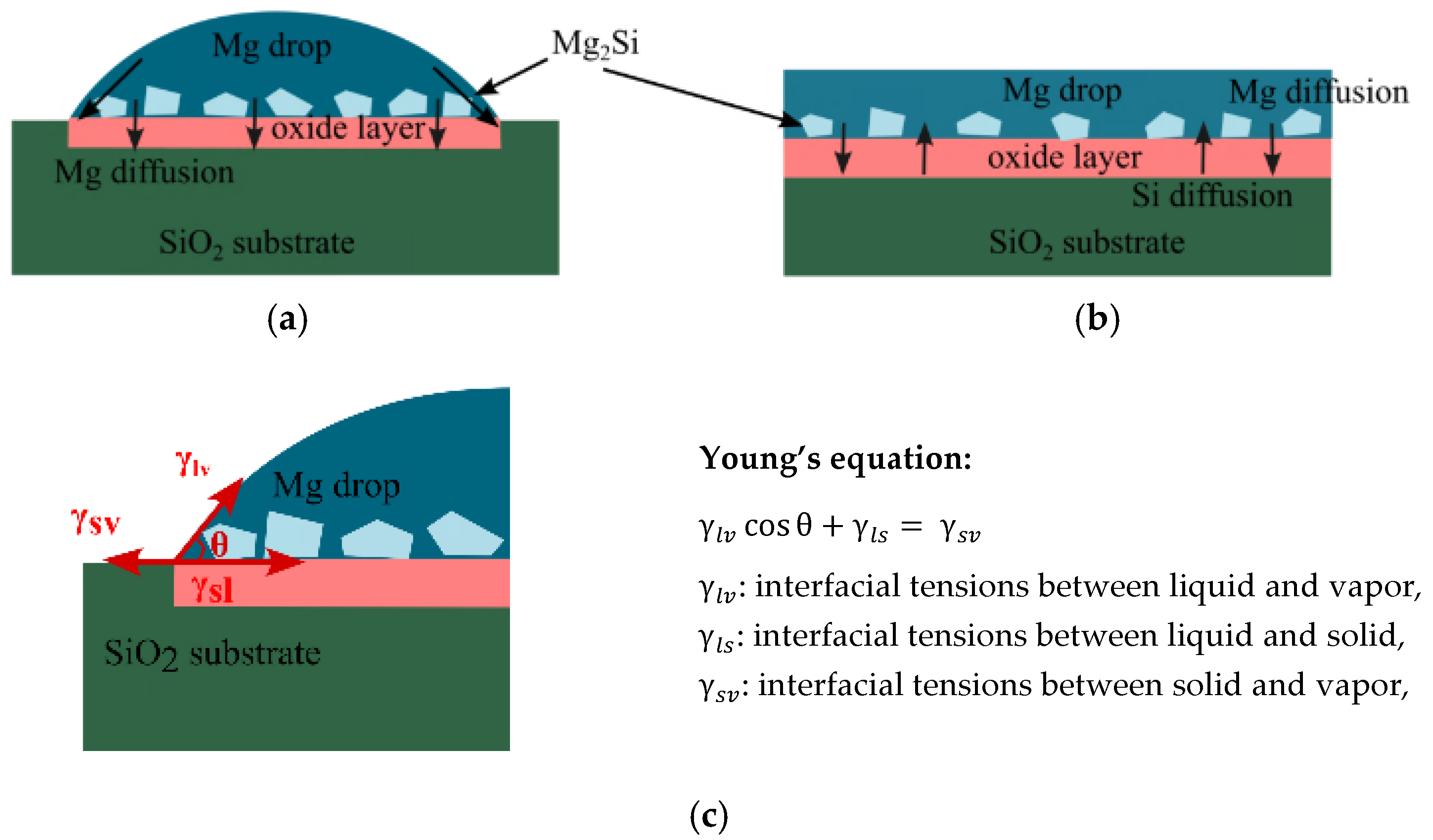
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tranell, G.; Wallin, M.; Safarian, J. SisAl-A New Process for Production of Silicon. In Proceedings of the Silicon for the Chemical and Solar Industry XV, Trondheim, Norway, 15–18 June 2020. [Google Scholar]
- Barati, M.; Sarder, S.; Mclean, A.; Roy, R. Recovery of Silicon from Silica Fume. J. Non-Cryst. Solids 2011, 357, 18–23. [Google Scholar] [CrossRef]
- Larbi, K.K.; Roy, R.; Barati, M.; Lakshmanan, V.I.; Sridhar, R.; McLean, A. Use of Rice Husk for Emission Neutral Energy Generation and Synthesis of Solar-Grade Silicon Feedstock. Biomass Convers. Biorefinery 2012, 2, 149–157. [Google Scholar] [CrossRef]
- Banerjee, H.D.; Sen, S.; Acharya, H.N. Investigations on the Production of Silicon from Rice Husks by the Magnesium Method. Mater. Sci. Eng. 1982, 52, 173–179. [Google Scholar] [CrossRef]
- Sandhage, K.H.; Dickerson, M.B.; Huseman, P.M.; Caranna, M.A.; Clifton, J.D.; Bull, T.A.; Heibel, T.J.; Overton, W.R.; Schoenwaelder, M.E.A. Novel, Bioclastic Route to Self-Assembled, 3D, Chemically Tailored Meso/Nanostructures: Shape-Preserving Reactive Conversion of Biosilica (Diatom) Microshells. Adv. Mater. 2002, 14, 429–433. [Google Scholar] [CrossRef]
- Cai, Y.; Allan, S.M.; Sandhage, K.H.; Zalar, F.M. Three-Dimensional Magnesia-Based Nanocrystal Assemblies via Low- Temperature Magnesiothermic Reaction of Diatom Microshells. J. Am. Ceram. Soc. 2005, 88, 2005–2010. [Google Scholar] [CrossRef]
- Bao, Z.; Weatherspoon, M.R.; Shian, S.; Cai, Y.; Graham, P.D.; Allan, S.M.; Ahmad, G.; Dickerson, M.B.; Church, B.C.; Kang, Z.; et al. Chemical Reduction of Three-Dimensional Silica Micro-Assemblies into Microporous Silicon Replicas. Nature 2007, 446, 172–175. [Google Scholar] [CrossRef]
- Liu, N.; Huo, K.; McDowell, M.T.; Zhao, J.; Cui, Y. Rice Husks as a Sustainable Source of Nanostructured Silicon for High Performance Li-Ion Battery Anodes. Sci. Rep. 2013, 3, 1919. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, J. High-Performance Silicon-Carbon Anode Material via Aerosol Spray Drying and Magnesiothermic Reduction. Nano Energy 2019, 63, 103845. [Google Scholar] [CrossRef]
