Thermal Properties and Drying Shrinkage Performance of Palm Kernel Shell Ash and Rice Husk Ash-Based Geopolymer Concrete
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
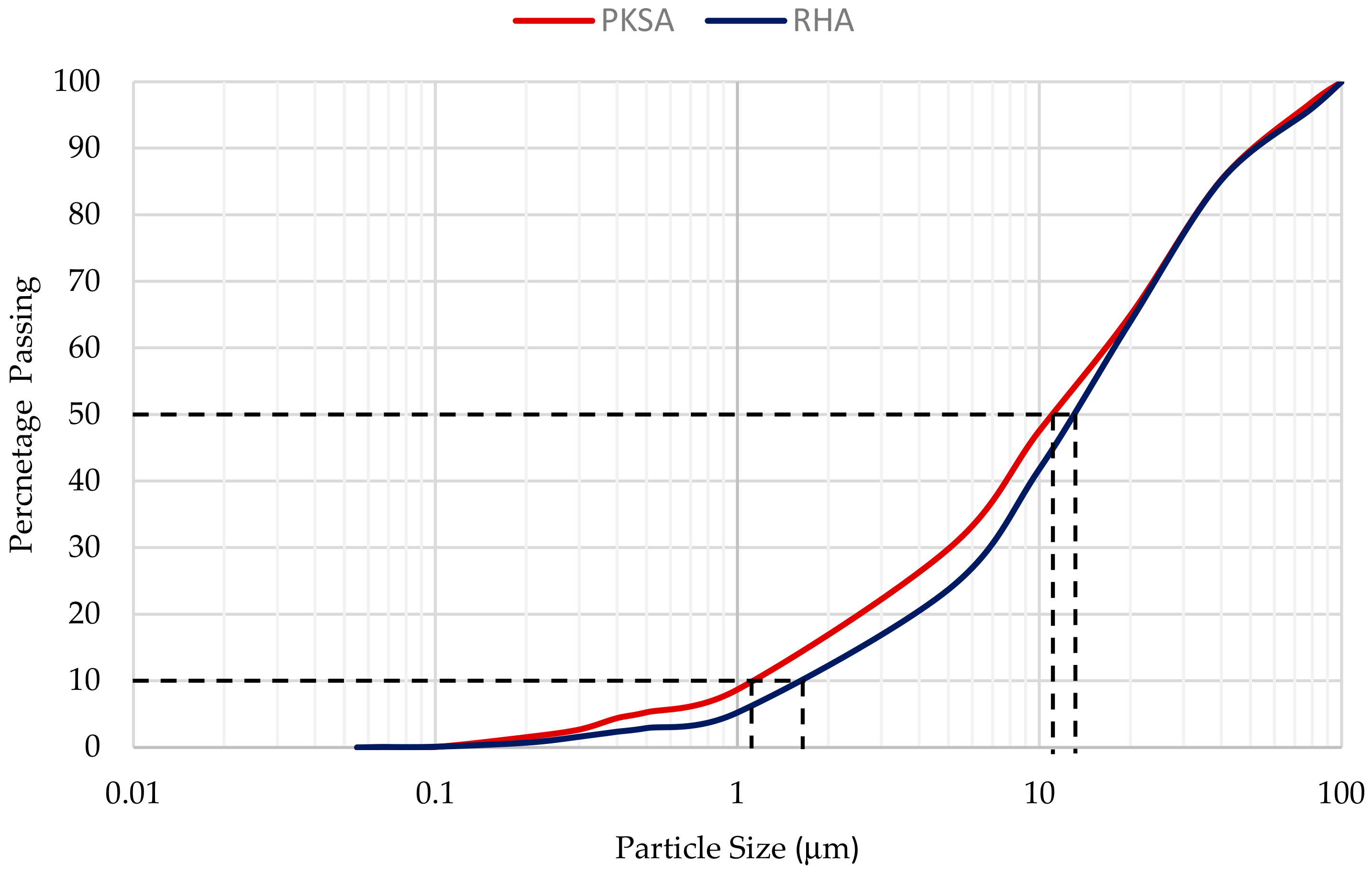
2.2. Ash Preparation
2.3. Geopolymer Concrete Preparation
2.4. Design of Experiments
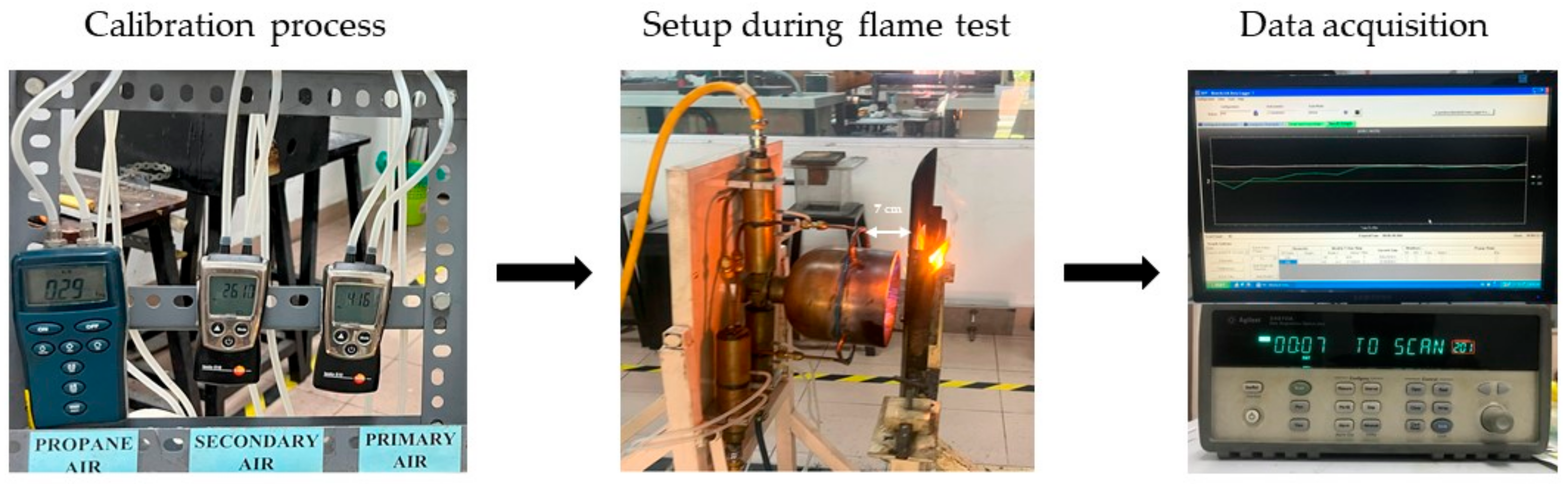
2.5. Direct Flame Test
2.6. Thermogravimetric Analysis (TGA)
2.7. Shrinkage Test
- shrinkage length;
- initial length;
- length at the given time.
