Degradation of RC Columns under Combined Exposure to Axial Loading, Stray Currents, and Chloride Ingress
Abstract
:1. Introduction
2. Research Scope and Goals
- Effect of the serviceability compressive axial load on the crack formation
- Examination of the threshold of chloride concentration
- Effect of the concrete cover thickness
3. Experimental Program
3.1. Materials
3.2. Casting and Curing
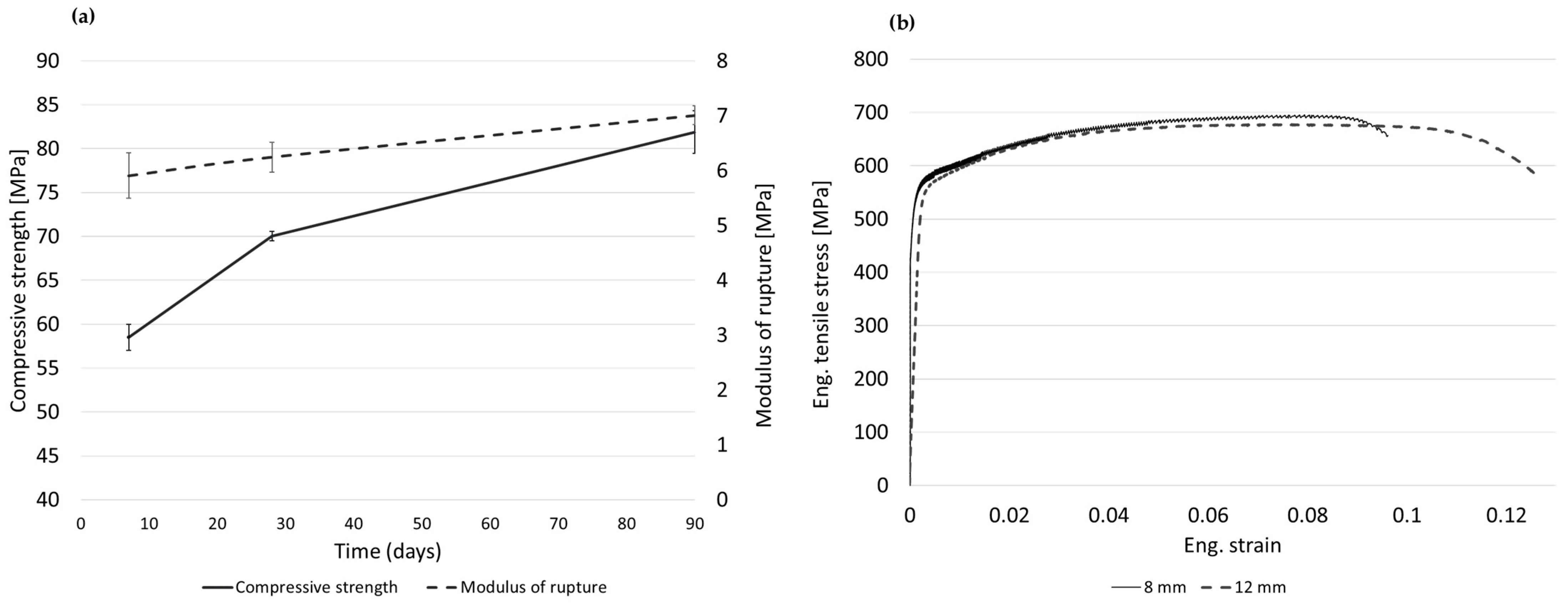
3.3. Mechanical Properties of Concrete and Steel Reinforcement
3.4. Methods
- Chemical reactions chloride-containing zone and chemical reactions in the chloride-free zone
- Reducing the concentration of free chloride near the reinforcing steel will reduce the risk of corrosion;
- Chloride binding will inhibit chloride penetration;
- The samples were initially manually ground to achieve a particle size of less than 75 microns.
- The XRD analysis utilized a Malvern Panalytical EMPYREAN X-ray diffractometer under specific measurement conditions: a CuKα1,2 X-ray source (λ = 1.5406 Å), with the X-ray generator operating at 45 kV and 40 mA, and a Goniometer radius of 240 mm. The incident beam optics comprised a ¼° divergence slit, 10 mm mask, 0.04 rad Soller slit, and 1° anti-scatter fixed slit. The diffracted beam optics included an 8 mm anti-scatter fixed slit and a 0.04 rad Soller slit. The detection system utilized a PIXcel 3D detector in a 1D continuous scan mode. Scanning was performed in Brag–Brentano geometry, covering a 2𝜃 range from 10° to 65° with 0.026° steps and a counting time of 84 s/step.
- The XRD data were analyzed using Panalytical HighScore Plus software (version 5.1), referencing ICDD PDF-4 Minerals and PAN-ICSD databases.