- Wynnyckyj, J.R.; Rao, D.B. The Mechanism of Reduction of Silica by Magnesium Vapor. High Temp. Sci. 1976, 8, 203–217. [Google Scholar]
- Shi, L.; Shen, P.; Zhang, D.; Jiang, Q. Reactive Wetting of Amorphous Silica by Molten Al-Mg Alloys and Their Interfacial Structures. Appl. Surf. Sci. 2016, 377, 340–348. [Google Scholar] [CrossRef]
- Shi, L.; Shen, P.; Zhang, D.; Dong, E.; Jiang, Q. Reactive Wetting in Liquid Magnesium/Silica and Magnesium/Silicon Systems. Appl. Surf. Sci. 2013, 274, 124–130. [Google Scholar] [CrossRef]
- Yuan, Z.; Huang, W.L.; Mukai, K. Wettability and Reactivity of Molten Silicon with Various Substrates. Appl. Phys. A 2004, 78, 617–622. [Google Scholar] [CrossRef]
- Shen, P.; Fujii, H.; Matsumoto, T.; Nogi, K. Wetting and Reaction of MgO Single Crystals by Molten Al at 1073–1473 K. Acta Mater. 2004, 52, 887–898. [Google Scholar] [CrossRef]
- Iyer, H.; Tafaghodi Khajavi, L.; Durlik, D.; Danaei, K.; Barati, M. Wettability of Al2O3, MgO, and TiB2 Inclusions with Liquid Silicon. Silicon 2018, 10, 2219–2226. [Google Scholar] [CrossRef]
- Fujii, H.; Yamamoto, M.; Hara, S.; Nogi, K. Effect of Gas Evolution at Solid-Liquid Interface on Contact Angle between Liquid Si and SiO2. J. Mater. Sci. 1999, 34, 3165–3168. [Google Scholar] [CrossRef]
- Li, J.G.; Hausner, H. Wetting and Adhesion in Liquid Silicon/Ceramic Systems. Mater. Lett. 1992, 14, 329–332. [Google Scholar] [CrossRef]
- Marumo, C.; Pask, J.A. Reactions and Wetting Behaviour in the Aluminium-Fused Silica System. J. Mater. Sci. 1977, 12, 223–233. [Google Scholar] [CrossRef]
- Shen, P.; Fujii, H.; Matsumoto, T.; Nogi, I. Reactive Wetting of SiO2 Substrates by Molten Al. Metall. Mater. Trans. A 2004, 35A, 583–588. [Google Scholar] [CrossRef]
- Zhou, X.B.; De Hosson, J.T.M. De Reactive Wetting of Liquid Metals on Ceramic Substrates. Acta Mater. 1996, 44, 421–426. [Google Scholar] [CrossRef]