3. Results and Discussions
3.1. Geopolymer Concrete Preliminary Design Characteristic
3.2. Thermal Performance of Geopolymer Concrete
3.2.1. Physical Changes Due to Elevated Temperature
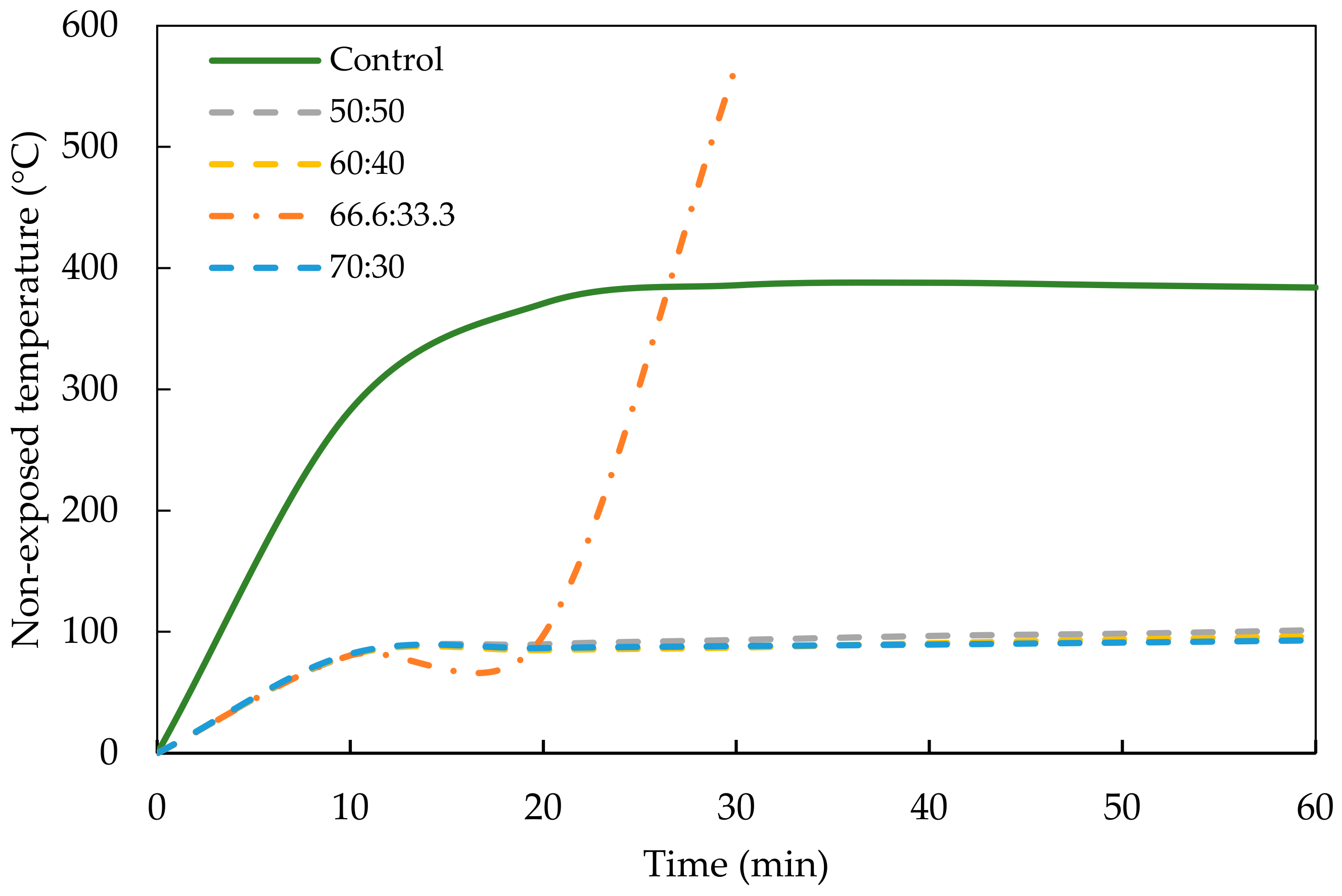
3.2.2. Fire Resistance Performance of Geopolymer Concrete
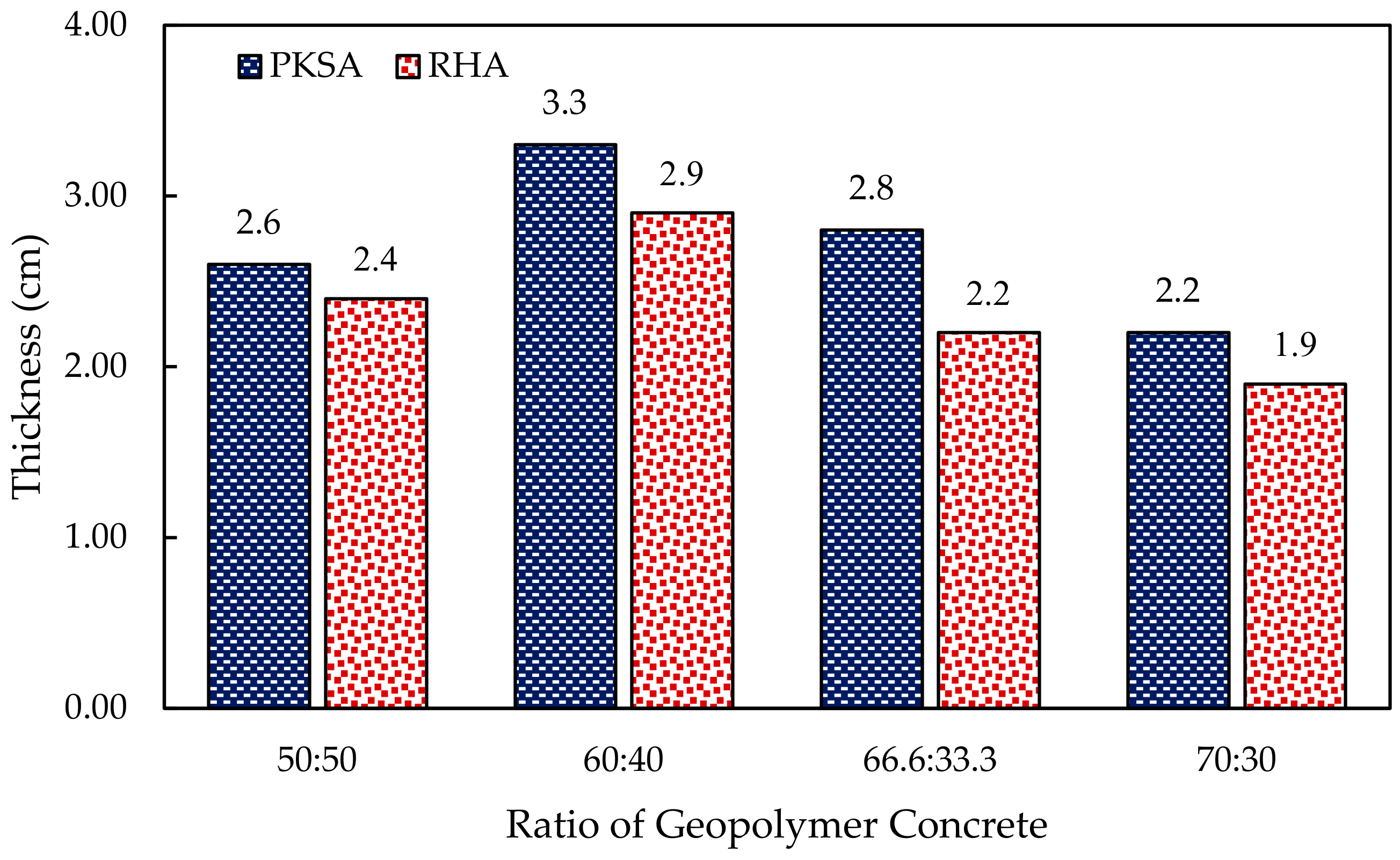
3.2.3. Thermal Expansion of the Geopolymer Concrete