4. Results
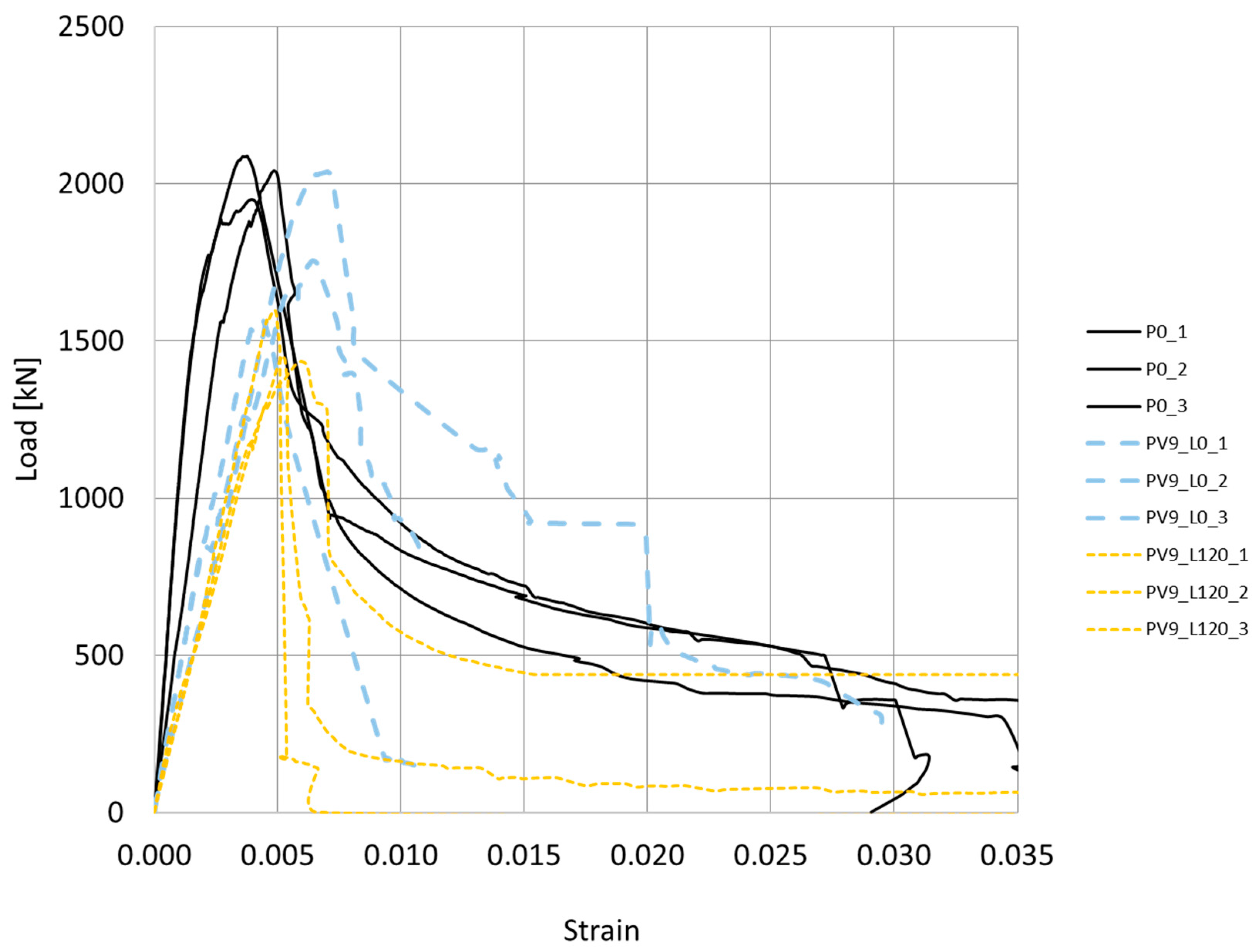
4.1. Reinforced Concrete Columns
4.1.1. Effect of Corrosion
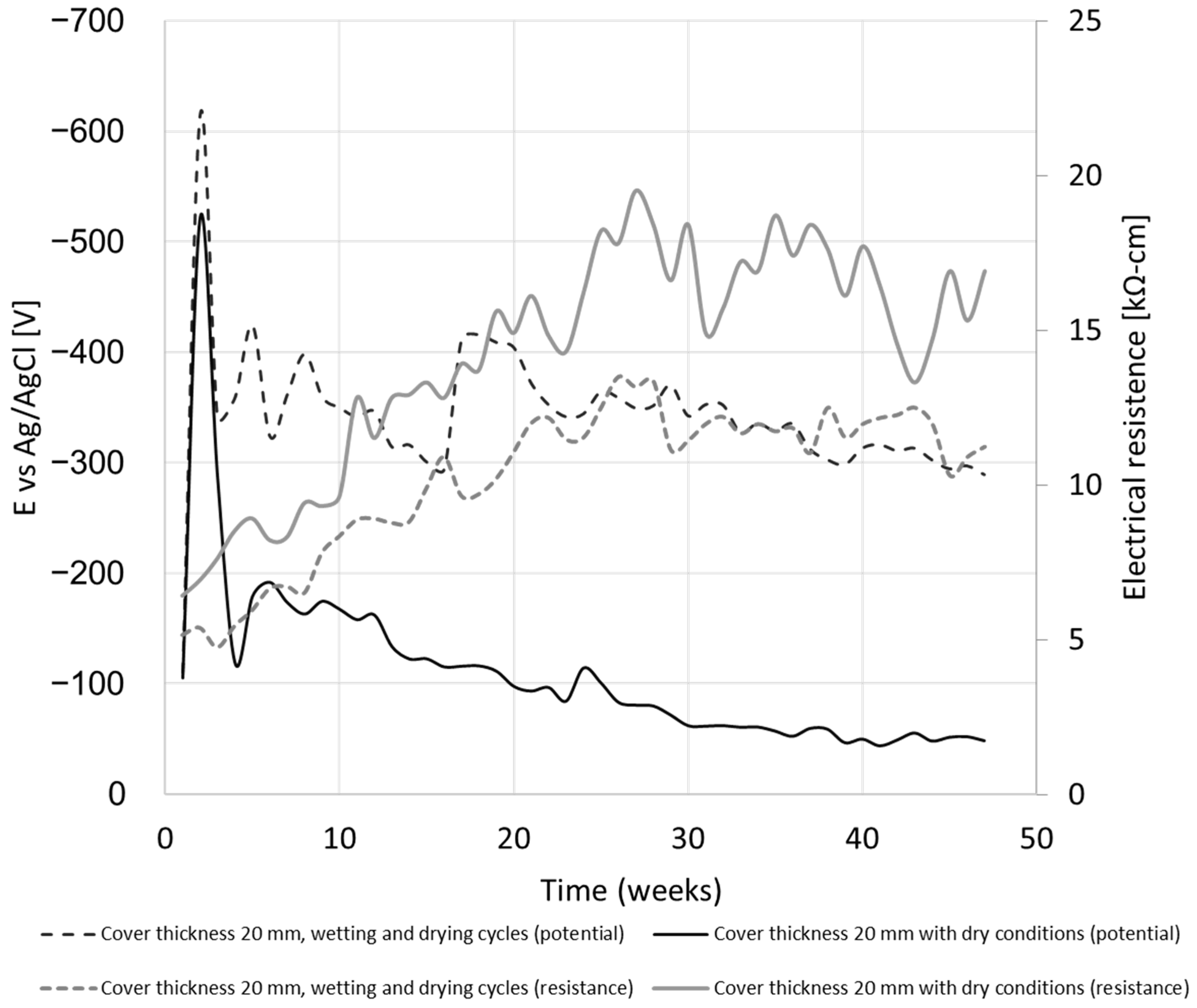
4.1.2. Effect of Wetting and Drying Cycles
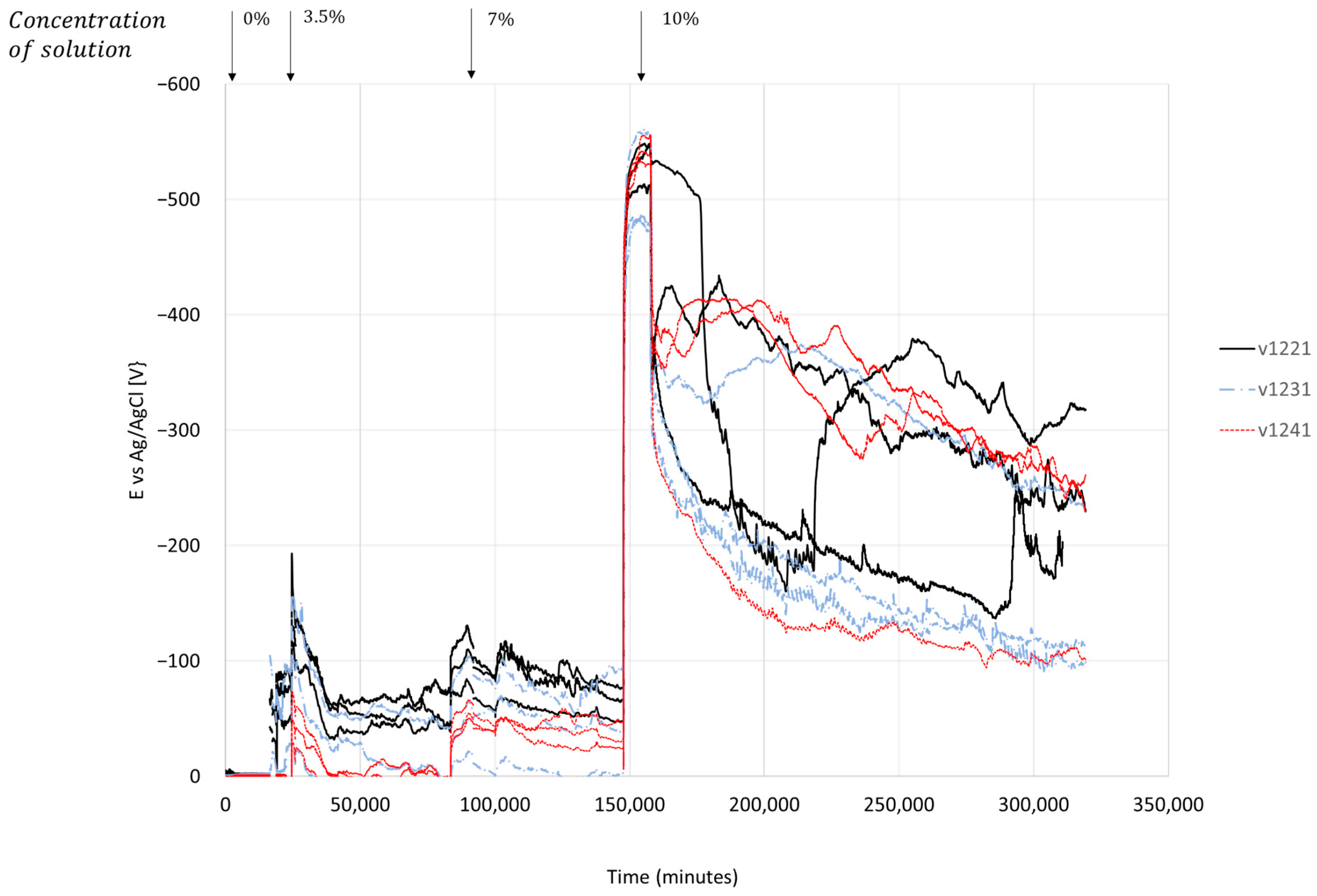