- Laurent, V.; Chatain, D.; Eustathopoulos, N. Wettability of SiO2 and Oxidized SiC by Aluminium. Mater. Sci. Eng. A 1991, 135, 89–94. [Google Scholar] [CrossRef]
- Aksay, I.A.; Hoge, C.E.; Pask, J.A. Wetting under Chemical Equilibrium and Nonequilibrium Conditions. J. Phys. Chem. 1974, 78, 1178–1183. [Google Scholar] [CrossRef]
- Rasouli, A.; Herstad, K.E.; Safarian, J.; Tranell, G. Magnesiothermic Reduction of Natural Quartz. Metall. Mater. Trans. B 2022, 53, 2132–2142. [Google Scholar] [CrossRef]
- Rasouli, A.; Tsoutsouva, M.; Safarian, J.; Tranell, G. Kinetics of Magnesiothermic Reduction of Natural Quartz. Materials 2022, 15, 6535. [Google Scholar] [CrossRef] [PubMed]
- Jusnes, K.F. Phase Transformations and Thermal Degradation in Industrial Quartz. Doctoral Dissertation, Norwegian University of Science and Tehnology, Trondheim, Norway, 2020. [Google Scholar]
- Kudyba, A.; Sobczak, N.; Budzioch, J.; Polkowski, W.; Giuranno, D. Improvements in Experimental Investigation of Molten Mg-Based Materials. Mater. Des. 2018, 160, 915–917. [Google Scholar] [CrossRef]
- Kudyba, A.; Sobczak, N.; Polkowski, W.; Bruzda, G.; Polkowska, A.; Giuranno, D. Improved Methodological Concepts for Processing Liquid Mg at High Temperature. J. Magnes. Alloys 2021, 9, 183–191. [Google Scholar] [CrossRef]
- Eustathopoulos, N.; Sobczak, N.; Passerone, A.; Nogi, K. Measurement of Contact Angle and Work of Adhesion at High Temperature. J. Mater. Sci. 2005, 40, 2271–2280. [Google Scholar] [CrossRef]
- Kudyba, A.; Polkowski, W.; Bruzda, G.; Polkowska, A.; Giuranno, D. Enhancement of Wettability in AZ91/B4C System by a Mechanical Purification of Liquid Alloy. J. Magnes. Alloys 2022, 10, 3133–3142. [Google Scholar] [CrossRef]
- Sobczak, N.; Singh, M.; Asthana, R. High-Temperature Wettability Measurements in Metal/Ceramic Systems—Some Methodological Issues. Curr. Opin. Solid State Mater. Sci. 2005, 9, 241–253. [Google Scholar] [CrossRef]
- Liggieri, L.; Passerone, A. ASTRA Reference Book, IENI Report; National Research Council of Italy: Genova, Italy, 2007. [Google Scholar]
- FactSage. Available online: https://www.factsage.com (accessed on 15 May 2023).