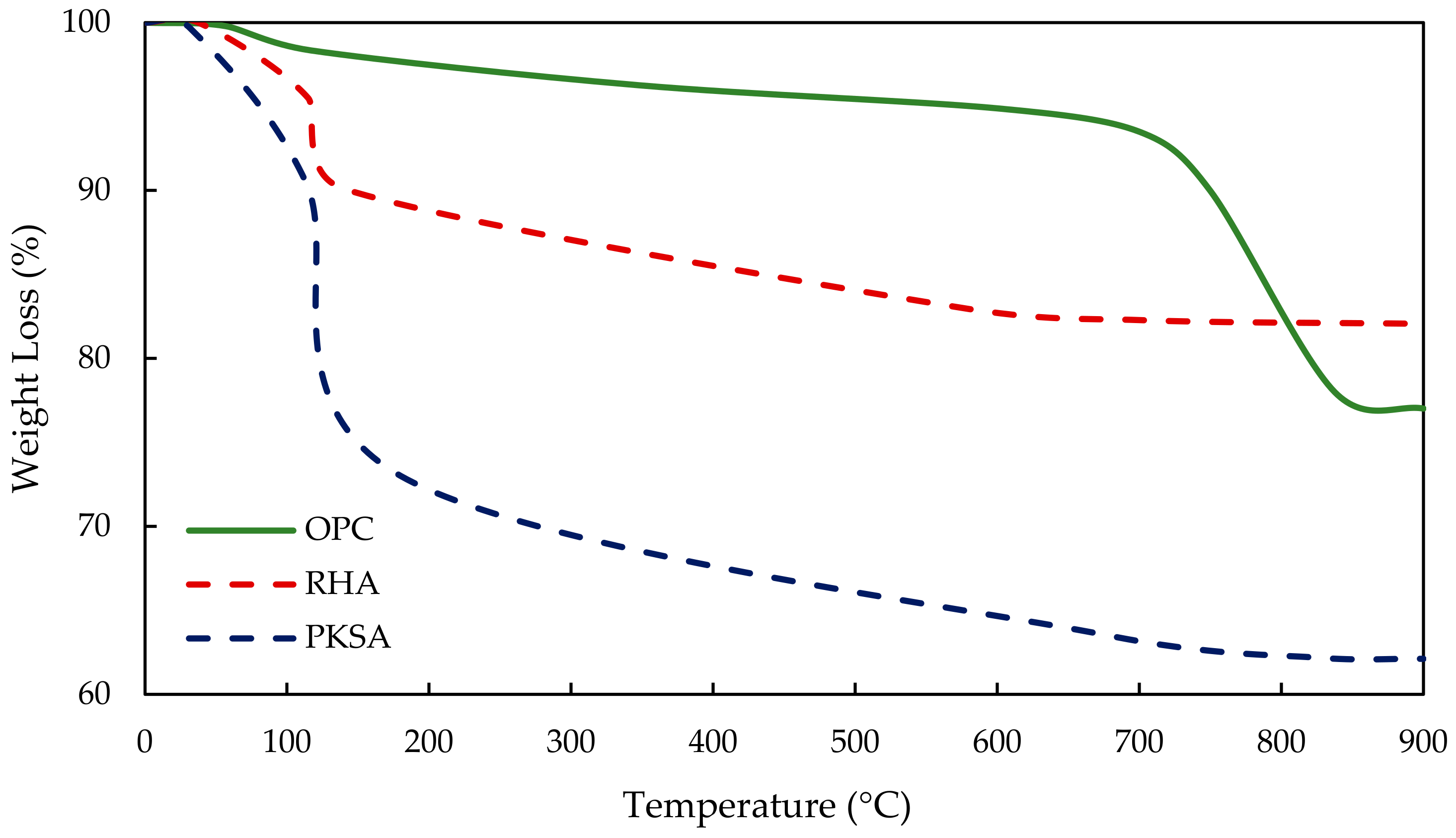
3.2.4. Thermal Stability of the Geopolymer Concrete and OPCC
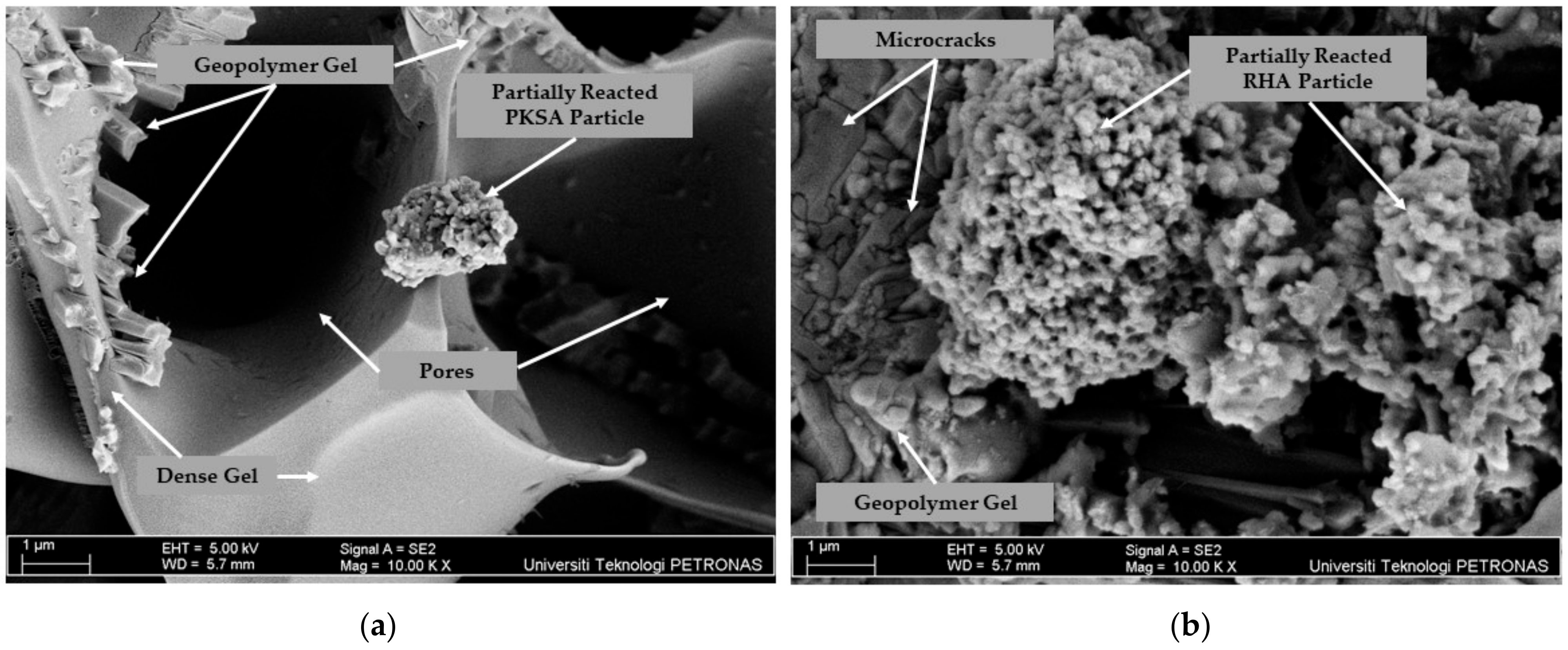
3.3. GPC Microstructure Analysis
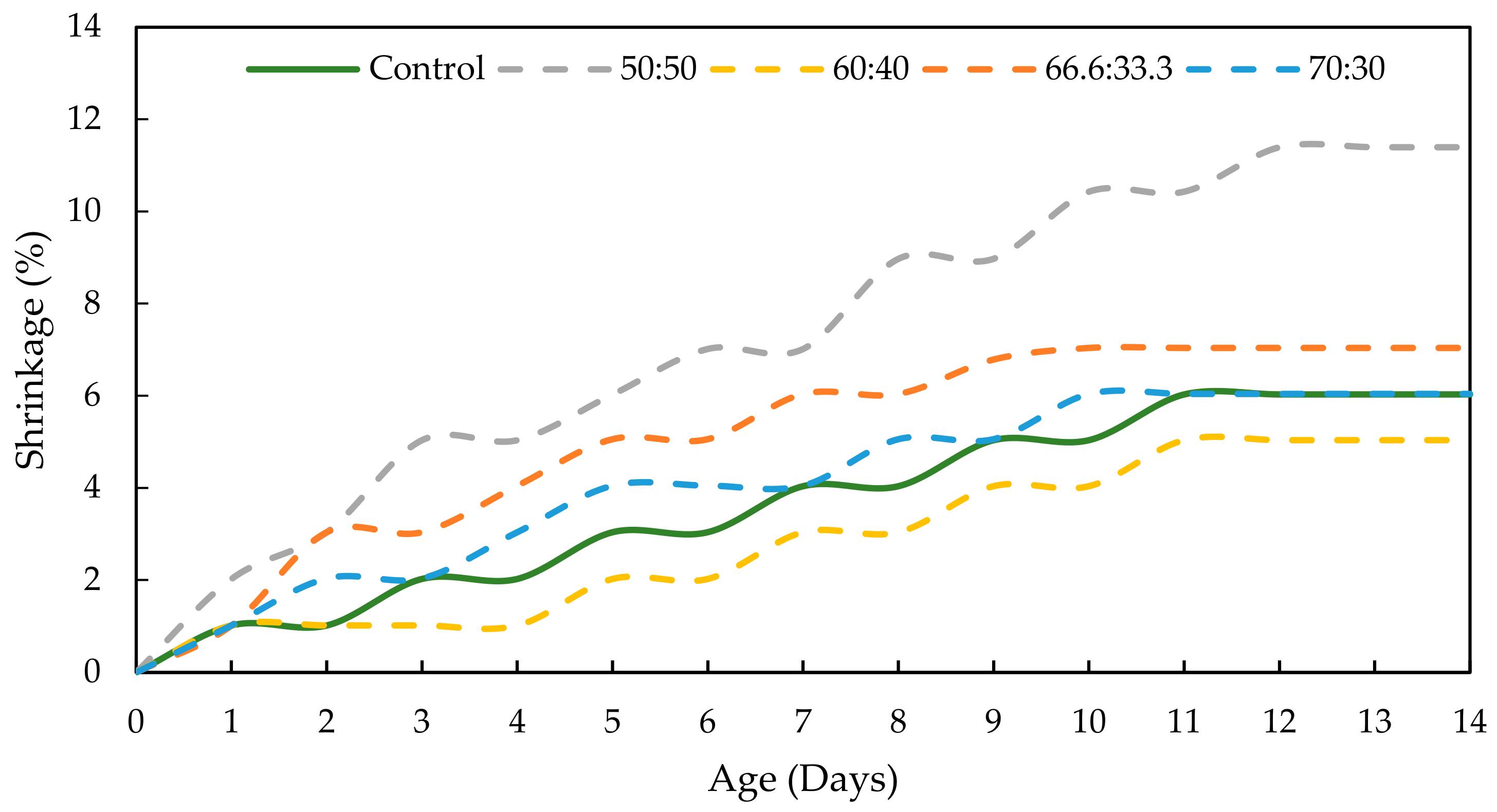
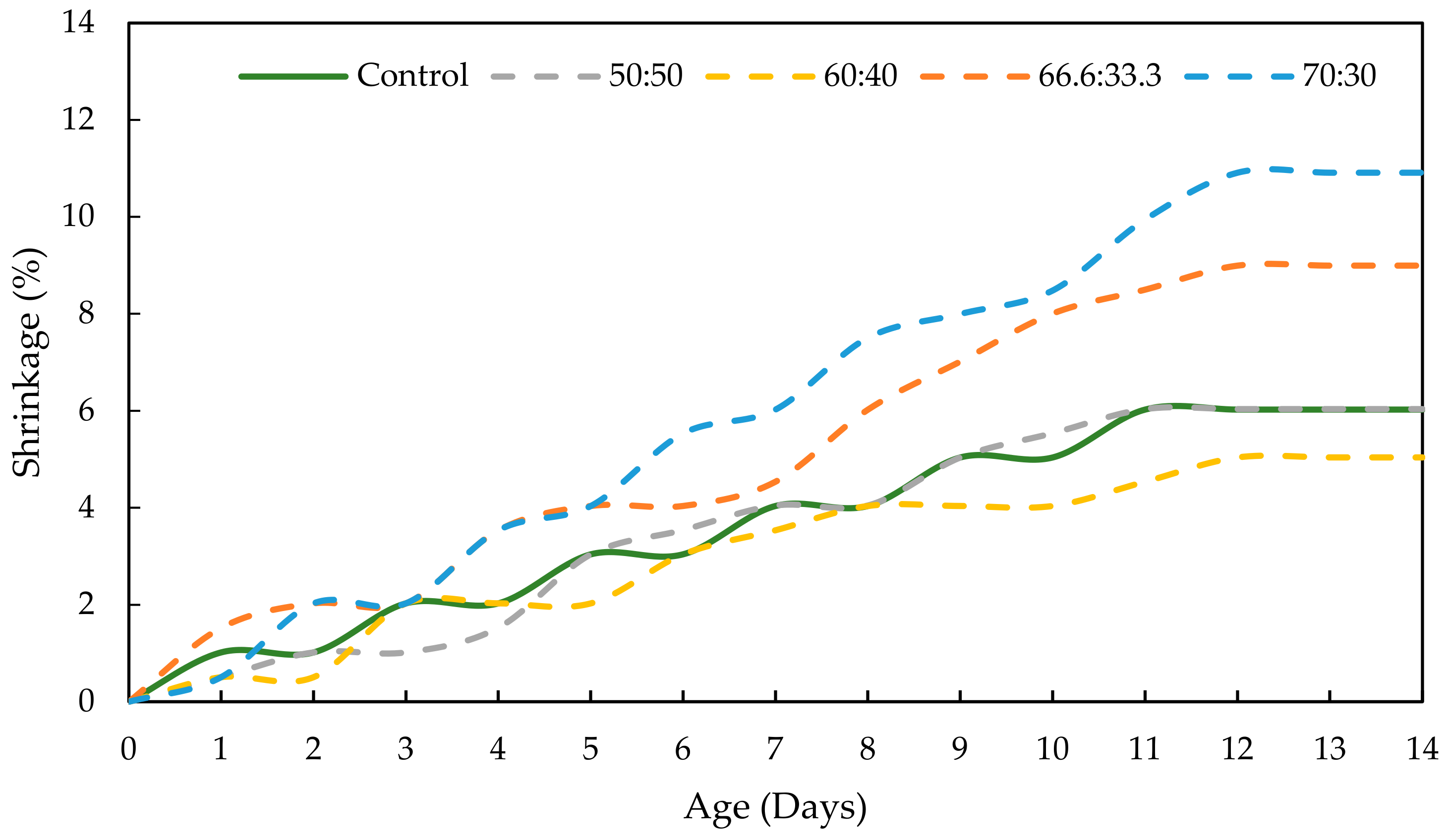
3.4. Drying Shrinkage of GPC
4. Conclusions
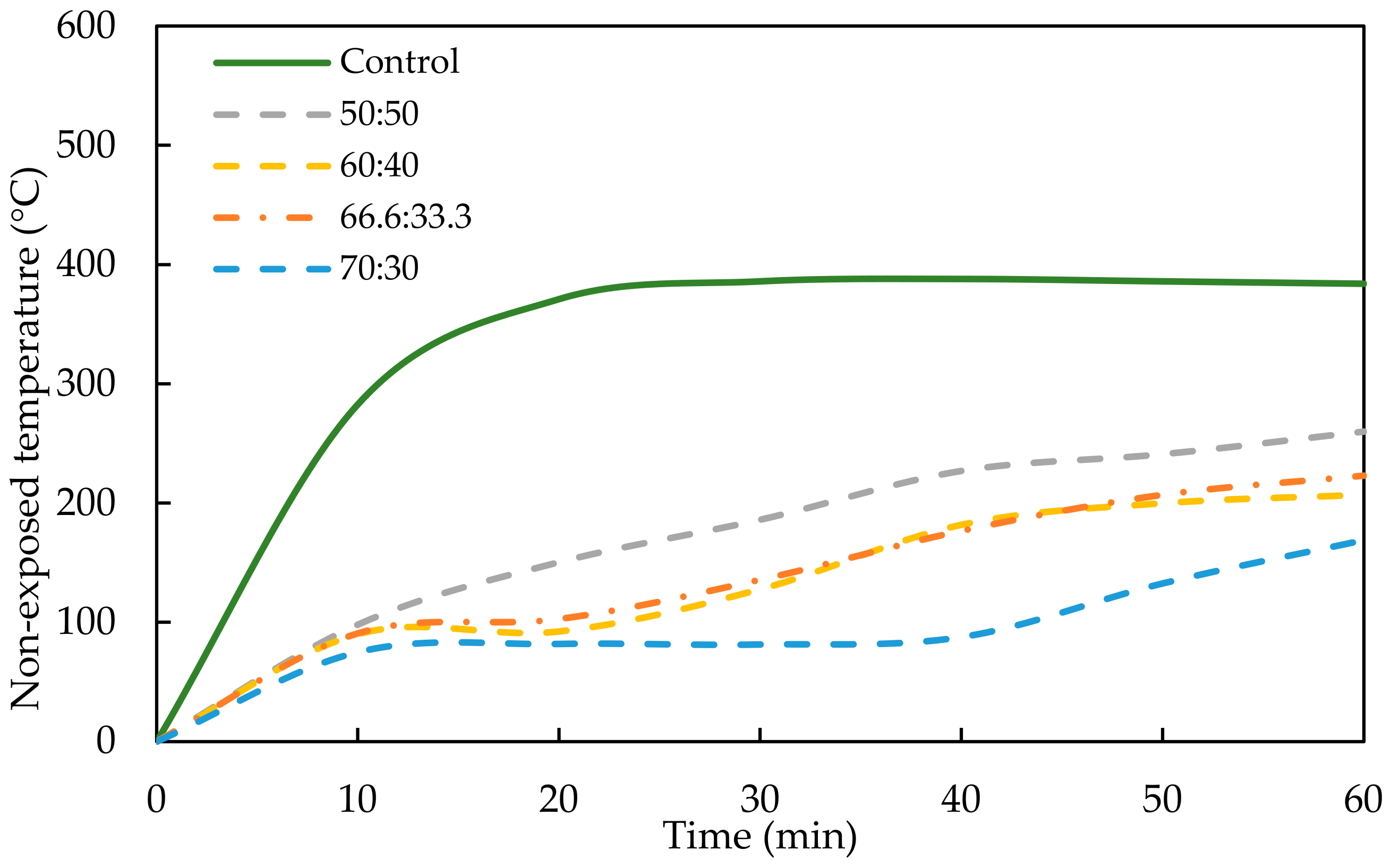
- It was found that PKSA-GPC had better fire performance than the RHA-GPC and OPCC. The OPCC showed a rapid temperature increment when exposed to the high flame temperature, which increased from ambient temperature to 350 °C within 60 min of the flame exposure. In contrast, the highest temperature increment for the PKSA-GPC (PKSA-GPC 50:50) was 169 °C after being exposed to the high flame temperature for 60 min.
- Although the RHA-GPC possessed lower temperature increments when exposed to the high temperature from ambient temperature to 93 °C, the RHA-GPC formed holes due to the collapse of the intumescent layer, indicating that the intumescent layer was weaker than the PKSA-GPC. This was verified in the microstructural analysis, which showed that the PKSA-GPC has a dense and compact intumescent layer compared to the RHA-GPC.