4.1.3. Electrochemical Behavior
4.2. Reinforced Concrete Disc Specimens
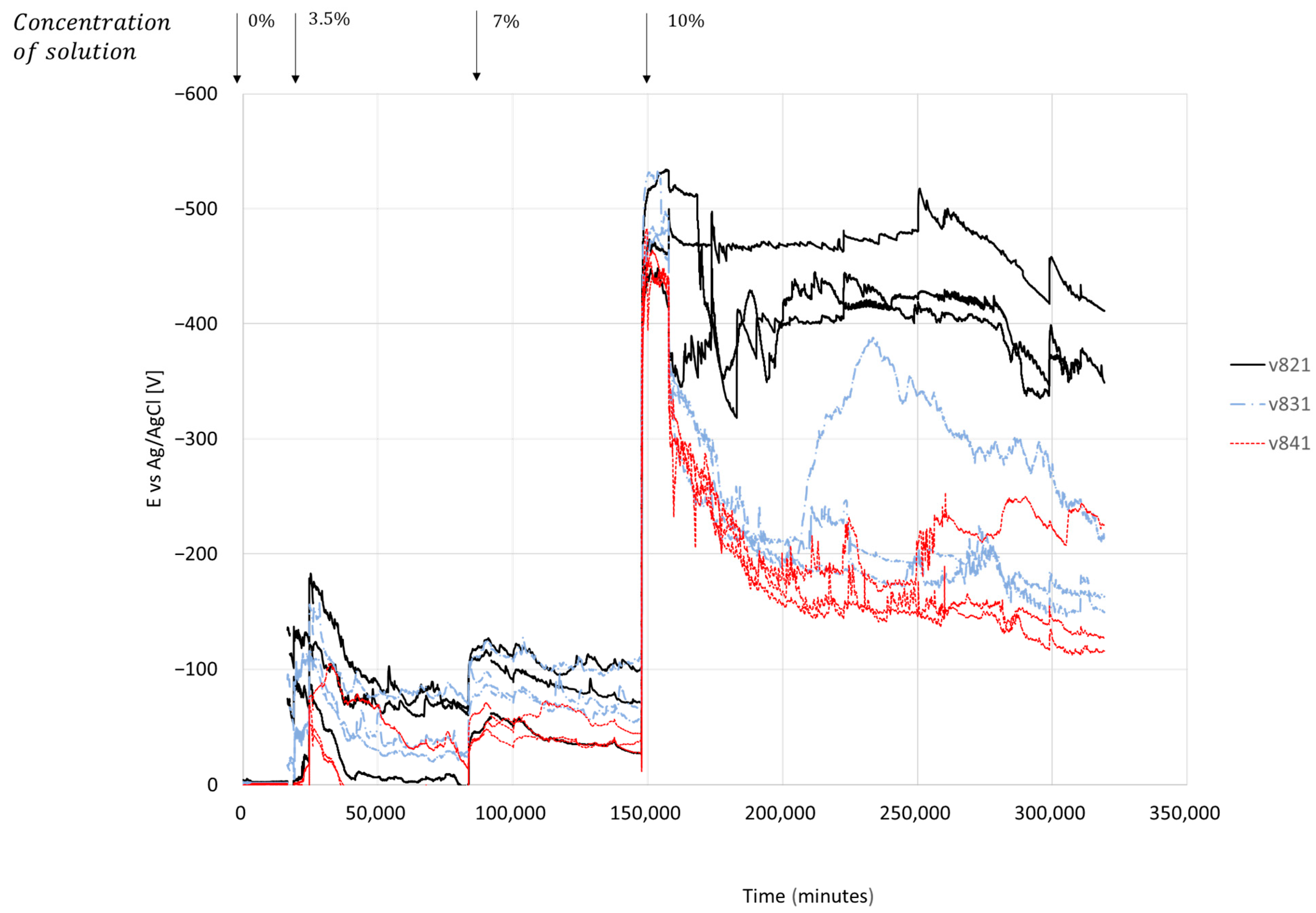
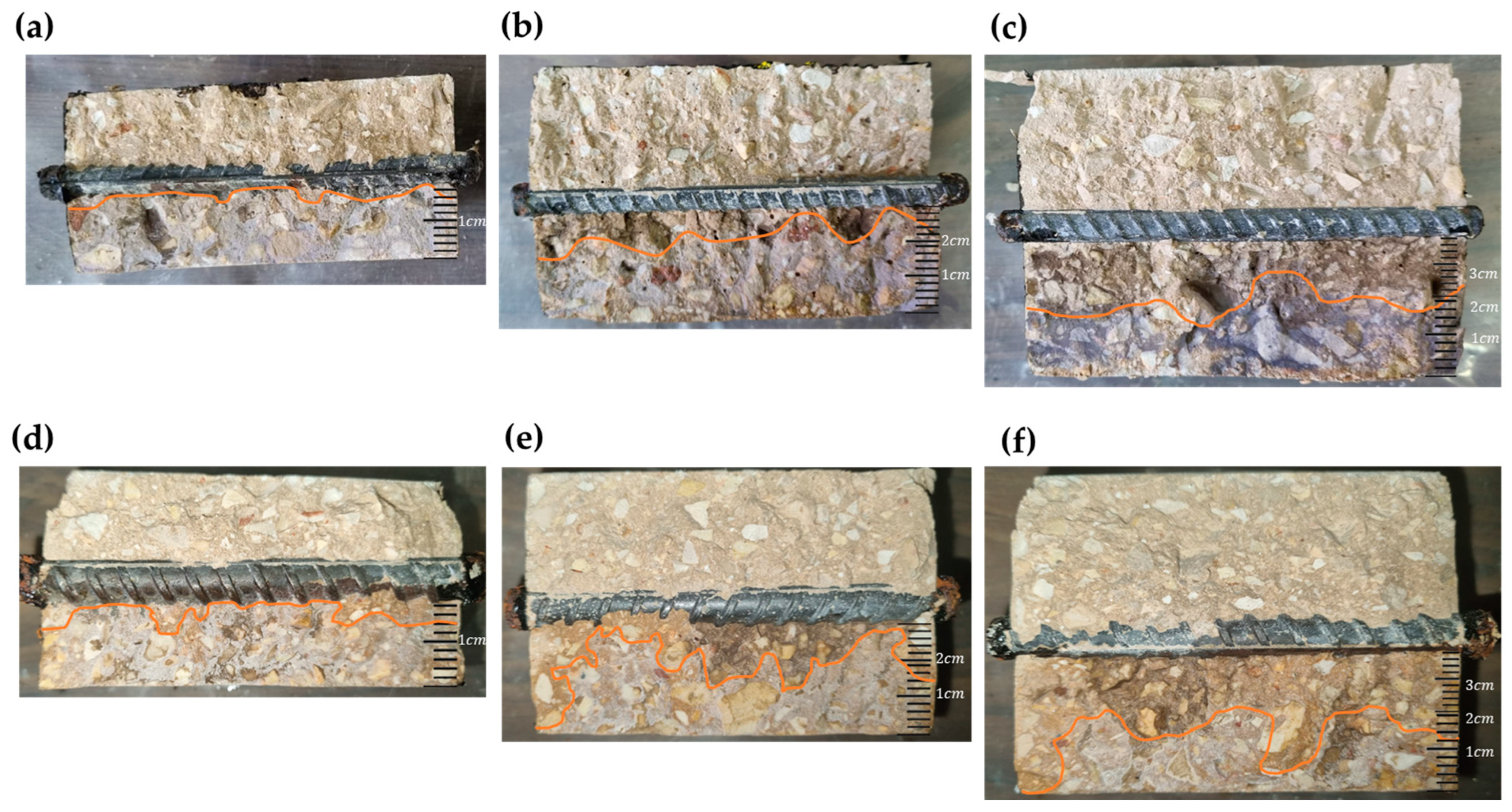
4.2.1. Effect of Concrete Cover Thickness and Surface Area of Steel Bars