- Gutman, I.; Klinger, L.; Gotman, I.; Shapiro, M. Experimental Observation of Periodic Structure Formation in SiO2 ± Mg System. Scr. Mater. 2001, 45, 363–367. [Google Scholar] [CrossRef]
- Gutman, I.; Gotman, I.; Shapiro, M. Kinetics and Mechanism of Periodic Structure Formation at SiO2/Mg Interface. Acta Mater. 2006, 54, 4677–4684. [Google Scholar] [CrossRef]
- Gutman, I.; Klinger, L.; Gotman, I.; Shapiro, M. Model for Evolution of Periodic Layered Structure in the SiO2/Mg System. Solid State Ion. 2009, 180, 1350–1355. [Google Scholar] [CrossRef]
- Chen, Y.C.; Xu, J.; Fan, X.H.; Zhang, X.F.; Han, L.; Lin, D.Y.; Li, Q.H.; Uher, C. The Mechanism of Periodic Layer Formation during Solid-State Reaction between Mg and SiO2. Intermetallics 2009, 17, 920–926. [Google Scholar] [CrossRef]
- Guo, X.L.; Liu, Z.G.; Chen, X.Y.; Zhu, S.N.; Xiong, S.B.; Hu, W.S.; Lin, C.Y. Pulsed Laser Deposition of Pulsed Laser Deposition of SrxBa1−x Nb2O6/MgO Bilayered Films on Si Wafer in Waveguide Form. J. Phys. D Appl. Phys. 1996, 29, 1632–1635. [Google Scholar] [CrossRef]
- Dezellus, O.; Eustathopoulos, N. Fundamental Issues of Reactive Wetting by Liquid Metals. J. Mater. Sci. 2010, 45, 4256–4264. [Google Scholar] [CrossRef]
- Gale, W.F.; Totemeier, T.C. (Eds.) Chapter 14. General Physical Properties. In Smithells Metals Reference Book; Butterworth-Heinemann: Oxford, UK, 2004; ISBN 9780750675093. [Google Scholar]
- Chase, M.W. Journal of Physical and Chemical Reference Data: No. 9 Pt. 2: Monograph NIST-JANAF Thermochemical Tables Cr-Zr: Vol. No. 9 Pt. 2, 4th ed.; American Chemical Society and the American Institute of Physics for the National Institute of Standards and Technology: New York, NY, USA, 1998. [Google Scholar]
- Young, T., III. An Essay on the Cohesion of Fluids. Philos. Trans. R. Soc. Lond. 1805, 95, 171–172. [Google Scholar] [CrossRef]


| Temperature (K) | 973 | 1073 | 1123 | 1173 | 1223 | 1273 |
|---|---|---|---|---|---|---|
| Vapor pressure (atm) | 9.4 × 10−3 | 4.3 × 10−2 | 8.4 × 10−2 | 1.5 × 10−1 | 2.6 × 10−1 | 4.32 × 10−1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rasouli, A.; Kudyba, A.; Bruzda, G.; Safarian, J.; Tranell, G. High-Temperature Reactive Wetting of Natural Quartz by Liquid Magnesium. Materials 2024, 17, 1302. https://doi.org/10.3390/ma17061302
Rasouli A, Kudyba A, Bruzda G, Safarian J, Tranell G. High-Temperature Reactive Wetting of Natural Quartz by Liquid Magnesium. Materials. 2024; 17(6):1302. https://doi.org/10.3390/ma17061302
Chicago/Turabian StyleRasouli, Azam, Artur Kudyba, Grzegorz Bruzda, Jafar Safarian, and Gabriella Tranell. 2024. "High-Temperature Reactive Wetting of Natural Quartz by Liquid Magnesium" Materials 17, no. 6: 1302. https://doi.org/10.3390/ma17061302