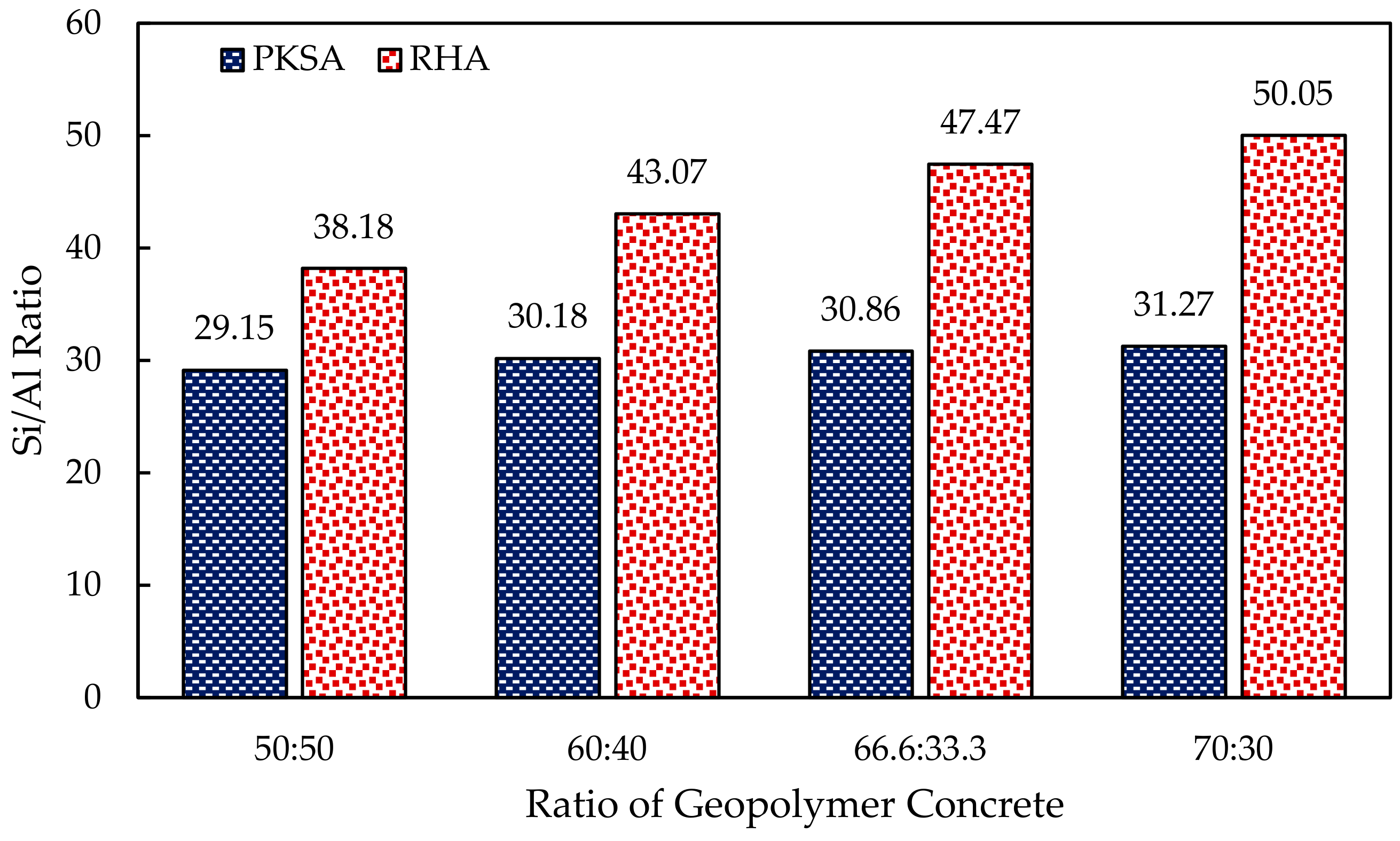
- The molar ratio of the Si/Al played an important role in the formation of the intumescent layer as lower molar Si/Al contributed to a rigid three-dimensional polymer network, which caused a denser and more compact intumescent layer.
- Moreover, both the mix designs of RHA-GPC 60:40 and PKSA-GPC 60:40 showed better drying shrinkage performance with a drying shrinkage of 5.040% compared to the OPCC, which experienced 8.996% drying shrinkage.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehta, P.K. High-performance, high-volume fly ash concrete for sustainable development. In Proceedings of the International Workshop on Sustainable Development and Concrete Technology, Beijing, China, 20–21 May 2004; Iowa State University: Ames, IA, USA, 2004; pp. 3–14. [Google Scholar]
- Wałach, D. Analysis of factors affecting the environmental impact of concrete structures. Sustainability 2020, 13, 204. [Google Scholar] [CrossRef]
- Kim, T.H.; Chae, C.U. Environmental impact analysis of acidification and eutrophication due to emissions from the production of concrete. Sustainability 2016, 8, 578. [Google Scholar] [CrossRef]
- Teh, S.H.; Wiedmann, T.; Castel, A.; de Burgh, J. Hybrid life cycle assessment of greenhouse gas emissions from cement, concrete and geopolymer concrete in Australia. J. Clean. Prod. 2017, 152, 312–320. [Google Scholar] [CrossRef]
- Abdul Rashid, M.K.; Ramli Sulong, N.H.; Alengaram, U.J. Fire resistance performance of composite coating with geopolymer-based bio-fillers for lightweight panel application. J. Appl. Polym. Sci. 2020, 137, 49558. [Google Scholar] [CrossRef]
- Suhendro, B. Toward green concrete for better sustainable environment. Procedia Eng. 2014, 95, 305–320. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.A.; de Gutiérrez, R.M.; Sulekar, S.; Davis, C.; Nino, J.C. Thermal properties of novel binary geopolymers based on metakaolin and alternative silica sources. Appl. Clay Sci. 2015, 118, 276–282. [Google Scholar] [CrossRef]
- So, H.S. Spalling prevention of high performance concrete at high temperatures. In High Performance Concrete Technology and Applications; IntechOpen: London, UK, 2016. [Google Scholar]
- Chen, K.; Wu, D.; Xia, L.; Cai, Q.; Zhang, Z. Geopolymer concrete durability subjected to aggressive environments–A review of influence factors and comparison with ordinary Portland cement. Constr. Build. Mater. 2021, 279, 122496. [Google Scholar] [CrossRef]
- Humad, A.M.; Kothari, A.; Provis, J.L.; Cwirzen, A. The effect of blast furnace slag/fly ash ratio on setting, strength, and shrinkage of alkali-activated pastes and concretes. Front. Mater. Sci. 2019, 6, 9. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- EN 206-1; Concrete-Specification, Performance, Production and Conformity. The Committee for Standarization: Brussels, Belgium, 2016.