Half-Cell Potential of Reinforcing Steel
Chloride Permeability by Colorimetric Method
Determination of Chloride Ion Concentration by Titration
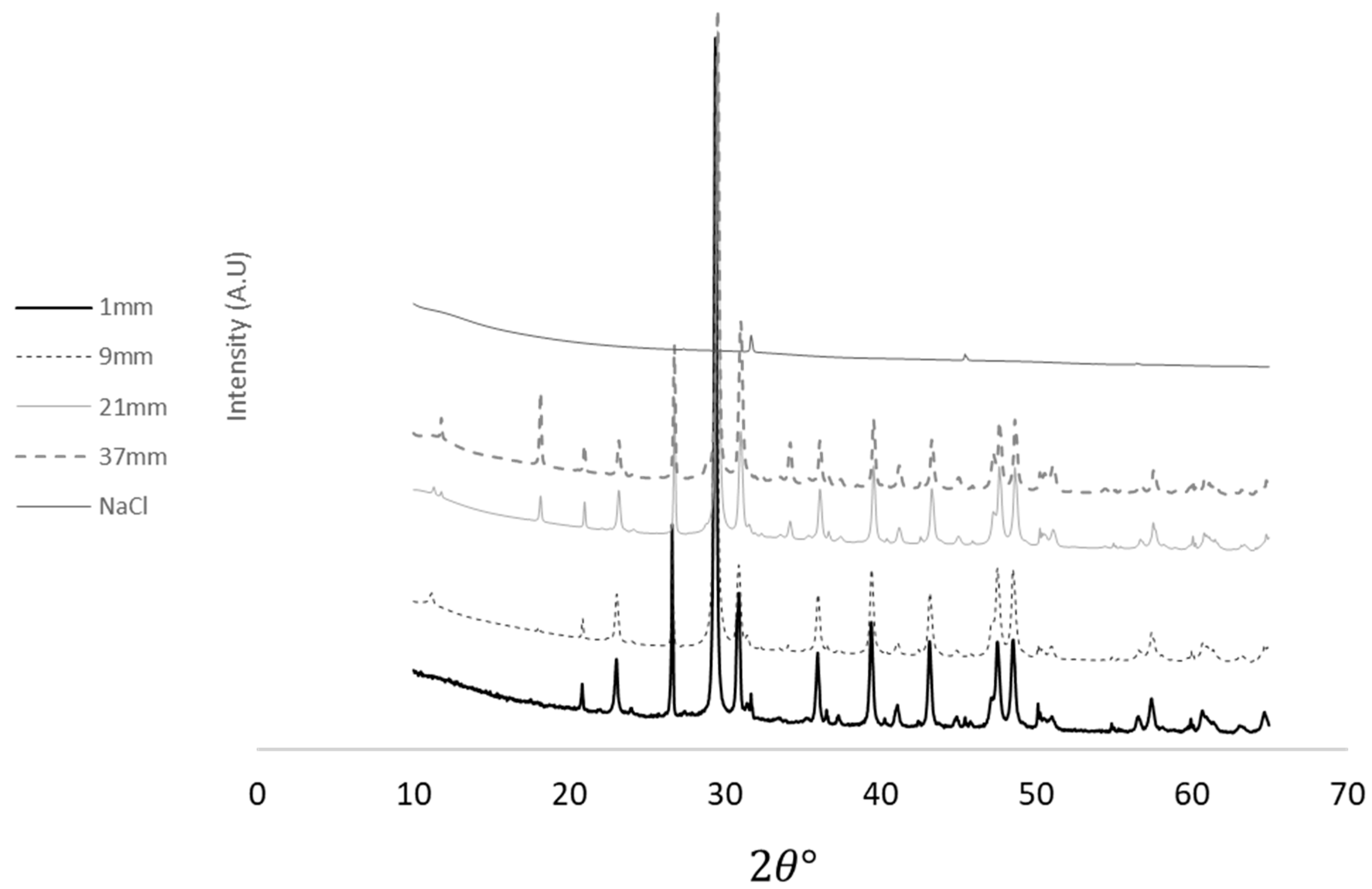
X-ray Diffraction Investigation
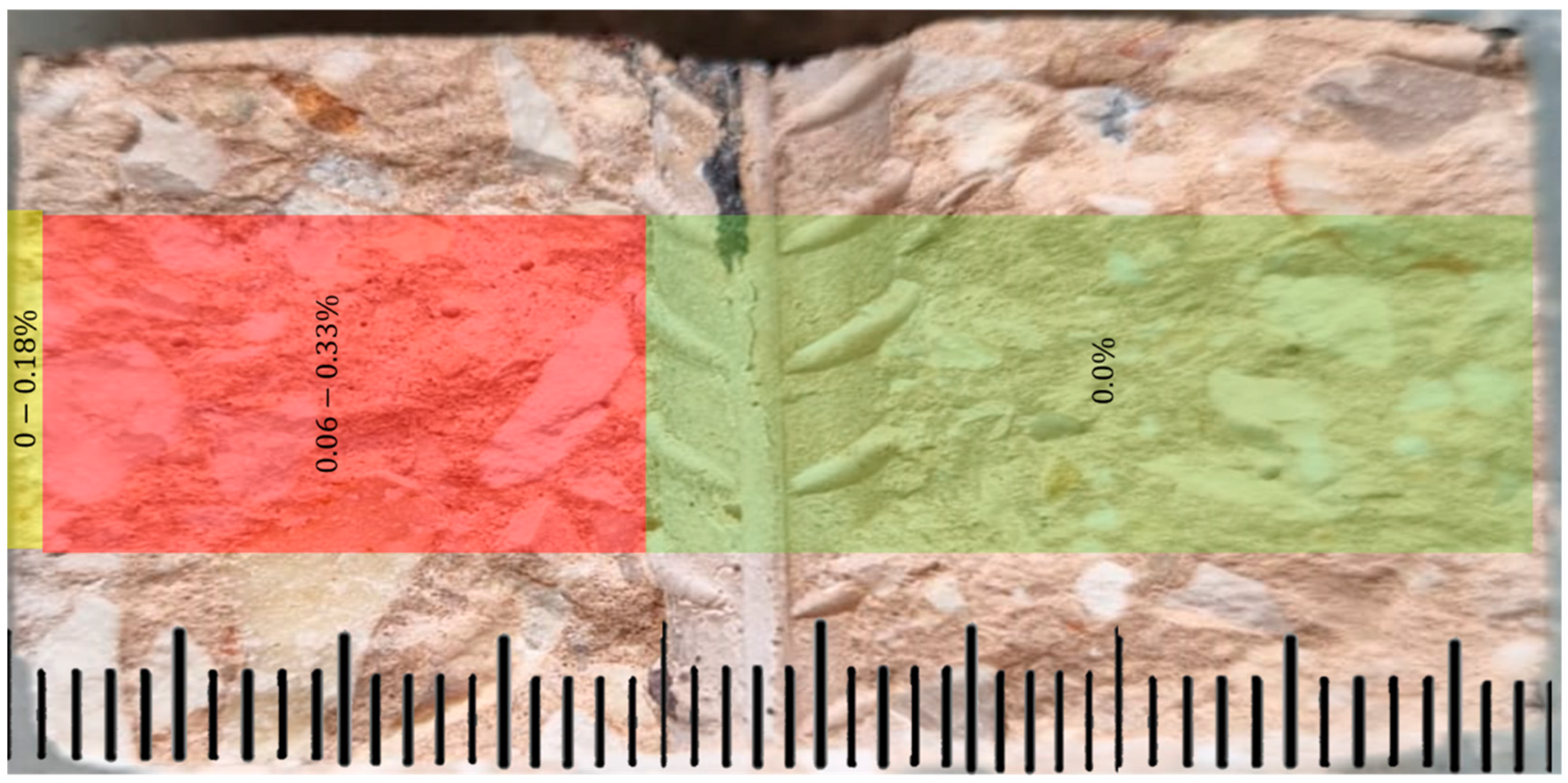
Analysis by EDS to Detect Chlorine Ions
5. Discussion
6. Conclusions
- The stray current affects the corrosion process, leading to a reduction in the structural properties of reinforced concrete columns in terms of strength and ductility.