- Nikoloutsopoulos, N.; Sotiropoulou, A.; Kakali, G.; Tsivilis, S. Physical and mechanical properties of fly ash based geopolymer concrete compared to conventional concrete. Buildings 2021, 11, 178. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, S.; Feng, W.; Wan, S.; Li, L.; Tang, Y. Flexural and compressive performance of BFRP-reinforced geopolymer sea-sand concrete beams and columns: Experimental and analytical investigation. Compos. Struct. 2023, 318, 117089. [Google Scholar] [CrossRef]
- Neupane, K.; Chalmers, D.; Kidd, P. High-Strength Geopolymer Concrete- Properties, Advantages and Challenges. Adv. Mater. 2018, 7, 15–25. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; García-Lodeiro, I.; Palomo, A. Durability of alkali-activated fly ash cementitious materials. J. Mater. Sci. 2007, 42, 3055–3065. [Google Scholar] [CrossRef]
- Azarsa, P.; Gupta, R. Durability and leach-ability evaluation of K-based geopolymer concrete in real environmental conditions. Case Stud. Constr. 2020, 13, e00366. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, J.; Nong, Y.; Yang, Y.; Zhang, H.; Tang, Y. Beyond time: Enhancing corrosion resistance of geopolymer concrete and BFRP bars in seawater. Compos. Struct. 2023, 322, 117439. [Google Scholar] [CrossRef]
- Lee, W.H.; Lin, K.L.; Chang, T.H.; Ding, Y.C.; Cheng, T.W. Sustainable development and performance evaluation of marble-waste-based geopolymer concrete. Polymers 2020, 12, 1924. [Google Scholar] [CrossRef] [PubMed]
- Onoja, E.; Attan, N.; Chandren, S.; Razak, F.I.A.; Keyon, A.S.A.; Mahat, N.A.; Wahab, R.A. Insights into the physicochemical properties of the Malaysian oil palm leaves as an alternative source of industrial materials and bioenergy. Malays. J. Fund. Appl. Sci. 2017, 13, 623–631. [Google Scholar] [CrossRef]
- Saba, N.; Jawaid, M.; Sultan, M.T.H. Thermal properties of oil palm biomass based composites. In Lignocellulosic Fibre and Biomass-Based Composite Materials: Processing, Properties and Applications; Woodhead Publishing: Cambridge, UK, 2017; pp. 95–122. [Google Scholar]
- Adnan, S.H.; Azemi, N.F.N.M.; Osman, M.H.; Jeni, M.L.A.; Ern, P.A.S.; Yassin, N.I.M.; Akasyah, W.M.N. Influence of Palm oil fuel ash (POFA) towards fire resistance performance of brick. J. Adv. Res. Fluid Mech. Therm. Sci. 2019, 64, 316–325. [Google Scholar]
- Hussin, M.W.; Abdullah, K. Properties of palm oil fuel ash cement based aerated concrete panel subjected to different curing regimes. Malays. J. Civ. Eng. 2009, 21, 17–31. [Google Scholar]
- Jong, L.Y.; Teo, D.C.L. Concrete Containing Palm Oil Fuel Ash (POFA) and Oil Palm Shell (OPS) Subjected to Elevated Temperatures. J. Civ. Eng. Sci. Technol. 2014, 5, 13–17. [Google Scholar] [CrossRef]
- Sulaiman, M.A.; Muthusamy, K.; Aris, S.M.; Rasid, M.M.; Paramasivam, R.; Othman, R. Effect of unground oil palm ash as mixing ingredient towards properties of concrete. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 140, p. 012150. [Google Scholar]
- Tam, V.W.; Le, K.N.; Evangelista, A.C.J.; Butera, A.; Tran, C.N.; Teara, A. Effect of fly ash and slag on concrete: Properties and emission analyses. Front. Eng. Manag. 2019, 6, 395–405. [Google Scholar] [CrossRef]
- He, P.; Wang, M.; Fu, S.; Jia, D.; Yan, S.; Yuan, J.; Zhou, Y. Effects of Si/Al ratio on the structure and properties of metakaolin based geopolymer. Ceram. Int. 2016, 42, 14416–14422. [Google Scholar] [CrossRef]
- ASTM E11-20; Standard Specification for Woven Wire Test Sieve Cloth and Test Sieves. ASTM International: Philadelphia, PA, USA, 2020. Available online: www.astm.org (accessed on 27 November 2023).
- Verma, M.; Dev, N. Effect of liquid to binder ratio and curing temperature on the engineering properties of the geopolymer concrete. Silicon 2022, 14, 1743–1757. [Google Scholar] [CrossRef]
- Abdullah, M.N.; Mustapha, M.; Sallih, N.; Ahmad, A.; Mustapha, F.; Dahliyanti, A. Study and use of rice husk ash as a source of aluminosilicate in refractory coating. Materials 2021, 14, 3440. [Google Scholar] [CrossRef]
- Amran, M.; Debbarma, S.; Ozbakkaloglu, T. Fly ash-based eco-friendly geopolymer concrete: A critical review of the long-term durability properties. Constr. Build. Mater. 2021, 270, 121857. [Google Scholar] [CrossRef]
- Gupta, N.; Gupta, A.; Saxena, K.K.; Shukla, A.; Goyal, S.K. Mechanical and durability properties of geopolymer concrete composite at varying superplasticizer dosage. Mater. Today Proc. 2021, 44, 12–16. [Google Scholar] [CrossRef]
- ASTM E119-19; Standard Test Methods for Fire Tests of Building Construction and Materials. ASTM International: West Conshohocken, PA, USA, 2019. Available online: www.astm.org (accessed on 27 November 2023).
- ASTM C426-16; Standard Test Method for Linear Drying Shrinkage of Concrete Masonry Units. ASTM International: West Conshohocken, PA, USA, 2016. Available online: www.astm.org (accessed on 27 November 2023).