- An electrical voltage of 9 V, simulating stray voltage, and an aggressive chloride environment were applied to test samples, resulting in an 11.85% decrease in bearing capacity and a 12.8% reduction in ductility (from μ = 1.94 to μ = 1.69). Further testing under a service load of 1200 kN showed more pronounced effects, with a 34.7% decrease in bearing capacity and a 41.7% reduction in ductility.
- For a combination of constant axial load alongside environmental conditions involving stray currents and chloride attacks, a reduction in the column’s maximum load-carrying capacity is observed. The reduction is less pronounced in terms of ductility (compared to the unloaded specimens). These results are attributed to the formation of early cracks and their subsequent closure upon the application of compressive axial loading.
- The amount of steel in the concrete and the surface area of the steel bar contribute to a higher concentration of chloride ions in the area at the interface between the concrete and the bar. It increases the electrical conductivity of the reinforced concrete element. This process accelerates the rate of corrosion while creating chemical bonds with the corrosion products and chlorine ions. Reinforced concrete samples with 12 mm diameter bars exhibit a 22% higher chloride penetration at the interface compared to samples with 8 mm diameter bars, with a cover thickness of 20 mm, as indicated by the results of the AgNO3 test.
- An electrochemical reaction initiates at the concrete-bar interface after a change in chloride concentration across the specimen surface. Consequently, under moist conditions, the onset of corrosion is not influenced by permeability time.
- Under the given conditions, no influence of the concrete cover thickness on the electrochemical reactions of the reinforcement bars inside the concrete was observed. Consequently, based on these findings, it does not provide adequate protection against stray currents.
- In future work, the aim is to investigate stray currents in a realistic scenario and to evaluate an existing model that can predict the service life of buildings near railway tracks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barlo, T.J.; Zdunek, A.D. Stray Current Corrosion in Electrified Rail Systems—Final Report; U.S. Department of Transportation: Washington, DC, USA, 1995.
- National Academies of Sciences, Engineering, and Medicine. Stray Current Control of Direct Current-Powered Rail Transit Systems: A TCRP Research Report 212 Stray Current Control of Direct Current-Powered Rail Transit Systems: A Guidebook; National Academic Press: Washington, DC, USA, 2020; ISBN 9780309481137. [Google Scholar]
- Creel, L. Ripple Effects: Population and Coastal Regions; Population Reference Bureau: Washington, DC, USA, 2003; pp. 1–8. [Google Scholar]
- Liu, J.; Jiang, Z.; Zhao, Y.; Zhou, H.; Wang, X.; Zhou, H.; Xing, F.; Li, S.; Zhu, J.; Liu, W. Chloride Distribution and Steel Corrosion in a Concrete Bridge after Long-Term Exposure to Natural Marine Environment. Materials 2020, 13, 3900. [Google Scholar] [CrossRef] [PubMed]
- Neville, A. Chloride Attack of Reinforced Concrete: An Overview. Mater. Struct. 1995, 28, 63–70. [Google Scholar] [CrossRef]
- Liu, F.; Zou, Y.; Wang, B.; Yuan, X. The Effect of Stray Current on Calcium Leaching of Cement-Based Materials. Materials 2022, 15, 2279. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, B.; Panesar, D.K. Effect of Stray Current on Cement-Based Materials under Sulfate Attack. J. Mater. Civ. Eng. 2022, 34, 4021439. [Google Scholar] [CrossRef]
- Ni, Y.; Zhu, E. Combined Effect of Stray Current and Sustained Compressive Loading on Chloride Transport in Concrete. Adv. Mater. Sci. Eng. 2021, 2021, 9975168. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical Chloride Content in Reinforced Concrete—A Review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- BS EN 50122-2:2010; Railway Applications. Fixed Installations. Electrical Safety, Earthing and the Return Circuit. Provisions against the Effects of Stray Currents Caused by d.c. Traction Systems. BSI: London, UK, 2010.
- ASTM-G165-99-R 2012; Standard Practice for Determining Rail-to-Earth Resistance. ASTM International: West Conshohocken, PA, USA, 2012.