- Xu, H.; Van Deventer, J.S.J. The geopolymerisation of alumino-silicate minerals. Int. J. Miner. Process. 2000, 59, 247–266. [Google Scholar] [CrossRef]
- Provis, J.L. Modelling the Formation of Geopolymers Modelling the Formation of Geopolymers. Ph.D. Dissertation, The University of Melbourne, Melbourne, VIC, Australia, March 2006. [Google Scholar]
- Bosenick, A.; Dove, M.T.; Myers, E.R.; Palin, E.J.; Sainz-Diaz, C.I.; Guiton, B.S.; Redfern, S.A.T. Computational methods for the study of energies of cation distributions: Applications to cation-ordering phase transitions and solid solutions. Miner. Mag. 2001, 65, 193–219. [Google Scholar] [CrossRef]
- Ling, Y.; Wang, K.; Wang, X.; Hua, S. Effects of mix design parameters on heat of geopolymerization, set time, and compressive strength of high calcium fly ash geopolymer. Constr. Build. Mater. 2019, 228, 116763. [Google Scholar] [CrossRef]
- Mohamed, R.; Abd Razak, R.; Abdullah, M.M.A.B.; Shuib, R.K.; Mortar, N.A.M.; Zailani, W.W.A. . Investigation of heat released during geopolymerization with fly ash based geopolymer. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; Volume 551, p. 012093. [Google Scholar]
- Nodehi, M.; Taghvaee, V.M. Alkali-activated materials and geopolymer: A review of common precursors and activators addressing circular economy. Circ. Econ. Sustain. 2022, 2, 165–196. [Google Scholar] [CrossRef]
- Autef, A.; Joussein, E.; Gasgnier, G.; Rossignol, S. Role of the silica source on the geopolymerization rate. J. Non-Cryst. 2012, 358, 2886–2893. [Google Scholar] [CrossRef]
- Bian, H.; Hannawi, K.; Takarli, M.; Molez, L.; Prince, W. Effects of thermal damage on physical properties and cracking behavior of ultrahigh-performance fiber-reinforced concrete. J. Mater. Sci. 2016, 51, 10066–10076. [Google Scholar] [CrossRef]
- Hager, I. Behaviour of cement concrete at high temperature. Bull. Pol. Acad. Sci. Tech. 2013, 60, 1–10. [Google Scholar] [CrossRef]
- Fletcher, I.A.; Welch, S.; Torero, J.L.; Carvel, R.O.; Usmani, A. Behaviour of concrete structures in fire. Therm. Sci. 2017, 11, 37–52. [Google Scholar] [CrossRef]
- Sakkas, K.; Panias, D.; Nomikos, P.; Sofianos, A. Comparison of fire resistant geopolymers for passive fire protection of concrete tunnel linings. Open Access Libr. J. 2017, 4, 1–15. [Google Scholar] [CrossRef]
- Abdullah, M.N.; Mustapha, F.; Ahmad, K.A.; Mustapha, M.; Khan, T.; Singh, B.; Sebaey, T.A. Effect of different pre-treatment on the microstructure and intumescent properties of rice husk ash-based geopolymer hybrid coating. Polymers 2022, 14, 2252. [Google Scholar] [CrossRef]
- Abdullah, M.M.A.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Ibrahim, W.M.W. Mechanism and chemical reaction of fly ash geopolymer cement—A review. Int. J. Pure Appl. Sci. Technol. 2011, 6, 35–44. [Google Scholar]
- Nijland, T.G.; Larbi, J.A. Microscopic examination of deteriorated concrete. In Non-Destructive Evaluation of Reinforced Concrete Structures; Woodhead Publishing: Cambridge, UK, 2010; pp. 137–179. [Google Scholar]
- He, R.; Dai, N.; Wang, Z. Thermal and mechanical properties of geopolymers exposed to high temperature: A literature review. Adv. Civ. Eng. 2020, 2020, 7532703. [Google Scholar] [CrossRef]
- Lahoti, M.; Wong, K.K.; Yang, E.H.; Tan, K.H. Effects of Si/Al molar ratio on strength endurance and volume stability of metakaolin geopolymers subject to elevated temperature. Ceram. Int. 2018, 44, 5726–5734. [Google Scholar] [CrossRef]
- Beh, J.H.; Yew, M.C.; Saw, L.H.; Yew, M.K. Fire resistance and mechanical properties of intumescent coating using novel bioash for steel. Coatings 2020, 10, 1117. [Google Scholar] [CrossRef]
- Baykara, H.; Cornejo, M.H.; Espinoza, A.; García, E.; Ulloa, N. Preparation, characterization, and evaluation of compressive strength of polypropylene fiber reinforced geopolymer mortars. Heliyon 2020, 6, e03755. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.K.; Kelly, S.; Yao, Z. Effect of fire exposure on cracking, spalling and residual strength of fly ash geopolymer concrete. Mater. Des. 2014, 63, 584–592. [Google Scholar] [CrossRef]
- Liew, K.M.; Sojobi, A.O.; Zhang, L.W. Green concrete: Prospects and challenges. Constr. Build. Mater. 2017, 156, 1063–1095. [Google Scholar] [CrossRef]





| Element | SiO₂ | Al₂O₃ | Fe₂O₃ | CaO | K2O | TiO2 | MgO | Others | LOI |
|---|---|---|---|---|---|---|---|---|---|
| PKSA | 46.41 | 7.74 | 0.89 | 11.83 | 13.87 | 1.95 | 5.93 | 2.12 | 9.26 |
| RHA | 87.40 | 3.00 | 0.03 | 0.02 | 0.82 | 0.08 | 0.05 | 0.13 | 8.47 |
| Ash | AA | NaOH | Na2SiO3 |
|---|---|---|---|
| 2 | 5 | 2 | 11 |
| Mix | Ratio (wt. in Gram) | |||||
|---|---|---|---|---|---|---|
| GP | Sand | PKSA/RHA | AA | NaOH | Na2SiO3 | |
| PKSA-GPC 40:60 | 80.00 | 120.00 | 22.86 | 57.14 | 8.78 | 48.36 |
| RHA-GPC 40:60 | ||||||
| PKSA-GPC 50:50 | 100.00 | 100.00 | 28.57 | 71.43 | 10.99 | 60.44 |
| RHA-GPC 50:50 | ||||||
| PKSA-GPC 60:40 | 120.00 | 80.00 | 34.29 | 85.71 | 13.19 | 72.52 |
| RHA-GPC 60:40 | ||||||
| PKSA-GPC 66.6:33.3 | 133.33 | 66.67 | 38.09 | 95.24 | 14.65 | 80.59 |
| RHA-GPC 66.6:33.3 | ||||||
| PKSA-GPC 70:30 | 140.00 | 60.00 | 40.00 | 100.00 | 15.38 | 84.62 |
| RHA-GPC 70:30 | ||||||
| PKSA-GPC 80:20 | 160.00 | 40.00 | 45.71 | 114.29 | 17.58 | 96.71 |
| RHA-GPC 80:20 | ||||||
| PKSA-GPC 90:10 | 180.00 | 20.00 | 51.43 | 128.57 | 19.78 | 108.79 |
| RHA-GPC 90:10 | ||||||
| Ratio (wt.%) | Observation | |||||
|---|---|---|---|---|---|---|
| GP | Sand | Mixing of GP | Mixing of GP and Sand | During Specimen Casting | ||
| Thermodynamic | Thermodynamic | Consistency | Rheology | Thixotropy | ||
| 90 | 10 | Exothermic | Endothermic | High | ✕ | ✕ |
| 80 | 20 | Exothermic | Endothermic | High | ✕ | ✕ |
| 70 | 30 | Exothermic | Endothermic | High | ✓ | ✓ |
| 66.6 | 33.3 | Exothermic | Endothermic | High | ✓ | ✓ |
| 60 | 40 | Exothermic | Endothermic | High | ✓ | ✓ |
| 50 | 50 | Exothermic | Endothermic | High | ✓ | ✓ |
| 40 | 60 | Exothermic | Endothermic | Low | ✕ | ✕ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, M.N.; Mustapha, F.; Yusof, N.‘I.; Khan, T.; Sebaey, T.A. Thermal Properties and Drying Shrinkage Performance of Palm Kernel Shell Ash and Rice Husk Ash-Based Geopolymer Concrete. Materials 2024, 17, 1298. https://doi.org/10.3390/ma17061298
Abdullah MN, Mustapha F, Yusof N‘I, Khan T, Sebaey TA. Thermal Properties and Drying Shrinkage Performance of Palm Kernel Shell Ash and Rice Husk Ash-Based Geopolymer Concrete. Materials. 2024; 17(6):1298. https://doi.org/10.3390/ma17061298
Chicago/Turabian StyleAbdullah, Mohd Na’im, Faizal Mustapha, Nurul ‘Izzati Yusof, Tabrej Khan, and Tamer A. Sebaey. 2024. "Thermal Properties and Drying Shrinkage Performance of Palm Kernel Shell Ash and Rice Husk Ash-Based Geopolymer Concrete" Materials 17, no. 6: 1298. https://doi.org/10.3390/ma17061298