- Bongiorno, J.; Mariscotti, A. Track Insulation Verification and Measurement. In Proceedings of the MATEC Web of Conferences, Abu Dhabi, United Arab Emirates, 20–22 November 2018; Volume 180, p. 01008. [Google Scholar]
- Markiewicz, H.; Klajn, A. Earthing & EMC Earthing Systems—Basic Constructional Aspects; Copper Development Association: Diepoldsau, Switzerland, 2004. [Google Scholar]
- Wu, P.; Zhu, X.; Xu, L.; Peng, W.; Zhao, G. Effect of Stray Current Coupled with Chloride Concentration and Temperature on the Corrosion Resistance of a Steel Passivation Film. Electrochem. Commun. 2020, 118, 106793. [Google Scholar] [CrossRef]
- Angst, U.M. Challenges and Opportunities in Corrosion of Steel in Concrete. Mater. Struct. Constr. 2018, 51, 1–20. [Google Scholar] [CrossRef]
- Aldea, C.-M.; Shah, S.P.; Karr, A. Effect of Cracking on Water and Chloride Permeability of Concrete. J. Mater. Civ. Eng. 1999, 11, 181–187. [Google Scholar] [CrossRef]
- Al-Mahaidi, R.; Kalfat, R. Introduction. In Rehabilitation of Concrete Structures with Fiber-Reinforced Polymer; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–5. [Google Scholar] [CrossRef]
- CEB-FIP. Model Code 2010; CEB-FIP: Lausanne, Switzerland, 2010; ISBN 9783433604090. [Google Scholar]
- Li, Q.; Dong, Z.; He, Q.; Fu, C.; Jin, X. Effects of Reinforcement Corrosion and Sustained Load on Mechanical Behavior of Reinforced Concrete Columns. Materials 2022, 15, 3590. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Zhang, C.; Pei, S.; Jin, Z. Modeling the Impact of Corrosion on Seismic Performance of Multi-Span Simply-Supported Bridges. Constr. Build. Mater. 2018, 185, 193–205. [Google Scholar] [CrossRef]
- De Domenico, D.; Messina, D.; Recupero, A. Seismic Vulnerability Assessment of Reinforced Concrete Bridge Piers with Corroded Bars. Struct. Concr. 2023, 24, 56–83. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, L.; Koleva, D.A. Evaluating the Stray Current Corrosion of Steel Rebar in Different Layouts. Meas. J. Int. Meas. Confed. 2022, 196, 111217. [Google Scholar] [CrossRef]
- Yao, W.; Xu, J. Current Distribution in Reinforced Concrete Cathodic Protection System. J. Tongji Univ. 2009, 37, 1014–1018. [Google Scholar]
- ACI Committee 222. Protection of Metals in Concrete Against Corrosion; ACI 222R-01; American Concrete Institute: Farmington Hills, MI, USA, 2001; pp. 1–41. [Google Scholar]
- ACI Committee 318. Building Code Requirements for Structural Concrete (ACI 318-19): An ACI Standard: Commentary on Building Code Requirements for Structural Concrete (ACI 318R-19); American Concrete Institute: Farmington Hills, MI, USA, 2019. [Google Scholar]
- The European Union. Eurocode 2: Design of Concrete Structures-Part 1–1: General Rules and Rules for Buildings; British Standards Institution: London, UK, 2005. [Google Scholar]
- ASTM C666-97; Standard Test Method for Resistance of Concrete to Rapid Freezing and Thawing. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM G109 (2013); Standard Test Method for Determining Effects of Chemical Admixtures on Corrosion of Embedded Steel Reinforcement in Concrete Exposed to Chloride Environments. ASTM International: West Conshohocken, PA, USA, 2013.
- BS EN 50122-1: 2022; Railway Applications. Fixed Installations. Protective Provisions Relating to Electrical Safety and Earthing. BSI: London, UK, 2022.
- Büchler, M. On the Mechanism of Cathodic Protection and Its Implications on Criteria Including AC and DC Interference Conditions. Corrosion 2020, 76, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, H.; Klajn, A. Power Quality Application Guide Earthing & EMC Earthing Systems-Fundamentals of Calculation and Design; Copper Development Association (IEE Endorsed Provider): McLean, VA, USA, 2003. [Google Scholar]
- Ontario Energy Board. Farm Stray Voltage: Issues and Regulatory Options; Staff Discussion Paper; Ontario Energy Board: Toronto, ON, Canada, 2008.
- Siranec, M.; Regula, M.; Otcenasova, A.; Altus, J. Measurement and Analysis of Stray Currents. In Proceedings of the 2019 20th International Scientific Conference on Electric Power Engineering (EPE 2019), Kouty nad Desnou, Czech Republic, 15–17 May 2019; pp. 1–6. [Google Scholar]
- Burke, J. The Confusion Surrounding “Stray Voltage”. In Proceedings of the Papers Presented at the Annual Conference—Rural Electric Power Conference, Rapid City, SD, USA, 6–8 May 2007; pp. 0–5. [Google Scholar]
- Denardo, C. Stray and Contact Voltage An Update on IEEE PES P1695 Working Group Activities. In Proceedings of the 2012 Rural Electric Power Conference, Milwaukee, WI, USA, 15–17 April 2012; pp. A3-1–A3-7. [Google Scholar] [CrossRef]
- SI 26; Part 2.1: Testing Concrete: Fresh Concrete—Consistency—Slump Test. The Standards Institution of Israel: Tel Aviv, Israel, 2022.
- BS EN 10080-2005; Steel for the Reinforcement of Concrete—Weldable Reinforcing Steel. British Standards: London, UK, 2005.
- BS EN 12390-1:2012; Testing Hardened Concrete—Part 1: Shape, Dimensions and Other Requirements for Specimens and Moulds. BSI: London, UK, 2012.
- ASTM C876-15; Standard Test Method for Corrosion Potentials of Uncoated Reinforcing Steel in Concrete. ASTM International: West Conshohocken, PA, USA, 2015; Volume 3.
- Elsener, B.; Andrade, C.; Gulikers, J.; Polder, R.; Raupach, M. Half-Cell Potential Measurements—Potential Mapping on Reinforced Concrete Structures. Mater. Struct. Constr. 2003, 36, 461–471. [Google Scholar] [CrossRef]
- Kendall, A.; Keoleian, G.A.; Helfund, G.E. Integrated Life Cycle Assessment and Life Cycle Cost Analysis Model for Concrete Bridge Deck Applications. J. Infrastruct. Syst. 2008, 14, 214–222. [Google Scholar] [CrossRef]
- AASHTO T 358; Surface Resistivity Indication of Concrete’s Ability to Resist Chloride Ion Penetration. AASHTO: Washington, DC, USA, 2015; pp. 1–9.
- Angst, U.M.; Boschmann, C.; Wagner, M.; Elsener, B. Experimental Protocol to Determine the Chloride Threshold Value for Corrosion in Samples Taken from Reinforced Concrete Structures. J. Vis. Exp. 2017, 2017, e56229. [Google Scholar] [CrossRef]
- He, F.; Shi, C.; Yuan, Q.; Chen, C.; Zheng, K. AgNO3-Based Colorimetric Methods for Measurement of Chloride Penetration in Concrete. Constr. Build. Mater. 2012, 26, 1–8. [Google Scholar] [CrossRef]
- Maes, M.; De Belie, N. Resistance of Concrete and Mortar against Combined Attack of Chloride and Sodium Sulphate. Cem. Concr. Compos. 2014, 53, 59–72. [Google Scholar] [CrossRef]
- Tex-617-J; Determining Chloride in Concrete. Texas Department of Transportation: Houston, TX, USA, 2005.
- BS EN 14629; Products and Systems for the Protection and Repair of Concrete the Sand Column Test. BSI: London, UK, 2004.
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781498738675. [Google Scholar]
- Lapiro, I.; Mezhov, A.; Kovler, K. Performance of Corrosion Inhibitors in Reinforced Concrete Elements under Electrical Voltage. Constr. Build. Mater. 2022, 342, 127656. [Google Scholar] [CrossRef]
- Paultre, P.; Asce, M.; Eid, R.; Langlois, Y.; Lévesque, Y. Behavior of Steel Fiber-Reinforced High-Strength Concrete Columns under Uniaxial Compression. J. Struct. Eng. 2010, 136, 1225–1235. [Google Scholar] [CrossRef]
- BS EN 50162:2004; Protection against Corrosion by Stray Current from Direct Current Systems. BSI: London, UK, 2004; pp. 1–3.
- Paul, D. DC Traction Power System Grounding. IEEE Trans. Ind. Appl. 2002, 38, 818–824. [Google Scholar] [CrossRef]
- Pham, K.D.; Thomas, R.S.; Stinger, W.E. Operational and Safety Considerations for Light Rail DC Traction Electrification System Design. In Proceedings of the 9th National Light Rail Transit Conference, Portland, OR, USA, 16–18 November 2003; pp. 650–668. [Google Scholar]
- Aminulai, H.O.; Robinson, A.F.; Ferguson, N.S.; Kashani, M.M. Impact of Corrosion on Axial Load Capacity of Ageing Low-Strength Reinforced Concrete Columns with Different Confinement Ratios. Constr. Build. Mater. 2023, 384, 131355. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Q.; Zhao, Y.; Li, P.; Ji, T.; Zou, G.; Qiao, Y.; Zhou, Z.; Wang, G.; Song, D. Research Progress of Macrocell Corrosion of Steel Rebar in Concrete. Coatings 2023, 13, 853. [Google Scholar] [CrossRef]
- Kim, J.K.; Kee, S.H.; Futalan, C.M.; Yee, J.J. Corrosion Monitoring of Reinforced Steel Embedded in Cement Mortar under Wet-and-Dry Cycles by Electrochemical Impedance Spectroscopy. Sensors 2020, 20, 199. [Google Scholar] [CrossRef]
- Sarja, A.; Vesikari, E. (Eds.) Durability Design of Concrete Structures (Rilem Report); CRC Press: Boca Raton, FL, USA, 1996; ISBN 0419214100. [Google Scholar]
- Zhao, Y.; Jin, W. Steel Corrosion in Concrete. Steel Corros. Concr. Crack. 2016, 19–29. [Google Scholar] [CrossRef]
- Feng, X.; Zuo, Y.; Tang, Y.; Zhao, X.; Zhao, J. The Influence of Strain on the Passive Behavior of Carbon Steel in Cement Extract. Corros. Sci. 2012, 65, 542–548. [Google Scholar] [CrossRef]





| Content (kg/m3) | W/C 3 | Air Content (%) | Unit Weight (kg/m3) | Slump 4 (mm) | |||||
|---|---|---|---|---|---|---|---|---|---|
| C 1 | W 1 | S 1 | Aggregates 2 | ||||||
| Fine | Coarse | Maximum Aggregate Size | |||||||
| (mm) | |||||||||
| 500 | 195 | 5 | 1100 | 500 | 9 | 0.39 | 1.9 | 2281 | S5 (138) |
| Chemical Component | Sample Distance from the Surface (mm) | ||||
|---|---|---|---|---|---|
| 1 | 9 | 21 | 37 | ||
| %Content | |||||
| Calicite | 76.7 | 82.2 | 71.9 | 58.1 | |
| Dolomite | 14.3 | 10.9 | 15.9 | 22.7 | |
| Quartz | 8.2 | 5.3 | 8 | 9.2 | |
| Halite | 0.8 | 0.1 | 0.1 | 0.1 | |
| Portlandite | Ca(OH)2 | 0.5 | 2.5 | 6.2 | |
| Hydrocalumite | - | 0.1 | 0.3 | - | |
| Forsterite | 0.9 | 0.6 | 0.8 | ||
| Tetracalcium Dialuminium Dodecahydroxide Carbonate Pentahydrate | - | 0.7 | 2.3 | ||
| Vesuvianite | - | - | 0.6 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapiro, I.; Eid, R.; Kovler, K. Degradation of RC Columns under Combined Exposure to Axial Loading, Stray Currents, and Chloride Ingress. Materials 2024, 17, 1295. https://doi.org/10.3390/ma17061295
Lapiro I, Eid R, Kovler K. Degradation of RC Columns under Combined Exposure to Axial Loading, Stray Currents, and Chloride Ingress. Materials. 2024; 17(6):1295. https://doi.org/10.3390/ma17061295
Chicago/Turabian StyleLapiro, Igor, Rami Eid, and Konstantin Kovler. 2024. "Degradation of RC Columns under Combined Exposure to Axial Loading, Stray Currents, and Chloride Ingress" Materials 17, no. 6: 1295. https://doi.org/10.3390/ma17061295






